Complement-targeted therapeutics (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 31.
Published in final edited form as: Nat Biotechnol. 2007 Nov;25(11):1265–1275. doi: 10.1038/nbt1342
Abstract
The complement system is a central component of innate immunity and bridges the innate to the adaptive immune response. However, it can also turn its destructive capabilities against host cells and is involved in numerous diseases and pathological conditions. Modulation of the complement system has been recognized as a promising strategy in drug discovery, and a large number of therapeutic modalities have been developed. However, successful marketing of complement-targeted drugs has proved to be more difficult than initially expected, and many strategies have been discontinued. The US Food and Drug Administration’s approval of the first complement-specific drug, an antibody against complement component C5 (eculizumab; Soliris), in March 2007, was a long-awaited breakthrough in the field. Approval of eculizumab validates the complement system as therapeutic target and might facilitate clinical development of other promising drug candidates.
When the bactericidal properties of certain heat-labile blood components were initially described more than a century ago1, no one could have imagined the considerable impact the complement system would have on both immunology and our understanding of many disease processes. Even the name “complement,” originally coined by Paul Ehrlich2, implied only a supplementary role of this system in the defense against microbial intruders. However, continued progress in research has led to a dramatic change in our knowledge about the complement system and moved it into the spotlight of basic and applied life sciences.
Today it is clear that complement is a key player of the innate immune system. However, this integral position in the maintenance and regulation of immune and inflammation reactions also makes it a trigger point for a variety of pathologic conditions. Erroneous activation or insufficient regulation of the complement cascade may turn its destructive actions against the host’s cells. As a consequence, many inflammatory and autoimmune diseases are thought to be caused, or at least supported, by unleashed complement. Inhibition or modulation of complement activity has therefore been recognized as a promising therapeutic strategy for many years.
Although many creative attempts to design complement-specific drugs have been made in recent decades, their development from experimental concept to clinical product has faced many obstacles. In light of these complications, most of the major pharmaceutical companies seem to have abandoned their initial efforts to develop drugs that target complement. Despite its bitter taste, this decision has encouraged a series of small startup companies in the biotechnology field to take up the challenge. The first results of this commitment are now visible and may well lead to a revival of this difficult but promising area of drug discovery. With its 2007 approval of an antibody against complement component C5 (eculizumab; Alexion, Cheshire, CT, USA), the US Food and Drug Administration (FDA) has now authorized the first complement-specific drug3. Many other promising drug candidates and therapeutic strategies are currently in the pipeline of various companies. The aim of this review is to provide an overview of current therapeutic strategies, with a focus on drug candidates that are in clinical trials (Table 1) or late preclinical development (Table 2).
Table 1.
Complement therapeutics on the market or in clinical trials
| Product (company) | Activity | Stage of development |
|---|---|---|
| Protease inhibitors (A) | ||
| C1-INH (Cetor/Sanquin, BerinertP/CSL Behring, Lev Pharma) | Purified plasma protein; inhibition of C1r/C1s, kallikrein and other proteases | Marketed (HAE)a; clinical phase 3 (HAE)a; preclinical/phase 1 for other indications (AMI, CABG) |
| Rhucin/rhC1INH (Pharming Group N.V.) | Recombinant human C1-INH from transgenic rabbits | Clinical phase 3 (HAE) |
| Soluble complement regulators (B) | ||
| sCR1/TP10 (Avant Immunotherapeutics) | Extracellular part of CR1; decay accelerator, factor I cofactor | Clinical phase 2 (CABG)b |
| CAB-2/MLN-2222 (Millenium Pharmaceuticals) | Chimera of DAF and MCP; decay accelerator, factor I cofactor | Clinical phase 1 (CABG)b |
| Therapeutic antibodies (C) | ||
| Eculizumab/Soliris (Alexion Pharmaceuticals) | Humanized long-acting mAb against C5 | Marketed (PNH), preclinical for other indications |
| Pexelizumab (Alexion Pharmaceuticals) | Humanized short-acting mAb against C5 | Clinical phase 3 (AMI, CABG)b |
| Ofatumumab (Genmab A/S) | Humanized anti-CD20 mAb | Clinical phase 2 |
| Complement component inhibitors (D) | ||
| Compstatin/POT-4 (Potentia Pharmaceuticals) | Peptidic C3 inhibitor; no generation of C3a/C3b | Clinical phase 1 (AMD) |
| Receptor antagonists (E) | ||
| PMX-53 (Peptech Ltd.) | Peptidic C5aR antagonist | Clinical phase 2 (RA, psoriasis) |
| Other | ||
| rhMBL (Enzon Pharmaceuticals) | Recombinant human MBL as substitution therapy | Clinical phase 1b (MBL deficiency) |
Table 2.
Complement therapeutics in pre-clinical development
| Product (company) | Activity | Stage of development |
|---|---|---|
| Protease inhibitors (A) | ||
| Factor D inhibitorsa (BCX1470 etc.) | Small molecule serine protease inhibitors | Mostly discontinued (due to lack of specificity and/or short half-life) |
| Soluble complement regulators (B) | ||
| sCR1-sLex/TP-20 (Avant Immunotherapeutics) | sCR1 with sLex-rich glycosylation to target sites of inflammation | Development/preclinical (stroke, heart attack) |
| Mirococept (Inflazyme Pharmaceuticals) | sCR1 with lipopeptide membrane linker | Preclinical (AMI, inflammatory diseases) |
| Therapeutic antibodies (C) | ||
| TNX-234 (Tanox) | Humanized antibody against factor D | Preclinical (wet AMD) |
| TNX-558 (Tanox) | Humanized antibody against C5a | Development/preclinical (inflammatory diseases) |
| TA106 (Taligen Therapeutics) | Antibody against factor B | Development/preclinical |
| Neutrazumab (G2 Therapies) | Antibody blocking the C5a receptor | Development/preclinical (RA, stroke) |
| Anti-properdin (Novelmed Therapeutics) | Antibody against properdin | Development/preclinical |
| HuMax-CD38 (Genmab A/S) | Humanized anti-CD38 mAb that triggers complement | Preclinical studies (cancer, multiple myeloma) |
| Complement component inhibitors (D) | ||
| ARC1905 (Archemix) | Aptamer-based C5 inhibitor (PEG-ylated) | Preclinical (AMD) |
| Receptor antagonists (E) | ||
| JPE-1375, JSM-7717 (Jerinib) | Small molecule/peptidomimetic antagonists for C5a receptor | Preclinical (inflammation, renal and ocular diseasesb) |
The complement system in innate and adaptive immunity
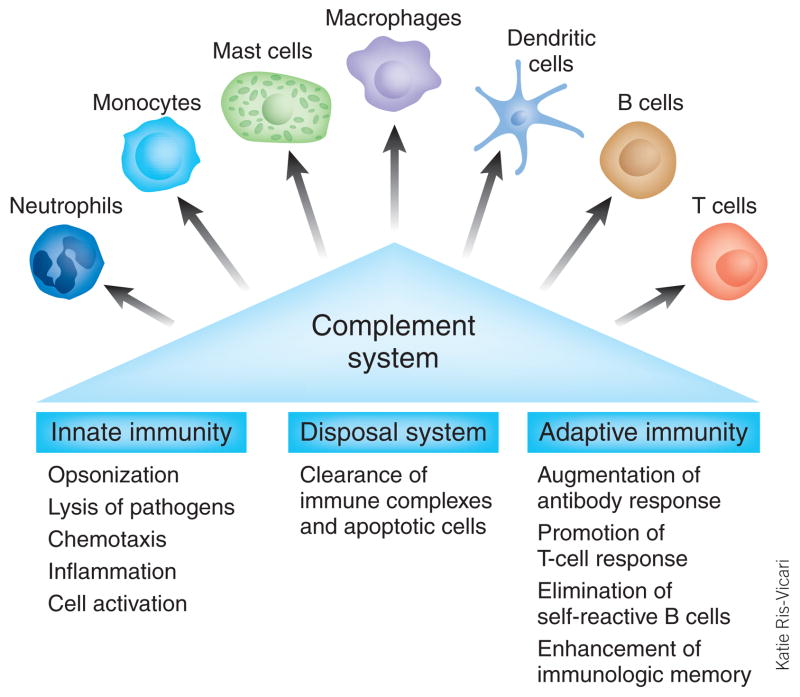
With an estimated age of 600–700 million years, the complement system is one of the most ancient defense strategies4, yet it represents far more than merely a simple protection mechanism against microbial infections. As an essential connection between adaptive and innate immunity, complement components are able to orchestrate immune reactions by communicating with multiple immune cells (Fig. 1). In addition, complement contributes to the maintenance of homeostasis by recognizing and eliminating apoptotic and necrotic cells. Furthermore, through its activity, the solubility of circulating immune complexes is maintained, thereby facilitating their elimination5–9.
