Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence (original) (raw)
. Author manuscript; available in PMC: 2011 Oct 1.
Published in final edited form as: Best Pract Res Clin Endocrinol Metab. 2010 Oct;24(5):731–743. doi: 10.1016/j.beem.2010.07.001
Abstract
Laboratory studies have found that short-term sleep restriction is associated with impairments in glucose metabolism, appetite regulation and blood pressure regulation. This chapter reviews the epidemiologic evidence for an association between habitual sleep duration and quality and risk of cardiometabolic diseases including obesity, diabetes and hypertension. Multiple studies observed a cross-sectional association between short sleep duration (generally <6 hours per night) and increased body mass index or obesity, prevalent diabetes and prevalent hypertension. Many studies also reported an association between self-reported long sleep duration (generally >8 hours per night) and cardiometabolic disease. There have been a few prospective studies and several, but not all, have found an association between short sleep and incident diabetes, hypertension and markers of cardiovascular disease. Future prospective epidemiologic studies need to include objective measures of sleep, and intervention studies are needed in order to establish a causal link between impaired or insufficient sleep and cardiometabolic disease risk.
Keywords: body mass index, obesity, diabetes mellitus, hypertension, cardiovascular diseases, epidemiologic studies
Introduction
Cardiometabolic risk has been defined as a cluster of metabolic and cardiovascular abnormalities, including abdominal obesity, insulin resistance, hypertension, dyslipidemia and atherosclerosis, that predispose individuals to cardiovascular disease (CVD) and type 2 diabetes [1, 2]. CVD, type 2 diabetes and overweight/obesity are closely linked conditions. For example, approximately 70% of total mortality in type 2 diabetes is due to CVD, and individuals with the metabolic syndrome, which is a clustering of risk factors including obesity, dyslipidemia, high blood pressure and insulin resistance, are at increased risk of developing type 2 diabetes and CVD [3]. The prevalence and impact of these cardiometabolic diseases is enormous. The World Health Organization estimates that worldwide in 2004–2005 approximately 1.6 billion adults were overweight, 400 million were obese, over 190 million people had diabetes and 17.1 million people died of cardiovascular disease [4]. In addition to increased mortality risk, CVD, diabetes, and obesity are associated with reduced quality of life and an increased economic burden on both the individual and on society [5–8]. Thus, an important goal of global public health is to reduce cardiometabolic risk.
Efforts to reduce the burden of these diseases require a better understanding of the mechanisms underlying increased cardiometabolic risk. Poor diet and limited physical activity certainly play an important role, but an additional possible explanation for the epidemic of cardiometabolic diseases is reduced sleep duration and quality. There is some evidence in the US that the number of adults obtaining insufficient sleep has increased over the same time period that the prevalence of obesity and diabetes has increased [9–13]. For example, a report from the National Health Interview Survey indicated the percentage of adults report sleeping six hours or less increased by approximately 5–6% between 1985 and 2004 [14]. Sleep loss may be the result of either a voluntary restriction of time spent in bed or as a result of a sleep disorder, such as insomnia and obstructive sleep apnea (OSA). Unfortunately, most epidemiologic studies cannot distinguish between voluntary sleep curtailment and sleep loss due to a pathological condition. Chapter 4 will discuss the impact of sleep disorders such as OSA on metabolism. Laboratory studies involving experimental restriction of time in bed provided the initial evidence that sleep loss can increase cardiometabolic risk and other papers in this issue will discuss these laboratory studies (see Chapter 1). One limitation of laboratory studies, however, is that they are short-term, lasting a few weeks at most. This raises the question of whether the associations observed in the laboratory persist in the real world when sleep loss is chronic. Epidemiologic studies provide some insight into the associations between sleep and health outside of the laboratory. This paper will review the epidemiologic evidence linking sleep duration and/or quality to cardiometabolic risk, including the relationship between sleep and body mass index (BMI) or obesity, appetite regulation, type 2 diabetes, hypertension and cardiovascular disease.
Sleep and BMI, obesity and appetite regulation
Several observational studies have examined the association between sleep and obesity or BMI. Over 65 published articles have presented cross-sectional analyses and most found a significant association between short sleep duration (generally < 6 hours per night) and increased prevalence of obesity or higher BMI in both adults and children from various countries (see [15–17] for reviews). Some of these studies also observed higher mean BMI associated with longer sleep durations (generally > 8 hours per night), suggesting a U-shaped association between sleep duration and BMI. Studies that examined self-reported measures of sleep quality have generally found worse sleep quality associated with higher BMI [18, 19]. Two recent metaanalyses analyzed data from some of these cross-sectional studies. Cappuccio et al [20] analyzed data from 17 studies and found that short sleep duration (<5 hours per night for adults, <10h per night for children) significantly predicted obesity in adults (pooled odds ratio [OR] was 1.55, 95% CI: 1.43–1.68) and in children (pooled OR was 1.89, 95% CI: 1.46–2.43) [20]. Sleep duration as a continuous variable was also significantly associated with BMI: the pooled regression indicated that on average BMI was a 0.35 kg/m2 lower for every additional hour of sleep [20]. A second meta-analysis examined sleep and obesity in children and the pooled OR predicting obesity from short sleep duration was 1.58 (95% CI: 1.26–1.98), which means that children who were short sleepers had 58% greater odds of being obese [21]. Thus, both meta-analyses confirmed that short sleep duration increased the odds of being obese. Most cross-sectional studies used self-reported sleep duration, but a few studies have used more objective measures. In a subset (n=612) of the Coronary Artery Risk Development in Young Adults (CARDIA) study in the US, wrist actigraphy was used to estimate sleep duration when participants were approximately 35–50 years old. Participants with shorter average sleep durations had higher BMI than those with longer sleep durations in cross-sectional analyses [22]. The Osteoporotic Fractures in Men (MrOS), which included over 2700 men aged 65 years and older, used polysomnography to determine which characteristics of sleep were associated with measures of body composition [23]. Those with the lowest percentage of slow-wave sleep (SWS) had the highest mean BMI and largest mean waist circumference, but the study did not find an association between SWS and percent body fat. In summary, most studies have reported a significant cross-sectional association where shorter sleep duration and in some studies longer sleep duration were associated with higher BMI or the presence of obesity. Of note, however, is that some studies have reported differences in these associations by age group. In particular, the association between sleep and BMI appears stronger at younger ages, and only one study in children has reported a U-shaped association [24].
