Structure of the Anaphase-Promoting Complex/Cyclosome Interacting with a Mitotic Checkpoint Complex (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 22.
Published in final edited form as: Science. 2009 Mar 13;323(5920):1477–1481. doi: 10.1126/science.1163300
Abstract
Once all chromosomes are connected to the mitotic spindle (bioriented), anaphase is initiated by the protein ubiquitylation activity of the anaphase-promoting complex/cyclosome (APC/C) and its coactivator Cdc20 (APC/CCdc20). Before chromosome biorientation, anaphase is delayed by a mitotic checkpoint complex (MCC) that inhibits APC/CCdc20. We used single-particle electron microscopy to obtain three-dimensional models of human APC/C in various functional states: bound to MCC, to Cdc20, or to neither (apo-APC/C). These experiments revealed that MCC associates with the Cdc20 binding site on APC/C, locks the otherwise flexible APC/C in a “closed” state, and prevents binding and ubiquitylation of a wide range of different APC/C substrates. These observations clarify the structural basis for the inhibition of APC/C by spindle checkpoint proteins.
Chromosome segregation is delayed by the spindle checkpoint until all chromosomes are connected to both poles (bioriented) of the mitotic or meiotic spindle. Defects in this checkpoint can lead to aneuploidy, which is associated with tumorigenesis, congenital trisomies, and aging (1). The spindle checkpoint is activated in prometaphase by the presence of kinetochores that are not properly attached to microtubules or not under tension by spindle-pulling forces (2, 3). The checkpoint inhibits Cdc20, which activates the ubiquitin-protein ligase APC/C in mitosis and helps to recruit substrates to APC/C. APC/C initiates chromosome segregation by ubiquitylating cyclin B, an activating subunit of cyclin-dependent kinase 1 (Cdk1), and securin, an inhibitor of the protease separase. After ubiquitin-mediated degradation of these proteins, separase becomes active and dissolves the cohesion between sister chromatids (4).
Inhibition of Cdc20 by the spindle checkpoint depends on mitotic arrest–deficient 2 (Mad2) (5). This protein associates with Cdc20 at unattached kinetochores (6–9). In animal cells, Cdc20 is also inhibited by association with budding uninhibited by benzimidazoles–related 1 (BubR1) and its binding partner budding uninhibited by benzimidazoles 3 (Bub3). Mad2 and BubR1-Bub3 can bind to Cdc20 either separately (10, 11) or simultaneously, forming a MCC (12), and these proteins can associate with APC/C (13–16). Similar complexes exist in yeast, in which a BubR1-related Mad3 protein might inhibit Cdc20 as a pseudosubstrate (17, 18).
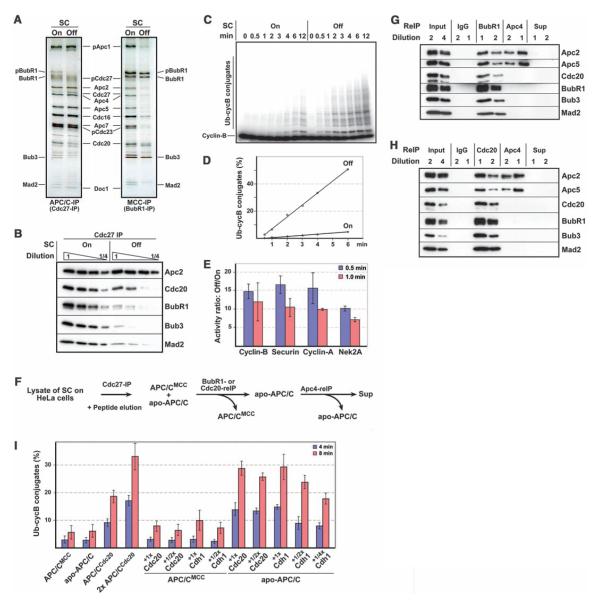
Structural information will be essential to understand how checkpoint proteins inhibit APC/CCdc20, but it is known only how Mad2 interacts with a short Cdc20 peptide (19, 20). We therefore analyzed with single-particle electron microscopy (EM) the structure of APC/C that is bound to checkpoint proteins. We first established conditions under which checkpoint-inhibited APC/C could be purified and characterized these complexes biochemically. We isolated APC/C from human HeLa cells that had been arrested in prometaphase by the microtubule-stabilizing agent Taxol, which keeps the checkpoint active. For