Control of Pancreas and Liver Gene Expression by HNF Transcription Factors (original) (raw)
. Author manuscript; available in PMC: 2010 Dec 30.
Published in final edited form as: Science. 2004 Feb 27;303(5662):1378–1381. doi: 10.1126/science.1089769
Abstract
The transcriptional regulatory networks that specify and maintain human tissue diversity are largely uncharted. To gain insight into this circuitry, we used chromatin immunoprecipitation combined with promoter microarrays to identify systematically the genes occupied by the transcriptional regulators HNF1α, HNF4α, and HNF6, together with RNA polymerase II, in human liver and pancreatic islets. We identified tissue-specific regulatory circuits formed by HNF1α, HNF4α, and HNF6 with other transcription factors, revealing how these factors function as master regulators of hepatocyte and islet transcription. Our results suggest how misregulation of HNF4α can contribute to type 2 diabetes.
Gene expression is controlled by transcriptional regulatory proteins, which bind specific DNA sequences and recruit cofactors and the transcription apparatus to promoters (1-3). Genome-wide analysis methods have been used recently to determine how most transcriptional regulators encoded in Saccharomyces cerevisiae are associated with the genome in living yeast cells and to model the transcriptional regulatory circuitry of these cells (4). These methods have also been used in human tissue-culture cells to identify target genes for several transcriptional regulators (5-7). Genome-scale analysis methods have yet to be used to determine how transcriptional regulators control the global gene expression programs that characterize specific tissues.
The liver and pancreas have long been the subject of studies to understand how organs develop and are regulated at the transcriptional level (8-12). The transcriptional regulators HNF1α (a homeodomain protein), HNF4α (a nuclear receptor), and HNF6 (a member of the onecut family) operate cooperatively in a connected network in the liver, but less is known about the structure of this regulatory network in human pancreatic islets. All three transcriptional regulators are required for normal function of liver and pancreatic islets (13-18). Mutations in HNF1α and HNF4α are the causes of the type 3 and type 1 forms of maturity-onset diabetes of the young (MODY3 and MODY1), a genetic disorder of the insulin-secreting pancreatic beta cells characterized by the onset of diabetes mellitus before 25 years of age and an autosomal dominant pattern of inheritance (19). Genome-scale analysis to find the pancreatic islet genes whose expression is regulated by these transcription factors in normal beta cells could provide insights into the molecular basis of the abnormal beta cell function that characterizes MODY. We identified the genes occupied by the transcription factors HNF1α, HNF4α, and HNF6 in hepatocytes and pancreatic islets, and we identified the genes transcribed in each tissue by determining the genomic occupancy of RNA polymerase II. We used this information to begin to map the transcriptional regulatory circuitry in these tissues.
We first used genome-scale location analysis (20) to identify the promoters bound by HNF1α in human hepatocytes and pancreatic islets isolated from tissue donors (Fig. 1A). For each tissue, HNF1α-DNA complexes were enriched by chromatin immunoprecipitation (ChIP) in three separate experiments. We constructed a custom DNA microarray containing portions of promoter regions of 13,000 human genes (Hu13K array). We targeted the region spanning 700 base pairs upstream and 200 base pairs downstream of transcription start sites for the genes whose start sites are best characterized on the basis of the National Center for Biotechnology Information (NCBI) annotation (20). Although many enhancers are present at more distant locations, most known transcription factor binding-site sequences occur within these start-site proximal regions.
Fig.1.
