Altered activity-rest patterns in mice with a human autosomal-dominant nocturnal frontal lobe epilepsy mutation in the β2 nicotinic receptor (original) (raw)
. Author manuscript; available in PMC: 2012 Apr 1.
Published in final edited form as: Mol Psychiatry. 2010 Jul 6;16(10):1048–1061. doi: 10.1038/mp.2010.78
Abstract
High-affinity nicotinic receptors containing beta2 subunits (β2*) are widely expressed in the brain, modulating many neuronal processes and contributing to neuropathologies such as Alzheimer’s disease, Parkinson’s disease and epilepsy. Mutations in both the α4 and β2 subunits are associated with a rare partial epilepsy, autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE). Here we introduced one such human missense mutation into the mouse genome to generate a knock-in strain carrying a valine-to-leucine mutation β2V287L.β2V287L mice were viable and born at an expected Mendelian ratio. Surprisingly, mice did not display an overt seizure phenotype; however homozygous mice did display significant alterations in their activity-rest patterns. This was manifest as an increase in activity during the light cycle suggestive of disturbances in the normal sleep patterns of mice; a parallel phenotype to that found in human ADNFLE patients. Consistent with the role of nicotinic receptors in reward pathways, we found that β2V287L mice did not develop a normal proclivity to voluntary wheel running, a model for natural reward. Anxiety-related behaviors were also affected by the V287L mutation. Mutant mice spent more time in the open arms on the elevated plus maze (EPM) suggesting that they had reduced levels of anxiety. Together, these findings emphasize several important roles of β2* nicotinic receptors in complex biological processes including the activity-rest cycle, natural reward, and anxiety.
Keywords: β2* nicotinic receptor, ADNFLE, knock-in mouse
Introduction
Neuronal nicotinic acetylcholine receptors (nAChRs) belong to a super family of Cys-loop ligand-gated ion channels whose members also include receptors for GABA, glycine and 5HT (1). Most nAChRs are pentameric heteromers which can be assembled from the known 11 mammalian subunits α2-10 and β2-4 leading to considerable functional heterogeneity (2). The majority of neuronal receptors are comprised of the α4 and β2 subunits (3, 4) and are widely expressed throughout the CNS (5). In particular the high-affinity β2-containing receptors (β2*) are ubiquitous in the brain (6) and their important neuromodulatory role has implicated them in several cognitive processes and pathophysiological conditions (7). Generation of a β2 knockout mouse greatly enhanced our understanding of β2* receptor function in vivo. These studies demonstrated that β2* receptors were necessary for all neuronal high-affinity nicotine binding sites in the CNS (8). Both the physiological effects on cellular excitability, and the behavioral effects of reinforcement by nicotine were abolished in knockout mice, demonstrating that β2 subunits are requisite members of functional receptors (9). Subsequent studies in β2 knockout mice have further uncovered several important phenotypes including an important role of β2* receptors in associative learning (8), locomotor activity (10, 11), exploration (12) and sleep (13, 14).
Recently, genetic association studies have linked the α2, α4 and the β2 subunit to autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE). In one family a single missense mutation in the gene encoding the β2 receptor, CHRNB2, leads to a valine to leucine substitution at position 287 (V287L), a residue which sits in the second transmembrane domain of the receptor subunit and contributes to receptor gating (15). Examination of the impact of the V287L in recombinant receptors has revealed several effects of this mutation on β2* receptor function; slowed channel desensitization (15), an increase in the apparent affinity of agonists (16), a decrease in Ca2+-dependent potentiation (17), and effects on channel assembly and trafficking (18). ADNFLE can occur in patients who are heterozygous for the missense mutation; however penetrance is incomplete, with only a proportion of carriers affected by seizures. While the genetic basis for these familial epilepsies have been uncovered, the environmental contribution to the disease, and the cellular and behavioral aspects are not as clear.
In the present study, we generated a novel mutant mouse harboring the human ADNFLE mutation in the Chrnb2 gene (β2V287L). This knock-in strain is potentially a valuable asset for examining the cellular mechanisms of the disease. In addition, given the widespread expression and high-affinity agonist binding toβ2* receptors in the brain, β2V287L mice can be used for further determining the role of β2* receptors in vivo by analyzing the endophenotypes generated by this “gain-of-function” mutation. We found that spontaneous motor seizures occurred only rarely in β2V287L mutant mice, however a more robust phenotype was a disequilibrium in activity-rest patterns in this strain. β2V287L mice were more active during the light cycle, suggesting that normal sleep patterns are disturbed. Consistent with a role for β2* receptors in reward pathways we also found that β2V287L mice had deficits in the development of voluntary wheel running. In addition, mutant mice displayed a reduction in anxiety-related behaviors. Taken together our findings demonstrate several important roles of β2* receptors and indicate that development of β2V287L mice provides a more concise view of the cellular and molecular basis for ADNFLE and the related phenotypes of the β2V287L missense mutation.
Materials and Methods
Generation of β2V287L mice
Conventional gene-targeting techniques were used to generate β2V287L knock-in mice (see supplementary Figure S1). A 20 kb genomic DNA fragment containing the Chrnb2 gene was isolated from a bacterial artificial chromosome (BAC) clone (RPCI-271-H24, obtained from BACPAC Resource Center at Children’s Hospital Oakland Research Institute in Oakland). A cassette containing a neomycin resistance (neo) gene, flanked by loxP sites, under the control of the phosphoglycerol kinase (PGK) promoter was introduced into the intron 720 bp downstream of exon 5 (Figure S1a). R1 mouse embryonic stem cells (19) were electroporated with the linearized targeting construct, maintained in G418 for positive selection, and screened by Southern blot for homologous recombination (Figure S1b). Chimeric animals produced by injection of these cells into C57BL/6 blastocysts were bred with C57BL/6 mice, and germ-line transmission of the mutation confirmed by Southern blot analysis of genomic DNA (Figure S1c). The neo cassette was removed in mice by crossing with a “Cre-deleter” line which expressed Cre recombinase in all tissues under the control of the protamine 1 promoter (20). Cre-mediated germline deletion of the neo cassette was confirmed by Southern blot and PCR genotyping. Mice heterozygous for the targeted allele were backcrossed greater than ten generations onto a C57BL/6 background. All wt and hom mutant mice used in these experiments were littermates derived from het crosses. Same sex littermates were housed 2–5 per cage, and maintained at 22°C, with a 12hr light/dark cycle.
Rotarod test
Motor coordination and balance were tested on an accelerating rotarod. Mice were placed on the rotarod and the speed accelerated at a rate of 0.1 rpm/s starting from 5 rpm. Mice were subjected to 3 trials per day for 3 consecutive days with ~30 minutes of inter-trial interval.
Wheel-running experiments
Wheel-running experiments were performed using the Wheel System (Lafayette Instruments) in a testing room with a 12-hr light, 12-hr dark light-dark (LD) cycle. In total experiments lasted for 37 days. 24-hr patterns of wheel-running activity were recorded and analyzed with ClockLab software (Coulbourn Instruments).