There have also been a few prospective studies of sleep and weight gain in both adults and children (see Table 1). A few of these studies found no statistically significant association between sleep duration and change in body size [22, 25, 26]. Many studies, however, did report a significant association. For example, in the Nurse’s Health Study women who slept ≤5 hours per night gained 1.14 kg (95% CI: 0.49, 1.79) and those sleeping 6 hours per night gained 0.71 kg (95% CI: 0.41, 1.00) more weight over 16 years than those sleeping 7 hours adjusting for age and baseline BMI [27]. In the Quebec Family Study, those who reported sleeping 5–6 hours per night gained approximately 1.84 kg (95% CI 1.08–2.61) more over 6 years than those reporting 7–8 hours per night, even after adjustment for numerous potential confounders [28]. A study in Spain also reported that women who reported sleeping ≤5 hours per night had increased odds of gaining 5 kg or more over 2 years compared to those who slept 7 hours per night (OR 3.41, 95%CI 1.34–8.69) [29]. This study also reported increased weight gain among women who reported sleeping 8 hours or 9 hours per night. No association was seen in men. A study in Japan found that mean BMI among men who were short (<6h) or long (≥9h/night) sleepers increased more than in men who slept 7-<8 hours per night, however no association was observed among women [30]. A recent study among two minority groups in the US, African-Americans and Hispanic adults, found that among those aged 18–39 years short self-reported sleep (≤5h/night) was significantly associated with a greater increase in BMI, visceral fat & subcutaneous fat over 5 years compared to those sleeping 6–7 hours per night [31]. Of note, the association between short sleep and change in visceral fat was similar to the association between short sleep and change in subcutaneous fat. No association between sleep and change in BMI or fat was observed for those aged 40 years or older [31]. Among children in the UK, the odds of becoming obese between 38 months and 7 years of age was higher for children sleeping <10.5 hours per night (OR 1.45, 95% CI 1.10–1.89) and children sleeping 10.5–11.4 hours per night (OR 1.32, 95% CI 1.02–1.79) relative to children sleeping 12 hours per night at 38 months of age [32]. A study that collected time diaries in children also found a small but significant association between sleep duration and 5-year change in BMI (beta = −.115, p<.01), however, when stratified by age group, the association was only significant among the younger children who were aged 3–7.9 years at baseline (beta = −.153, p<.010) [33]. Finally, a study in the US collected parental reports of sleep duration when the children were 6 months, 1 year and 2 years of age and sleep duration was averaged over the three time points [34]. Average sleep duration<12 hours per night (versus >12 hours) was positively associated with BMI z score, sum of skinfolds and odds of being overweight at 3 years of age, even after multiple adjustments [34]. These prospective studies together suggest that sleep duration is associated with changes in body size, which could increase the risk of developing obesity and associated cardiometabolic disease.
Table 1.
Summary of prospective observational studies that examine association between sleep and changes in body size.
| Authors | Sample | SleepMeasure | Follow-upPeriod | Results |
|---|---|---|---|---|
| ADULTS | ||||
| Hasler et al,2004 [76] | n=496 men & women inSwitzerland aged 19y atbaseline | Self-report | 11 years | Adjusted OR for sleep duration predicting obesity was 0.50 (p<.01) |
| Gangwisch etal, 2005 [25] | n=9588 men & women inUS aged 32–86 y | Self-report | 8–10 years | No significant association between sleep duration and BMI change. |
| Patel et al,2006 [27] | n=68,183 women in USaged 39–65y | Self-report | 16 years | Those who reported sleeping ≤5h/night & 6h/night gained moreweight than those sleeping 7h/night. |
| Stranges et al,2008 [26] | n=10308 men & womenin UK aged 44–65 y | Self-report | 5–6 years | No significant association between sleep duration and change inBMI or waist circumference. |
| Lopez-Garciaet al, 2008 [29] | n=3235 men & women inSpain aged ≥60 years | Self-report | 2 years | Increased odds for gaining ≥5 kg associated with sleep≤5h/night,8h/night and 9h/night compared to 7h/night in women only. |
| Chaput et al,2008 [28] | n=276 men & women inCanada aged 21–64y | Self-report | 6 years | Short sleepers (5–6h/night) gained more weight than those sleeping7–8h/night. |
| Gunderson etal 2008 [77] | n=940 post-partumwomen in US, mean age33.0 (SD 4.7) y | Self-report | 6 months | Women who slept ≤5h/night at 6 months post-partum had greaterodds of substantial weight gain by 1 year post partum compared to7h/night (OR: 3.13, 95% CI 1.42–6.94) |
| Hairston et al,2010 [31] | n=1107 men & women inthe US aged 18–81y | Self-report | 5 years | Among 18–39 year olds, increase in BMI, subcutaneous fat andvisceral fat was grater among those sleeping ≤5h/night compared to6–7h/night. No significant associations for those aged ≥40 years.. |
| CHILDREN | ||||
| Agras et al,2004 [78] | n=150 boys & girls in USaged 3–5 y at baseline | Parentalreport | 6 years | Mean sleep at ages 3–5 y was 30 minutes less for those whobecame overweight compared to those who did not, most of whichwas daytime sleep. |
| Sugimori et al,2004 [79] | n=8170 boys & girls inJapan aged 3 years atbaseline | Parentalreport | 3 years | Children obese at both 3 years & 6 years of age had largest % ofshort sleepers. Association was statistically significant in boys butnot girls. |
| Reilly et al,2005 [32] | n=7758 boys & girls inUK aged 38 months atbaseline | Parentalreport | 4 years | Children sleeping <10.5h/night and 10.5–11.4h/night were morelikely to be obese at age 7 years than children sleeping ≥12h pernight. |