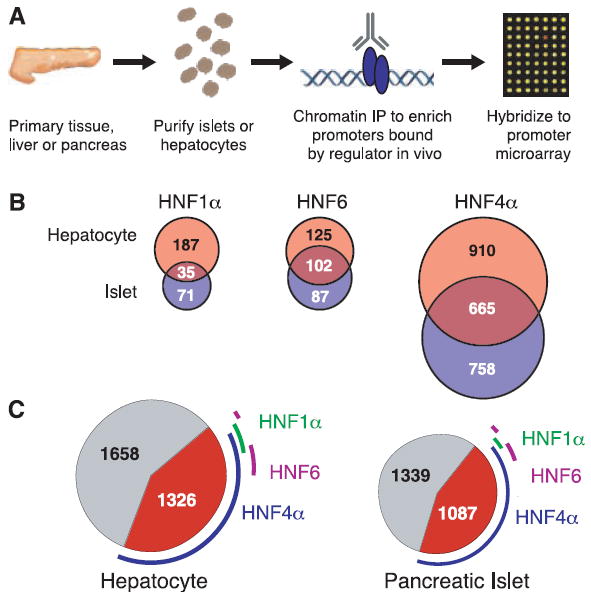
Genome-scale location analysis of HNF regulators in human tissues. (A) Hepatocytes and pancreatic islets were obtained from tissue distribution programs. These cells were treated with formaldehyde to covalently link transcription factors to DNA sites of interaction. Cells were harvested, and chromatin in cell lysates was sheared by sonication. The regulator-DNA complexes were enriched by ChIP with specific antibodies, the cross-links were reversed, and enriched DNA fragments and control genomic DNA fragments were amplified with ligation-mediated polymerase chain reaction. The amplified DNA preparations, labeled with distinct fluorophores, were mixed and hybridized onto a promoter array. (B) Venn diagram showing the overlap of HNF1α-, HNF6-, and HNF4α-bound promoters in hepatocytes (top, red circles) and pancreatic islets (bottom, blue circles). (C) The collection of genes occupied by RNA polymerase II in hepatocytes is displayed as a circle, with the genes bound by HNF1α, HNF6, and/or HNF4α outlined collectively in red as a fraction of the chart. The relative contributions of HNF1α (green), HNF6 (purple), and HNF4α (blue) are shown as framing arcs.
The results of these genome location experiments revealed that HNF1α is bound to at least 222 target genes in hepatocytes, representing 1.6% of the genes on the Hu13K array (Table 1) (20). This result was verified with independent, conventional ChIP experiments, which suggest that the frequency of false positives in genome-scale location data with gene-specific regulators is no more than 16% when our threshold criteria were used (20). The genes that we found to be occupied by HNF1α in primary human hepatocyates encode products whose functions represent a substantial cross section of hepatocyte biochemistry. The results confirm that HNF1α contributes to the transcriptional regulation of many of the central rate-limiting steps in gluconeogenesis and associated pathways. HNF1α also binds to genes whose products are central to normal hepatic function, including carbohydrate synthesis and storage, lipid metabolism (synthesis of cholesterol and apolipoproteins), detoxification (synthesis of cytochrome P450 monooxygenases), and synthesis of serum proteins (albumin, complements, and coagulation factors).
Table 1.
Partial list of proximal promoters occupied by HNF1α in human hepatocytes and pancreatic islets. These genes were assigned to functional categories using the program ProtoGo (www.protogo.cs.huji.ac.il). Genes not in this automated Gene Ontology database were assigned on the basis of NCBI information. Four genes are shown for each tissue/category combination; for some combinations, fewer than four promoters qualified as targets. Hypothetical and functionally uncharacterized genes are not shown. A complete list of bound genes, including their accession numbers, is available (20) (tables S2 and S3). HGF, hepatocyte growth factor; Ubiq.-cyt., ubiquinol-cytochrome; IGF insulin-like growth factor; GTP, guanosine 5′-triphosphate; CREB, cyclic adenosine monophosphate response element– binding protein; RAR, retinoic acid receptor; OAT, organic anion transporter; SNAP, N-ethylmaleimide–sensitive factor attachment protein.
