Cellular strategies for regulating DNA supercoiling: A single-molecule perspective (original) (raw)
. Author manuscript; available in PMC: 2011 Aug 20.
Summary
Excess entangling and twisting of cellular DNA (i.e., DNA supercoiling) are problems inherent to the helical structure of double-stranded DNA. Supercoiling affects transcription, DNA replication, and chromosomal segregation. Consequently the cell must fine-tune supercoiling to optimize these key processes. Here, we summarize how supercoiling is generated and review experimental and theoretical insights into supercoil relaxation. We distinguish between the passive dissipation of supercoils by diffusion and the active removal of supercoils by topoisomerase enzymes. We also review single-molecule studies that elucidate the timescales and mechanisms of supercoil removal.
The helical structure of double-stranded DNA allows for the faithful deciphering and transmission of genetic information, whereby each DNA strand serves as a template for the synthesis of a complementary polynucleotide chain. The intertwining of the strands promotes genome stability by physically linking the polynucleotides in a structure stabilized by hydrogen bonding and base stacking. However, these features impede free rotation of the DNA strands, so that, in the context of circular bacterial chromosomes or eukaryotic chromatin, the DNA is topologically constrained. As a result, any local changes in the number of twists about the helical axis due to duplex unwinding or protein tracking result in compensatory topological changes (such as supercoiling) elsewhere in the DNA molecule (Box 1 and Figure 1). Excessive local supercoiling may impede the movement of RNA or DNA polymerases along the DNA template or entangle daughter DNA chromosomes during genome replication and cell division. Thus, the cell must carefully control DNA supercoiling to ensure that these processes proceed smoothly.
DNA Topology Basics.
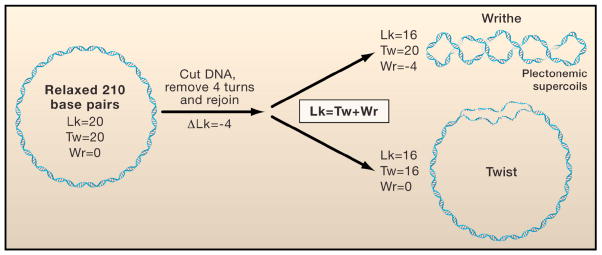
For closed circular DNA or linear duplex DNA that is topologically constrained (as in the case of chromosome loops), the linking number (Lk) is a quantitative descriptor of DNA topology that includes the number of times the helix winds around its central axis and the number of times the helix crosses itself (Figure 1). The linking number can only be altered by breaking and then rejoining a strand of DNA. Lk0 designates the linking number of DNA when it is relaxed, and ΔLk designates the difference between Lk and Lk0 under the same experimental conditions. “Twist” (Tw) is the number of helical turns in the duplex DNA. When the helix is overwound or underwound, ΔLk is positive or negative, respectively. Alternatively, “writhe” (Wr) occurs when the DNA helix buckles into loop-like structures called plectonemic supercoils, or when the DNA wraps around proteins complexes, such as nucleosomes. Lk is the sum of Tw and Wr (Lk = Tw + Wr), and thus changes in the value of ΔLk may partition between changes in Tw and Wr. As illustrated in Figure 1, for a relaxed 210-basepair DNA circle with an average 10.5 helical turns per basepair, Tw = 20, Wr = 0, and Lk = Lk0 = Tw. A ΔLk of −4 could be accommodated at the two extremes by (i) a pure change in Tw, leading to local denaturation of 4 helical turns; or (ii) a pure change in Wr with the formation of 4 plectonemic supercoils (Figure 1, right).
Figure 1. DNA topology.
The topology of a double-stranded DNA is described by its linking number (Lk), which is the sum of twist (Tw) and writhe (Wr). (Left) A torsionally relaxed DNA molecule with a length of 210 basepairs contains 20 turns (10.5 bp/turn) or Tw=20. Hypothetically, if the DNA were cut, then one end was twisted by four turns in the direction opposite to the natural helicity of the DNA, and subsequently resealed, the resulting linking number of the DNA would equal Lk=20−4=16. (Right) The upper and lower panels show the topology of the DNA molecule when the removal of these turns is at the expense of twist (Tw=20−4=16) and writhe (Wr=0−4=−4), respectively.
In this Review, we discuss how the cell modulates supercoiling through spontaneous and enzyme-catalyzed mechanisms. We compare results from classical ensemble methods with those derived from single-molecule force spectroscopy studies performed with magnetic and optical tweezers. Ensemble techniques average the behavior of a large number of molecules, and thus mask transient intermediates or rare events. By contrast, single-molecule methods can detect such events and yield otherwise unattainable insights into how supercoiling is modulated. Where possible, we indicate which specific insights rely on the dynamic control afforded by single-molecule methods. We also discuss how controlled mechanical manipulation of the topology of single DNA molecules has enhanced our understanding of basic DNA biophysics and how enzymes, such as DNA topoisomerases, influence DNA topology. We conclude by discussing how extensions of single-molecule techniques might inspire and impact the next generation of experiments on DNA topology and its control in the cell.
Cellular Processes Affected by Supercoiling
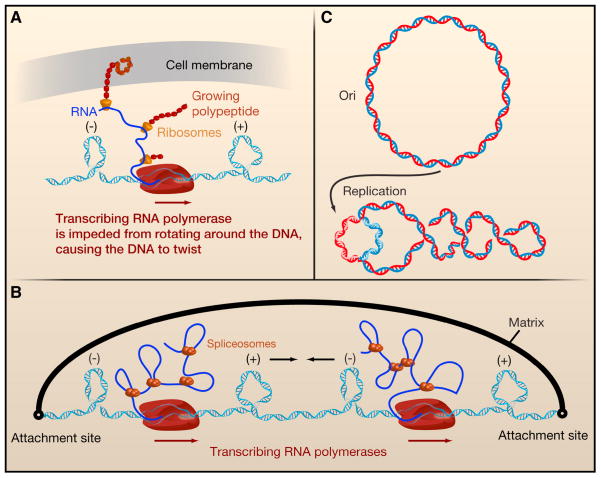
Transcription by RNA polymerase (RNAP) has profound effects on DNA topology. Processes that increase the rotational drag of RNAP or anchor it to a cell surface prevent RNAP from rotating around the DNA as it tracks along the helix. For example, in bacteria the mRNA is rapidly loaded with ribosomes. If, as in the case of membrane protein synthesis, the nascent polypeptide on the ribosome becomes embedded in the cell membrane, the drag on RNAP is enhanced further (Figure 2A). Alternatively, the mRNA may hybridize with the negatively supercoiled DNA that is formed in the wake of the advancing polymerase, generating an R-loop consisting of a DNA-RNA hybrid (Drolet, 2006). In eukaryotic cells, the rotational drag on RNAP stems from large macromolecular complexes, such as spliceosomes, that bind to nascent pre-mRNAs (Figure 2B). Because RNAP is prevented from rotating around the helix, it is the DNA that is forced to rotate, which generates positive supercoils ahead of the transcription machinery and compensatory negative supercoils behind it (Figure 2A and 2B; Box 1). This scenario is known as the twin-supercoiled domain (Liu and Wang, 1987).
Figure 2. DNA topology and its relevance in transcription and replication.
A. When RNA polymerase (RNAP) is prevented from rotating along the helical axis of the DNA during transcription, positive and negative supercoils accumulate ahead and behind the enzyme, respectively. Multiple factors impede the rotation of RNAP by increasing its hydrodynamic drag. In bacteria, these factors include the nascent RNA strand (blue solid line), ribosomes on the mRNA (yellow), and even the growing peptide itself. Furthermore, the nascent protein might insert itself into the cell membrane (gray sheet), providing an anchor point. B. In eukaryotes, the nascent RNA and its processing factors, such as spliceosomes, increase the rotational drag on RNAP and impede its rotation around DNA’s helical axis, leading to supercoiling behind and ahead of the enzyme. When tandem genes are transcribed, RNAP complexes progress in the same direction on duplex DNA. The DNA domain between them contains both negative and positive supercoils that could diffuse towards each other and subsequently annihilate. The rate at which this process occurs depends on the timescales at which DNA can spin around its own helical axis and the axis defined by plectonemes. C. When a circular DNA (top) is replicated, two origins move in opposite directions, unwinding the parental DNA. By conservation of linking number, this generates positive supercoils ahead of the forks (bottom).
DNA replication also impacts DNA topology. As the strands of duplex DNA unwind to allow each single strand of DNA to serve as a template for the synthesis of a complementary strand (Wang, 2002), a replisome that is prevented from rotating will accumulate positive supercoils in front of the replication fork. Alternatively, the replisome may follow the helical path of the template strands, but this will produce interwound DNA helices that must be unlinked or decatenated during cell division (Figure 2C).