Open-field experiments
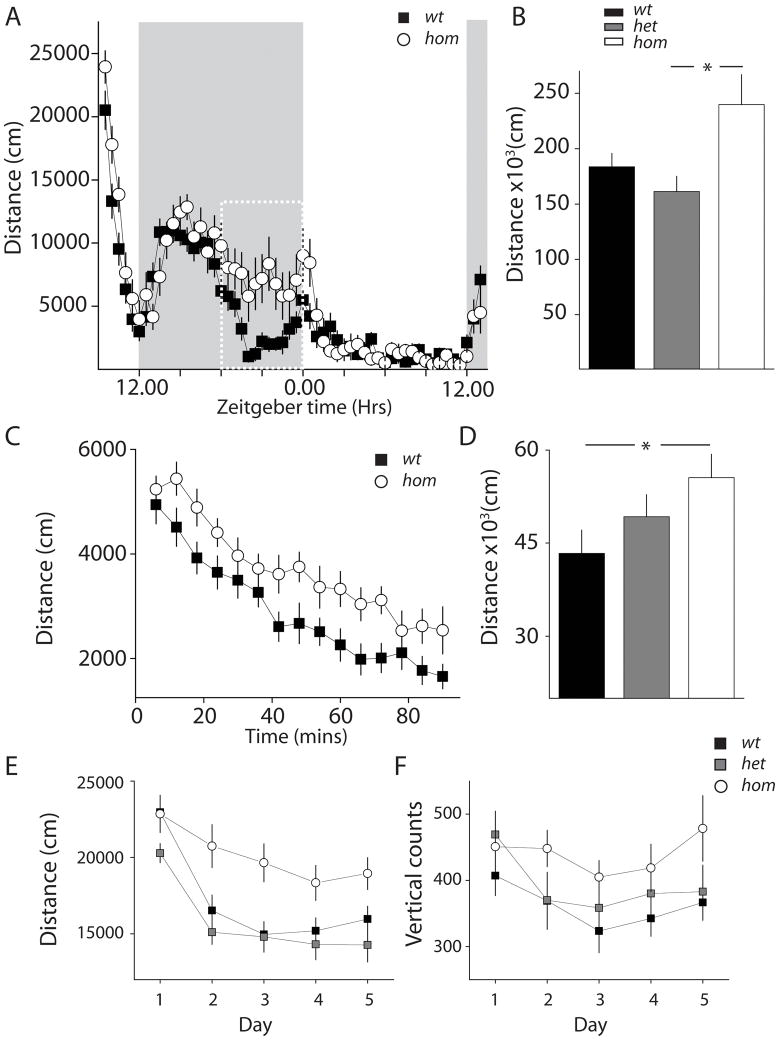
Open-field tests were performed in 42 cm × 42 cm behavioral chambers (Medical Associates). Movement was detected by infrared beam breaks. Animals were housed in a 12-hr:12-hr LD cycle prior to testing. The room for the open-field test had the same 12-hr:12-hr LD cycle. For experiments in Figure 4A–D, mice were introduced to chambers 3 hrs before lights were switched off. Their activity in the chamber was monitored for 28 hr. Food and water was provided in the testing chamber. Different cohorts of mice were used in the experiments in Figure 4E–F. The tests were conducted during the light cycle, ~3 hrs before dark onset. Each mouse was placed in the chamber for 30 minutes each day for 5 consecutive days.
Figure 4. β2V287L mice demonstrate increased locomotor activity and decreased habituation.
(A) Time series (28 hrs) of distance traveled during a single session open-field test. Animals (wt, n=14; het, n=15; hom, n =13) were introduced to open-field test chambers at 09:00 h on day1 with lights on. Lights were switched off at 12:00 h day 1, switched on at 0:00 h day 2 and off at 12:00 h day 2. Experiments were stopped at 13:00 h day 2. (B) Total distance traveled during a full light/dark cycle (12:00 h day 1 to 12:00 h day 2). *p<0.05; Tukey’s multiple-comparison test. (C), Time series and (D) total distance traveled for the initial 1.5 hour. The effect of genotype was not significant by Tukey’s multiple comparison test. However, a significant difference between wt and hom was detected by t-test. *p<0.05, t-test. (E&F) Open-field inter-session habituation test. Animals (wt, n=14; het, n=13; hom, n =12) were tested for 30 minutes each day over 5 consecutive days. Intersession-habituation was evaluated by horizontal distance traveled (E) and rearing assessed by vertical counts (F) across the testing sessions.
Anxiety-related behaviors
The elevated plus maze (EPM) test was performed using a maze consisting of two open arms (30 × 5 cm) and two closed arms (30 × 5 cm) constructed of black Plexiglas that extended from a center platform. The closed arms had sides of clear Plexiglas (15 cm tall, 0.5 cm thick). The entire apparatus was mounted 33 cm above the surface of the floor. Tests were performed during the dark phase of the LD cycle (~ 2 to 5 hrs after dark onset). Each test was begun by placing the mouse in the center of the maze facing one of the open arms. Each mouse was allowed to stay on the EPM for 5 minutes, and data was acquired by video tape. Subjects were scored for the total amount of time spent, and the number of entries into the open and closed arms, determined by placement of all four paws into that area. For open-field anxiety tests mice were placed in the center of a behavioral chamber (42 cm × 42 cm) and allowed to explore the whole field for 30 mins. Time spent in the inner squares, which is defined as a 30 × 30 cm central area that covers ~52% of open-field area, and inner-square crossings were used as a measure of anxiety-related behavior. Tests were performed during the light phase of the LD cycle (~3 hrs before the dark onset). L/D exploration tests were performed in the same behavioral chambers during the dark phase of the LD cycle (~ 2 to 5 hrs after dark onset). An L/D insert was used which divided the chamber into two compartments; one relatively large and bright, and the other small and dark. Mice were initially placed in the lit side, and transitions between the two sides and the time spent in each compartment were recorded for 5 min.
Nicotine-Induced seizures
Prior to experiments, mice were housed under a 12:12-hr L/D cycle. Mice were subjected to intraperitoneal injections with vehicle (phosphate buffer), or various doses of nicotine (n ≥ 5 for each group). Animals received a single i.p. injection and were observed for the ensuing 5–10 min for seizure activity and scored for their overall sensitivity to nicotine on a scale of 1 to 7. Seizure classification was based on previously published systems with slight modifications (21, 22). Class 0: no visible response. Class 1: sedation and loss of locomotion. Class 2: circling, head bobbing, Straub tail (with tail bending > 60° angle, lasting > 10s), fast breathing, slight back hunch, head bending. Class 3: loss of balance (wobbling), slight tremors, moderate back hunch. Class 4: severe tremors, severe back hunch, very slight convulsion, body jerking, tail swing, forelimb clonus. Class 5: wild running, clonic body movement, falling with clonic forelimb and hindlimb movement. Class 6: clonic-tonic convulsion (both limbs), falling with tonic movement. Class 7: tonic hyperextension of the hindlimb, death.
Morris Water Maze
Experiments were conducted as previously described (23). Briefly a 120 cm diameter tank filled with opaque water and containing a transparent platform submerged 1cm below the surface was used in combination with an automated video tracking system to record the swim path, velocity and time taken to reach the platform (latency) or the time spent in each zone. Mice were first trained to find a visible platform for 3 days (3 trials per day). Hidden platform training was conducted one day after the completion of the visible platform training. Mice were trained for 6 days (3 trials per day) to find the submerged platform at a fixed position. Distal cues in the testing room were provided as spatial references. Each trial lasted either until the mouse found the platform, or for 60s. Starting points were changed every trial. Mice were allowed to rest on the platform for 15s after each trial. For the reverse test, the hidden platform was moved to the opposite quadrant without changing any distal visual cues. Mice were then trained to find this new platform location for 3 days.
Statistical analysis
Statistical analyses were conducted with Graphpad Prism. Data of all three genotypes, wt, het and hom, were analyzed. One-way ANOVA was used when genotype was the only grouping variable, and when data were collected in a single trial or single session. Differences between two means were assessed with Tukey’s multiple-comparison tests, unless specified otherwise. Student’s _t-_test was used to assess significance between 2 groups in Figure 4D. For the multiple trial experiments, two-way RM ANOVA was conducted to assess the effects of both genotype and sessions/trials. Genotype comparisons at individual time points/trials were assessed by Bonferroni post-tests. One-way RM ANOVA was conducted to assess the effects of training blocks/trials within the same genotype group. Post-test for linear trend was carried out to determine whether there is an increasing/deceasing trend during habituation tests (Figure 4F). All data are presented as mean ± SEM. Differences were considered significant if p values were < 0.05.
Results
Using standard gene-targeting techniques we introduced the V287L mutation into the mouse Chrnb2 gene (Figure S1). Mutant β2V287L mice were born at the expected Mendelian ratio, suggesting that there was little, if any, problem with embryo development, or even any negative impact of the mutation on perinatal survival. Spontaneous motor seizures rarely occurred in the mutant mice. However when mice were followed for longer periods, we found that homozygous mice (hom) had a significantly more pronounced mortality rate (Figure S1 F & G). It is possible that an undetected seizure endophenotype contributed this increased mortality (24), or alternatively, as high affinity nAChRs are known to be important to respiratory patterns (25), alterations in breathing and arousal responses during sleep, could contribute to increased death in these animals.
