Global capacity for emerging infectious disease detection (original) (raw)
Abstract
The increasing number of emerging infectious disease events that have spread internationally, such as severe acute respiratory syndrome (SARS) and the 2009 pandemic A/H1N1, highlight the need for improvements in global outbreak surveillance. It is expected that the proliferation of Internet-based reports has resulted in greater communication and improved surveillance and reporting frameworks, especially with the revision of the World Health Organization's (WHO) International Health Regulations (IHR 2005), which went into force in 2007. However, there has been no global quantitative assessment of whether and how outbreak detection and communication processes have actually changed over time. In this study, we analyzed the entire WHO public record of Disease Outbreak News reports from 1996 to 2009 to characterize spatial-temporal trends in the timeliness of outbreak discovery and public communication about the outbreak relative to the estimated outbreak start date. Cox proportional hazards regression analyses show that overall, the timeliness of outbreak discovery improved by 7.3% [hazard ratio (HR) = 1.073, 95% CI (1.038; 1.110)] per year, and public communication improved by 6.2% [HR = 1.062, 95% CI (1.028; 1.096)] per year. However, the degree of improvement varied by geographic region; the only WHO region with statistically significant (α = 0.05) improvement in outbreak discovery was the Western Pacific region [HR = 1.102 per year, 95% CI (1.008; 1.205)], whereas the Eastern Mediterranean [HR = 1.201 per year, 95% CI (1.066; 1.353)] and Western Pacific regions [HR = 1.119 per year, 95% CI (1.025; 1.221)] showed improvement in public communication. These findings provide quantitative historical assessment of timeliness in infectious disease detection and public reporting of outbreaks.
Keywords: disease reporting, disease surveillance, epidemiology, disease outbreaks, public health
Infectious disease events, especially those resulting from novel emerging pathogens, have significantly increased over the past few decades, possibly as a result of alterations in various environmental, biological, socioeconomic, and political factors (1–4). Trends in globalization, including expansion in international travel and trade, have also extended the reach and increased the pace at which infectious diseases spread (5, 6), prompting the need for more rapid outbreak detection and reporting along with improved transparency to minimize the burden on global health and the economy.
Historically, outbreaks have been reported through a structured, multilevel public health infrastructure that can involve lengthy delays in information transmission. After event onset, it takes an average of 15 d before the event is detected, another 12–24 h before the World Health Organization (WHO) is notified, and then another 7 d before the event is verified,* with longer delays where public health infrastructure is lacking or weak, or where political pressure or fear of economic repercussions may suppress information from being relayed beyond local boundaries (7).
The increasing number of emerging infectious disease events of international concern, such as severe acute respiratory syndrome (SARS) and the 2009 pandemic influenza A/H1N1, dictate a specific need to increase bidirectional communication between local governments and the international community. Recognizing this need, the Global Outbreak Alert and Response Network (GOARN) was formed in 2000 as a global collaboration to consolidate technical support for outbreak surveillance and response efforts (8), and the WHO's International Health Regulations (IHR 2005) were revised to update surveillance capacity standards and mandate reporting of disease events that may constitute “public health emergencies of international concern” (9).
The rapid expansion in Internet access and utilization over the past decade has also potentially provided a more open route for reporting that could push local governments toward greater transparency. Internet data therefore may serve as a valuable, timely, and informative data source that complements traditional public health infrastructure. There now exist several early warning systems that collect disease-related information from informal sources, examples being the International Society for Infectious Diseases’ Program for Monitoring Emerging Diseases (ProMED-mail) (10), the Public Health Agency of Canada's Global Public Health Intelligence Network (GPHIN) (11, 12), HealthMap (13, 14), Argus (15), MedISys (15), and BioCaster (16).
Although there have been many changes and developments over the course of time that are expected to improve epidemic surveillance, there has been no widescale quantitative assessment of the trends in outbreak discovery and public communication processes, and aside from one study (2), little effort has gone toward a detailed historical record of confirmed outbreaks. In this study, we analyzed the entire WHO public record of Disease Outbreak News reports and created a catalog of selected WHO-confirmed outbreaks that occurred during 1996–2009. This dataset was supplemented with information from corresponding informal reports found by searching three Web-based informal outbreak-reporting systems (ProMED, GPHIN, and HealthMap). Characterizing spatial-temporal trends over the past 14 y, we provide a quantitative historical assessment of timeliness of global infectious disease detection and public reporting of outbreaks.
