Indian Hedgehog and Its Targets in Human Endometrium: Menstrual Cycle Expression and Response to CDB-2914 (original) (raw)
Abstract
Context: Progesterone is critical for secretory endometrial differentiation in women, but its downstream mediators are poorly understood.
Objective: Our objective was to investigate endometrial expression of Indian Hedgehog (IHH) and genes involved in its signaling [smoothened (SMO), patched-1 (PTCH1), glioma-associated oncogene homolog 1 (GLI1), and _GLI2_] during the menstrual cycle and the effects of the selective progesterone receptor modulator CDB-2914 on its expression.
Design and Setting: Comparisons between normally cycling volunteers and women with symptomatic fibroids who received CDB-2914 or placebo were made at a clinical research center.
Patients and Interventions: Endometrial biopsy was performed on 34 volunteers, 17 additional women with fibroids.
Main Outcome Measures: Endometrial expression of IHH, SMO, PTCH1, GLI1, and GLI2 by in situ hybridization and/or RT-PCR and IHH, GLI1, and PTCH1 immunohistochemistry were evaluated.
Results: RT-PCR showed expression of IHH, SMO, PTCH1, GLI1, and GLI2, with significant increases in IHH (5.2-fold) and GLI1 (3.6-fold) in endometrium exposed to CDB-2914 compared with placebo. In situ hybridization showed IHH mRNA expression in glands and stroma that was stronger in secretory samples. Among volunteers, IHH and GLI1 immunohistochemistry scores were higher in the secretory than proliferative phase in the nuclei and cytoplasm of glands and stroma (P = 0.0002–0.04). Compared with follicular-phase controls, women exposed to CDB-2914 showed increased IHH expression in all compartments except stromal cytoplasm (P = 0.0199–0.0423); GLI1 was up-regulated in glandular nuclei and cytoplasm compared with both volunteers and women receiving placebo (P ≤ 0.0416).
Conclusions: The temporal increase in endometrial IHH and GLI1 during the secretory phase, and their modulation by CDB-2914, suggests progestin regulation and a potential role in endometrial differentiation and implantation.
In human endometrium, Indian hedgehog increases in both nuclei and cytoplasm of glands and stroma in the secretory phase; GLI1 increases in nuclei.
The secreted protein hedgehog (HH), first discovered in a search for modulators of Drosophila embryonic patterning, is highly conserved in vertebrates. Although Drosophila exhibit a single HH gene, multiple HH homologs, including Sonic hedgehog (SHH), Desert hedgehog (DHH), and Indian hedgehog (IHH), have been identified in mammals and likewise appear to be important in the developmental events of many tissues and structures (1).
The classical model of HH signaling maintains that it interacts with a transmembrane tumor suppressor protein, known as patched-1 (PTCH1) (2). Although many details of the HH-PTCH1 interaction remain unclear, the end result is the reversal of PTCH1-mediated inhibition of smoothened (SMO), which occurs via a conformational change in the SMO protein. This conformational change releases SMO, which then activates downstream transcription factors of the glioma-associated oncogene homolog (GLI) family (3).
Although well known for its role in embryonic development, HH signaling recently has been recognized as controlling critical aspects of postnatal development in tissues requiring cell renewal by directing cell proliferation, promoting cell survival, and regulating differentiation (4,5,6). Evidence has emerged that Ihh is expressed in the murine uterus and is influenced by gonadal steroids (6,7). In particular, progesterone has been shown to rapidly induce Ihh expression in murine endometrium, thus stimulating endometrial cell proliferation and differentiation via its downstream targets SMO, PTCH1, GLI1, and GLI2 (8). This progesterone-dependent up-regulation of Ihh expression localizes to the luminal and glandular epithelium of murine endometrium (9) and may underlie an important role in murine implantation (6,10). Similarly, IHH and GLI1 are increased at proestrus and during early pregnancy in the rat (11). By contrast, estrogen exposure down-regulates Ihh and Gli1 expression in the immature rat (7).
Current evidence supports a possible role of IHH in rodent endometrium, yet little is known about its expression or regulation in human endometrium. Thus, the goal of this study was to determine whether IHH was differentially expressed during the menstrual cycle, because cyclic expression may indicate hormonal regulation and suggest a specific function. Furthermore, because progesterone regulates murine endometrial IHH expression, we evaluated women receiving long-term administration of CDB-2914, a selective progesterone receptor modulator, to characterize its effect on endometrial IHH (12).
