Adult onset asymmetric upper limb tremor misdiagnosed as Parkinson’s disease: A clinical and electrophysiological study (original) (raw)
. Author manuscript; available in PMC: 2010 Dec 6.
Published in final edited form as: Mov Disord. 2010 Apr 15;25(5):560–569. doi: 10.1002/mds.23019
Summary
Approximately 10% of subjects thought clinically to have early Parkinson’s disease (PD) have normal dopaminergic functional imaging (SWEDDs – Scans Without Evidence of Dopaminergic Deficit). SWEDDs are a heterogeneous group. Here we aimed to delineate clinical and electrophysiological characteristics of a distinct subgroup of SWEDDs patients from PD and to clarify the underlying pathophysiology of this subgroup as a form of parkinsonism or dystonia. Therefore we compared clinical details of 25 patients referred with a diagnosis of tremor-dominant PD but with normal DaT SPECT scans (SWEDDs) with 12 tremor-dominant PD patients with abnormal DaT SPECT scans. We performed tremor analysis using accelerometry in the following patients with 1) SWEDDs, 2) PD, 3) primary segmental dystonia with dystonic limb tremor and 4) essential tremor (ET). We used transcranial magnetic stimulation with a facilitatory paired associative stimulation (PAS) paradigm to test if sensorimotor plasticity in SWEDDs resembled the pattern seen in PD, dystonia or ET. Although PD and SWEDDs patients shared several clinical features, the lack of true bradykinesia, occurrence of dystonia, and position- and task-specificity of tremor favoured a diagnosis of SWEDDs, whereas re-emergent tremor, true fatiguing or decrement, good response to dopaminergic drugs as well as presence of nonmotor symptoms made PD more likely. Basic tremor parameters overlapped between SWEDDs, PD, segmental dystonia and ET. However, a combination of re-emergent tremor and highest tremor amplitude in the resting condition was characteristic of PD tremor, while SWEDDs, dystonia and ET subjects had the highest tremor amplitude during action. Both SWEDDs and segmental dystonia patients exhibited an exaggerated pattern of sensorimotor plasticity in response to the PAS paradigm, with spread of excitation to an adjacent hand muscle. In contrast, PD patients showed no response to PAS, and the response of ET patients was no different from controls. Taken together, these results may help differentiate these SWEDDs patients from PD and support our hypothesis that adult-onset dystonia is the underlying diagnosis in this sub-group of patients with SWEDDs.
Keywords: SWEDDs, benign tremulous Parkinson’s disease, dystonic tremor, accelerometry, paired associative stimulation
Introduction
Recent drug trials in idiopathic Parkinson’s disease (PD) have used functional dopaminergic imaging ([18F]dopa PET, DaT SPECT) as surrogate markers of disease progression. An unexpected consequence of this study design has been that 4%-14.7% of recruited patients, all of whom apparently fulfilled clinical diagnostic criteria for PD, had normal dopaminergic functional imaging ((Marek and Seibyl, 2003; Whone et al., 2003). These individuals have been referred to as having SWEDDs (“Scans without evidence of dopaminergic deficit”). There has been considerable debate as to the exact diagnosis in these patients; could they have very early PD, some previously unrecognized form of PD, or not have PD at all? The latter possibility seems most likely given that these patients failed to respond to levodopa in the ELLDOPA study (Fahn, 2005) and repeat dopamine transporter scans after an interval of 4 years in a proportion of patients still showed no nigrostriatal degeneration (Marek et al., 2005). SWEDDs are a heterogeneous group, and alternative diagnoses that have been suggested include essential tremor (ET), depression, vascular or psychogenic parkinsonism, dopa-responsive dystonia, and rare causes of supranigral parkinsonism (Fahn, 2005).
We have previously reported a small clinical case series of 10 patients with SWEDDs. (Schneider et al., 2007). These patients had all been referred with a diagnosis of PD and had asymmetric resting arm tremor as their dominant feature, as well as increased muscle tone, impaired arm swing and sometimes also facial hypomimia and jaw tremor. We identified signs of dystonia, or components of their arm tremor that were compatible with dystonic tremor and hence made the suggestion that this group had primary adult-onset dystonic tremor and that this entity may be one cause of SWEDDs.
There are a number of potential criticisms of this proposition. Firstly, there is no definite diagnostic test for dystonia/ dystonic tremor and the diagnosis of dystonia depends entirely on clinical acumen. The dystonic features we described in our previous group of 10 patients were subtle in some cases and more importantly, they were not assessed by a clinician blinded to the results of the DaT SPECT scans (Schneider et al., 2007). Dystonia is also known to be a feature of PD itself (particularly in those with young disease onset), and therefore its presence does not automatically indicate a diagnosis of primary dystonia. More recently, a group of SWEDDs subjects was reported to have a normal sense of smell(Silveira-Moriyama et al., 2009). These patients have not yet been compared with PD patients regarding other non motor symptoms. Also, apart from the normal dopamine transporter scans, there has been no attempt to investigate in detail the pathophysiology of this condition and in particular no use of experimental methods that might help to distinguish PD from dystonia. The tremor characteristics of these patients have not been compared with PD, clinically definite dystonic tremor or essential tremor (ET). Also, one would predict that if these patients have dystonia that their response to a plasticity probing protocol, such as paired associative stimulation (PAS), might be different from PD. The dynamic changes observed in motor cortex after PAS represent a form of motor cortex plasticity (Stefan et al., 2000; Wolters et al., 2003; Wolters et al., 2005). In healthy subjects plasticity induced by PAS evolves rapidly (within 30 min), is persistent (minimum duration 30–60 min) yet reversible, and is topographically specific (Stefan et al., 2000). When median nerve stimulation is paired with TMS pulses at an interstimulus interval of 25ms the result is a long term potentiation (LTP)-like effect. Previous studies have demonstrated that patients with dystonia have abnormal spread of the effects of PAS outside the target muscle compared with healthy subjects (Quartarone et al., 2003; Quartarone et al., 2007). In contrast patients with PD off treatment show no response to PAS conditioning (Morgante et al., 2006); (Ueki et al., 2006). So far, no data have been published describing the response to PAS in patients with ET.
We have therefore prospectively collected a new group of 25 consecutive patients with a previous diagnosis of PD, an asymmetric resting tremor and a normal DaT SPECT scan with the following aims. 1) To look at the clinical evidence of dystonia, parkinsonian signs, and specific tremor characteristics in these patients compared to a group of PD patients using blinded video rating. 2) To compare these patients with PD patients using a nonmotor symptoms screening questionnaire. 3) To compare the tremor of these patients using tremor analysis with the tremor of patients with PD, dystonic tremor, and ET. 4) To compare motor cortex plasticity using PAS in these patients and patients with PD, segmental dystonia, ET, and healthy controls.