Results
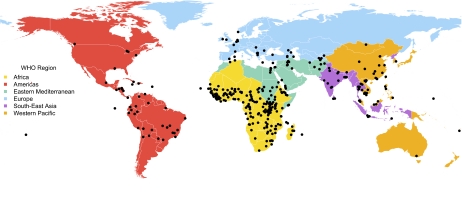
The final dataset consisted of 398 WHO-verified outbreaks that occurred between 1996 and 2009, although only 281 remained for the analyses once those without information indicating outbreak start dates were eliminated. Broken down by WHO region, 53% of the outbreaks occurred in Africa, 11% in the Eastern Mediterranean, 11% in the Western Pacific, 10% in the Americas, 7% in Europe, and 7% in South-East Asia; 2% fell within jurisdictions not classified into WHO regions (Fig. 1). The most common diseases in the dataset were cholera (29%), yellow fever (12%), meningitis (9%), avian influenza (9%), and dengue (5%).
Fig. 1.
Geographical distribution of a subset of outbreaks confirmed and reported by WHO, 1996–2009. Points mark the reported origin of the outbreak, or if unknown, where the highest reported morbidity and mortality rates were reported. (World borders dataset downloaded from http://thematicmapping.org/.)
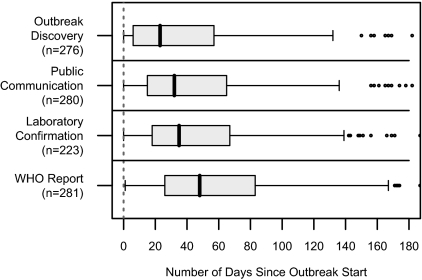
Fig. 2 shows the distribution of the time difference between estimated outbreak start date and various key dates of interest. Median time to these “milestones” were
- earliest reported date of a case being detected (outbreak discovery): 23 d [95% confidence interval, CI (18; 30)];
- earliest date of a public communication (local or international, informal or official, verbal or written) about the outbreak: 32 d [95% CI (28; 38.5)];
- date of an official laboratory confirmation: 35 d [95% CI (32; 47)]; and
- date of the WHO's Disease Outbreak News report about the outbreak: 48 d [95% CI (40; 56)].
Fig. 2.
Box plots of the median time between estimated outbreak start and various outbreak milestones for a subset of WHO-confirmed outbreaks, 1996–2009. Public communication refers to the earliest date of the public being informed about the existence of cases. WHO report refers to the date of WHO's Disease Outbreak News report about the outbreak. Some extreme outliers are not shown. n, sample size.
Looking at our milestones of interest, median time from outbreak start to outbreak discovery and to public communication about the outbreak generally decreased over time, from 29.5 d [95% CI (13.5; 59.0)] in 1996 to 13.5 d [95% CI (3.5; 44.5)] in 2009 for outbreak discovery, and from 40 d [95% CI (23.5; 80)] in 1996 to 19 d [95% CI (11.5; 56.5)] in 2009 for public communication (Fig. 3). With respect to when the revised IHR went into force in 2007, median time from outbreak start to outbreak discovery was 28 d [95% CI (20; 32)] before their implementation and 7 d [95% CI (4; 14)] after implementation, whereas for public communication, it was 33 d [95% CI (29; 40)] and 23 d [95% CI (17; 43)], respectively.
Fig. 3.
Box plots of the temporal trends in the yearly median time between estimated outbreak start and (A) outbreak discovery and (B) public communication about the outbreak for selected WHO-verified outbreaks, 1996–2009. The revised International Health Regulations (IHR 2005) went into effect in 2007.
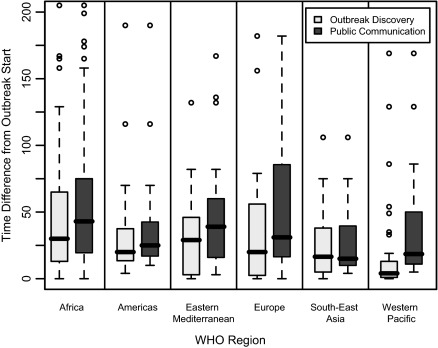
These lags from outbreak start also varied by geographic region, with the longest delays, on average, in Africa [30 d, 95% CI (24; 41), and 43 d, 95% CI (31; 51), for outbreak discovery and public communication, respectively] and the Eastern Mediterranean [29 d, 95% CI (10; 44), and 39 d, 95% CI (20; 54)], and the shortest delays in South-East Asia [16.5 d, 95% CI (6; 34), and 15 d, 95% CI (11; 38)] and the Western Pacific [4 d, 95% CI (3; 7), and 18.5 d, 95% CI (12.5; 30.5)] (Fig. 4).
