IL-27 regulates IL-12 responsiveness of naïve CD4+ T cells through Stat1-dependent and -independent mechanisms (original) (raw)
Abstract
IL-27, a novel heterodimeric cytokine produced by antigen-presenting cells, signals through the T cell cytokine receptor (TCCR)/WSX-1 expressed on naïve CD4+ T cells and natural killer cells. TCCR/WSX-1 deficiency results in delayed T helper type 1 (TH1) development through an unresolved mechanism. We report here that IL-27 stimulation in developing murine T helper cells potently induces the expression of the major TH1-specific transcription factor T-bet and its downstream target IL-12R β2, independently of IFNγ. In addition, IL-27 suppresses basal expression of GATA-3, the critical TH2-specific transcription factor that inhibits TH1 development by down-regulating signal transducer and activator of transcription (Stat) 4. IL-27 signaling through TCCR/WSX-1 induces phosphorylation of Stat1, Stat3, Stat4, and Stat5. Stat1 is required for suppression of GATA-3, but T-bet induction by IL-27 can also be mediated through a Stat1-independent pathway. Despite its TH1-like signaling profile, IL-27 is not sufficient to drive the differentiation of CD4+ T cells into IFNγ-producing cells. Similarly, IL-27 induces T-bet expression in primary natural killer cells, but this does not result in an increase of IFNγ production or cytotoxic activity. Therefore, although IL-27 is unable to drive IFNγ production on its own, it plays an important role in the early steps of TH1 commitment by contributing in a paracrine manner to the control of IL-12 responsiveness.
The differentiation of naïve CD4+ T cells into T helper (TH) type 1 (TH1) or TH2 effector cells is a critical process of the adaptive immune responses. TH1 cells produce IFNγ and promote cellular immunity, which is critical to control infections by intracellular pathogens. In contrast, TH2 effector cells produce IL-4, IL-5, and IL-13 and promote humoral immunity, allergic reactions, and resistance to helmintic infections (1–3). Many factors influence the differentiation process of CD4+ T cells into TH1 or TH2 effector cells, including the dose of antigen, strength of signal through the T cell receptor, and costimulation, but cytokines have emerged as key determinants of the outcome (4). IL-12 promotes TH1 development by means of a signaling pathway that involves activation of signal transducer and activator of transcription (Stat) 4 (5). IL-4 induces Stat6 activation and drives naïve CD4+ T cells down a TH2 differentiation pathway. In addition, transcription factors playing causal roles in the establishment of gene expression programs specific for TH1 and TH2 cells have been identified. The T-box transcription factor T-bet was shown to play a central role in TH1 cell development (6, 7). An important function of T-bet is to maintain expression of the IL-12 receptor (IL-12R) β2 chain after activation of CD4+ T cells (7–9). In contrast, the zinc-finger transcription factor GATA-3 is crucial for inducing key attributes of TH2 cells (10–13). GATA-3 also inhibits TH1 development through suppression of IL-12R β2 and Stat4 expression, leading to the loss of IL-12 signaling (12, 14).
T cell cytokine receptor (TCCR)/WSX-1 is a type I cytokine receptor that was identified on the basis of sequence homology with gp130 and the IL-12R β2 chain (15, 16). TCCR/WSX-1 expression is restricted to lymphoid tissues and is highest in naïve CD4+ T cells and natural killer (NK) cells. TCCR/WSX-1 knockout mice develop impaired TH1 responses to infections by intracellular pathogens (16, 17), but further analysis of the functions of this new cytokine receptor was hampered by the lack of an identified ligand. Recently, a new heterodimeric cytokine named IL-27 (18) was shown to bind to TCCR/WSX-1. A product of activated antigen-presenting cells, IL-27 is formed by the association of EBI3, an IL-12p40-related polypeptide, and p28, a protein related to IL-12p35. IL-27 promotes the growth of naïve CD4+ T cells and was suggested to play a role in the differentiation of TH1 cells in vitro (18).
We sought to further define the mechanisms by which IL-27 can influence the differentiation of TH cells. As a first step, we analyzed the signaling pathways that are activated by IL-27. We next evaluated the consequences of IL-27 stimulation on the expression of T-bet, GATA-3, and IL-12R β2, and the production of IFNγ and IL-4 by TH cells. Finally, we examined the effects of IL-27 stimulation on NK cells, which express the TCCR/WSX-1 chain of the IL-27 receptor.
Methods
Mice, Primary Cells, Cytokines, and Antibodies. _TCCR_-/- and TCCR+/+ mice in C57BL/6 background (backcross 12) were bred in pathogen-free facilities at Genentech (16). _Stat1_-/- mice in 129Sv/Ev background and 129Sv/Ev (Stat1+/+) mice were purchased from Taconic Transgenics (Oxnard, CA).
Murine mononuclear cells were isolated from total splenocytes on a density gradient (Lympholyte, Cedarlane Laboratories). Primary CD4+ T cells and NK cells were purified from splenic mononuclear cells by magnetic cell sorting with a Mouse CD4+ T Cell Isolation Kit or anti-DX5 microbeads (Miltenyi Biotec, Auburn, CA). Purity of sorted cells was verified by fluorescence-activated cell sorting (FACS) analysis and ranged from 92% to 95% for CD4+ T cells and from 60% to 70% for DX5+ NK cells. Contamination of purified DX5+ NK cells with CD4+ T cells was <3.8%.
Human peripheral blood mononuclear cells from healthy donors were purified by density gradient (Lymphocyte Separation Medium, ICN).
Recombinant murine IL-2 (mIL-2), mIL-12, mIL-4, and human IL-15 (hIL-15) were purchased from R & D Systems. To produce mIL-27 and hIL-27, we constructed a plasmid with two cytomegalovirus promoter-driven expression cassettes. The first cassette contained murine or human p28 with a C-terminal gD-tag, and C-terminally FLAG-tagged murine or human EBI3 was cloned into the second cassette. These plasmids were transfected into mammalian cells, and recombinant mIL-27 or hIL-27 was purified from the supernatants.