Patients and Methods
Participants
We recruited five age-matched groups of participants who took part in various aspects of the study. These groups were: 1) 25 consecutive patients seen in our movement disorder clinics (tertiary referral centre) between April 2007 and December 2008 who fulfilled the following inclusion criteria: adult-onset (>40 years) of asymmetric arm tremor with a rest component; a previous clinical diagnosis of PD made by their physician or neurologist; a subsequent normal DaT SPECT and structural MRI. Patients who had possible other causes for their asymmetric rest tremor (psychogenic tremor, neuropathic tremor, use of medication associated with drug-induced tremor/parkinsonism) were excluded. We refer to this group below as SWEDDs, however we do appreciate that this phenotype may not be representative of the whole clinical spectrum of SWEDDs. None of the patients included in this study has been previously reported. 2) 12 patients with tremor-dominant PD as defined in the DATATOP study (Jankovic et al., 1990). All had disease onset after age 40 and all had abnormal DaT SPECT scans and normal MRI brain scans. An additional 28 tremor-dominant PD patients completed the assessment of nonmotor symptoms including anxiety and depression (total of 40 patients). 3) 7 patients with segmental primary dystonia recruited from our botulinum toxin injection clinic. These patients all had a combination of clear cervical and limb dystonia associated with dystonic limb tremor. 4) 8 patients with classic (definite) ET previously diagnosed according to the TRIG criteria (Deuschl et al., 1998). 5) 7 healthy control participants with no history of neurological or severe general medical disease. Informed consent was obtained from all subjects and the study was approved by the local ethics committee in accordance with the Declaration of Helsinki on the use of human subjects in experiments.
Clinical study
Information regarding demographics, medical and family history, disease course and treatment were collected during a face-to-face interview in 25 patients with SWEDDs and 12 patients with tremor-dominant PD. All patients were asked to rate the efficacy of current and previous medications for the tremor (worsening, no improvement, slight improvement, moderate improvement, marked improvement, or complete alleviation of tremor). They also had to rate if withdrawal of any drug had altered their tremor. Answers were cross-checked with information from medical records. Overall treatment response (benefit versus no benefit from any drug) was compared between the groups (Fisher’s Exact Test).
Tremor was assessed by the Fahn-Tolosa-Marin (FTM) rating scale (Fahn et al., 1988). Patients underwent an extensive standardised videotaped examination. The videotaped examinations were edited in random order onto a CD-Rom for subsequent rating by an experienced movement disorder neurologist blinded to the DaT SPECT findings and to the working diagnosis. The blinded rater (MJE) assessed motor disability due to parkinsonian symptoms using the Unified Parkinson’s Disease Rating Scale motor examination, UPDRS-III (on-site findings for UPDRS rigidity were provided)(Fahn and Elton, 1987), and the severity of dystonia by applying the Unified Dystonia Rating Scale (UDRS) (Comella et al., 2003). In addition the blinded rater was asked to evaluate the presence of specific tremor/ dystonia and other clinical characteristics (simple YES/NO choice): 1.) Tremor regions (head, jaw, voice, tongue, upper limb, lower limb); 2.) Upper limb tremor (unilateral/ asymmetric/ symmetric); 3.) Improvement of tremor with use of a “sensory trick”; 4.) Postural tremor aggravated in particular positions; 5.) Task specificity/ aggravation of tremor during writing/ drawing/ pouring water; 6.) Jerkiness; 7.) Flurries of tremor; 8.) Thumb hyperextension tremor; 9.) Dominant tremor position (rest, posture, or action); 10.) Re-emergent tremor; 11.) Hypomimia; 13.) Slowness, reduced amplitude, true fatiguing, true decrement on repetitive finger and leg tasks; 14.) Gait disturbance; 15.) Reduced arm swing; 16.) Postural stability on pull test; 17.) Presence of dystonia; 18) Distribution of dystonia (eyes/ upper face, lower face, jaw/ tongue, larynx, neck, shoulder/ proximal arm, distal arm/ hand, pelvis/ proximal leg, distal leg/ foot).
In the whole group of 25 SWEDDs and in all 40 PD patients, nonmotor symptoms, anxiety and depression scores were evaluated using the nonmotor symptoms questionnaire for PD (NMSQuest) (Chaudhuri et al., 2006) and the Hospital Anxiety And Depression Scale (HADS) (Zigmond and Snaith, 1983).
Electrophysiological studies
We included 9 SWEDDs patients, 9 tremor-dominant PD patients with abnormal DaT SPECT scans, 7 segmental dystonia patients, 8 ET patients, and 7 healthy age-matched controls in this aspect of the study. All patients had stopped any drugs that would potentially act on the central nervous system for at least 24 hours (including dopaminergic drugs), and in patients with segmental dystonia the last injection with botulinum toxin had been given at least three months before the study. Patients underwent both tremor analysis and a PAS protocol (see below).
Tremor analysis was undertaken using accelerometry. The patient was comfortably seated upright in a chair and the more tremulous upper limb was selected for the recording. A triaxial accelerometer transducer (Entran Sensors and Electronics®, Les Clayes-sous-Bois, France; Sensitivity 13.44/12.88/10.21 mV/g) was attached to the dorsal surface of the first proximal phalanx of the thumb. Recordings were performed: a) with the hands/wrists relaxed and forearms supported by an armrest [rest condition (R)], (b) with the entire upper limbs extended and pronated at shoulder level [action/ postural condition(P)], and (c) while performing a goal-directed movement (repetitive finger-to-nose test) [action/ kinetic condition (K)]. The tremor was recorded for at least 1 minute in each condition. The data were captured and analyzed using Spike2 version 6 (Cambridge Electronic Design). Representative continuous recordings for 30 seconds of each condition were used for analysis. The epochs were windowed using Hanning window and a Fast Fourier Transformation (FFT, size 1024) was computed on each epoch and the tremor spectra power calculated. This procedure was performed for each accelerometer axis separately. Tremor frequency was determined by the frequency of the first dominant peak of the spectrum. The total power between 1 and 30 Hz was calculated for each axis. After showing that the main frequency was the same in each axis the values of total power were averaged and used as a surrogate measure of tremor amplitude. We compared the dominant frequency peaks and amplitudes in the three different conditions using two separate ANOVAs for frequency and amplitude. We had GROUP (SWEDDs, PD, segmental dystonia, ET) as between subject factors, and CONDITION (R,P,K) as within subject factors. The results were corrected for multiple comparisons (Bonferroni). Where appropriate, additional one-way ANOVAs and post hoc t tests were applied, as outlined in detail in Results. We also inspected the raw accelerometry data for a pause of tremor after changing from rest to posture to ascertain the presence of a re-emergent tremor.
For the PAS protocol, subjects were seated in a comfortable reclining chair. Electromyographic (EMG) recordings were made from the abductor pollicis brevis (APB) and first dorsal interosseous (FDI) muscles with Ag-AgCl surface electrodes using a bipolar belly-tendon montage. Recordings were made from the less tremulous hand in patients. Subjects received auditory (speakers) and visual (oscilloscope) feedback of EMG activity. Active traces were deleted online. EMG signals were amplified and filtered using a time constant of 3 ms and a highpass filter set at 2.5 kHz (Neurolog System, Digitimer Ltd., Welwyn Garden City, Herts, UK). EMG signals were digitized at 5 kHz using an analog-digital interface and stored on a personal computer for off-line analysis (CED 1401 interface and Signal software, Cambridge Electronic Design, Cambridge, UK).