Activity-rest patterns are altered in β2 V287L mice
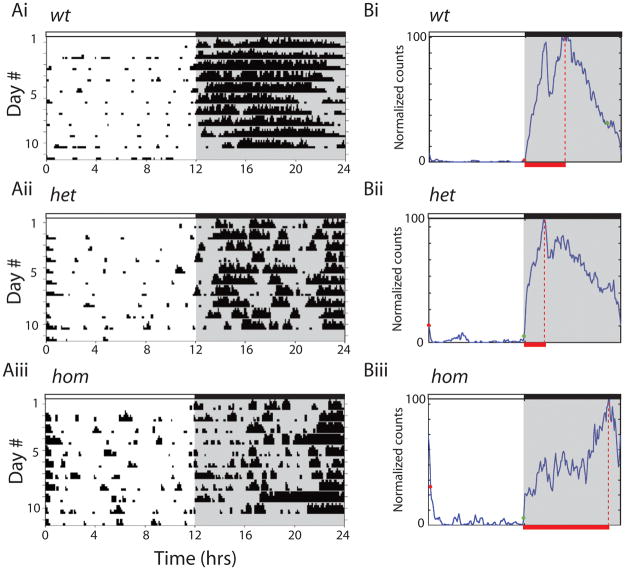
In the human disease, ADNFLE seizures occur predominantly during sleep (26). Even in the absence of seizures, many ADNFLE patients endure severe sleep disruption and excessive daytime fatigue (27, 28). These clinical observations suggest that ADNFLE mutations may affect activity-rest rhythms. We tested this idea in animals using wheel-running experiments by following animals continuously for 37 days (n=10 for each group). In the first set of experiments we analyzed wheel-running using actograms created during the final 10 days, once mice had become habituated to the wheel apparatus (Figure 1). We found that the activity-rest profile in hom mice was significantly altered compared to wt animals. Wt animals exhibited typical activity consolidation during the dark cycle. Their activity onset was coincident with the onset of the dark cycle and activity peaked to the maximal activity levels early in the dark period (Figure 1Ai and Bi). However, in hom mutant mice the individual activity-rest cycles were less consolidated into the dark cycle as indicated by a high level of fragmentation during both dark and light periods (Figure 1Aiii). The activity of wt animals peaked 3–5 hrs into the dark cycle, and then gradually declined to low levels during the remainder of the dark period, with much reduced activity present at the onset of the light cycle (Figure 1Bi). The het group had an activity profile similar to wt mice but with some subtle differences such as elevated activity at the end of the dark cycle. In contrast, β2V287L hom mutant mice had a grossly different activity pattern. Hom mice did not have a rapid transition to high activity with the onset of the dark cycle, the latency to peak activity during the dark cycle was lengthened, and activity remained elevated at the end of the dark cycle and beginning of the light phase (Figure 1Biii).
Figure 1. Activity profiles of β2V287L mice are altered.
(Ai-iii) Representative actograms of a wt, het and hom mutant mice. Observations were made during a 12-h light:12-h dark (LD) cycle for ~40 days. 24-h patterns of wheel-running activity recorded over the last 10 days of the test were analyzed for all three genotypes (n = 10 for each group). (Bi-iii) Normalized activity profiles of wt, het and hom animals for 24 hour LD cycle (n = 10 for each group).
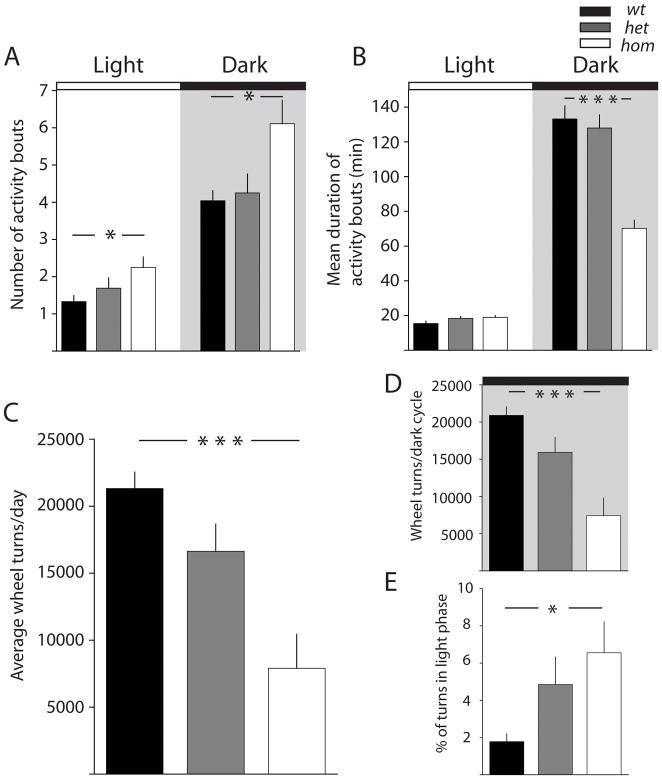
A closer examination of the individual activity bouts more acutely revealed disturbances of activity-rest consolidation in β2V287L mutant mice. During the light cycle, wt mice exhibited few activity bouts (1.3 ± 0.2) which were of short average duration (15.4 ± 1.4 min) (Figure 2A & B). During the dark phase of the cycle, wt mice had an increase in the number of activity bouts which were consolidated into longer episodes of sustained activity (133 ± 7.5 min). In contrast mutant hom mice performed significantly more activity bouts both during the light (2.3 ± 0.3; F2, 27 = 3.56 p= 0.043) and dark cycle (6.1 ± 0.6; F 2,27 = 5.30, p= 0.012) compared to wt mice (Figure 2A & B). The duration of these activity bouts was not different during the light cycle (wt: 15.4 ± 1.4 min; hom: 19.0 ± 1.0 min, F 2,523 = 2.57, p > 0.05). However the average duration of bouts during the dark cycle was significantly reduced in hom mice (70 ± 4.7 min, F 2,1428 = 32.98, p< 0.0001) compared to wt animals. The increased number of activity bouts during the light phase is indicative of frequent sleep interruptions in the β2V287L mutant mice. In addition, the increase in the number of activity bouts of relatively shorter duration during the dark phase suggest that β2V287L rest more frequently during the dark cycle, and are unable to maintain consolidated individual activity episodes for the same duration as wt and het mice.
Figure 2. Disruption of activity-rest rhythm by the V287L mutation.
(A) Number of activity bouts during the light phase and dark phase and (B) the average duration of activity bouts during each phase. (C) Average wheel turns per day for each genotype (D) Number of wheel turns during the dark cycle and (E) fraction of wheel turns during light phase. Data are presented as the mean ± SEM. *p<0.05,***p<0.001; Tukey’s multiple-comparison test.
When we calculated the activity of mice by measuring the total number of wheel turns we found that hom mutants exhibited ~40% of the running activity as compared to wt mice (F2,27=11.15, p=0.0002) (Figure 2C). This overall decrease in activity was primarily due to a significant decline in number of wheel turns during the dark phase (Figure 2D, F2,27=12.61, p<0.0001) with no effect of genotype observed on the total wheel turns during the light cycle (_wt_: 414 ± 110; _hom_: 496 ± 219, F2,27 =0.73, p>0.05). Therefore only 1.8 ± 0.4 % of the total activity of wildtype mice was during the light cycle, whereas a significantly higher portion of the activity of β2V287L was in the same period (6.6 ± 1.7 %, F2, 27 = 3.434, p = 0.047) (Figure 2E).