| Name | Description | Hepatocytes | Islets |
|---|---|---|---|
| Chaperone | |||
| C4BPA | Complement 4 binding protein α | x | x |
| APCS | Amyloid P component | x | |
| F11 | Coagulation factor XI | x | |
| C1S | Complement component 1s | x | |
| VTN | Somatomedin B | x | |
| Enzyme—hydrolase | |||
| PGCP | Glutamate carboxypeptidase | x | |
| GLA | Galactosidase, α | x | |
| LIPA | Lipase A | x | |
| SPO11 | SPO11-like | x | |
| PAFAH2 | Platelet-activating factor 2 | x | x |
| AADAC | Arylacetamide deacetylase | x | x |
| PS-PLA1 | Phospholipase A1α | x | x |
| VNN3 | Vanin 3 | x | x |
| CPB2 | Carboxypeptidase B2 | x | |
| ANPEP | Alanyl aminopeptidase | x | |
| HGFAC | HGF activator | x | |
| ENPEP | Glutamyl aminopeptidase | x | |
| Enzyme—ligase | |||
| MCCC1 | Methylcrotonoyl-CoA carboxylase | x | |
| GARS | Glycyl-tRNA synthetase | x | |
| TARS | Threonyl-tRNA synthetase | x | |
| Enzyme—lyase | |||
| UROD | Uroporphyrinogen decarboxylase | x | |
| PCK1 | Phosphoenolpyruvate carboxykinase 1 | x | |
| HPCL2 | 2-hydroxyphytanoyl-CoA lyase | x | |
| HAL | Histidine ammonia-lyase | x | |
| FH | Fumarate hydratase | x | |
| Enzyme—oxidoreductase | |||
| COQ7 | COQ7 coenzyme Q, 7 | x | |
| ADH4 | Alcohol dehydrogenase 4 | x | |
| UQCRC2 | Ubiq.-cyt. c reductase core protein II | x | x |
| CYB5-M | Cytochrome b5 | x | x |
| CYP2E | Cytochrome P450, IIE | x | |
| CYB5 | Cytochrome b-5 | x | |
| HSD17B2 | Hydroxysteroid dehydrogenase 2 | x | |
| ADH1A | Alcohol dehydrogenase 1A | x | |
| Enzyme—transferase | |||
| GCNT3 | Glucosaminyl transferase 3 | x | |
| FNTB | Farnesyltransferase β | x | x |
| HNMT | Histamine N-methyltransferase | x | |
| GOT1 | Aspartate aminotransferase 1 | x | |
| UGT2B15 | UDP glycosyltransferase 2B15 | x | |
| GBE1 | Glycogen branching enzyme | x | |
| Enzyme regulator | |||
| SERPING1 | C1-inhibitor | x | |
| SERPINA1 | α-1-antitrypsin | x | |
| ITIH4 | Inter-α inhibitor H4 | x | |
| AHSG | α-2-HS-glycoprotein | x | |
| Ligand binding | |||
| TMOD2 | Tropomodulin 2 | x | |
| IGFBP1 | IGF binding protein 1 | x | |
| MT1X | Metallothionein 1X | x | |
| CRP | C-reactive protein | x | |
| APOA2 | Apolipoprotein A-II | x | |
| Signal transduction—other | |||
| BIKE | BMP-2 inducible kinase | x | |
| SGK2 | Serum/glucocorticoid reg. kinase 2 | x | x |
| SEL1L | Suppressor of lin-12-like | x | x |
| SCYE1 | Small cytokine E1 | x | |
| ANGPTL3 | Angiopoietin-like 3 | x | |
| Signal transduction—receptor | |||
| HAVCR-1 | Hepatitis A virus cellular receptor 1 | x | |
| TACR3 | Tachykinin receptor 3 | x | |
| GNB2L1 | GTP-binding protein, β2-like 1 | x | |
| INSR | Insulin receptor | x | |
| SSTR1 | Somatostatin receptor 1 | x | x |
| TM4SF4 | Transmembrane 4-4 | x | x |
| ASGR2 | Asialoglycoprotein receptor 2 | x | |
| GPR39 | G protein–coupled receptor 39 | x | |
| IFNAR1 | Interferon receptor 1 | x | |
| TFRC | Transferrin receptor | x | |
| Transcription Regulation | |||
| ZNF300 | Kruppel-like zinc finger protein | x | |
| BCL6 | B cell CLL/lymphoma 6 | x | |
| ZNF155 | Zinc finger protein 155 | x | |
| FBX08 | F-box only protein 8 | x | |