These local changes in topology have functional consequences in the cell, such as the activation or repression of transcription (Drolet, 2006; Fisher, 1984; Gartenberg and Wang, 1992; Peter et al., 2004; Travers and Muskhelishvili, 2005). For example, the local melting of duplex DNA at promoters or replication origins in response to negative supercoiling facilitates the initiation of transcription or DNA replication, respectively. Conversely, positive supercoiling can impede mRNA synthesis (Gartenberg and Wang, 1992) and arrest the progression of the replication fork (Wang, 2002).
Given the biological consequences of alterations in DNA topology, cells possess several mechanisms to tune the degree of supercoiling. In eukaryotic chromatin, the solenoidal wrapping of DNA around histone protein complexes absorbs one negative supercoil per nucleosome core particle, such that the DNA is subjected to less superhelical strain (Worcel et al., 1981). Local DNA unwinding or transitions from B-form to Z-form DNA can also absorb local changes in supercoiling (i.e., linking number or Lk) (Frank-Kamenetskii, 1990). In addition, supercoils can “diffuse” towards each other along the DNA and cancel when supercoils of opposite signs collide. The cell also has an elaborate toolkit of enzymes, called topisomerases, which modulate DNA topology without changing the chemical structure of the DNA (Champoux, 2001; Schoeffler and Berger, 2008). Present in all organisms and many DNA viruses, topoisomerases use a mechanism of reiterative DNA strand cleavage and religation to alter the degree of supercoiling in vivo (Box 3).
BOX 3. The cellular roles of the various topoisomerases.
All topoisomerases catalyze changes in the linkage of DNA strands or helices by a conserved mechanism of transient DNA strand cleavage and religation. Yet, the different types of topoisomerases described in this Review carry out distinct roles inside the cell. In the bacterium E. coli, for example, the antagonist actions of the type IA topoisomerase TopA (i.e., removal of negative supercoils to increase Lk) and the type II enzyme gyrase (i.e., introduction of negative supercoils to decrease Lk) provide a homeostatic mechanism to regulate global DNA supercoiling in chromosomes. The other type II enzyme in this organism, Topoisomerase IV (TopIV), acts to remove positive supercoils in advance of the replication fork and is a potent decatenase to resolve chromosomal intertwining. In eukaryotes, TopIB and TopII enzymes provide the major DNA relaxation activities during transcription and replication to remove positive and negative supercoils. TopIB uses a mechanism with a protein-linked DNA swivel whereas TopII enzymes act through a strand passage mechanism. The type II enzymes also act during mitosis to decatenate newly replicated sister chromatids. Topoisomerase III (TopIII), a type IA enzyme, resolves recombination intermediates and acts as a decatenase on nicked DNA during replication. Reverse gyrase is a type I topoisomerase occurring predominantly in archaea, where it has the ability to introduce positive supercoils. Reverse gyrase can thus act in concert with TopIA, a function that may be particularly useful for maintaining genomic stability at the high environmental temperatures at which most archaea thrive.
Methods to study supercoiling
Detecting global supercoiling by gel electrophoresis
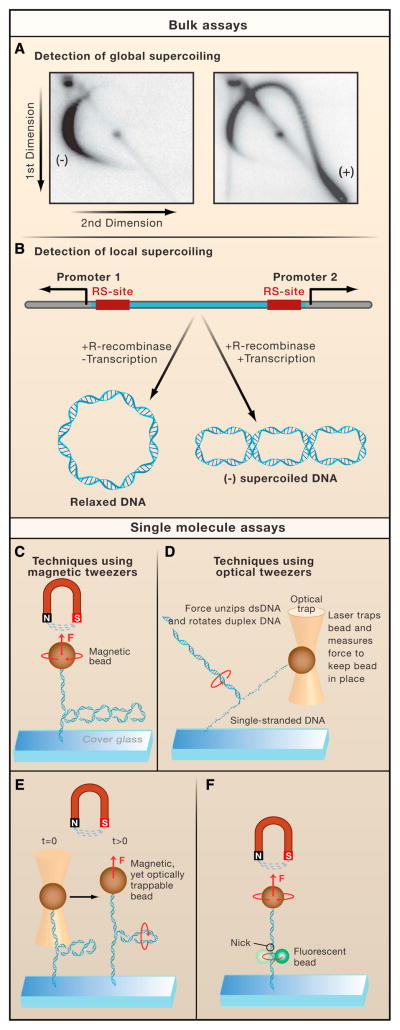
Techniques such as equilibrium and velocity sedimentation (Supplemental Materials II) and electron microscopy (Supplemental Materials III) can be used to analyze the extent of DNA supercoiling. However, agarose gel electrophoresis (Supplemental Materials I) is currently the most widely used method for determining the distribution of the degree of supercoiling in an ensemble of DNA plasmids. By running one-dimensional agarose gels before and after the addition of a topoisomerase, one can derive rudimentary kinetic parameters for a particular enzyme and gain insights to its substrate specificity and catalytic mechanism (Figure S1 in Supplemental Materials I). For instance, the unique ability of the type II topoisomerase DNA gyrase to introduce negative supercoils into relaxed DNA and the specificity of bacterial topoisomerase I for relaxing only negative supercoils were both determined using such techniques (Gellert et al., 1976; Kirkegaard and Wang, 1985). Furthermore, the TopIA and the type II topoisomerases were shown by these techniques to remove supercoils in increments of one and two turns respectively, (i.e. |ΔLk| = 1 and 2) (Brown and Cozzarelli, 1979) and (Brown and Cozzarelli, 1981)). Two-dimensional gel electrophoresis with different concentrations of intercalator in each dimension (Supplemental Materials I) resolves a heterogeneous population of closed DNA molecules distributed in an arc-shaped pattern, wherein DNA topoisomers are separated as a function of writhe (Figure 3A).
Figure 3. Assays for measuring supercoiling.
A. Global supercoiling can be detected using two-dimensional gel electrophoresis, in which plasmid DNA is first resolved in the gel in one direction, followed by electrophoresis in a perpendicular direction in the presence of an increased concentration of an intercalating agent. As a result, an arc-shaped pattern emerges, consisting of spots where the degree of supercoiling (i.e., linking number or Lk) increases in a clockwise orientation (Left panel). Plasmid DNA isolated from yeast cells expressing TopIB yields a population of negatively supercoiled topoisomers because the DNA is wrapped around nucleosomes in vivo (Right panel). By contrast, in cells that lack TopIB or TopII but express E. coli TopIA, the preferential relaxation of negatively supercoiled DNA induced by transcription, results in the accumulation of positively supercoiled DNA (right panel). B. The ring excision assay can detect local supercoiling in a predefined region on the DNA. When a recombinase excises a stretch of DNA between two recombinase sites (RS), the resulting ring of DNA retains the supercoil density it had when it was still integrated. This assay was used to characterize the negative supercoils generated between two RNAP complexes moving away from divergent promoters. C. Magnetic tweezers exert a magnetic force (F) on a magnetic bead, which is attached to a double-stranded DNA molecule. The other end of the molecule is linked to a microscope coverslip. By translating the magnets up or down, one increases or decreases the pulling force, respectively. The DNA can be supercoiled by twisting the magnets. D. A focused laser can trap a transparent bead at a fixed position in space and measure the force exerted on the bead. These optical tweezers can measure the rotational drag of non-plectonemic duplex DNA with one strand tethered to the glass slide and the other strand held stationary in the optical trap. As the stage is moved away from the trap, the duplex DNA starts to unzip, forcing the double-stranded portion of the DNA to spin around its axis. The optical trap measures the force required to start unzipping the DNA. E. A combination of magnetic and optical tweezers has been used to measure the rotational drag of plectonemic DNA. Here, the pulling force on a supercoiled DNA molecule is suddenly increased. During this process, the plectonemes will rotate as indicated, generating viscous drag that opposes the motion of the bead. F. The rotor bead assay consists of a standard magnetic tweezers setup augmented with a strategically positioned small fluorescent “rotor bead”. This rotor bead serves as an indicator for the rotational motion of DNA as it spins along the axis of the DNA concurrently with the DNA.
Detecting local supercoiling by electrophoresis
Measuring the average global supercoiling masks transient supercoiling generated at specific positions (e.g., directly ahead of or directly behind the transcription and replication machinery). Gartenberg and Wang developed a technique that determines the levels of DNA supercoiling in vivo between two precisely positioned recombinase recognition sites (Gartenberg and Wang, 1993) that are subsequently excised while maintaining the linking number of the intervening DNA fragment (Figure 3B). This technique has been used to identify factors that inhibit free rotation of DNA in vivo, which contribute to the formation of twin-supercoiled domains (Gartenberg and Wang, 1993).