Fig. 4.
Box plots of the median time difference from estimated outbreak start to outbreak discovery and public communication about the outbreak for selected WHO-verified outbreaks,1996–2009, across various WHO regions. Extreme outliers are not shown.
The results of the univariate Cox proportional hazards regression analyses show that overall, the timeliness of outbreak discovery improved by 7.3% [hazard ratio (HR) = 1.073, 95% CI (1.038; 1.110)] per year, whereas the timeliness of public communication improved by 6.2% [HR = 1.062, 95% CI (1.028; 1.096)] per year (Table 1). Excluding outbreaks in Africa, which constitute half of the dataset, the hazard ratio per year increases to 1.111 [95% CI (1.057; 1.167)] and 1.113 [95% CI (1.061; 1.168)], respectively. However, stratified by WHO region, the only region with statistically significant (α = 0.05) improvement of outbreak discovery was the Western Pacific region [HR = 1.102 per year, 95% CI (1.008; 1.205)], whereas the Eastern Mediterranean [HR = 1.201 per year, 95% CI (1.066; 1.353)] and Western Pacific regions [HR = 1.119 per year, 95% CI (1.025; 1.221)] showed improved public communication. Other regions with large, but not statistically significant, hazard ratios were South-East Asia [HR = 1.169 per year, 95% CI (0.972; 1.406)] and the Eastern Mediterranean [HR = 1.119 per year, 95% CI (0.996; 1.256)] for outbreak discovery, and South-East Asia [HR = 1.128 per year, 95% CI (0.948; 1.342)] for public communication.
Table 1.
Results of univariate Cox proportional hazards regression analyses of WHO-verified outbreaks during 1996–2009, for all regions and for specific WHO regions
| Outbreak discovery | Public communication | |||
|---|---|---|---|---|
| Sample size | Hazard ratio per year (95% CI) | Sample size | Hazard ratio per year (95% CI) | |
| Overall* | 276 | 1.073 (1.038;1.110)† | 280 | 1.062 (1.028;1.096)† |
| Excluding Africa | 139 | 1.111 (1.057;1.167)† | 141 | 1.113 (1.061;1.168)† |
| Africa | 137 | 1.047 (1.000;1.098) | 139 | 1.028 (0.983;1.074) |
| Americas | 24 | 1.059 (0.938;1.195) | 24 | 1.077 (0.947;1.226) |
| South-East Asia | 18 | 1.169 (0.972;1.406) | 19 | 1.128 (0.948;1.342) |
| Europe | 19 | 1.016 (0.890;1.159) | 19 | 0.993 (0.864;1.141) |
| Eastern Mediterranean | 33 | 1.119 (0.996;1.256) | 33 | 1.201 (1.066;1.353)† |
| Western Pacific | 37 | 1.102 (1.008;1.205)† | 38 | 1.119 (1.025;1.221)† |
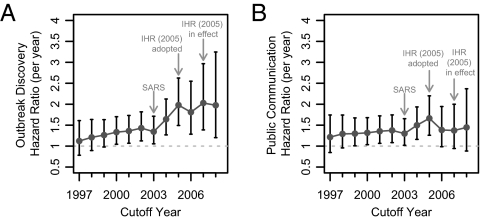
For the sensitivity analysis, periods before and after a sequentially changed cutoff year were compared using Cox proportional hazards regression. The per-year hazard ratios were statistically significant (α = 0.05) for the cutoff years 2000 onwards for outbreak discovery and for 1999–2005 for public communication. The per-year hazard ratio for outbreak discovery started increasing after 2003, with peaks in 2007 [HR = 2.0289, 95% CI (1.387; 2.968)] and in 2005 [HR = 1.979, 95% CI (1.492; 2.625)]. The per-year hazard ratio for public communication also started increasing after 2003, and peaked in 2005 [HR = 1.664, 95% CI (1.259; 2.199)] (Fig. 5).