Development of wheel-running activity as a natural reward
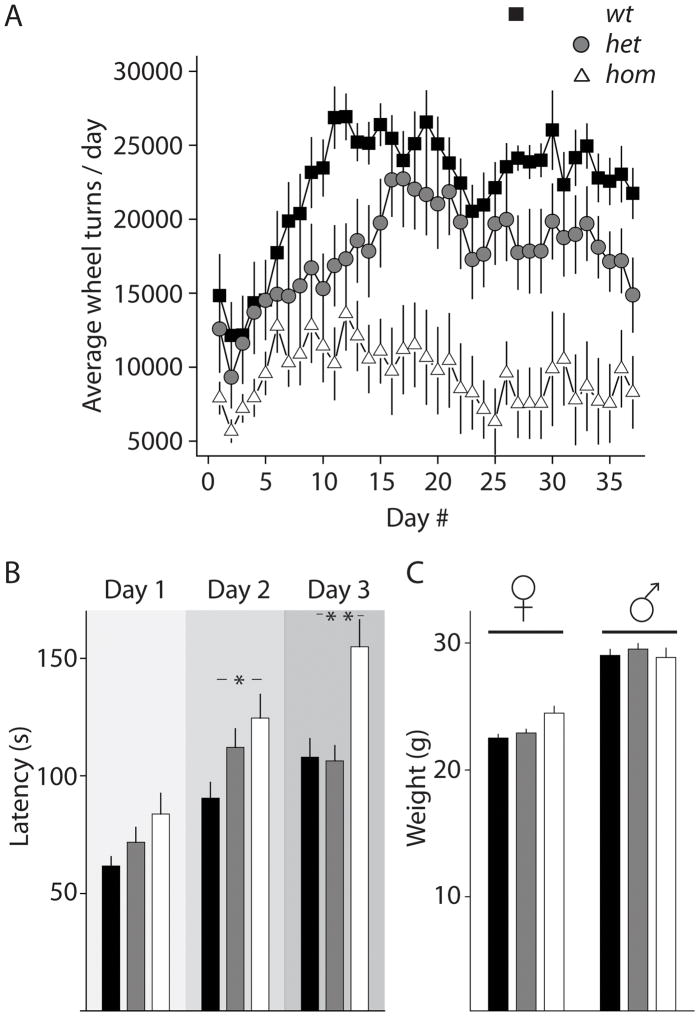
The initial aim of the wheel-running experiments was to determine activity-rest patterns in β2V287L mice. However time-course analysis of daily wheel-running activities revealed a surprising effect of the mutation on the development of voluntary wheel-running (Figure 3A). Animals were allowed free access to wheels and experiments were conducted during a 12:12-hr LD cycle for a total of 37 days. After wheels were introduced to the home cage, wt mice increased their daily running activity over the first 12 days. After this initial elevation running activity was maintained at a near plateau state for the remainder of the 37 day period, similar to what has been reported previously for voluntary running behavior (29)(Figure 3A). Het mice showed a similar pattern in running behavior, with a steady increase in daily running that peaked at day 17, 5 days later than wt mice. Running activity was subsequently maintained close to this maximum peak activity (Figure 3A). In contrast, hom mice performed considerably less wheel-running than their het and wt littermate controls throughout the duration of the test. While there was an initial increase of activity lasting for approximately 6 days, hom mice never reached the high activity of the wt and het mice. Analysis of the running activities during the first 20 days revealed a significant gene-dosage effects (F2,513=7.66, p=0.0023) and genotype-day interaction (F38,513=3.42, p<0.0001). During the last 10 days of the test when the running activities of all three cohorts were stable (F18,243=0.85, p=0.65, for genotype-day interaction), the gene-dosage effect of the β2V287L mutation on daily running was significant (main genotype effect: F2,243=12.25, p=0.0002)(Figure 3A). The development of voluntary wheel running by rodents, as measured by the increase of total wheel running by day, has been established as a model for natural reward (29, 30). Therefore, it is possible that that the β2V287L mutation in vivo affects reward pathways, consistent with the known involvement of β2* receptors in other reward and addiction processes (31). In order to rule out the possibility that the reduced running in mutant mice was instead caused by a general motor deficit, we conducted rotarod tests to examine if motor coordination/balance and motor learning were altered in β2V287L mutant mice (Figure 3B). Trials were performed over three consecutive days (3 trials/day) and the daily averages for the latency to fall from the accelerating rotarod were measured. Each cohort (wt, n= 23; het, n= 25; hom, n=19) showed improvement in their ability to remain on the rotarod across days (F2,128 = 61.2; p< 0.0001); however hom mutants outperformed het and wt groups on each day of testing. Overall the hom mutant mice were able to remain on the rotarod significantly longer during the test than wt and het mice (main effect of genotype, F2,128=7.46, p=0.0012). There was no difference in the body weights of wt, het, or hom mice, eliminating the possibility that size or weight differences could contribute to the ability of mice to remain on the rotarod (Figure 3C). Therefore, surprisingly motor coordination and learning was actually improved by the β2V287L hom mutation, and the significant reduction in voluntary wheel-running in hom mutant mice is unlikely to be caused by a general motor deficit.
Figure 3. Deficits in development of voluntary wheel-running, but enhanced performance in motor coordination of β2V287L mutant mice.
(A) Time-course of voluntary wheel running. Mice had free access to running wheels for 37 days. Average daily wheel turns increased in wt and het mice over the test period. Hom mice showed little proclivity towards increased running behaviors (n = 10 mice for each genotype). (B) Performance on an accelerating rotarod task. Mice (wt, n= 23; het, n= 25; hom, n=19) were evaluated on the rotarod test three times a day for 3 consecutive days. Latency to fall of the rotating rotarod was measured on each day. Data are presented as the mean ± SEM of the daily averages. *p<0.05, **p < 0.01; Tukey’s multiple-comparison test. (C) Comparison of body weights of female (♀) and male (♂) mice (10–30 week animals).
Open-field activity and locomotor habituation in β2V287L mice
In the experiments just described, activity-rest patterns were monitored by wheel-running activity of mice in a well-habituated environment. In order to further assess locomotor activity and daily activity-rest rhythms, we performed open-field tests. Naïve animals (wt, n=14; het, n=15; hom, n =13) were introduced to activity chambers at 09:00 hr (Zeitgeber time, 3 hrs before light-off) and were monitored until 13:00 hr on the second day. Mice of all three genotype groups exhibited activity-rest patterns that are consistent with normal daily circadian rhythms. Activity of all mouse cohorts was consolidated during the dark phase, and the onset of increased activity was coincident with the dark onset time. All three groups reached the maximal level of activity 3 – 4 hrs after dark onset, and subsequently activity gradually declined for all three groups (Figure 4A)(het data not shown). About 8 hrs into the dark phase, wt (and het) mice displayed an abrupt reduction in activity level, which then remained low until the final hour of the dark phase (Figure 4A). In contrast, hom mutant mice maintained their activity levels during the same time period. All mouse cohorts demonstrated a brief increase in activity around light-onset, followed by very low levels of activity during the entire light-phase. Overall, there was no significant difference in the total distance run by wt and hom mice (Figure 4B). However, the difference in distance traveled by hom and het mice did achieve significance (F2,29= 4.80, P = 0.0137) (Figure 4b). This small increase in overall activity over the 28 hour period is due to the sustained activity of hom mice during the last six hours in the dark phase (Figure 4A white box). There was no difference in the activity between any of the genotypes in this open field test during the 12-h light phase (Distance traveled; wt: 37000 ± 4300 cm; het: 31000 ± 2800 cm; hom: 38000 ± 3100 cm, F2,39 =1.23, p=0.30). This is in contrast to the increased activity of hom mice that we observed during the light phase in the well-habituated environment (Figure 1).
The same experiment also allowed us to determine the initial exploratory locomotor activity of β2V287L mice by measuring the distance traveled during the first 90 minutes of the open field test. Hom mutant mice demonstrated increased levels of activity, travelling over the greatest distance in this novel environment (overall effect of genotype: F2,39 =2.64 P=0.08; wt vs. hom, p=0.030, _t_-test) (Figure 4C & D). Over the course of the experiment each mouse cohort habituated to the novel environment, with activity declining over time; however, hom mutant mice habituated significantly more slowly (Figure 4C) (F8,156=2.12, p=0.037 for time-genotype interaction). Taken together, these results suggest that that intra-session habituation to a novel environment is partially impaired in hom β2V287L mutant mice.
