A Comparative View of Face Perception (original) (raw)
. Author manuscript; available in PMC: 2010 Dec 7.
Published in final edited form as: J Comp Psychol. 2010 Aug;124(3):233–251. doi: 10.1037/a0019460
Abstract
Face perception serves as the basis for much of human social exchange. Diverse information can be extracted about an individual from a single glance at their face, including their identity, emotional state, and direction of attention. Neuropsychological and fMRI experiments reveal a complex network of specialized areas in the human brain supporting these face-reading skills. Here we consider the evolutionary roots of human face perception by exploring the manner in which different animal species view and respond to faces. We focus on behavioral experiments collected from both primates and non-primates, assessing the types of information that animals are able to extract from the faces of their conspecifics, human experimenters, and natural predators. These experiments reveal that faces are an important category of visual stimuli for animals in all major vertebrate taxa, possibly reflecting the early emergence of neural specialization for faces in vertebrate evolution. At the same time, some aspects of facial perception are only evident in primates and a few other social mammals, and may therefore have evolved to suit the needs of complex social communication. Since the human brain likely utilizes both primitive and recently evolved neural specializations for the processing of faces, comparative studies may hold the key to understanding how these parallel circuits emerged during human evolution.
For humans, faces are among the most important visual stimuli, a fact that becomes apparent in social settings – as a species we are constantly, almost obsessively, monitoring each other's faces, paying close attention to subtle details that can give some insight into the emotional state, level of engagement, or object of attention of our associates. Fluency with faces offers great social advantages, allowing one to glean aspects of another's internal thought processes and to predict their behavior. But how did humans come to place so much emphasis upon this particular aspect of personal appearance? Is the capacity to read faces a product of our society, finely tuned to meet the needs of human culture? Already in the nineteenth century, Darwin suggested that this is not the case, and that human facial expressions share much in common with those of many animals (Darwin, 1872). For Darwin the study of faces fell naturally into a comparative and evolutionary context, a perspective adopted by only a small group of contemporary researchers (e.g. Parr, Waller, & Fugate, 2005; Pascalis & Kelly, 2009).
Here we take a first step in exploring the evolution of face perception by reviewing a wide range of behavioral studies that provide insight into the following questions: (1) To what extent do faces constitute a “special” category of visual stimuli for nonhuman primates as well as other mammalian and vertebrate species? That is, to what extent are they important stimuli, eliciting specific behavioral and neural responses? (2) What types of information are various animals able to extract from a face? (3) How and when might have different aspects of face perception emerged during evolution? Our review covers behavioral experiments conducted in animals from many taxa related to visual individual recognition, predator detection, gaze following, and reaction to emotional expressions. While avoiding detailed descriptions of brain anatomy and physiology, we do refer to relevant neurophysiological data where available. In the first section, we set the stage by breaking down face perception, based on data from human studies, into several dissociable components that provide a conceptual framework to guide our review of animal face perception and social vision more generally.
Components of face perception
What kinds of information can be extracted from a face? Numerous studies have demonstrated that face perception is multi-faceted: not only do we recognize individuals, but also monitor their faces to obtain a continuous stream of social information, ranging from communicative gestures to emotional and attentive states (for reviews see Bruce & Young, 1998; Kanwisher & Yovel, 2006; Peterson & Rhodes, 2003; Tsao & Livingstone, 2008). In our comparative review, we separately analyze different aspects of face perception, described briefly here for humans:
1) Identity
The recognition of individual faces is in some ways the pinnacle of human visual performance. Since all faces have the same basic configural appearance (e.g. two eyes above a nose and mouth, some times called the first-order configuration), individuals must be identified by subtle deviations from this prototypic pattern, sometimes referred to as second-order relational information or configuration (Diamond & Carey, 1986). Human face recognition is highly efficient, involving a parallel integration of information over the entire face (for reviews, see Farah, Wilson, Drain, & Tanaka, 1998; McKone, Kanwisher, & Duchaine, 2007). This form of recognition appears to rely on specialized areas in the brain that are selectively engaged by faces that are upright and of normal contrast polarity (Kanwisher, 2000; Rossion & Gauthier, 2002; Thompson, 1980; Tsao & Livingstone, 2008; Yin, 1969).
2) Emotional Expression
Humans communicate their emotional states to others through the stereotypic posturing of facial elements. Elaborated facial musculature contributes to a large repertoire of expressions involving the display of the teeth, the furrowing of the brow, and the closure of the eyes, some of which are uniquely human and some not (Darwin, 1872). The appearance of an emotional expression can directly influence the observer's own emotional state. The appearance of the eyes serves as a particularly salient emotional cue (Adolphs et al., 2005; Whalen et al., 2004). Some basic facial expressions seem to be consistent across human populations (Ekman, Sorenson, & Friesen, 1969), possibly owing to their adaptive value in evolution (Schmidt & Cohn, 2001).
3) Gaze
In addition to its role in signaling emotional state, the appearance of the eyes also provides insight into an individual's attentive state, including their level of engagement, intentions, or focus of interest. This aspect of face perception may be particularly well developed in humans, since the sclera of the human eye is more visible than in other primates (Kobayashi & Kohshima, 1997). Indeed, human infants primarily use the orientation of the eyes, rather than the head, to determine another's direction of gaze (Tomasello, Hare, Lehmann, & Call, 2007). Gaze interplay is a salient feature of social interaction and is abnormal in a number of psychiatric conditions, in which patients typically avoid looking into the eyes of others (Coss, 1978b; Johnson et al., 2005).
4) Attraction
Faces play an important role in human sexual attraction, which has been linked to face averageness, symmetry, and sexual dimorphism (masculinity in male faces, femininity in female faces) (Rhodes & Simmons, 2007). These attributes may in turn be related to mate quality, so that our preferences for them could have been sexually selected (Rhodes, 2006). Similarly, subtle coloration of the face affects perceived health, which is important in attractiveness (Stephen, Coetzee, Law, & Perrett, 2009).
5) Development
The developmental trajectory of face perception is complex and only partly understood. Newborn infants detect and visually orient to face-like patterns in preference to other complex patterns (Goren, Sarty, & Wu, 1975; Johnson, Dziurawiec, Ellis, & Morton, 1991; for a review see Johnson, 2005), and form a preference for their mother's face within days of birth (Bushnell, Sai, & Mullin, 1989; Pascalis, de Schonen, Morton, Deruelle, & Fabre-Grenet, 1995). They are also able to discriminate and imitate a few basic facial expressions in the hours after birth (Meltzoff & Moore, 1983). During the first year of life, face processing mechanisms are “tuned” by exposure to faces (Kelly et al., 2007; Pascalis, de Haan, & Nelson, 2002; Pascalis et al., 2005), and recognition performance continues to improve through childhood and adolescence (Carey, 1992; Carey, Diamond, & Woods, 1980; Mondloch, Geldart, Maurer, & Le Grand, 2003; Mondloch, Le Grand, & Maurer, 2002). The basis of this improvement remains controversial, with some arguing that sensitivity to second-order configuration improves throughout childhood (Mondloch et al., 2003) and others attributing it to the development of domain-general skills in attention and executive functions (Crookes & McKone, 2009).
6) Neural Specialization
Different circuits in the brain support the perception of different kinds of information, a principle that is evident in both brain damaged patients and functional imaging studies (Adolphs, Tranel, & Damasio, 1998; Calder & Young, 2005; Hoffman & Haxby, 2000; Humphreys, Donnelly, & Riddoch, 1993). The most prominent face-specialized area in the human brain is on the fusiform gyrus, where responses are stronger to faces than to any other category of stimuli (Haxby, Hoffman, & Gobbini, 2000; Kanwisher, McDermott, & Chun, 1997; Kanwisher & Yovel, 2006). This region may receive basic perceptual representations of faces from a more posterior occipital face-selective area (Fairhall & Ishai, 2007), and damage in the vicinity of these areas can lead to the inability to recognize individual faces (Bouvier & Engel, 2006; Damasio, Damasio, & Van Hoesen, 1982). Some neurons in the medial temporal lobe are thought to represent people at a very general, abstract level, and respond robustly and selectively to faces (Quiroga, Reddy, Kreiman, Koch, & Fried, 2005). By contrast, the superior temporal sulcus (STS) appears sensitive to changeable aspects of the face, as it responds selectively to emotional expression and eye gaze (Allison, Puce, & McCarthy, 2000; Engell & Haxby, 2007; Hoffman & Haxby, 2000). Eye gaze perception additionally activates the intraparietal sulcus, and its coding can be dissociated from that of identity and expression (Pelphrey & Vander Wyk, 2010). Other neural structures respond to specific emotional expressions. For example, the amygdala responds selectively to fearful or unhappy facial expressions (Morris et al., 1996), whereas the orbitofrontal cortex responds to angry facial expressions (Blair, Morris, Frith, Perrett, & Dolan, 1999).
Thus research in humans has shown that face perception entails a diverse set of skills that are supported by multiple specialized neural circuits and that require years to fully develop. Based on this framework we now turn to face perception by nonhuman species, reviewing the extent to which different animals look at faces to extract different types of information, and referring to the developmental course and neural basis of face processing where information is available. We ask to what extent faces are a special category of visual stimuli for nonhuman primates, other mammals, and other vertebrates. We survey a wide range of experiments investigating the specific types of visual information various animals are and are not able to extract from faces. Then, based on the shared capacities of different animals, we speculate on the evolutionary history of different aspects of face perception, and how this history might be informative about our own face perception.
Nonhuman primates
Like humans, other primates are highly developed in their use of faces for social purposes, such as recognition, communication, and mate selection. Primates have been tested extensively on how they perceive faces, primarily because of their evolutionary proximity to humans and human-like capacity to read faces, but also because they can be easily trained to respond to images and videos in a laboratory setting (see Figure 1A)
Figure 1.
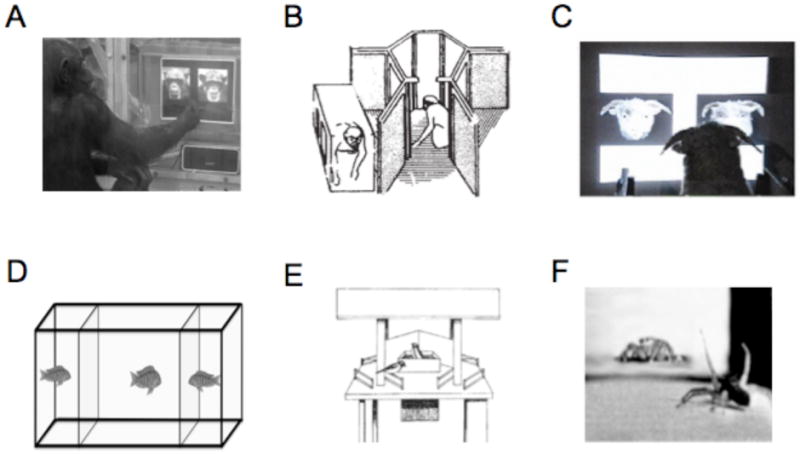
Methods for testing animals on visual conspecific perception. A. A chimp indicates its recognition of a face by pressing on a touch-screen [1]. B. Macaque monkeys tested for capacity to visually recognize kin with whom they had no prior experience [2]. C. A sheep discriminates between two faces by pressing one of two panels in exchange for a food reward [3]. D. A fish inspects two neighbors in order to test whether it can subsequently recognize them [4]. E. Reactions of individual great tits to a radio-controlled maneuverable dummy was used to investigate the contribution of breast-stripe width to establishing social dominance [5]. F. A male jumping spider courts the video image of a female [6].
Figure 1 Citations
[1] adapted from Martinez and Matsuzawa (2009). Animal Cognition, Suppl 1:S71-75.
[2] adapted from Wu, H. M., Holmes, et al. (1980). Nature, 285(5762), 225-227.
[3] adapted from Kendrick, K.M. (2008), in The Welfare of Sheep, (C. Dwyer, Ed.), Springer, Netherlands.
[4] adapted from Balshine-Earn and Lotem (1997) Behaviour 135:369-386.
[5] adapted from Jarvi and Bakken (1984) Animal Behaviour 32: 590-596.
[6] adapted from Clark and Uetz (1990) Animal Behaviour. 40:884-890.
Identity
All primate species tested experimentally are able to recognize familiar individuals by the appearance of their faces. Nonetheless, careful testing has revealed some differences between species. Chimp face perception seems to be most similar to humans. They are able to readily discriminate photos of unfamiliar conspecifics, and when doing so rely primarily on the eyes (Parr, Heintz, & Akamagwuna, 2006; Parr, Winslow, Hopkins, & de Waal, 2000; Tomonaga, Itakura, & Matsuzawa, 1993). Chimps are also sensitive to familial similarity in unfamiliar faces (Parr & de Waal, 1999) (Figure 2A). In fact, a number of experiments indicate that chimps use configural processing in a manner similar to humans, since their recognition is sensitive to the same manipulations that disrupt face recognition in humans, such as rotational inversion (Parr, Dove, & Hopkins, 1998; Tomonaga, 2007), and second-order spatial distortions (Parr et al., 2006).
Figure 2.
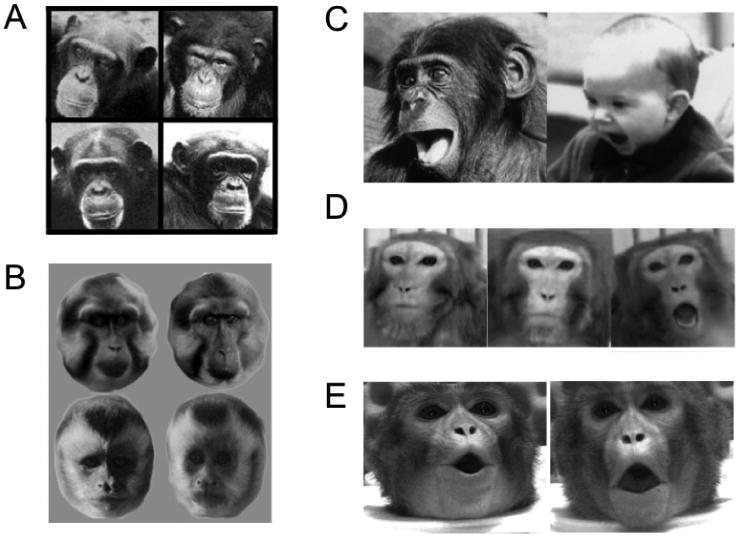
The recognition and interpretation of conspecific primate faces. A. Chimps can recognize familial face similarity between mothers (left column) and their sons (right column) [1]. B. Tonkean macaques and brown-faced capuchins are better able to discriminate between members of their own species (top and bottom rows, respectively) than between members of other species [2]. C. Homologous play face in bonobo and human [3]. D. Lip-smacking, neutral, and threat expressions from a rhesus macaque, used as stimuli for neurophysiological experiments [4]. E. Images taken from video stimuli shown to macaques with either matching or nonmatching acoustic vocalizations [5].
Figure 2 Citations
[1] adapted from Parr and de Waal (1999) Nature, 399: 647-8.
[2] adapted from DuFour et al (2006) Behavioral Processes, 73: 107-13.
[3] adapted from Schmidt and Cohn (2001) Yearbook of Physical Anthropology, 44:8-24.
[4] adapted from Gothard et al (2007) J Neurophysiology, 97:1671-1683.
[5] adapted from Ghazanfar and Logothetis (2003) Nature, 423:937-8.