Fig. 5.
A sensitivity analysis where serial Cox proportional hazards regression analyses were performed to determine the hazard ratio comparing the hazard for (A) outbreak discovery and (B) public communication about the outbreak before and after a cutoff date that was sequentially changed to June 15 of each year from 1997 to 2008. WHO's revised International Health Regulations (IHR 2005) officially went into force on June 15, 2007.
Discussion
In this study we explore temporal and spatial trends in the outbreak discovery and public communication processes over the past 14 y. Our analyses show that the average interval between estimated outbreak start to the earliest reported date of outbreak discovery, and to the earliest date of a public communication about the outbreak, both decreased over this period, although there was geographic variation, with the greatest gains in the Eastern Mediterranean and the Western Pacific regions.
Although reporting delays in public health surveillance systems have been evaluated previously (17), we know of no large-scale studies that have quantitatively looked at how these lags have changed over the course of an extended period worldwide. Studies have tended to be systems-based or country- or disease-specific evaluations and have focused on the delay in reporting to public health units, whereas here we look at lags in communicating to the general public.
Possible explanations for the improvement in time to outbreak discovery and public communication over this period include the formalization of international collaborations and regulations to mediate prompt detection of and response to public health emergencies of international concern, as well as technological advancements that facilitate the capacity for surveillance, detection, and reporting.
A major advancement has been the formation of GOARN in 2000 (8). A voluntary collaboration coordinated through the WHO, GOARN provides an operational network through which human and technical resources from over 140 existing institutions and networks in global epidemic surveillance are pooled, with aims of “combating the international spread of outbreaks; ensuring that appropriate technical assistance reaches affected states rapidly and contributing to long-term epidemic preparedness and capacity building.”
The revision of the WHO's IHR, adopted at the World Health Assembly in 2005 and in effect since 2007, also marks a major milestone by adapting the regulations to modern realities (9). Core to the functioning of GOARN, the IHR (2005) set forth regulations for strengthening core surveillance and response capacities (18). Changes include requiring state parties to notify the WHO of any disease event that may constitute a “public health emergency of international concern” (PHEIC) occurring within their territory, defined as “an extraordinary event which is determined … (i) to constitute a public health risk to other States through the international spread of disease and (ii) to potentially require a coordinated international response” (18). This “all-risks” approach (9) contrasts with the short list of notifiable diseases specified in the previous IHR, although we have restricted this study to infectious disease events, excluding other events of a radiological or chemical nature, for example.
The revised IHR also set minimum requirements for developing and maintaining core capacities for detecting and responding to PHEIC, to be fulfilled by 2012 (19). Our findings show that outbreak discovery and public communication have improved over time, particularly in the Eastern Mediterranean and Western Pacific regions, and possibly also in South-East Asia (which did not reach statistical significance, perhaps due to lack of statistical power). The concentration of avian influenza outbreaks emerging from these regions may have fueled particular global scrutiny and development of stronger surveillance infrastructure over the past several years (20). This would be a promising improvement, as these regions include many of the world's developing nations, which have faced challenges with newly emerging and reemerging infectious diseases (1), with surveillance capacity and reporting (8), and with potential economic consequences of reporting (21, 22). However, our findings show that some of the longest delays in outbreak discovery and public communication occurred in Africa. Africa also comprised half of our dataset, confirming its continued status as an infectious disease hotspot (1). These findings reiterate the continued need for development of public health infrastructure in Africa, assisted by efforts such as GOARN.
Though official electronic reporting systems (including automated ones) have generally helped improve the completeness and timeliness of reporting (13, 23, 24), informal Web-based media reports, blogs, and discussion groups also have opened alternate channels for reporting (25), and could provide potentially earlier outbreak signals, as was demonstrated retrospectively with SARS (11) and pandemic influenza A/H1N1 (26). The Internet can propel local issues to greater exposure at the global level by casting a spotlight that effectively pressures governments toward greater transparency and compliance with international reporting standards, and may well provide an explanation for the significant improvements in timeliness of outbreak discovery and public communications. In fact, the majority of current WHO investigations of ultimately confirmed outbreaks were prompted by reports from unofficial sources such as the media (1, 27). The revised IHR allow the WHO to use nonofficial information sources for the first time as a basis for requesting verification from the affected state parties. They also require the WHO to share such information with all relevant state parties and organizations when necessary to initiate a public health response (9).