The above results suggest that β2V287L have a more robust exploratory response in a novel environment. To test this further we performed further test on different cohorts of mice in the open field chamber. In this case, mice were introduced to the open field for only 30 minutes each day for 5 consecutive days to test inter-session habituation. Both locomotion (horizontal distance traveled) and rearing (vertical counts measured by beam breaks) were quantified. The distance traveled on Day 1 of the experiment was the same for each genotype group (Figure 4E). However, on the Day 2 trial het and wt mice exhibited a large decline in locomotion whereas hom mice exploratory behavior was still elevated (Figure 4E). A two-way RM ANOVA revealed a significant effect of genotype (F2,144=6.40, p=0.0042) and genotype-day interaction (F8,144=2.92, p=0.0047). The habituation pattern was less apparent for rearing behavior with no significant effects of genotype and genotype-day interaction detected by two-way RM ANOVA on vertical counts (Figure 4F). However, closer examination of this data revealed a trend of decrease in vertical counts in wt (slope = −10.76, p= 0.06) and het (slope= −116.24, p=0.01) mice, but not in hom mutants. These data are further support for a habituation phenotype in β2V287L mice.
Decreased anxiety-like behaviors in β2V287L mice
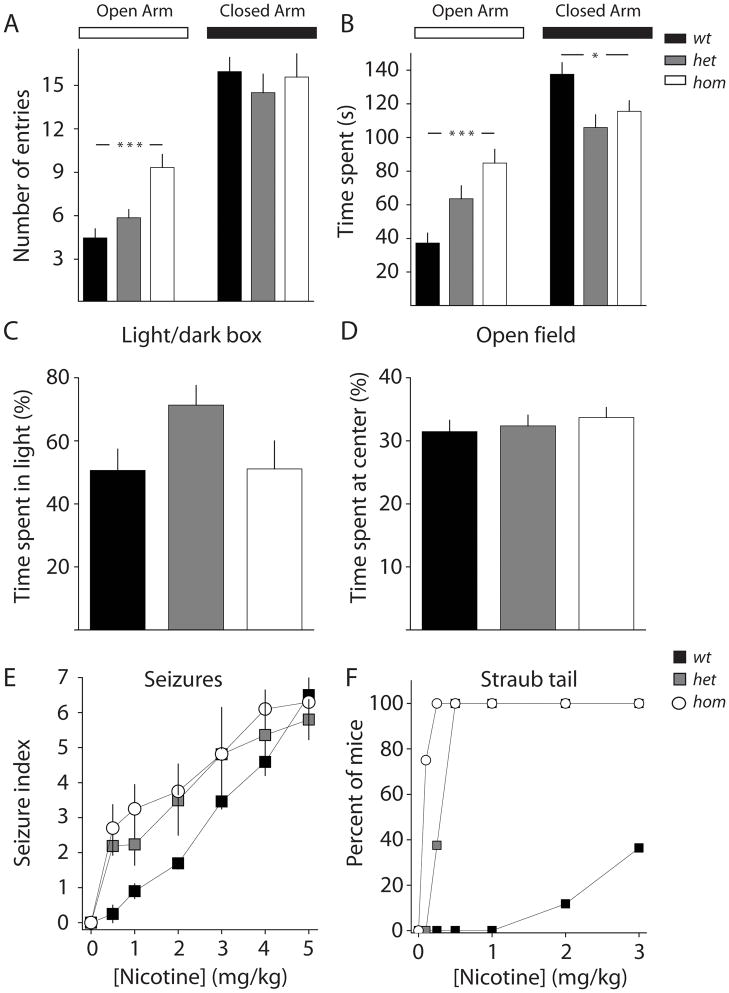
nAChRs are known to be involved in pathways that control anxiety-related behaviors (32–34). In fact some of the increased locomotor activity and lack of habituation found in these mice may be due to a decrease in anxiety. To directly test the effects of the V287L mutation on anxiety-like behaviors we used three behavioral paradigms which test different aspects of anxiety; the Elevated Plus Maze (EPM), the open-field test and the light/dark test. In the EPM test, hom and het mutant mice displayed significantly less anxiety than wt mice (wt, n= 19; het, n= 22; hom, n=21). Mutant animals entered the open arms of the EPM more often than wt mice (F2,59=12.04, p<0.0001) (Figure 5A) and spent more time in the open arms than wt animals (F2,59=9.80, p=0.0002), while wt mice spent more time in the closed arms than mutant animals (F2,59=5.27, p=0.0079)(Figure 5B). There was no overall difference in activity between the different genotypes in the EPM, as evidenced by a lack of difference in entries into the closed arms (F2,59=0.32, p=0.73), a common index of locomotor activity (Figure 5A). Together, these measures are consistent with overall less anxiety in β2V287L mutant mice in EPM tests. We also tested mice (wt: n=23; het: n=32; hom: n=19) in a second test of anxiety; the light/dark box. In this test het and hom did not demonstrate any difference from wt mice in the total transitions between the light and dark compartments (wt: 6.87 ± 0.81; het: 7.44 ± 0.59; hom: 6.21 ± 1.21; F2,71= 0.55, p=0.58) and there was no difference in the time spent in the light compartment between any of the genotypes (Figure 5C). Similarly, in an additional test of anxiety, the open-field test, no genotype differences were observed in the proportion of time spent by mice (wt, n=14; het, n=13; hom, n=13) in the center regions of the field (F2,37=0.44, p=0.65) (Figure 5D).
Figure 5. Effects of V287L mutation on anxiety-related behaviors and response to nicotine.
(A) Elevated-plus maze test (wt, n= 19; het, n= 22; hom, n=21). Number of entries into the open arms and closed arm, and (B) time spent in each arm *p<0.05, ***p<0.001; Tukey’s multiple-comparison test. (C) Light/dark box test (wt, n= 23; het, n=32; hom n=19). Percentage of total time spent in light compartment. Effect of genotype was not significant by Tukey’s multiple-comparison test. (D) Open-field tests (wt, n=14; het, n=13; hom, n=13). Total time spent in the center square. Effect of genotype was not significant by Tukey’s multiple-comparison test. Data are presented as the mean ± SEM. (E) Susceptibility to nicotine induced seizures is reduced in β2V287L mice. Seizure severity is plotted against nicotine dose. (F) Quantification of straub tail in response to I/P injection of nicotine.
It is possible that the performance of mutant animals in these tests could be influenced by alterations in vision. However, in the Morris Water Maze test β2V287L mice did not display any deficits in the visible platform testing or in the hidden platform portion of the test (which relies on distal visual cues), suggesting that mice had no gross deficits in vision as well as normal spatial learning (Figure S2). Similarly average swim speeds were not different between genotypes (Figure S2C).
Enhanced sensitivity to nicotine-induced seizures in β2V287L mice
High doses of nicotine induce seizures in rodents by acting on nAChRs (35, 36). Prior studies in ADFNLE knock-in strains with mutated α4 subunits displayed higher sensitivity to nicotine-induced seizures and it has been suggested that increased sensitivity to nicotinic agonists might be related to ADNFLE (17, 22, 37, 38). To determine if β2V287L mice had an altered response to nicotine, we injected mice in the C57Bl/6 background with several doses of nicotine. Both het and hom mutant mice exhibited enhanced nicotine-induced seizure sensitivity compared to their wt littermates (Figure 5E). β2V287L mice also displayed hypersensitivity to nicotine-induced dorsiflexion (Straub tail) (22, 39) in response to 0.5–3 mg/kg nicotine (Figure 5F). All hom mice injected with 0.25 mg/kg nicotine and all het mice injected with 0.5 mg/kg nicotine exhibited Straub tail, while a dose of 3 mg/kg nicotine elicited this response in fewer than 40% of wt mice. Susceptibility to nicotine-induced seizures depends on genetic background (36, 37), therefore to determine that the effects observed in β2V287L mice were not strain-associated, we crossed het mutants in the C57Bl/6 background to wildtype 129/Svj mice to yield F1 offspring of C57/Bl6×129/SvJ. Het mutants in this mixed background also exhibited higher sensitivity to nicotine-induced seizures and dorsiflexion than their wt littermates (Data not shown). Thus, the β2V287L mutation increases an animal’s sensitivity to nicotine-induced seizures in two distinct genetic backgrounds.