| NR0B2 | Small heterodimer protein | x | x |
| HNF4A7 | HNF4α, alternate splice | x | x |
| NR5A2 | LRH-1/FTZ-F1 | x | x |
| ELF3 | E74-like factor 3 | x | x |
| NR1D1 | THRA1 | x | |
| ATF2 | Activating transcription factor 2 | x | |
| CREBL2 | CREB-like 2 | x | |
| RARB | RAR-β | x | |
| Transporter—channel/pore | |||
| SLC17A2 | Vesicular glutamate transporter | x | |
| AQP3 | Aquaporin 3 | x | |
| SLC22A11 | hOAT4 | x | |
| GJB1 | Gap junction protein, β 1 | x | |
| Transporter—lipids and small molecules | |||
| APOH | Apolipoprotein H | x | x |
| ALB | Albumin | x | |
| ABCC2 | Canalicular OAT | x | |
| G6PT1 | Glucose-6-phosphatase, transport | x | |
| Transporter—proteins | |||
| RAB6KIFL | RAB6 interacting, kinesin-like | x | |
| PEX13 | Peroxisome biogenesis factor 13 | x | |
| TMP21 | Transmembrane trafficking protein | x | |
| RAB33B | RAS oncogene | x | x |
| NAPA | α-SNAP | x | |
| AP3M1 | Adaptor-related protein complex | x | |
| SNX17 | Sorting nexin 17 | x |
We next identified HNF1α target genes in human pancreatic islets (Table 1) (20). HNF1α occupied the promoter regions of 106 genes (0.8% of the Hu13K array promoters) in islets, 30% of which were also bound by HNF1α in hepatocytes (Fig. 1B). In islets, fewer chaperones and enzymes are bound by HNF1α than in hepatocytes, and the receptors and signal transduction machinery regulated by HNF1α vary between the two tissues.
HNF1α has previously been implicated in the regulation of many genes in hepatocytes and islets (13, 16, 20) (table S4). The direct genome binding data reported here confirmed many but not all of these genes. The difference may be due, at least in part, to our stringent criteria for binding in the genome-scale data, which enhances our confidence in the direct target genes identified by location analysis, but likely underestimates the actual number of targets in vivo. Furthermore, although the proximal promoter regions printed on the array contain a number of transcription factor binding sequences, many genes are also regulated by more distal promoter elements and enhancers that are not present on the Hu13K array.
We also identified the promoters bound by HNF6 in human hepatocytes and pancreatic islets using genome-scale location analysis (Fig. 1B and tables S5 and S6) (20). HNF6 was bound to at least 227 genes in hepatocytes and 189 genes in pancreatic islets, representing 1.7 and 1.4% of the promoters on the array, respectively. About half of the promoters occupied by HNF6 were common to the two tissues, including a number of important cell cycle regulators such as cyclin-dependent kinase 2 (20).
Genome-scale location analysis revealed surprising results for HNF4α in hepatocytes and pancreatic islets (Fig. 1B). The number of genes enriched in HNF4α ChIPs was much larger than observed with typical site-specific regulators. HNF4α was bound to about 12% of the genes represented on the Hu13K DNA microarray in hepatocytes and 11% in pancreatic islets. No other transcription factor that we have profiled in human cells has been observed to bind more than 2.5% of the promoter regions represented on the Hu13K array.