Neutralizing antibodies against mIL-12, murine IFNγ, mIL-4, and mIL-2 were purchased from R & D Systems, and anti-mCD3ε and mCD28 antibodies were from Pharmingen.
Anti-Stat1 antibodies for immunoprecipitation (ATO-2F5) and for Western blot (ATO-1D6) were provided by R. D. Schreiber (Washington University, St. Louis). Anti-phosphoStat1 (Tyr-701), phosphoStat3 (Tyr-705), Stat3, phosphoStat5 (Tyr-694), and phosphoStat6 (Tyr-641) antibodies were from Cell Signaling Technology. Anti-Stat4 (C-20 sc-486) and Stat5b (C-17 sc-835) were purchased from Santa Cruz Biotechnology. Anti-phosphoStat4 (Tyr-693) was from Zymed.
Antibodies used for FACS analysis were purchased from Pharmingen.
Analysis of Stat Protein Phosphorylation. A total of 1.3–1.7 × 107 freshly isolated murine CD4+ T cells were treated for 15 min at 37°C with cytokines (200 ng/ml mIL-27, mIL-2, mIL-4, or mIL-12 or 100 ng/ml murine IFNγ) in serum-free AIM-V medium (Invitrogen). Cells were lysed in cold buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.1% SDS, 1% Triton X-100, 2 mM Na molybdate, 2 mM NaVo4, and Complete protease inhibitors (Roche Molecular Biochemicals). Lysates were immunoprecipitated with antibodies directed against Stat proteins and Protein A Sepharose CL-4B (Amersham Pharmacia Biotech). Immunoprecipitates or cell lysates were analyzed by Western blot with antibodies against specific phosphorylated Stat proteins. To control for equal protein loading, blots were stripped with Restore Western Blot Stripping Buffer (Pierce) and reprobed with antibodies specific for Stat proteins.
Primary Cell Cultures. Primary cells were cultivated in Iscove's modified Dulbecco's medium (Invitrogen) supplemented with 10% heat-inactivated FBS (HyClone PerBio), 1 mM l-glutamine, 1% penicillin/streptomycin (Invitrogen), and 0.6 μM 2-mercaptoethanol.
Murine CD4+ T cells were seeded at 6 × 105 per ml in 48-well plates coated with anti-mCD3ε (5 μg/ml) and anti-CD28 (1 μg/ml) antibodies, in complete medium with or without mIL-27 (200 ng/ml), with cocktails of cytokines and neutralizing antibodies (TH1 conditions: 3.5 ng/ml IL-12, 1 μg/ml anti-IL-4, 3.3 ng/ml IL-2; TH2 conditions: 3.5 ng/ml IL-4, 5 μg/ml anti-IL-12, 5 μg/ml anti-IFNγ, 3.3 ng/ml IL-2; neutral: 5 μg/ml anti-IL-12, 5 μg/ml anti-IFNγ, 1 μg/ml anti-IL-4, 3.3 ng/ml IL-2). Cells were expanded on day 3 in IL-2-containing complete medium. On day 6 or 7, cells were collected, washed, and seeded at 106 cells per ml in 48-well plates coated with anti-mCD3ε antibody (5 μg/ml).
For proliferation assays, murine CD4+ T cells were seeded at 8 × 104 cells per well in 96-well plates coated with anti-mCD3ε (5 μg/ml) and anti-CD28 (1 μg/ml) antibodies, in complete medium containing anti-mIL-2 antibodies (7.5 μg/ml), with or without mIL-27 (200 ng/ml).
Murine DX5+ NK cells were seeded in 48-well plates at 0.6 to 1 × 106 per ml in complete medium supplemented with cytokines (200 ng/ml mIL-27, 20 ng/ml mIL-12, or 20 ng/ml hIL-15).
Human peripheral blood mononuclear cells were seeded in 24-well plates at 106 cells per ml in complete medium (without 2-mercaptoethanol) supplemented with cytokines (200 ng/ml hIL-27 or 100 ng/ml hIL-15). Cells were collected and counted on day 7, and percentages of NK cells (CD3-/CD56+) at the start and at the end of the culture were determined by FACS analysis.
Real-Time RT-PCR. Total RNA was isolated with the RNeasy Mini kit and submitted to RNase-free DNase I digestion (Qiagen). TaqMan quantitative RT-PCR using a Sequence Detector 7700 instrument was carried out according to the manufacturer's instructions (Applied Biosystems). For each sample, duplicate test reactions and a control reaction into which no reverse transcriptase had been added were analyzed for expression of T-bet, IL-12R β2, or GATA-3 mRNAs and a housekeeper mRNA, rpl-19. No signal was observed in the control reactions. Expression levels are calculated by comparison to serial dilutions of a standard RNA and are expressed as nanograms of standard RNA, by using SEQUENCE DETECTOR software (Applied Biosystems). Arbitrary expression units were calculated by dividing T-bet, IL-12R β2, or GATA-3 expression by rpl-19 expression. Probes and primers were designed with PRIMER EXPRESS software (Applied Biosystems). The primer triplets were 5′-CCAGAGCGGCAAGTGGG-3′ (sense), 5′-CATATAAGCGGT TCCCTGGC-3′ (antisense), and 5′-TGCTGCCT TCTGCCTTTCCACACTG-3′ (probe) for mouse T-bet; 5′-CAAGCATTTGCATCGCTATCA-3′ (sense), 5′-AATGCCTTTTGCCGGAAGT-3′ (antisense), and 5′-ACGAATTGAGAACGTGCCCACCGT-3′ (probe) for mouse IL-12R β2; 5′-CCTACCGGGTTCGGATGTAA-3′ (sense), 5′-CACACACTCCCTGCCTTCTGT-3′ (antisense), and 5′-TCGAGGCCCAAGGCACGATCC-3′ (probe) for mouse GATA-3; and 5′-ATCCGCAAGCCTGTGACTGT-3′ (sense), 5′-TCGGGCCAGGGTGTTTTT-3′ (antisense), and 5′-T TCCCGGGCTCGTTGCCG-3′ (probe) for mouse rpl-19.