Rhesus macaques also recognize each other by their faces, with individual recognition being an important feature of their dominance hierarchy (Bovet & Washburn, 2003; Deaner, Khera, & Platt, 2005; Parr et al., 2000). They also appear sensitive to familial resemblance, as they spend more time looking at their kin than at other conspecifics, even when lacking previous visual experience with them due to early postnatal separation (Wu, Holmes, Medina, & Sackett, 1980) (testing apparatus shown in Figure 1B). Compared to chimps, macaques pay more attention to the component parts of a face and their first-order, rather than second-order, spatial relations (Parr, Heintz, & Pradhan, 2008; Parron & Fagot, 2007). Also, the effect of rotational inversion on face recognition in monkeys has been mixed (Gothard, Brooks, & Peterson, 2009; Parr et al., 2008; Parr, Winslow, & Hopkins, 1999; Phelps & Roberts, 1994; Tomonaga, 1994; Weiss, Kralik, & Hauser, 2001; Wright & Roberts, 1996), suggesting that if they do use configural processing, it is to a lesser degree than humans and chimps.
The primates that have been tested also appear to exhibit a conspecific advantage; that is, they recognize and discriminate members of their own species more readily than other species. This advantage has been likened to the human other-race effect, where faces from one's own race are more easily recognized than faces from another race (Malpass & Kravitz, 1969; Meissner & Brigham, 2001). One comparative behavioral study used viewing preferences as a measure of face recognition in humans, Tonkean macaques (Old World monkeys), and capuchins (New World monkeys). Each species was shown photographs of a large number of faces, from their own species and from 2-3 other primate species. New faces from the same-species engendered reliably longer looking times than new faces from other species, suggesting better individual recognition of conspecific than heterospecific faces (Dufour, Pascalis, & Petit, 2006) (Figure 2B). A similar advantage for conspecifics was also demonstrated in rhesus macaques (Fujita, 1987, 1993; Pascalis & Bachevalier, 1998). Interestingly, the reduced recognition of heterospecifics may involve a change in the way faces are analyzed. For example, rhesus macaques, while showing some signs of configural analysis for conspecific faces, appear to switch to a feature-based mode of analysis when viewing human faces (Gothard et al., 2009).
The capacity to efficiently recognize heterospecific faces appears to be, at least in part, shaped by experience. Chimps raised in a human environment, having more exposure to human than chimp faces, were better at discriminating pictures of unknown human faces than unknown chimp faces (Martin-Malivel & Okada, 2007). Similarly, rhesus macaques explicitly trained for several months to discriminate between four human faces with reduced identity cues scored nearly as well as humans performing the same task (Leopold & Bondar, 2005). Under normal conditions, experience may determine which categories of faces will be analyzed in a configural manner and which will not (Pascalis et al., 2002; Sugita, 2008) (see Development section below).
Finally, tests of self-recognition in a mirror can be used to assess individual recognition to some extent. This paradigm allows for testing in a wide range of species, and typically involves observing whether or not an animal, upon observing a mark on its face in the mirror, attempts to remove it by touching its own face. Using this test, many (but not all) chimps are able to recognize themselves in the mirror (Gallup, 1970; Povinelli, Rulf, Landau, & Bierschwale, 1993). While this appears to be a shared cognitive capability of the great apes (Swartz, 1997), there is minimal evidence for self-recognition in other primate species (Gallup, 1977; Hauser, Miller, Liu, & Gupta, 2001).
Emotional Expression
Facial expression in primates is a difficult topic to summarize. Whereas some universal facial gestures appear to be shared across a broad range of primate species (see Figure 2C), others are distinctly species-specific and highly stereotyped (Gaspar, 2006; Preuschoft & van Hooff, 1995). Similarly, whereas aspects of facial expression perception may be inborn, others can be learned socially. In an example of inborn expression recognition, rhesus macaques raised in isolation of their conspecifics respond immediately to facial threats without any prior experience with faces (Sackett, 1966).
Emotional expressions are tightly linked to the mobility of the face, which increased during primate evolution, possibly reflecting the increasing importance of social exchange (Andrew, 1963; Burrows, 2008; Huber, 1931). To communicate anything, faces must be configurable into a range of postures. Primates, including apes, Old World monkeys and New World monkeys, are endowed with a broad repertoire of facial expressions involving the lips and eyes (Hauser, 1993; Maestripieri & Wallen, 1997; Preuschoft & van Hooff, 1995; Thierry, Demaria, Preuschoft, & Desportes, 1989; Tomonaga et al., 2004; van Hooff, 1962; Weigel, 1979), owing to an elaboration of the mimetic facial musculature compared to other mammals (Burrows, 2008; Burrows, Waller, Parr, & Bonar, 2006; Waller, Parr, Gothard, Burrows, & Fuglevand, 2008).
Experimental testing has shown that chimps can accurately interpret photographed expressions (Parr, 2003; Parr, Hopkins, & de Waal, 1998) and can, through experience, learn to categorize expressions of both monkeys and humans (Dittrich, 1990; Kanazawa, 1996). As with humans, the eyes, including the closure of the lid and position of the brow, are important components of expression for many nonhuman primates (Andrew, 1963; Ghazanfar, Nielsen, & Logothetis, 2006), as are some aspects of mouth behavior. For example yawning can serve as a signal to conspecifics (Smith, 1999), with the exposure of canines serving as a low-grade threat among Old World monkeys (Hadidian, 1980). In both chimps and macaques, facial signals are coordinated with acoustic vocalizations. In extracting the social meaning of these signals, primates are therefore sensitive to the congruency of visual and acoustic facial signals (Ghazanfar & Logothetis, 2003; Parr, 2004) (Figure 2E). Accurate interpretation of facial and bodily expressions facilitates social learning in monkeys, as shown by macaques' acquisition of the fear of toy snakes and crocodiles after observing videos of their conspecifics reacting fearfully to them (Cook & Mineka, 1989).
Gaze
In addition to their role in emotional expression, eyes serve as important cues to gaze direction and objects of interest in the environment. Eyes, which are often high-contrast features, highlighted by coloration or patterning such as eye-rings to enhance visibility (Coss & Goldthwaite, 1995), attract primates' attention. For example, macaques focus their gaze on the eye region in pictures of monkeys (Guo, Robertson, Mahmoodi, Tadmor, & Young, 2003; Keating & Keating, 1982; Kyes & Candland, 1987) and other animals (Demaria & Thierry, 1988). The importance of eyes and eye-like patterns is also evident in at least one prosimian species, as mouse lemurs were shown to have a selective aversion to looking at pairs of horizontally spaced dots (Coss, 1978c).
Accurate gaze monitoring is important in primate society, in which rules often govern who may look at whom. Prolonged gaze sometimes serves as an aggressive gesture to reinforce submissive gaze aversion in lower ranking group members (Coss, Marks, & Ramakrishnan, 2002; Deaner et al., 2005; Emery, 2000). Some primates can additionally infer the direction of another's gaze to an object or individual of interest (Emery, Lorincz, Perrett, Oram, & Baker, 1997; Tomasello, Call, & Hare, 1998). Rhesus monkeys rely primarily on head orientation for this, but follow eye-based direction of gaze under some conditions (Deaner & Platt, 2003; Ferrari, Kohler, Fogassi, & Gallese, 2000; Lorincz, Baker, Perrett, & Fagot, 1999). Chimps and bonobos are able to follow head orientation of a human experimenter to a target. Interestingly, they cease to do this when the object of attention is deemed to be out of the experimenter's line of sight (Okamoto-Barth, Call, & Tomasello, 2007). Humans are able to follow their mothers' gaze within the first year of life (Butterworth & Jarrett, 1991). In contrast to monkeys and apes, human infants rely primarily on the eyes rather than the head (Tomasello et al., 2007) (for a review, see Emery & Clayton, 2009).
Attraction
Like humans, rhesus macaques appear to find symmetry attractive, preferring to gaze at artificially symmetric photos of conspecific faces over asymmetric ones (Waitt & Little, 2006) (Figure 3B). Preference for symmetry may be related to phenotypic quality, as suggested by a study of fluctuating asymmetry (random deviations from perfect bilateral symmetry) of canine teeth in a wide range of primates (Manning & Chamberlain, 1993). It is not known whether non-human primates or other animals find averageness attractive, although it has been suggested that they might (Koeslag, 1990). It would be particularly interesting to know whether apes and monkeys share the human preference for averaged composite faces.
Figure 3.
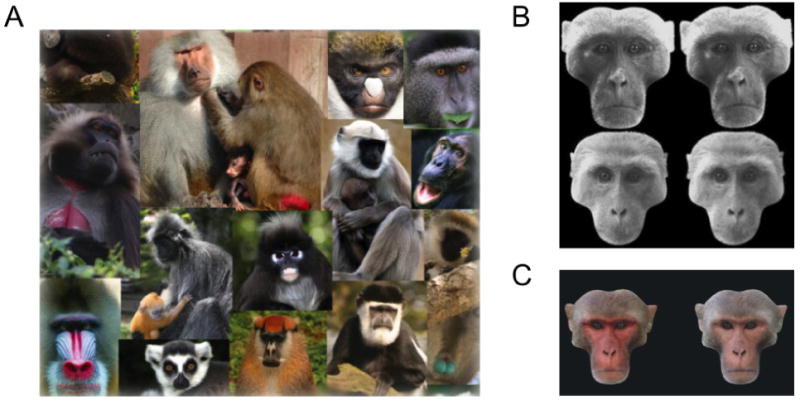
Face attributes associated with sexual selection in primates. A. Primate faces are often high contrast or colored, often with highlights around the eyes [1]. B. Both male and females rhesus monkeys prefer symmetrical (left column) over asymmetrical (right column) faces [2]. C. Male facial coloration in rhesus macaques affects viewing preference of females, with artificially reddened faces attracting longer periods of inspection [3].
Figure 3 Citations
[1] adapted from Bradley B and Mundy N (2008). Evolutionary Anthropology, 17(2), 97-111.
[2] adapted from Waitt C and Little AC (2006) International Journal of Primatology, 27(1),133-45.
[3] adapted from Waitt C et al (2003) Proceedings of the Royal Society B: Biological Sciences, 270, S144-6.
A particularly important facial cue for nonhuman primate mate selection is skin color. Primate faces are often decorated with patterns and colors that appear to play a role in mate selection (Figure 3A, for a review, see Bradley & Mundy, 2008). Trichromatic primates are usually bare-faced, underscoring the potential role of color vision in primate face perception (Changizi, Zhang, & Shimojo, 2006). Facial skin coloration in chimps is variable within a social group (Bradley & Mundy, 2008), but its relationship to dominance and sexual selection is unknown. In monkeys, several studies have explored the connection between coloration and various social factors. Following computer graphic manipulation of redness in the coloration of faces, female rhesus macaques spent more time looking at artificially reddened male faces than the original versions of the same face, with increased looking times taken as a possible indicator of sexual attraction (Waitt et al., 2003) (Figure 3C). This preference may be because redness can signal male dominance rank and fitness (Setchell & Wickings, 2005). Their male counterparts showed no such preference for artificially reddened faces, but did prefer artificially reddened hindquarters (Waitt, Gerald, Little, & Kraiselburd, 2006). Both males and females attended to female facial coloration signaling pregnancy (Gerald, Waitt, & Little, 2009).
It is important to note that attractiveness in monkeys is often inferred by observing their gaze behavior when they are presented with multiple visual stimuli. This paradigm, which is similar to that used to assess novelty and recognition, must be interpreted with caution, as diverse social and non-social factors also contribute to gaze behavior. For example, male rhesus monkeys will spend more time looking at images containing sexual content than nonsexual images, and similarly spend more time looking at dominant than submissive male faces (Deaner et al., 2005; Sackett, 1965). Whether these stimuli should be considered attractive in the same sense as symmetrical and reddened faces, and how these factors contribute to paradigms using preferred looking to assess attraction, novelty, or recognition, remains an open question.
Development
The development of primate face processing is complex, involving an innate predisposition to look at the mother's face, along with a strong learning component. Chimpanzees can recognize their mother's face within a month of birth, with an increase in mutual gaze in the following month (Myowa-Yamakoshi, Yamaguchi, Tomonaga, Tanaka, & Matsuzawa, 2005; Tomonaga et al., 2004). Subsequent exposure is also important for their developing face recognition skills, as evidenced by the fact that chimps brought up primarily among humans become experts with human faces (Martin-Malivel & Okada, 2007).
Like humans, infant monkeys prefer schematic faces to other stimuli, with spatial configuration being the important factor for the first month, and feature details becoming important later (Kuwahata, Adachi, Fujita, Tomonaga, & Matsuzawa, 2004). Macaques deprived of any exposure to faces over the first 6-24 months of life showed immediate interest in subsequently shown faces, and gained expertise with either human or macaque faces thereafter, depending on subsequent experience (Sugita, 2008). Thus macaques exhibit both an innate preference for faces, along with a window of plasticity (abnormally extended in that case) in which their perceptual skills for a given category of faces are sharpened. A comparable developmental sharpening has also been observed in humans, whose capacity to discriminate monkey faces declines during development, presumably in part due to extensive selective exposure to human faces (Pascalis et al., 2002).
Neural Specialization
Most of our information about neural specialization for faces in nonhuman primates comes from electrophysiological single-unit experiments in macaques (see Gross, 2008 for a review). These studies reveal a core of face processing circuitry in the lower bank and fundus of the superior temporal sulcus and the adjacent surfaces of the inferior temporal cortex, in cortical area TE (Desimone, Albright, Gross, & Bruce, 1984; Gross, Rocha-Miranda, & Bender, 1972; Perrett, Rolls, & Caan, 1982; Tanaka, Saito, Fukada, & Moriya, 1991). Face-selective responses have also been observed elsewhere in the cortex, including the superior temporal polysensory (Bruce, Desimone, & Gross, 1981), orbitofrontal (Rolls, Critchley, Browning, & Inoue, 2006) and ventrolateral prefrontal (Wilson, Scalaidhe, & Goldman-Rakic, 1993) cortical areas. More recent fMRI studies have supported and extended these initial findings (Logothetis, Guggenberger, Peled, & Pauls, 1999; Pinsk, DeSimone, Moore, Gross, & Kastner, 2005; Tsao, Freiwald, Knutsen, Mandeville, & Tootell, 2003), with combined fMRI/microstimulation demonstrating that patches of face responsive areas are functionally linked (Moeller, Freiwald, & Tsao, 2008). Other work suggests that the analysis of bodies takes place in regions of temporal cortex directly adjacent to face patches (Bell, Hadj-Bouziane, Frihauf, Tootell, & Ungerleider, 2009; Hasselmo, Rolls, & Baylis, 1989; Jellema & Perrett, 2003). And, as in humans, face processing shows hemispheric asymmetry, with strongest activation in the right hemisphere of both macaques (Pinsk et al., 2005) and vervets (Zangenehpour & Chaudhuri, 2005).
Some subcortical structures also show selective neural responses to faces. The macaque amygdala, for example, is sensitive to facial expressions (Gothard, Battaglia, Erickson, Spitler, & Amaral, 2007; Hoffman, Gothard, Schmid, & Logothetis, 2007), with the eyes playing a particularly important role in its responses (Gothard et al., 2007; Hoffman et al., 2007; Leonard, Rolls, Wilson, & Baylis, 1985). Amygdala responses probably primarily reflect input from high-level visual cortex. Direct subcortical input to the amygdala might also contribute to face-selective responses passing through the superior colliculus and pulvinar (Adolphs, 2002; Johnson, 2005; Morris, Ohman, & Dolan, 1999; Palermo & Rhodes, 2007), though the existence of a viable functional pathway has not yet been firmly established in either humans or macaques (for a discussion, see Pessoa & Ungerleider, 2004 and Andino, Menendez, Khateb, Landis, & Pegna, 2009). Face-selective single-unit responses have also been reported in the macaque medial dorsal thalamus (Tanibuchi & Goldman-Rakic, 2003), striatum (Logothetis et al., 1999), superior colliculus (Arendes, 1994), and hippocampus (Hampson, Pons, Stanford, & Deadwyler, 2004).