Six independent lines of evidence indicate that the HNF4α results are not due to poor antibody specificity or errors in the microarray analysis; this evidence supports the view that HNF4α is associated with an unusually large number of promoters in hepatocytes and pancreatic islets (20). First, essentially identical results were obtained with two different antibodies that recognize different portions of HNF4α. Second, Western blots showed that the HNF4α antibodies are highly specific. Third, we verified binding at more than 50 randomly selected targets of HNF4α in hepatocytes by conventional gene-specific ChIP. Fourth, when antibodies against HNF4α were used for ChIP in control experiments with Jurkat, U937, and BJT cells (which do not express HNF4α), no more than 17 promoters were identified in each cell line by our criteria, which is well within the noise inherent in this system. Fifth, when pre-immune antibodies from rabbit and goat (the two different antibodies to HNF4α came from rabbit and goat) were used in control experiments in hepatocytes, the number of targets identified was within the noise. Finally, if the HNF4α results are correct, then we would expect that the set of promoters bound by HNF4α should be largely a subset of those bound by RNA polymerase II in each tissue; we found that this is the case. We conclude that HNF4α is a widely acting transcription factor in these tissues, consistent with the observation that it is an unusually abundant and constitutively active transcription factor (11).
We next identified the genes represented on the Hu13K microarray that are actively transcribed in hepatocytes and pancreatic islets to determine the fraction of actively transcribed genes that are bound by HNF4α (Fig. 1C). It is difficult to determine the transcriptome of these tissues accurately by profiling transcript levels with DNA microarrays. Transcript profiling requires a reference RNA population against which a tissue RNA population can be compared, and there are limitations to generating appropriate reference RNA. To circumvent this limitation, we exploited the fact that RNA polymerase II occupies the set of protein-coding genes that are actively transcribed in eukaryotic cells. Location analysis with RNA polymerase II antibodies can identify these actively transcribed genes (7, 21). We found that 23% of the genes on the Hu13K array (2984 genes) were bound by RNA polymerase II in hepatocytes, and 19% (2426 genes) were bound by RNA polymerase II in islets (20). The sets of genes occupied by RNA polymerase II in hepatocytes and islets overlapped substantially (81% overlap, relative to islets), consistent with the relatedness of the two tissues (22). As expected, the majority of genes occupied by HNF4α in hepatocytes and pancreatic islets (80 and 73%, respectively) were also occupied by RNA polymerase II. Notably, of the genes occupied by RNA polymerase II, 42% (1262 out of 2984) were bound by HNF4α in hepatocytes and 43% (1047 out of 2426) were bound by HNF4α in islets (Fig. 1C). By comparison, only 6 and 2% of RNA polymerase II–enriched promoters were also bound by HNF1α in hepatocytes and islets, respectively. The occupancy by HNF4α of a substantial fraction of expressed genes suggests that abnormal levels of HNF4α could impair glucose-stimulated insulin secretion in pancreatic beta cells by affecting a large portion of the transcriptome rather than through misregulation of a specific downstream target gene.
Previous studies indicate that HNF1α, HNF4α, and HNF6 are at the center of a network of transcription factors that cooperatively regulate numerous developmental and metabolic functions in hepatocytes and islets (9, 15, 17). Our systematic analysis of the direct in vivo targets of these factors considerably expands our understanding of the regulatory network in primary human tissues (Fig. 2A). A comparison of the regulatory network in these two tissues reveals that HNF1α, HNF4α, and HNF6 occupy the promoters of genes encoding a large population of transcription factors and cofactors (20). The precise set of transcription factor genes occupied by HNF1α, HNF4α, and HNF6, and the extent to which they are co-occupied by the HNF regulators, differed substantially between these two tissues. For example, the HNF4-A gene has two different promoters, which are differentially utilized in hepatocytes and islets (23, 24), and which are differentially occupied by HNF1α, HNF6, and HNF4α in human tissues (Fig. 2A). Because both promoters are used in hepatocytes but only the promoter for HNF4α7 is used in pancreatic islets, it is possible that defects in the regulatory network disproportionately affect HNF4α expression in islets.