| Lumeng et al,2007 [80] | n=785 boys & girls in theUS aged 9–10 y atbaseline | Maternalreport | 3 years | Sleep duration as continuous variable significantly predictedoverweight (OR 0.60 per hour, 95%CI 0.36–0.99).Sleep problems were not associated with overweight. |
| Al Mamun etal, 2007 [81] | n=2494 boys & girls inAustralia. Sleepassessed at 6 mos & 2–4y of age. | Parentalreport | Obesity at 21years of age | Obesity was not associated with sleeping problem at 6 monthsIncreasing sleep problems at 2–4 years of age was associated withincreased BMI and overweight/obesity at age 21. |
| Snell et al,2007 [33] | n=1441 boys & girls inUS aged 3–13y atbaseline | Time Diaries | 5 years | Statistically significant association between sleep from time diarypredicting BMI. Only significant among children aged 3–7.9 atbaseline. |
| Taveras et al,2008 [34] | n=915 boys & girls in US.Sleep assessed at 6 mos,1 year & 2 years of age | Parentalreport | BMI at 3 yearsof age | Average sleep duration<12 (vs>12h) was positively associated withBMI z score, sum of skinfolds & odds of being overweight, aftermultiple adjustments. |
| Touchette etal, 2008 [82] | n=1138 boys & girls inCanada. Sleep assessedyearly from 2.5 to 6years of age | Parentalreport | BMI at 6 yearsof age | Short persistent sleepers (<10h) had higher risk ofoverweight/obesity (OR 4.2) compared to 11-hour persistentsleepers. |
Two hormones that are involved in appetite regulation are leptin, a satiety factor, and ghrelin, an appetite stimulant. Previous laboratory studies found that sleep restriction resulted in decreased leptin and increased ghrelin in peripheral blood [35, 36]. A few observational studies have also examined levels of leptin and ghrelin in relation to habitual sleep duration. The Wisconsin Sleep Cohort Study, a population-based study that enrolled Wisconsin State employees aged 30–60 years, collected sleep diaries to assess habitual sleep, conducted one night of polysomnography (PSG) in the laboratory, and in the morning following the PSG, obtained a single blood sample [37]. Total sleep time from PSG was inversely associated with ghrelin levels (beta = −0.69, p=0.008) while average habitual sleep duration was positively associated with leptin levels independently of BMI (beta = 0.11, p=0.01) [37]. Data from the Quebec Family Study of 740 men and women aged 21–64 years indicated that leptin levels in those sleeping 5–6 hours per night were approximately 15–17% lower than predicted based on body fat alone [38]. These two studies are consistent with some of the laboratory studies but two other studies in women did not observe similar associations. The Nurse’s Health Study, which asked participants to return a blood sample through the mail, did not observe a significant association between self-reported sleep duration and leptin levels [39]. Also, a randomized trial of moderate-intensity exercise among 173 obese, sedentary postmenopausal women aged 50–74 found no cross-sectional associations between self-reported sleep duration and leptin or total ghrelin levels nor any significant associations between change in sleep duration and changes in leptin or ghrelin after the exercise intervention [40]. These discrepant results may be due to differences in the association between sleep and appetite regulation in women, particularly obese older women, or may be due to methodological issues such as self-reported sleep duration, sample collection or assay procedures. Finally, the Women’s Health Initiative study examined the relationship between sleep and dietary intake in 459 postmenopausal women aged 50–81 years [41]. Average sleep duration from 1 week of wrist actigraphy was negatively correlated with dietary fat intake and total calories, which suggests that short sleepers have a greater food intake particularly in the form of fat. A study in adolescents did not find an association between hunger ratings and average nocturnal sleep from 7-day sleep diaries, however, those who slept 3 hours or more during the daytime reported greater caloric intake and food cravings and this association did not appear to be confounded by nocturnal sleep duration [42]. More research is required to assess whether habitual insufficient sleep is truly associated with greater appetite and greater food intake.
Sleep and diabetes
Several large observational studies have reported cross-sectional associations between short sleep duration or impaired sleep and greater prevalence of diabetes or impaired glucose tolerance (see [17] for a review). Most of these studies found an increased odds of diabetes associated with short sleep durations (≤5h or ≤6h per night) and some also found an increased odds of diabetes among long sleepers (≥8 or ≥9 hours). One study found stronger associations in older people [43] and another observed a significant association in women only [44]. Most of these studies relied on self-reported sleep duration and quality, but one study used wrist actigraphy and found greater sleep fragmentation in those with type 2 diabetes compared to healthy controls but no difference in total sleep time [45]. A survey study among African-Americans with type 2 diabetes found a significant association between poor subjective sleep quality or insufficient sleep and worse glycemic control as indicated by higher HbA1c levels [46]. Finally, a study of 60 patients with type 2 diabetes found that 77% of the patients had an apnea-hypopnea index above 5, which indicates sleep-disordered breathing (SDB) [47].
Furthermore, a greater degree of SDB was associated with worse glycemic control [47]. The results of these studies suggest an association between diabetes and short sleep duration or poor sleep quality, however, the direction of causality cannot be determined. Poor or insufficient sleep may increase the risk of developing diabetes, as the laboratory studies suggest, or, conversely, having diabetes could impair sleep.