Summary and Discussion
Primates extract a rich amount of social information from faces of their conspecifics. For the three species studied in the greatest detail, humans, chimps, and macaques, the many shared features of face processing, along with the apparently similar face-selective responses in the brain, suggests that our most recent common ancestor, living more than 20 million years ago, may have enjoyed a similar fluency with faces. The functional and anatomical similarities raise the possibility of evolutionary homology (similarity due to shared ancestry), between the primary face selective cortical areas in the macaque and human, albeit with a ventral shift in their anatomical location in humans (Pinsk et al., 2009; Tsao, Moeller, & Freiwald, 2008). However, to distinguish between homology and convergent (independent) evolution would require a detailed phylogenetic analysis of the species involved (Brooks & McLennan, 1991). While neural specialization for faces has not been investigated in New World monkeys, the few behavioral studies suggest that they also possess sophisticated face perception (Dufour et al., 2006; Phelps & Roberts, 1994), potentially pushing the origins of complex face processing back as far as stem anthropoid primates, who lived more than 40 million years ago (Janecka et al., 2007).
Did the exquisite face perception abilities we share with chimps and macaques evolve to fit the social needs of interactive primates, or did primate sociality exploit an existing fluency with faces? While evolutionary cause/effect relationships are notoriously difficult to establish, it is interesting to consider that high spatial acuity may have been the primary driver for the evolution of primate social vision. Nearly all anthropoid primates, including apes, New World and Old World monkeys, have excellent acuity that stems from their large, forward-facing eyes and foveas. One theory holds that these features may, in part, reflect adaptations acquired during a nocturnal phase of their evolutionary history (Ross, 2000). A different theory attributes advances in the primate visual system to selection pressure arising from predation, in particular from snakes (Isbell, 2009). Whatever its basis, a pre-adaptation resulting in a high-resolution fovea that could be quickly directed to objects of interest would have greatly enhanced visual social communication (Allman, Sprague, & Epstein, 1977). It is interesting to note that extant primate species with larger eyes, and hence better acuity, have a particularly wide range of facial expressions (Dobson, 2009; Kiltie, 2000), consistent with this conjecture. In short, primate face perception and consequent aspects of sociality depend upon making quick and accurate visual discriminations at a distance. The emergence of our complex, vision-based sociality may ultimately be traced to a prior adaptation for visual acuity that emerged for reasons unrelated to social interaction.
Mammals
Compared to primates, other mammals rely less on vision and more on olfaction and audition for social communication. Nonetheless, many mammals have good visual acuity, though the use of vision in face perception has only been evaluated in a few species. Curiously, the most studied non-primate species, sheep, shows a pronounced visual specialization for faces at both behavioral and neural levels. In the mid 1980s, Keith Kendrick and colleagues began a series of landmark studies aimed at understanding social communication in sheep (for a review see (Tate, Fischer, Leigh, & Kendrick, 2006), and found that their face processing closely resembles that of humans and monkeys.
Identity
Sheep have excellent visual acuity (Kendrick, 2008), advanced social perception, and the ability to visually recognize faces of their conspecifics in photographs (Kendrick, da Costa, Leigh, Hinton, & Peirce, 2001)(Figure 1C, Figure 4A). They can recognize individual sheep on a computer screen even when identity information is reduced using morphing techniques, or when photographs are presented at a small scale (Tate et al., 2006). They can also recognize the faces of individual human caretakers and sheep dogs. Like primates, they prefer to look at pictures of their conspecifics over heterospecifics (Da Costa, Leigh, Man, & Kendrick, 2004).
Figure 4.
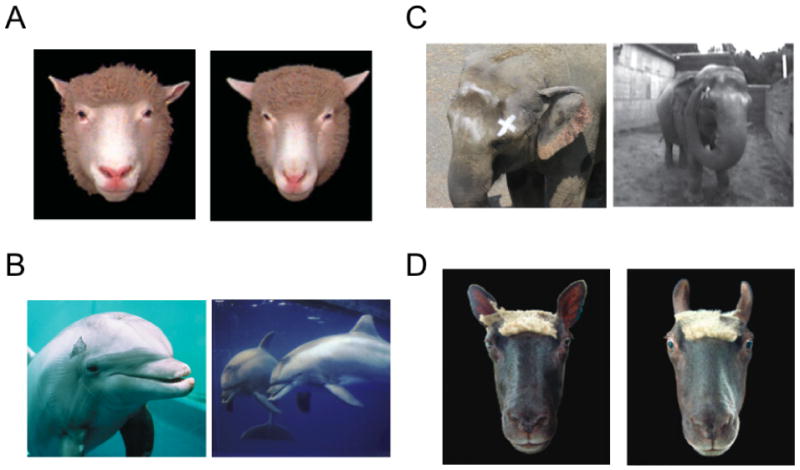
The recognition of identity, expression, and self in mammals other than primates. A. Sheep (superorder Laurasiatheria) use the variation in the structure of the face to recognize one another [1]. B. Bottlenose dolphins (superorder Laurasiatheria) pass tests of self-recognition. When marked on the skin (left), they position themselves in front of the mirror in order to see the mark on their body (right) [2]. C. Similarly, elephants (superorder Afrotheria) pass tests of self-recognition. After receiving a mark on their face (left), they use their trunk in front of a mirror in an attempt to remove it (right) [3]. D. Sheep communicate emotion with their face, in this case indicating fear by drawing back the ears and opening the eyes [1].
Figure 4 Citations
[1] adapted from Tate AJ et al (2006) Philosophical Transactions of the Royal Society B: Biological Sciences, 361: 2155-2172.
[2] adapted from Marino et al (2007) PLOS Biology 5(5):966-972 and Reiss and Marino (2001) Proceedings of the National Academy of Sciences of the United States of America, 98:5937-42.
[3] adapted from Plotnick et al (2006) Proceedings of the National Academy of Sciences of the United States of America, 103(45):17053-57.
Key features of sheep face perception are similar to those of primates. For example, the eyes seem to be a particularly important cue for discriminating individuals (Kendrick et al., 1995). Also, like humans, sheep exhibit a right hemisphere (left visual field) advantage for identifying other sheep based on photographs of their faces (Peirce, Leigh, & Kendrick, 2000), with lateralized activity in the right temporal cortex (Broad, Mimmack, & Kendrick, 2000; Peirce & Kendrick, 2002). Their individual recognition is dependent upon the correct configuration of facial features (Peirce et al., 2000) and their expertise appears greatest for their own species (Peirce, Leigh, daCosta, & Kendrick, 2001).
Recent work shows that sheep are not the only mammals capable of face recognition. Cattle are also able to recognize one another from photographs of their faces (Coulon, Deputte, Heyman, & Baudoin, 2009; Coulon et al., 2007). Domestic dogs can also recognize photographs of their owners' faces (Adachi & Fujita, 2007). These results suggest that the capacity for face recognition may, in fact, be common among mammals.
Several mammals have been tested for self-recognition in a mirror. The bottlenose dolphin exhibits some capacity for self-recognition (Marino et al., 2007; Marten & Psarakos, 1995; Reiss, 2001) (Figure 4B), though the interpretations of such experiments are not always straightforward (Gallup, 1995). Killer whales also show signs of self inspection in a mirror, whereas sea lions do not (Delfour & Marten, 2001). Likewise, elephants have been reported to inspect marks on their face in front of a large mirror (Plotnik, de Waal, & Reiss, 2006) (but see (Nissani & Hoefler-Nissani, 2007)) (Figure 4C).
Emotional Expression
Some non-primate mammals convey their emotions using dynamic postures of the face and body, as documented by Darwin (Darwin, 1872). Muscles in the mammalian face permit a range of facial expressions involving the mouth, eyes, and ears, which are usually complemented by posturing of the body. Sheep readily communicate their facial expression of fear to one another, and can recognize emotion in photographs of their conspecifics (Da Costa et al., 2004; Tate et al., 2006) (Figure 4D). When given a choice between a distressed facial expression and a calm one, they choose the latter in exchange for food reward, even if it is displayed by a less familiar individual (overriding their preference for viewing familiar conspecifics). Again, the eyes appear to be the most important stimulus feature (Tate et al., 2006). In cattle, the percentage of eye white has been shown to be an indicator of stress, and may serve as an emotional signal for conspecifics (Sandem, Janczak, Salte, & Braastad, 2006).
In general, mammals display a wide range of facial behavior. Canids' expressive faces can signal both fear and aggression (Fox, 1969, 1970; Lorenz, 1966). Dogs, bears and related animals frequently engage in play, which involves gesturing with both the face and body (Bekoff, 1977; Henry & Herrero, 1974). Elephants use movements of the ears, head, and jaw to signal aggression or submission (Langbauer, 2000). Brow lowering, ear flattening, whisker movements, and the flehmen response (the stereotyped curling of the upper lip) are present in many mammalian species, and serve as salient cues for conspecifics (Andrew, 1963). However, little systematic work has been done to examine the mechanisms by which such visual expressions are processed and interpreted.
Gaze
The direction of gaze is likely to be important for many mammals, particularly those living in social hierarchies, though different species may use gaze differently. In canids, the use of direct gaze resembles that in primates: passive submission is associated with aversion of the eyes accompanied by flattening of the ears and a slight grimace, whereas aggression is associated with a direct stare, erect ears, and a slight pucker (Fox, 1970). In contrast, staring in domestic cats is associated with both offensive and defensive behaviors (van den Bos & de Vries, 1996).
Several mammalian species have been tested on their ability use gaze to infer a human's or conspecific's focus of attention. Testing often begins with asking whether an animal is able to find a hidden reward based on cues provided by the experimenter (for a review, see Emery & Clayton, 2009). Under such conditions, domestic dogs can follow their owner's head orientation and eye position to find a reward (Hare & Tomasello, 2005). This ability may be related to domestication, as the results in human-raised wolves are mixed (Hare, 2002; Virányi et al., 2008). However, the use of gaze cues is not strictly limited to domestication, since cetaceans, which are not the product of human domestication, can also follow human gaze (Pack & Herman, 2006, 2007; Tschudin, Call, Dunbar, Harris, & van der, 2001). Domestic goats, while unable to use either eye or head orientation to follow the gaze of humans, are able to use head orientation to follow the gaze of their conspecifics (Kaminski, Riedel, Call, & Tomasello, 2005). In summary, some non-primate mammal species that have been studied seem able to extract cues from the orientation of the eyes and head to determine the direction of attention. Testing of additional species is needed to determine how widely this capacity is present.
A related issue is whether animals alter their gaze-following based on whether they believe an object is visible to the gazer. As mentioned above, chimps have this capability, though there is no evidence that monkeys do. While most species have not been tested in this regard, recent work demonstrated that domestic dogs are sensitive to the experimenter's field of visual attention (Miklosi, 2007). It is possible that this sensitivity is linked to dogs' general attentiveness to the human face and eyes (Gacsi, Varga, TopaL, & Csanyi, 2004).
Attraction
Mammals typically use multiple sensory modalities for mate selection, kin recognition, and the assessment of dominance. Vision is often of minor importance, playing a secondary role to acoustic, and particularly chemical, signaling (Brennan & Kendrick, 2006; Wyatt, 2003). For species that do rely on vision, the key features influencing mate selection are often aspects of the body, such as size, ornamentation, and posture. Nevertheless, some looking preferences for facial feature have been observed. For example, sheep prefer to look at faces of familiar over unfamiliar conspecifics, and conspecifics over heterospecifics (Da Costa et al., 2004). These preferences could be related to perceptions of attractiveness and mate choice, although they might simply be driven by a general preference for familiarity.
Development
It is unknown whether other non-primate mammals display innate preferences to face-like patterns, or whether their face processing improves with experience. There is a small amount of evidence for visual imprinting in mammals. Newborn guinea pigs, who, like some birds, are mobile and self-sufficient shortly after birth, will imprint upon and follow individual humans, though it is unknown which visual cues are used (Hess, 1959). It is clear, however, that at least in some non-primate mammals early experience with faces can significantly shape later behavior. In an ingenious cross-fostering experiment, baby goats were raised by sheep mothers, and vice-versa. As adults, when cross-fostered males from each species were shown photographs of sheep and goat faces, they preferred to look at females of the foster mother's species (rather than their own). Moreover, when placed in the pen with females of both species, they preferentially attempted to mate with the foster mother's species (Kendrick, Hinton, Atkins, Haupt, & Skinner, 1998), demonstrating that early facial experience determines sexual preference.
Neural Specialization
Aside from primates, neural specialization for faces has only been studied in sheep, whose brains display a striking similarity to those of monkeys in this regard. Single-cell recordings showed that neurons display a range of selectivity for faces, ranging from those selective for individuals, to those selective for stimulus categories (Kendrick, 1991, 2008; Kendrick & Baldwin, 1987). The demonstration of such face cells in the sheep's temporal cortex (Kendrick & Baldwin, 1987) is the sole piece of evidence that the brain's specialization for face processing extends outside the sphere of primates. Photographs of familiar faces elicited stronger neural responses than those of unfamiliar faces (Kendrick et al., 2001). Moreover, familiarity-based neural responses were not restricted to sheep faces, but extended to humans and sheepdogs. Interestingly, the tuning for “horn length” in one class of neurons (Kendrick, 1994) in the sheep temporal cortex bears more than a superficial resemblance to tuning for face distinctiveness by neurons in the monkey (Leopold, Bondar, & Giese, 2006) and human (Loffler, Yourganov, Wilkinson, & Wilson, 2005) temporal cortex (Figure 5). Finally, like neurons in the monkey temporal cortex, neurons in the sheep temporal cortex respond to faces in a view-dependent manner (Perrett et al., 1985; Tate et al., 2006).
Figure 5.
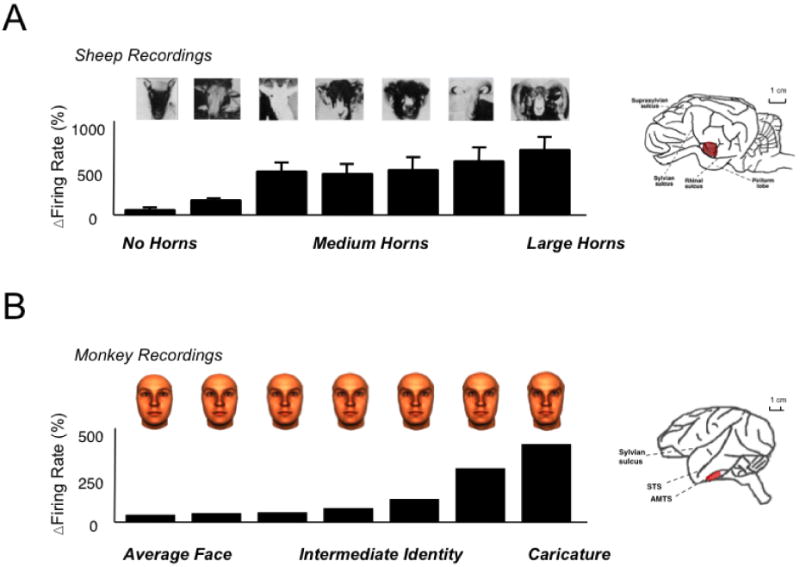
Face processing in sheep and monkeys. (a) A subset of neurons in the sheep temporal cortex respond as a function of the visible length of the horns, a distinguishing feature for recognition of individual and species, as well as for social dominance [1]. (b) Neurons in the monkey temporal cortex respond to images of human faces, in this case responding monotonically to increasing amounts of “identity” level, or individuating feature information [2].