Transcranial magnetic stimulation (TMS) was given via a figure-of-eight coil (mean loop diameter 9cm) connected to a Magstim 200 stimulator (Magstim, Whitland, Dyfed, UK). The coil was held tangentially to the skull with the handle pointing backwards and laterally at an angle of 45° to the sagittal plane and was optimally positioned to obtain motor-evoked potentials (MEPs) in the APB. TMS was used to probe corticospinal excitability before and after PAS. The coil position and orientation and the intensity of the stimulator were kept constant throughout all the experimental sessions. In each testing block, we assessed the mean MEP amplitude for the APB and FDI muscles. Thirty consecutive MEPs were recorded using an interstimulus interval of 5 s. Stimulus intensity was defined at the beginning of the experiment at a stimulator output that induced MEPs of approximately 1mV in the APB muscle. These recordings were performed before PAS (baseline) and immediately (T0), 15 min (T15) and 30 min (T30) after the end of PAS. Trials with voluntary EMG contamination were discarded from further analyses. In addition we measured input-output curves (IO curves) to test stimulus intensity-dependent recruitment of corticospinal projections to the small hand muscles at baseline (Ridding and Rothwell, 1997). IO curves were recorded with 5 trials in 9 steps of intensity from 10–90% TMS output (Tisch et al., 2007). Trials with any ongoing EMG artefact were rejected online.
PAS consisted of 200 electrical stimuli of median nerve at the wrist paired with TMS over the hot spot of the APB muscle area. The rate of paired stimulation was 0.25 Hz. Electrical stimulation was applied through a bipolar electrode (cathode proximal). The stimulus duration was 0.2 ms, the intensity was 300% of perceptual threshold. The intensity of the TMS pulse was adjusted to produce a MEP of ~1 mV in peak to peak amplitude in the resting APB when given without the preceding median nerve stimulus. Stimuli were given 25ms apart with the electrical stimulus given first in each pair. Subjects were instructed to look at their stimulated hand and count the peripheral electrical stimuli they perceived in order to ensure comparable attention levels.
Repeated measures analyses of variance (ANOVA) were performed for MEP amplitudes. For group comparisons we computed repeated measures ANOVA with TIME (baseline, T0, T15, T30) and MUSCLE (APB, FDI) as within-subjects factors and GROUP (SWEDDs, PD, segmental dystonia, ET, controls) as between-subjects factors.). The results were corrected for multiple comparisons (Bonferroni). If the main factors showed significant main effects or interaction, the effect of time and muscle in each group was explored with separate one-way repeated measures ANOVA. The Greenhouse-Geisser method was used to correct for nonsphericity. For the ANOVA, a non-corrected p-value of < 0.05 was considered significant. Conditional on a significant F-value, post hoc t-tests were used.
IO curves were analyzed for each subject for the APB and the FDI muscle separately. The mean MEP amplitude was calculated for each stimulus intensity and a curve was plotted of MEP amplitude versus stimulus intensity. The IO curve gradient was determined by fitting a linear regression line through the steepest part of each curve. IO curve gradients between the 5 groups were compared using one-way ANOVA.
Results
Clinical study
Demographics and history
Clinical details of 25 subjects with SWEDDs (15 males, 10 females; 22 right-handed, 3 left-handed) and 12 patients with tremor-dominant PD (all males; 11 right-handed, 1 left-handed) were obtained. Both groups had a similar age at onset (SWEDDs: mean 57.0, SD 15.3, range 41-83 years; PD: mean 60.6, SD 9.5, range 46-76 years; independent-samples t-test: p=0.5). Disease duration was not different (p=0.3) in the SWEDDs (mean 9.3, SD 9.2 years) versus the PD group (mean 6.5, SD 3.2 years).
Fifty-two percent of the SWEDDs subjects reported a positive family history of tremor (n=4) or parkinsonism (n=9) in one or more first degree relatives. Only 17% of the PD group reported a positive family history of tremor (n=1) or parkinsonism (n=1). No patient with PD had been diagnosed with an alternative cause of their movement disorder. All 25 subjects with SWEDDs had by definition had received a previous diagnosis of PD, but 16% had at one point also been diagnosed with ET, and one each with multiple system atrophy and psychogenic tremor. Alcohol responsiveness of the tremor was reported by 32% of SWEDDs and none of the PD patients.
Therapy
Only 40% of the SWEDDs group had benefit from any therapy. In contrast 92% of the PD patients had noted response to treatment of their tremor (Fisher’s Exact Test, p=0.004). Among the 25 SWEDDs patients, 10 had received levodopa (range 150-900mg total daily dose). All patients reported no improvement. Three patients who had received a dopamine agonist reported no response. Withdrawal of the dopaminergic drugs had not worsened the tremor. Other drugs that were tried without benefit were amantadine in one, gabapentin in one, primidone in three, and topiramate in three subjects. Six patients had been given trihexyphenidyl (range 3-6 mg total daily dose). No improvement was reported by 1, slight improvement by 1, and moderate improvement by 4. Of 7 patients receiving propranolol (range 30-100 mg total daily dose), 3 had no response, 3 had slight improvement, and 1 had moderate improvement. Of 5 patients receiving clonazepam (range 1-6 mg total daily dose), 4 had no response, and 1 reported slight improvement.
Among 12 patients with PD, 7 had tried levodopa (range 300-1200 mg total daily dose). Two reported moderate and five marked improvement regarding tremor amelioration. Six patients were tried on a dopamine agonist. This was of no benefit in 2, slight benefit in 3, and moderate benefit in 1. Other drugs tried for PD tremor were trihexyphenidyl in 2 (moderate and marked benefit in one patient each), and propranolol in 4 cases (3 reported no, and 1 reported slight improvement).
Clinical characteristics
The group clinical characteristics of subjects with SWEDDs versus PD and the results of blinded video rating are summarized in Table 1.
Table 1.