comparison, we purified APC/C from Taxol-arrested cells in which the checkpoint had been inactivated by hesperadin, an inhibitor of Aurora B kinase (15, 21). Quantitative fluorescence microscopy of chromosome morphology, cyclin B levels, and kinetochore association of Mad2, BubR1, and Bub1 confirmed that cells had been arrested in prometaphase with either an active or inhibited spindle checkpoint (fig. S1). Mass spectrometry (table S1) and SDS–polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining (Fig. 1A) or semiquantitative immunoblotting (Fig. 1B) revealed that four times more BubR1, Bub3, and Mad2 were associated with APC/C in cells in which the checkpoint was active than in hesperadin-treated cells. The amount of Cdc20 bound to APC/C was twofold greater when the checkpoint was active, perhaps because checkpoint proteins stabilize APC/C–Cdc20 interactions. Immunoprecipitation of BubR1 confirmed that more BubR1, Bub3, Mad2, and Cdc20 were bound to APC/C when the checkpoint was active (Fig. 1A and table S1). In a reconstituted system, ubiquitylation of cyclin B and securin was reduced to 17% when APC/C was isolated from cells with an active checkpoint as compared with reactions mediated by APC/C from cells with an inactive checkpoint. This difference was even greater when samples were normalized to the amounts of APC/C–associated Cdc20, which is rate-limiting for APC/C activity (Fig. 1, C and D). Surprisingly, APC/C from cells with an active checkpoint was similarly less active toward cyclin A and never-in-mitosis A (NIMA)–related kinase 2A (Nek2A), which are normally degraded in prometaphase when the checkpoint is active (Fig. 1E and fig. S2). The checkpoint appears not to inhibit APC/CCdc20 in a substrate-specific manner, as was proposed to explain Nek2A degradation in prometaphase (22), but by recruiting substrates before checkpoint activation, as shown for cyclin A (23).
Fig. 1.
Biochemical characterization of APC/CMCC and apo-APC/C. (A) SDS-PAGE–silver-staining analysis of APC/C and MCC from cells in which the checkpoint was active (SC on) or inhibited (SC off). (B) Semiquantitative immunoblot analysis of APC/C from cells in which the checkpoint was on or off. (C and D) Cyclin B ubiquitylation activity of APC/C isolated from cells in which the checkpoint was on or off, analyzed with SDS-PAGE and autoradiography (C) and quantified (D). (E)Checkpoint activation reduces APC/C activity similarly toward prometaphase (cyclin A and Nek2A) and metaphase substrates (cyclin B and securin). Ratios of ubiquitiylation by the two APC/C samples were calculated for each substrate in three independent experiments. Error bars indicate SD of the mean. (F) Schematic representation of APC/CMCC and apo-APC/C isolation from cells with an active checkpoint (reIP, reimmunoprecipitation). (G and H) Immunoblot analyses of complexes obtained in (F). (I) Cyclin B ubiquitylation activities of APC/CCdc20 from cells with an inactive checkpoint and of APC/CMCC and apo-APC/C from cells with an active checkpoint. APC/C levels were normalized to Apc2. APC/CMCC and apo-APC/C were preincubated with various concentrations of recombinant Cdc20 or Cdh1. Cyclin B ubiquitylation was quantified after 4- and 8-min reaction times. Data represent three independent experiments and error bars indicate SD of the mean.