Cytotoxic Assay. Purifed murine DX5+ NK cells cultivated 18 h in the presence of cytokines were incubated in duplicate wells for 4 h with Na(51Cr)O4-labeled YAC-1 target cells at the indicated NK-to-target ratios. The amount of radioactivity released in the supernatants was measured with a γ counter. Spontaneous and maximum lysis were determined by incubating target cells in medium and medium containing 0.1% Triton X-100, respectively. Specific lysis was calculated as (test 51Cr released - spontaneous 51Cr released)/(maximum 51Cr released - spontaneous 51Cr released).
Results
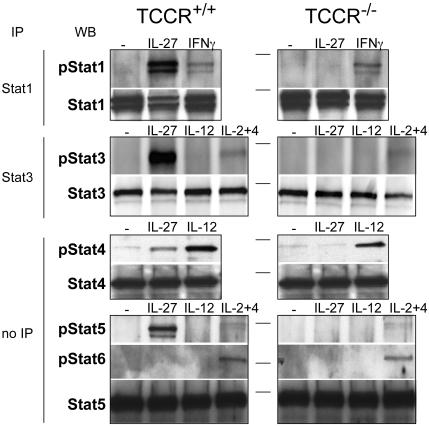
IL-27 Activates Stat1, but also Stat3, Stat4, and Stat5. Cytokines that bind to cytokine receptors of the type I family transduce their signal through the Janus kinase/Stat pathway. To determine which Stat proteins are activated in response to IL-27 binding to its receptor, freshly isolated CD4+ T cells from TCCR+/+ and TCCR_-/_- mice were treated for 15 min with 200 ng/ml mIL-27. We subsequently detected phosphorylation of Stat proteins by immunoprecipitation and/or Western blot with anti-phosphorylated Stat antibodies (Fig. 1). IL-27 strongly induces the phosphorylation of Stat1, -3, and -5, and, to a lesser extent, Stat4. IL-27 does not induce Stat6 phosphorylation. No phosphorylation of Stat proteins was observed in TCCR_-/_- cells in response to IL-27, indicating that TCCR is an essential component of the IL-27 receptor with regard to activation of Stat proteins.
Fig. 1.
IL-27 induces Stat1, -3, -4, and -5 phosphorylation. CD4+ cells purified from TCCR+/+ or TCCR_-/_- splenocytes were treated with the indicated cytokines for 15 min in serum-free medium. Cell lysates were immunoprecipitated (IP) with Stat1 or Stat3 antibodies. Immunoprecipitates or cell lysates (for Stat4, -5, and -6 detection) were submitted to SDS/PAGE, followed by Western blotting (WB) with antibodies specific for the indicated phosphorylated Stat proteins. To control for equal protein loading, blots were stripped and reprobed with antibodies specific for the indicated total Stat proteins. Horizontal lines indicate the position of a 98-kDa marker.
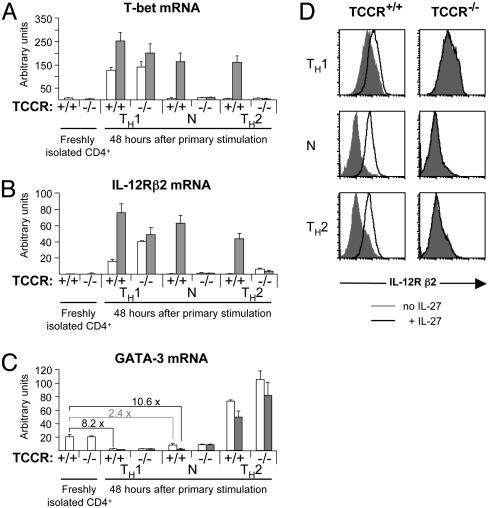
IL-27 Induces T-bet and IL-12R β2 Expression and Suppresses GATA-3 Expression. To dissect the function of IL-27 in TH differentiation, we next tested whether IL-27 is able to induce expression of the TH1-specific transcription factor T-bet under various conditions. Purified CD4+ T cells from TCCR+/+ and TCCR_-/- mice were stimulated in the presence or absence of IL-27 with plate-bound anti-CD3ε and anti-CD28 antibodies, in conditions that drive the differentiation process toward a TH1 phenotype or a TH2 phenotype, or in neutral conditions where IL-12, IFNγ, and IL-4 are all neutralized by antibodies. T-bet mRNA levels were measured by real-time RT-PCR (Fig. 2_A_). In the absence of IL-27, T-bet expression is induced 48 h after primary stimulation under TH1 conditions as a result of autocrine IFNγ signaling (8), but it is not induced in neutral and TH2 conditions, in which IFNγ is efficiently neutralized by antibodies. IL-27 moderately increases the IFNγ-mediated T-bet induction that is observed in TH1 conditions. More importantly, IL-27 induces T-bet expression independent of IFNγ, in neutral and TH2 conditions (32-fold inductions in TCCR+/_+ cells in the presence of IL-27 compared with the absence of IL-27). These inductions are specific for IL-27 because they are not observed in TCCR_-/_- CD4+ cells.
Fig. 2.