Several prospective studies have examined the association between sleep duration or impaired sleep and incident diabetes. The results are summarized in Table 2. Most of these studies reported increased odds of diabetes associated with short sleep duration (≤5h and/or ≤6h) and many observed a U-shaped association. Furthermore, impaired sleep, such as difficulty initiating or maintaining sleep, was associated with increased odds of developing diabetes in multiple studies. A meta-analysis of 10 prospective studies examined the association between the incidence of diabetes and either short sleep duration, long sleep duration or sleep disturbances [48]. The estimated pooled OR for short sleep was 1.28 (95% CI 1.03–1.6), however, there was a significant gender difference. The OR was 2.07 (1.16–3.72) for men and 1.07 (0.90–1.28) for women, indicating a stronger association among men. The pooled OR for long sleep was 1.48 (1.13–1.96), indicating that overall there is a significant U-shaped association between sleep duration and incident diabetes. Finally, both difficulty initiating sleep (pooled OR 1.57, 95% CI 1.25–1.97) and difficulty maintaining sleep (pooled OR 1.84, 95% CI 1.39–2.43) significantly predicted incident diabetes. Thus, taken together, prospective epidemiologic studies have suggested that subjective short or long sleep duration and poor sleep quality predict the development of diabetes.
Table 2.
Summary of prospective observational studies that examine association between sleep and incident diabetes.
| Authors | Sample | Sleep.measure | Follow-upPeriod | Results |
|---|---|---|---|---|
| Ayas et al,2003 [83] | n=70,026 women in theUS aged 30–55 years atbaseline. | Self-report | 10 years | When adjusting for BMI, only those reporting sleeping 9h/night ormore had significantly higher odds of incident diabetes compared tothose sleeping 8h/night (OR 1.29, 95%CI 1.05–1.59)When predicting incident symptomatic diabetes, both those reportingsleeping ≤5h/night (OR 1.37, 95%CI 1.07–1.77) and those reporting≥9h/night (OR 1.36, 95%CI 1.04–1.73) had significantly higher oddsthan those sleeping 8h/night. |
| Kawakami etal, 2004 [84] | n=2649 men in Japanaged 18–53 years atbaseline. | Self-report | 8 years | Difficulty initiating sleep (OR 2.98, , 95%CI 1.36–6.53) and difficultymaintaining sleep (OR 2.23, 95%CI 1.08–4.61) both predictedincident diabetes. |
| Nilsson et al,2004 [85] | n=6599 men in Swedenaged 35–51 years atbaseline | Self-report | Mean 14.8years | Increased odds of incident diabetes among those who reporteddifficulty falling asleep or use of sleeping pills (OR 1.52, 95% CI:1.05, 2.20). |
| Meisinger etal, 2005 [86] | n=8269 men & women inGermany aged 24–74years at baseline. | Self-report | Mean 7.5 years | Difficulty maintaining sleep predicted incident diabetes in both men(OR 1.60, 95% CI 1.05–2.45) and women (OR 1.98, 95% CI 1.20-3.29). Difficulty initiating sleep was not associated with incidentdiabetes. |
| Mallon et al,2005 [87]. | n=1244 men & women inSweden aged 45–65years at baseline | Self-report | 12 years | In men, difficulty maintaining sleep (OR 4.8, 95% CI 1.9–12.5) andsleeping≤5h (OR 2.8, 95% CI 1.1–7.3) predicted incident diabetes.There were no significant associations between sleep and diabetesin women. |
| Bjorkelund etal, 2005 [88] | n=1447 women inSweden aged 38–60years at baseline | Self-report | 32 years | No association between incident diabetes & sleep problems or sleepduration. |
| Yaggi et al,2006 [89] | n=1139 men in US aged40–70 years at baseline | Self-report | 15–17 years | Odds of incident diabetes was higher for those reporting sleeping6h/night (OR 1.95, 95% CI 1.06–3.58) and 9h (OR 3.12, 95% CI1.53–6.37) compared to those sleeping 7h/night. |
| Gangwisch etal, 2007 [90] | n=8992 men & womenaged 32–86 years | Self-report | 8–10 years | Odds of incident diabetes was higher for those reporting sleeping≤5h (OR 1.47, 95% CI 1.03–2.09) and ≥9h (OR 1.52, 95% CI 1.06-2.17)compared to those sleeping 7h/night |
| Hayashino etal, 2007 [91] | n=6509 men & womenaged 19–69 years. | Self-report | Median 4.2years | Odds of incident diabetes was higher for those reporting difficultyinitiating sleep “sometimes” (OR 1.39, 95% CI 1.04–1.88) and “often”(OR 1.63, 95% CI 1.04–2.59) compared to those with no difficulty.Sleep duration and difficulty maintaining sleep were not related toincident diabetes. |
| Chaput et al,2009 [92] | n=276 men & women inCanada aged 21–64years | Self-report | Mean 6 years | Odds of incident impaired glucose tolerance/type 2 diabetes(combined) were higher for those reporting sleeping ≤6h (OR 2.42,95% CI 1.49–3.33) and ≥9h (OR 2.31, 95% CI 1.41–3.15) comparedto those reporting sleeping 7–8h/night. |
| Biehl et al,2009 [93] | n= 900 men & women inUS aged 40–69 years atbaseline. | Self-report | 5 years | Among non-Hispanic whites and Hispanics (combined), odds forincident diabetes was higher among those reporting sleeping ≤7h(OR 2.36, 95% CI 1.11–5.00) compared to those sleeping 8h/night.No association between sleep duration and incident diabetes wasobserved in African-Americans |
| Xu et al, 2010[94] | n=174,542 men & womenin US aged 50–71 yearsage baseline | Self-report | 3–10 years | Odds of incident diabetes was higher in those reporting sleeping <5h(OR 1.46, 95% CI 1.31–1.63) and 5–6h (OR 1.11, 95% CI 1.06–1.16)compared to those sleeping 7–8h/night. Longer day napping wasalso associated with increased odds of incident diabetes. |
Sleep and cardiovascular disease
Sleep duration and quality have also been associated with blood pressure in epidemiologic studies [49–54]. Cross-sectional studies have generally found that self-reported short sleep durations or subjectively poor sleep quality are associated with higher blood pressure or higher prevalence of hypertension [43, 49, 51–53, 55, 56]. Some of these studies also observed higher blood pressure among long sleepers [43, 49]. Two of these studies observed a significant association in women but not men [53, 56]. A few studies found no association between sleep and blood pressure, including two among elderly adults [57, 58] and one among children aged 3–10 years [59], which suggests that associations between sleep and blood pressure may be modified by age. Two studies used wrist actigraphy, an objective measure, to estimate sleep duration and quality. One of these studies was conducted among 238 adolescents and found that low sleep efficiency, but not sleep duration, was significantly associated with prevalent prehypertension [60]. In a subset of participants in the CARDIA study, sleep duration and quality was estimated from 3–6 days of wrist actigraphy when participants were approximately 35–50 years old [61]. Shorter sleep duration and lower sleep maintenance (the percentage of time between sleep onset and sleep end that was spent sleeping) were both associated cross-sectionally with higher blood pressure. Thus, cross-sectional studies generally support a relationship between disturbed or insufficient sleep and higher blood pressure, but the causal direction cannot be determined and the strength of these associations may vary by gender and age.