Similar results were obtained when APC/C was isolated from cells with active or inactive checkpoints that were obtained with a different protocol in which cells had not been treated with hesperadin (fig. S3), indicating that hesperadin caused changes in APC/C’s subunit composition and activity by checkpoint inhibition and not by an unrelated mechanism. Our results therefore indicated that checkpoint activation leads to association of BubR1, Bub3, and Mad2 with APC/C and to the general inhibition of APC/C’s ubiquitylation activity. However, our SDS-PAGE–silver staining analysis of APC/C from cells in which the checkpoint is active suggested that BubR1, Bub3, and Mad2 were present in substoichiometric amounts relative to APC/C (Fig. 1A). We therefore separated APC/C purified from cells with an active checkpoint into different subpopulations (Fig. 1F). We first captured a subpopulation of APC/C with BubR1, Cdc20, or Mad2 antibodies and then isolated the remaining particles with antibodies to the APC/C subunit Apc4 (Fig.1, G and H,and fig. S4). These experiments revealed that cells with an active checkpoint contained two forms of APC/C. One contained BubR1, Bub3, Cdc20, and Mad2, whereas the other one lacked all of these proteins. We refer to these forms as APC/CMCC and apo-APC/C, respectively. In cyclin B–ubiquitylation assays, the specific activities of APC/CMCC and apo-APC/C were similarly low (Fig. 1I). Purified Cdc20 and the related coactivator Cdc20 homolog 1 (Cdh1) activated apo-APC/C in a dose-dependent manner, suggesting that this form of APC/C is inactive because it is lacking a coactivator. In contrast, APC/CMCC could be stimulated by coactivators only to a small degree, indicating that this form of APC/C represents a truly inhibited state (Fig. 1I).
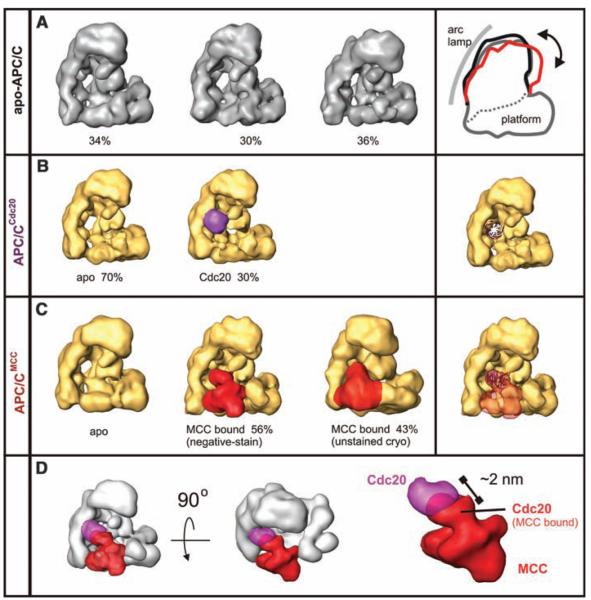
To understand how checkpoint proteins inhibit APC/C, we determined the structures of APC/CMCC, apo-APC/C, and APC/CCdc20.We isolated APC/CMCC by means of sequential immunoprecipitation with antibodies to Cdc27 and BubR1. We isolated apo-APC/C with antibodies to Apc4 from the supernatant of the BubR1 immunoprecipitation. After a peptide elution, both samples were further purified by use of glycerol density-gradient centrifugation (24) and analyzed by use of both negative staining at low temperatures and unstained cryo-EM in a vitrified buffer (Fig. 2, figs. S5 and S6, and table S2). We used a technique based on three-dimensional (3D) alignment of random conical tilt (RCT) reconstructions and subsequent 3D multivariate statistical analysis for initial structure determination and simultaneous unsupervised analysis of the heterogeneity in the data set. For apo-APC/C, we identified three conformers that differ mainly in the relative orientation of their two major domains: the platform and the arc lamp domains (Fig. 2A and 3D-PDF S1) (25). The initial RCT 3D structures were used to determine the handedness (fig. S7) and as templates for further refinement of the existing subpopulations of apo-APC/C (Fig. 2A). We suspect that these conformers represent the structural variability of apo-APC/C in solution and represent a continuum of flexible states (for an animation of the corresponding movements, see movie S1).
Fig. 2.
Localization of Cdc20 and MCC bound to APC/C, analyzed by negative-staining EM. (A) Distinct conformers of apo-APC/C isolated from cells with an active checkpoint. (B) Localization of recombinant Cdc20 (purple) bound to apo-APC/C. A model of Cdc20’s WD40 propeller is projected onto APC/C. (C) 3D structure of APC/CMCC. MCC is labeled in red. (D) Superposition of the MCC (red) and Cdc20 (purple) densities shown from orthogonal directions.