Subjects and Methods
The Institutional Review Board of the National Institute of Child Health and Human Development approved the experimental protocols of this study [95-CH-0110, 02-CH-0287 (NCT00044876), and 06-CH-0090 (NCT00290251)], and all subjects gave written and oral informed consent. Exclusion criteria included the use of glucocorticoids or megesterol within 1 yr, use of hormonal compounds within 8 wk, or therapy affecting ovarian or hepatic function. All women charted menses for 1–3 months before endometrial biopsy.
Premenopausal women with fibroids received CDB-2914 at a daily dose of 10 or 20 mg, or inert placebo, for approximately 3 months before hysterectomy (13). Blood was obtained approximately every 2 wk for estradiol and progesterone measurement, and women charted vaginal bleeding days. A gene microarray analysis of endometria from these women [Affymetrix (Santa Clara, CA) U133 Plus 2.0, data not shown], showed differential expression of IHH and GLI1, based on a 2-fold cutoff change and P values <0.02. To validate and extend these findings, we evaluated the mRNA expression of IHH in endometrium of women given CDB-2914 and evaluated protein expression in these and normally cycling women.
Endometrial samples
Endometrial samples were obtained from healthy premenopausal women with normal menstrual function (n = 34). None had a history suggestive of endocrinological or gynecological disorders or subfertility. Each woman had normal menstrual cycle dynamics, defined as a cycle length of 24–35 d, with a secretory phase of at least 12 d based on in-home detection of an LH surge (OvuQUICK; Quidel, San Diego, CA). Women were randomly assigned to undergo endometrial biopsy in the proliferative or secretory phase of the menstrual cycle based on timing of the last menses and the LH surge. Samples were obtained using the Pipelle curette (Unimar, Wilton, CT). Serum estradiol and progesterone were measured on that day.
Endometrial samples from premenopausal women receiving CDB-2914 at 10 mg (n = 6) or 20 mg (n = 6) or placebo (n = 5) were collected at the time of scheduled hysterectomy at the National Institutes of Health Clinical Center, as previously described (13). A portion of endometrium from fibroid patients was placed in RNAlater (QIAGEN, Valencia, CA) before isolation of total RNA.
For all women, endometrial samples were placed in 4% paraformaldehyde before paraffin embedding. Paraffin blocks were sectioned, and representative slides were stained with hematoxylin and eosin and dated according to the Noyes criteria by clinical pathology staff (14).
Endometria from normally cycling women were grouped by the histological date as early (d 2–5), mid (d 6–10), or late (d 11–14) proliferative or early (cycle d 15–18), mid (cycle d 19–23), or late (cycle d 24–28) secretory phase. Samples from women with fibroids were characterized as proliferative, secretory, or hyperplastic. Menstrual calendars and estradiol and progesterone levels were assessed to determine anovulation (progesterone <3 ng/ml) and amenorrhea.
Quantitative RT-PCR methods
RT-PCR was performed on proliferative-phase endometria from fibroid patients. Total RNA was isolated from tissue homogenates using TRIzol reagent (Invitrogen, Carlsbad, CA). The quality of the purified RNA was verified on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA), and concentrations were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Gene-specific TaqMan probe/primers and TaqMan universal master mix (Applied Biosystems Inc., Foster City, CA) were used to measure transcript levels. Briefly, 3 μg total RNA was subjected to reverse transcription using a first-strand cDNA synthesis kit (Invitrogen) according to the manufacturer’s instructions. One tenth of the reaction mixture was used as template for each PCR using a 7500 real-time PCR system (Applied Biosystems, Foster City, CA). Each sample was assayed in triplicate. Specific 6-carboxyfluorescein (FAM)-labeled probes and forward and reverse primers for IHH, PTCH1, SMO, and GLI2 were selected from Assay on Demand (Applied Biosystems, Branchburg, NJ). Negative controls included the use of RNA without reverse transcriptase (for reverse transcription) and water instead of cDNA (for PCR). With primers synthesized in the laboratory, the SYBR (Applied Biosystems, Foster City, CA) method was used to measure GLI1 (sense 5′-TTCCTACCAGAAGTCCAAGT-3′ and antisense 5′-CCCTATGTGAAGCCCTATTT-3′), progesterone receptors A and B (PR-A&B) (antisense 5′-AGC CCA CAA TAC AGC TTC GAG-3′ and sense 5′-TTT CGA CCT CCA AGG ACC AT-3′) and PR-B (antisense 5′-ACT GAG CTG AAG GCA AAG GGT-3′ and sense 5′-GTC CTG TCC CTG GCA GGG C-3′). The primers for PR-A&B are located in the 3′ common region and those for PR-B are located in the 5′ region of the gene that is unique to PR-B. The PCR thermal cycling conditions were 50 C for 2 min, 95 C for 10 min, and then 40 cycles of 95 C for 1 min and 60 C for 1 min. The values of genes of interest were normalized to those of β-actin.