Discussion
Here we report the generation of the first mouse strain with a human ADNFLE knock-in mutation in the Chrnb2 gene. Phenotypic analysis revealed several important effects of this mutation on animal behavior, including alterations in activity-rest patterns, impairment in the development of wheel-running behavior, altered motor learning and locomotor habituation, and effects on anxiety-like behaviors and increased sensitivity to nicotine induced seizure and tail straub. These results demonstrate that the β2V287L mutation in mice recapitulates some of the known disruptions found in human ADNFLE patients, but in addition this knock-in mutation is informative of the neurobiology of high affinity β2* receptors.
ADNFLE
ADNFLE was the first group of familial monogenic epilepsies for which specific mutations were found (40). The first families described had mutations in the gene encoding the α4 receptor subunit CHRNA4 (40, 41), and these were later followed by evidence of mutations in the gene encoding β2 (CHRNB2) (15, 42, 43). The importance of these linkage studies provided the impetus to introduce the human mutations into the mouse genome to provide mouse models of the human disease. Thus far, several mice harboring mutations in the α4 subunit have been generated and described. Two strains α4 ADNFLE mice, the _Chrna4_S252F and _Chrna4_S248F lines, were found to have frequent spontaneous seizures associated with high-voltage EEG spikes (38). However, a separate mouse line created by another group harboring the same α4S252F did not have spontaneous seizures, but instead responded to low-dose nicotine injection with distinct stereotypic behaviors collectively termed dystonic activation complex (DAC) (37). A transgenic line (not a targeted mutation) expressing the same β2V287L mutant receptors, was found to have frequent spontaneous seizures (44); although the seizure phenotype in transgenic animals can be caused by artificial overexpression of β2 receptors. In the new line of knock-in mice reported here, we observed a very low probability of spontaneous motor seizures, which because they occurred so rarely, were difficult to quantify. A similar low instance of seizures were reported for α4 knock-in lines carrying the ADNFLE mutation or hypersensitive mutations (17, 22, 37). Interestingly, in families with the human mutation, the penetrance of the seizure phenotype is also not complete, suggesting that other genetic or environmental factors play a role (15, 45). However, consistent with the role of nicotinic receptors in modulating diverse brain functions, patients with ADNFLE mutations in the nicotinic receptor genes often suffer from multiple neurological disorders including mental retardation, memory deficits and central respiratory problems (46–49). Thus, mouse models provide uniquely powerful tools to understand the disease phenotype and improve future treatment.
Activity-rest patterns
The majority of ADNFLE seizures occur during non-REM sleep (50). However even in the absence of seizure activity, ADNFLE patients often endure severe sleep disruption and excessive daytime sleepiness (51, 52). These clinical observations suggest that ADNFLE mutations may affect sleep patterns and activity-rest rhythms. Furthermore, β2 mRNA levels have been demonstrated to be under circadian regulation with peak expression in the subjective evening; suggesting that β2* receptors might be involved in regulating daily rhythm (53). The most prominent finding in our current study linking the knock-in mutation in mouse to human ADFNLE is the extensive alteration in activity-rest patterns in β2V287L mutant mice. These alterations were manifest in multiple ways: 1. hom mutant mice demonstrated an increase in the number of activity bouts, and a higher percentage of total activity during the light phase, suggesting more sleep disruption. 2. hom mice demonstrated more activity bouts with shorter average duration during the dark phase, suggesting that mutant mice slept more frequently during the dark cycle. 3. The activity patterns of hom mice during the dark cycle were significantly altered. Peak activity of wt animals occurred ~3–5 hrs into the dark cycle then gradually declined. In contrast activity of hom mutant animals only slowly increased at the onset of the dark cycle but continued to increase throughout the dark phase. Together, these results indicate the involvement of β2* in the regulation of daily rhythms of activity-rest patterns. There is considerable evidence for the involvement of the cholinergic system in circadian rhythms. In particular, high affinity β2* receptors in the suprachiasmatic nucleus (SCN) have been implicated in modulating sleep-wake patterns (54, 55). ACh levels fluctuate with the light/dark cycle in rodents, with a substantial increase at lights out (56) and β2 knockout mice have altered REM and non-REM sleep patterns (14). Additionally activation of presynaptic β2-containing receptors in the ventral preoptic area of the hypothalamus promotes arousal by inhibiting sleep-promoting GABAergic neurons (57). The β2V287L mutation could therefore disrupt sleep by disturbing the regulation of these neurons. Thus, altered activity-rest patterns in β2V287L knock-in mice are consistent with a role for high affinity nAChRs in sleep regulation.
Natural reward
The roles of β2* receptors in nicotine addiction has been well established, and the involvement of nAChRs in drug reinforcement pathways are well documented (9, 58). However, it is not known if β2* receptors also play a role in the natural reward system in the brain which involves such behaviors as consumption of food, mating behaviors, and exercise. We found a severe deficit in the development of voluntary wheel-running in β2V287L mice providing the first evidence that β2* nAChRs are also important for natural reward-seeking behaviors. Coincidentally, we found that matings with male hom mice did not produce litters as regularly as matings involving het or wt male mice. This observation may also be due to a decreased response to natural reward in male hom mutant mice. Reward-seeking behaviors, either reinforced by natural reward or by drug stimuli, depend critically on dopamine signaling (59). The altered function of β2V287L receptors might therefore have an impact on the dopaminergic system. Activation or desensitization of β2* nAChRs by agonists, such as nicotine or ACh, can affect DA release through several mechanisms. First, agonists can act directly on β2* nAChRs expressed on cell bodies of mid-brain dopaminergic neurons to increase their firing rate (9). Second, activation of β2* at the presynaptic terminals of DA neurons can lead to enhanced dopamine release in striatum (60). Third, activation or desensitization of β2* nAChRs on midbrain GABAergic interneurons can modulate the firing rate of DA neurons by regulating GABA release (61). Fourth, agonists can act viaβ2* nAChRs in cholinergic interneurons in striatum to regulate dopamine release by midbrain DA neurons (62). Thus altered activation and desensitization kinetics of β2* receptors in β2V287L mice are likely to have a significant effect on reward pathways.
Anxiety-like behaviors
Nicotine has complex effects on anxiety. Previous studies have shown that nicotine can be anxiolytic and anxiogenic, depending on multiple factors including the behavioral paradigm, the nicotine dose, and the animal’s gender (34). Anxiolytic and anxiogenic actions of nicotine may be mediated by a specific subunit configuration of the nAChRs. Knockout mice lacking β4 or β3 subunits demonstrated reduced anxiety-like behaviors on the EPM (33, 63). In contrast, increased anxiety is observed by ablation of α4 (64), or in a knock-in mouse with a hypersensitive α4 mutation (65). Despite its widespread expression and involvement in several important neuronal processes the role of the β2 subunit in anxiety-related behaviors has not been conclusively demonstrated. A number of agonists that bind to the α4β2* nAChRs are known to have effects on anxiety (66). However, β2−/− mice did not have any alteration in the EPM test (67). In our study we found that the β2V287L mutation has a gene-dose dependant anxiolytic effect in the EPM. This observation provides the first strong evidence for the involvement of β2* nAChRs in modulating some aspects of anxiety-related behaviors. However, we performed two further anxiety tests in these mice and found no alterations. Anxiety is a multidimensional behavior and different tests likely evaluate different aspects of anxiety (68). Therefore it is likely that this divergence in findings in the light/dark box test and open-field test compared with the altered response in EPM test, suggests that the cholinergic system might be particularly important in regulating certain modes of anxiety.
Motor coordination, motor learning, locomotor activity and habituation
Nicotine administration affects motor skills in humans (69) and in rodent models (70). Ablation of either of the α7 or β2 receptor subunits individually does not have any effect on motor learning in mice. However, in double mutant α7 and β2 knockout mice, motor coordination and learning are enhanced (71). β2V287L hom mutant mice performed significantly better than the wt and het mice on the rotarod test further supporting a role of these receptors in motor learning.