In the APC/CMCC sample, a large additional density was found inserted into the “front” side of the platform domain (Fig. 2C, red, and 3D-PDF S2). The 3D volume of this density element corresponds to a mass of 180 to 200 kD, slightly less than MCC’s theoretical mass of 236 kD (if every subunit is present once). We confirmed that this mass represents MCC by identifying the location of BubR1 by means of antibody labeling (fig. S8). To obtain a 3D model of APC/CCdc20,we bound recombinant Cdc20 to purified apo-APC/C and determined the structures as above. In 30% of APC/C molecules, we found an additional globular 50-kD mass on the front side of APC/C in a location that overlaps with the site to which MCC is bound in APC/CMCC (Fig. 2B and fig. S9). The Cdc20 density does not fully overlap with the MCC density and is ~20 Å away from a perfect fit. The precise position of Cdc20 may thus change upon MCC binding to APC/C (Fig. 2D). APC/CCdc20 showed similar conformational flexibility as apo-APC/C.
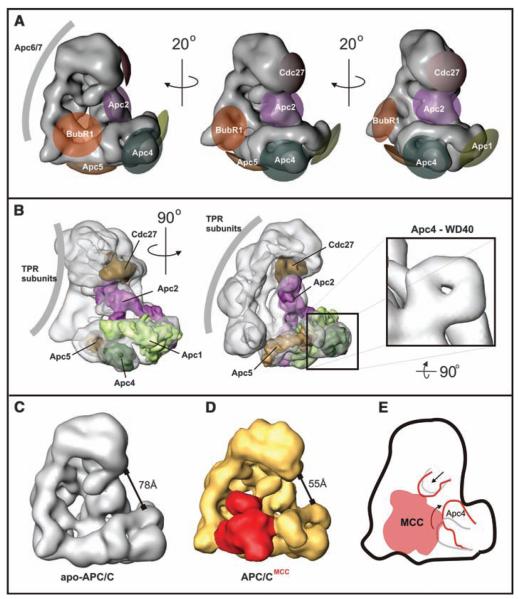
Comparison of the apo-APC/C and APC/CMCC structures revealed a number of differences in addition to the presence of an extra mass in APC/CMCC. To interpret these changes, we mapped the 3D location of APC/C core subunits with antibody labeling (fig. S8). Antibodies to Apc1, Apc4, and Apc5 bound to distinct sites in the platform domain with Apc5 at the bottom, Apc4 at the front, and Apc1 at the right-hand side (Fig. 3, A and B). The volumes of the labeled domains are consistent with the predicted masses of Apc1, Apc4, and Apc5 (Fig. 3B). The modeling of these subunits into the APC/C 3D structure suggests that Apc5 is close to the interface of the platform and arc lamp domains, and the mass accommodated by Apc1 spans from the front to the inner mass (Fig. 3B). Part of the subunit that we identified as Apc4 has a ring-shaped structure (Fig. 3B, right), which is consistent with bioinformatic analyses that Apc4 contains an N-terminal propeller-shaped WD40 domain (4). Cdc27 antibodies bound to the headlike protrusion that is located at the top end of the arc lamp domain (Fig. 3A). We confirmed that Apc2 is located at the inner mass on the front side of APC/C (25), residing in close vicinity to Apc1 and Cdc27 (Fig. 3, A and B). Antibodies to Cdc16 and Apc7 were bound at multiple locations on the arc lamp domain (fig. S8), presumably because these subunits are present in more than one copy per APC/C molecule (25). The arc lamp domain is bent and has an unusual repetitive regularity (Fig. 3B), features that are characteristic of tetra-tricopeptide repeat (TPR) domains present in Cdc16 and Apc7 and also in Cdc27 and Cdc23. These observations indicate that a major part of the arc lamp domain consists of TPR subunits. With the exception of Apc2 and Apc4 topology, our 3D model of subunit topology is consistent with the 2D positions of subunits determined in fission yeast APC/C (26).
Fig. 3.
APC/C subunit topology and structural changes upon MCC binding. (A) APC/C subunits were localized with antibody labeling. Antibody epitopes are marked on the surface of the APC/C 3D model, and the labeling accuracy is indicated by the size of the differently colored areas. (B)Models of Apc1, Apc2, Apc4, Apc5, and Cdc27 were docked into the 3D density of the APC/C reconstruction. (C)The apo-APC/Cstructure in its most open conformation. (D)TheAPC/CMCC structure with a closed conformation. (E) Conformational changes induced by MCC binding to APC/C.