IL-27 induces T-bet and IL-12R β2 and suppresses GATA-3 in developing CD4+ T cells. (A_–_C) CD4+ T cells purified from TCCR+/+ or TCCR-/- splenocytes were activated with plate-bound anti-CD3ε and anti-CD28 antibodies in the absence (white bars) or presence (gray bars) of IL-27 in TH1 conditions (IL-12 plus anti-IL-4 plus IL-2), TH2 conditions (IL-4 plus anti-IL-12 plus anti-IFNγ plus IL-2), or neutral (N) conditions (anti-IL-12 plus anti-IFNγ plus anti-IL-4 plus IL-2). Before activation or 48 h later, cells were collected for total RNA preparation. Levels of rpl19 (a ribosomal housekeeping gene), T-bet, IL-12R β2, and GATA-3 mRNAs were determined in duplicate by real-time RT-PCR and compared with levels measured in serial dilutions of a reference RNA. Results shown are arbitrary units normalized for rpl19 expression and are representative of three independent experiments. Error bars represent 1 SD. P values between data points that are discussed in the text as significantly different are <0.02. (A) T-bet mRNA levels. (B) IL-12R β2 mRNA levels. (C) GATA-3 mRNA levels. Fold suppressions between relevant samples are indicated. (D) CD4+ T cells purified from TCCR+/+ or TCCR-/- splenocytes were activated as in A_–_C. Forty-eight hours after activation, cells activated in the absence (filled gray histograms) or presence (open histograms) of mIL-27 were stained with anti-mCD4 and anti-mIL-12R β2 antibodies. Histograms are gated on live CD4+ cells.
The maintenance of high levels of IL-12R β2 chain expression after T cell receptor activation is a consequence of T-bet mRNA induction and is important for maintaining IL-12 responsiveness in developing TH1 cells (9, 19). Therefore, we examined whether IL-27-mediated induction of T-bet also leads to high IL-12R β2 mRNA expression (Fig. 2_B_). In the absence of IL-27, IL-12R β2 mRNA is induced 50-fold after 48 h of culture in TH1 conditions. IL-27 stimulation further increases this induction in TH1 conditions. IL-27 treatment is also able to strongly induce IL-12R β2 expression independent of IFNγ in neutral conditions (150-fold induction in TCCR+/+ cells in the presence of IL-27 compared with the absence of IL-27). During TH2 development, IL-12R β2 expression is normally suppressed by GATA-3 (12). Nevertheless, IL-27 treatment of developing TH2 cells leads to high levels of IL-12R β2 expression (89-fold induction in the presence of IL-27 compared with its absence), although GATA-3 mRNA is strongly induced by IL-4 in these conditions (Fig. 2 B and C). To further confirm the ability of IL-27 to induce the expression of IL-12R β2, we used FACS to examine surface expression of IL-12R β2 on activated CD4+ T cells (Fig. 2_D_). In the absence of IL-27, surface expression of the IL-12R β2 chain is observed on CD4+ T cells activated in TH1 conditions only. As predicted by the analysis of mRNA levels, IL-27 treatment further increases surface expression of IL-12R β2 in TH1 conditions and strongly induces it in neutral and TH2 conditions independently of IFNγ.
We next examined the effect of IL-27 on the expression of the transcription factor GATA-3, considered to be the master switch in TH2 development (13). IL-12 and IFNγ were previously shown to inhibit GATA-3 expression, and the complete suppression of GATA-3 during TH1 development requires IL-12 (12). As shown in Fig. 2_C_, GATA-3 expression is indeed completely suppressed in the presence of IL-12 (8.2-fold reduction in TCCR+/+ cells 48 h after activation in TH1 conditions compared with freshly isolated cells). In neutral conditions where IL-12, IFNγ, and IL-4 are all neutralized by antibodies, IL-27 strongly suppresses GATA-3 (10.6-fold reduction in TCCR+/+ cells 48 h after activation compared with freshly isolated cells). This IL-27-mediated suppression of GATA-3 is comparable to that mediated by IL-12 and is not observed in TCCR_-/_- cells. Therefore, we conclude that IL-27 is able to repress GATA-3 expression independent of IFNγ and IL-12. However, IL-27 is not able to significantly inhibit the high level of GATA-3 expression induced by IL-4 in TH2 conditions (Fig. 2_C_).
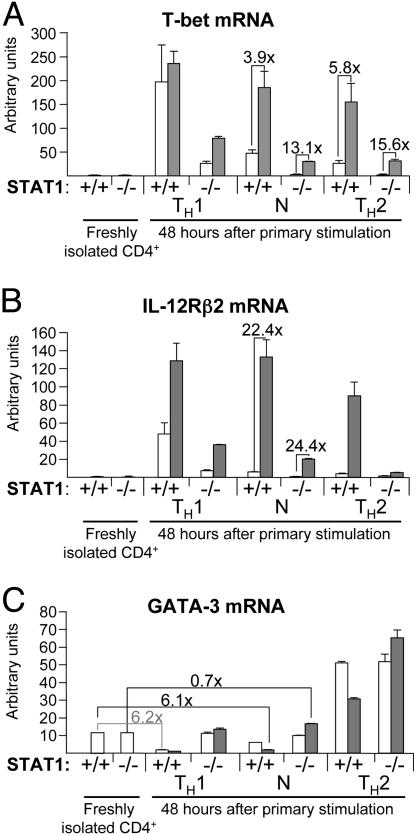
Stat1 Is Not Required for the IL-27-Mediated Induction of T-bet but Is Required for the IL-27-Mediated Suppression of GATA-3. During in vitro TH1 development, the induction of T-bet expression through IFNγ was shown to depend strongly on Stat1 (8). We therefore sought to determine whether the IL-27-dependent induction of T-bet was also mediated by Stat1. To this end, CD4+ T cells isolated from _Stat1_-/- mice or isogenic wild-type animals were stimulated with plate-bound anti-CD3ε and anti-CD28 antibodies, and T-bet mRNA levels were measured 48 h after activation by real-time RT-PCR (Fig. 3_A_). We found that, although T-bet levels were generally lower in _Stat1_-/- cells, T-bet is still induced in Stat1-deficient cells under TH1 conditions, although to a lower extent than in wild-type cells (24- and 126-fold inductions in _Stat1_-/- and Stat1+/+ CD4+ cells, respectively, 48 h after activation compared with the corresponding freshly isolated CD4+ cells). Therefore, although IFNγ-dependent T-bet induction depends on Stat1, alternative mechanisms of T-bet induction exist in TH1 conditions. Similarly, IL-27-mediated induction of T-bet is observed in _Stat1_-/- cells (Fig. 3_A_), demonstrating the existence of a Stat1-independent pathway for the induction of T-bet in response to IL-27.