Single-molecule methods - Magnetic tweezers
Magnetic tweezers enable controlled, real-time twisting and stretching of a single linear DNA molecule, which makes them well suited for investigations of DNA topology (Figure 3C) and changes in supercoiling or Lk induced by enzymes. In the magnetic tweezers technique, a single linear DNA molecule is attached at one extremity to the bottom glass slide of a flow cell whereas the other extremity is bound to a magnetic bead. Magnets positioned above the flow cell pull on the magnetic bead and stretch the DNA. The Lk of the DNA molecule is tightly controlled: twisting the magnets any number of turns changes the Lk of the DNA molecule by exactly the same number. This change in Lk is initially accommodated as a change in twist, but as one continues to twist the DNA, at some point plectonemic supercoils (a form of writhe) form. After this point, the height of the bead above the surface drops with an increase in the number of of plectonemes (~50 nm per plectoneme). In this manner, the molecule’s partitioning between twist and writhe can be set precisely (Supplemental materials IV) and monitored in real time as topoisomerases act (Figure 5A–D) (Strick et al., 1996). Even the behavior of an enzyme that indirectly affects the degree of supercoiling can be monitored, as illustrated for DNA unwinding by E. coli RNAP during transcription initiation (Revyakin et al., 2004) and for DNA transport enzymes, such as Type I restriction enzymes (Seidel et al., 2004) and FtsK (Saleh et al., 2005)..
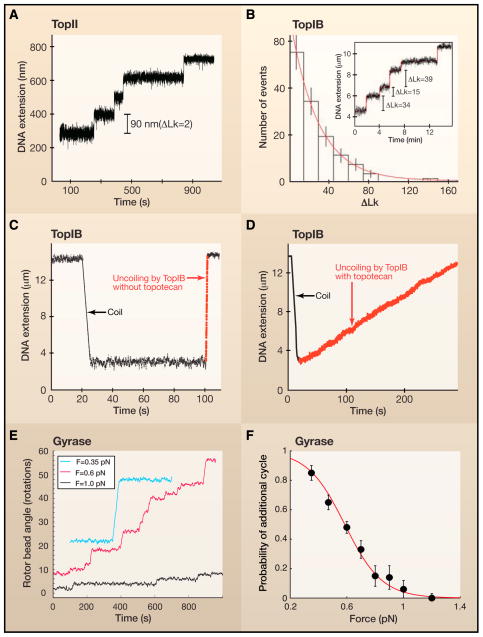
Figure 5. Single-molecule studies of topoisomerase dynamics.
A. In a magnetic tweezer experiment, the length or extension of the DNA provides a measure of its supercoil density at a fixed force. In the presence of TopII, the DNA extension increases with time in equally-sized steps of 90 nm which is equivalent to the removal of two supercoils for each catalytic cycle. B. In contrast to TopII, TopIB does not remove a fixed number of supercoils with each catalytic event (inset). Rather, the distribution of the number of supercoils removed is exponential, with a mean far above unity. This result agrees with the proposed swivel mechanism for supercoil relaxation by TopIB. C. The instantaneous relaxation rate of supercoils by TopIB in the absence of the chemotherapeutic drug topotecan is relatively fast. In this trace, the rate is ~100 supercoils per second (dashed red line) D. The chemotherapeutic drug topotecan slows down relaxation dramatically, to the extent that the typical rates are now ~5 supercoils per second. E. Rotor bead assays showed that DNA gyrase introduces two supercoils with each catalytic cycle. F. The processivity of gyrase activity decreases markedly with increasing force, suggesting the presence of a force-sensitive step in the enzyme’s catalytic cycle. It is thought that the enzyme performs work against the externally applied force when wrapping a DNA segment around itself. The solid line is a fit to a model that includes competition between this wrapping step and enzyme dissociation from the DNA.
Single-molecule methods - Optical tweezers
Optical tweezers allow for the accurate study of DNA dynamics. In the context of DNA supercoiling, they have been used to unzip DNA (Figure 3D). Optical tweezers are true “tweezers:” beads can be picked up and moved around by means of a laser beam. One can suspend individual biomolecules, such as DNA between two beads, and stretch the molecule by moving the beads away from each other (Neuman and Block, 2004). Optical tweezers can attain ultra-high spatial resolution, as demonstrated by the observation of individual, base-pair-sized (3.7 ± 0.6 Å) steps of RNA polymerases along DNA (Abbondanzieri et al., 2005). To study the fast dynamics of DNA molecules, one uses the ability of lasers to rapidly change the position of the beads to which DNA is attached (Bohbot-Raviv et al., 2004; Coelho Neto et al., 2005; Feingold, 2001; Goshen et al., 2005). Recently, the ability to twist DNA with optical tweezers has been developed, and the impact of this development for studies of DNA topology is discussed in the Outlook section below.
Single-molecule methods - Combined magneto-optical trap
To study the dynamics of supercoiled DNA at the single-molecule level, a magneto-optical trap was developed that combined the speed of optical tweezers with the ease of twisting of the magnetic tweezers (Figure 3E) (Crut et al., 2007; Romano et al., 2003; Sacconi et al., 2001). The key to this technique is the use of a magnetic bead that is also amenable to optical trapping, allowing the tethered DNA to be held by both the optical trap and the magnetic tweezers. As such, one can introduce plectonemes in the DNA using magnetic tweezers and then rapidly “pull them out” by suddenly increasing the force using the optical trap. This permits one to probe the inherent resistance of DNA to the dissipation of plectonemes, either in the absence or presence of proteins on the DNA.
Single-molecule methods - Rotor bead assay
To monitor direct changes in DNA twist, the rotor bead assay (Figure 3F) was developed (Bryant et al., 2003; Gore et al., 2006). As with magnetic tweezers, a DNA molecule is fixed between a magnetic bead and a surface, but in this technique, a small fluorescent “rotor bead” is also attached in the vicinity of a nick artificially induced in the DNA. Whenever the DNA swivels about the nick in order to dissipate excess twist, the rotor bead also swivels, and its rotation is easily observed with standard fluorescent microscopy. Rotor bead techniques have been used to quantify the torque in the DNA as a function of the number of helical turns per basepair (i.e., twist) (Bryant et al., 2003) and to track the activity of a single DNA gyrase enzyme in real time as it induces supercoils in a DNA molecule (Figure 5E) (Gore et al., 2006).
The dissipation of DNA supercoils
The positive and negative supercoils that coexist as a result of transcription and replication determine, to a large degree, the steady state level of DNA supercoiling in the cell (Figures 2). Local domains of DNA supercoiling (described by the twin-supercoiled domain model (Liu and Wang, 1987)) have been demonstrated in vitro in bacterial and yeast cells (Figure 3A) (Giaever and Wang, 1988; Tsao et al., 1989; Wu et al., 1988). They result from a competition between the rate at which the supercoils are generated and the rate at which they are removed. Removal can occur as a result of annihilation of the positive supercoils with the negative supercoils by diffusion along the DNA and subsequent merging (Figure 2B) or by the enzymatic activity of topoisomerases (Figure 4). In the following sections, we ask two questions: what factors limit diffusion of supercoils along the DNA to the extent that they do not merge, and what are the mechanisms and kinetics of the enzymatic removal of supercoils by topoisomerases?
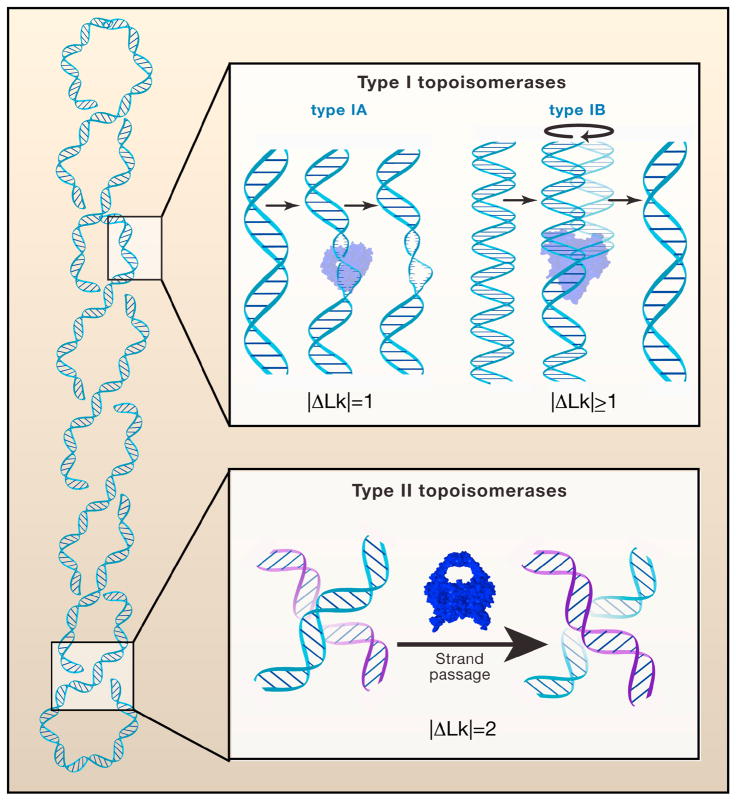
Figure 4. Types of topoisomerases.