Figure 1.
The complement system as a bridge between innate and adaptive immunity. Although the complement system has traditionally been considered part of the innate immune system, research in recent decades has revealed that complement is able to activate cells involved in both the adaptive and innate immune response. Complement triggers and modulates a variety of immune activities and acts as a linker between the two branches of the immune response. In addition, the complement system maintains cell homeostasis by eliminatiing cellular debris and immune complexes.
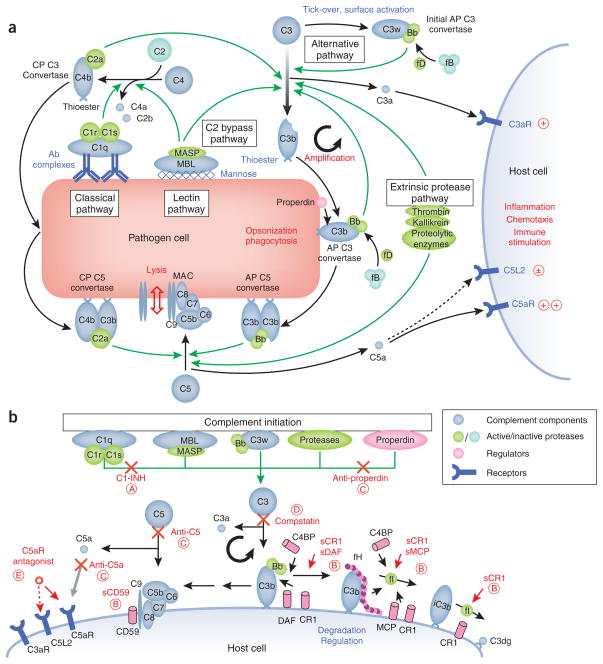
As a first line of defense against pathogens, complement has to quickly detect, tag, destroy and eliminate microbes. This task can be accomplished only by a complex and tightly regulated assembly of proteins involving various soluble and surface-bound complement components, receptors and regulators (Fig. 2). Complement activation is usually initiated by the interaction of several pattern-recognition receptors with foreign surface structures. Depending on the activation trigger, the complement cascade follows one of three pathways: the classical, lectin or alternative pathway.
Figure 2. Activation, regulation and therapeutic modulation of the complement system.
(a) The complement cascade after activation by pathogens. In addition to a low level of constant activation by the alternative pathway (AP; tick-over via hydrolyzed C3 (C3w) results in the formation of the initial C3 convertase C3wBb), the complement cascade is usually activated by antibody complexes (classical pathway) or high-density mannose (lectin pathway) on the surface of pathogens. This activation leads to the formation of the C3 convertases C4bC2a and C3wBb, which cleave native C3 to C3b and C3a. Deposition of C3b on cell surfaces via its thioester group initiates the cleavage of more C3 (the amplification loop via the final AP C3 convertase C3bBb), opsonization and phagocytosis, as well as lysis as a result of the formation of the membrane attack complex (MAC). In addition, the anaphylatoxins C3a and C5a are released and trigger further immune reactions upon binding to their receptors (C3aR, C5aR, C5L2). These combined actions of complement lead to the elimination of pathogenic cells. Recently, studies have shown that some steps of the cascade can be directly initiated by certain proteolytic enzymes (the extrinsic protease pathway) or by MBL/MASP (the C2 bypass pathway). Furthermore, the regulatory protein properdin may propagate and stabilize the formation of C3 convertases on the surface of the cell. Protein conversions are shown as black arrows and enzymatic reactions as green arrows. (b) Regulation, deactivation and inhibition of the complement cascade on host cells by natural regulators and complement-specific therapeutics. Several pathogenic processes and diseases are the result of an erroneous activation or insufficient downregulation of the complement cascade. Under normal conditions, any host-associated C3 convertase (C3bBb) undergoes an accelerated decay mediated by complement receptor 1 (CR1), decay accelerating factor (DAF), C4b-binding protein (C4BP) or factor H (fH). C3b is degraded to inactive iC3b by factor I in a reaction that requires as cofactor CR1, fH, C4BP or membrane cofactor protein (MCP). In addition, CD59 prevents the formation of the MAC. Some of the therapeutic interventions focus on increasing this downregulation by using soluble forms of these regulators (that is, sCR1, sDAF, sMCP, sCD59). Other approaches involve the substitution of the natural C1 inhibitor (C1-INH), the inhibition of the central conversion of C3 to C3b and C3a (compstatin), blockage of C5 or C5a by antibodies, and the suppression of anaphylatoxic signaling by C5a receptor antagonists. For clarity, only the regulation of the alternative pathway, which may contribute up to ~80% of all complement activity, is shown here. The C3 convertase of the classical pathway (C4bC2a), as well as the C5 convertases (C4bC2aC3b and C3bC3bBb), can be modulated by the same regulators and drug compounds. Therapeutic modulators in clinical trials or late preclinical development are indicated in red, with the circled letter referring to the corresponding drug class in Tables 1 and 2 and the main text.
The recognition of antibody complexes on the surface of pathogens, mediated by the multimeric collectin C1q, was early recognized as initiator of an enzymatic cascade that was later termed classical pathway. In the related lectin pathway, complement activation is initiated by an interaction of mannose binding lectin (MBL) with high-density arrays of terminal mannose on bacterial surfaces. In both cases, these pattern-recognition proteins form complexes with serine proteases (C1r/C1s and MASP, respectively) that lead to the cleavage of C4 to its fragments C4b and C4a. After binding of C2 to C4b, the same protease complexes are responsible for generating the active C2a fragment, which is part of the classical pathway C3 convertase (C4bC2a; Fig. 2a). To guarantee instant response to pathogens, the cascade is constantly kept at a low level of activity—‘tick-over’—by the alternative pathway. Spontaneous hydrolysis of C3 to C3w induces a conformational change that enables binding to factor B and formation of the initial alternative pathway C3 convertase (C3wBb; Fig. 2a). The alternative pathway may represent up to 80% of complement activation in some instances and can also be triggered by various reactions with foreign surfaces.
All three pathways converge in the cleavage of C3 into its active fragments, C3a and C3b, by the C3 convertases. Formation of C3b exposes a previously hidden thioester group that covalently binds to patches of hydroxyl and amino groups on the pathogen surface. This process, termed opsonization, is critical for all subsequent steps in the complement cascade and the elimination of pathogens and apoptotic host cells by phagocytes. In an amplification loop, binding of factor B to surface-bound C3b and its cleavage by factor D generates the alternative pathway C3 convertase C3bBb, which causes a gradual acceleration of C3b production. Surface-tethered C3b also plays a central role in the formation of the two C5 convertases (C4bC2aC3b and C3bC3bBb), which cleave C5 into C5a and C5b. C5b initiates the assembly of the membrane attack complex (MAC), a pore that is formed by components C5b, C6, C7, C8 and multiple units of C9 and ultimately leads to cell lysis. The anaphylatoxins C3a and C5a, generated as a result of proteolytic cleavage of C3 and C5, respectively, are potent inflammatory mediators that trigger a plethora of processes, which culminate in the stimulation of immune cells and the elimination of the pathogen5.
Despite years of intensive research in this field, our picture of complement activation seems to be far from complete. Very recently, additional activation pathways have been identified: for example, MBL-associated protease 2 (MASP-2) of the lectin pathway seems to directly attack and cleave C3 without formation of the corresponding C3 convertase (C2 bypass pathway; Fig. 2a)10. Similarly, direct cleavage of C3 and C5 by a series of extrinsic proteases such as kallikrein or thrombin (extrinsic protease pathway; Fig. 2a), has also been proposed. This activity points to an interesting connection to other essential plasma cascades such as the coagulation system11. Finally, properdin seems to not only stabilize the C3 convertase as a positive complement regulator but may also act as a pattern-recognition molecule and actively induce convertase formation on foreign surfaces12,13.
One of the most fascinating aspects of complement is the sophisticated regulation mechanism that allows a rapid reaction to pathogenic intruders while protecting host cells from its destructive potential. After activation, the opsonization rates often reach several thousand C3b molecules per cell per minute. In principle, this attack is not specifically directed against foreign cells. However, interplay of time, location and molecule-based regulation mechanisms generally prevents the complement cascade from attacking host tissues. Both the thioester group of C3b and the C3 convertases have short half-lives, limiting their action to the site of activation. Whereas the convertase is actively stabilized on foreign cells by properdin (Fig. 2a), a set of soluble and membrane-bound negative regulators inactivates and degrades active molecules on host cells (Fig. 2b). These structurally related regulators of complement activation include factor H, decay accelerating factor (DAF/CD55), membrane cofactor protein (MCP/CD46), C4-binding protein (C4BP) and complement receptor 1 (CR1/CD35). These regulators either induce an accelerated decay of the convertases (DAF, CR1, factor H) or act as cofactors for factor I, which degrades C3b to its inactive form iC3b (MCP, CR1, factor H). An additional regulator of complement activation, CD59, prevents the formation of MAC by intercalating between C8 and C9 subunits (Fig. 2b)5,14,15.