Figure 5 Citations.
[1] adapted from Kendrick, KM (1994) Behavioral Processes 33:89-112.
[2] Leopold DA et al (2006) Nature 442:572-575.
Discussion: Mammals
Many mammals readily produce and interpret facial gestures. Is this behavior the result of a shared and evolutionarily conserved cortical face processing circuitry? It is possible that at least some aspects of face perception were inherited from a common ancestor rather than that all aspects of face expertise emerged independently in all mammalian superorders. However, it is by no means obvious how shared modules for face processing could have been preserved: Much of mammalian evolution occurred during the age of the dinosaurs, where most mammals occupied nocturnal niches, thus diminishing the usefulness of visual cues for social signaling. Nonetheless, it is possible that some conspicuous facial markings such as eye-ring patterns that are commonly found in extant mammals (Caro, 2005; Ortolani, 1999) first evolved to make faces more conspicuous in moonlight, when some extant nocturnal prosimians are most active (Nash, 2007). During this nocturnal era, critical adaptations for facial signaling, such as moveable ears and elaborated mimetic musculature, became permanent fixtures of the mammalian face, though perhaps at first having little to do with visual signaling. Following the extinction of the dinosaurs, as some mammals became diurnal, these facial patterns and natural movements may have increasingly served as the basis for individual recognition and social signaling.
Clearly, there are many open questions regarding mammalian face processing and its evolutionary relationship to face processing in primates. Since neither brains nor cognitive processes leave behind a fossil record, the evolutionary history of face perception must be inferred from the similarity of behaviors, anatomy, and neural responses across different species. Distinguishing between homology and convergent evolution is particularly difficult (Wenzel, 1992). Homology would require functionally and anatomically similar face-responsive regions to be present in the common ancestor of mammals and primates, a mammal living with dinosaurs during the Cretaceous period (Springer, Murphy, Eizirik, & O'Brien, 2003). For some critical aspects of face processing, this intriguing possibility cannot be ruled out. Alternatively, given that both sheep and macaques are diurnal and social, their face-selective cortical machinery might have evolved independently, as parallel adaptations of a general visual processing system, driven by similar sources of natural and sexual selection. In either case, the functional and neural similarities of mammalian and primate face perception highlight the probable ancient evolutionary origins of face perception.
In the future, neurophysiology and imaging experiments in a wider range of mammalian species may shed light on the origins of primate face perception. For example, the presence of face selective cortical responses in a large proportion of mammals with widely varying different ecologies, perhaps diurnal and nocturnal carnivores, rodents, and marsupials, would be consistent with specialization for face processing as early as the Cretaceous period. If, on the other hand, the cortical specialization for faces is present in only a few species of mammals with very similar ecologies (e.g., highly social and diurnal) then it would seem more likely that face perception in primates and non-primate mammals are examples of convergent evolution arising in response to similar sources of natural selection. Given the multiple, interacting aspects of face processing in the primate brain, it may only be through comparative and behavioral studies that scientists are able to disentangle the recently evolved neural circuits specialized for primate sociality from older, more general mammalian circuits involved in the more basic visual analysis of faces.
Vertebrates
Reptiles, birds, and fish lack the elaborated facial musculature of mammals, and therefore have a limited repertoire of facial behavior. Nonetheless, many species depend on their vision for social interaction, with some paying particular attention to the face and head.
Identity
Visual conspecific recognition has been demonstrated in birds (Bird & Emery, 2008; D'Eath & Stone, 1999; Ryan & Lea, 1994; Thorpe, 1968), reptiles (Olsson, 1994; Van Dyk & Evans, 2007) and fish (Bshary, Wickler, & Fricke, 2002; Grosenick, Clement, & Fernald, 2007) (see Figure 1D,E). Some fish are able not only to visually recognize their conspecifics, but can even infer and remember their relative dominance rank based on appearance (Grosenick et al., 2007). Heterospecific individual recognition has also been demonstrated in some species of birds. For example, mockingbirds can recognize individual humans after repeated exposures (Levey et al., 2009). In crows, individual recognition of humans seems to be based on the face, since a familiar human wearing a mask is treated as a stranger (Cornell, Marzluff, & Peccorro, 2009).
Unfortunately, little is known about the specific cues underlying visual conspecific recognition in vertebrates, though a few studies suggest the face is sometimes important. For example, pigeons can discriminate pictures of conspecific faces (Nakamura, Croft, & Westbrook, 2003; Watanabe & Ito, 1991). In birds, plumage on the face supports individual recognition of conspecifics in white-throated sparrows (Whitfield, 1987), male ruffs (Dale, Lank, & Reeve, 2001), and budgerigars (Brown & Dooling, 1992). Experiments using parametrically manipulated stimuli have shown that budgerigars, like humans, rely on second order configural cues in their recognition (Brown & Dooling, 1993) (Figure 6A). Magpies, like chimps, and a few other mammalian species mentioned above, have also been reported to recognize their reflection, removing a mark on their face in front of a mirror (Prior, Schwarz, & Gunturkun, 2008).
Figure 6.
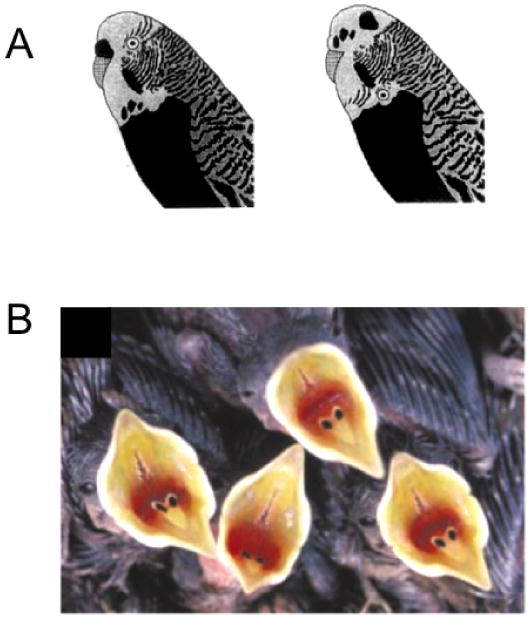
Visual analysis of conspecific faces in birds. (a) Budgerigars can discriminate pairs of real and synthetic conspecific faces. They are quicker at discriminating pairs of individuals when faces are configurally intact (left) than when they are scrambled (right) [1]. (b) The appearance of a nestling's open mouth determines the parental feeding response. Visual features include the size of the gape, internal patterning, and coloration [2].
Figure 6 Citations
[1] adapted from Brown SD and Dooling RJ (1993) Journal of Comparative Psychology. 107(1):48-60.
[2] adapted from Kilner RM et al (1999) Nature, 397:667-72.
Emotional Expression
For non-mammals, most communicative gestures involve displays of the body, which have been analyzed and quantified in birds (Davies, 1978; van Rhijn, 1981) and reptiles (Carpenter, 1977). Video playback testing has shown that such conspecific displays elicit appropriate behavioral responses (Ord & Evans, 2002). Though birds, lizards, and fish lack the capacity to make complex facial expressions, some basic facial musculature, such as the that involved in moving the eyes and jaw, is shared (Noden & Francis-West, 2006). This leaves open the possibility, albeit only weakly supported, that mouth and eye movements play a role in social signaling in non-mammalian vertebrates. There are some obvious facial gestures, such as stereotypical begging signals in nestling birds (Figure 6B) (Hunt, Kilner, Langmore, & Bennett, 2003; Kilner & Davies, 1998). In addition, communicative gestures of the head, mouth, and throat have been reported in some species of frogs (Hodl, Amezquita, & Ryan, 2001), lizards (Jennsen, 1977), birds (Andrew, 1961), and fish (Baerends & Baeronds-Van Roon, 1950).
Gaze
Eyes are an important stimulus for a wide range of non-mammalian vertebrates (reviewed in Emery, 2000). Previous work has shown that representatives of all major vertebrate taxa are capable of reacting to a pair of dark eyes. In some cases eye sensitivity is important for conspecific interactions, such as gaze aversion. In young jewelfish, for example, a pair of horizontally spaced black disks elicits an evasive response, while other spatial configurations do not (Coss, 1979). This eye-specific reaction is thought to be innate and important for establishing social dominance (Coss, 1978a).
In other cases eye sensitivity appears to be more strongly geared for interspecific interactions, such as predator-avoidance. Eyes, as visual stimuli, serve as a basis for recognizing predators. For example, pied flycatchers respond to “dummy” birds shaped like natural predators (a shrike or owl) with anti-predator behavior in the form of mobbing, vocalization, freezing, and tail-flicks (Curio, 1975). However, when the eye stripe or eyes themselves were altered in the dummy, the incidence of these behaviors decreased significantly. Lizards and snakes also react with avoidance or defensive behavior to the presence of eyes, either the direct gaze of an experimenter or detached glass eyes (Bern & Herzog, 1994; Burger, 1998; Hennig, 1977). White Leghorn chicks avoided eye-like patterns compared to control patterns (Scaife, 1976b), and were more likely to approach a model of a stuffed hawk when its eyes were obscured with feathers (Scaife, 1976a). In addition, some birds and snakes are able to detect when they are the object of an experimenter's (or predator's) gaze, and adjust their behavior accordingly (Burghardt & Greene, 1988; Carter, Lyons, Cole, & Goldsmith, 2008; Hampton, 1994). This widespread sensitivity to eyes may stem from a simple principle of natural selection: those able to notice and avoid a pair of eyes fixed upon them are more likely to survive. At the same time, certain predators use their heterospecific eye perception as a target for attack, such as the spitting cobras, who consistently aim for the eyes in both natural and laboratory conditions (Westhoff, Tzschatzch, & Bleckmann, 2005).
In following visual gaze, birds show a striking, and somewhat surprising, convergence with primates. Like primates, ravens can follow shifts in combined head- and eye-gaze direction in humans. When a barrier is present, they move into a position in which they can determine the object of the experimenter's gaze (Bugnyar, Stowe, & Heinrich, 2004), with this latter skill requiring several months to develop (Schloegl, Kotrschal, & Bugnyar, 2008a). Interestingly, despite having the requisite perceptual skills to compute and follow gaze direction, ravens do not naturally seem to exploit such information to locate a food reward in an object choice task (Schloegl, Kotrschal, & Bugnyar, 2008b). In one experiment, however, jackdaws could do this, although only when the experimenter's gaze alternated back and forth between the bird and the reward providing a strong cuing signal (von Bayern & Emery, 2009).
Attraction
Social attraction and dominance of many non-mammalian vertebrates is often based on visual appearance. This is apparent in the beautiful displays of many birds, which are commonly used to illustrate principles of sexual selection (Pruett-Jones & Pruett-Jones, 1990; Trainor & Basolo, 2000). Some such displays involve markings on the face and head, such as the status marks on white crowned sparrows and great tits (Whitfield, 1987), as well as the markings on the cheeks and chest of the zebra finch (Brazas & Shimizu, 2002). Symmetry is also an important feature for sexual selection, although little is known about preferences for facial symmetry in vertebrates (Swaddle & Cuthill, 1994; Thornhill & Moller, 1998; Waas & Wordsworth, 1999).
Development
Like humans, some non-mammalian vertebrates are born with innate predispositions to react to certain visual patterns. These predispositions can serve as a basis for attraction, or as a signal of danger that causes them to flee. An example of the latter is in jewel fish, who, as early as 13-days post-spawning, instinctively avoid pairs of black dots resembling eyes, even in the absence of previous experience with real eyes (Coss, 1978a). Juvenile and adult fish become highly sensitive to this cue and use it to guide their aggressive or avoidance behavior in the dominance hierarchy.
One aspect of avian visual development that resembles the development of face perception in primates is the innate preference to orient to a particular configural stimulus shortly after birth. In some birds, filial imprinting undergoes a similar temporal sequence to that seen in humans. For example, chicks are initially attracted to any stimulus with eyes and/or head and neck shape (Bolhuis & Honey, 1998). Then, following a relatively short period of experience, the preference becomes more narrowly focused on a single object or individual, a critical step in filial imprinting (Bolhuis & Honey, 1998). These two stages are thought to represent a transition from processing in the mesencephalon (e.g. optic tectum) to processing in the telencephalon (e.g. the mesopallium, formerly called hyperstriatum ventrale). Damage to the latter structure abolishes filial imprinting (Bolhuis & Honey, 1998; Johnson & Horn, 1987). Note that these two sequential mechanisms of orientation to conspecifics resembles those observed in human newborns and infants, and may represent a homologous ontogenic transition (Johnson, 2005).
Neural Specialization
To our knowledge, there are no reports of selective neural responses in the non-mammalian vertebrate brain to faces or other complex forms. It is presently difficult to assess whether this absence is because form-selective neurons do not exist, or simply because complex stimuli have not been systematically tested. Lesion studies in birds suggest that high-level specialization related to social aspects of vision may exist in the avian brain. In pigeons, damage to the entopallium (formerly called the ectostriatum) causes a deficit in complex form vision (Bessette & Hodos, 1989), including conspecific recognition, while damage to the Wulst does not. This dissociation is interesting, since the Wulst receives retinofugal input, and is thought to be homologous to mammalian primary visual cortex (Medina & Reiner, 2000; Pettigrew & Konishi, 1976), whereas the entopallium in birds and lizards receives tectofugal input, and may be homologous to mammalian extrastriate visual cortex (or possibly only to its input layers) (Krutzfeldt & Wild, 2005). Recent studies in the zebra finch further support the interpretation that the entopallium supports high-level vision (Watanabe, Maier, & Bischof, 2008).
Discussion: Vertebrates
Vision is abundantly used for social interplay among nonmammalian vertebrates, and particularly birds, although the role of the face is unclear and probably minor compared to mammals. The eyes appear to be important stimuli for all major verterbrate taxa, and the capacity of corvids to read human gaze is remarkable. Given that birds are so distantly related to primates and have such different brain structures, it seems likely that their gaze perception mechanisms evolved independently of those in primates. Nevertheless, it remains possible that while their common ancestors, who lived roughly 320 million years ago (Butler & Hodos, 2005), may not have had complex gaze following abilities, they had some sensitivity to eyes. Ancient circuits in the midbrain, diencepalon, and pallium mediating this sensitivity may have then evolved into more specialized face-processing circuitry in both primates and birds, and possibly many other vertebrates.
Invertebrates
For completeness, we also consider complex visual processing in invertebrates, asking whether there could be any connection to human face perception. Some invertebrates can learn and remember complex forms (Heisenberg, 1995) and vision is an important social sense for some invertebrate species, including butterflies (Vane-Wright & Boppre, 1993), spiders (Clark & Uetz, 1990) (see Figure 1F), and horseshoe crabs (Barlow, Ireland, & Kass, 1982). In spiders, eyes seem to be a salient feature for visually mediated social interaction (Harland & Jackson, 2002). While there is good evidence that octopi can recognize objects, conspecific recognition in cephalopods has not yet been tested rigorously (Boal, 2006). In cuttlefish, five experiments failed to show vision-based individual recognition (Boal, 1996), despite rich intraspecific interaction, visual displays, and mate-guarding.