Several prospective epidemiologic studies have examined cardiovascular outcomes in relation to sleep duration. Analysis of over 4500 adults who participated in the National Health and Nutrition Examination Survey (NHANES) in the US found that those who reported sleeping ≤5 hours per night had increased odds of incident hypertension (OR 1.32, 95% CI 1.02–1.71) compared to those reporting sleeping 7–8 hours per night after adjusting for numerous potential confounders [50]. In the CARDIA study of adults aged 35–50 years, sleep duration estimated from 3–6 days of wrist actigraphy was significantly associated with incident hypertension over five years [61]. The odds ratio for shorter average sleep duration predicting hypertension was 1.37 (95% CI, 1.05–1.78), which means for every hour less sleep there is a 37% higher odds of incident hypertension. The CARDIA study also examined incident coronary artery calcification (CAC), which is a predictor of the development of coronary heart disease. Results indicated a significant negative association between sleep duration and incidence of CAC. Longer measured sleep duration was associated with a decreased adjusted odds of incident calcification over 5 years (OR = 0.67 per hour, p=.011; 95% CI 0.49–0.91), which means every extra hour of sleep was associated with a 33% lower odds of CAC [62]. The Nurses’ Health Study examined incident coronary heart disease (CHD) over 10 years and found a U-shaped association with self-reported sleep duration [63]. The risk ratio (RR) was 1.45 (95%CI: 1.1–1.92) for those reporting ≤5 hours sleep per night and 1.38 (95%CI: 1.03–1.86) for those reporting ≥9 hours per night compared to those sleeping 8 hours per night. A few studies have examined cardiovascular disease mortality in relation to sleep duration, but none found an association in fully adjusted models [64–66]. Subjective insomnia or insomnia symptoms have been associated with increased cardiovascular disease events or mortality. For example, a study in Sweden reported that coronary artery disease mortality was higher for men reporting difficulty initiating sleep (RR: 3.1, 95%CI 1.5–6.3), but no association was observed in women [64] A study in the US of over 3400 men and women 35 years of age or older reported a significant increase of a cardiovascular disease event in those who complained of insomnia every day compared to those without any insomnia complaint (RR: 1.78, 95%CI 1.03–3.08) [66]. Overall, there is some evidence that insufficient or impaired sleep is associated with cardiovascular disease and hypertension, but more rigorous studies are required to fully understand this association.
Limitations
The majority of the observational studies described above had consistent findings, but we must still consider the methodological limitations of these studies. First, the vast majority of these studies relied on a self-reported measure of sleep duration, which may not be very accurate. Recent analysis comparing sleep durations estimated from wrist actigraphy to self-reported sleep in a sample of over 600 middle-aged adults indicated only moderate agreement between these measures (r=0.47) [67]. In addition, there may be important confounders that are not taken into account in these analyses, including race, socioeconomic status, physical activity, alcohol and caffeine consumption, and psychological disorders [67, 68]. Future studies need to incorporate objective measures of sleep and include detailed measures of potential confounding variables.