Group clinical characteristics of patients with SWEDDs (n=25) in comparison to tremor-dominant PD (n=12). Except item “increased muscle tome”, all items are derived from blinded video rating. The frequency of the presence of the tremor/ dystonia/ other clinical characteristics in each group is given as percentage (%). To compare the frequency of each clinical criterion between the two groups we used Fisher’s exact test. A P-value<0.05 indicates significant difference between the groups. For subitems 13.5, 13.6, and 19 we also calculated sensitivity, specificity, and positive/ negative predictive value
| SWEDDs(%) | PD(%) | Fisher’sExact Test(Exact Sign.2-sided) | Sensitivity/specificity(%) | Positive/negativepredictivevalue (%) | ||
|---|---|---|---|---|---|---|
| Tremor characteristics | ||||||
| 1 | Body regions affected | |||||
| 1.1 | Head | 32 | 0 | P=0.036 | ||
| 1.2 | Jaw | 16 | 33 | n.s. | ||
| 1.3 | Voice | 8 | 0 | n.s. | ||
| 1.4 | Tongue | 4 | 0 | n.s. | ||
| 1.5 | UL | 100 | 100 | n.s. | ||
| 1.6 | LL | 24 | 83 | P=0.001 | ||
| 2 | Upper limb tremor | |||||
| 2.1 | Unilateral | 32 | 33 | n.s. | ||
| 2.2 | Highly asymmetrical | 64 | 33 | n.s. | ||
| 2.3 | Symmetrical or onlymild asymmetry | 4 | 33 | P=0.030 | ||
| 3 | Sensory trick | 20 | 0 | n.s. | ||
| 4 | Postural tremoraggravated in particularpositions | 60 | 58 | n.s. | ||
| 5 | Task-specificity/aggravation (writing,drawing, pouringwater) | 68 | 33 | n.s. | ||
| 6 | Jerkiness | 68 | 33 | n.s. | ||
| 7 | Flurries | 44 | 25 | n.s. | ||
| 8 | Thumb hyperextensiontremor | 48 | 42 | n.s. | ||
| 9.1 | Dominant tremorposition: rest | 24 | 83 | P=0.001 | ||
| 9.2 | Dominant tremorposition: posture | 76 | 17 | P=0.001 | ||
| 9.3 | Dominant tremorposition: action | 0 | 0 | n.s. | ||
| 10 | Re-emergent tremor | 0 | 25 | P=0.028 | ||
| Other clinical characteristics | ||||||
| 11 | Hypomimia | 48 | 83 | n.s. | ||
| 12 | Increased tone | 48 | 100 | P=0.002 | ||
| 13 | Repetitive finger andleg tasks | |||||
| 13.1 | Slowness | 20 | 50 | n.s. | ||
| 13.2 | Reduced amplitude | 44 | 58 | n.s. | ||
| 13.3 | Decrement | 20 | 83 | P=0.001 | ||
| 13.4 | Fatiguing | 4 | 92 | P<0.001 | ||
| 13.5 | Decrement or fatiguing | 20 | 100 | P<0.001 | 100/ 80 | 70.6/100 |
| 13.6 | Decrement andfatiguing | 4 | 75 | P<0.001 | 75/ 96 | 90/ 88.9 |
| 14 | Gait disturbance | 32 | 50 | n.s. | ||
| 15 | Reduced arm swing | 64 | 83 | n.s. | ||
| 16 | Postural stability | 24 | 17 | n.s. | ||
| Dystonia characteristics | ||||||
| 17 | Presence of dystonia | 84 | 33 | P=0.006 | 84/ 66.7 | 84/ 66.7 |
| 18 | Dystonia distribution: | |||||
| 18.1 | Eyes/ upper face | 0 | 0 | n.s. | ||
| 18.2 | Lower face | 4 | 0 | n.s. | ||
| 18.3 | Jaw/ tongue | 4 | 0 | n.s. | ||
| 18.4 | Larynx | 16 | 0 | n.s. | ||
| 18.5 | Neck | 44 | 25 | n.s. | ||
| 18.6 | Shoulder/ proximal arm | 48 | 25 | n.s. | ||
| 18.7 | Distal arm/ hand | 56 | 25 | n.s. | ||
| 18.8 | Pelvis/ proximal leg | 0 | 0 | n.s. | ||
| 18.9 | Distal leg/ foot | 0 | 0 | n.s. | ||
| 18.10 | trunk | 4 | 0 | n.s. | ||
| 19 | At least 2 out of items4,5,6,7,8,17 | 96 | 58 | P=0.009 | 96/20 | 77.4/83.3 |
Rating scales
Scores on the various rating scales are given in Table 2. The NMSQuest and the HADS were completed by 40 tremor-dominant PD patients and 25 SWEDDs patients, whereas other rating scales were completed by 12 tremor-dominant PD patients and 25 SWEDDs patients.
Table 2.
Results of Rating Scales (UPDRS-III and UDRS derived from blinded rating; FTM assessed un-blinded onsite; NMSQuest and HADS completed by patients as self screening questionnaires)
| PD | SWEDDs | p-value | |
|---|---|---|---|
| NMSQuest (max. score 30) | 12.2 (6.3) | 6.4 (4.1) | <0.001** |
| FTM severity (%) | 11.6 (5.6) | 22.1 (10.0) | 0.097 |
| UPDRS-III (max. score 108) | 27.5 (11.7) | 12.5 (8.5) | <0.001** |
| UDRS (max. score 112) | 1.7 (2.1) | 5.8 (4.9) | 0.001** |
| HADS anxiety (max. score 21) | 6.5 (4.4) | 6.0 (3.6) | 0.611 |
| HADS depression (max. score 21) | 6.1 (3.3) | 3.7 (2.7) | 0.004** |
For the NMSQuest scores we additionally compared each single item between the groups (Fisher’s Exact Test, Exact Sig. 2-sided). The difference between items was significant for items 1 (Dribbling of saliva during the daytime; PD 38%, SWEDDs 12%, p=0.044), 3 (Difficulty swallowing food or drink or problems with choking; PD 28%, SWEDDs 0%, p=0.005), 5 (Constipation or having to strain to pass stool; PD 58%, SWEDDs 12%, p<0.001), 10 (Unexplained pains; PD 48%, SWEDDs 16%, p=0.016), 11 (Unexplained change in weight; PD=20%, SWEDDs=0%, p=0.019), 12 (Problems remembering things that have happened recently or forgetting to do things; PD=50%, SWEDDs=24%, p=0.043), 13 (Loss of interest in what is happening around you or in doing things; PD=33%, SWEDDs=8%, p=0.033), 19 (Finding it difficult to have sex when you try; PD 53%, SWEDDs 16%, p=0.003), 22 (Finding it difficult to stay awake during activities such as working, driving, or eating; PD 43%, SWEDDs 8%, p=0.004), 23 (Difficulty getting to sleep at night or staying asleep at night; PD=63%, SWEDDs=32%, p=0.023), 25 (Talking or moving about in your sleep as if you are ‘acting’ out a dream); PD 43%, SWEDDs 16%, p=0.029) and 29 (Double vision; PD=30%, SWEDDs=0%, p=0.002).
Tremor analysis
A tremor was recorded under all 3 conditions (R, P, K) in all patients with SWEDDs and segmental dystonia investigated. All 9 PD patients had a rest-, and kinetic tremor, but only 8 had a tremor on posture. All 8 ET patients had a postural and kinetic tremor, but only in 3 a tremor at rest was recorded.