Some invertebrates can recognize each other visually using “facial” cues (Detto, Backwell, Hemmi, & Zeil, 2006; Tibbetts, 2002; Van Der Velden, Zheng, Patullo, Macmillan, & Brembs, 2008). In one study wasps treated familiar conspecifics as strangers when artificial markings were added to their faces or abdomens, effectively changing their visual identity (Tibbetts, 2002). In another, crayfish remembered the appearance of their opponents' faces following a direct conflict (Van Der Velden et al., 2008). Bees can discriminate and remember human faces, even interpolating between studied views to recognize novel views of these faces (Dyer, 2005; Dyer & Vuong, 2008). These observations demonstrate that comparatively simple visual systems are able to perform complex discriminations to support individual recognition (but see Pascalis, 2006).
Finally, in an ironic evolutionary twist, the sensitivity of vertebrates to faces has been unknowingly exploited by some invertebrates (Figure 7). For example, some Lepidoptera possess eye-like patterning on their wings, which is thought to protect them against predator attacks (Stevens, 2005) (although they may also be used in intraspecific visual mate selection (Robertson & Monteiro, 2005)). A remarkable example of eye patterning is found in cuttlefish, who dynamically create high contrast eyespots on their body before fleeing from visually oriented teleost fish, but not from crabs and dogfish, which are chemosensory predators (Langridge, Broom, & Osorio, 2007). Thus while invertebrates themselves may be somewhat indifferent to the visual appearance of faces, they exploit the fact that their predators are not!
Figure 7.
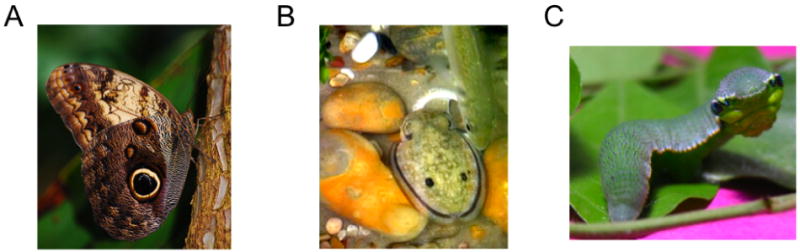
Examples of invertebrate species with eyespot markings. (a) Owl butterfly with a conspicuous eyespot on its wing [1]. (b) Eyespot displays on the dorsum of a cuttlefish appear according to what type of predator is approaching [2]. (c) Caterpillar of the Great Orange Tip butterfly has false eyespots and the facial appearance of a green vine snake [3].
[1] adapted from http://en.wikipedia.org/wiki/Caligo_memnon.
[2] adapted from Langridge KV et al (2007) Current Biology 17:R1044-R1045
[3] http://en.wikipedia.org/wiki/Hebomoia_glaucippe
Conclusions
This review has focused on the behavioral importance of faces in a wide range of animals. We first asked to what extent faces represent a special category of stimuli. We conclude that faces are important for a wide range of animals, as representative species from all vertebrate classes exhibit behavioral responses to faces that differ from those to non-face stimuli. In primates and sheep, for which face perception is highly developed and serves as the foundation of their social exchange, the brain contains neurons in multiple areas that are dedicated to the analysis of faces. Thus for these species, which are the only ones to have been systematically tested, the behavioral importance of faces appears to have fundamentally shaped processing in the visual pathway.
We next asked what types of visual information different animals extract from a face. At the most basic level, horizontally paired dark eyes elicit specific behavioral responses from a wide range of species, including primates, other mammals, birds, reptiles and fish. For visually oriented animals, the importance of eye detection may be understood in an evolutionary context, as it might indicate the approach of a competitor, predator, or potential mate, all of which have important implications for survival. In addition, some species of primates, sheep and birds were shown to extract more complex information from faces, such as species or individual identity, the direction of gaze, or the level of aggression, with primates showing the most sophisticated face-reading behavior. An intriguing possibility is that the shared perceptual basis reviewed here may be particularly suited to facilitate some forms of heterospecific interaction. This can include recognition of threatening species, which important for survival (Coss, Ramakrishnan, & Schank, 2005), as well as the playful or nurturing behavior sometimes observed between members of different species living together under unnatural conditions.
Finally, we asked how different aspects of face processing might have evolved. We can speculate that paired dark eyes served as the earliest “face” cue, providing visible markers of sighted conspecifics to all sighted creatures. This important cue may have led to the evolution of eye-selective neural responses in animals living hundreds of millions of years ago, before the first dinosaur, and perhaps even before the first vertebrate. Other important aspects of face perception, such as the detection of an open mouth or a fixed gaze involved in predator detection, may also have very ancient origins and may have further shaped the evolution of visual processing in the brain. We can speculate that recognition based on faces came later, and probably first pertained to the identification of the species rather than the individual. It is possible that visual species recognition in mammals emerged while they were predominantly nocturnal, aided by highly visible and stereotyped patterns on the face and head. After the extinction of the dinosaurs, diurnal mammals may have then capitalized on this pre-existing recognition system and adapted it to identify and remember individuals based on more subtle cues. For many primates, including humans, face perception takes center stage in social signaling, and the origin of such signaling may lie in the enhanced sensitivity to detail afforded by high visual acuity. In addition, the learning of subtle facial gestures and the capacity to discriminate highly similar faces require a wide network of brain areas whose division of labor is presently under intense investigation along with an extended period of experience-dependent development. The preeminence face recognition in primates over that of other animals, which is exemplified by the use configural cues to recognize unfamiliar faces, appears to hinge on neural encoding that is both systematic and adaptable. Through experiential learning, primates including humans optimize their own personal face interpreting machinery, as both psychophysical and physiological experiments have demonstrated that, over time, the brain automatically tunes itself the natural statistical variation of the faces it has experienced (for a review, see Rhodes & Leopold, 2010).
In choosing animal models for biological research, there is a trade-off between depth and breadth of study. Systems and behavioral neuroscience has opted for depth, and has invested monumental effort into a small number of animal models. While this approach has uncovered a wealth of information about face processing in the (typically rhesus macaque) monkey and human brain, it has not led to the proper biological or evolutionary contextualization of this information. Dobzhansky's admonition that nothing makes sense except in the light of evolution can be applied just as well to face perception as it can to any other biological feature (Dobzhansky, 1973). If not for Kendrick's investigation in sheep, there would be no evidence that non-primate brains were specialized for perceiving complex visual stimuli, let alone faces. In the future, exploration of face processing in other animals, such as prosimians, carnivores, diurnal rodents, and marsupials, would be of great value for interpreting the currently available neurophysiological data from the temporal cortex of primates and sheep. Similarly, discovering whether vertebrates such as birds and lizards have specialized neural machinery responding to eyes and other natural stimuli would be highly informative, and might lead primate neurophysiologists to discover other circuits, perhaps subcortical ones that are homologous to circuits in the human brain, that would otherwise remain hidden. Discovery is, by definition, a process that is impossible to predict. When the experiments are too narrowly focused on a single species, however, some forms of discovery are ruled out from the start.
Acknowledgments
The authors would like to thank Drs. Keith Kendrick, Sarah Brosnan, Chris Baker, and Asif Ghazanfar for comments on an earlier version of the manuscript, Drs. Evan Balaban and William Hodos for discussion, and two anonymous reviewers for many helpful suggestions. This work was supported by the Intramural Research Programs of the National Institute of Mental Health, National Eye Institute, and National Institute of Nervous Disorders and Stroke and an Australian Research Council Discovery Grant and Professorial Fellowship to G. Rhodes.
References
- Adachi I, Fujita K. Cross-modal representation of human caretakers in squirrel monkeys. Behavioural Processes. 2007;74(1):27–32. doi: 10.1016/j.beproc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Current Opinions in Neurobiology. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Allman JM. Evolution of the visual system in early primates. In: Sprague JM, Epstein AN, editors. Progress in Psychobiology and Physiological Psychology. Vol. 7. New York: Academic Press; 1977. pp. 1–53. [Google Scholar]
- Andino SL, Menendez RG, Khateb A, Landis T, Pegna AJ. Electrophysiological correlates of affective blindsight. Neuroimage. 2009;44(2):581–589. doi: 10.1016/j.neuroimage.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Andrew RJ. The display given by passerines in courtship and reproductive fighting: a review. Ibis. 1961;103a(3):315–348. [Google Scholar]
- Andrew RJ. Evolution of facial expression. Science. 1963;142:1034–1041. doi: 10.1126/science.142.3595.1034. [DOI] [PubMed] [Google Scholar]
- Arendes L. Superior colliculus activity related to attention and to connotative stimulus meaning. Cognitive Brain Research. 1994;2(1):65–69. doi: 10.1016/0926-6410(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Baerends GP, Baeronds-Van Roon JM. An introduction to the study of the ethology of the cichlid fishes. Behaviour. 1950 1:1–243. [Google Scholar]
- Barlow RB, Jr, Ireland LC, Kass L. Vision has a role in Limulus mating behaviour. Nature. 1982;296(5852):65–66. doi: 10.1038/296065a0. [DOI] [PubMed] [Google Scholar]
- Bekoff M. Social Communication in Canids: Evidence for the Evolution of a Stereotyped Mammalian Display. Science. 1977;197(4308):1097–1099. doi: 10.1126/science.197.4308.1097. [DOI] [PubMed] [Google Scholar]
- Bell AH, Hadj-Bouziane F, Frihauf JB, Tootell RB, Ungerleider LG. Object representations in the temporal cortex of monkeys and humans as revealed by functional magnetic resonance imaging. Journal of Neurophysiology. 2009;101(2):688–700. doi: 10.1152/jn.90657.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C, Herzog HA. Stimulus control of defensive behaviors in garter snakes (Thamnofis sirtalis): Effects of eye spots and movement. Journal of Comparative Psychology. 1994;108(4):353–357. [Google Scholar]
- Bessette BB, Hodos W. Intensity, color, and pattern discrimination deficits after lesions of the core and belt regions of the ectostriatum. Visual Neuroscience. 1989;2(1):27–34. doi: 10.1017/s0952523800004296. [DOI] [PubMed] [Google Scholar]
- Bird CD, Emery NJ. Using video playback to investigate the social preferences of rooks, Corvus frugilegus. Animal Behaviour. 2008;76(3):679–687. [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(Pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Boal JG. A review of simultaneous visual discrimination as a method of training octopuses. Biological Reviews of the Cambridge Philosophical Society. 1996;71(2):157–190. doi: 10.1111/j.1469-185x.1996.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Boal JG. Social recognition: a top down view of cephalopod behavior. Vie et Milieu. 2006;56(2):69–79. [Google Scholar]
- Bolhuis JJ, Honey RC. Imprinting, learning and development: from behaviour to brain and back. Trends in Neurosciences. 1998;21(7):306–311. doi: 10.1016/s0166-2236(98)01258-2. [DOI] [PubMed] [Google Scholar]
- Bouvier SE, Engel SA. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cerebral Cortex. 2006;16(2):183–191. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- Bovet D, Washburn D. Rhesus Macaques (Macaca mulatta) Categorize Unknown Conspecifics According to Their Dominance Relations. Journal of Comparative Psychology. 2003;117(4):400–405. doi: 10.1037/0735-7036.117.4.400. [DOI] [PubMed] [Google Scholar]
- Bradley B, Mundy N. The primate palette: The evolution of primate coloration. Evolutionary Anthropology. 2008;17(2):97–111. [Google Scholar]
- Brazas ML, Shimizu T. Significance of visual cues in choice behavior in the female zebra finch (Taeniopygia guttata castanotis) Animinal Cognition. 2002;5(2):91–95. doi: 10.1007/s10071-002-0136-9. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361(1476):2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Mimmack ML, Kendrick KM. Is right hemisphere specialization for face discrimination specific to humans? European Journal of Neuroscience. 2000;12(2):731–741. doi: 10.1046/j.1460-9568.2000.00934.x. [DOI] [PubMed] [Google Scholar]
- Brooks DR, McLennan DA. Phylogeny, ecology, and behavior: a research program in comparative biology. Chicago: The University of Chicago Press; 1991. [Google Scholar]
- Brown SD, Dooling RJ. Perception of conspecific faces by budgerigars (Melopsittacus undulatus): I. Natural faces. Journal of Comparative Psychology. 1992;106(3):203–216. doi: 10.1037/0735-7036.106.3.203. [DOI] [PubMed] [Google Scholar]
- Brown SD, Dooling RJ. Perception of conspecific faces by budgerigars (Melopsittacus undulatus): II. Synthetic models. Journal of Comparative Psychology. 1993;107(1):48–60. doi: 10.1037/0735-7036.107.1.48. [DOI] [PubMed] [Google Scholar]
- Bruce C, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. Journal of Neurophysiology. 1981;46(2):369–384. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young A. In the eye of the beholder: The science of face perception. New York, NY, USA: Oxford University Press; 1998. [Google Scholar]
- Bshary R, Wickler W, Fricke H. Fish cognition: a primate's eye view. Animal Cognition. 2002;5(1):1–13. doi: 10.1007/s10071-001-0116-5. [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Stowe M, Heinrich B. Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc Biol Sci. 2004;271(1546):1331–1336. doi: 10.1098/rspb.2004.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J. Antipredator behaviour of hatchling snakes: effects of incubation temperature and simulated predators. Animal Behaviour. 1998;56(3):547–553. doi: 10.1006/anbe.1998.0809. [DOI] [PubMed] [Google Scholar]
- Burghardt GM, Greene HW. Predator simulation and duration of death feigning in neonate hognose snakes. Animal Behaviour. 1988;36:1842–1844. [Google Scholar]
- Burrows A. The facial expression musculature in primates and its evolutionary significance. Bioessays. 2008;30(3) doi: 10.1002/bies.20719. [DOI] [PubMed] [Google Scholar]
- Burrows AM, Waller BM, Parr LA, Bonar CJ. Muscles of facial expression in the chimpanzee (Pan troglodytes): descriptive, comparative and phylogenetic contexts 2. Journal of Anatomy. 2006;208(2):153–167. doi: 10.1111/j.1469-7580.2006.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell IW, Sai F, Mullin JT. Neonatal recognition of the mother's face. British Journal of Developmental Psychology. 1989;7(1):3–15. [Google Scholar]
- Butler AB, Hodos W. Comparative vertebrate neuroanatomy. Hoboken, NJ: John Wiley and Sons; 2005. [Google Scholar]
- Butterworth G, Jarrett N. What minds have in common in space: spatial mechanisms serving joint visual attention in infancy. British Journal of Developmental Psychology. 1991;9(1):55–72. [Google Scholar]
- Calder A, Young A. Understanding the recognition of facial identity and facial expression. Nature Reviews Neuroscience. 2005;6(8):641–651. doi: 10.1038/nrn1724. [DOI] [PubMed] [Google Scholar]
- Carey S. Becoming a face expert. Philosophical Transactions of the Royal Society B: Biological Sciences. 1992;335:95–103. doi: 10.1098/rstb.1992.0012. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamond R, Woods B. Development of face recognition: A maturational component? Developmental Psychology. 1980;16(4):269. [Google Scholar]