Complement’s involvement in disease
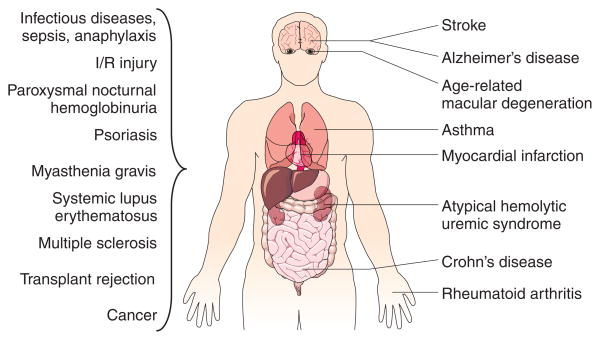
The central role of complement in the orchestration of immune reactions inevitably renders it a target for immune evasion or attack by pathogens and also a contributor to many diseases. Even small disruptions in the delicate balance of activation and regulation can lead to an overreaction of complement that triggers inflammation and cell lysis. Many inflammatory, autoimmune, neurodegenerative and infectious diseases have been shown to be associated with excessive complement activity (Fig. 3). Complement involvement is usually complex and may include both an inappropriate initiation of the cascade and deficiencies in specific components or regulators6,16,17.
Figure 3.
Examples of pathological conditions involving the complement system. Erroneous activation or insufficient regulation of the complement cascade may lead to an attack by the immune system against self-tissue. Many autoimmune, inflammatory and ischemia/reperfusion (I/R) injury-related diseases are therefore connected with complement. Whereas some of these pathological conditions are localized to specific organs and tissues, many of them are systemic. In addition, some pathogens have found ways to evade or even misuse the complement system, thereby contributing to infectious diseases and their consequences.
Ischemia/reperfusion (I/R) injury is a severe consequence of conditions that lead to a deprivation and subsequent restoration of the tissues’ blood supply; I/R injury can occur in a number of clinical conditions, including acute myocardial infarction, stroke, and hemorrhagic and septic shock, and may also be a complication of coronary artery bypass graft surgery. Paradoxically, it appears that the tissue reperfusion and not the ischemia activates complement and leads to inflammation-induced damage. Even though the exact involvement of complement activation in I/R injury is still unclear, several experimental studies have indicated a connection between complement and the pathogenesis of I/R injury, and have suggested complement inhibition as a potential therapy18.
Complement seems to be a major contributor to a number of autoimmune diseases. For example, deposition of immune complexes during systemic lupus erythematosus initiates the classical pathway by binding of C1q (Fig. 2b) to these complexes19. A similar mechanism may also be involved in the pathogenesis of rheumatoid arthritis, and C5a (Fig. 2b) seems to have a major role in psoriasis and complement-mediated inflammatory diseases including asthma20. Complement activation by C1q, which acts as a pattern-recognition receptor for amyloidal plaques, has been correlated with the pathology of Alzheimer’s disease21. Although complement also seems to be involved in other neurodegenerative diseases such as Huntington’s disease or Parkinson’s, the exact connection is less clear21.
Alterations in the expression and functions of complement regulators may be another cause of complement-related diseases. For example, polymorphism and mutations in genes encoding factor H (Fig. 2b) are associated with age-related macular degeneration (AMD), the major cause of blindness in industrial nations, and atypical hemolytic uremic syndrome (aHUS), respectively22. Low plasma levels of C1 inhibitor have been found to be responsible for hereditary angioedema (HAE)23,24, and a lack of CD59 (Fig. 2b) on erythrocyte surfaces causes paroxysmal nocturnal hemoglobinuria (PNH)25.
Finally, a series of pathogens have found ways to evade or even hijack complement proteins. Microbial proteins that either inhibit complement components or mimic regulators have been identified in both viruses (e.g., HIV, smallpox, Epstein-Barr virus) and bacteria (e.g., Staphylococcus aureus, Streptococcus pneumoniae)5,26–30.
Druggability of complement
The central role of complement in the pathophysiology of major diseases makes it an interesting target for the pharmaceutical industry. However, despite numerous attempts in recent years to inhibit or modulate complement therapeutically17,31–34, the success rate has been disappointingly low. Before the recent approval of complement-specific drugs, many promising candidates encountered insuperable obstacles while in clinical development. Inevitably, this high attrition rate raises questions about what renders complement drug discovery such a difficult task.
The multifaceted nature of both the cascade and its disease involvement may be one central problem. Many questions about the exact disease-related mechanisms of complement, both at the molecular and clinical level, are still unresolved, and this lack of clarity complicates specific targeting. “Druggability” (Box 1) is another major issue. Traditional drug discovery aims for small molecular entities with the potential for oral administration, yet many critical steps in the complement cascade are fundamentally based on large protein-protein interactions, which are challenging to influence with small molecules35. Anaphylatoxin receptors and serine proteases are the only complement targets with an auspicious druggability potential. This limitation may also explain the exceptionally high number of biopharmaceuticals (proteins, antibodies, or peptides, nucleotides) and biotechnology startups that are involved in the current drug development process (Tables 1 and 2). Finally, the optimal target within the complement cascade (Fig. 2b) and the extent of inhibition has to be critically defined for each indication. Suppressing pathogenic complement activity without compromising its defensive and immunomodulatory functions might be one of the most delicate challenges when treating chronic conditions. Intervention at the activation level may require a highly specific approach, for example, inhibiting the recognition of amyloid plaques by C1q in the case of Alzheimer’s disease. However, inhibition of a single pathway may be insufficient for many diseases and can still put pathogen defense at risk. Inhibition of C3 and its convertase potentially has the broadest effect, because it blocks all terminal activity of complement as well as the amplification loop. However, this nearly complete blockage may also lead to adverse effects and therefore favors local or targeted applications. Prevention of terminal complement action, for example, by inhibiting C5a formation and binding, is highly specific but requires the most information about the target involved. Finally, supplementation or inhibition of complement regulators may offer a more physiologic and specific way to modulate complement activity, especially in the treatment of chronic diseases.
Box 1. Drugs and druggability.
For the pharmaceutical industry, the ideal drug is a small molecule featuring high potency, selectivity, few adverse effects, low production costs and administration in a single daily oral dose. However, identifying compounds with these properties is challenging, and drug companies have been confronted with an ever-growing drug attrition rate97. During the drug discovery process, molecules that fulfill a set of molecular guidelines for oral bioavailability, known as the “Rule of 5”98 (RO5; e.g., a molecular weight below 500 Da or a partition coefficient [logP] below 5), are referred to as “drug-like” compounds. Similarly, this concept can be transferred to the targets of therapeutic intervention. Druggability refers to disease-related proteins that have the potential to be modulated by drug-like compounds97. Several attempts have been made to predict the druggability of protein families and to identify promising drug targets. Using the “druggable genome” approach, the targets of currently marketed drugs have been analyzed and mapped to protein families, assuming persistent druggability within the family99. Enzymes are the predominant class (nearly 50% of the total), followed by G-protein-coupled receptors (30%) and ion channels (7%). Within the complement cascade, the only targets with acceptable druggability potential are therefore the serine proteases and the anaphylatoxin receptors. Considering the large post-translational variability of protein targets and the increasing market for biopharmaceuticals, expansions of this model, such as the “pharmaceutically tractable genome,” have recently been suggested100.
Current complement therapeutics
A number of promising approaches for the clinical substitution, inhibition or modulation of complement have been developed and are presented in the following sections. We have classified complement therapeutics that either have been in clinical trials (Table 1) or are in the late steps of preclinical development (Table 2) into five major categories according to the mechanism of action (labeled a–e in Tables 1 and 2, and Fig. 2b).
Serine protease inhibitors
The consecutive cleavage and activation of several proteases constitutes the driving force behind complement function. Eight serine proteases are integral elements of the complement cascade itself (C1r, C1s, C2a, MASP-1, MASP-2, factor D, factor B, factor I)36, whereas others are involved through the extrinsic protease pathway (Fig. 2)11. Because proteases are among the most druggable targets in the complement system (Box 1), it is not surprising that early drug development efforts focused on protease inhibitors37. Although screening- and structure-based approaches resulted in drug candidates for factor D (BCX-1470, BioCryst, Birmingham, AL, USA)38 and C1s (C1s-INH-248, Knoll/Abbott, Abbott Park, IL, USA)39, short half-lives and a lack of specificity have thus far prevented the successful development of small-molecule protease inhibitors for the treatment of complement-related disorders.