Potential Mechanisms
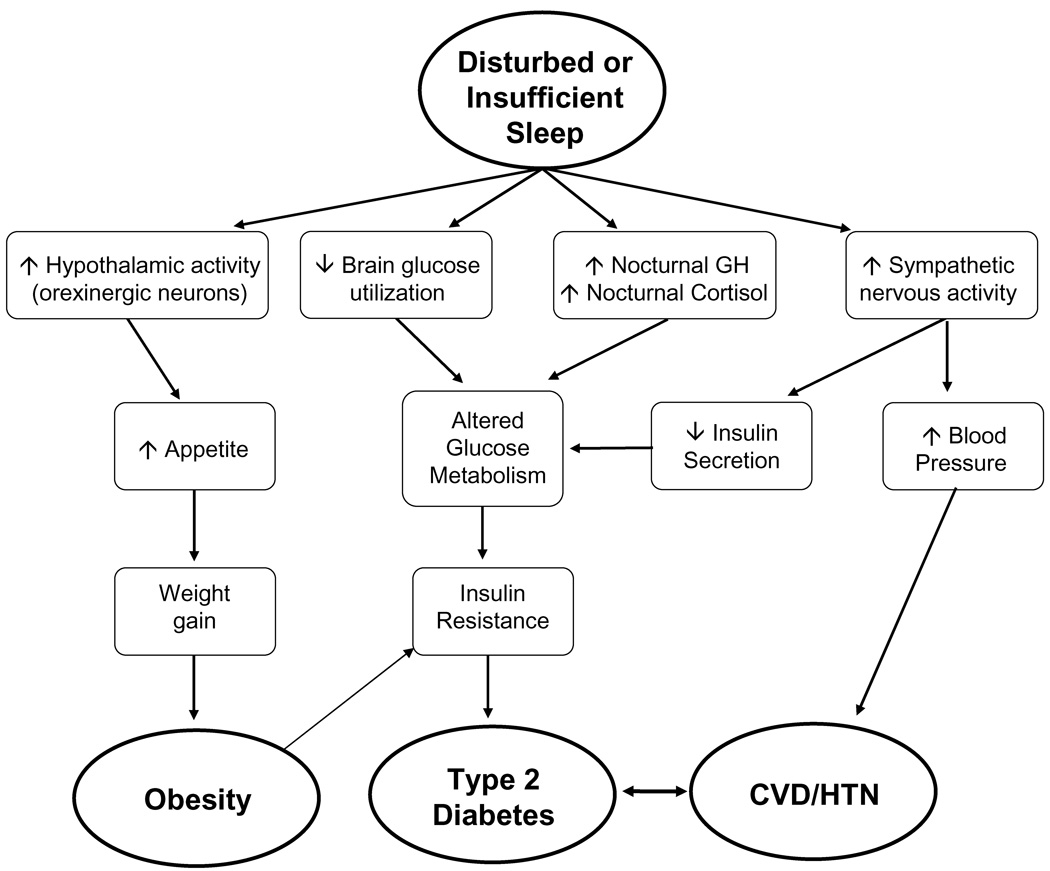
The mechanisms underlying the association between cardiometabolic disease risk and sleep duration or quality are not fully understood. However, laboratory studies have provided some clues about potential pathways leading from insufficient or disturbed sleep to diabetes and obesity. The figure below presents potential pathways linking disturbed or insufficient sleep to the development of obesity, type 2 diabetes and cardiovascular disease (CVD) or hypertension (HTN). It is unlikely that any single pathway is responsible, but rather a combination of mechanisms leads to increased cardiometabolic risk. Furthermore, there are probably other pathways that remain to be identified, including effects of sleep disturbances on physical activity and energy expenditure.
Figure.
Schematic representation of possible mechanistic pathways linking disturbed or insufficient sleep to obesity, diabetes, cardiovascular disease (CVD) and hypertension (HTN).
One pathway from short sleep to cardiometabolic disease involves an increase in appetite. The subjective increase in hunger after sleep restriction may be a result of decreased inhibition of hypothalamic activity in appetite centers and a loss of inhibition of the activity of orexigenic neurons in the hypothalamus, leading to increased hunger [69, 70]. If increased hunger led to increased food intake without compensatory increases in physical activity, weight gain would result and could eventually lead to obesity. The second pathway indicates that sleep loss results in a decrease in brain glucose utilization, which would promote reduced glucose tolerance. Studies of total sleep deprivation that used positron emission tomography (PET) did in fact observe decreased brain glucose utilization after sleep deprivation [70]. The third pathway involves increases in daytime release of growth hormone (GH) and ghrelin, as well as evening release of cortisol. A laboratory study of six days of sleep restriction observed an extended duration of elevated nighttime GH concentrations [71] and an increase in evening cortisol levels [72]. Elevated evening cortisol concentrations are likely to result in reduced insulin sensitivity on the following morning, an alteration that can further impair glucose tolerance following sleep restriction [73]. Increased levels of GH can lead to an induction of a transient insulin resistance in muscle cells, resulting in decreased glucose uptake, elevated blood glucose levels and subsequent increases in insulin resistance in other tissues. Thus, both the second and third pathways can have a deleterious impact on glucose metabolism. The fourth pathway in the figure proposes that disturbed and insufficient sleep are associated with an increase in sympathetic nervous activity. Laboratory studies observed that sleep restriction was associated with elevated cardiac sympatho-vagal balance estimated from heart rate variability, which likely reflects an increased influence of sympathetic tone [35, 72]. Increased sympathetic nervous activity at the level of the pancreas could result in a reduction of insulin secretion from pancreatic beta-cells, but sympatho-vagal balance at the level of the pancreas has not yet been assessed in any sleep restriction study. Deleterious alterations in glucose metabolism could lead to the development of insulin resistance, which is a risk factor for the development of type 2 diabetes. In addition, increased sympathetic nervous activity would also be associated with increased blood pressure, which could predispose individuals to the development of hypertension and cardiovascular disease. Finally, obesity is a risk factor for insulin resistance and diabetes and diabetes and CVD are closely linked conditions.
As described above, numerous observational studies have also documented an association between long sleep duration and obesity, diabetes or hypertension. To date, no biological mechanisms have been identified to explain this association. Several explanations have been postulated, however. An analysis of the Nurses’ Health study indicated that the presence of depression and low socioeconomic status were the most likely explanations for the association between long sleep and mortality [74]. It is important to note that in all of the studies that observed a U-shaped association, sleep duration was based on self-report. Therefore, it is not clear if long sleepers are actually obtaining more physiologic sleep or if they are just spending more time in bed. A study that recruited individuals who self-reported sleep duration was ≥9 hours per night provides some evidence for the latter explanation. In this study, the self-reported long sleepers only obtained an average of 7–7.5 hours of sleep per night based on wrist actigraphy monitoring despite reporting sleeping more than 9 hours per night [75]. The large discrepancy between self-reported and objectively-measured sleep duration is not explained, but could represent either a sleep disorder or other pathological condition(s). Given the large number of studies that have found an association between long sleep duration and morbidity risk, it is important that more research into possible mechanisms or explanations be conducted.