- Caro T. Antipredator defenses in birds and mammals. Chicago: University of Chicago Press; 2005. [Google Scholar]
- Carpenter CC. Communication and displays in snakes. American Zoologist. 1977;17:217–223. [Google Scholar]
- Carter J, Lyons NJ, Cole HL, Goldsmith AR. Subtle cues of predation risk: starlings respond to a predator's direction of eye-gaze. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1644):1709–1715. doi: 10.1098/rspb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changizi MA, Zhang Q, Shimojo S. Bare skin, blood and the evolution of primate colour vision. Biological Letters. 2006;2(2):217–221. doi: 10.1098/rsbl.2006.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Uetz GW. Video image recognition by the jumping spider, Maevia inclemens (Araneae: Salticidae) Animal Behaviour. 1990;40:884–890. [Google Scholar]
- Cook M, Mineka S. Observational conditioning of fear to fear-relevant versus fear-irrelevant stimuli in rhesus monkeys. Journal of Abnormal Psychology. 1989;98(4):448–459. doi: 10.1037//0021-843x.98.4.448. [DOI] [PubMed] [Google Scholar]
- Cornell HN, Marzluff JM, Peccorro S. Individual and social learning enable american crows to discriminate dangerous from safe humans. Animal Behaviour. 2009 in press. [Google Scholar]
- Coss RG. Development of face aversion by the jewel fish (Hemichromis bimaculatus, Gill 1862) Zeitschrift für Tierpsychologie. 1978a;48:28–46. [Google Scholar]
- Coss RG. Perceptual determinants of gaze aversion by normal and psychotic children: the role of two facing eyes. Behaviour. 1978b;LXIX(3-4):228–254. doi: 10.1163/156853979x00494. [DOI] [PubMed] [Google Scholar]
- Coss RG. Perceptual determinants of gaze aversion by the lesser mouse lemur (Microcebus murinus), the role of two facing eyes. Behaviour. 1978c;LXIV(3-4):248–270. doi: 10.1163/156853979x00494. [DOI] [PubMed] [Google Scholar]
- Coss RG. Delayed plasticity of an instinct: recognition and avoidance of 2 facing eyes by the jewel fish. Developmental Psychobiolology. 1979;12(4):335–345. doi: 10.1002/dev.420120408. [DOI] [PubMed] [Google Scholar]
- Coss RG, Goldthwaite RO. The persistence of old designs for perception. In: Thompson NS, editor. Perspectives in Ethology 11: Behavioural Design. New York: Plenum Press; 1995. [Google Scholar]
- Coss RG, Marks S, Ramakrishnan U. Early environment shapes the development of gaze aversion by wild bonnet macaques (Macaca radiata) Primates. 2002;43(3):217–222. doi: 10.1007/BF02629649. [DOI] [PubMed] [Google Scholar]
- Coss RG, Ramakrishnan U, Schank J. Recognition of partialy concealed leopards by wild bonnet macaques (Macaca radiata). The role of the spotted coat. Behavioural Processes. 2005;68:145–163. doi: 10.1016/j.beproc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Coulon M, Deputte B, Heyman Y, Baudoin C. Individual recognition in domestic cattle (Bos taurus): evidence from 2D-images of heads from different breeds. PLoS One. 2009;4(2):e4441. doi: 10.1371/journal.pone.0004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon M, Deputte BL, Heyman Y, Delatouce L, Richard C, Baudoin C. Visual discrimination by heifers (Bos taurus) of their own species. Journal of Comparative Psychology. 2007;121(2):198–204. doi: 10.1037/0735-7036.121.2.198. [DOI] [PubMed] [Google Scholar]
- Crookes K, McKone E. Early maturity of face recognition: no childhood development of holistic processing, novel face encoding, or face-space. Cognition. 2009;111(2):219–247. doi: 10.1016/j.cognition.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Curio E. The functional organization of anti-predator behaviour in the pied flycatcher: a study of avian visual perception. Animal Behaviour. 1975;23:1–115s. doi: 10.1016/0003-3472(75)90056-1. [DOI] [PubMed] [Google Scholar]
- D'Eath RB, Stone RJ. Chickens use visual cues in social discrimination: an experiment with coloured lighting. Applied Animal Behavioral Science. 1999;62:233–242. [Google Scholar]
- Da Costa A, Leigh A, Man M, Kendrick K. Face pictures reduce behavioural, autonomic, endocrine and neural indices of stress and fear in sheep. Proceedings of the Royal Society B: Biological Sciences. 2004;271(1552):2077–2084. doi: 10.1098/rspb.2004.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J, Lank DB, Reeve HK. Signaling individual identity versus quality: a model and case studeis with ruffs, queleas, and house finches. The American Naturalist. 2001;158(1):75–86. doi: 10.1086/320861. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Van Hoesen GW. Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology. 1982;32(4):331–341. doi: 10.1212/wnl.32.4.331. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of emotions in man and animals. Chicago and London: The University of Chicago Press; 1872. [Google Scholar]
- Davies WG. Cluster analysis applied to the classification of postures in the Chilean flamingo (Phoenicopterus chilensis) Animal Behaviour. 1978;26:381–388. [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Current Biology. 2005;15(6):543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Platt ML. Reflexive social attention in monkeys and humans. Current Biology. 2003;13(18):1609–1613. doi: 10.1016/j.cub.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Delfour F, Marten K. Mirror image processing in three marine mammal species: killer whales (Orcinus orca), false killer whales (Pseudorca crassidens) and California sea lions (Zalophus californianus) 2. Behavioural Processes. 2001;53(3):181–190. doi: 10.1016/s0376-6357(01)00134-6. [DOI] [PubMed] [Google Scholar]
- Demaria C, Thierry B. Responses to animal stimulus photographs in stumptailed macaques (Macaca arctoides) Primates. 1988;29:237–244. [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. Journal of Neuroscience. 1984;4(8):2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detto T, Backwell PR, Hemmi JM, Zeil J. Visually mediated species and neighbour recognition in fiddler crabs (Uca mjoebergi and Uca capricornis) Proceedings of the Royal Soceity B: Biological Sciences. 2006;273(1594):1661–1666. doi: 10.1098/rspb.2006.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: An effect of expertise. Journal of Experimental Psychology: General. 1986;115(2):107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Dittrich WH. Rerpresentation of faces in longtailed macaques (Macaca fascicularis) Ethology. 1990;85:265–278. [Google Scholar]
- Dobson S. Allometry of facial mobility in anthropoid primates: Implications for the evolution of facial expression. American Journal Physical Anthropology. 2009;138(1):70–81. doi: 10.1002/ajpa.20902. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Nothing in biology makes sense except in the light of evolution. The American Biology Teacher. 1973;35:125–129. [Google Scholar]
- Dufour V, Pascalis O, Petit O. Face processing limitation to own species in primates: a comparative study in brown capuchins, Tonkean macaques and humans 18. Behavioural Processes. 2006;73(1):107–113. doi: 10.1016/j.beproc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Dyer A. Honeybee (Apis mellifera) vision can discriminate between and recognise images of human faces. Journal of Experimental Biology. 2005;208(24):4709–4714. doi: 10.1242/jeb.01929. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Vuong QC. Insect brains use image interpolation mechanisms to recognise rotated objects. PLoS ONE. 2008;3(12):e4086. doi: 10.1371/journal.pone.0004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Sorenson ER, Friesen WV. Pan-cultural elements in facial displays of emotion 19. Science. 1969;164(875):86–88. doi: 10.1126/science.164.3875.86. [DOI] [PubMed] [Google Scholar]
- Emery N. The eyes have it: the neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24(6):581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Emery N, Clayton N. Comparative Social Cognition. Annual Review Psycholology. 2009;60:87–113. doi: 10.1146/annurev.psych.60.110707.163526. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI. Gaze following and joint attention in rhesus monkeys (Macaca mulatta) Journal of Comparative Psychology. 1997;111(3):286–293. doi: 10.1037/0735-7036.111.3.286. [DOI] [PubMed] [Google Scholar]
- Engell AD, Haxby JV. Facial expression and gaze-direction in human superior temporal sulcus. Neuropsychologia. 2007;45(14):3234–3241. doi: 10.1016/j.neuropsychologia.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17(10):2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychological Review. 1998;105(3):482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Kohler E, Fogassi L, Gallese V. The ability to follow eye gaze and its emergence during development in macaque monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13997–14002. doi: 10.1073/pnas.250241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MW. The anatomy of aggression and its ritualization in Canidae: A developmental and comparative study. Behaviour. 1969;35:242–258. [Google Scholar]
- Fox MW. A comparative study of facial expressions in canids; Wolf, coyote, and foxes. Behaviour. 1970;36(1-2):177–191. [Google Scholar]
- Fujita K. Species recogntion by five macaque monkeys. Primates. 1987;28(3):353–366. [Google Scholar]
- Fujita K. Development of visual preference for closely related species by infant and juvenile macaques with restricted social experience. Primates. 1993;34(2):141–150. [Google Scholar]
- Gacsi M, Varga O, TopaL J, Csanyi V. Are readers of our face readers of our minds? Dogs (Canis familiaris) show situation-dependent recognition of human?s attention. Animal Cognition. 2004;7(3):1–10. doi: 10.1007/s10071-003-0205-8. [DOI] [PubMed] [Google Scholar]
- Gallup GG. Chimpanzees: self-recognition. Science. 1970;167(3914):86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- Gallup GG., Jr Absence of self-recognition in a monkey (Macaca fascicularis) following prolonged exposure to a mirror 38. Developmental Psychobiology. 1977;10(3):281–284. doi: 10.1002/dev.420100312. [DOI] [PubMed] [Google Scholar]
- Gallup J. Mirrors, minds, and cetaceans. Consciousness and Cognition. 1995;4(2):226–228. doi: 10.1006/ccog.1995.1028. [DOI] [PubMed] [Google Scholar]
- Gaspar A. Universals and individuality in facial behavior—past and future of an evolutionary perspective. Acta Ethologica. 2006;9(1):1–14. [Google Scholar]
- Gerald M, Waitt C, Little AC. Pregnancy coloration in macaques may act as a warning signal to reduce antagonism by conspecifics. Behavioural Processes. 2009;80(1):7–11. doi: 10.1016/j.beproc.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Logothetis NK. Neuroperception: facial expressions linked to monkey calls. Nature. 2003;423(6943):937–938. doi: 10.1038/423937a. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Nielsen K, Logothetis NK. Eye movements of monkey observers viewing vocalizing conspecifics 4. Cognition. 2006;101(3):515–529. doi: 10.1016/j.cognition.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Goren CC, Sarty M, Wu PY. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56(4):544–549. [PubMed] [Google Scholar]
- Gothard K, Battaglia F, Erickson C, Spitler K, Amaral D. Neural responses to facial expression and face identity in the monkey amygdala. Journal of Neurophysiology. 2007;97(2):1671. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Gothard K, Brooks K, Peterson M. Multiple perceptual strategies used by macaque monkeys for face recognition. Animal Cognition. 2009;12(1):155–167. doi: 10.1007/s10071-008-0179-7. [DOI] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445(7126):429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Gross CG. Single neuron studies of inferior temporal cortex. Neuropsychologia. 2008;46(3):841–852. doi: 10.1016/j.neuropsychologia.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Gross CG, Rocha-Miranda CE, Bender DB. Visual properties of neurons in inferotemporal cortex of the Macaque. Journal of Neurophysiology. 1972;35(1):96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- Guo K, Robertson RG, Mahmoodi S, Tadmor Y, Young MP. How do monkeys view faces?--A study of eye movements. Experimental Brain Research. 2003;150(3):363–374. doi: 10.1007/s00221-003-1429-1. [DOI] [PubMed] [Google Scholar]
- Hadidian J. Yawning in an Old World monkey, Macaca nigra (Primates: cercopithecidae) Behaviour. 1980;75(3/4):133–147. [Google Scholar]
- Hampson RE, Pons TP, Stanford TR, Deadwyler SA. Categorization in the monkey hippocampus: a possible mechanism for encoding information into memory. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3184–3189. doi: 10.1073/pnas.0400162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR. Sensitivity to information specifying the line of gaze of humans in sparrows (Passer domesticus) Behaviour. 1994;130(1-2):41–51. [Google Scholar]
- Hare B. The Domestication of Social Cognition in Dogs. Science. 2002;298(5598):1634–1636. doi: 10.1126/science.1072702. [DOI] [PubMed] [Google Scholar]
- Hare B, Tomasello M. Human-like social skills in dogs? Trends i Cognitive Sciences. 2005;9(9):439–444. doi: 10.1016/j.tics.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Harland DP, Jackson RR. Influence of cues from the anterior medial eyes of virtual prey on Portia fimbriata, an araneophagic jumping spider. Journal of Experimental Biology. 2002;205(Pt 13):1861–1868. doi: 10.1242/jeb.205.13.1861. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Rolls ET, Baylis GC. The role of expression and identity in the face-selective responses of neurons in the temporal visual cortex of the monkey. Behavioural Brain Research. 1989;32(3):203–218. doi: 10.1016/s0166-4328(89)80054-3. [DOI] [PubMed] [Google Scholar]
- Hauser MD. Right hemisphere dominance for the production of facial expression in monkeys 46. Science. 1993;261(5120):475–477. doi: 10.1126/science.8332914. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Miller CT, Liu K, Gupta R. Cotton-top tamarins (Saguinus oedipus) fail to show mirror-guided self-exploration 25. American Journal of Primatology. 2001;53(3):131–137. doi: 10.1002/1098-2345(200103)53:3<131::AID-AJP4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Pattern recognition in insects. Current Opinions in Neurobiology. 1995;5(4):475–481. doi: 10.1016/0959-4388(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Hennig CW. Effects of simulated predation on tonic immobility in Anolis carolinensis: The role of eye contact. Bulletin of the Psychonomic Society. 1977;9:239–242. [Google Scholar]
- Henry JD, Herrero SM. Social play and the american black bear: its similarity to canid social play and an examination of its identifying characteristics. American Zoologist. 1974;14(1):371–389. [Google Scholar]
- Hess EH. Imprinting. Science. 1959;130(3377):733. doi: 10.1126/science.130.3377.733. [DOI] [PubMed] [Google Scholar]
- Hödl W, Amezquita A, Ryan MJ. Visual signaling in anuran amphibians. In: Ryan MJ, editor. Anuran Communication. Washington, DC: Smithsonian Institute Press; 2001. pp. 121–141. [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3(1):80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Current Biology. 2007;17(9):766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Huber E. Evolution of facial musculature and facial expresasion. London: Oxford University Press; 1931. [Google Scholar]
- Humphreys GW, Donnelly N, Riddoch MJ. Expression is computed separately from facial identity, and it is computed separately for moving and static faces: neuropsychological evidence. Neuropsychologia. 1993;31(2):173–181. doi: 10.1016/0028-3932(93)90045-2. [DOI] [PubMed] [Google Scholar]
- Hunt S, Kilner RM, Langmore NE, Bennett AT. Conspicuous, ultraviolet-rich mouth colours in begging chicks. Proceedings of the Royal Society B: Biological Sciences (Supplement) 2003;1:S25–S28. doi: 10.1098/rsbl.2003.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell L. The fruit, the tree, and the serpent: why we see so well. Cambridge, Massachussetts: Harvard University Press; 2009. [Google Scholar]
- Janecka JE, Miller W, Pringle TH, Wiens F, Zitzmann A, Helgen KM, et al. Molecular and genomic data identify the closest living relative of primates. Science. 2007;318(5851):792–794. doi: 10.1126/science.1147555. [DOI] [PubMed] [Google Scholar]