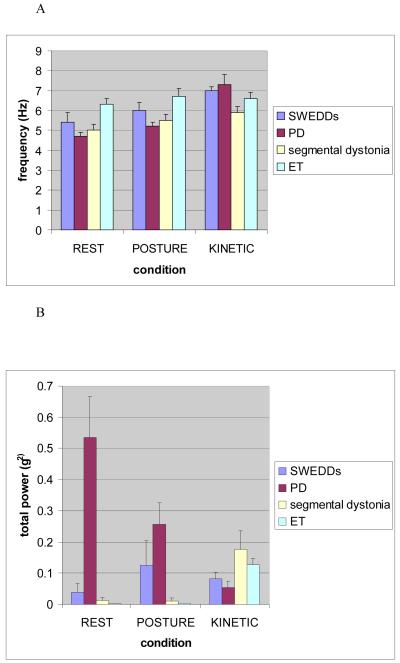
Tremor frequency
Mean peak tremor frequencies in the 3 different conditions for each group are shown in Fig. 1A. ANOVA revealed a significant effect for the factor CONDITION [F(2,58)=28.04; p<0.001] and the interaction between factors GROUP and CONDITION [F(6,58)=4.77; p=0.001], but not for the factor GROUP, indicating that tremor frequency is not a discriminative parameter. Post hoc paired t-tests revealed the significant effect for CONDITION to be due to a significant lower frequency for resting (mean 5.3 Hz, SE 0.2) compared to postural (mean 5.8 Hz, SE 0.2; p=0.002) and kinetic tremor (mean 6.8 Hz, SE 0.2; p<0.001). The frequency of postural tremor was significantly lower than the frequency of kinetic tremor (p=0.001) in the total group of patients.
Fig.1.
Mean peak tremor frequencies (1A) and mean total power (1B) under the conditions “resting”, “posture” and “kinetic”in patients with SWEDDs, PD, segmental dystonia, and ET. Error bars represent standard error. Means were calculated from patients in which a tremor was recorded.
Tremor amplitude
Mean tremor amplitudes for each group and position are given in Fig. 1B. ANOVA revealed a significant effect for the factor GROUP [F(3,29)=8.03; p<0.001] and the interaction between GROUP and CONDITION [F(4,58)=10.69; p<0.001], but not for the factor CONDITION. Post hoc multiple comparisons with Bonferroni correction revealed that the PD group had a significantly higher overall tremor amplitude compared to SWEDDs (p=0.005), segmental dystonia (p=0.005) and ET (p=0.001). To explore the interaction between factors GROUP and CONDITION we performed a 1-way-ANOVA which showed a significant effect for the factors REST [F(3,12)=6.31; p=0.008] and POSTURE [F(3,12.5)=4.58; p=0.022]. Posthoc multiple comparisons with Bonferroni correction revealed this to be due to significantly higher amplitude of resting tremor in PD patients compared to SWEDDs (p<0.001), segmental dystonia (p<0.001) and ET (p<0.001). Tremor amplitudes at posture were significantly higher in the PD group compared to segmental dystonia (p=0.036) and ET (p=0.023). Other comparisons were not statistically significant.
Post hoc paired t-tests showed that PD patients had their highest amplitude at rest (mean 0.535 g2, SE 0.13), followed significantly lower tremor amplitudes during posture (mean 0.256 g2, SE 0.07; p=0.041), and movement (mean 0.054 g2, SE 0.02; p=0.004). There was also a significant difference between postural and kinetic tremor (p=0.030). In contrast, the kinetic tremor component in patients with segmental dystonia had the highest amplitude (mean 0.176 g2, SE 0.06), whereas tremor amplitudes at rest (mean 0.012 g2, SE 0.01) and posture (mean 0.010 g2, SE 0.01) were significantly lower (p=0.021; p=0.022). The same was true for ET patients, with the amplitude being highest in the kinetic component (mean 0.127 g2, SE 0.02), and a significantly lower tremor amplitude at posture (mean 0.004 g2, SE 0.00; p=0.001) and at rest (mean 0.001 g2, SE 0.00; p=0.001). In the SWEDDs group the two action tremor components had a higher tremor amplitude (postural tremor: mean 0.125 g2, SE 0.08; kinetic tremor: mean 0.082 g2, SE 0.02) compared to the rest component (mean 0.038 g2; SE 0.03). These differences did not reach statistical significance.
Re-emergent tremor
All patients with SWEDDs and segmental dystonia had continuous tremor when changing from resting to a position with arm outstretched at shoulder level. Eight out of 9 PD patients had a postural tremor component. In six of these patients there was a pause in tremor when patients went from rest to posture (mean duration 2.8 seconds; range 2.6-6.4).
TMS
One-way ANOVAs revealed no significant difference between the groups for baseline RMT and IO curve gradients.
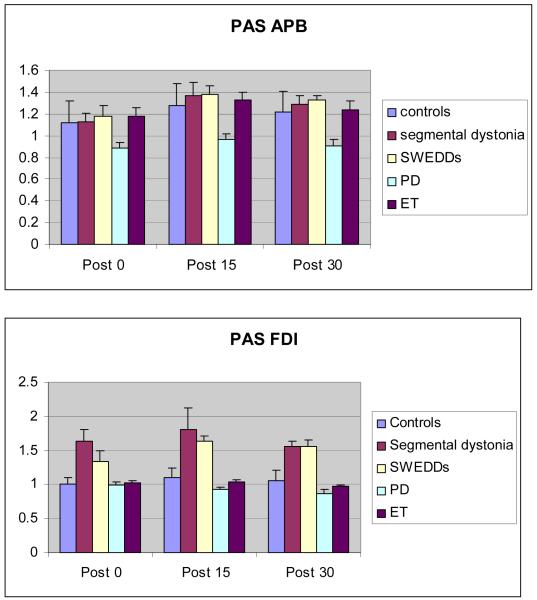
PAS
Data on the effects of PAS conditioning are shown in Fig. 2A and 2B. ANOVA with TIME (baseline, T0, T15, T30) and MUSCLE (two levels: APB, FDI) as within-subjects factors and GROUP (SWEDDs, PD, segmental dystonia, ET, controls) as between-subjects factors revealed a significant interaction between the factors TIME, MUSCLE and GROUP [F(9.1,74.8)= 2.12; p=0.038]. There was a strong main effect of the factor TIME [F(2.6,84.4)= 17.93; p<0.001]. The magnitude of MEP facilitation differed between groups as indicated by a significant interaction between TIME and GROUP [F(10.2,84.4)= 4.82; p<0.001]. The effect of PAS in the two muscles differed between the groups as demonstrated by a significant interaction between the factors MUSCLE and GROUP [F(4,33)=6.11; p=0.001]. The results of post hoc multiple comparisons with Bonferroni correction are given in table 3.
Fig. 2.
Effect of PAS on mean MEP amplitudes in healthy controls (black), patients with segmental dystonia (white), SWEDDs (light-grey) and PD (dark-grey). The data are plotted as a ratio to the baseline MEP amplitude. Error bars represent standard error. Ratios higher than 1 indicate facilitation and ratios below 1 indicate inhibition of MEP amplitude. Fig. 2A shows the effect of PAS on MEP amplitude for the APB (target) muscle. Fig. 2B shows the effect of PAS on MEP amplitude for the FDI muscle. *P < 0.05, ** P < 0.01 paired t-test comparing MEP amplitude with baseline.
Table 3.