In situ hybridization
A 720-bp cDNA fragment at the 3′ region of IHH was generated from total human endometrial RNA by PCR and was cloned into pCRII-Topo vector (Invitrogen). Sense and antisense riboprobes were synthesized and labeled with digoxigenin-UTP by in vitro transcription [Dig RNA labeling kit (SP6/T7); Roche Applied Science Inc., Indianapolis, IN] according to the manufacturer’s instructions. Secretory-phase slides from healthy volunteers were deparaffinized, and the tissue sections were permeabilized in 0.1 N HCl, neutralized in 1× PBS, and acetylated in 0.1 m triethanolamine (pH 8.0) buffer containing fresh acetic anhydride. They were then incubated with no probe, poly-dT probe, sense probe, or antisense probe at 52 C overnight. Next, the alkaline phosphatase-conjugated anti-Dig antibody (Roche) was added to the slides after blocking, and the slides were incubated with nitroblue tetrazolium chloride/5-bromo-4-chloro-3′-indolylphosphate _p_-toluidine salt (NBT/BCIP) substrate at 4 C overnight. Slides were counterstained with nuclear fast red for 4 min.
Immunohistochemistry (IHC)
IHC was performed on specimens from fibroid patients and volunteers. Slides were deparaffinized, hydrated, and quenched in 5% hydrogen peroxide. They were then heated in a microwave in 0.01 m citric acid (pH 6.0) for 20 min at 95 C for antigen retrieval. After cooling, slides were blocked for 60 min using normal horse serum (4% for IHH, 5% for GLI1). Primary goat antihuman IHH polyclonal antibody (sc-1196; Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit antihuman PTCH1 polyclonal antibody (ab39266; Abcam Inc., Cambridge, MA), or a rabbit antihuman GLI1 polyclonal antibody (sc-20687; Santa Cruz) was applied, and the slides were incubated overnight at 4 C. Two washings with PBS (PBS-Tween 20 for Gli1) were performed before incubating for 30 min at room temperature with the secondary antibody (IHH: biotinylated antigoat; GLI1 and PTCH1: biotinylated antirabbit IgG; Vector Laboratories, Burlingame, CA). The slides were developed for 3.5 min in peroxidase substrate solution (Vector Laboratories), counterstained for 1 min with hematoxylin, and then washed in running tap water for 5 min. The slides were then dehydrated in successive dilutions of ethanol and finally in three xylene washes. Anonymized cervical tissue was used as negative control.
Data capture and analysis
The RT-PCR results were converted into fold change based on a doubling of PCR product in each cycle, according to the manufacturer’s guidelines. The ΔΔCt was used for statistical analysis. Wilcoxon rank sum two-sample tests were used to compare groups.
Glandular and stromal cytoplasm and nuclei IHC results were evaluated independently, based on at least 200 cells per specimen. Because cytoplasmic staining was consistent, the relative staining intensity (i) was scored by two independent examiners on a 0–3 scale with 0.5 increments. Because the nuclear staining varied across a field, an H-score = ΣPi(i + 1) was calculated for each specimen, where Pi is the percentage of cells for each intensity, varying from 0–100%. Differences in H-scores and staining intensity were analyzed by the Kruskal-Wallis test followed by Dunette’s or Tukey’s post hoc procedure for comparisons. For all statistical interpretations, a P value <0.05 was considered significant.
Results are presented as mean ± se unless otherwise specified. Statistical analyses were performed using JMP version 7 (SAS Institute Inc., Cary, NC).