- Jellema T, Perrett DI. Cells in monkey STS responsive to articulated body motions and consequent static posture: a case of implied motion? Neuropsychologia. 2003;41(13):1728–1737. doi: 10.1016/s0028-3932(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Jennsen TA. Evolution of anoline lizard display behavior. American Zoologist. 1977;17:203–215. [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6(10):766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns' preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40(1-2):1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, de HM, et al. The emergence of the social brain network: evidence from typical and atypical development. Developmental Psychopathology. 2005;17(3):599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Horn G. The role of a restricted region of the chick forebrain in the recognition of individual conspecifics. Behavioural Brain Research. 1987;23:269–275. doi: 10.1016/0166-4328(87)90027-1. [DOI] [PubMed] [Google Scholar]
- Kaminski J, Riedel J, Call J, Tomasello M. Domestic goats, Capra hircus, follow gaze direction and use social cues in an object choice task. Animal Behaviour. 2005;69:11–18. [Google Scholar]
- Kanazawa S. Recognition of facial expressions in a Japanese monkey. Primates. 1996;37:25–38. [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nature Neuroscience. 2000;3(8):759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361(1476):2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating CF, Keating EG. Visual scan paterns of rhesus monkeys viewing faces. Perception. 1982;11:211–219. doi: 10.1068/p110211. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: evidence of perceptual narrowing. Psychological Science. 2007;18(12):1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM. How the sheep's brain controls the visual recognition of animals and humans. Journal of Animal Science. 1991;69(12):5008–5016. doi: 10.2527/1991.69125008x. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Neurobiological correlates of visual and olfactory recognition in sheep. Behavioural Processes. 1994;33:89–112. doi: 10.1016/0376-6357(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Sheep senses, social cognition and capacity for consciousness. The Welfare of Sheep. 2008;6:135–157. [Google Scholar]
- Kendrick KM, Atkins K, Hinton M, Broad KD, Fabre-Nys C, Keverne B. Facial and vocal discrimination in sheep. Animal Behaviour. 1995;49:1665–1676. [Google Scholar]
- Kendrick KM, Baldwin BA. Cells in temporal cortex of conscious sheep can respond preferentially to the sight of faces 57. Science. 1987;236(4800):448–450. doi: 10.1126/science.3563521. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, da Costa AP, Leigh AE, Hinton MR, Peirce JW. Sheep don't forget a face. Nature. 2001;414(6860):165–166. doi: 10.1038/35102669. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Hinton MR, Atkins K, Haupt MA, Skinner JD. Mothers determine sexual preferences 19. Nature. 1998;395(6699):229–230. doi: 10.1038/26129. [DOI] [PubMed] [Google Scholar]
- Kilner R, Davies NB. Nestling mouth colour: ecological correlates of a begging signal. Animal Behaviour. 1998;56(3):705–712. doi: 10.1006/anbe.1998.0785. [DOI] [PubMed] [Google Scholar]
- Kiltie RA. Scaling of visual acuity with body size in mammals and birds. Functional Ecology. 2000;14(2):226–234. [Google Scholar]
- Kobayashi H, Kohshima S. Unique morphology of the human eye. Nature. 1997;387(6635):767–768. doi: 10.1038/42842. [DOI] [PubMed] [Google Scholar]
- Koeslag JH. Koinophilia groups sexual creatures into species, promotes stasis, and stabilizes social behaviour. Journal of Theoretical Biology. 1990;144(1):15–35. doi: 10.1016/s0022-5193(05)80297-8. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt NO, Wild JM. Definition and novel connections of the entopallium in the pigeon (Columba livia) Journal of Comparative Neurology. 2005;490(1):40–56. doi: 10.1002/cne.20627. [DOI] [PubMed] [Google Scholar]
- Kuwahata H, Adachi I, Fujita K, Tomonaga M, Matsuzawa T. Development of schematic face preference in macaque monkeys. Behavioural Processes. 2004;66(1):17–21. doi: 10.1016/j.beproc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kyes RC, Candland DK. Baboon (Papio hamadryas) visual preferences for regions of the face. Journal of Comp Psychology. 1987;101(4):345–348. [PubMed] [Google Scholar]
- Langbauer W. Elephant communication. Zoo Biology. 2000;19(5):425–445. [Google Scholar]
- Langridge KV, Broom M, Osorio D. Selective signalling by cuttlefish to predators. Current Biology. 2007;17(24):R1044–R1045. doi: 10.1016/j.cub.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Leary MR, Britt TW, Cutlip WD, Templeton JL. Social blushing. Psychological Bulletin. 1992;112(3):446–460. doi: 10.1037/0033-2909.112.3.446. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Rolls ET, Wilson FA, Baylis GC. Neurons in the amygdala of the monkey with responses selective for faces. Behavioural Brain Research. 1985;15(2):159–176. doi: 10.1016/0166-4328(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Bondar I. Adaptation to complex visual patterns in humans and monkeys. In: Clifford CW, Rhodes G, editors. Fitting the mind to the world: adaptation and aftereffects in high-level vision. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
- Leopold DA, Bondar IV, Giese MA. Norm-based face encoding by single neurons in the monkey inferotemporal cortex. Nature. 2006;442(7102):572–575. doi: 10.1038/nature04951. [DOI] [PubMed] [Google Scholar]
- Levey DJ, Londono GA, Ungvari-Martin J, Hiersoux MR, Jankowski JE, Poulsen JR, et al. Urban mockingbirds quickly learn to identify individual humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(22):8959–62. doi: 10.1073/pnas.0811422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler G, Yourganov G, Wilkinson F, Wilson H. fMRI evidence for the neural representation of faces. Nature Neuroscience. 2005;8(10):1386–1391. doi: 10.1038/nn1538. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nature Neuroscience. 1999;2(6):555–562. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- Lorenz K. On Aggression. New York: Harcourt Brace Jovanovich; 1966. [Google Scholar]
- Lorincz EN, Baker CI, Perrett DI. Visual cues for attention following in rhesus monkeys. In: Fagot J, editor. Picture Perception in Animals. East Sussex, UK: Psychology Press; 1999. [Google Scholar]
- Maestripieri D, Wallen K. Affiliative and submissive communication in rhesus macaques. Primates. 1997;38(2):127–138. [Google Scholar]
- Malpass RS, Kravitz J. Recognition for faces of own and other race. Journal of Personality and Socical Psychology. 1969;13(4):330–334. doi: 10.1037/h0028434. [DOI] [PubMed] [Google Scholar]
- Manning JT, Chamberlain AT. Fluctuating asymmetry, sexual selection and canine teeth in primates. Proceedings of the Royal Society B: Biological Sciences. 1993;251(1331):83–87. doi: 10.1098/rspb.1993.0012. [DOI] [PubMed] [Google Scholar]
- Marino L, Connor R, Fordyce R, Herman L, Hof P, Lefebvre L, et al. Cetaceans Have Complex Brains for Complex Cognition. PLoS Biology. 2007;5(5):e139. doi: 10.1371/journal.pbio.0050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten K, Psarakos S. Using self-view television to distinguish between self-examination and social behavior in the bottlenose dolphin (Tursiops truncatus) Consciousness and Cognition. 1995;4(2):205–224. doi: 10.1006/ccog.1995.1026. [DOI] [PubMed] [Google Scholar]
- Martin-Malivel J, Okada K. Human and chimpanzee face recognition in chimpanzees (Pan troglodytes): Role of exposure and impact on categorical perception. Behavioral Neuroscience. 2007;121(6):1145–1155. doi: 10.1037/0735-7044.121.6.1145. [DOI] [PubMed] [Google Scholar]
- McKone E, Kanwisher N, Duchaine BC. Can generic expertise explain special processing for faces? Trends Cognitive Sciences. 2007;11(1):8–15. doi: 10.1016/j.tics.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Medina L, Reiner A. Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends in Neurosciences. 2000;23(1):1–12. doi: 10.1016/s0166-2236(99)01486-1. [DOI] [PubMed] [Google Scholar]
- Meissner C, Brigham J. Thirty years of investigating the own-race bias in memory for faces: A meta-analytic review. Psychology, Public Policy, and Law. 2001;7(1):3–35. [Google Scholar]
- Meltzoff AN, Moore MK. Newborn infants imitate adult facial gestures. Child Development. 1983;54(3):702–709. [PubMed] [Google Scholar]
- Miklosi A. Dog: behavior, evolution, and cognition. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- Moeller S, Freiwald W, Tsao D. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science. 2008;320(5881):1355. doi: 10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondloch CJ, Geldart S, Maurer D, Le Grand R. Developmental changes in face processing skills. Journal of Experimental Child Psychology. 2003;86(1):67–84. doi: 10.1016/s0022-0965(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Le Grand R, Maurer D. Configural face processing develops more slowly than featural face processing. Perception. 2002;31(5):553–566. doi: 10.1068/p3339. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(4):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myowa-Yamakoshi M, Yamaguchi MK, Tomonaga M, Tanaka M, Matsuzawa T. Development of face recognition in infant chimpanzees (Pan troglodytes) Cognitive Development. 2005;20:49–63. [Google Scholar]
- Nakamura T, Croft DB, Westbrook RF. Domestic pigeons (Columba livia) discriminate between photographs of individual pigeons. Learning and Behavior. 2003;31(4):307–317. doi: 10.3758/bf03195993. [DOI] [PubMed] [Google Scholar]
- Nash LT. Moonlight and behavior in nocturnal and cathemeral primates, especially Lepilemur leucopus: illuminating possible anti-predator efforts. In: Gursky S, Nekaris KAI, editors. Primate antipredator strategies. New York: Springer; 2007. [Google Scholar]
- Nissani M, Hoefler-Nissani D. Absence of Mirror Self-Referential Behavior in Two Asian Elephants. scientificjournals.org 2007 [Google Scholar]
- Noden D, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Developmental Dynamics. 2006;235(5):1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Okamoto-Barth S, Call J, Tomasello M. Great apes' understanding of other individuals' line of sight. Psychological Science. 2007;18(5):462–468. doi: 10.1111/j.1467-9280.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- Olsson M. Rival recognition affects male contest behavior in sand lizards (Lacerta agilis) Behavioral Ecology and Sociobiology. 1994;35(4):249–252. [Google Scholar]
- Ord T, Evans C. Interactive video playback and opponent assessment in lizards. Behavioural Processes. 2002;59(2):55. doi: 10.1016/s0376-6357(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Ortolani A. Spots, stripes, tail tips and dark eyes: predicting the function of carnivore colour patterns using the comparative method. Biological Journal of the Linnean Society. 1999;67:433–476. [Google Scholar]
- Pack A, Herman L. Dolphin social cognition and joint attention: Our current understanding. Aquatic Mammals. 2006;32(4):443. [Google Scholar]
- Pack A, Herman L. The dolphin's (Tursiops truncatus) understanding of human gazing and pointing: Knowing what and where. Journal of Comparative Psychology. 2007;121(1):34–45. doi: 10.1037/0735-7036.121.1.34. [DOI] [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45(1):75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Parr L. The Discrimination of Faces and Their Emotional Content by Chimpanzees (Pan troglodytes) Annals of the New York Academy of Sciences. 2003;1000(1):56–78. doi: 10.1196/annals.1280.005. [DOI] [PubMed] [Google Scholar]
- Parr L. Perceptual biases for multimodal cues in chimpanzee (Pan troglodytes) affect recognition. Animal Cognition. 2004;7(3):1–8. doi: 10.1007/s10071-004-0207-1. [DOI] [PubMed] [Google Scholar]
- Parr L, Heintz M, Pradhan G. Rhesus monkeys (Macaca mulatta) lack expertise in face processing. Journal of Comparative Psychology. 2008;122(4):390–402. doi: 10.1037/0735-7036.122.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, de Waal FB. Visual kin recognition in chimpanzees. Nature. 1999;399(6737):647–648. doi: 10.1038/21345. [DOI] [PubMed] [Google Scholar]
- Parr LA, Dove T, Hopkins WD. Why faces may be special: evidence of the inversion effect in chimpanzees. Journal of Cognitive Neuroscience. 1998;10(5):615–622. doi: 10.1162/089892998563013. [DOI] [PubMed] [Google Scholar]
- Parr LA, Heintz M, Akamagwuna U. Three studies on configural face processing by chimpanzees 21. Brain and Cognition. 2006;62(1):30–42. doi: 10.1016/j.bandc.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Hopkins WD, de Waal FB. The perception of facial expressions in chimpanzees (Pan troglodytes) Evolution of Communication. 1998;2:1–23. [Google Scholar]
- Parr LA, Waller BM, Fugate J. Emotional communication in primates: implications for neurobiology 4. Current Opinions in Neurobiology. 2005;15(6):716–720. doi: 10.1016/j.conb.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Hopkins WD. Is the inversion effect in rhesus monkeys face-specific? Animal Cognition. 1999;2:123–129. [Google Scholar]
- Parr LA, Winslow JT, Hopkins WD, de Waal FB. Recognizing facial cues: individual discrimination by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta) Journal of Comparative Psychology. 2000;114(1):47–60. doi: 10.1037/0735-7036.114.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parron C, Fagot J. Baboons (Papio papio) spontaneously process the first-order but not second-order configural properties of faces. American Journal of Primatology. 2007;70(5):415–422. doi: 10.1002/ajp.20503. [DOI] [PubMed] [Google Scholar]
- Pascalis O. What can bees really tell us about the face processing system in humans? Journal of Experimental Biology. 2006;209(16):3266–3266. doi: 10.1242/jeb.02411. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Bachevalier J. Face recognition in primates: a cross-species study. Behavioural Processes. 1998;43:87–96. doi: 10.1016/s0376-6357(97)00090-9. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is Face Processing Species-Specific During the First Year of Life? Science. 2002;296(5571):1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Schonen S, Morton J, Deruelle C, Fabre-Grenet M. Mother's face recognition by neonates: a replication and an extension. Infant Behavior and Development. 1995;18:79–85. [Google Scholar]
- Pascalis O, Kelly DJ. The origins of face processing in humans: phylogeny and ontogeny. Perspectives on Psychological Science. 2009;4(2):200–209. doi: 10.1111/j.1745-6924.2009.01119.x. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, et al. Plasticity of face processing in infancy. Proceedings of the National Academy of Science of the United States of America. 2005;102(14):5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW, Kendrick KM. Functional asymmetry in sheep temporal cortex. Neuroreport. 2002;13(18):2395–2399. doi: 10.1097/00001756-200212200-00004. [DOI] [PubMed] [Google Scholar]
- Peirce JW, Leigh A, Kendrick K. Configurational coding, familiarity and the right hemisphere advantage for face recognition in sheep. Neuropsychologia. 2000;38(4):475–483. doi: 10.1016/s0028-3932(99)00088-3. [DOI] [PubMed] [Google Scholar]
- Peirce JW, Leigh AE, daCosta AP, Kendrick KM. Human face recognition in sheep: lack of configurational coding and right hemisphere advantage. Behavioural Processes. 2001;55(1):13–26. doi: 10.1016/s0376-6357(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Vander Wyk BC. Functional and neural mechanisms for eye gaze processing. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. Handbook of Face Perception. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Experimental Brain Research. 1982;47(3):329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Potter DD, Mistlin AJ, Head AS, Milner AD, et al. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proceedings of the Royal Society B: Biological Sciences. 1985;223(1232):293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider L. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Progress in Brain Research. 2004;144:171–82. doi: 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- Peterson MA, Rhodes G. Perception of faces, objects, and scenes: analytic and holistic processes. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- Pettigrew JD, Konishi M. Neurons selective for orientation and binocular disparity in the visual Wulst of the barn owl (Tyto alba) Science. 1976;193(4254):675–678. doi: 10.1126/science.948741. [DOI] [PubMed] [Google Scholar]