Fig. 3.
IL-27-mediated induction of T-bet does not require Stat1, but IL-27-mediated suppression of GATA-3 is strictly dependent on Stat1. CD4+ T cells purified from Stat1+/+ or Stat1-/- splenocytes were activated as in Fig. 2 in the absence (white bars) or presence (gray bars) of IL-27. Before activation or 48 h later, cells were collected for total RNA preparation. Levels of rpl19, T-bet, IL-12R β2, and GATA-3 mRNAs were determined as indicated in Fig. 2. Results shown are arbitrary units normalized for rpl19 expression and are representative of two independent experiments. Fold inductions (for T-bet expression and IL-12R β2) or suppressions (for GATA-3 expression) between relevant samples are indicated. Error bars represent 1 SD. P values between data points that are discussed in the text as significantly different are <0.05. (A) T-bet mRNA levels. (B) IL-12R β2 mRNA levels. (C) GATA-3 mRNA levels.
As shown in Fig. 3_B_, IL-27 treatment also induces IL-12R β2 expression in _Stat1_-/- cells activated in neutral conditions. Therefore, the level of IL-27-mediated Stat1-independent induction of T-bet is sufficient to lead to increased IL-12R β2 expression.
We next evaluated the Stat1 dependence of the IL-27-mediated GATA-3 suppression. As shown in Fig. 3_C_, IL-27 is not able to suppress GATA-3 expression in CD4+ T cells activated in neutral conditions if Stat1 is absent. Therefore, unlike IL-27-mediated T-bet induction, IL-27-mediated GATA-3 suppression depends is fully dependent on Stat1 activation.
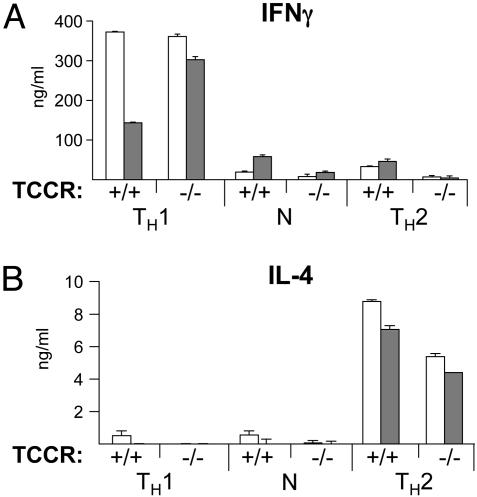
IL-27 Alone Is Not Able to Induce the Differentiation of CD4+ T Cells into IFNγ-Producing Cells. Because IL-27 induces the expression of T-bet and is able to moderately activate Stat4, we next sought to determine whether it can drive the differentiation of CD4+ T cells into fully competent TH1 cells by recapitulating the combined functions of IFNγ and IL-12. Freshly isolated CD4+ cells were stimulated with anti-CD3ε and anti-CD28 in the presence or absence of IL-27 in various conditions. Activated cells were collected 7 days after activation, a time frame sufficient to allow the differentiation of CD4+ T cells into TH1 or TH2 cells in the appropriate conditions, and 106 differentiated cells were restimulated on anti-CD3ε-coated wells in the absence of any cytokine for 24 h. The production of IFNγ and IL-4 was measured by ELISA in the culture supernatants (Fig. 4). In this classical in vitro differentiation experimental setting, the effect of IL-27 on the differentiation of CD4+ T cells into IFNγ-producing cells can be distinguished from its effect on proliferation. In the absence of IL-12 (neutral and TH2 conditions), the addition of IL-27 at the time of activation was not sufficient to significantly drive the differentiation of CD4+ T cells into IFNγ-producing cells (Fig. 4_A_). Noticeably, in these conditions, IL-27 is not able to synergize with IL-12 to increase the production of IFNγ by TH1 CD4+ cells (Fig. 4_A_,TH1 conditions). The moderately reduced amount of IFNγ secreted by TH1 cells activated in the presence of IL-27 (Fig. 4_A_) was not repeatedly observed in other independent experiments and is not significant. IL-27 had no significant effect on IL-4 secretion (Fig. 4_B_).
Fig. 4.
IL-27 does not induce significantly the production of IFNγ by differentiated CD4+ T cells. CD4+ T cells purified from TCCR+/+ or TCCR-/- splenocytes were activated as in Fig. 2 in the indicated conditions in the absence (white bars) or presence (gray bars) of IL-27. Three days after activation, cells were expanded in medium containing mIL-2 only. Cells were collected on day 6 or 7 and washed, and 106 cells were restimulated with plate-bound anti-CD3ε antibodies. Cytokines secreted in the supernatant 24 h after restimulation were measured in duplicate by ELISA. (A) IFNγ production. (B) IL-4 production. Results shown are representative of three independent experiments.
The IL-27-Mediated Proliferative Signal on CD4+ Cells Is Not Transduced by Stat1. IL-27 induces the proliferation of activated naïve CD4+ T cells (18). We sought to determine whether Stat1 is implicated in transducing the IL-27 proliferative signal. As shown in Fig. 5, activated Stat1-deficient CD4+ T cells proliferate in response to IL-27 at least as well as their wild-type counterparts, thereby demonstrating that Stat1 is not required to mediate the IL-27-proliferative signal. On the contrary, Stat1 might mediate an antiproliferative signal in response to IL-27, similar to the antiproliferative effects that it mediates in response to IFNs (20–22). Disruption of the IL-27-mediated activation of Stat1 could explain the observed hyperproliferation of TCCR/wsx-1 knockout splenocytes (17).
Fig. 5.