Overall, determining the exact role of the implementation of the IHR (2005) in the improvement in time to outbreak discovery and public communication in this study is challenging. Although we obtained larger and statistically significant per year hazard ratios comparing the “hazard” before and after IHR (2005) adoption in 2005 for both outbreak discovery and public communication, and before and after IHR (2005) implementation in 2007 for outbreak discovery, we cannot with certainty attribute the improvement exclusively to the revised IHR because of potential confounding with the passage of time or other factors also associated with time such as technological improvements. Models were unable to accommodate both an IHR variable and a time variable to control for time because the two variables are highly correlated. Our sensitivity analysis also shows that significant improvements in outbreak discovery and public communications about outbreaks started occurring as early as 2003, in the aftermath of SARS and several years before the implementation of the revised IHR in 2007. SARS may have had an immediate effect by encouraging diligence in surveillance and reporting and certainly was a critical factor in pushing the final revision and adoption of the IHR (9). However, though the improvement in outbreak discovery appears to have been sustained, the hazard ratio for public communications dropped to the pre-2003 levels after 2005.
There may be debate as to whether public communication of an event is always necessary. Restricting reported sensitive information to the relevant organizations might encourage transparency and official reporting from countries concerned about potential economic consequences due to the presence of an outbreak. However, astute clinicians have been a cornerstone of public health surveillance, and many control measures require informing both clinicians and the general public of the risk to encourage their participation in prevention measures, such as staying home from school/work, boiling water, removing stagnant water, or getting immunized. Although there is a balance in deciding when to report outbreaks publicly, for this study we chose to analyze time to public communication with the second scenario in mind.
There are several limitations in this study. The WHO receives and posts communications about public health events on a private internal website, and although this site is accessible by National Focal Points (required by the IHR to be established by member states as a means to communicate to and from the WHO), not all of these events are necessarily made known to the public (28). Difficulties also arose in choosing and applying, consistently, a set of exclusion criteria to arrive at our selected subset. For example, it was challenging to concretely define endemic disease, or to determine when isolated clusters of illness constituted outbreaks. To fill in information gaps, we used informal media sources and estimated dates. In addition, we may have overlooked information if it was not captured by the reports analyzed. These limitations are compounded by difficulties in interpreting wording in reports and translating this information into concrete “outbreak milestones.”
In addition, there are inherent reporting biases in the data sources used in this study, including language and regional biases (29). Although the three systems from which we obtained data for this study have all made increasing efforts to incorporate more local-language media sources, the reports collected were still predominantly from English-language sources. Biases may also arise from heightened surveillance for certain diseases in regions for which there is a predisposition for disease activity, although we attempted to minimize this bias by excluding endemic/seasonal diseases.
Despite these limitations, we provide reasonable estimates of the improvement in surveillance over time across a variety of diseases and geographic regions. Accurate identification of disease outbreaks is crucial for expediting implementation of appropriate control measures. Therefore, there is a need to identify geographic regions where the outbreak detection and public communication processes could be improved, as well as where they have improved to identify and learn from successful strategies. Future studies could look into outbreak milestones by type of disease, estimating specificity of informal disease reports, and delve deeper into the advantages of different surveillance strategies using a systems comparison. With the collection of additional years of data, it would be possible to better assess the effectiveness of the revised IHR, increase statistical power for analyses stratifying by region and disease, and identify other explanatory factors associated with delayed or lack of improvement in the disease surveillance and reporting processes.
Methods
Data Sources.
Official reports.
The WHO disseminates Disease Outbreak News reports online at http://www.who.int/csr/don/en/. These reports describe confirmed public health events deemed of international concern (27).
Informal reports.
The informal online reports analyzed in our evaluation were collected from three systems that monitor both formal and informal online sources for disease reports.
ProMED-mail.
The Program for Monitoring Emerging Diseases (ProMED)-mail is an expert-moderated global electronic reporting system that collects information about infectious disease outbreaks and acute toxin exposures from local media, regional observers, and official reports (10, 30). ProMED distributes summary reports through http://www.promedmail.org/ and via e-mail to subscribers.
GPHIN.