- Phelps MT, Roberts WA. Memory for pictures of upright and inverted primate faces in humans (Homo sapiens), squirrel monkeys (Saimiri sciureus), and pigeons (Columba livia) Journal of Comparative Psychology. 1994;108(2):114–125. doi: 10.1037/0735-7036.108.2.114. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, Gross CG, et al. Neural Representations of Faces and Body Parts in Macaque and Human Cortex: A Comparative fMRI Study. Journal of Neurophysiology. 2009;101(5):2581–600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsk MA, DeSimone K, Moore T, Gross CG, Kastner S. Representations of faces and body parts in macaque temporal cortex: a functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(19):6996–7001. doi: 10.1073/pnas.0502605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnik JM, de Waal FB, Reiss D. Self-recognition in an Asian elephant. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):17053–17057. doi: 10.1073/pnas.0608062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povinelli DJ, Rulf AB, Landau KR, Bierschwale DT. Self-recognition in chimpanzees (Pan troglodytes): distribution, ontogeny, and patterns of emergence. Journal of Comparative Psychology. 1993;107(4):347–372. doi: 10.1037/0735-7036.107.4.347. [DOI] [PubMed] [Google Scholar]
- Preuschoft S, van Hooff JA. Homologizing primate facial displays: a critical review of methods. Folia Primatologica. 1995;65(3):121–137. doi: 10.1159/000156878. [DOI] [PubMed] [Google Scholar]
- Prior H, Schwarz A, Gunturkun O. Mirror-induced behavior in the magpie (Pica pica): evidence of self-recognition. PLoS Biology. 2008;6(8):e202. doi: 10.1371/journal.pbio.0060202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett-Jones SG, Pruett-Jones MA. Sexual selection through female choice in Lawes' Parotia, a lek-mating bird of paradise. Evolution. 1990;44(3):486–501. doi: 10.1111/j.1558-5646.1990.tb05934.x. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435(7045):1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Reiss D. Mirror self-recognition in the bottlenose dolphin: A case of cognitive convergence. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(10):5937–5942. doi: 10.1073/pnas.101086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G. The Evolutionary Psychology of Facial Beauty. Annual Review of Psychology. 2006;57(1):199–226. doi: 10.1146/annurev.psych.57.102904.190208. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Leopold DA. Adaptive norm-based coding of face identity. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. Handbook of Face Perception. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- Rhodes G, Simmons L. Symmetry, attractiveness, and sexual selection. In: Dunbar R, Barrett L, editors. Oxford handbook of evolutionary psychology. New York: Oxford University Press; 2007. [Google Scholar]
- Robertson KA, Monteiro A. Female Bicyclus anynana butterflies choose males on the basis of their dorsal UV-reflective eyespot pupils. Proceedings of the Royal Soceity B: Biological Sciences. 2005;272(1572):1541–1546. doi: 10.1098/rspb.2005.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning AS, Inoue K. Face-selective and auditory neurons in the primate orbitofrontal cortex. Experimental Brain Research. 2006;170(1):74–87. doi: 10.1007/s00221-005-0191-y. [DOI] [PubMed] [Google Scholar]
- Ross CF. Into the light: the origin of anthropoidea. Annual Review of Anthropology. 2000;29:147–194. [Google Scholar]
- Rossion B, Gauthier I. How does the brain process upright and inverted faces? Behavioral and Cognitive Neuroscience Reviews. 2002;1(1):63–75. doi: 10.1177/1534582302001001004. [DOI] [PubMed] [Google Scholar]
- Ryan CME, Lea SEG. Images of conspecifics as categories to be discriminated by pigeons and chickens: slides, video tapes, stuffed birds and live bireds. Behavioural Processes. 1994;33:155–176. doi: 10.1016/0376-6357(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Sackett GP. Response of rhesus monkeys to social stimulation presented by means of colored slides. Perceptual and Motor Skills. 1965;20 8 doi: 10.2466/pms.1965.20.3c.1027. [DOI] [PubMed] [Google Scholar]
- Sackett GP. Monkeys reared in isolation with pictures as visual input: evidence for an innate releasing mechanism 58. Science. 1966;154(755):1468–1473. doi: 10.1126/science.154.3755.1468. [DOI] [PubMed] [Google Scholar]
- Sandem AI, Janczak AM, Salte R, Braastad BO. The use of diazepam as a pharmacological validation of eye white as an indicator of emotional state in dairy cows. Applied Animal Behaviour Science. 2006;96:177–183. [Google Scholar]
- Scaife M. The response to eye-like shapes by birds. I. The effect of context: a predator and a strange bird. Animal Behaviour. 1976a;24:195–199. [Google Scholar]
- Scaife M. The response to eye-like shapes by birds. II. The importance of staring, pairedness and shape. Animal Behaviour. 1976b;24(1):200–206. [Google Scholar]
- Schloegl C, Kotrschal K, Bugnyar T. Do common ravens (Corvus corax) rely on human or conspecific gaze cues to detect hidden food? Animal Cognition. 2008a;11(2):231–241. doi: 10.1007/s10071-007-0105-4. [DOI] [PubMed] [Google Scholar]
- Schloegl C, Kotrschal K, Bugnyar T. Modifying the object-choice task: is the way you look important for ravens? Behavioural Processes. 2008b;77(1):61–65. doi: 10.1016/j.beproc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Cohn JF. Human facial expressions as adaptations: Evolutionary questions in facial expression research. Americal Journal of Physical Anthropology. 2001 33:3–24. doi: 10.1002/ajpa.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell JM, Wickings EJ. Dominance, status signals, and coloration in male mandrills (Mandrillus sphinx) Ethology. 2005;111:25–50. [Google Scholar]
- Smith EO. Yawning: an evolutionary perspective. Human Evolution. 1999;14(3):191–198. [Google Scholar]
- Springer MS, Murphy WJ, Eizirik E, O'Brien SJ. Placental mammal diversification and the Cretaceous-Tertiary boundary. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1056–1061. doi: 10.1073/pnas.0334222100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen ID, Coetzee V, Law SM, Perrett DI. Skin blood perfusion and oxygenation colour affect perceived human health. PLoS One. 2009;4(4):e5083. doi: 10.1371/journal.pone.0005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. The role of eyespots as anti-predator mechanisms, principally demonstrated in the Lepidoptera. Biological Reviews. 2005;80(04):573–588. doi: 10.1017/S1464793105006810. [DOI] [PubMed] [Google Scholar]
- Sugita Y. Face perception in monkeys reared with no exposure to faces. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaddle JP, Cuthill IC. Female zebra finches prefer males with symmetric chest plumage. Proceedings of the Royal Society B: Biological Sciences. 1994;258(1353):267–271. [Google Scholar]
- Swartz KB. What is mirror self-recognition in nonhuman primates, and what is it not? Annals of the New York Academy of Sciences. 1997;818:64–71. doi: 10.1111/j.1749-6632.1997.tb48246.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Saito H, Fukada Y, Moriya M. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. Journal of Neurophysiology. 1991;66(1):170–189. doi: 10.1152/jn.1991.66.1.170. [DOI] [PubMed] [Google Scholar]
- Tanibuchi I, Goldman-Rakic PS. Dissociation of spatial-, object-, and sound-coding neurons in the mediodorsal nucleus of the primate thalamus. Journal of Neurophysiology. 2003;89(2):1067–1077. doi: 10.1152/jn.00207.2002. [DOI] [PubMed] [Google Scholar]
- Tate AJ, Fischer H, Leigh AE, Kendrick KM. Behavioural and neurophysiological evidence for face identity and face emotion processing in animals. Philosophical Transacations of the Royal Society B: Biological Sciences. 2006;361(1476):2155–2172. doi: 10.1098/rstb.2006.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B, Demaria C, Preuschoft S, Desportes C. Structural convergence between silent bared-teeth display and relaxed open-mouth display in the Tonkean macaque (Macaca tonkeana) Folia Primatologica. 1989;52(3-4):178–184. doi: 10.1159/000156396. [DOI] [PubMed] [Google Scholar]
- Thompson P. Margaret Thatcher: a new illusion. Perception. 1980;9:483–484. doi: 10.1068/p090483. [DOI] [PubMed] [Google Scholar]
- Thornhill R, Moller AP. The relative importance of size and symmetry in sexual selection. Behavioral Ecology. 1998;9(6):546–551. [Google Scholar]
- Thorpe WH. Perceptual basis for group organization in social vertebrates, especially birds. Nature. 1968;220(5163):124–128. doi: 10.1038/220124a0. [DOI] [PubMed] [Google Scholar]
- Tibbetts EA. Visual signals of individual identity in the wasp Polistes fuscatus. Proceedings of the Royal Society B: Biological Sciences. 2002;269(1499):1423–1428. doi: 10.1098/rspb.2002.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, Call J, Hare B. Five primate species follow the visual gaze of conspecifics. Animal Behaviour. 1998;55(4):1063–1069. doi: 10.1006/anbe.1997.0636. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Lehmann H, Call J. Reliance on head versus eyes in the gaze following of great apes and human infants: the cooperative eye hypothesis. Journal of Human Evolution. 2007;52(3):314–320. doi: 10.1016/j.jhevol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Tomonaga M. How laboratory-raised Japanese monkeys (Macaca fuscata) perceive rotated photographs of monkeys. Primates. 1994;35(2):155–165. [Google Scholar]
- Tomonaga M. Visual search for orientation of faces by a chimpanzee (Pan troglodytes): face-specific upright superiority and the role of facial configural properties. Primates. 2007;48(1):1–12. doi: 10.1007/s10329-006-0011-4. [DOI] [PubMed] [Google Scholar]
- Tomonaga M, Itakura S, Matsuzawa T. Superiority of conspecific faces and reduced inversion effect in face perception by a chimpanzee. Folia Primatologica. 1993;61(2):110–114. doi: 10.1159/000156737. [DOI] [PubMed] [Google Scholar]
- Tomonaga M, Tanaka M, Matsuzawa T, Myowa-Yamakoshi M, Kosugi D, Mizuno Y, et al. Development of social cognition in infant chimpanzees (Pan troglodytes): Face recognition, smiling, gaze, and the lack of triadic interactions. Japanese Psychological Research. 2004;46(3):227–235. [Google Scholar]
- Trainor BC, Basolo AL. An evaluation of video playback using Xiphophorus helleri. Animal Behaviour. 2000;59(83):89. doi: 10.1006/anbe.1999.1289. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nature Neuroscience. 2003;6(9):989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Livingstone MS. Mechanisms of face perception. Annual Review of Neuroscience. 2008;31:411–437. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudin A, Call J, Dunbar RI, Harris G, van der EC. Comprehension of signs by dolphins (Tursiops truncatus) Journal of Comparative Psychology. 2001;115(1):100–105. doi: 10.1037/0735-7036.115.1.100. [DOI] [PubMed] [Google Scholar]
- van den Bos R, de Vries H. Clusters in social behavior of female domestic cats (Felis silvestris catus) living in confinement. Journal of Ethology. 1996;14:123–131. [Google Scholar]
- Van Der Velden J, Zheng Y, Patullo B, Macmillan D, Brembs B. Crayfish Recognize the Faces of Fight Opponents. PLoS One. 2008;3(2):e1695. doi: 10.1371/journal.pone.0001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyk D, Evans C. Familiar–unfamiliar discrimination based on visual cues in the Jacky dragon, Amphibolurus muricatus. Animal Behaviour. 2007;74(1):33–44. [Google Scholar]
- van Hooff JARAM. Facial expressions in higher primates. Symposia of the Zoological Society of London. 1962;8:97–125. [Google Scholar]
- van Rhijn JG. Units of behavior in the black-headed gull, Larus ridibundus L. Animal Behaviour. 1981;29(586):597. [Google Scholar]
- Vane-Wright RI, Boppre M. Visual and chemical signalling in butterflies: functional and phylogentic perspectives. Philosophical Transactions of the Royal Society B: Biological Sciences. 1993;340(1292):197–205. [Google Scholar]
- Virányi Z, Gácsi M, Kubinyi E, Topál J, Belényi B, Ujfalussy D, et al. Comprehension of human pointing gestures in young human-reared wolves (Canis lupus) and dogs (Canis familiaris) Animal Cognition. 2008;11(3):373–387. doi: 10.1007/s10071-007-0127-y. [DOI] [PubMed] [Google Scholar]
- von Bayern AM, Emery NJ. Jackdaws respond to human attentional states and communicative cues in different contexts. Current Biology. 2009;19(7):602–606. doi: 10.1016/j.cub.2009.02.062. [DOI] [PubMed] [Google Scholar]
- Waas JR, Wordsworth AF. Female zebra finches prefer symmetrically banded males, but only during interactive mate choice tests. Animal Behaviour. 1999;57(5):1113–1119. doi: 10.1006/anbe.1998.1055. [DOI] [PubMed] [Google Scholar]
- Waitt C, Gerald M, Little A, Kraiselburd E. Selective attention toward female secondary sexual color in male rhesus macaques. Americal Journal of Primatology. 2006;68(7):738–744. doi: 10.1002/ajp.20264. [DOI] [PubMed] [Google Scholar]
- Waitt C, Little A. Preferences for Symmetry in Conspecific Facial Shape Among Macaca mulatta. International Journal of Primatology. 2006;27(1):133–145. [Google Scholar]
- Waitt C, Little A, Wolfensohn S, Honess P, Brown A, Buchanan-Smith H, et al. Evidence from rhesus macaques suggests that male coloration plays a role in female primate mate choice. Proceedings of the Royal Society B: Biological Sciences. 2003;270(0):S144–S146. doi: 10.1098/rsbl.2003.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller B, Parr L, Gothard K, Burrows A, Fuglevand A. Mapping the contribution of single muscles to facial movements in the rhesus macaque. Physiology and Behavior. 2008;95(1-2):93–100. doi: 10.1016/j.physbeh.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Ito Y. Discrimination of individuals in pigeons. Bird Behavior. 1991;9:20–29. [Google Scholar]
- Watanabe S, Maier U, Bischof H. Pattern discrimination is affected by entopallial but not by hippocampal lesions in zebra finches. Behavioural Brain Research. 2008;190(2):201–205. doi: 10.1016/j.bbr.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Weigel RM. The facial expressions of the brown capuchin monkey (Cebus apella) Behaviour. 1979;68(3/4):250–276. [Google Scholar]
- Weiss D, Kralik J, Hauser M. Face processing in cotton-top tamarins (Saguinus oedipus) Animal Cognition. 2001;3(4):191–205. [Google Scholar]
- Wenzel JW. Behavioral homology and phylogeny. Annual Review of Ecology and Systematics. 1992;23:361–381. [Google Scholar]
- Westhoff G, Tzschatzch K, Bleckmann H. The spitting behavior of two species of spitting cobras. Journal of Comparative Physiology A. 2005;191:873–881. doi: 10.1007/s00359-005-0010-8. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306(5704):2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Whitfield DP. Plumage variability, status signalling, and indivdiual recognition in avian flocks. Tree. 1987;2(1):13–18. doi: 10.1016/0169-5347(87)90194-7. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260(5116):1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Wright AA, Roberts WA. Monkey and human face perception: Inversion effects for human faces but not for monkey faces or scenes. Journal of Cognitive Neuroscience. 1996;8:278–290. doi: 10.1162/jocn.1996.8.3.278. [DOI] [PubMed] [Google Scholar]
- Wu HM, Holmes WG, Medina SR, Sackett GP. Kin preference in infant Macaca nemestrina. Nature. 1980;285(5762):225–227. doi: 10.1038/285225a0. [DOI] [PubMed] [Google Scholar]
- Wyatt TD. Pheromones and animal behavior: communication by smell and taste. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- Yin RK. Looking at upside-down faces. Journal of Experimental Psychology. 1969;81:141–145. [Google Scholar]
- Zangenehpour S, Chaudhuri A. Patchy organization and asymmetric distribution of the neural correlates of face processing in monkey inferotemporal cortex. Current Biology. 2005;15(11):993–1005. doi: 10.1016/j.cub.2005.04.031. [DOI] [PubMed] [Google Scholar]