Results
There were five proliferative-phase specimens from the fibroid patients who received placebo and 12 from those who received CDB-2914. One CDB-2914-treated patient demonstrated complex hyperplasia without atypia, consistent with progestin modulator-associated endometrial changes (PAEC) (12). Each CDB-2914-treated fibroid patient had amenorrhea and was anovulatory, whereas women who received placebo were ovulatory and had menses, as previously described (13). As a result, none of the CDB-2914 samples had secretory histology.
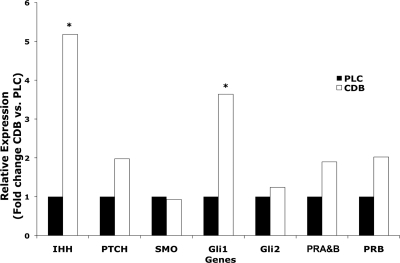
RT-PCR demonstrated IHH expression, and the expression of genes involved in its signaling, PTCH1, SMO, GLI1, and GLI2, in proliferative-phase endometria from patients (n = 12). Compared with placebo (n = 5), CDB-2914 significantly up-regulated IHH and GLI1 (P ≤ 0.035) but did not affect PTCH1, SMO, or GLI2. By RT-PCR, there was a trend to increased PR-A&B (1.9-fold change, P = 0.0618) and PR-B (2.0-fold change, P = 0.14) in CDB-2914-exposed endometria, but these were not statistically significant (Fig. 1).
Figure 1.
Relative expression of IHH, PTCH1, SMO, GLI1, GLI2, PR-A&B, and PR-B in proliferative-phase endometrium as determined by RT-PCR. Samples were obtained after 3 months administration of placebo (black bars) or CDB-2914 (white bars). *, Administration of CDB-2914 resulted in differential gene expression of IHH and GLI1 (P < 0.035) compared with placebo.
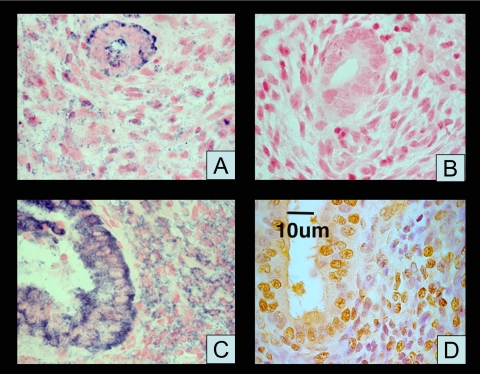
In situ hybridization showed moderate staining in stromal and strong staining in glandular compartments in the CDB-2914-treated proliferative-phase endometrium and normal secretory-phase endometrium (Fig. 2). The sense and no-probe (data not shown) results were indistinguishable.
Figure 2.
In situ hybridization for IHH mRNA and IHC for IHH in representative endometrium. A, Purple staining in proliferative-phase glands and stroma with antisense probe; B, lack of staining with sense probe; C, purple staining in secretory glands and stroma with antisense probe; D, IHC for IHH; brown indicates positive staining.
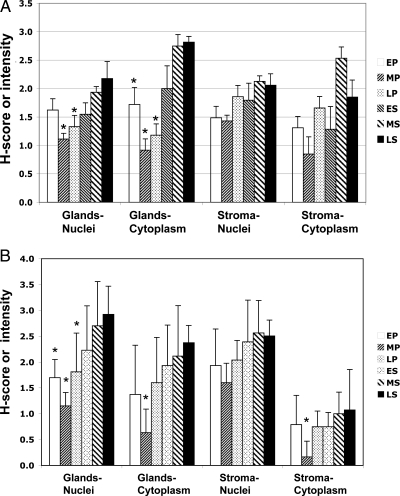
In healthy volunteers, proliferative-phase endometrial samples were identified as early (n = 5), middle (n = 6), or late (n = 5). Likewise, secretory-phase samples were dated as early, mid, and late (each n = 6). IHC showed differential expression of both IHH and GLI1 during the normal menstrual cycle. Compared with follicular samples, IHH increased during the secretory phase, which had significantly higher mean scores in all tissue compartments (glandular nuclei P = 0.0042; glandular cytoplasm P < 0.001; stromal nuclei P = 0.0178; stromal cytoplasm P = 0.0256) (Table 1). Staining intensity was similar among the early, middle, and late secretory-phase tissues. In comparison with the late secretory phase, the proliferative-phase scores were significantly lower in the glandular compartment (Figs. 3A and 4A). In sections containing both glandular and luminal epithelium, IHH staining was similar in both areas.