Fig.2.
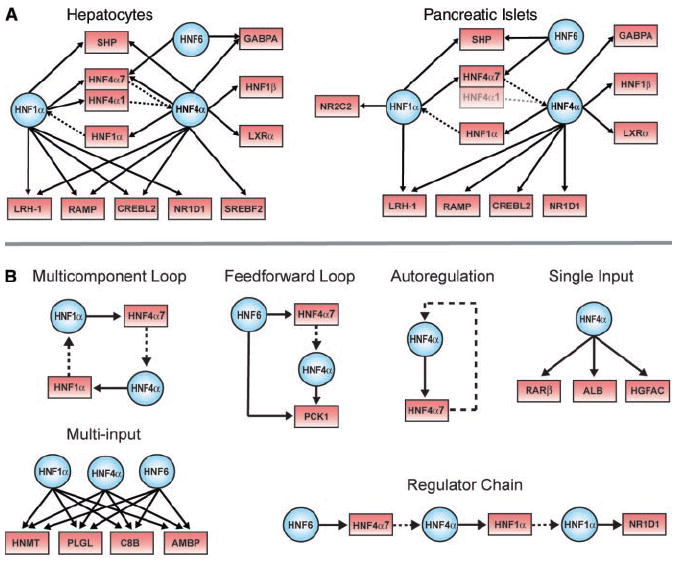
Transcriptional regulatory networks and motifs. (A) HNF1α, HNF6, and HNF4α are at the center of tissue-specific transcriptional regulatory networks. In these examples selected for illustration, regulatory proteins and their gene targets are represented as blue circles and red boxes, respectively. Solid arrows indicate protein-DNA interactions, and genes encoding regulators are linked to their protein products by dashed lines. The HNF4α1 promoter is poorly expressed in pancreatic islets and is thus shaded to reflect this. The HNF4α7 promoter, also known as the P2 promoter (23, 24), is the predominant promoter in pancreatic islets and was recently implicated as a major human diabetes susceptibility locus. For clarity, some gene promoters have been designated by the names of their protein products (e.g., HNF1α for TCF1, SHP for NR0B2, HNF4α7 for HNF4A P2, and HNF1β for TCF2). (B) Examples of regulatory network motifs in hepatocytes. For instance, in the multicomponent loop, HNF1α protein binds to the promoter of the HNF4α gene, and the HNF4α protein binds to the promoter of the HNF1α gene. These network motifs were uncovered by searching binding data with various algorithms; details on the algorithms used and a full list of motifs found are available in (20).
The transcription factor binding data were used to identify regulatory network motifs, simple units of transcriptional regulatory network architecture that suggest mechanistic models (Fig. 2B) (4, 25). Our data confirm previous reports that HNF1α and HNF4α occupy one another’s promoters in both hepatocytes and islets, forming a multicomponent loop (23, 24, 26). Multicomponent loops provide the capacity for feedback control and produce bistable systems that can switch between two alternate states (25), and it has been suggested that the multicomponent loop present between HNF1α and HNF4α is responsible for stabilization of the terminal phenotype in pancreatic beta cells (26). We also found that HNF6 serves as a master regulator for feedforward motifs in hepatocytes and pancreatic islets involving more than 80 genes in each tissue (tables S9 and S11). For example, in hepatocytes, HNF6 binds the promoter for HNF4α7, and HNF6 and HNF4α together bind PCK1, which encodes phosphoenolpyruvate carboxykinase, an enzyme key to gluconeogenesis (Fig. 2B). A feedforward loop can act as a switch designed to be sensitive to sustained inputs rather than transient inputs (25). HNF1α, HNF4α, and HNF6 were also found to form multi-input motifs by collectively binding to sets of genes in hepatocytes and islets. This regulatory motif suggests coordination of gene expression through multiple input signals. We also found that HNF6, HNF4α, and HNF1α form a regulator chain motif with THRA (NR1D1); regulator chain motifs represent the simplest circuit logic for ordering transcriptional events in a temporal sequence (4, 25). Additional examples of these regulatory motifs can be found in tables S9 to S12 (20).