One major function of nAChRs in the brain is to modulate motor activity through the dopaminergic system. Alterations in motor activity after nicotine exposure have been described in humans. Several nAChR mutant mice display altered locomotor activity and locomotor habituation in the open-field test (10, 72). β2−/− mice have a striking hyperactivity in the open-field test, presumably caused by an imbalance in DA neurotransmission (10). We found that β2V287L mutant mice were more active in the open-field test; however activity changes were more nuanced than those reported in β2−/− mice. Increased activity in β2V287L mice was most evident during the last several hours of the dark phase in a normal light/dark cycle (Figure 4A), suggesting that this change in activity was related to the activity-rest pattern rather than the generalized hyperactivity seen in β2−/− mice.
We also found that the V287L mutation had larger effects on locomotor habituation than on the initial exploratory activity. During the first trial of the open-field test, hom mice did not perform differently than het and wt littermates in the distance traveled. However, V287L hom mutant mice demonstrated a decreased habituation response both within the same test session (intra-session) and across multiple sessions (inter-session). Overall, these results provide additional evidence that β2* receptors have important roles in the habituation response of mice to a novel environment. This is consistent with previous findings that open-field habituation learning can be improved by nicotine and attenuated by the nAChR antagonists mecamylamine, which specifically blocks β2* nAChRs (73). Interestingly, defective habituation has been associated with Attention Deficit Hyperactivity Disorder (ADHD), and agonists of nAChRs can produce significant improvements in adults with ADHD (74, 75).
In summary, β2V287L knock-in mice provide a unique model to examine the roles of β2* nAChRs in vivo. The β2V287L mutation caused several behavioral alterations related both to the ADNFLE phenotype and to more general processes involving β2* receptors. The development of these mice also suggests that ADNFLE mutations may produce more complex biological disturbances than the manifestation of seizures. Therefore, understanding these complex behavioral consequences of ADNFLE mutations in the nicotinic receptor genes will provide better insight into the diagnosis and treatment of ADNFLE.
Supplementary Material
1
Acknowledgments
Supported by grants from NIH/NIDA (R01DA018247 to SFH) and NIH/NINDS (R01NS058894 to AC). We thank Drs Michael Marks, Sharon Grady and Allan Collins for their suggestions.
References
- 1.Karlin A, Akabas MH. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995 Dec;15(6):1231–44. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 2.McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–46. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 3.Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–43. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 4.Whiting P, Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci U S A. 1987 Jan;84(2):595–9. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–35. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 6.Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998 Jun 15;18(12):4461–72. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 8.Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995 Mar 2;374(6517):65–7. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- 9.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998 Jan 8;391(6663):173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 10.Avale ME, Faure P, Pons S, Robledo P, Deltheil T, David DJ, et al. Interplay of beta2* nicotinic receptors and dopamine pathways in the control of spontaneous locomotion. Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15991–6. doi: 10.1073/pnas.0807635105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granon S, Faure P, Changeux JP. Executive and social behaviors under nicotinic receptor regulation. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9596–601. doi: 10.1073/pnas.1533498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maubourguet N, Lesne A, Changeux JP, Maskos U, Faure P. Behavioral sequence analysis reveals a novel role for beta2* nicotinic receptors in exploration. PLoS Comput Biol. 2008 Nov;4(11):e1000229. doi: 10.1371/journal.pcbi.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen G, Han ZY, Grailhe R, Gallego J, Gaultier C, Changeux JP, et al. beta 2 nicotinic acetylcholine receptor subunit modulates protective responses to stress: A receptor basis for sleep-disordered breathing after nicotine exposure. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13272–7. doi: 10.1073/pnas.192463599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lena C, Popa D, Grailhe R, Escourrou P, Changeux JP, Adrien J. Beta2-containing nicotinic receptors contribute to the organization of sleep and regulate putative micro-arousals in mice. J Neurosci. 2004 Jun 23;24(25):5711–8. doi: 10.1523/JNEUROSCI.3882-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, Quattrone A, et al. The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet. 2000 Nov;26(3):275–6. doi: 10.1038/81566. [DOI] [PubMed] [Google Scholar]
- 16.Bertrand D, Picard F, Le Hellard S, Weiland S, Favre I, Phillips H, et al. How mutations in the nAChRs can cause ADNFLE epilepsy. Epilepsia. 2002;43(Suppl 5):112–22. doi: 10.1046/j.1528-1157.43.s.5.16.x. [DOI] [PubMed] [Google Scholar]
- 17.Fonck C, Cohen BN, Nashmi R, Whiteaker P, Wagenaar DA, Rodrigues-Pinguet N, et al. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive alpha 4* nicotinic receptors. J Neurosci. 2005 Dec 7;25(49):11396–411. doi: 10.1523/JNEUROSCI.3597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son CD, Moss FJ, Cohen BN, Lester HA. Nicotine normalizes intracellular subunit stoichiometry of nicotinic receptors carrying mutations linked to autosomal dominant nocturnal frontal lobe epilepsy. Mol Pharmacol. 2009 May;75(5):1137–48. doi: 10.1124/mol.108.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8424–8. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14602–7. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobelis P, Hutton S, Lu Y, Collins AC. GABAergic systems modulate nicotinic receptor-mediated seizures in mice. J Pharmacol Exp Ther. 2003 Sep;306(3):1159–66. doi: 10.1124/jpet.103.053066. [DOI] [PubMed] [Google Scholar]
- 22.Fonck C, Nashmi R, Deshpande P, Damaj MI, Marks MJ, Riedel A, et al. Increased sensitivity to agonist-induced seizures, straub tail, and hippocampal theta rhythm in knock-in mice carrying hypersensitive alpha 4 nicotinic receptors. J Neurosci. 2003 Apr 1;23(7):2582–90. doi: 10.1523/JNEUROSCI.23-07-02582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009 Mar 25;29(12):3676–84. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annegers JF, Coan SP. SUDEP: overview of definitions and review of incidence data. Seizure. 1999 Sep;8(6):347–52. doi: 10.1053/seiz.1999.0306. [DOI] [PubMed] [Google Scholar]
- 25.Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, et al. Alpha4* nicotinic receptors in preBotzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J Neurosci. 2008 Jan 9;28(2):519–528. doi: 10.1523/JNEUROSCI.3666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marini C, Guerrini R. The role of the nicotinic acetylcholine receptors in sleep-related epilepsy. Biochem Pharmacol. 2007 Oct 15;74(8):1308–14. doi: 10.1016/j.bcp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Vignatelli L, Bisulli F, Naldi I, Ferioli S, Pittau F, Provini F, et al. Excessive daytime sleepiness and subjective sleep quality in patients with nocturnal frontal lobe epilepsy: a case-control study. Epilepsia. 2006;47(Suppl 5):73–7. doi: 10.1111/j.1528-1167.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 28.Zucconi M, Oldani A, Smirne S, Ferini-Strambi L. The macrostructure and microstructure of sleep in patients with autosomal dominant nocturnal frontal lobe epilepsy. J Clin Neurophysiol. 2000 Jan;17(1):77–86. doi: 10.1097/00004691-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, et al. Delta FosB regulates wheel running. J Neurosci. 2002 Sep 15;22(18):8133–8. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J Exp Anal Behav. 1993 Jul;60(1):219–38. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol. 2008 Sep;19(5–6):461–84. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Booker TK, Butt CM, Wehner JM, Heinemann SF, Collins AC. Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacol Biochem Behav. 2007 May;87(1):146–57. doi: 10.1016/j.pbb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003 Jul 16;23(15):6255–63. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002 Jul 2;13(9):1097–106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 35.Damaj MI, Glassco W, Dukat M, Martin BR. Pharmacological characterization of nicotine-induced seizures in mice. J Pharmacol Exp Ther. 1999 Dec;291(3):1284–91. [PubMed] [Google Scholar]