Post hoc multiple comparisons (Bonferroni correction) of the PAS effect on MEP amplitudes between the groups (p<0.05 indicates a significant difference)
| controls | Segmental dystonia | SWEDDs | PD | ET |
|---|---|---|---|---|
| controls | p=0.001 | p=0.005 | p=0.020 | p=1.000 |
| Segmental dystonia | p=0.001 | p=1.000 | p<0.001 | p=0.001 |
| SWEDDs | p=0.005 | p=1.000 | p<0.001 | p=0.006 |
| PD | p=0.020 | p<0.001 | p<0.001 | p=0.018 |
| ET | p=1.000 | p=0.001 | p=0.006 | p=0.018 |
To further explore the conditioning effects of PAS on the MEP amplitude, we computed separate repeated 1-way-ANOVAs for each group and each muscle, treating TIME as a within-subject factor. In healthy controls, PAS induced an increase in mean MEP amplitude compared with baseline values in the APB muscle with a significant main effect of TIME [F(3,18)=4.93; p=0.011], without a significant effect in the FDI muscle. In patients with segmental dystonia there was a main effect of TIME in both the APB [F(3,18)=4.63; p=0.014] and the FDI [F(1.6,9.4)=4.64; p=0.046] muscles. Similarly, SWEDDs subjects showed a facilitatory effect in both the APB [F(3,21)=9.76; p<0.001] and FDI [F(3,21)=8.58; p=0.001]. The pattern of response in ET patients resembled the one of controls with a significant effect in the APB muscle [F(3,18)=5.03; p=0.010] without a significant effect in the FDI muscle. In contrast, PD patients exhibited no increase in MEP amplitudes after PAS in both muscles (no main effect of TIME). These findings were further explored by post-hoc paired t-tests revealing that PAS stimulation induced a significant and persistent increase in MEP amplitudes at T15 and T30 (p < 0.05) compared to baseline in the APB muscle in segmental dystonia (T15: p=0.027, T30: p=0.014), SWEDDs (T15: p=0.002, T30: p<0.001) and ET (T15: p=0.005, T30: p=0.020), but not in PD patients. In healthy controls and increase in MEP amplitude was only found at T15 (p=0.006). A significant facilitation of the MEP amplitude in the FDI muscle was only observed in patients with segmental dystonia (T15: p=0.044, T30: p<0.001) and SWEDDs (T15: p<0.001, T30: p<0.001).
Discussion
Here we have described a group of 25 patients with an adult onset asymmetric arm tremor with a rest component, all of whom had a previous diagnosis of PD but subsequently had normal DaT SPECT scans. It is obvious why neurologists had difficulties in distinguishing these patients from tremor-dominant PD patients with abnormal DaT SPECT scans since there is a major clinical overlap as also shown here by blinded video ratings (Table 1). Our study was designed to explore various hypotheses as to the underlying pathophysiology in this group of patients.
1.) What is the evidence that these patients do not have PD?
All 25 patients had normal DaT SPECT scans. The clinical significance of normal presynaptic dopaminergic imaging in a patient with a clinical diagnosis of PD is still controversial but follow-up studies suggest that only a minority of SWEDDs patients actually have nigrostriatal denervation or are due to supranigral causes of parkinsonism (e.g. cerebrovascular disease or cryptic neuroleptic use) (Marshall et al., 2006) (Whone et al., 2003) (Fahn et al., 2004). A recent 3 year prospective study comparing the results of baseline DaT SPECT scans with the clinical diagnosis after 3 years showed that PD is overdiagnosed clinically at baseline in diagnostically uncertain cases, and only in 1 out of 99 patient the SPECT finding was changed from normal at baseline to abnormal after 3 years [Ref: Marshall et al]. The DaT SPECT scans in our patients were performed on average 6.5 years into the disease course (5 patients even had a repeat scan on average 2 years after the first one), so we feel confident in assuming that there are no “false negative” results.
There were clear differences in treatment response between the PD and SWEDDs groups. Ninety-two percent of the PD patients in this study noted response to treatment of their tremor, with levodopa reported to be most effective. Tremor in the SWEDDs subjects had a poorer response with only 40% of patients having at least a slight benefit to any therapy that was given to them and no SWEDD subject responded to levodopa treatment. All had been brought off dopaminergic drugs without clinical deterioration prior to inclusion in this study, which is in concordance with a previous report of successful antiparkinsonian therapy withdrawal in 11 patients with SWEDDs [Marshall, 2006 #157]. 94%-100% of autopsy proven PD cases had responded to levodopa during life (Hughes et al., 1992) (Louis et al., 1997) (Colosimo et al., 1995) (Rajput et al., 1990). Although tremor may sometimes be more difficult to treat than other parkinsonian signs, total refractoriness to therapeutic doses of levodopa therapy, in the absence of malabsorption, would argue strongly against the diagnosis of PD.
The UK PDSBB diagnostic criteria for PD (Gibb and Lees, 1988) specify bradykinesia as the obligatory feature for a parkinsonian syndrome. Moreover, these criteria further define bradykinesia not just as the presence of “slowness of initiation of voluntary movement”, but also “with progressive reduction in speed (fatiguing) and amplitude (decrement) of repetitive actions.” The severity of bradykinesia loosely correlates with the extent of the nigrostriatal dopaminergic lesion assessed in vivo using dopamine transporter SPECT (DaTSCAN) or F-DOPA PET imaging (Scherfler et al., 2007). We therefore investigated as part of the blinded clinical rating if the presence of true bradykinesia can discriminate between SWEDDs and tremor-dominant PD. Both items “fatiguing” and “decrement” were significantly more frequent in the PD group. The specificity of the combination “fatiguing and decrement” for the diagnosis of PD with abnormal DaT SPECT scan was 75% with a sensitivity of 96%. Only one SWEDDs case was rated as a false positive. This also shows however, that a proportion of tremor-dominant PD patients, (25% in this study) may fail to fulfill such a strict definition of bradykinesia. Importantly, these data also indicate that the pure presence of slowness or reduced amplitude cannot distinguish between the two groups.
Nonmotor symptoms (NMS) are a common feature in PD and sometimes may be present before the diagnosis can be made (O’Sullivan et al., 2008). The clinical picture of advanced PD is often dominated by NMS, which contribute to disability, impaired quality of life, and shortened life expectancy (Chaudhuri et al., 2006). Our group of 40 tremor-dominant PD patients had a mean NMS total score of 12.2, resembling previously reported results in PD (Chaudhuri et al., 2006). The SWEDDs group however had an average total score of 6.1, significantly lower than the PD group, although slightly higher than the frequency of NMS in healthy controls reported in the literature (mean 4.0) (Chaudhuri et al., 2006). A previous study has also shown that the mean UPSIT score for a group of SWEDDs patients was higher than in a PD group (p<0.001) and not different from the mean UPSIT in the controls (p=0.7), ET (p=0.4), or dystonia patients (p=0.9) (Silveira-Moriyama et al., 2009).