This subunit map indicates that MCC binds in the vicinity of Apc2, Apc4, and Apc5, which are located toward the right-hand side of MCC (Fig. 3, B, D, and E, and 3D-PDFs S1 and S2). On the left-hand side, MCC contacts the arc lamp domain, which suggests that MCC also interacts with TPR subunits. Little if any conformational flexibility was observed among populations of APC/CMCC. Instead, APC/CMCC adopts a closed conformation (Fig. 3D). In addition, Apc4 is bent upward by 35° in APC/CMCC as compared with its position in apo-APC/C, and the position of a more centrally located subunit, possibly Apc2, is also changed (Fig. 3E). This rearrangement might be required for MCC binding and, together with a higher curvature of the arc lamp, results in a more compact appearance of APC/CMCC as compared with that of apo-APC/C.
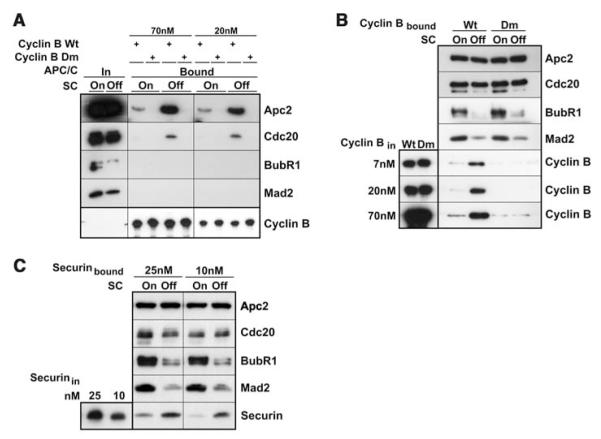
To understand the functional consequences of these structural changes, we compared the interactions of different forms of APC/C with the ubiquitin-conjugating enzyme UbcH10 and substrate proteins. Binding of UbcH10 to APC/C was reduced to 50% when the checkpoint was active (fig. S10). It is unlikely that this small change can explain the almost complete inhibition of APC/C by checkpoint proteins. In contrast, we saw a substantial reduction in substrate binding to checkpoint-inhibited APC/C. When APC/C was isolated from cells with an active checkpoint, much less binding of an N-terminal fragment of fission yeast cyclin B or of full-length human securin was detected as compared with the binding that was observed with APC/C from cells in which the checkpoint was inhibited (Fig. 4, A to C). As reported (27), binding of cyclin B to APC/C required the presence of a destruction box (D box) in the substrate (Fig. 4, A and B). Similar results were obtained when a Dbox–containing fragment of budding yeast Hsl1 was used as a substrate (fig. S11). Even a 35-fold increase in substrate concentration resulted in less Hsl1 binding to APC/C from cells with an active checkpoint, as compared with Hsl1 binding to APC/C from cells in which the checkpoint was inactive.
Fig. 4.
MCC inhibits the binding of substrates to APC/C. (A) A tandem fragment of wild-type (wt) or D-box mutant (dm) fission yeast cyclin B was immobilized on glutathione beads at the indicated concentrations, and binding of APC/C from cells with checkpoints on or off was analyzed with SDS-PAGE immunoblotting. (B) APC/C from cells with checkpoints on or off was bound to beads coupled with antibody to Cdc27, and binding of soluble wild-type or D-box mutant cyclin B was analyzed in the same manner as in (A). (C) The binding of fulllength human securin to APC/C was analyzed in the same manner as in (B).
Our data indicate that activation of the spindle checkpoint leads to association of BubR1, Bub3, and Mad2 with APC/CCdc20, in which the checkpoint proteins occupy a site that partially overlaps with the Cdc20 binding site. The association of checkpoint proteins with APC/C coincides with inhibition of APC/C toward a broad range of prometaphase and metaphase substrates, indicating that checkpoint activation inhibits APC/C generally and not in a substrate-specific manner. This inhibition is at least in part caused by the prevention of substrate binding, which agrees well with the proposed role of Cdc20 as a substrate adaptor (28, 29) and of yeast Mad3 as a pseudosubstrate inhibitor (17). Partial overlap of positions of Cdc20 binding to APC/CCdc20 and APC/CMCC raises the possibility that APC/C inhibition may be mediated by MCC-induced repositioning of Cdc20. MCC binding also induces conformational changes in APC/C itself and appears to lock it in a closed conformational state.