The only complement-associated protease inhibitor currently on the market is C1-inhibitor (C1-INH), a heavily glycosylated plasma protein that is used in the treatment of HAE23,24,40,41. This disease is characterized by recurrent episodes of severe skin and mucous membrane edema and is caused by either an insufficient production of C1-INH (type 1) or a mutation around its binding site that leads to inactivity (type 2). In both types, therapeutic supplementation of this protein has proven to be an effective and safe treatment for HAE and helps prevent severe disease relapses and life-threatening complications42,43. A third HAE type of unknown etiology and low prevalence does not benefit from supplementation, because it is not dependent on C1-INH activity44. Although C1-INH gained its name from its ability to block the serine esterase activities of C1r and C1s45, its specificity includes other proteases, such as MASP, kallikrein and coagulation factors XI and XII. Although plasma levels of C2 and C4 are clearly affected by HAE, the primary mechanism of C1-INH seems to be more closely associated with bra-dykinin-kallikrein than with the complement system23. However, there is increasing evidence that therapeutic administration of C1-INH may also be beneficial in other disorders with a direct connection to complement, such as I/R injury41,46–48.
Whereas pasteurized C1-INH concentrates for the treatment of HAE have been available since 1985, a lack of licensed products still limits the accessibility of this treatment in several countries, including the United States43. Fortunately, annotation of orphan drug status by the FDA (Box 2) and licensing agreements with established providers have boosted the development and registration of this important drug, and its approval in the United States is imminent49. Current C1-INH formulations are still being improved50, for example, by the use of nanofiltered concentrates that feature better safety with regard to viral contamination. The high production costs and extensive quality control requirements for plasma-derived C1-INH still makes it an expensive treatment option and will certainly limit its availability in poorer countries. Development of a recombinant form, which is isolated as a glycosylated protein from the milk of transgenic animals, may be an important step in overcoming these limitations51. Experience with C1-INH in the clinic and its good safety profile are expected to lead to an extension of its indications. For example, clinical trials regarding the use of C1-INH for the prevention of I/R injury after acute myocardial infarction are currently planned.
Box 2. Supporting the niches—the Orphan Drug Act.
Development of a new drug compound is a challenging and expensive endeavor. The cost for a newly marketed drug is currently estimated to be in the range of 500milliontomorethan500 million to more than 500milliontomorethan2,000 million101. It is therefore understandable that pharmaceutical companies focus primarily on drugs to treat major diseases with a large market potential (that is, “blockbuster drugs”). This focus often leaves a painful therapeutic gap for rare diseases, niche markets or biopharmaceuticals with patent limitations (e.g., natural proteins). Compounds that are unprofitable to develop are usually referred to as “orphan drugs”102. In an effort to encourage the development of such drugs, several drug regulation authorities, including the US Food and Drug Administration (FDA), have initiated special programs such as the Orphan Drug Act (ODA) of 1983 (ref. 103). Designation of orphan drug status is coupled to a series of financial incentives, the most important of which is marketing exclusivity for 7 years after drug approval. In addition, orphan product development grants sponsor clinical trials and close the gap between basic research and clinical development. Undeniably, the ODA is one of the most successful recent United States legislative actions and has had a major impact on the drug market. Twenty years after its enactment, over 1,000 drugs had been accorded orphan drug status, and more than 200 had been approved for marketing, thereby improving the clinical situation of an estimated 11 million patients in the United States alone102. Meanwhile, these numbers have grown to 1,750 designated and 315 marketed orphan drugs (as of October 4, 2007, http://www.fda.gov/orphan/designat/list.htm). Similar initiatives also exist in other countries and regions (e.g., at the European Medicines Agency). In the complement field, eculizumab, for the treatment of paroxysmal nocturnal hemoglobinuria, and C1 inhibitor concentrate, for treatment of hereditary angioedema, are covered by the ODA. For more information, see http://www.fda.gov/orphan/.
Soluble complement regulators
Because regulators of complement activation are natural modifiers of complement activities and prevent a host cell from being attacked by its own defense system, they have been considered for therapeutic use since the early stages of complement drug discovery52. A first breakthrough was reached with the expression of a soluble form of complement receptor 1 (sCR1)53. This molecule featured both decay accelerator and cofactor activity and had a high potency in inhibiting both the classical and alternative pathways. sCR1 showed promising results in the treatment of I/R injury and various other conditions in experimental animal models. Based on these encouraging results, sCR1 was developed as a therapeutic (TP10; Avant Immunotherapeutics, Needham, MA, USA) for use after coronary artery bypass graft surgery. TP10 is expressed as a 240-kDa glycoprotein in Chinese hamster ovary cells, has a plasma half-life of ~55 hours and is safe and well-tolerated in both adult and infant patients54. In a large placebo-controlled phase 2 trial, comprising 564 high-risk patients undergoing cardiac surgery, a single intravenous bolus of TP10 immediately before surgery was found to inhibit complement activation for up to 3 days postoperatively. Whereas TP10 treatment resulted in a significant improvement of the clinical end-points in males, no such benefits were observed in female participants. This surprising gender specificity was confirmed by a subsequent phase 2b study comprising 297 women. Whereas the mechanism governing these differences is still unclear, effects other than complement inhibition are likely to be involved. As a consequence, a male-only indication of TP10 for bypass surgery was considered for clinical phase 3. TP10 also showed promising results in smaller studies of adults undergoing lung transplants and infants undergoing coronary artery bypass graft surgery54. However, Avant Immune has recently decided not to continue investing in clinical trials of TP10 (ref. 55). It remains to be seen whether this development will mean the end of sCR1 as a therapeutic compound. Improvements in the structure (e.g., a reduction of its size) and formulation of sCR1 or an extension of its indications may well lead to its resurrection. Although the first steps in this direction have already been taken, neither TP20 (sCR1 with sialyl LewisX-glycosylation for targeting sites of inflammation56, Avant Immune) nor Mirococept/APT070 (a truncated sCR1 with a membrane-tethering motif57, Inflazyme Pharmaceuticals, Richmond, BC, Canada) has been tested in clinical trials.
Soluble forms of MCP, DAF and CD59 have also been considered as therapeutics. Whereas DAF and MCP each offer only a single regulatory activity, a recombinant chimera of their extracellular parts has been developed17. The resulting sDAF-sMCP hybrid was initially named “complement activity blocker 2” (CAB-2; Xoma, Berkeley, CA, USA) but entered clinical trials with a new name and licensing partner (MLN-2222; Millennium, Cambridge, MA, USA). However, no further studies have been initiated recently. Like Mirococept/APT070, a membrane-tethering sCD59 has been developed by Inflazyme Pharmaceuticals. Although not listed in the company’s pipeline, experimental studies were recently performed to investigate its potential therapeutic usefulness as a treatment option for PNH58. This rare, genetic, life-threatening blood disorder leads to decreased expression of membrane-anchored proteins, including CD59 and DAF, on erythrocytes. Substitution and membrane tethering of recombinant CD59 may develop into a promising therapy for PNH58.
Therapeutic antibodies
Following a general trend in the pharmaceutical industry, antibody-based therapeutics appear to be the most rapidly growing drug class against complement-related diseases. Crucial developments in their screening, production and humanization have led to a remarkable boost in the number of therapeutic antibodies59. By targeting specific components of the complement system, all stages from the initiation and activation process to single terminal actions can hypothetically be blocked in a selective manner. Current drug candidates in the pipeline focus primarily on the inhibition of downstream processes around C5 and its cleavage fragment, the anaphylatoxin C5a.
Selective inhibition of C5 using monoclonal antibodies (mAb) has been considered a promising therapeutic option for many years. A highly selective mAb against mouse C5 was introduced by Frei et al. two decades ago60 and was later demonstrated to be effective in a mouse model of rheumatoid arthritis by Alexion Pharmaceuticals61. Continuous improvement and clinical testing finally led to the FDA approval of eculizumab3, which is currently the only complement-specific antibody on the market62. Eculizumab is the first and only approved therapy for PNH, a rare but life-threatening disorder that is characterized by a chronic destruction of red blood cells. A mutation on the X chromosomes of hematopoietic stem cells prevents the proper biosynthesis of the glycosylphosphatidylinositol (GPI) anchor, which leads to a deficiency in membrane-anchored proteins, including DAF and CD59. The lack of efficient inhibition of complement activation on red blood cells results in an increase in MAC formation on erythrocyte membranes and cell lysis. The survival of PNH erythrocytes is dramatically reduced in PNH patients, to 10% of normal red blood cells. On platelets, the absence of CD59 and increased MAC formation can lead to morphological changes of their surface. These platelets show enhanced susceptibility for activation, contributing to a high risk of thrombosis in PNH patients. Until very recently, only supportive therapies (blood transfusions, iron therapy, anticoagulation and others) or allogeneic stem cell transplantation had been available for the clinical management of this disease25.