(Top) Type I topoisomerases cleave a single-strand of DNA and relax a supercoil by either passing the other strand through an enzyme-DNA linked intermediate (type IA enzymes) or by a strand-swivel mechanism (type IB enzymes). (Bottom) Type II topoisomerases cleave duplex DNA and then relax the supercoil by passing a second duplex DNA through the transient enzyme-DNA linked intermediate.
In the simplest scenario, the rate of supercoil relaxation by diffusion is set by the rotational drag of bare DNA. The “speedometer” model (Levinthal and Crane, 1956) proposes that linear DNA can relax supercoils simply by spinning about its own helical axis. This model predicts that DNA can relax supercoils with a maximal speed of 5·106 rotations per second for a DNA fragment 50 nm in length (Box 2).
Box 2. Speedometer model.
The “speedometer” model (Levinthal and Crane, 1956) assumes that DNA can rotate as a rigid, rod-like structure with a rotational drag ζ equal to 4_πη R2L_, where η is the viscosity of water (10−3 kg.m−1.s−1), R is the DNA radius, and L is its length. The drag torque τ opposing motion then equals τ=ςω . Given a typical driving torque of ~20 pN·nm, torque balance requires that DNA can maximally rotate about its axis at a frequency equal to τ2πς=τ8π2ηR2L=5·106 rotations/s for L = 50 nm, or at 2.5·105 rotations/s for L = 1 μm.
To test the “speedometer” model, Thomen et al. measured the rotational drag of a single DNA molecule using optical tweezers (Thomen et al., 2002). The authors progressively unzipped a DNA molecule with the aim of spinning the zipped double-stranded portion of the DNA while simultaneously measuring the force required (Figure 3D). In this way, they found that the rotational drag of DNA exceeds the prediction of the naïve “speedometer” model by only 10-fold, which would allow for high rates of supercoil dissipation. However, typically the diffusion of supercoils requires the rotation of plectonemic DNA, which is significantly larger in diameter than a single DNA duplex [~50 nm (the order of the persistence length) versus 2 nm, respectively]. Experiments using the magneto-optical tweezers (Crut et al., 2007) measured the rate at which pre-formed plectonemes were “stretched out,” resulting in rotation about their axis and the formation of helical twist (Figure 3E). In these experiments, the contribution of the rotational drag of plectonemes was too small to be observed, suggesting that plectoneme removal is fast, with an upper bound of 100 μs per individual plectoneme. Thus, single-molecule experiments indicate that bare DNA, either as a single duplex or in plectonemic supercoils, does not significantly slow down DNA dynamics.
However, the observation that domains of positive and negative supercoils do not rapidly disappear in cellular processes is incompatible with high diffusion rates (Droge and Nordheim, 1991; Liu and Wang, 1987; Tsao et al., 1989). Several factors have been proposed to hinder DNA rotation under biological conditions, including anchoring of DNA to the nuclear envelope or chromosome matrix, protein binding, or higher-order chromatin structure. All of these factors are depicted schematically as an “attachment site” in Figure 2B.
Indeed, the anchoring of DNA to cellular structures (induced in Saccharomyces cerevisiae by the binding of REP1 and REP2 proteins to DNA containing a REP3 sequence) increases in vivo supercoiling (Gartenberg and Wang, 1993). The sharp bends in DNA caused by DNA-binding proteins, such as Lac repressor (Lewis et al., 1996), IHF (Rice et al., 1996), HU (Swinger et al., 2003) or FIS (Pan et al., 1996), might also increase resistance to rotation. Indeed, the in vitro binding to DNA of sequence-specific proteins, such as the bacteriophage λ O replication initiator or the E. coli lactose or galactose repressors, increases transcription-coupled supercoiling (Leng and McMacken, 2002) as well. The deliberate insertion of persistent bends in DNA was not by itself, however, sufficient to induce a large accumulation of transcriptional supercoiling (Stupina and Wang, 2004). Thus, the rotational drag of DNA with bound proteins merits further quantitative investigation.
Overview of DNA topoisomerase mechanisms
Topoisomerases, enzymes that modulate the degree of DNA supercoiling, are divided broadly into two families: type I enzymes transiently cleave and reseal one strand of duplex DNA in the absence of ATP (Figure 4, top), and type II enzymes cleave and religate both DNA strands in the presence of ATP (Figure 4, bottom). These two families are divided further into subfamilies, which can be distinguished on the basis of protein architecture (monomer versus oligomer), DNA substrate preference (duplex versus single-strand), reaction outcomes (net loss or gain of supercoils; complete or partial supercoil removal), and requirements for metals and ATP. Here we provide a brief overview of the catalytic mechanism for each family of topoisomerase enzymes.
Type I topoisomerases
TopIA enzymes transiently cleave a single strand of supercoiled DNA to form a 5′-phosphotyrosyl intermediate (Schoeffler and Berger, 2008). These enzymes include E. coli TopA, which preferentially relaxes negatively supercoiled DNA, and Topoisomerase III, which efficiently unknots and decatenates single-stranded or nicked DNA. TopIA enzymes have a clamp-like structure with a large central cavity in which DNA binds. Cleavage yields a covalent enzyme-DNA intermediate, in which TopA bridges the nick that it created in the DNA. The intact strand is then passed through the nick which results in a change of Lk by one unit per cleavage-religation cycle (Figure 4, top). Single-molecule experiments with E. coli TopA showed these unitary changes in Lk in real time (Dekker et al., 2002), which substantiated the previously proposed enzyme-bridged strand passage mechanism for TopIA (Lima et al., 1994).
In contrast, TopIB enzymes form a 3′-phosphotyrosyl intermediate and are structurally unrelated to TopIA. Mutational analysis of the Top1B from vaccinia virus, combined with studies of the effects of chemical modifications of the DNA cleavage site (Krogh and Shuman, 2000; Tian et al., 2004; Tian et al., 2005), provided a functional map of the TopIB active site at atomic resolution. Crystal structures of DNA-bound topoisomerases, captured at sequential steps along the reaction pathway (pre-cleavage, transition-state, and post-cleavage covalent complex), illuminated the DNA-protein interactions and reaction chemistry of Top1B (Davies et al., 2006; Perry et al., 2010; Redinbo et al., 1998). These studies revealed that nucleophilic attack of the active-site tyrosine hydroxyl on the DNA phosphodiester bond is catalyzed by two arginines, one lysine, and one histidine that together stabilize the pentacoordinate transition-state and expel the 5′ leading strand. This creates the 3′-phosphotyrosyl intermediate and nicked DNA. The reversibility of the reaction allows TopIB to switch the DNA back and forth between a nicked and a religated state, with a preference for the religated state over the nicked state. When the DNA is cleaved, torsional energy present in the molecule dissipates by rotation of the DNA about its intact strand (Figure 4, top). This mechanism is generally referred to as the “swivel” mechanism.
TopIB enzymes engage the DNA duplex as a C-shaped protein clamp (Figure 4, top) (Redinbo et al., 1998; Sekiguchi and Shuman, 1994). The crystal structure of human TopIB suggested that the tightly closed clamp may hinder DNA swiveling (Stewart et al., 1998). Indeed, when two reversible disulfide bonds were engineered into human TopIB close to the active site with the aim to lock the protein clamp tightly around the duplex DNA, rotation was impeded (Woo et al., 2003). DNA rotation was not noticeably impeded when locking occurred through disulfide bonds located further away (Carey et al., 2003). Thus, DNA strand rotation within the covalent enzyme-DNA complex requires at least some opening of the flexible TopIB protein clamp (Carey et al., 2003; Stewart et al., 1998; Stivers et al., 1997).
Prior to single-molecule investigations, the dynamics of the swivel action was poorly understood, including the rate at which the DNA spins during supercoil relaxation, the number of supercoils removed per cleavage/religation cycle, and in particular, how these parameters change as a function of DNA torque. In addition, the camptothecin-class of anti-tumor drugs was known to reversibly stabilize the human TopIB-DNA covalent complex (Hsiang et al., 1985; Porter and Champoux, 1989), but how these drugs influence the swivel dynamics of Top1B was largely unknown.