The Global Public Health Intelligence Network (GPHIN) is a restricted early warning network operated by the Public Health Agency of Canada (11, 12). Through an automated process, the system continuously retrieves and categorizes online news articles about any health hazards (diseases, toxin exposures, tsunamis, etc.) across nine languages currently. Analysts then review these classifications for relevancy and importance, and alerts are sent to subscribers.
HealthMap.
HealthMap is an Internet-based, multilingual, and largely automated disease surveillance system that collects infectious disease information from a variety of official and informal (news media, personal accounts) electronic sources (13, 14). Using natural language processing tools, each report is automatically categorized by geographic location and disease. Human curators review these classifications, and aggregated alerts are displayed on a freely available interactive map at http://www.healthmap.org/.
Database Assembly.
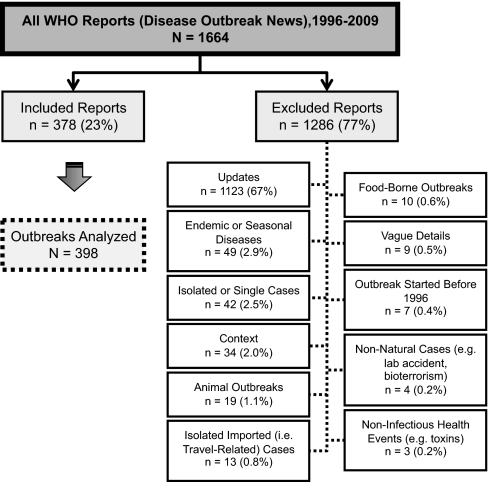
Using the WHO's set of Disease Outbreak News reports as a gold standard of outbreak reporting, a database of selected distinct WHO-confirmed outbreaks that occurred during 1996–2009 was created. Outbreak reports of endemic or seasonally recurrent diseases, isolated or single cases, diseases occurring in animals, food-borne outbreaks, nonnatural cases (e.g., acts of bioterrorism and laboratory accidents), and noninfectious health events were excluded according to predetermined exclusion criteria (Fig. 6). Further details are provided in SI Methods. A visualization of the distribution of the included outbreaks can be found at http://www.healthmap.org/globalbaseline/.
Fig. 6.
The exclusion criteria applied in selecting a subset of WHO-confirmed outbreaks reported in Disease Outbreak News (1996–2009). A single report may describe more than one outbreak, and may fall under more than one exclusion criterion category.
For the selected set of WHO-confirmed outbreaks in this database, corresponding ProMED (English only), GPHIN, and HealthMap reports were identified, and the issue date of the earliest electronic report among these disease reporting systems was noted for each outbreak. ProMED data were available for all years of our study period (1996–2009) but matching GPHIN and HealthMap reports were reviewed only for outbreaks with a WHO report date of 2007 or later. For each outbreak, key dates such as the date that the outbreak started or the earliest date of hospitalization or medical visit were identified from both formal (WHO) and informal (ProMED, GPHIN, HealthMap) reports. A full itemization of this database is provided in SI Methods.
Analysis.
In our study, we were particularly interested in three “outbreak milestones”: (i) date of outbreak start; (ii) earliest reported date of a case being detected (outbreak discovery); and (iii) earliest date of a public communication (local or international, informal or official, verbal or written) about the outbreak. Where these dates were not explicitly mentioned in the reports analyzed, estimates were obtained using an approach based on taking the earliest of several available dates (see SI Methods for details). Generally, the earliest date that we used for estimated date of outbreak start was date of symptom onset. Date of outbreak discovery ideally referred to the earliest reported date that authorities became aware of an ill individual (e.g., date of hospitalization or medical visit). Date of a public communication includes dates of informal or official reports and dates of announcements made by medical or government authorities. A total of 31% of the outbreaks in the dataset were excluded from the following analyses for one of these reasons: (i) estimated date of outbreak start was not available (29.4%, n = 117); (ii) estimated date of outbreak discovery was earlier than the estimated date of outbreak start (1.3%, n = 5); or (iii) estimated date of public communication was earlier than the estimated date of outbreak start (0.3%, n = 1).
Timeline of outbreak progression.
To characterize the progression of an outbreak, we calculated the median time difference between the estimated outbreak start date and the earliest reported dates of four outbreak milestones: (i) outbreak discovery; (ii) public communication about the outbreak; (iii) laboratory confirmation; and (iv) WHO Disease Outbreak News report about the outbreak. The 95th-percentile confidence intervals (CIs) for the median values were also determined via the bootstrapping method (with 1,000 replicates).