Table 1.
IHC scores in proliferative endometrium tissue compartments of healthy cycling women and women receiving CDB-2914 for 3 months
| Gland nuclei (H-scores) | Gland cytoplasm (intensity) | Stroma nuclei (H-scores) | Stroma cytoplasm (intensity) | |
|---|---|---|---|---|
| IHH (n) | ||||
| Healthy volunteers proliferative (16) | 1.3 ± 0.1 | 1.3 ± 0.2 | 1.6 ± 0.1 | 1.3 ± 0.2 |
| Healthy volunteers secretory (18) | 1.9 ± 0.1c | 2.5 ± 0.2d | 2.0 ± 0.1a | 1.9 ± 0.2a |
| CDB-2914 proliferative (10) | 2.3 ± 0.3c | 2.1 ± 0.2c | 2.3 ± 0.2c | 0.9 ± 0.1 |
| Placebo proliferative (5) | 1.4 ± 0.2 | 1.2 ± 0.3 | 1.5 ± 0.2 | 0.8 ± 0.1 |
| GLI1 (n) | ||||
| Healthy volunteers proliferative (16) | 1.5 ± 0.1 | 1.2 ± 0.2 | 1.8 ± 0.2 | 0.6 ± 0.1 |
| Healthy volunteers secretory (18) | 2.6 ± 0.2d | 2.2 ± 0.2c | 2.5 ± 0.1b | 1 ± 0.1a |
| CDB-2914 proliferative (10) | 2.5 ± 0.3a | 1.7 ± 0.3a | 2.1 ± 0.3 | 0.6 ± 0.1 |
| Placebo proliferative (5) | 1.8 ± 0.1 | 1.0 ± 0.2 | 1.5 ± 0.2 | 0.2 ± 0.1 |
Figure 3.
IHC scores for IHH (A) and GLI1 (B) during the menstrual cycle in normally cycling volunteers. Histological dating included early (EP), mid (MP), and late (LP) proliferative phase and early (ES), mid (MS), and late (LS) secretory phase. Numbers represent mean ± se H-scores for nuclei and average stain intensity for cytoplasm. Asterisks indicate a significant difference between one or more proliferative-phase results compared with the late secretory phase (for IHH: glandular nuclei all P < 0.02, glandular cytoplasm P < 0.001 for mid- and late-proliferative and 0.032 for early proliferative; for GLI1: glandular nuclei P ≤ 0.02, glandular cytoplasm P = 0.0018, stromal cytoplasm P < 0.02).
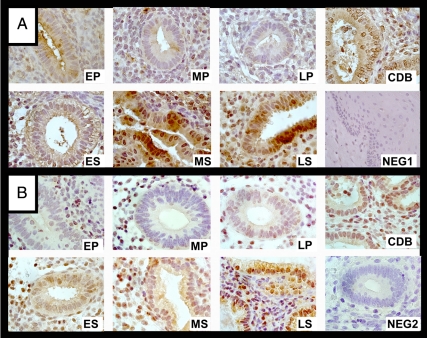
Figure 4.
Representative photomicrographs of endometrial IHC results for IHH (A) and GLI1 (B) in a woman treated with CDB-2914 (CDB), in normal cervix (NEG1, negative control), in endometrium without primary antibody (NEG2), and in normally cycling women in the early (EP), mid- (MP), and late (LP) proliferative phase and the early (ES), mid- (MS), and late (LS) secretory phase. Brown indicates positive staining. Magnification, ×100 for all images.
In volunteer subjects, GLI1 also was up-regulated in all compartments in the secretory phase compared with the proliferative phase (glandular nuclei P = 0.0002; glandular cytoplasm P = 0.0015; stromal nuclei P = 0.0099; stromal cytoplasm P = 0.0375) (Table 1). As with IHH, the early, middle, and late secretory-phase values were not significantly different, but when compared with the late-secretory values, IHC scores for all of the proliferative-phase glandular nuclei were lower, as were the mid-proliferative-phase glandular and stromal cytoplasm (Figs. 3B and 4B). Cervical samples did not show IHH or GLI1 staining (Fig. 3).