Archaeal topoisomerase V is the sole member of the newly established TopIC family (Gadelle et al., 2003). Although structurally distinct, this enzyme functionally resembles TopIB in terms of its mechanism of action; it forms a 3′-phosphotyrosyl intermediate and relaxes positive and negative supercoils.
Type II topoisomerases
Type II topoisomerases transiently cleave both strands of a DNA duplex to allow the unidirectional passage of another DNA duplex through the protein-linked DNA gate (Figure 4, bottom) (Schoeffler and Berger, 2008). Cleavage of the phosphodiester backbone in one segment of duplex DNA (termed the gate or G-segment) by the two active site tyrosines is accomplished by the formation of a covalent 5′-phosphotyrosyl-enzyme adduct on each DNA strand, separated by 4 nucleotides. A second duplex (the transfer or T-segment) is captured by the ATP-bound enzyme and passed along the dimer interface of the enzyme through the double-strand break. The broken DNA strands are then religated. Depending on whether the captured DNA derives from the same duplex cleaved by the enzyme or from a separate DNA molecule, type II enzymes can catalyze changes in DNA supercoiling (in steps of 2 Lk), knotting, or catenation. ATP hydrolysis is not required for DNA cleavage or religation per se. Rather, type II enzymes use ATP binding and hydrolysis to drive conformational changes in the dimeric enzyme that are required to change the linkage of the DNA strands or duplexes.
Type II topoisomerases are divided into two subfamilies: IIA and IIB. All TopIIA enzymes possess a similar two-fold symmetrical structure and reaction mechanism. In eukaryotes, IIA enzymes catalyze the relaxation of positively or negatively supercoiled DNA, as well as the decatenation and unknotting of DNA helices.
Bacterial IIA topoisomerases, including DNA gyrase and topoisomerase IV (TopIV), can also decatenate and unknot DNA, but they also possess unique activities. Gyrase catalyzes a reduction in Lk, such as the removal of positive supercoils, and it can also introduce negative supercoils into DNA. TopIV preferentially relaxes positive supercoils and is a potent decatenase. These specialized enzymatic activities apparently derive from the unique C-terminal DNA binding domains not found in eukaryotic IIA enzymes (reviewed in Schoeffler and Berger, 2008). However, the physical basis for the preferential binding of TopIV for positive versus negative supercoils, as well as the role of DNA wrapping in the introduction of negative supercoils by gyrase, are not well understood.
Topoisomerase VI (TopVI) exemplifies the IIB subfamily, which is found in archaea, plants, and algae. It is distinguished from the IIA enzymes on the basis of its primary structure and domain architecture. In particular, TopVI lacks the third dimerization interface present in IIA enzymes. Nevertheless, TopVI still bears some structural and mechanistic similarities to TopIIA (Corbett et al., 2007; Graille et al., 2008). TopVI is a homodimer, cleaves two strands of DNA by staggered 5′-phosphotyrosyl linkages, and uses ATP to drive a second DNA duplex through the protein-linked DNA gate to change Lk by steps of two. TopVI can also catalyze DNA decatenation and the relaxation of positive and negative supercoils.
Single-molecule studies of topoisomerase mechanisms
Single-molecule experiments are ideally suited to mimic topological substrates that are biologically relevant, such as the positive supercoils generated ahead of the replication fork or the entangled DNA present prior to chromosome segregation. Furthermore, single-molecule techniques can accurately study the dynamics of topoisomerase activity on these DNA substrates. Here we describe how these approaches uncovered new mechanistic information about topoisomerases.
By following the action of TopIA enzymes on supercoiled DNA in real time with magnetic tweezers, it was demonstrated that these enzymes display a uniform step-size of one change in Lk (i.e., ΔLk = 1) per round of catalysis (Dekker et al., 2002; Dekker et al., 2003). Indeed, this is what one would predict given the formation of an enzyme-bridged DNA gate during catalysis by Top1A (Figure 4). Furthermore, the average relaxation rate decreased as a function of the applied force on the DNA, indicating a force-sensitive and rate-limiting step possibly associated with DNA strand-passage.
Magnetic tweezer experiments with type IB topoisomerases revealed quite different behavior. These enzymes do not relax supercoils in homogeneous steps. Rather, the number of supercoils removed from the DNA varied widely between relaxation cycles (Figure 5B) (Koster et al., 2005). The distribution of this number was exponential with a mean that increased with increasing torque in the DNA. In other words, the harder one pulls on the DNA, the more supercoils TopIB removes. Apparently, TopIB does not perform work against the externally applied force as type II toposiomerases or gyrases do, but instead Top1B is aided by it. These observations point to a swivel mechanism in which the free 5′ end of the DNA spins about the intact DNA strand to remove supercoils. DNA religation subsequently arrests supercoil removal. The spinning of the 5′ end of DNA can be described as a random walk over an energy landscape that was tilted due to the torque stored in the DNA. The rate of supercoil removal was slowed by the tight clamping of the topoisomerase about its DNA substrate (Figure 6, right panel). Similar results were obtained for type IC topoisomerases (Taneja et al., 2007), indicating that these topoisomerases use similar mechanisms of hindered DNA rotation.
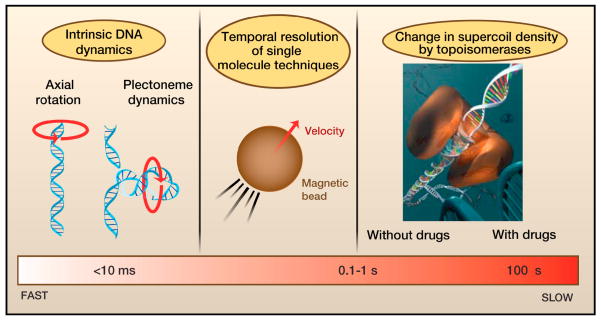
Figure 6. Timescales for removing supercoils.
From fast (left) to slow (right): The rotation of DNA around its own axis (as described by the “speedometer” model) and the rotation of plectonemes about their central axis are estimated to occur on timescales faster than 100 μs per one unit change in Lk (i.e., |ΔLk|=1). The temporal resolution for the measurement of supercoil removal using single-molecule techniques is ~10 ms and is limited by the dynamics of the force sensors (e.g. micron-sized beads). Much slower processes are the addition or removal of a few supercoils by enzymes, such as gyrase and Type IB topoisomerases (typically ~10 ms per supercoil). For the latter, inhibitor drugs have been shown to dramatically slow down supercoil removal to approximately 0.2 s per supercoil for the removal of positive supercoils.
Human TopIB is the target of the camptothecin-class of anti-tumor drugs. These drugs impede religation by intercalating into the nick in the DNA generated by Top1B (Hsiang et al., 1985; Porter and Champoux, 1989; Staker et al., 2002). Single-molecule analyses of the dynamics of the swivel in the presence of topotecan showed that the instantaneous velocity with which TopIB removes positive supercoils is decreased approximately 20-fold (Figure 5D, Figure 6) in comparison to removal in the absence of drug (Figure 5C, Figure 6) (Koster et al., 2007). Interestingly, positive supercoils were removed even more slowly than negative supercoils.
To investigate potential biological ramifications of the imbalance of supercoil removal rates in the presence of camptothecin-based drugs, the supercoiling of plasmid DNA in yeast cells was monitored in the presence or absence of camptothecin during various phases of the cell cycle (Koster et al., 2007). The presence of camptothecin skewed the topoisomer distribution towards higher Lk (i.e., positive supercoiling) independent of cell cycle distribution, signifying that drug-induced accumulation of positive supercoils may also result from DNA transactions other than replication (e.g., transcription). Positive supercoils did not accumulate in drug-treated cells expressing a mutant Top1B enzyme that is resistant to camptothecin, suggesting that positive supercoils might contribute to the drug’s cytotoxic or antitumor activity.
Magnetic tweezers experiments and rotor bead assays have also been performed on several type IIA enzymes. For Drosophila IIA topoisomerase, Strick et al. (2000) found that the end-to-end extension of a supercoiled DNA increased in discrete steps of 90 nm (Figure 5A), a distance that is associated with the removal of two supercoils from the DNA during a single catalytic cycle (Strick et al., 2000). The average rate of supercoil removal depended on the force acting on the DNA. Again, this implies the existence of a force-sensitive and rate-limiting step in the passage of a DNA strand through the gate of the enzyme. Using the rotor-bead assay, Gore et al. (2006) found that DNA gyrase adds negative supercoils in steps of two (Figure 5E), confirming the gyrase mechanism imputed from ensemble studies. Moreover, they found that gyrase, like other type II topoisomerases), performs work against the externally applied force. Based on the decrease of the enzyme’s processivity with increasing force (Figure 5F), they devised a reaction scheme that includes a competition between a force-sensitive DNA wrapping action and enzyme dissociation from the DNA.