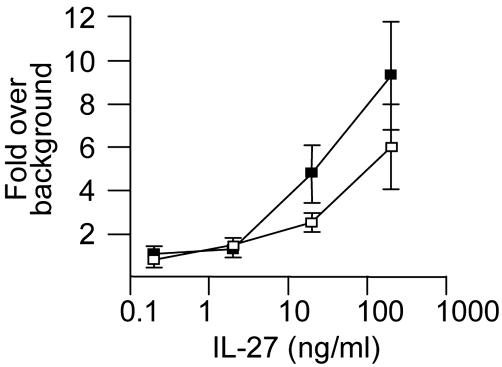
Stat1 is not required for the IL-27-induced proliferation of CD4+ cells. CD4+ cells purified from Stat1+/+ (□) or Stat1-/- (▪) splenocytes were activated in triplicate wells with plate-bound anti-CD3ε and anti-CD28 antibodies in complete medium supplemented with anti-mIL-2 antibodies and increasing concentrations of mIL-27. [3H]Thymidine (1 μCi per well; 1 Ci = 37 GBq) was added during the last 24 h of this 3-day assay. Results are shown as the fold increase in [3H]thymidine incorporation in the presence of IL-27 compared with its absence (16,478 and 22,857 cpm in Stat1+/+ and Stat1-/- cells, respectively, in the absence of IL-27).
IL-27 Induces T-bet Expression in NK Cells but Does Not Increase NK Effector Functions. The TCCR chain of the IL-27 receptor is expressed on NK cells (16), and IL-27 was shown to synergize with IL-2 and IL-12 to increase IFNγ production by human NK cells (18). Therefore, we further investigated the effect of IL-27 on NK cells in vitro. IL-27, unlike IL-15, does not promote the proliferation of human or murine primary NK cells (Fig. 6_A_ and data not shown).
Fig. 6.
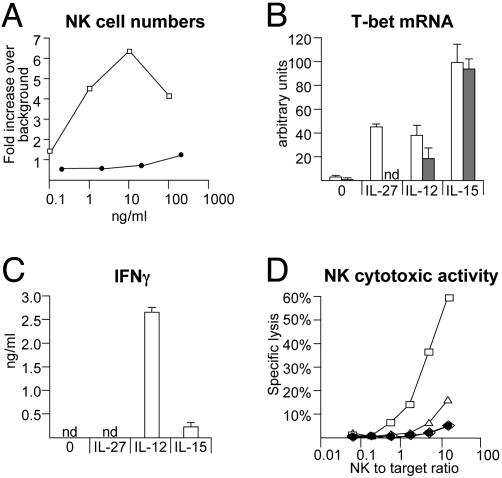
Effects of IL-27 on primary NK cells. (A) IL-27 does not induce the proliferation of primary NK cells. A total of 2 × 106 peripheral blood mononuclear cells from a healthy human donor were seeded in complete medium in the absence or presence of increasing concentrations of hIL-15 (□) or hIL-27 (•). Percentages of NK cells (CD56+/CD3- cells) were determined by FACS analysis before the initiation of the cultures and 7 days later, when cells were collected and counted. Results are indicated as the fold increase in total NK cell numbers in the presence of cytokines over the fold increase in the absence of cytokine (background: 0.54-fold in the absence of cytokine). (B) IL-27, like IL-12 and IL-15, induces T-bet expression in primary NK cells. DX5+ NK cells purified from TCCR+/+ (white bars) or TCCR-/- (gray bars) splenocytes were cultured in vitro for 44 h in the absence or presence of the indicated cytokines. Cells were collected for total RNA preparation, and rpl19 and T-bet mRNA levels were measured by real-time RT-PCR as indicated in Fig. 2. nd, not detectable. (C) IL-27, unlike IL-12 and IL-15, does not induce IFNγ production by primary NK cells. Supernatants from cultures of DX5+ TCCR+/+ NK cells described in B were collected after 44 h, and IFNγ production was measured by ELISA. nd, not detectable. (D) IL-27, unlike IL-12 and IL-15, does not increase the cytotoxic activity of primary NK cells. DX5+ NK cells purified from TCCR+/+ splenocytes were cultured in vitro for 18 h in the absence (⋄) or presence of mIL-27 (•), hIL-15 (□), or mIL-12 (▵). Cells were collected and incubated for 4 h with 51Cr-labeled NK-sensitive YAC-1 target cells at the indicated NK-to-target ratios. Specific lysis of YAC-1 cells was determined by measuring 51Cr released in the supernatants.
Because T-bet expression is induced in NK cell lines by cytokines such as IL-15 and IL-21 (23), we examined whether IL-27 can induce T-bet expression in primary mouse NK cells. IL-27, like IL-12 and IL-15, induces T-bet expression in primary TCCR+/+ NK cells, but not in TCCR_-/_- NK cells (Fig. 6_B_). _T-bet_-/- NK cells were shown to produce reduced amounts of IFNγ and have reduced spontaneous cytotoxic activities (6). However, IL-27-mediated induction of T-bet does not result in increased IFNγ production by NK cells (Fig. 6_C_), nor is it able to stimulate the cytolytic activity of primary NK cells (Fig. 6_D_), in contrast to the direct effects of IL-12 and IL-15.
Discussion
The differentiation of CD4+ T cells into distinct TH subsets is regulated by strongly polarizing cytokines such as IL-12 and IL-4, through signaling by means of Stat4 and Stat6, respectively (4). IL-27, a newly described member of the IL-12 family, has been implicated in TH1 development (16–18, 24), but the exact mechanisms by which IL-27 influences the differentiation of TH cells are unclear.