IHH and Gli1 staining were relatively consistent within each tissue compartment at each time point. In some serial sections in the mid- and late-secretory phase, it appears that the staining intensity of Gli1 was proportional to that of IHH in the same cells (Supplemental Data, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). As a result, there is not strong evidence regarding whether IHH might act as an autocrine or paracrine factor.
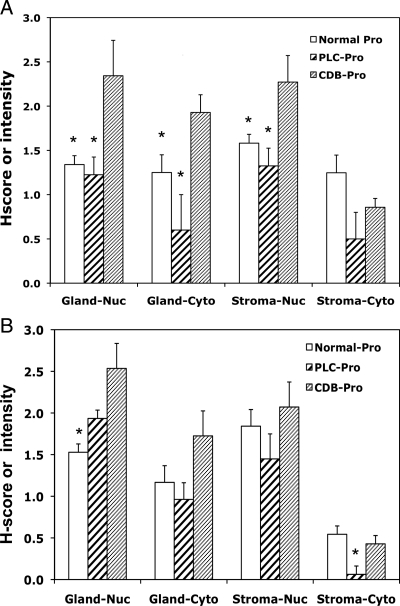
Mean proliferative-phase IHH and GLI1 staining intensity scores were not statistically different in healthy volunteers and fibroid patients who received placebo (Fig. 5). In proliferative phase samples, women receiving CDB-2914 had significantly higher IHH protein expression compared with healthy volunteers or the placebo group in all tissue compartments except stromal cytoplasm (CDB-2914 vs. volunteers: glandular nuclei P = 0.0029, glandular cytoplasm P = 0.0025, stromal nuclei P = 0.0040; CDB-2914 vs. placebo: glandular nuclei P = 0.0358, glandular cytoplasm P = 0.0242, stromal nuclei P = 0.0167) (Table 1 and Fig. 4A). The CDB-2914 group also had significantly up-regulated GLI1 H-scores in the glandular nuclei and cytoplasm compared with both volunteers and women receiving placebo (P ≤ 0.0416) (Table 1 and Fig. 5).
Figure 5.
IHC scores for glandular and stroma cytoplasm and nuclei for IHH (A) and GLI1 (B) in normally cycling volunteers (normal) and women with fibroids who received CDB-2914 (CDB) or placebo (PLC) for 3 months. All biopsies had proliferative-phase (Pro) histological dating. Numbers represent mean ± se H-scores for nuclei (nuc) and average stain intensity for cytoplasm (cyto). Asterisks indicate a significant difference between the CDB-exposed specimens compared with either the normal or PLC group.
PTCH1 expression was similar in each compartment of endometrium in women who received CDB or placebo (P ≥ 0.4611, data not shown).
Discussion
Although it is well known that progesterone is critical for secretory endometrial differentiation in women, the downstream mediators of its action have only recently begun to be established. In the mouse, endometrial Ihh expression is modulated by progesterone and plays a vital role in murine embryonic development and in tumor growth, and endometrial null Ihh mice do not respond to progesterone (10). To our knowledge, this study is the first to demonstrate progesterone-associated IHH endometrial expression in women at both the transcriptional and translational level. In addition, surprisingly, the selective progesterone receptor modulator (SPRM) CDB-2914 enhanced IHH expression in proliferative endometrium.
As in the mouse, endometrial IHH expression in women was up-regulated during progesterone exposure. GLI1 has been reported to be present (15) and absent (16) in human endometria that were not characterized by cycle phase. In this study, GLI1 expression paralleled the IHH increase from the proliferative to secretory phases. There are two progesterone-response elements in the human IHH promoter but none in the GLI1 promoter. This provides a potential mechanism by which progesterone could up-regulate IHH, whereas the increase in GLI1 expression likely reflects modulation by IHH. However, a report using an endometrial-specific progesterone receptor knockout demonstrated that stromal, and not epithelial, progesterone receptor is required for glandular Ihh induction (10). Thus, a progesterone-responsive stromal factor may be involved.
By analogy with the mouse, we speculate that IHH might be important in human implantation, and studies of endometrium from women with impaired fertility would be of interest. Women with endometriosis appear to have a defect in progesterone-dependent peptides during the window of implantation, and we speculate that IHH also may be abnormal in this setting (17).
In contrast to the restricted glandular expression in the mouse (8) and the hedgehog (18), we found that IHH mRNA and protein were present in both the stromal and epithelial compartments. GLI1 increased in the secretory phase, especially in the glandular nuclei, suggesting a role as a paracrine downstream target of IHH in that compartment, but not in the stroma. Because IHH and Gli1 staining was similar within any tissue compartment, this study does not provide evidence regarding whether IHH might act as an autocrine factor as well.