Single-molecule techniques have also helped to resolve a paradox involving the handedness preference of bacterial TopIV (Kato et al., 1990). Unlike eukaryotic TopIIA, which relaxes positive and negative supercoils equally well, TopIV removes positive supercoils quickly and processively, but it removes negative supercoils only slowly and distributively (Charvin et al., 2005; Crisona et al., 2000). Inside a cell, TopIV removes the positive supercoils that are generated ahead of an advancing replication fork or transcription complex (Khodursky et al., 2000), and it also decatenates newly replicated daughter chromosomes from each other (Peng and Marians, 1993; Zechiedrich and Cozzarelli, 1993). However, positive supercoils and intertwined daughter chromosomes have the opposite handedness. Positive supercoils form a left-handed superhelix whereas linked chromosomes are right-handed. How can TopIV remove the right-handed superhelices that make up the intertwined chromosomes given that it does not readily remove the right-handed superhelices of negative supercoils?
To resolve this paradox, a series of single-molecule measurements were performed with either positive or negative supercoiled DNA and either positive or negative braids of two DNA molecules. The key feature of the single-molecule techniques used (i.e., magnetic tweezers and a combination of an optical trap and a micropipette) was their ability to introduce positive or negative supercoils with arbitrary degree of supercoiling. As TopIV binds both types of supercoils equally well (Stone et al., 2003), it was inferred that TopIV discriminates between positive and negative supercoils in a step that occurs after binding.
Specifically, it was hypothesized that a preferred crossing angle exists between the two duplex DNA segments at which TopIV acts most effectively. Monte-Carlo simulations predicted that positive supercoils on average cross at 60 degrees whereas negative supercoils cross at 120 degrees (Neuman et al., 2009; Stone et al., 2003). This large difference in crossing angles could be the structural feature that TopIV uses to “read” the local sign of supercoiling. If this were the case, the preferred crossing angle for TopIV should be significantly smaller than 90 degrees, the angle at which TopIV would be “blind” to the 180 degrees difference between positive and negative supercoils. The crossing angle hypothesis also addresses the issue of how TopIV could remove right-handed catenates. Monte-Carlo simulations of right-handed catenates showed that their angle distribution is broad and allows right-handed catenates to switch over to left-handed catenates quite readily, allowing their subsequent resolution by TopIV (Stone et al., 2003; Vologodskii and Cozzarelli, 1996).
To test the crossing angle hypothesis, Neuman et al. (2009) used magnetic tweezers that could impose different crossing angles on a DNA braid. Surprisingly, the preferred crossing angle measured for TopIV equalled 86 degrees, too close to 90 degrees for the topoisomerase to use the crossing angle as a basis for distinguishing between positive and negative supercoils. Subsequent measurements revealed that all positive supercoils are removed rapidly and processively (Neuman et al., 2009). This is different than the removal of negative supercoils, for which pauses recur after the removal of each single crossing. In other words, the relaxation of negative supercoils is perfectly distributive and likely involves dissociation and rebinding of TopIV after the relaxation of each crossing. Neuman et al. propose that the enzyme’s C-terminal domains (CTDs) stabilize positive supercoils over negative supercoils, leading to dissociation of TopIV from negative supercoils and not from positive supercoils. This hypothesis is consistent with earlier work showing that CTDs are required for TopIV’s chiral discrimination (Corbett et al., 2005). Thus, it is the difference in processivity in the removal of positive and negative supercoils, not the crossing angle, that allows TopIV to distinguish between the two types of supercoils.
Interestingly, several additional puzzles involving asymmetries in the behavior of topoisomerases now present themselves. It will be interesting to test whether the asymmetry in the relaxation of positive and negative supercoils remains even after deleting the CTDs. A further puzzle is how does type II topoisomerase, when faced with entangled DNA molecules, decatenate rather than catenate DNA further. Here, CTDs may also play a central role. We expect single-molecule experiments to play a critical part in dissecting these intriguing phenomena.
Outlook
By integrating results from several single-molecule studies, we have provided an overview of the timescales in which supercoiling can be removed (Figure 6). Enzyme-free relaxation of supercoiling occurs at the current time resolution with which magnetic tweezers can detect supercoil removal, a few tens of microsecond per unit of supercoiling. By contrast, removal of supercoils by topoisomerases is typically much slower. For example, TopIB, removes individual supercoils on a timescale of 0.01 to 0.2 s, where the longer timescales are induced by chemotherapeutic drugs such as camptothecins.
Several future improvements could enable more accurate analyses of enzyme mechanisms and DNA dynamics during transcription and replication. First, the use of stiffer systems in single-molecule experiments will shift the noise amplitude to higher frequencies, allowing one to obtain more detailed snapshots of enzyme activity. Second, alternative fluorescence-based single-molecule experiments, such as fluorescence correlation spectroscopy, allow one to probe faster supercoil dynamics (Shusterman et al., 2008). Future measurements with increased time resolution will be able to shed light on the fastest of twist propagation processes. In addition, it has recently become possible to measure torque in DNA (Bishop et al., 2003; Oroszi et al., 2006) Capitanio et al., 2004); Forth et al., 2008). This is an exciting development because it enables the study of the direct effects of torque on enzyme activity and on the progression of transcription and replication complexes.
Lastly, combining fluorescence measurements, such as fluorescence resonance energy transfer (FRET), with magnetic and optical tweezers should be a powerful combination. One could then observe nm-scale conformational changes in enzymes while simultaneously observing their effect on DNA topology as a whole. For instance, one can imagine using FRET to observe conformational changes in TopII or gyrase, such as the opening of the gates, simultaneously with the direct observation of supercoil removal. In this context, a challenge is to marry the relatively long timescales of force spectroscopy experiments with the relatively short lifetimes of fluorophores. This might be achieved by exciting the fluorophores periodically or by observing a large number of DNA molecules simultaneously while illuminating them.
Ultimately, measurements with high spatial and temporal resolution operating inside living cells could directly dissect the dynamic interplay between supercoiling and critical cellular processes, such as transcription and replication (Xie et al., 2008). Such advances, will undoubtedly inform genetic and cell-based analyses of the biological consequences of alterations in DNA topology.
Supplementary Material
1
Acknowledgments
We thank Vincent Croquette and Elise Praly for providing us with an unpublished trace of TopII, Elisa Bot and Komaraiah Palle for contributions to earlier work, and Jan Lipfert, Keir Neuman, and John Marko for useful discussions. NHD acknowledges financial support from TU Delft, the Dutch Foundation for Research on Matter (FOM), and the European Science Foundation. MAB acknowledges support from NIH (CA058755 and CA111542). SS is supported by NIH grant GM46330.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbondanzieri EA, Greenleaf WJ, Shaevitz JW, Landick R, Block SM. Direct observation of base-pair stepping by RNA polymerase. Nature. 2005;438:460–465. doi: 10.1038/nature04268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AI, Nieminen TA, Heckenberg NR, Rubinsztein-Dunlop H. Optical application and measurement of torque on microparticles of isotropic nonabsorbing material. Phys Rev A. 2003;68:033802. [Google Scholar]