Spatial temporal trends.
Temporal and spatial analyses were conducted to assess trends and relationships in the duration between outbreak start date to (i) outbreak discovery and (ii) public communication. The median (with the bootstrapped 95th-percentile CI) for these time differences was calculated for each year during 1996–2009, for the periods before and after the WHO's revised IHR (2005) went into force on June 15, 2007, and for each WHO geographic region (i.e., Africa, the Americas, South-East Asia, Europe, Eastern Mediterranean, and Western Pacific).
Univariate Cox proportional hazards regression analysis was performed with outbreak discovery and public communication as outcomes and the date that the outbreak started as the predictor variable. These analyses were repeated after stratifying the data by WHO region. In addition, because outbreaks in the African region constituted half of the dataset, the analyses were repeated after excluding outbreaks within this region. Cox proportional hazards regression produces estimates of the hazard ratio. A hazard ratio compares the “hazard” of an outcome for one stratum of the covariate relative to that of the reference stratum. In our analyses, our two outcomes of interest (outbreak discovery and public communication) must have occurred due to how we defined our dataset. Our findings are presented as estimated 1-y hazard ratios with 95% CI.
A sensitivity analysis was conducted where Cox proportional hazards regression analyses were again performed, but instead of having outbreak start date as the covariate, a binary variable was used, coded as 1 if the outbreak was reported by the WHO after a certain cutoff date or 0 if before. Therefore, the hazard ratio compares the hazard for outbreak discovery and public communication regarding the outbreak before and after the cutoff date. In each reiteration, the cutoff date was sequentially changed to June 15 of each year from 1997 to 2008 (the revised IHR went into force on June 15, 2007).
Supplementary Material
Supporting Information
Acknowledgments
We are grateful to Anne Gatewood-Hoen (Harvard, Cambridge, MA) and Johannes Schnitzier (World Health Organization, Geneva) for their insightful discussion; to Qiyuan Li and Peter Park for sharing their statistical expertise; and to Chris Mahlke for his help with map imaging. This work was supported by research grants from Google.org and the National Library of Medicine, National Institutes of Health (1R01LM01812-01).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Rodier G, Global Health Information Forum, Prince Mahidol Award Conference, January 27–30, 2010, Bangkok, Thailand.
References
- 1.Heymann DL, Rodier GR WHO Operational Support Team to the Global Outbreak Alert and Response Network. Hot spots in a wired world: WHO surveillance of emerging and re-emerging infectious diseases. Lancet Infect Dis. 2001;1:345–353. doi: 10.1016/S1473-3099(01)00148-7. [DOI] [PubMed] [Google Scholar]
- 2.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morse SS. Factors and determinants of disease emergence. Rev Sci Tech. 2004;23:443–451. doi: 10.20506/rst.23.2.1494. [DOI] [PubMed] [Google Scholar]
- 5.Khan K, et al. Spread of a novel influenza A (H1N1) virus via global airline transportation. N Engl J Med. 2009;361:212–214. doi: 10.1056/NEJMc0904559. [DOI] [PubMed] [Google Scholar]
- 6.Wilson ME. The traveller and emerging infections: Sentinel, courier, transmitter. J Appl Microbiol. 2003;94(Suppl):1S–11S. doi: 10.1046/j.1365-2672.94.s1.1.x. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine and National Research Council (U.S.) In: Incentives for Disease Surveillance, Reporting, and Response. Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases. Keusch GT, Pappaioanou M, González MC, Scott KA, Tsai P, editors. Washington, DC: National Academies Press; 2009. pp. 165–186. [PubMed] [Google Scholar]
- 8.World Health Organization . World Health Report 2007: A Safer Future: Global Public Health Security in the 21st Century. Geneva: World Health Organization; 2007. [Google Scholar]
- 9.Fidler DP, Gostin LO. The new International Health Regulations: An historic development for international law and public health. J Law Med Ethics. 2006;34:85–94. doi: 10.1111/j.1748-720X.2006.00011.x. [DOI] [PubMed] [Google Scholar]
- 10.Madoff LC. ProMED-mail: An early warning system for emerging diseases. Clin Infect Dis. 2004;39:227–232. doi: 10.1086/422003. [DOI] [PubMed] [Google Scholar]