Given the complement-focused molecular mechanism of PNH, prevention of MAC activity evolved as the most promising treatment strategy. Two possible approaches were identified: substitution of defective CD59 or neutralization of complement proteins involved in MAC formation itself, as in the case of eculizumab. By tightly binding to C5 and preventing its cleavage to C5b, this new drug suppresses the formation of the MAC and reduces the clinical symptoms of PNH. Eculizumab (5G1.1-SC) is a humanized version of the anti-C5 antibody h5G1.1, which was first described in 1996 (ref. 63). Eculizumab received orphan drug status for PNH from both the FDA and the EU in 2003, and Alexion Pharmaceuticals subsequently initiated two phase 3 trials. In both studies, eculizumab was safe and well-tolerated, and no development of antibodies against the drug was detected. Because complement inhibition is expected to lead to a higher susceptibility to infection, particularly with Neisseria meningitidis, all patients had been vaccinated with a meningococcal vaccine before beginning treatment. The antibody was clearly effective: 51% of those who received eculizumab gained independence from blood transfusions (versus 0% in the placebo group). Both the endogenous erythrocyte mass and the hemoglobin levels were remarkably higher during treatment than before, and many symptoms related to quality of life (e.g., fatigue, abdominal pain) were attenuated. Whereas the preliminary data also indicated a lower risk for thrombosis, this benefit needs to be confirmed by ongoing clinical trials25,64,65.
As an anti-C5 antibody that inhibits the generation of both C5b and the anaphylatoxin C5a, the potential indications for eculizumab are certainly not limited to PNH. Consequently, eculizumab has undergone several preclinical and clinical studies for a variety of conditions (e.g., psoriasis, rheumatoid arthritis, systemic lupus erythematosus and transplant rejection). Even though some of these paths seem to have been discontinued65, Alexion Pharmaceuticals still performs preclinical development of eculizumab for autoimmune diseases and transplant rejection. Furthermore, nebulized eculizumab for the treatment of asthma and an intravitreal application to treat age-related macular degeneration are in the company’s pipeline. In the case of pexelizumab, the short-acting sc-Fv fragment of eculizumab, several phase 3 trials have been done to test its potential for use in coronary artery bypass graft surgery and acute myocardial infarction66. Despite promising pre-clinical results, however, data from these clinical trials did not show significant improvement in the treatment group when compared to the placebo group67,68. Alexion Pharmaceuticals has therefore announced that it plans to focus its development efforts on eculizumab69.
The concept of using humanized antibodies (and their fragments) to inhibit certain steps of the complement cascade seems to be a very productive strategy. Several companies list anticomplement antibodies in their pipelines (Table 2). Two of them target the various actions of the anaphylatoxin C5a. Neutrazumab (G2 Therapies, Darlinghurst, NSW, Australia) binds to extracellular loops of C5aR and thereby inhibits the binding of C5a to its major signaling receptor. G2 Therapies recently announced a partnership with Novo Nordisk (Bagsværd, Denmark) with regard to the development of their anti-C5aR antibodies. Another approach has been taken by Tanox (now part of Genentech, S. San Francisco, CA, USA) with their antibody TNX-558, which neutralizes C5a by binding to the anaphylatoxin itself. Interestingly, TNX-558 binds to a C5a epitope in native C5 but does not prevent its cleavage. Tanox has also developed an antibody directed against factor D (TNX-234). Together with the anti–factor B (TA106 for age-related macular degeneration and asthma70; Taligen, Aurora, CO, USA) and anti-properdin (Novelmed, Cleveland, OH, USA) antibodies in development, these biologics may offer additional approaches for inhibiting complement at the C3 convertase level.
Whereas inhibition of complement activity is the desired outcome in the vast majority of therapeutic approaches, its local stimulation may be beneficial in some malignant diseases, such as cancer. Experience with monoclonal antibodies in cancer therapy suggests that induction of cell death through antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity (CDC) may be a major driving force behind their effectiveness (e.g., in the case of the anti-CD20 mAb rituximab from Roche). However, relatively high antigen densities are required for an efficient initiation of the classical pathway by C1q. Genmab (Copenhagen) recently described anti-CD20 antibodies with exceptionally high CDC potency71,72. Both the location of the epitope as well as the dissociation rate are apparently responsible for this high level of effectiveness. Ofatumumab/HuMax-CD20 has been selected for clinical development and has recently been evaluated in terms of its effectiveness in treating acute rheumatoid arthritis (phase 2), B-cell chronic lymphocytic leukemia (phase 3) and follicular lymphoma (phase 3). Other antibodies with high CDC potency against multiple myeloma (HuMax-CD38) or colon, pancreatic and prostate cancer (HuMax-ZP3) are currently in preclinical development (http://www.genmab.com/). This strategy is a good example of how to make use of a more general, nonspecific property and select and optimize it to obtain the desired clinical effect. Because CDC may generally be induced by therapeutic IgGs (and IgMs), the CDC activity of these reagents may become a crucial parameter in the development of therapeutic antibodies.
Complement component inhibitors
Although antibodies may be considered the archetype of site-blocking compounds, smaller molecules such as peptides, nucleotides and synthetic molecules may also have the potential to interrupt protein functions by steric hindrance or the induction of conformational changes. Small functional inhibitors of complement activity are expected to have drug-like properties with enhanced pharmacokinetic profiles (Box 1). These advantages may make them suitable for drug development efforts related to oral bioavailability and better administration routes.
Compstatin is the most developed candidate in this class of substances and recently entered clinical trials. This cyclic tridecapeptide has been discovered through screening of combinatorial phage-display libraries73 in the research group led by one of the authors of this review. It effectively prevents the cleavage of C3 to its active fragments C3a and C3b and therefore inhibits the most central step in the complement cascade. Although the exact mechanism of compstatin’s inhibitory action has not yet been resolved, the recent publication of the cocrystal structure between compstatin and C3c suggests that a disruption of protein-protein interactions during convertase formation may be an important determinant74. This finding has led to the hypothesis that a conformational change or an interruption in protein-protein interactions is responsible for its activity. Compstatin has shown effective complement inhibition in a variety of experimental disease models34. Despite a narrow selectivity for primate C3 that may affect preclinical animal experiments75, its high efficacy and rather small size make it a promising drug candidate. Its inhibitory efficacy has been increased by 264-fold34,76 through the use of rational and combinatorial synthesis, structural analysis, and computational approaches to identify active analogs. Potentia (Louisville, K Y, USA) has announced the inauguration of clinical trials for the treatment of neo-vascular age-related macular degeneration by intravitreal application of a compstatin analog (POT-4).
Combinatorial approaches have also been used to identify a short aptamer molecule directed against C5 (ref. 78). Aptamers are single-stranded nucleotide stretches that have molecular recognition properties similar to those of antibodies, but offer better options for derivatization. They can be selected in an automated high-throughput process known as systematic evolution of ligands by exponential enrichment (SELEX)79. More than 15 years after the initial description of this method, the first aptamer-based drug pegaptanib (Macugen; Eyetech Pharmaceuticals/Pfizer, New York) a PEG-ylated anti–vascular endothelial growth factor (VEGF) aptamer for the treatment of wet age-related macular degeneration, was approved by the FDA80. The optimized and heavily modified form of the anti-C5 RNA aptamer (ARC1905; Archemix, Cambridge, MA, USA) features a subnanomolar binding affinity for human C5. It provides complete blockage of downstream complement activation by inhibiting the cleavage of C5 into C5a and C5b. A 40-kDa PEG group has been added to the 5′ end to improve its pharmacokinetic properties. Although ARC1905 was initially developed for use during coronary artery bypass graft surgery, the recent failure of pexelizumab for this indication has had a direct impact on the fate of ARC1905. Like compstatin (POT-4) and Macugen, the compound is now positioned as an intravitreal treatment option for wet and dry age-related macular degeneration and is awaiting clinical trials (P. Bouchard, Archemix Corp., personal communication).
Anaphylatoxin receptor antagonists
The proinflammatory activity of the anaphylatoxin C5a is the driving force behind many complement-associated disorders. After its liberation from C5, this hormone-like glycoprotein binds to two high-affinity receptors, the C5a receptor (C5aR) and C5L2 (Fig. 2)81. Most of the proinflammatory signaling seems to be induced by C5aR, a G-protein-coupled receptor that is expressed on numerous myeloid (e.g., neutrophils, macrophages) and nonmyeloid cells. Despite its similar structure and affinity for C5a, the C5L2 receptor produces a completely different signaling pattern. In plasma, the activity of C5a is controlled through the removal of the C-terminal arginyl residue by the plasma enzyme carboxypeptidase N. The resulting C5adesArg binds poorly to C5aR but with a high affinity to C5L2. Earlier reports about binding of C3a and C3adesArg to C5L2 have recently been questioned and require further investigation81. The differential binding pattern, as well as reports of the anti-inflammatory activity mediated by C5L2, has led to the hypothesis that the regulation of C5a involves a sophisticated feedback mechanism20,64. However, other studies have reported a proinflammatory or C5aR-supporting role for C5L2 (ref. 82), leaving its exact function a matter of scientific debate.