- 36.Miner LL, Collins AC. Strain comparison of nicotine-induced seizure sensitivity and nicotinic receptors. Pharmacol Biochem Behav. 1989 Jun;33(2):469–75. doi: 10.1016/0091-3057(89)90532-7. [DOI] [PubMed] [Google Scholar]
- 37.Teper Y, Whyte D, Cahir E, Lester HA, Grady SR, Marks MJ, et al. Nicotine-induced dystonic arousal complex in a mouse line harboring a human autosomal-dominant nocturnal frontal lobe epilepsy mutation. J Neurosci. 2007 Sep 19;27(38):10128–42. doi: 10.1523/JNEUROSCI.3042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klaassen A, Glykys J, Maguire J, Labarca C, Mody I, Boulter J. Seizures and enhanced cortical GABAergic inhibition in two mouse models of human autosomal dominant nocturnal frontal lobe epilepsy. Proc Natl Acad Sci U S A. 2006 Dec 12;103(50):19152–7. doi: 10.1073/pnas.0608215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keil W, Kluge A. Über die anwendung des mäuseschwanzphänomens zur auswertung von morphin- und skopolaminpräparaten. Naunyn Schmiedebers Arch Pharmakol. 1934;174:493–501. [Google Scholar]
- 40.Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995 Oct;11(2):201–3. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- 41.Hirose S, Iwata H, Akiyoshi H, Kobayashi K, Ito M, Wada K, et al. A novel mutation of CHRNA4 responsible for autosomal dominant nocturnal frontal lobe epilepsy. Neurology. 1999 Nov 10;53(8):1749–53. doi: 10.1212/wnl.53.8.1749. [DOI] [PubMed] [Google Scholar]
- 42.Diaz-Otero F, Quesada M, Morales-Corraliza J, Martinez-Parra C, Gomez-Garre P, Serratosa JM. Autosomal dominant nocturnal frontal lobe epilepsy with a mutation in the CHRNB2 gene. Epilepsia. 2008 Mar;49(3):516–20. doi: 10.1111/j.1528-1167.2007.01328.x. [DOI] [PubMed] [Google Scholar]
- 43.Phillips HA, Favre I, Kirkpatrick M, Zuberi SM, Goudie D, Heron SE, et al. CHRNB2 is the second acetylcholine receptor subunit associated with autosomal dominant nocturnal frontal lobe epilepsy. Am J Hum Genet. 2001 Jan;68(1):225–31. doi: 10.1086/316946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manfredi I, Zani AD, Rampoldi L, Pegorini S, Bernascone I, Moretti M, et al. Expression of mutant beta2 nicotinic receptors during development is crucial for epileptogenesis. Hum Mol Genet. 2009 Mar 15;18(6):1075–88. doi: 10.1093/hmg/ddp004. [DOI] [PubMed] [Google Scholar]
- 45.Leniger T, Kananura C, Hufnagel A, Bertrand S, Bertrand D, Steinlein OK. A new Chrna4 mutation with low penetrance in nocturnal frontal lobe epilepsy. Epilepsia. 2003 Jul;44(7):981–5. doi: 10.1046/j.1528-1157.2003.61102.x. [DOI] [PubMed] [Google Scholar]
- 46.McLellan A, Phillips HA, Rittey C, Kirkpatrick M, Mulley JC, Goudie D, et al. Phenotypic comparison of two Scottish families with mutations in different genes causing autosomal dominant nocturnal frontal lobe epilepsy. Epilepsia. 2003 Apr;44(4):613–7. doi: 10.1046/j.1528-1157.2003.20102.x. [DOI] [PubMed] [Google Scholar]
- 47.Cho YW, Motamedi GK, Laufenberg I, Sohn SI, Lim JG, Lee H, et al. A Korean kindred with autosomal dominant nocturnal frontal lobe epilepsy and mental retardation. Arch Neurol. 2003 Nov;60(11):1625–32. doi: 10.1001/archneur.60.11.1625. [DOI] [PubMed] [Google Scholar]
- 48.Magnusson A, Stordal E, Brodtkorb E, Steinlein O. Schizophrenia, psychotic illness and other psychiatric symptoms in families with autosomal dominant nocturnal frontal lobe epilepsy caused by different mutations. Psychiatr Genet. 2003 Jun;13(2):91–5. doi: 10.1097/01.ypg.0000056173.32550.b0. [DOI] [PubMed] [Google Scholar]
- 49.Derry CP, Heron SE, Phillips F, Howell S, MacMahon J, Phillips HA, et al. Severe autosomal dominant nocturnal frontal lobe epilepsy associated with psychiatric disorders and intellectual disability. Epilepsia. 2008 Dec;49(12):2125–9. doi: 10.1111/j.1528-1167.2008.01652.x. [DOI] [PubMed] [Google Scholar]
- 50.Combi R, Dalpra L, Tenchini ML, Ferini-Strambi L. Autosomal dominant nocturnal frontal lobe epilepsy--a critical overview. J Neurol. 2004 Aug;251(8):923–34. doi: 10.1007/s00415-004-0541-x. [DOI] [PubMed] [Google Scholar]
- 51.Oldani A, Zucconi M, Asselta R, Modugno M, Bonati MT, Dalpra L, et al. Autosomal dominant nocturnal frontal lobe epilepsy. A video-polysomnographic and genetic appraisal of 40 patients and delineation of the epileptic syndrome. Brain. 1998 Feb;121(Pt 2):205–23. doi: 10.1093/brain/121.2.205. [DOI] [PubMed] [Google Scholar]
- 52.Ryvlin P, Rheims S, Risse G. Nocturnal frontal lobe epilepsy. Epilepsia. 2006;47(Suppl 2):83–6. doi: 10.1111/j.1528-1167.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 53.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002 May 3;109(3):307–20. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 54.O’Hara BF, Edgar DM, Cao VH, Wiler SW, Heller HC, Kilduff TS, et al. Nicotine and nicotinic receptors in the circadian system. Psychoneuroendocrinology. 1998 Feb;23(2):161–73. doi: 10.1016/s0306-4530(97)00077-2. [DOI] [PubMed] [Google Scholar]
- 55.Miller JD, Murakami DM, Fuller CA. The response of suprachiasmatic neurons of the rat hypothalamus to photic and nicotinic stimuli. J Neurosci. 1987 Apr;7(4):978–986. doi: 10.1523/JNEUROSCI.07-04-00978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marrosu F, Portas C, Mascia MS, Casu MA, Fa M, Giagheddu M, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995 Feb 13;671(2):329–32. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 57.Saint-Mleux B, Eggermann E, Bisetti A, Bayer L, Machard D, Jones BE, et al. Nicotinic enhancement of the noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic area. J Neurosci. 2004 Jan 7;24(1):63–7. doi: 10.1523/JNEUROSCI.0232-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005 Jul 7;436(7047):103–7. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- 59.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002 May 1;22(9):3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004 Jun;65(6):1526–35. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 61.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002 Mar 14;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 62.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001 Dec;4(12):1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 63.Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, et al. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003 Dec 3;23(35):11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, et al. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000 Sep 1;20(17):6431–41. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, et al. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2786–91. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brioni JD, O’Neill AB, Kim DJ, Decker MW. Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. Eur J Pharmacol. 1993 Jul 6;238(1):1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- 67.Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett. 2008 Jul 11;439(2):187–91. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belzung C, Le Pape G. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiol Behav. 1994 Sep;56(3):623–8. doi: 10.1016/0031-9384(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 69.West RJ, Jarvis MJ. Effects of nicotine on finger tapping rate in non-smokers. Pharmacol Biochem Behav. 1986 Oct;25(4):727–31. doi: 10.1016/0091-3057(86)90377-1. [DOI] [PubMed] [Google Scholar]
- 70.Marks MJ, Burch JB, Collins AC. Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther. 1983 Jul;226(1):291–302. [PubMed] [Google Scholar]
- 71.Marubio LM, Paylor R. Impaired passive avoidance learning in mice lacking central neuronal nicotinic acetylcholine receptors. Neuroscience. 2004;129(3):575–82. doi: 10.1016/j.neuroscience.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron. 2008 Oct 9;60(1):123–36. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schildein S, Huston JP, Schwarting RK. Open field habituation learning is improved by nicotine and attenuated by mecamylamine administered posttrial into the nucleus accumbens. Neurobiol Learn Mem. 2002 May;77(3):277–90. doi: 10.1006/nlme.2001.4017. [DOI] [PubMed] [Google Scholar]
- 74.Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32(1):67–73. [PubMed] [Google Scholar]
- 75.Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 1996 Jan;123(1):55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1