To better define the roles of IL-27 in TH development, we examined the signaling pathways and, in particular, which Stat proteins are activated by IL-27 in primary CD4+ T cells. We found that IL-27 stimulation leads to phosphorylation of Stat1, -3, -4, and -5. This pattern of Stat activation is more complex than that reported in a recent study in which, in an in vitro system testing direct interactions, Stat1 was the only Stat factor found to bind to the phosphorylated TCCR/WSX-1 chain of the IL-27 receptor (24). However, like most other type I cytokine receptors, the functional IL-27 receptor is probably a heterodimer, as demonstrated by the inability of the TCCR/WSX-1 chain alone to transduce an IL-27 signal in transfected cells (ref. 18 and our unpublished data). Therefore, one possible interpretation of our data is that, upon stimulation with IL-27, Stat1 binds to the TCCR/WSX-1 chain of the IL-27 receptor, whereas binding and/or activation of Stat3, -4, and -5 requires the second, as-yet-unidentified chain of the IL-27 receptor. Only a few cytokine receptor chains of the type I family, including IL-12R β2 and IL-23 receptor, are able to activate Stat4. However, we did not observe any transduction of the IL-27 signal in cells transfected with TCCR/WSX-1 and IL-12R β2 or IL-23 receptor (data not shown), and the identity of the second chain of the IL-27 receptor remains to be determined.
T-bet, GATA-3, and IL-12R β2 represent key regulators of TH cell development. T-bet is a TH1-specific transcription factor whose crucial importance for the development of TH1 responses in vivo is underscored by the susceptibility of T-bet knockout mice to challenge with Leishmania major (25). The expression of T-bet is induced readily in CD4+ T cells by IFNγ signaling mediated by Stat1 (8, 26). However, induction of T-bet expression was observed in splenocytes derived from _IFN_γ knockout mice (26), implying the existence of IFNγ-independent mechanisms of T-bet induction. We demonstrate here that IL-27 provides an IFNγ-independent signal for the induction of T-bet in developing TH cells. Similarly, we show that IL-27 induces T-bet expression in primary NK cells. Although IFNγ-mediated induction of T-bet was shown to strongly depend on Stat1 activation (8), we show that T-bet can be induced in a Stat1-independent manner by IL-27 and under classical TH1 culture conditions in which IL-12 induces IFNγ secretion. The nature of the alternative signaling pathway implicated in the Stat1-independent induction of T-bet expression remains to be determined.
A critical downstream effect of T-bet expression in CD4+ T cells consists in the induction of IL-12R β2 expression (8) and, consequently, acquisition of IL-12 responsiveness by developing TH cells. Consistently, IL-27-mediated induction of T-bet in CD4+ T cells also leads to high levels of IL-12R β2 expression.
GATA-3 is a transcription factor that regulates the expression of a broad array of TH2 cytokines (10, 11) and represents a master switch for TH2 development (13). The expression of GATA-3 is induced rapidly by IL-4, increasing from a low basal level in naïve T cells to a high level in TH2 cells (12). Along with its direct role in promoting TH2 development, GATA-3 inhibits TH1 development by repressing IL-12R β2 expression (12) and, more importantly, by directly down-regulating Stat4 mRNA and protein levels (14). Therefore, suppression of basal GATA-3 expression represents a critical event during TH1 development, necessary to maintain IL-12 responsiveness of developing TH1 cells. IL-12 and IFNγ can suppress GATA-3 expression, and complete suppression requires both Stat1 and Stat4 (12). We show here that IL-27 provides an alternative signal for GATA-3 suppression, which depends on Stat1.
T-bet induction in CD4+ T cells leads to maintenance of high levels of IL-12R β2 expression, but the exact role of T-bet in inducing IFNγ production by TH1 cells is still unclear. Initial studies reported that retroviral overexpression of T-bet can induce IFNγ secretion, independent of IL-12, by CD4+ T cells restimulated with phorbol 12-myristate 13-acetate/ionomycin (6, 7). A later report showed that, in response to more physiological stimuli such as antigen-loaded antigen-presenting cells, retroviral T-bet expression is not able to induce IFNγ production by CD4+ T cells in the absence of IL-12 signaling (8). Our results obtained by stimulating CD4+ T cells with anti-CD3 antibodies are in accordance with the latter study, because IL-27, which up-regulates T-bet expression, does not induce IFNγ production in the absence of IL-12. Comparably, IL-27 can induce T-bet expression in primary NK cells but does not stimulate terminal effector functions such as IFNγ production or cytotoxic activity. Our results supporting the inability of IL-27 to induce IFNγ production on its own might appear contradictory to previous reports, in which IL-27 was initially shown to induce IFNγ production by CD4+ T cells in the absence of exogenous IL-12 (18), or more recently to synergize with IL-12 to increase IFNγ production (24). One major caveat of these two studies is that both analyze IFNγ production directly in the culture supernatants of CD4+ T cells, 3 days after their activation. Because IL-27 potently induces the proliferation of activated naïve CD4+ T cells (ref. 18 and Fig. 5), and because neither IL-12 nor IFNγ was neutralized in the culture conditions described, it is possible that the increase of IFNγ production that was observed in the presence of IL-27 is exclusively due to the proliferative effect of this cytokine on CD4+ T cells, which differentiate into IFNγ-producing cells in response to added or contaminating IL-12.
Although IL-27 does not drive the differentiation process of CD4+ cells toward an IFNγ-secreting phenotype on its own, it certainly represents an important early trigger for TH1 differentiation in vivo, as demonstrated by the increased initial susceptibility of TCCR/WSX-1 knockout mice to infection with intracellular pathogens (16, 17). Our in vitro studies show that IL-27, an early product of activated antigen-presenting cells (18), acts on naïve CD4+ T cells by inducing IL-12 responsiveness by means of induction of T-bet and suppression of GATA-3. Therefore, at the time of activation of naïve CD4+ T cell by antigen-presenting cells in vivo, IL-27 functions in a paracrine manner to establish IL-12 responsiveness of early developing TH cells, and consequently contributes to bias the T cell response toward a TH1 outcome.
Acknowledgments
We thank Dr. R. D. Schreiber for anti-Stat1 antibodies and Fred Arellano and Phil Hass for purification of human and mouse IL-27. Sophie Lucas is supported in part by a fellowship from the Fonds National de la Recherche Scientifique, Belgium.