- Bohbot-Raviv Y, Zhao WZ, Feingold M, Wiggins CH, Granek R. Relaxation dynamics of semiflexible polymers. Phys Rev Lett. 2004;92:098101. doi: 10.1103/PhysRevLett.92.098101. [DOI] [PubMed] [Google Scholar]
- Brown PO, Cozzarelli NR. Sign Inversion Mechanism for Enzymatic Supercoiling of DNA. Science. 1979;206:1081–1083. doi: 10.1126/science.227059. [DOI] [PubMed] [Google Scholar]
- Brown PO, Cozzarelli NR. Catenation and Knotting of Duplex DNA by Type-1 Topoisomerases - a Mechanistic Parallel with Type-2 Topoisomerases. P Natl Acad Sci-Biol. 1981;78:843–847. doi: 10.1073/pnas.78.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant Z, Stone MD, Gore J, Smith SB, Cozzarelli NR, Bustamante C. Structural transitions and elasticity from torque measurements on DNA. Nature. 2003;424:338–341. doi: 10.1038/nature01810. [DOI] [PubMed] [Google Scholar]
- Capitanio M, Normanno D, Pavone FS. High-precision measurements of light-induced torque on absorbing microspheres. Opt Lett. 2004;29:2231–2233. doi: 10.1364/ol.29.002231. [DOI] [PubMed] [Google Scholar]
- Carey JF, Schultz SJ, Sisson L, Fazzio TG, Champoux JJ. DNA relaxation by human topoisomerase I occurs in the closed clamp conformation of the protein. P Natl Acad Sci USA. 2003;100:5640–5645. doi: 10.1073/pnas.1031537100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux JJ. DNA topoisomerases: Structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- Charvin G, Strick TR, Bensimon D, Croquette V. Tracking topoisomerase activity at the single-molecule level. Annu Rev Bioph Biom. 2005;34:201–219. doi: 10.1146/annurev.biophys.34.040204.144433. [DOI] [PubMed] [Google Scholar]
- Coelho Neto J, Dickman R, Mesquita ON. Recoiling DNA molecule: simulation and experiment. Physica A. 2005;345:173–184. [Google Scholar]
- Corbett KD, Benedetti P, Berger JM. Holoenzyme assembly and ATP-mediated conformational dynamics of topoisomerase VI. Nat Struct Mol Biol. 2007;14:611–619. doi: 10.1038/nsmb1264. [DOI] [PubMed] [Google Scholar]
- Corbett KD, Schoeffler AJ, Thomsen ND, Berger JM. The structural basis for substrate specificity in DNA topoisomerase IV. J Mol Biol. 2005;351:545–561. doi: 10.1016/j.jmb.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Crisona NJ, Strick TR, Bensimon D, Croquette V, Cozzarelli NR. Preferential relaxation of positively supercoiled DNA by E-coli topoisomerase IV in single-molecule and ensemble measurements. Gene Dev. 2000;14:2881–2892. doi: 10.1101/gad.838900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crut A, Koster DA, Seidel R, Wiggins CH, Dekker NH. Fast dynamics of supercoiled DNA revealed by single-molecule experiments. P Natl Acad Sci USA. 2007;104:11957–11962. doi: 10.1073/pnas.0700333104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DR, Mushtaq A, Interthal H, Champoux JJ, Hol WGJ. The structure of the transition state of the heterodimeric topoisomerase I of Leishmania donovani as a vanadate complex with nicked DNA. J Mol Biol. 2006;357:1202–1210. doi: 10.1016/j.jmb.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Dekker NH, Rybenkov VV, Duguet M, Crisona NJ, Cozzarelli NR, Bensimon D, Croquette V. The mechanism of type IA topoisomerases. P Natl Acad Sci USA. 2002;99:12126–12131. doi: 10.1073/pnas.132378799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker NH, Viard T, de La Tour CB, Duguet M, Bensimon D, Croquette V. Thermophilic topoisomerase I on a single DNA molecule. J Mol Biol. 2003;329:271–282. doi: 10.1016/s0022-2836(03)00320-6. [DOI] [PubMed] [Google Scholar]
- Droge P, Nordheim A. Transcription-Induced Conformational Change in a Topologically Closed DNA Domain. Nucleic Acids Res. 1991;19:2941–2946. doi: 10.1093/nar/19.11.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet M. Growth inhibition mediated by excess negative supercoiling: the interplay between transcription elongation, R-loop formation and DNA topology. Mol Microbiol. 2006;59:723–730. doi: 10.1111/j.1365-2958.2005.05006.x. [DOI] [PubMed] [Google Scholar]
- Feingold M. Single-molecule studies of DNA and DNA-protein interactions. Physica E. 2001;9:616–620. [Google Scholar]
- Fisher LM. DNA Supercoiling and Gene-Expression. Nature. 1984;307:686–687. doi: 10.1038/307686a0. [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetskii MD. DNA supercoiling and unusual structures. In: Cozzarelli NR, Wang JC, editors. DNA topology and its biological effects. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 1990. pp. 185–215. [Google Scholar]
- Gadelle D, Filee J, Buhler C, Forterre P. Phylogenomics of type II DNA topoisomerases. Bioessays. 2003;25:232–242. doi: 10.1002/bies.10245. [DOI] [PubMed] [Google Scholar]
- Gartenberg MR, Wang JC. Positive Supercoiling of DNA Greatly Diminishes Messenger-Rna Synthesis in Yeast. P Natl Acad Sci USA. 1992;89:11461–11465. doi: 10.1073/pnas.89.23.11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartenberg MR, Wang JC. Identification of Barriers to Rotation of DNA Segments in Yeast from the Topology of DNA Rings Excised by an Inducible Site-Specific Recombinase. P Natl Acad Sci USA. 1993;90:10514–10518. doi: 10.1073/pnas.90.22.10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M, Mizuuchi K, Odea MH, Nash HA. DNA Gyrase - Enzyme That Introduces Superhelical Turns into DNA. P Natl Acad Sci USA. 1976;73:3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever GN, Wang JC. Supercoiling of Intracellular DNA Can Occur in Eukaryotic Cells. Cell. 1988;55:849–856. doi: 10.1016/0092-8674(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Gore J, Bryant Z, Stone MD, Nollmann MN, Cozzarelli NR, Bustamante C. Mechanochemical analysis of DNA gyrase using rotor bead tracking. Nature. 2006;439:100–104. doi: 10.1038/nature04319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen E, Zhao WZ, Carmon G, Rosen S, Granek R, Feingold M. Relaxation dynamics of a single DNA molecule. Phys Rev E. 2005;71:061920. doi: 10.1103/PhysRevE.71.061920. [DOI] [PubMed] [Google Scholar]
- Graille M, Cladiere L, Durand D, Lecointe F, Gadelle D, Quevillon-Cheruel S, Vachette P, Forterre P, van Tilbeurgh H. Crystal structure of an intact type II DNA topoisomerase: Insights into DNA transfer mechanisms. Structure. 2008;16:360–370. doi: 10.1016/j.str.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin Induces Protein-Linked DNA Breaks Via Mammalian DNA Topoisomerase-I. J Biol Chem. 1985;260:4873–4878. [PubMed] [Google Scholar]
- Kato J, Nishimura Y, Imamura R, Niki H, Hiraga S, Suzuki H. New Topoisomerase Essential for Chromosome Segregation in Escherichia-Coli. Cell. 1990;63:393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- Khodursky AB, Peter BJ, Schmidt MB, DeRisi J, Botstein D, Brown PO, Cozzarelli NR. Analysis of topoisomerase function in bacterial replication fork movement: Use of DNA microarrays. P Natl Acad Sci USA. 2000;97:9419–9424. doi: 10.1073/pnas.97.17.9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard K, Wang JC. Bacterial-DNA Topoisomerase-I Can Relax Positively Supercoiled DNA Containing a Single-Stranded Loop. J Mol Biol. 1985;185:625–637. doi: 10.1016/0022-2836(85)90075-0. [DOI] [PubMed] [Google Scholar]
- Koster DA, Croquette V, Dekker C, Shuman S, Dekker NH. Friction and torque govern the relaxation of DNA supercoils by eukaryotic topoisomerase IB. Nature. 2005;434:671–674. doi: 10.1038/nature03395. [DOI] [PubMed] [Google Scholar]
- Koster DA, Palle K, Bot ESM, Bjornsti MA, Dekker NH. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature. 2007;448:213–217. doi: 10.1038/nature05938. [DOI] [PubMed] [Google Scholar]
- Krogh BO, Shuman S. Catalytic mechanism of DNA topoisomerase IB. Mol Cell. 2000;5:1035–1041. doi: 10.1016/s1097-2765(00)80268-3. [DOI] [PubMed] [Google Scholar]
- Leng FF, McMacken R. Potent stimulation of transcription-coupled DNA supercoiling by sequence-specific DNA-binding proteins. P Natl Acad Sci USA. 2002;99:9139–9144. doi: 10.1073/pnas.142002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinthal C, Crane HR. On the Unwinding of DNA. Proc Natl Acad Sci U S A. 1956;42:436–438. doi: 10.1073/pnas.42.7.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Chang G, Horton NC, Kercher MA, Pace HC, Schumacher MA, Brennan RG, Lu PZ. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996;271:1247–1254. doi: 10.1126/science.271.5253.1247. [DOI] [PubMed] [Google Scholar]
- Lima CD, Wang JC, Mondragon A. 3-Dimensional Structure of the 67k N-Terminal Fragment of Escherichia-Coli DNA Topoisomerase-I. Nature. 1994;367:138–146. doi: 10.1038/367138a0. [DOI] [PubMed] [Google Scholar]