There were some differences between the clinical tremor characteristics of the PD and SWEDDS groups. The blinded rating showed that no PD patient had head tremor, compared with 32% of the SWEDDs group. Re-emergent tremor was present in some PD patients (20%) but in none of the SWEDDs group. However, all other clinical tremor characteristics overlapped between the groups. Accelerometry showed that a single basic tremor parameter cannot distinguish between SWEDDs, PD, ET, and segmental dystonia. However a combination of re-emergent tremor and highest tremor amplitude in the resting condition was characteristic of the tremor in the PD group, and this was different from the tremor in the SWEDDs group who had the highest tremor amplitude on posture.
This study shows a clear difference in response to PAS between the PD and the SWEDDs group. We confirmed previous findings that PD patients off dopaminergic therapy fail to respond to PAS conditioning (Morgante et al., 2006; Ueki et al., 2006). It has been suggested that dopaminergic deficiency in PD prevents the motor cortex from changing the strength of synaptic connections when it is primed by a repetitive, low-frequency stimulation (Morgante et al., 2006). Strikingly, despite their clinical resemblance to PD, our SWEDDs patients exhibited an exaggerated response with the same spread of excitation to the FDI muscle following PAS that was seen in patients with segmental dystonia.
2) What evidence is there to support the hypothesis that the SWEDDs group have dystonia?
Blinded clinical rating identified signs of dystonia in 84% of SWEDDs patients. In general, the dystonia was focal/segmental in distribution and was scored at a mean of 5.8 on the UDRS. “Features of dystonic tremor” such as thumb extension tremor, jerkiness, “flurries” or task/ position-specificity of tremor were also common in the SWEDDs group. A combination of at least 2 features indicative of a dystonic tremor was present in 96% of SWEDDs patients. Therefore, clinically, our data support the hypothesis that this group of SWEDDs patients may have dystonic tremor. However, dystonia (albeit subtle; mean UDRS score 1.7/112) was also noted on blinded video rating in one third of the patients with tremor-dominant PD. It is well known that dystonia can be found in PD, especially in patients with early onset disease. It is however uncommon in untreated patients and is more frequently seen as a complication of its treatment (Tolosa and Compta, 2006).
The slowness and reduction in amplitude of repetitive movement is a clinical feature that is in keeping with limb dystonia. Crucially, true bradykinesia (with fatiguing and decrement), which would be atypical for primary dystonia, was only seen in one SWEDDs patient. Tremor analysis, while showing overlap between all groups, indicated that in common with segmental dystonia (and ET) patients the tremor in the SWEDDs group was primarily an action tremor.
Our PAS data confirmed the results of previous studies in dystonia (Quartarone et al., 2003; Quartarone et al., 2007) by finding that our patients with segmental dystonia had exaggerated facilitation of MEPs not only in the target APB muscle, but also in the FDI,. This is in line with studies using other techniques to probe motor cortical plasticity in dystonia which have also shown an excessive response compared to normal subjects (Edwards et al., 2006). This was identical to the finding in our SWEDDs group who also showed an excessive response to PAS with spread of excitation outside the target muscle into adjacent hand muscles.
3.) Is there evidence that these patients have ET?
Both alcohol responsiveness and a positive family history (seen in 32% and 52% respectively of our SWEDDs group), could be seen as supportive clinical pointers that the tremor in these patients might be due to ET. However both alcohol responsiveness and a positive family history are commonly reported in primary dystonia too.
Although ET may be responsible for a number of SWEDDs subjects reported in clinical trials, we do not think that the distinctive subgroup of SWEDDs patients included in this study has ET. Inclusion criteria for ET include presence of bilateral, largely symmetrical postural or kinetic tremor involving hands and forearms that is visible and persistent. The SWEDDs subjects investigated in this study all had a unilateral or highly asymmetric tremor that was present at posture and action but also at rest. The presence of dystonia is an exclusion criterion for ET (Deuschl et al., 1998), and therefore 84% of our SWEDDs group would be excluded on these grounds alone. We also, for the first time to our knowledge, recorded response to PAS in ET patients and compared this with our SWEDDs group. The response to this PAS protocol was significantly different between our SWEDDs and ET group, with the former resembling the response observed in dystonia, the latter resembling healthy controls.
Clinical implications and conclusions
SWEDDs represent a heterogeneous group of patients. This study did not aim to investigate the whole range of underlying diagnoses responsible for all SWEDDs patients, but instead we concentrated on a specific subgroup, namely patients presenting with an asymmetric resting tremor with normal dopamine transporter / presynaptic dopamine ligand scans. In this group, our data (clinical, non-motor scales, tremor analysis, PAS response) all support the hypothesis that these patients have an alternative diagnosis to idiopathic PD. Having excluded patients with psychogenic tremor, drug-induced tremor and neuropathic tremor on clinical grounds at entry into the study, we believe that our data are supportive (but not conclusively so) of the hypothesis that adult-onset dystonic tremor is the most likely cause of the clinical syndrome in this sub-group of SWEDDs patients. This would represent an expansion of the range of clinical phenotypes within the current classification of primary dystonia to include a group of patients presenting with late-onset asymmetric mainly upper limb tremor with mild additional dystonic postures. There are unusual clinical features in this group (presence of facial hypomimia, reduced arm swing (although this can be seen in primary cervical dystonia too (Kagi et al., 2008), occurrence of postural instability in some). Distinguishing these patients from PD is important. These patients do not develop the typical long-term motor and nonmotor complications of advanced PD, and many of the 25 SWEDDs patients described here had received unnecessary and inappropriate treatment for many years. In addition to the personal benefit to patients from clarity in the diagnosis, there are also pharmaco-economic reasons for trying to distinguish these patients from PD. For example the cost of ropinirole (9mg/day) and levodopa/benserazide (300mg/d) which one patient received as her treatment for 5 years was £9715. It is important to raise awareness of the existence of this entity and the clinical features which may help to distinguish these patients from PD both in a general clinical setting and for those planning clinical trials in PD. However, the differentiation of these patients from tremor-dominant PD is clearly difficult on clinical grounds alone, and if diagnostic doubt remains despite careful clinical examination, a DaT SPECT scan in such patients is an appropriate investigation. Its cost of approximately £800 is likely to outweigh the long-term financial burden of inappropriate dopaminergic drug therapy in these patients.
Acknowledgements
We are grateful to the patients for participating in our research. This work was supported by the Austrian Science Fund (FWF) [Erwin Schrödinger Grant J 2764 to PS], the National Institutes of Health Research (NIHR) [Clinician Scientist award to MJE], and the Brain Research Trust UK [Lady Astor Prize to SAS].