Summary
The accumulated evidence from numerous observational studies suggests that insufficient or disturbed sleep may play a role in the development of cardiometabolic disease risk. Potential pathways through which sleep could lead to the development of obesity, diabetes, cardiovascular disease and hypertension have been discussed. In particular, these pathways involve impairments in glucose metabolism, appetite regulation, and sympatho-vagal balance. However, more research is required to better understand how sleep impacts cardiometabolic risk (see Research Agenda). In particular, whether gender or age modifies the association between sleep and cardiometabolic diseases needs to be investigated further. Obesity, diabetes and cardiovascular disease have enormous negative impacts on quality of life, life expectancy and financial burden.
Therefore, a better understanding of the factors that can influence the development or prognosis of these conditions could help improve the lives of millions of people. Evidence reviewed here suggests that sleep duration or quality may be a novel risk factor that is potentially modifiable. Future research should test whether a sleep intervention could ameliorate the cardiometabolic consequences of impaired or insufficient sleep.
Practice Points
- Short sleep duration is associated with increased prevalence of obesity in adults and children.
- Associations between sleep and body mass index/obesity appear stronger at younger ages.
- Short sleep duration is associated with increased risk of diabetes and hypertension in adults.
- Long sleep duration has also been associated with increased obesity, diabetes and hypertension in some studies.
- Possible mechanisms for these observations include alterations in hypothalamic activity that relate to appetite regulation, alterations in hormonal profiles, such as GH and cortisol, and increased sympathetic nervous activity.
Research Agenda
- The potential impact of sleep disturbances on energy expenditure warrants further examination.
- More research is required to assess the relationship between habitual sleep duration and quality and food intake.
- Intervention studies to examine the effect of improving or extending sleep on cardiometabolic risk factors are important for establishing a causal link.
- Future observational studies should be prospective and include objective measures of habitual sleep.
- More research is needed to understand the association between long sleep and morbidity risk.
- Future studies should investigate whether the associations between sleep duration and morbidity vary by gender or age.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Kahn R, et al. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2005;48:1684–1699. doi: 10.1007/s00125-005-1876-2. [DOI] [PubMed] [Google Scholar]
- 3.Vasudevan AR, Ballantyne CM. Cardiometabolic risk assessment: an approach to the prevention of cardiovascular disease and diabetes mellitus. Clin Cornerstone. 2005;7:7–16. doi: 10.1016/s1098-3597(05)80063-8. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. [Last Accessed: July 8, 2010]; Available from: http://www.who.int/en/
- 5.Solomon CG, Manson JE. Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr. 1997;66:1044S–1050S. doi: 10.1093/ajcn/66.4.1044S. [DOI] [PubMed] [Google Scholar]
- 6.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 7.Ettaro L, et al. Cost-of-illness studies in diabetes mellitus. Pharmacoeconomics. 2004;22:149–164. doi: 10.2165/00019053-200422030-00002. [DOI] [PubMed] [Google Scholar]
- 8.Franco OH, et al. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167:1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 9.Gallup Organization. The Gallup Study of Sleeping Habits. Princeton, NJ.: Gallup Organization; 1979. [Google Scholar]
- 10.Gallup Organization. Sleep in America. Princeton, NJ.: Gallup Organization; 1995. [Google Scholar]
- 11.Breslau N, et al. Daytime sleepiness: An epidemiological study of young adults. American Journal of Public Health. 1997;87:1649–1653. doi: 10.2105/ajph.87.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kripke D, et al. Short and long sleep and sleeping pills. Is increased mortality associated? Archives of General Psychiatry. 1979;36:103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 13.National Sleep Foundation. 2001 "Sleep in America" Poll. Washington, DC.: National Sleep Foundation; 2001. pp. 1–113. [Google Scholar]
- 14.National Center for Health Statistics. QuickStats: Percentage of adults who reported an average of ≤ 6 hours of sleep per 24-hour period, by sex and age group - United States, 1985 and 2004. MMWR. Morbidity and Mortality Weekly Report. 2005;54:933. [Google Scholar]
- *15.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–298. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- *16.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asplund R, Aberg H. Sleep complaints in women of ages 40–64 years in relation to sleep in their parents. Sleep Med. 2001;2:233–237. doi: 10.1016/s1389-9457(00)00055-1. [DOI] [PubMed] [Google Scholar]
- 19.Jennings JR, et al. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–223. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- *20.Cappuccio FP, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 22.Lauderdale DS, et al. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. American Journal of Epidemiology. 2009;170:805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Rao MN, et al. Association between sleep architecture and measures of body composition. Sleep. 2009;32:483–490. doi: 10.1093/sleep/32.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danielsen YS, et al. The relationship between school day sleep duration and body mass index in Norwegian children (aged 10–12) Int J Pediatr Obes. 2010 doi: 10.3109/17477160903473739. [DOI] [PubMed] [Google Scholar]
- 25.Gangwisch JE, et al. Inadequate Sleep as a Risk Factor for Obesity: Analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 26.Stranges S, et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol. 2008;167:321–329. doi: 10.1093/aje/kwm302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel SR, et al. Association between Reduced Sleep and Weight Gain in Women. Am J Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaput JP, et al. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–523. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Garcia E, et al. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87:310–316. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe M, et al. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33:161–167. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Hairston KG, et al. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep. 2010;33:289–295. doi: 10.1093/sleep/33.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reilly J, et al. Early life risk factors for obesity in childhood: cohort study. British Medical Journal. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007;78:309–323. doi: 10.1111/j.1467-8624.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 34.Taveras EM, et al. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–311. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiegel K, et al. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology and Metabolism. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel K, et al. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels and increased hunger and appetite. Annals of Internal Medicine. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- *37.Taheri S, et al. Short Sleep Duration Is Associated With Reduced Leptin, Elevated Ghrelin, And Increased Body Mass Index (BMI) Sleep. 2004;27:A146–A147. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaput JP, et al. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 39.Williams CJ, et al. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30:1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 40.Littman AJ, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2006;31:466–475. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- 41.Grandner MA, et al. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010;11:180–184. doi: 10.1016/j.sleep.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landis AM, Parker KP, Dunbar SB. Sleep, hunger, satiety, food cravings, and caloric intake in adolescents. J Nurs Scholarsh. 2009;41:115–123. doi: 10.1111/j.1547-5069.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 43.Choi KM, et al. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes (Lond) 2008;32:1091–1097. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]