Abbreviations: TH, T helper; Stat, signal transducer and activator of transcription; NK, natural killer; FACS, fluorescence-activated cell sorting; TCCR, T cell cytokine receptor; mIL, murine IL; hIL, human IL; IL-12R, IL-12 receptor.
References
- 1.Abbas, A. K., Murphy, K. M. & Sher, A. (1996) Nature 383**,** 787-793. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann, T. R. & Sad, S. (1996) Immunol. Today 17**,** 138-146. [DOI] [PubMed] [Google Scholar]
- 3.O'Garra, A. (1998) Immunity 8**,** 275-283. [DOI] [PubMed] [Google Scholar]
- 4.Murphy, K. M. & Reiner, S. L. (2002) Nat. Rev. Immunol. 2**,** 933-944. [DOI] [PubMed] [Google Scholar]
- 5.Wurster, A. L., Tanaka, T. & Grusby, M. J. (2000) Oncogene 19**,** 2577-2584. [DOI] [PubMed] [Google Scholar]
- 6.Szabo, S. J., Kim, S. T., Costa, G. L., Zhang, X., Fathman, C. G. & Glimcher, L. H. (2000) Cell 100**,** 655-669. [DOI] [PubMed] [Google Scholar]
- 7.Mullen, A. C., High, F. A., Hutchins, A. S., Lee, H. W., Villarino, A. V., Livingston, D. M., Kung, A. L., Cereb, N., Yao, T. P., Yang, S. Y. & Reiner, S. L. (2001) Science 292**,** 1907-1910. [DOI] [PubMed] [Google Scholar]
- 8.Afkarian, M., Sedy, J. R., Yang, J., Jacobson, N. G., Cereb, N., Yang, S. Y., Murphy, T. L. & Murphy, K. M. (2002) Nat. Immunol. 3**,** 549-557. [DOI] [PubMed] [Google Scholar]
- 9.Szabo, S. J., Dighe, A. S., Gubler, U. & Murphy, K. M. (1997) J. Exp. Med. 185**,** 817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng, W. & Flavell, R. A. (1997) Cell 89**,** 587-596. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, D. H., Cohn, L., Ray, P., Bottomly, K. & Ray, A. (1997) J. Biol. Chem. 272**,** 21597-21603. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang, W., Ranganath, S. H., Weindel, K., Bhattacharya, D., Murphy, T. L., Sha, W. C. & Murphy, K. M. (1998) Immunity 9**,** 745-755. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang, W., Lohning, M., Gao, Z., Assenmacher, M., Ranganath, S., Radbruch, A. & Murphy, K. M. (2000) Immunity 12**,** 27-37. [DOI] [PubMed] [Google Scholar]
- 14.Usui, T., Nishikomori, R., Kitani, A. & Strober, W. (2003) Immunity 18**,** 415-428. [DOI] [PubMed] [Google Scholar]
- 15.Sprecher, C. A., Grant, F. J., Baumgartner, J. W., Presnell, S. R., Schrader, S. K., Yamagiwa, T., Whitmore, T. E., O'Hara, P. J. & Foster, D. F. (1998) Biochem. Biophys. Res. Commun. 246**,** 82-90. [DOI] [PubMed] [Google Scholar]
- 16.Chen, Q., Ghilardi, N., Wang, H., Baker, T., Xie, M. H., Gurney, A., Grewal, I. S. & de Sauvage, F. J. (2000) Nature 407**,** 916-920. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida, H., Hamano, S., Senaldi, G., Covey, T., Faggioni, R., Mu, S., Xia, M., Wakeham, A. C., Nishina, H., Potter, J., et al. (2001) Immunity 15**,** 569-578. [DOI] [PubMed] [Google Scholar]
- 18.Pflanz, S., Timans, J. C., Cheung, J., Rosales, R., Kanzler, H., Gilbert, J., Hibbert, L., Churakova, T., Travis, M., Vaisberg, E., et al. (2002) Immunity 16**,** 779-790. [DOI] [PubMed] [Google Scholar]
- 19.Rogge, L., Barberis-Maino, L., Biffi, M., Passini, N., Presky, D. H., Gubler, U. & Sinigaglia, F. (1997) J. Exp. Med. 185**,** 825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromberg, J. F., Horvath, C. M., Wen, Z., Schreiber, R. D. & Darnell, J. E., Jr. (1996) Proc. Natl. Acad. Sci. USA 93**,** 7673-7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihle, J. N. (2001) Curr. Opin. Cell Biol. 13**,** 211-217. [DOI] [PubMed] [Google Scholar]
- 22.O'Shea, J. J., Gadina, M. & Schreiber, R. D. (2002) Cell 109**,** Suppl., S121-S131. [DOI] [PubMed] [Google Scholar]
- 23.Strengell, M., Sareneva, T., Foster, D., Julkunen, I. & Matikainen, S. (2002) J. Immunol. 169**,** 3600-3605. [DOI] [PubMed] [Google Scholar]
- 24.Takeda, A., Hamano, S., Yamanaka, A., Hanada, T., Ishibashi, T., Mak, T. W., Yoshimura, A. & Yoshida, H. (2003) J. Immunol. 170**,** 4886-4890. [DOI] [PubMed] [Google Scholar]
- 25.Szabo, S. J., Sullivan, B. M., Stemmann, C., Satoskar, A. R., Sleckman, B. P. & Glimcher, L. H. (2002) Science 295**,** 338-342. [DOI] [PubMed] [Google Scholar]
- 26.Lighvani, A. A., Frucht, D. M., Jankovic, D., Yamane, H., Aliberti, J., Hissong, B. D., Nguyen, B. V., Gadina, M., Sher, A., Paul, W. E. & O'Shea, J. J. (2001) Proc. Natl. Acad. Sci. USA 98**,** 15137-15142. [DOI] [PMC free article] [PubMed] [Google Scholar]