Supplementary Material
supplementary figures and tables
Acknowledgments
We are grateful to M. Madalinski, M. Gmachl, and G. Kohlmaier for reagents; J. Deckert and R. Lührmann for support; and S. Westermann for comments on the manuscript. Research in the laboratory of H.S. was supported by grants from the Federal Ministry of Education and Research, Germany, and the Sixth Framework Programme of the European Union via the Integrated Project 3DRepertoire. Research in the laboratories of J.-M.P. and K.M. were supported by Boehringer Ingelheim, the Vienna Spots of Excellence Programme, and the Austrian Science Fund. The structural coordinates have been deposited in the Electron Microscopy Data Bank Database at the European Molecular Biology Laboratory–European Bioinformatics Institute. The structures of APC/CMCC and apo-APC/C can be accessed under the codes EMD-1591 and EMD-1592, respectively.
Footnotes
References and Notes
- 1.Baker DJ, Chen J, van Deursen JM. Curr. Opin. Cell Biol. 2005;17:583. doi: 10.1016/j.ceb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Rieder CL, Cole RW, Khodjakov A, Sluder G. J. Cell Biol. 1995;130:941. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern BM, Murray AW. Curr. Biol. 2001;11:1462. doi: 10.1016/s0960-9822(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 4.Peters JM. Mol. Cell. 2002;9:931. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 5.Li R, Murray AW. Cell. 1991;66:519. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 6.Fang G, Yu H, Kirschner MW. Genes Dev. 1998;12:1871. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang LH, et al. Science. 1998;279:1041. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Lin DP, Matsumoto S, Kitazono A, Matsumoto T. Science. 1998;279:1045. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- 9.DeAntoni A, Sala V, Musacchio A. Philos. Trans. R. Soc. London Ser. B. 2005;360:637. doi: 10.1098/rstb.2004.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Z, Bharadwaj R, Li B, Yu H. Dev. Cell. 2001;1:227. doi: 10.1016/s1534-5807(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 11.Fang G. Mol. Biol. Cell. 2002;13:755. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudakin V, Chan GK, Yen TJ. J. Cell Biol. 2001;154:925. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ. J. Cell Biol. 1998;141:1393. doi: 10.1083/jcb.141.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wassmann K, Benezra R. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11193. doi: 10.1073/pnas.95.19.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow CJ, et al. J. Cell Sci. 2005;118:3639. doi: 10.1242/jcs.02487. [DOI] [PubMed] [Google Scholar]
- 16.Braunstein I, Miniowitz S, Moshe Y, Hershko A. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4870. doi: 10.1073/pnas.0700523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton JL, Solomon MJ. Genes Dev. 2007;21:655. doi: 10.1101/gad.1511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sczaniecka M, et al. J. Biol. Chem. 2008;283:23039. doi: 10.1074/jbc.M803594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo X, Tang Z, Rizo J, Yu H. Mol. Cell. 2002;9:59. doi: 10.1016/s1097-2765(01)00435-x. [DOI] [PubMed] [Google Scholar]
- 20.Sironi L, et al. EMBO J. 2002;21:2496. doi: 10.1093/emboj/21.10.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauf S, et al. J. Cell Biol. 2003;161:281. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes MJ, et al. Nat. Cell Biol. 2006;8:607. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- 23.Wolthuis R, et al. Mol. Cell. 2008;30:290. doi: 10.1016/j.molcel.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Materials and methods are available as supporting material on Science Online.
- 25.Dube P, et al. Mol. Cell. 2005;20:867. doi: 10.1016/j.molcel.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Ohi MD, et al. Mol. Cell. 2007;28:871. doi: 10.1016/j.molcel.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamano H, Gannon J, Mahbubani H, Hunt T. Mol. Cell. 2004;13:137. doi: 10.1016/s1097-2765(03)00480-5. [DOI] [PubMed] [Google Scholar]
- 28.Burton JL, Tsakraklides V, Solomon MJ. Mol. Cell. 2005;18:533. doi: 10.1016/j.molcel.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM. Mol. Cell. 2005;18:543. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary figures and tables