- Liu LF, Wang JC. Supercoiling of the DNA-Template During Transcription. P Natl Acad Sci USA. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman KC, Block SM. Optical trapping. Rev Sci Instrum. 2004;75:2787–2809. doi: 10.1063/1.1785844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman KC, Charvin G, Bensimon D, Croquette V. Mechanisms of chiral discrimination by topoisomerase IV. P Natl Acad Sci USA. 2009;106:6986–6991. doi: 10.1073/pnas.0900574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszi L, Galajda P, Kirei H, Bottka S, Ormos P. Direct measurement of torque in an optical trap and its application to double-strand DNA. Phys Rev Lett. 2006;97:058301. doi: 10.1103/PhysRevLett.97.058301. [DOI] [PubMed] [Google Scholar]
- Pan CQ, Finkel SE, Cramton SE, Feng JA, Sigman DS, Johnson RC. Variable structures of Fis-DNA complexes determined by flanking DNA-protein contacts. J Mol Biol. 1996;264:675–695. doi: 10.1006/jmbi.1996.0669. [DOI] [PubMed] [Google Scholar]
- Peng H, Marians KJ. Decatenation Activity of Topoisomerase-Iv During Oric and Pbr322 DNA-Replication in-Vitro. P Natl Acad Sci USA. 1993;90:8571–8575. doi: 10.1073/pnas.90.18.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry K, Hwang Y, Bushman FD, Van Duyne GD. Insights from the Structure of a Smallpox Virus Topoisomerase-DNA Transition State Mimic. Structure. 2010;18:127–137. doi: 10.1016/j.str.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Arsuaga J, Breier AM, Khodursky AB, Brown PO, Cozzarelli NR. Genomic transcriptional response to loss of chromosomal supercoiling in Escherichia coli. Genome Biol. 2004;5:R87. doi: 10.1186/gb-2004-5-11-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SE, Champoux JJ. The Basis for Camptothecin Enhancement of DNA Breakage by Eukaryotic Topoisomerase-I. Nucleic Acids Res. 1989;17:8521–8532. doi: 10.1093/nar/17.21.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WGJ. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998;279:1504–1513. doi: 10.1126/science.279.5356.1504. [DOI] [PubMed] [Google Scholar]
- Revyakin A, Ebright RH, Strick TR. Promoter unwinding and promoter clearance by RNA polymerase: Detection by single-molecule DNA nanomanipulation. P Natl Acad Sci USA. 2004;101:4776–4780. doi: 10.1073/pnas.0307241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice PA, Yang SW, Mizuuchi K, NAsh HA. Crystal structure of an IHF-DNA complex: A protein-induced DNA u-turn. Cell. 1996;87:1295–1306. doi: 10.1016/s0092-8674(00)81824-3. [DOI] [PubMed] [Google Scholar]
- Romano G, Sacconi L, Capitanio M, Pavone FS. Force and torque measurements using magnetic micro beads for single molecule biophysics. Opt Commun. 2003;215:323–331. [Google Scholar]
- Sacconi L, Romano G, Ballerini R, Capitanio M, De Pas M, Giuntini M, Dunlap D, Finzi L, Pavone FS. Three-dimensional magneto-optic trap for micro-object manipulation. Opt Lett. 2001;26:1359–1361. doi: 10.1364/ol.26.001359. [DOI] [PubMed] [Google Scholar]
- Saleh OA, Bigot S, Barre FX, Allemand JF. Analysis of DNA supercoil induction by FtsK indicates translocation without groove-tracking. Nat Struct Mol Biol. 2005;12:436–440. doi: 10.1038/nsmb926. [DOI] [PubMed] [Google Scholar]
- Schoeffler AJ, Berger JM. DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q Rev Biophys. 2008;41:41–101. doi: 10.1017/S003358350800468X. [DOI] [PubMed] [Google Scholar]
- Seidel R, van Noort J, van der Scheer C, Bloom JGP, Dekker NH, Dutta CF, Blundell A, Robinson T, Firman K, Dekker C. Real-time observation of DNA translocation by the type I restriction modification enzyme EcoR124I. Nat Struct Mol Biol. 2004;11:838–843. doi: 10.1038/nsmb816. [DOI] [PubMed] [Google Scholar]
- Sekiguchi J, Shuman S. Vaccinia Topoisomerase Binds Circumferentially to DNA. J Biol Chem. 1994;269:31731–31734. [PubMed] [Google Scholar]
- Shusterman R, Gavrinyov T, Krichevsky O. Internal dynamics of superhelical DNA. Phys Rev Lett. 2008;100:098102. doi: 10.1103/PhysRevLett.100.098102. [DOI] [PubMed] [Google Scholar]
- Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. P Natl Acad Sci USA. 2002;99:15387–15392. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L, Redinbo MR, Qiu XY, Hol WGJ, Champoux JJ. A model for the mechanism of human topoisomerase I. Science. 1998;279:1534–1541. doi: 10.1126/science.279.5356.1534. [DOI] [PubMed] [Google Scholar]
- Stivers JT, Harris TK, Mildvan AS. Vaccinia DNA topoisomerase I: Evidence supporting a free rotation mechanism for DNA supercoil relaxation. Biochemistry-Us. 1997;36:5212–5222. doi: 10.1021/bi962880t. [DOI] [PubMed] [Google Scholar]
- Stone MD, Bryant Z, Crisona NJ, Smith SB, Vologodskii A, Bustamante C, Cozzarelli NR. Chirality sensing by Escherichia coli topoisomerase IV and the mechanism of type II topoisomerases. P Natl Acad Sci USA. 2003;100:8654–8659. doi: 10.1073/pnas.1133178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick TR, Allemand JF, Bensimon D, Bensimon A, Croquette V. The elasticity of a single supercoiled DNA molecule. Science. 1996;271:1835–1837. doi: 10.1126/science.271.5257.1835. [DOI] [PubMed] [Google Scholar]
- Strick TR, Croquette V, Bensimon D. Single-molecule analysis of DNA uncoiling by a type II topoisomerase. Nature. 2000;404:901–904. doi: 10.1038/35009144. [DOI] [PubMed] [Google Scholar]
- Stupina VA, Wang JC. DNA axial rotation and the merge of oppositely supercoiled DNA domains in Escherichia coli: Effects of DNA bends. P Natl Acad Sci USA. 2004;101:8608–8613. doi: 10.1073/pnas.0402849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinger KK, Lemberg KM, Zhang Y, Rice PA. Flexible DNA bending in HU-DNA cocrystal structures. Embo J. 2003;22:3749–3760. doi: 10.1093/emboj/cdg351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja B, Schnurr B, Slesarev A, Marko JF, Mondragon A. Topoisomerase V relaxes supercoiled DNA by a constrained swiveling mechanism. P Natl Acad Sci USA. 2007;104:14670–14675. doi: 10.1073/pnas.0701989104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomen P, Bockelmann U, Heslot F. Rotational drag on DNA: A single molecule experiment. Phys Rev Lett. 2002;88:248102. doi: 10.1103/PhysRevLett.88.248102. [DOI] [PubMed] [Google Scholar]
- Tian LG, Claeboe CD, Hecht SM, Shuman S. Remote phosphate contacts trigger assembly of the active site of DNA topoisomerase IB. Structure. 2004;12:31–40. doi: 10.1016/j.str.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Tian LG, Claeboe CD, Hecht SM, Shuman S. Mechanistic plasticity of DNA topolsomerase IB: Phosphate electrostatics dictate the need for a catalytic arginine. Structure. 2005;13:513–520. doi: 10.1016/j.str.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Travers A, Muskhelishvili G. DNA supercoiling - A global transcriptional regulator for enterobacterial growth? Nat Rev Microbiol. 2005;3:157–169. doi: 10.1038/nrmicro1088. [DOI] [PubMed] [Google Scholar]
- Tsao YP, Wu HY, Liu LF. Transcription-Driven Supercoiling of DNA - Direct Biochemical-Evidence from Invitro Studies. Cell. 1989;56:111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- Vologodskii A, Cozzarelli NR. Effect of supercoiling on the juxtaposition and relative orientation of DNA sites. Biophys J. 1996;70:2548–2556. doi: 10.1016/S0006-3495(96)79826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC. Cellular roles of DNA topoisomerases: A molecular perspective. Nat Rev Mol Cell Bio. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- Woo MH, Losasso C, Guo H, Pattarello L, Benedetti P, Bjornsti MA. Locking the DNA topoisomerase I protein clamp inhibits DNA rotation and induces cell lethality. P Natl Acad Sci USA. 2003;100:13767–13772. doi: 10.1073/pnas.2235886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A, Strogatz S, Riley D. Structure of Chromatin and the Linking Number of DNA. P Natl Acad Sci-Biol. 1981;78:1461–1465. doi: 10.1073/pnas.78.3.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Shyy S, Wang JC, Liu LF. Transcription Generates Positively and Negatively Supercoiled Domains in the Template. Cell. 1988;53:433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Xie XS, Choi PJ, Li GW, Lee NK, Lia G. Single-molecule approach to molecular biology in living bacterial cells. Ann Rev Biophys. 2008;37:417–444. doi: 10.1146/annurev.biophys.37.092607.174640. [DOI] [PubMed] [Google Scholar]
- Zechiedrich EL, Cozzarelli NR. Comparative DNA Topology and Cytology of Gyrase and Topoisomerase-Iv Mutants in Escherichia-Coli. J Cell Biochem. 1993:309–309. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1