- *44.Tuomilehto H, et al. Sleep duration is associated with an increased risk for the prevalence of type 2 diabetes in middle-aged women - The FIN-D2D survey. Sleep Med. 2008;9:221–227. doi: 10.1016/j.sleep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Trento M, et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45:225–229. doi: 10.1007/s00592-008-0047-6. [DOI] [PubMed] [Google Scholar]
- 46.Knutson KL, et al. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 47.Aronsohn RS, et al. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. American Journal of Respiratory and Critical Care Medicine. 2010;181:507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cappuccio FP, et al. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009 doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottlieb DJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 50.Gangwisch JE, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 51.Houyez F, et al. Sommeil et hypertension artérielle. Arch Mal Coeur Vaiss. 1990;83:1085–1088. [PubMed] [Google Scholar]
- 52.Kotani K, et al. Sleep status and blood pressure in a healthy normotensive female population. Int J Cardiol. 2007;125:425–427. doi: 10.1016/j.ijcard.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 53.Cappuccio FP, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells JC, et al. Sleep patterns and television viewing in relation to obesity and blood pressure: evidence from an adolescent Brazilian birth cohort. Int J Obes (Lond) 2008;32:1042–1049. doi: 10.1038/ijo.2008.37. [DOI] [PubMed] [Google Scholar]
- 55.Kawabe H, Saito I. Does short sleep duration in daily life affect morning home blood pressure? Evaluation in Japanese people. Clinical and Experimental Hypertension. 2008;30:183–190. doi: 10.1080/10641960802064575. [DOI] [PubMed] [Google Scholar]
- 56.Stang A, et al. Gender-specific associations of short sleep duration with prevalent hypertension. Hypertension. 2008;51:e15–e16. doi: 10.1161/HYPERTENSIONAHA.107.108456. author reply e17. [DOI] [PubMed] [Google Scholar]
- 57.van den Berg JF, et al. Sleep duration and hypertension are not associated in the elderly. Hypertension. 2007;50:585–589. doi: 10.1161/HYPERTENSIONAHA.107.092585. [DOI] [PubMed] [Google Scholar]
- 58.Lima-Costa MF, Peixoto SV, Rocha FL. Usual sleep duration is not associated with hypertension in Brazilian elderly: The Bambui Health Aging Study (BHAS) Sleep Med. 2008;9:806–807. doi: 10.1016/j.sleep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Bayer O, Neuhauser H, von Kries R. Sleep duration and blood pressure in children: a cross-sectional study. Journal of Hypertension. 2009;27:1789–1793. doi: 10.1097/HJH.0b013e32832e49ef. [DOI] [PubMed] [Google Scholar]
- 60.Javaheri S, et al. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–1040. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knutson KL, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Archives of Internal Medicine. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King CR, et al. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayas NT, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 64.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 65.Heslop P, et al. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Medicine. 2002;3:305–314. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 66.Chien KL, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–184. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lauderdale DS, et al. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magee CA, et al. A link between chronic sleep restriction and obesity: methodological considerations. Public Health. 2008;122:1373–1381. doi: 10.1016/j.puhe.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Gautier JF, et al. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 70.Thomas M, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 71.Spiegel K, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2000;279:R874–R883. doi: 10.1152/ajpregu.2000.279.3.R874. [DOI] [PubMed] [Google Scholar]
- 72.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 73.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocrine Reviews. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- *74.Patel SR, et al. Correlates of long sleep duration. Sleep. 2006 doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zielinski MR, et al. No effect of 8-week time in bed restriction on glucose tolerance in older long sleepers. J Sleep Res. 2008;17:412–419. doi: 10.1111/j.1365-2869.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hasler G, et al. The association between short sleep duration and obesity in young adults: a 13-Year prospective study. Sleep. 2004;27:661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 77.Gunderson EP, et al. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol. 2008;167:178–187. doi: 10.1093/aje/kwm298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agras WS, et al. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004;145:20–145. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 79.Sugimori H, et al. Analysis of factors that influence body mass index from ages 3 to 6 years: A study based on the Toyama cohort study. Pediatr Int. 2004;46:302–310. doi: 10.1111/j.1442-200x.2004.01895.x. [DOI] [PubMed] [Google Scholar]
- 80.Lumeng JC, et al. Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics. 2007;120:1020–1029. doi: 10.1542/peds.2006-3295. [DOI] [PubMed] [Google Scholar]
- 81.Al Mamun A, et al. Do childhood sleeping problems predict obesity in young adulthood? Evidence from a prospective birth cohort study. Am J Epidemiol. 2007;166:1368–1373. doi: 10.1093/aje/kwm224. [DOI] [PubMed] [Google Scholar]
- 82.Touchette E, et al. Associations between sleep duration patterns and overweight/obesity at age 6. Sleep. 2008;31:1507–1514. doi: 10.1093/sleep/31.11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ayas NT, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 84.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27:282–283. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 85.Nilsson PM, et al. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 86.Meisinger C, Heier M, Loewel H. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235–241. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 87.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 88.Bjorkelund C, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care. 2005;28:2739–2744. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- *89.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 90.Gangwisch JE, et al. Sleep Duration as a Risk Factor for Diabetes Incidence in a Large US Sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hayashino Y, et al. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Public Health. 2007;7:129. doi: 10.1186/1471-2458-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaput JP, et al. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family Study. Sleep Med. 2009;10:919–924. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 93.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19:351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 94.Xu Q, et al. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]