Selective inhibition of the binding of C5a to its receptors offers a very promising opportunity for dampening the inflammatory response without depleting the defensive potential of complement64,83,84. The macromolecular bio-pharmaceuticals we have previously discussed either inhibit C5a generation (anti-C5 antibodies and aptamers) or neutralize the C5a-C5aR interaction by shielding the relevant binding site. C5aR antagonists, on the other hand, are designed to bind to the receptor with high affinity without inducing any signaling activity (Fig. 2b). Because only one of the two proposed binding sites on C5aR has to be targeted for this purpose81,84, these antagonists are expected to be rather small. Simultaneous efforts by many companies have focused on reducing the functional fragment of C5a to a C-terminal hexapeptide, while replacing individual residues to shift its activity from agonistic to antagonistic. Cyclization of these hexapeptides not only allows them to be locked into the desired turn-conformation but also gives them higher stability with regard to proteolytic degradation84.
The most promising candidate to emerge, the cyclic peptidomimetic PMX-53, is currently under development by Peptech85. With a molecular size under 1 kDa and a high oral bioavailability, PMX-53 has many of the desired features of a designed drug. However, its short half-life of only 70 min may mean a requirement for frequent, if not continuous, administration. Although the drug showed high stability in blood, gastric and intestinal contents, it was rapidly degraded by proteolysis in intestinal mucosa washings. In a phase 1a/2b trial in 21 patients with rheumatoid arthritis, PMX-53 was orally administered and showed positive trends in many disease measures. An equally positive result was observed in a phase 1b/2a trial of topical application of the drug in ten people with psoriasis. In both studies, PMX-53 was found to be safe and well-tolerated85. Despite these encouraging results, the short half-life and rapid degradation in intestinal mucosa of this drug may severely limit its use. It remains to be seen if Peptech will further develop PMX-53 or initiate new trials with PMX-205, a similar yet more hydrophobic compound that shows high potency but a much lower susceptibility to proteolysis than that of PMX-53 (refs. 86,87).
Given the current uncertainty about the role of the C5L2 receptor, a critical evaluation of the binding specificity may be an important requirement for the development of successful C5aR antagonists. Several companies seem to have promising candidates in preclinical testing. For example, Jerini has a series of peptidomimetics and small organic molecules (JPE-1375, JSM-7717) for the treatment of inflammatory, renal and ocular diseases in preclinical stages (http://www.jerini.com/).
Other concepts
Whereas most of the previously discussed concepts are designed to inhibit or decrease complement activation, one compound is intended to boost low complement activity. Approximately 30% of the human population shows low plasma levels of MBL, making them more susceptible to infections under immunosuppressive conditions. Recombinant human MBL (rhMBL; Enzon, Bridgewater NJ, USA) restores the activity of the lectin pathway and has been found to be safe and well tolerated88. It is currently being tested in phase 1b trials as replacement therapy in people with multiple myeloma who are deficient in MBL and undergoing high-dose chemotherapy and stem cell transplants, and in MBL-deficient people undergoing liver transplantation. Additional approaches such as quenching of exposed thioester groups of C3b (thioester inhibitors)17 or inhibition of the C1q-IgG interaction by anionic small molecules (e.g., steroid/triterpenoid sulfates) have been described31,89 but have not appeared in preclinical settings thus far.
Perspectives and outlook
As is true for complement research itself, the quest for complement-directed therapeutics has made dramatic changes in direction and encountered difficult challenges; initial euphoria has given way to a more realistic view of strategies and aims. Whereas some first-generation approaches of complement drug discovery have either failed (e.g., small-molecule serine protease inhibitors) or are severely struggling (e.g., soluble regulators), there are many promising concepts on the horizon that represent a second generation of complement drugs. Current indications for complement drugs, such as HAE or PNH, certainly represent niche markets far away from blockbuster areas. Both eculizumab and the C1-INH concentrates are protected under the Orphan Drug Act (Box 2), and their approval is an important step in establishing the concept and accumulating clinical experience before expanding their indications.
Undeniably, the unsatisfactory results obtained for TP10 and pexelizumab have left a bitter taste and may have had a negative impact on the field. These disappointments underscore the limitations inherent in broadly applying complement-specific drugs in inflammatory diseases. Many of the compounds that have been discontinued were designed to target multifaceted disorders such as I/R injury. In these disorders, many parallel mechanisms are involved, and the influence of complement is still a matter of debate. For such complex indications, the key to success may be to use complement drugs not as single therapies but (in consonance with their name) as complementary therapies. Clearly, more basic research and additional tests are required in this context. In cases in which clear complement targets can be identified (as in HAE or PNH), complement therapeutics have already been successfully applied.
Several factors are expected to boost the development of complement therapeutics. In particular, our accumulating knowledge about the exact role of complement in various diseases will greatly facilitate the identification of relevant targets. Even more importantly, crystal structures of a number of crucial complement components (e.g., C3 (ref. 90), C3b91,92, C3c90, C3d93, factor B94, factor D95) have recently been solved and are available for structure-based design. Genentech has reported a co-crystal structure of C3b and C3c with the extracellular domain of the macrophage complement receptor CRIg92. The same group recently demonstrated that soluble murine CRIg selectively inhibits the alternative pathway by competing with C5 for convertase binding, and that it was effective in mouse models of arthritis96. Its high selectivity for the alternative pathway and the availability of a detailed crystal structure make CRIg an attractive lead structure and render its further clinical development highly likely. Along with the continuous development of established approaches, the quest for complement therapeutics may get help from an unexpected source: human pathogens. Staphylococcus aureus (S. aureus) alone offers several interesting candidates. Among them, the extracellular fibrinogen-binding protein28 and the staphylococcal complement inhibitor30 of S. aureus have been reported to inhibit the conversion of C3 to C3a and C3b. An understanding of their interaction sites and mechanisms could be applied to developing either potent complement inhibitors or new antibiotics targeting S. aureus. In addition, the chemotaxis inhibitory protein of S. aureus29 has been found to antagonize C5aR and may therefore serve as a lead structure for novel antagonists.
In many aspects, complement drug discovery illustrates general trends that are observed in the pharmaceutical industry. Limitations in the development of small compounds (e.g., lack of specificity or inability to disrupt protein-protein interactions) and improved technology and safety in the production of natural macromolecules has led to a growing market for these biopharmaceuticals. An analysis of the compounds indicates that they almost exclusively represent large proteins and nucleotides, or that they have been developed from peptides. Furthermore, major pharmaceutical companies tend to outsource early drug discovery phases to academic institutions and small startup companies. Partnerships with major pharmaceutical companies are usually only sealed after clinical trials have begun (often after phase 1/2). Indeed, many of the promising complement drug candidates (e.g., compstatin and PMX-53) were initially developed in the academic field and further developed by biotechnology companies. Such partnerships have already been established in the case of ofatumumab (Genmab/Glaxo), pexelizumab (Alexion/Procter & Gamble, Cincinnati, OH, USA) and neutrazumab (G2 Therapies/Novo Nordisk).
Acknowledgments
We thank Peter Ward, Wenchao Song and Maciej Markiewski for their input and for critically reading the manuscript and Deborah McClellan for editorial assistance. This work was supported by National Institutes of Health grants GM-069736, GM-62134, AI-30040, EB003968, CA112162 and AI-068730.