The nuclear localization of IHH was surprising, because it is thought to act as a secreted autocrine or paracrine factor that ultimately induces transcription factors such as Gli1 after receptor binding. Most previous reports with high-magnification IHC demonstrate cytoplasmic and not nuclear expression (5). However, one report localized IHH and PTCH1 C terminus to the nucleus and cytoplasm in human and mouse sebaceous cell tumors (19).
CDB-2914 increased the transcriptional and translational expression of IHH and its downstream target GLI1 after 3 months treatment in vivo. This up-regulation of IHH associated with CDB-2914 administration presumably represents a progesterone-like effect. However, the endometrial histology did not show a progesterone agonist (secretory) effect, but rather an estrogen-dependent proliferative pattern. Thus, in a single tissue, this SPRM seemed to show both an agonist effect and a lack of effect in these anovulatory, amenorrheic women.
Mixed agonist-antagonist actions of CDB-2914 have been reported from in vitro studies of the estrogen receptorα- and PR-negative breast cancer cell line MDA-MB-231 transfected with PR-A or PR-B. In those studies, CDB-2914 inhibited cell growth, an agonist effect, through PR-A and PR-B. CDB-2914 also had agonist activity (increased cell motility and induction of cell spreading) through PR-B. By contrast, it had an antagonist effect on cell morphology and cell migration through PR-A (20). More recently, the agonist activity of a chemically similar compound, mifepristone, has been shown to be dependent on N-terminal domain coactivators and phosphorylation (20,21).
Taken together, these data suggest that increased PR-B relative to PR-A might cause an agonist effect. However, although there was a trend toward increased PR-A&B and PR-B expression with CDB-2914 exposure, this did not reach statistical significance, and the proportional increase was similar (about 2-fold). Thus, the effect on IHH was not explained by a change in PR expression. Possible differences in receptor phosphorylation or coactivator availability in CDB-2914-exposed endometria were not evaluated.
Some women who receive SPRM display an unusual type of endometrial hyperplasia, without atypia (PAEC), whose etiology is not understood (12). The association of endometrial proliferation with IHH expression in the mouse suggests the hypothesis that abnormal expression of IHH might be linked to PAEC in women (22). The single woman with PAEC in this study had IHH tissue expression similar to others with normal histology. Additional studies are needed to evaluate further the potential relationship of IHH to PAEC.
In conclusion, we demonstrate the presence of IHH and its downstream partner GLI1 in the endometrium of normally cycling women, with an increase during the secretory phase that suggests progesterone modulation. Surprisingly, the SPRM CDB-2914 had a progestin-like ability to increase IHH in proliferative-phase endometrium. Thus, this major progesterone-dependent signaling pathway is conserved in the mouse and human, with species-specific differences in cell distribution. We speculate that IHH may play an important role in cellular proliferation, differentiation, and implantation in the human, as it does in the mouse.
Supplementary Material
[Supplemental Data]
Footnotes
This work was supported by the Program in Reproductive and Adult Endocrinology, of the intramural program of National Institute of Child Health and Human Development, National Institutes of Health (Bethesda, MD), and by funds provided through a Cooperative Research and Development Agreement with Laboratoire HRA-Pharma, Paris, France.
This work was presented in part at the 63rd Annual Meeting of The American Society for Reproductive Medicine, Washington, D.C., October 13–17, 2007.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 29, 2010
Abbreviations: GLI, Glioma-associated oncogene homolog; HH, hedgehog; IHC, immunohistochemistry; IHH, Indian hedgehog; PAEC, progestin modulator-associated endometrial changes; PR-A&B, progesterone receptor A and B; PTCH1, patched-1; SMO, smoothened; SPRM, selective progesterone receptor modulator.