Abbreviations
PD
Parkinson’s disease
SWEDDs
scans without evidence of dopaminergic deficit
DaT SPECT
dopamine transporter single photon emission computed tomography
PAS
paired associative stimulation paradigm
References
- Bain PG. The management of tremor. J Neurol Neurosurg Psychiatry. 2002;72(Suppl 1):I3–I9. doi: 10.1136/jnnp.72.suppl_1.i3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain PG, Findley LJ, Britton TC, Rothwell JC, Gresty MA, Thompson PD, et al. Primary writing tremor. Brain. 1995;118(Pt 6):1461–72. doi: 10.1093/brain/118.6.1461. [DOI] [PubMed] [Google Scholar]
- Bain PG, Findley LJ, Thompson PD, Gresty MA, Rothwell JC, Harding AE, et al. A study of hereditary essential tremor. Brain. 1994;117(Pt 4):805–24. doi: 10.1093/brain/117.4.805. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–45. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord. 2006;21:916–23. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
- Colosimo C, Albanese A, Hughes AJ, de Bruin VM, Lees AJ. Some specific clinical features differentiate multiple system atrophy (striatonigral variety) from Parkinson’s disease. Arch Neurol. 1995;52:294–8. doi: 10.1001/archneur.1995.00540270090024. [DOI] [PubMed] [Google Scholar]
- Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T. Rating scales for dystonia: a multicenter assessment. Mov Disord. 2003;18:303–12. doi: 10.1002/mds.10377. [DOI] [PubMed] [Google Scholar]
- de Carvalho Aguiar PM, Ozelius LJ. Classification and genetics of dystonia. Lancet Neurol. 2002;1:316–25. doi: 10.1016/s1474-4422(02)00137-0. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Bain P, Brin M, Ad Hoc Scientific Committee Consensus statement of the Movement Disorder Society on Tremor. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Huang YZ, Mir P, Rothwell JC, Bhatia KP. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Mov Disord. 2006;21:2181–6. doi: 10.1002/mds.21160. [DOI] [PubMed] [Google Scholar]
- Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol. 2005;252(Suppl 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Unified Parkinson’s disease rating scale. MacMillan Healthcare Information; Florham Park, NJ: 1987. [Google Scholar]
- Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. Williams & Wilkins; Baltimore: 1988. pp. 225–234. [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–91. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. The Parkinson Study Group Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. Neurology. 1990;40:1529–34. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- Kagi G, Schwingenschuh P, Bhatia KP. Arm swing is reduced in idiopathic cervical dystonia. Mov Disord. 2008 doi: 10.1002/mds.22216. [DOI] [PubMed] [Google Scholar]
- Koller WC, Busenbark K, Miner K, Essential Tremor Study Group The relationship of essential tremor to other movement disorders: report on 678 patients. Ann Neurol. 1994;35:717–23. doi: 10.1002/ana.410350613. [DOI] [PubMed] [Google Scholar]
- Louis ED, Klatka LA, Liu Y, Fahn S. Comparison of extrapyramidal features in 31 pathologically confirmed cases of diffuse Lewy body disease and 34 pathologically confirmed cases of Parkinson’s disease. Neurology. 1997;48:376–80. doi: 10.1212/wnl.48.2.376. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ottman R. Study of possible factors associated with age of onset in essential tremor. Mov Disord. 2006;21:1980–6. doi: 10.1002/mds.21102. [DOI] [PubMed] [Google Scholar]
- Marek K, Jennings D, Seibyl J. Long-term follow-up of patients with scans without evidence of dopaminergic deficit (SWEDD) in the ELLDOPA study. Neurology. 2005;64(suppl1):A274. [Google Scholar]
- Marek K, Seibyl J. Beta-CIT Scans without Evidemce of Dopaminergic Deficit (SWEDD) in the ELLDOPA-CIT and CALM-CIT Study: Long-Term Imaging Assessment. Neurology. 2003;60:A293. [Google Scholar]
- Marshall VL, Patterson J, Hadley DM, Grosset KA, Grosset DG. Two-year follow-up in 150 consecutive cases with normal dopamine transporter imaging. Nucl Med Commun. 2006;27:933–7. doi: 10.1097/01.mnm.0000243374.11260.5b. [DOI] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–69. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SS, Williams DR, Gallagher DA, Massey LA, Silveira-Moriyama L, Lees AJ. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov Disord. 2008;23:101–6. doi: 10.1002/mds.21813. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, et al. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain. 2003;126:2586–96. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant’angelo A, Rizzo V, Bagnato S, Terranova C, et al. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2007 doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant’Angelo A, Romano M, et al. Homeostatic-like plasticity of the primary motor hand area is impaired in focal hand dystonia. Brain. 2005;128:1943–50. doi: 10.1093/brain/awh527. [DOI] [PubMed] [Google Scholar]
- Quinn NP, Rothwell JC, Thompson PD, Marsden CD. Hereditary myoclonic dystonia, hereditary torsion dystonia and hereditary essential myoclonus: an area of confusion. Adv Neurol. 1988;50:391–401. [PubMed] [Google Scholar]
- Rajput AH, Rozdilsky B, Rajput A, Ang L. Levodopa efficacy and pathological basis of Parkinson syndrome. Clin Neuropharmacol. 1990;13:553–8. doi: 10.1097/00002826-199012000-00007. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol. 1997;105:340–4. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Rocca WA, McDonnell SK, Strain KJ, Bower JH, Ahlskog JE, Elbaz A, et al. Familial aggregation of Parkinson’s disease: The Mayo Clinic family study. Ann Neurol. 2004;56:495–502. doi: 10.1002/ana.20228. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Williamon A, Rothwell JC. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci. 2007;27:5200–6. doi: 10.1523/JNEUROSCI.0836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfler C, Schwarz J, Antonini A, Grosset D, Valldeoriola F, Marek K, et al. Role of DAT-SPECT in the diagnostic work up of parkinsonism. Mov Disord. 2007;22:1229–38. doi: 10.1002/mds.21505. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Edwards MJ, Mir P, Cordivari C, Hooker J, Dickson J, et al. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs) Mov Disord. 2007 doi: 10.1002/mds.21685. [DOI] [PubMed] [Google Scholar]
- Silveira-Moriyama L, Schwingenschuh P, O’Donnell A, Schneider SA, Mir P, Carrillo F, et al. Olfaction in patients with suspected Parkinsonism and scans without evidence of dopaminergic deficit (SWEDDs) J Neurol Neurosurg Psychiatry. 2009 doi: 10.1136/jnnp.2009.172825. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–84. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Sullivan KL, Hauser RA, Zesiewicz TA. Essential tremor. Epidemiology, diagnosis, and treatment. Neurologist. 2004;10:250–8. doi: 10.1097/01.nrl.0000138736.07840.b2. [DOI] [PubMed] [Google Scholar]
- Tisch S, Rothwell JC, Bhatia KP, Quinn N, Zrinzo L, Jahanshahi M, et al. Pallidal stimulation modifies after-effects of paired associative stimulation on motor cortex excitability in primary generalised dystonia. Exp Neurol. 2007;206:80–5. doi: 10.1016/j.expneurol.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Tolosa E, Compta Y. Dystonia in Parkinson’s disease. J Neurol. 2006;253(Suppl 7):VII7–13. doi: 10.1007/s00415-006-7003-6. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A, et al. Altered plasticity of the human motor cortex in Parkinson’s disease. Ann Neurol. 2006;59:60–71. doi: 10.1002/ana.20692. [DOI] [PubMed] [Google Scholar]
- Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54:93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, et al. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–45. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, et al. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–52. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]