Footnotes
References
- 1.Nuttall G. Experimente über die bacterienfeindlichen Einflüsse des thierischen Körpers. Z Hyg Infektionskr. 1888;4:353–394. [Google Scholar]
- 2.Ehrlich P, Morgenroth J. Ueber haemolysine—zweite mittheilung. Berl Klin Wochenschr. 1899:481–486. [Google Scholar]
- 3.US Food and Drug Administration. FDA approves first-of-its-kind drug to treat rare blood disorder. USFDA; Rockville, MD, USA: Mar 16, 2007. ( http://www.fda.gov/bbs/topics/NEWS/2007/NEW01589.html) [Google Scholar]
- 4.Sunyer JO, Zarkadis IK, Lambris JD. Complement diversity: a mechanism for generating immune diversity? Immunol Today. 1998;19:519–523. doi: 10.1016/s0167-5699(98)01341-3. [DOI] [PubMed] [Google Scholar]
- 5.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 6.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 7.Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check–a new role for complement regulators? Trends Immunol. 2006;27:102–108. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Morgan BP, Marchbank KJ, Longhi MP, Harris CL, Gallimore AM. Complement: central to innate immunity and bridging to adaptive responses. Immunol Lett. 2005;97:171–179. doi: 10.1016/j.imlet.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Mastellos D, Lambris JD. Complement: more than a ‘guard’ against invading pathogens? Trends Immunol. 2002;23:485–491. doi: 10.1016/s1471-4906(02)02287-1. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson JP, Frank MM. Bypassing complement: evolutionary lessons and future implications. J Clin Invest. 2006;116:1215–1218. doi: 10.1172/JCI28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markiewski MM, Nilsson B, Nilsson Ekdahl K, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Hourcade DE. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem. 2006;281:2128–2132. doi: 10.1074/jbc.M508928200. [DOI] [PubMed] [Google Scholar]
- 13.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 14.Kirkitadze MD, Barlow PN. Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol Rev. 2001;180:146–161. doi: 10.1034/j.1600-065x.2001.1800113.x. [DOI] [PubMed] [Google Scholar]
- 15.Soares DC, Barlow PN. Complement control protein modules in the regulators of complement activation. In: Morikis D, Lambris JD, editors. Structural Biology of the Complement System. CRC Press; Boca Raton, Florida: 2005. pp. 19–62. [Google Scholar]
- 16.Volanakis JE, Frank M, editors. The Human Complement System in Health and Disease. Marcel Dekker, Inc; New York: 1998. [Google Scholar]
- 17.Sahu A, Lambris JD. Complement inhibitors: a resurgent concept in anti-inflammatory therapeutics. Immunopharmacology. 2000;49:133–148. doi: 10.1016/s0162-3109(00)80299-4. [DOI] [PubMed] [Google Scholar]
- 18.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases—from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 20.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 21.Bonifati DM, Kishore U. Role of complement in neurodegeneration and neuroinflammation. Mol Immunol. 2007;44:999–1010. doi: 10.1016/j.molimm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Zipfel PF, Heinen S, Jozsi M, Skerka C. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol. 2006;43:97–106. doi: 10.1016/j.molimm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Davis AE., III The pathophysiology of hereditary angioedema. Clin Immunol. 2005;114:3–9. doi: 10.1016/j.clim.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Wagenaar-Bos IG, Hack CE. Structure and function of C1-inhibitor. Immunol Allergy Clin North Am. 2006;26:615–632. doi: 10.1016/j.iac.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Hill A, Richards SJ, Hillmen P. Recent developments in the understanding and management of paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2007;137:181–192. doi: 10.1111/j.1365-2141.2007.06554.x. [DOI] [PubMed] [Google Scholar]
- 26.Rooijakkers SH, van Strijp JA. Bacterial complement evasion. Mol Immunol. 2007;44:23–32. doi: 10.1016/j.molimm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Datta PK, Rappaport J. HIV and complement: hijacking an immune defense. Biomed Pharmacother. 2006;60:561–568. doi: 10.1016/j.biopha.2006.07.087. [DOI] [PubMed] [Google Scholar]
- 28.Hammel M, et al. A structural basis for complement inhibition by Staphylococcus aureus. Nat Immunol. 2007;8:430–437. doi: 10.1038/ni1450. [DOI] [PubMed] [Google Scholar]
- 29.de Haas CJ, et al. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med. 2004;199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooijakkers SH, et al. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6:920–927. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- 31.Bureeva S, Andia-Pravdivy J, Kaplun A. Drug design using the example of the complement system inhibitors’ development. Drug Discov Today. 2005;10:1535–1542. doi: 10.1016/S1359-6446(05)03592-0. [DOI] [PubMed] [Google Scholar]
- 32.Makrides SC. Therapeutic inhibition of the complement system. Pharmacol Rev. 1998;50:59–87. [PubMed] [Google Scholar]
- 33.Lambris JD, Holers VM, editors. Therapeutic Interventions in the Complement System. Humana Press; Totowa, NJ, USA: 2000. [Google Scholar]
- 34.Holland MC, Morikis D, Lambris JD. Synthetic small-molecule complement inhibitors. Curr Opin Investig Drugs. 2004;5:1164–1173. [PubMed] [Google Scholar]
- 35.Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004;3:301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 36.Sim RB, Tsiftsoglou SA. Proteases of the complement system. Biochem Soc Trans. 2004;32:21–27. doi: 10.1042/bst0320021. [DOI] [PubMed] [Google Scholar]
- 37.Narayana SV, Babu YS, Volanakis JE. Inhibition of complement serine proteases as a therapeutic strategy. In: Lambris JD, Holers VM, editors. Therapeutic Interventions in the Complement System. Humana Press; Totowa, New Jersey: 2000. pp. 57–74. [Google Scholar]
- 38.Szalai AJ, et al. The Arthus reaction in rodents: species-specific requirement of complement. J Immunol. 2000;164:463–468. doi: 10.4049/jimmunol.164.1.463. [DOI] [PubMed] [Google Scholar]
- 39.Buerke M, Schwertz H, Seitz W, Meyer J, Darius H. Novel small molecule inhibitor of C1s exerts cardioprotective effects in ischemia-reperfusion injury in rabbits. J Immunol. 2001;167:5375–5380. doi: 10.4049/jimmunol.167.9.5375. [DOI] [PubMed] [Google Scholar]
- 40.Agostoni A, Cicardi M, Bergamaschini L, Boccassini G, Tucci A. C1-inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 1980;303:527. [PubMed] [Google Scholar]
- 41.Kirschfink M, Mollnes TE. C1-inhibitor: an anti-inflammatory reagent with therapeutic potential. Expert Opin Pharmacother. 2001;2:1073–1083. doi: 10.1517/14656566.2.7.1073. [DOI] [PubMed] [Google Scholar]
- 42.De Serres J, Groner A, Lindner J. Safety and efficacy of pasteurized C1 inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus Apher Sci. 2003;29:247–254. doi: 10.1016/j.transci.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Longhurst HJ, Carr S, Khair K. C1-inhibitor concentrate home therapy for hereditary angioedema: a viable, effective treatment option. Clin Exp Immunol. 2007;147:11–17. doi: 10.1111/j.1365-2249.2006.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bork K, Barnstedt SE, Koch P, Traupe H. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet. 2000;356:213–217. doi: 10.1016/S0140-6736(00)02483-1. [DOI] [PubMed] [Google Scholar]
- 45.Pensky J, Levy LR, Lepow IH. Partial purification of a serum inhibitor of C′1-esterase. J Biol Chem. 1961;236:1674–1679. [PubMed] [Google Scholar]
- 46.Caliezi C, et al. C1-Esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol Rev. 2000;52:91–112. [PubMed] [Google Scholar]
- 47.Nielsen EW, et al. Effect of supraphysiologic levels of C1-inhibitor on the classical, lectin and alternative pathways of complement. Mol Immunol. 2007;44:1819–1826. doi: 10.1016/j.molimm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Lauterbach M, et al. C1-esterase inhibitor reverses functional consequences of superior mesenteric artery ischemia/reperfusion by limiting reperfusion injury and restoring microcirculatory perfusion. Shock. 2007;27:75–83. doi: 10.1097/01.shk.0000235093.83915.0b. [DOI] [PubMed] [Google Scholar]
- 49.Lev Pharmaceuticals. Lev Pharmaceuticals; New York: Mar 14, 2007. Lev Pharmaceuticals reports positive results in pivotal phase III trial for hereditary angioedema. ( http://www.levpharma.com/investors.news.3.14.07.aspx) [Google Scholar]
- 50.Zuraw BL. Novel therapies for hereditary angioedema. Immunol Allergy Clin North Am. 2006;26:691–708. doi: 10.1016/j.iac.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 51.van Doorn MB, et al. A phase I study of recombinant human C1 inhibitor in asymptomatic patients with hereditary angioedema. J Allergy Clin Immunol. 2005;116:876–883. doi: 10.1016/j.jaci.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Brook E, Herbert AP, Jenkins HT, Soares DC, Barlow PN. Opportunities for new therapies based on the natural regulators of complement activation. Ann NY Acad Sci. 2005;1056:176–188. doi: 10.1196/annals.1352.033. [DOI] [PubMed] [Google Scholar]