- 11.Mawudeku A, Lemay R, Werker D, Andraghetti R, St. John R. The Global Public Health Intelligence Network. In: M'ikanatha NM, Lynfield R, Van Beneden CA, de Valk H, editors. Infectious Disease Surveillance. Lynn, MA: Wiley-Blackwell; 2007. pp. 304–317. [Google Scholar]
- 12.Mykhalovskiy E, Weir L. The Global Public Health Intelligence Network and early warning outbreak detection: A Canadian contribution to global public health. Can J Public Health. 2006;97:42–44. doi: 10.1007/BF03405213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownstein JS, Freifeld CC, Reis BY, Mandl KD. Surveillance Sans Frontières: Internet-based emerging infectious disease intelligence and the HealthMap project. PLoS Med. 2008;5:e151. doi: 10.1371/journal.pmed.0050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freifeld CC, Mandl KD, Reis BY, Brownstein JS. HealthMap: Global infectious disease monitoring through automated classification and visualization of Internet media reports. J Am Med Inform Assoc. 2008;15:150–157. doi: 10.1197/jamia.M2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartley D, et al. The landscape of international event-based biosurveillance. Emerging Health Threats J. 2010;3:e3. doi: 10.3134/ehtj.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collier N, et al. BioCaster: Detecting public health rumors with a Web-based text mining system. Bioinformatics. 2008;24:2940–2941. doi: 10.1093/bioinformatics/btn534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jajosky RA, Groseclose SL. Evaluation of reporting timeliness of public health surveillance systems for infectious diseases. BMC Public Health. 2004;4:29. doi: 10.1186/1471-2458-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . 2nd Ed. Geneva: WHO; 2008. International Health Regulations (2005) [Google Scholar]
- 19.Wilson K, Brownstein JS, Fidler DP. Strengthening the International Health Regulations: Lessons from the H1N1 pandemic. Health Policy Plan. 2010;25:505–509. doi: 10.1093/heapol/czq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eagles D, et al. H5N1 highly pathogenic avian influenza in Southeast Asia. Rev Sci Tech. 2009;28:341–348. doi: 10.20506/rst.28.1.1864. [DOI] [PubMed] [Google Scholar]
- 21.Cash RA, Narasimhan V. Impediments to global surveillance of infectious diseases: Consequences of open reporting in a global economy. Bull World Health Organ. 2000;78:1358–1367. [PMC free article] [PubMed] [Google Scholar]
- 22.Sturtevant JL, Anema A, Brownstein JS. The new International Health Regulations: Considerations for global public health surveillance. Disaster Med Public Health Prep. 2007;1:117–121. doi: 10.1097/DMP.0b013e318159cbae. [DOI] [PubMed] [Google Scholar]
- 23.Overhage JM, Grannis S, McDonald CJ. A comparison of the completeness and timeliness of automated electronic laboratory reporting and spontaneous reporting of notifiable conditions. Am J Public Health. 2008;98:344–350. doi: 10.2105/AJPH.2006.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward M, Brandsema P, van Straten E, Bosman A. Electronic reporting improves timeliness and completeness of infectious disease notification, The Netherlands, 2003. Euro Surveill. 2005;10:27–30. [PubMed] [Google Scholar]
- 25.Keller M, et al. Use of unstructured event-based reports for global infectious disease surveillance. Emerg Infect Dis. 2009;15:689–695. doi: 10.3201/eid1505.081114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brownstein JS, Freifeld CC, Madoff LC. Digital disease detection—harnessing the Web for public health surveillance. N Engl J Med. 2009;360:2153–2155. doi: 10.1056/NEJMp0900702. 2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grein TW, et al. Rumors of disease in the global village: Outbreak verification. Emerg Infect Dis. 2000;6:97–102. doi: 10.3201/eid0602.000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz R, Fischer J. The Revised International Health Regulations: A Framework for Global Pandemic Response. Global Health Governance. 2010;3(2) Spring. [Google Scholar]
- 29.Wu H. Systemic determinants of international news coverage: A comparison of 38 countries. J Commun. 2000;50:110–130. [Google Scholar]
- 30.Madoff LC, Woodall JP. The internet and the global monitoring of emerging diseases: Lessons from the first 10 years of ProMED-mail. Arch Med Res. 2005;36:724–730. doi: 10.1016/j.arcmed.2005.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information