References
- McMahon AP, Ingham PW, Tabin CJ 2003 Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 53:1–114 [DOI] [PubMed] [Google Scholar]
- Cohen Jr MM 2003 The hedgehog signaling network. Am J Med Genet A 123A:5–28 [DOI] [PubMed] [Google Scholar]
- Katoh Y, Katoh M 2008 Hedgehog signaling, epithelial-to-mesenchymal transition and miRNA. Int J Mol Med 22:271–275 [PubMed] [Google Scholar]
- Ingham PW, McMahon AP 2001 Hedgehog signaling in animal development: Paradigms and principles. Genes Dev 15:3059–3087 [DOI] [PubMed] [Google Scholar]
- Kang DH, Han ME, Song MH, Lee YS, Kim EH, Kim HJ, Kim GH, Kim DH, Yoon S, Baek SY, Kim BS, Kim JB, Oh SO 2009 The role of hedgehog signaling during gastric regeneration. J Gastroenterol 44:372–379 [DOI] [PubMed] [Google Scholar]
- Walterhouse DO, Lamm ML, Villavicencio E, Iannaccone PM 2003 Emerging roles for hedgehog-patched-Gli signal transduction in reproduction. Biol Reprod 69:8–14 [DOI] [PubMed] [Google Scholar]
- Katayama S, Ashizawa K, Gohma H, Fukuhara T, Narumi K, Tsuzuki Y, Tatemoto H, Nakada T, Nagai K 2006 The expression of hedgehog genes (Ihh, Dhh) and hedgehog target genes (Ptc1, Gli1, Coup-Tfii) is affected by estrogenic stimuli in the uterus of immature female rats. Toxicol Appl Pharmacol 217:375–383 [DOI] [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY, DeMayo FJ 2002 Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol 16:2338–2348 [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK 2002 Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol 245:280–290 [DOI] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ 2006 Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 38:1204–1209 [DOI] [PubMed] [Google Scholar]
- Kubota K, Yamauchi N, Matsumoto K, Watanabe R, Oozono S, Aramaki S, Wood C, Soh T, Hattori MA 2008 Expression of hedgehog family genes in the rat uterus during early pregnancy. J Reprod Dev 54:340–345 [DOI] [PubMed] [Google Scholar]
- Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, Williams AR, Blithe DL 2008 The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol 21:591–598 [DOI] [PubMed] [Google Scholar]
- Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, Blocker W, Nieman LK 2008 CDB-2914 for uterine leiomyomata treatment: A randomized controlled trial. Obstet Gynecol 111:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J 1975 Dating the endometrial biopsy. Am J Obstet Gynecol 122:262–263 [DOI] [PubMed] [Google Scholar]
- Liao X, Siu MK, Au CW, Chan QK, Chan HY, Wong ES, Ip PP, Ngan HY, Cheung AN 2009 Aberrant activation of hedgehog signaling pathway contributes to endometrial carcinogenesis through β-catenin. Mod Pathol 22:839–847 [DOI] [PubMed] [Google Scholar]
- Feng YZ, Shiozawa T, Miyamoto T, Kashima H, Kurai M, Suzuki A, Ying-Song J, Konishi I 2007 Overexpression of hedgehog signaling molecules and its involvement in the proliferation of endometrial carcinoma cells. Clin Cancer Res 13:1389–1398 [DOI] [PubMed] [Google Scholar]
- Wei Q, St Clair JB, Fu T, Stratton P, Nieman LK 2009 Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertil Steril 91:1686–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatua A, Wang X, Ding T, Zhang Q, Reese J, DeMayo FJ, Paria BC 2006 Indian hedgehog, but not histidine decarboxylase or amphiregulin, is a progesterone-regulated uterine gene in hamsters. Endocrinology 147:4079–4092 [DOI] [PubMed] [Google Scholar]
- Niemann C, Unden AB, Lyle S, Zouboulis Ch C, Toftgard R, Watt FM 2003 Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci USA 100(Suppl 1):11873–11880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo JC, Lin VC 2008 The activities of progesterone receptor isoform A and B are differentially modulated by their ligands in a gene-selective manner. Int J Cancer 122:230–243 [DOI] [PubMed] [Google Scholar]
- Wardell SE, Narayanan R, Weigel NL, Edwards DP 2010 Partial agonist activity of the progesterone receptor antagonist RU486 mediated by an amino-terminal domain coactivator and phosphorylation of serine400. Mol Endocrinol 24:335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Lee KY, Broaddus RR, White LD, Lanske B, Lydon JP, Jeong JW, DeMayo FJ 2010 Ablation of Indian hedgehog in the murine uterus results in decreased cell cycle progression, aberrant epidermal growth factor signaling, and increased estrogen signaling. Biol Reprod 82:783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]