- 53.Weisman HF, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 54.Li JS, Jaggers J, Anderson PA. The use of TP10, soluble complement receptor 1, in cardiopulmonary bypass. Expert Rev Cardiovasc Ther. 2006;4:649–654. doi: 10.1586/14779072.4.5.649. [DOI] [PubMed] [Google Scholar]
- 55.Avant Immunotherapeutics, Inc. Avant Immunotherapeutics; Needham, MA: Apr 16, 2007. Press Release: AVANT Restructures Organization to Focus Resources on Core Programs and Operations. http://phx.corporate-ir.net/phoenix.zhtml?c=93243&p=irol-newsArticle&t=Regular&id=985098&. [Google Scholar]
- 56.Rittershaus CW, et al. Recombinant glycoproteins that inhibit complement activation and also bind the selectin adhesion molecules. J Biol Chem. 1999;274:11237–11244. doi: 10.1074/jbc.274.16.11237. [DOI] [PubMed] [Google Scholar]
- 57.Smith RA. Targeting anticomplement agents. Biochem Soc Trans. 2002;30:1037–1041. doi: 10.1042/bst0301037. [DOI] [PubMed] [Google Scholar]
- 58.Hill A, et al. Protection of erythrocytes from human complement-mediated lysis by membrane-targeted recombinant soluble CD59: a new approach to PNH therapy. Blood. 2006;107:2131–2137. doi: 10.1182/blood-2005-02-0782. [DOI] [PubMed] [Google Scholar]
- 59.Presta LG. Selection, design, and engineering of therapeutic antibodies. J Allergy Clin Immunol. 2005;116:731–736. doi: 10.1016/j.jaci.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Frei Y, Lambris JD, Stockinger B. Generation of a monoclonal antibody to mouse C5 application in an ELISA assay for detection of anti-C5 antibodies. Mol Cell Probes. 1987;1:141–149. doi: 10.1016/0890-8508(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc Natl Acad Sci USA. 1995;92:8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexion Pharmaceuticals, Inc. Alexion; Cheshire, CT: Mar 16, 2007. FDA approves Alexion’s Soliris(™) for all patients with PNH—first therapy approved for this rare and life-threatening blood disease. http://ir.alexionpharm.com/releasedetail.cfm?ReleaseID=234156. [Google Scholar]
- 63.Thomas TC, et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33:1389–1401. doi: 10.1016/s0161-5890(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 64.Proctor LM, Woodruff TM, Taylor SM. Recent developments in C5/C5a inhibitors. Expert Opin Ther Pat. 2006;16:445–458. [Google Scholar]
- 65.Adis International Ltd. Eculizumab. Drugs R D. 2007;8:61–68. [Google Scholar]
- 66.Whiss PA. Pexelizumab Alexion. Curr Opin Investig Drugs. 2002;3:870–877. [PubMed] [Google Scholar]
- 67.Armstrong PW, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. J Am Med Assoc. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 68.Morrow T. A promising theory stumbles in clinical trials. Manag Care. 2007;16:69–70. [PubMed] [Google Scholar]
- 69.Mitchell Steve. Analysis: Alexion’s pexelizumab fails. 2007 January 2; http://www.upi.com/Health_Business/Analysis/2007/01/02/analysis_alexions_pexelizumab_fails/6113/
- 70.Taube C, et al. Factor B of the alternative complement pathway regulates development of airway hyperresponsiveness and inflammation. Proc Natl Acad Sci USA. 2006;103:8084–8089. doi: 10.1073/pnas.0602357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teeling JL, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 72.Teeling JL, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 73.Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-bind-ing peptide isolated from a phage-displayed random peptide library. J Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- 74.Janssen BJ, Halff EF, Lambris JD, Gros P. Structure of compstatin in complex with complement component C3c reveals a new mechanism of complement inhibition. J Biol Chem. 2007;282:29241–29247. doi: 10.1074/jbc.M704587200. [DOI] [PubMed] [Google Scholar]
- 75.Sahu A, Morikis D, Lambris JD. Compstatin, a peptide inhibitor of complement, exhibits species-specific binding to complement component C3. Mol Immunol. 2003;39:557–566. doi: 10.1016/s0161-5890(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 76.Katragadda M, Magotti P, Sfyroera G, Lambris JD. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, comp-statin. J Med Chem. 2006;49:4616–4622. doi: 10.1021/jm0603419. [DOI] [PubMed] [Google Scholar]
- 77.Potentia Pharmaceuticals, Inc. Potentia; Louisville, KY: Mar 20, 2007. Potentia Pharmaceuticals announces initiation of phase I clinical trials to evaluate its lead compound for age-related macular degeneration. http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/03-20-2007/0004549227&EDATE= [Google Scholar]
- 78.Biesecker G, Dihel L, Enney K, Bendele RA. Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology. 1999;42:219–230. doi: 10.1016/s0162-3109(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 79.Bunka DH, Stockley PG. Aptamers come of age—at last. Nat Rev Microbiol. 2006;4:588–596. doi: 10.1038/nrmicro1458. [DOI] [PubMed] [Google Scholar]
- 80.Ng EW, et al. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 81.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen NJ, et al. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- 83.Allegretti M, et al. Targeting C5a: recent advances in drug discovery. Curr Med Chem. 2005;12:217–236. doi: 10.2174/0929867053363379. [DOI] [PubMed] [Google Scholar]
- 84.Wong AK, Taylor SM, Fairlie DP. Development of C5a receptor antagonists. IDrugs. 1999;2:686–693. [PubMed] [Google Scholar]
- 85.Kohl J. Drug evaluation: the C5a receptor antagonist PMX-53. Curr Opin Mol Ther. 2006;8:529–538. [PubMed] [Google Scholar]
- 86.March DR, et al. Potent cyclic antagonists of the complement C5a receptor on human polymorphonuclear leukocytes. Relationships between structures and activity. Mol Pharmacol. 2004;65:868–879. doi: 10.1124/mol.65.4.868. [DOI] [PubMed] [Google Scholar]
- 87.Woodruff TM, et al. Therapeutic activity of C5a receptor antagonists in a rat model of neurodegeneration. FASEB J. 2006;20:1407–1417. doi: 10.1096/fj.05-5814com. [DOI] [PubMed] [Google Scholar]
- 88.Petersen KA, et al. Phase I safety, tolerability, and pharmacokinetic study of recombinant human mannan-binding lectin. J Clin Immunol. 2006;26:465–475. doi: 10.1007/s10875-006-9037-z. [DOI] [PubMed] [Google Scholar]
- 89.Bureeva S, et al. Selective inhibition of the interaction of C1q with immunoglobulins and the classical pathway of complement activation by steroids and triterpenoids sulfates. Bioorg Med Chem. 2007;15:3489–3498. doi: 10.1016/j.bmc.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 90.Janssen BJ, et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437:505–511. doi: 10.1038/nature04005. [DOI] [PubMed] [Google Scholar]
- 91.Janssen BJ, Christodoulidou A, McCarthy A, Lambris JD, Gros P. Structure of C3b reveals conformational changes that underlie complement activity. Nature. 2006;444:213–216. doi: 10.1038/nature05172. [DOI] [PubMed] [Google Scholar]
- 92.Wiesmann C, et al. Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature. 2006;444:217–220. doi: 10.1038/nature05263. [DOI] [PubMed] [Google Scholar]
- 93.Nagar B, Jones RG, Diefenbach RJ, Isenman DE, Rini JM. X-ray crystal structure of C3d: a C3 fragment and ligand for complement receptor 2. Science. 1998;280:1277–1281. doi: 10.1126/science.280.5367.1277. [DOI] [PubMed] [Google Scholar]
- 94.Milder FJ, et al. Factor B structure provides insights into activation of the central protease of the complement system. Nat Struct Mol Biol. 2007;14:224–228. doi: 10.1038/nsmb1210. [DOI] [PubMed] [Google Scholar]
- 95.Narayana SV, et al. Structure of human factor D. A complement system protein at 2.0 A resolution. J Mol Biol. 1994;235:695–708. doi: 10.1006/jmbi.1994.1021. [DOI] [PubMed] [Google Scholar]
- 96.Katschke KJ, Jr, et al. A novel inhibitor of the alternative pathway of complement reverses inflammation and bone destruction in experimental arthritis. J Exp Med. 2007;204:1319–1325. doi: 10.1084/jem.20070432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keller TH, Pichota A, Yin Z. A practical view of ‘druggability’. Curr Opin Chem Biol. 2006;10:357–361. doi: 10.1016/j.cbpa.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 98.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 99.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 100.Gould Rothberg BE, Pena CEA, Rothberg JM. A systems biology approach to target identification and validation for human chronic disease drug discovery. In: Knäblein J, editor. Modern Biopharmaceuticals. Vol. 1. Wiley-VCH Verlag; 2005. pp. 99–125. [Google Scholar]
- 101.Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 2006;25:420–428. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- 102.Haffner ME, Whitley J, Moses M. Two decades of orphan product development. Nat Rev Drug Discov. 2002;1:821–825. doi: 10.1038/nrd919. [DOI] [PubMed] [Google Scholar]
- 103.Title 21. United States Code (USC) Section 360ee.