Our results suggest that the nuclear receptor HNF4α contributes to regulation of a large fraction of the liver and pancreatic islet transcriptomes by binding directly to almost half of the actively transcribed genes. This likely explains why HNF4α is crucial for development and proper function of these tissues (12-18). Furthermore, our results suggest a mechanistic explanation for the recent discovery that polymorphisms in and near HNF4A, including those near the promoter used in islets, can increase the risk of type II diabetes (27-30). We found that multiple HNF factors bind directly to the P2 promoter in primary healthy human islets. Alterations in the binding sites for these factors could cause misregulation of HNF4α expression and thus its downstream targets, leading to beta cell malfunction and diabetes.
Supplementary Material
Supplementary data
Acknowledgments
We thank the anonymous donors who made this research possible. We are grateful to G. Weir, S. Bonner-Weir, and J. Kushner for helpful discussions and access to the resources of the Joslin Diabetes Center. Tissue distribution programs were supported by the Juvenile Diabetes Research Foundation and the Liver Tissue Procurement and Distribution System (National Institute of Diabetes and Digestive and Kidney Diseases contract N01-DK-9-2310). We thank the Whitehead Institute Center for Microarray Technology and Whitehead Institute Biocomputing for technical support, and S. Strom and A. Omer for experimental support. Supported by a fellowship from the Sloan Foundation/U.S. Department of Energy Program for Computational Molecular Biology (D.T.O.), and grants from the Howard Hughes Medical Institute (G.I.B.) and the NIH (G.I.B. and R.A.Y.).
Footnotes
References and Notes
- 1.Roeder RG. Cold Spring Harbor Symp Quant Biol. 1998;63:201. doi: 10.1101/sqb.1998.63.201. [DOI] [PubMed] [Google Scholar]
- 2.Lee TI, Young RA. Annu Rev Genet. 2000;34:77. doi: 10.1146/annurev.genet.34.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Orphanides G, Reinberg D. Cell. 2002;108:439. doi: 10.1016/s0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 4.Lee TI, et al. Science. 2002;298:799. doi: 10.1126/science.1075090. [DOI] [PubMed] [Google Scholar]
- 5.Ren B, et al. Genes Dev. 2002;16:245. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinmann AS, et al. Genes Dev. 2002;16:235. doi: 10.1101/gad.943102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, et al. Proc Natl Acad Sci U S A. 2003;100:8164. doi: 10.1073/pnas.1332764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai E, Darnell JE., Jr Trends Biochem Sci. 1991;16:427. doi: 10.1016/0968-0004(91)90169-v. [DOI] [PubMed] [Google Scholar]
- 9.Kuo CJ, et al. Nature. 1992;355:457. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- 10.Pontoglio M, et al. Cell. 1996;84:575. doi: 10.1016/s0092-8674(00)81033-8. [DOI] [PubMed] [Google Scholar]
- 11.Sladek FM, Seidel SD. In: Nuclear Receptors and Genetic Disease. Burris TP, editor. Academic Press; New York: 2001. pp. 309–361. [Google Scholar]
- 12.Parviz F, et al. Nature Genet. 2003;34:292. doi: 10.1038/ng1175. [DOI] [PubMed] [Google Scholar]
- 13.Costa RH, et al. Hepatology. 2003;38:1331. doi: 10.1016/j.hep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Bell GI, Polonsky KS. Nature. 2001;414:788. doi: 10.1038/414788a. [DOI] [PubMed] [Google Scholar]
- 15.Shih DQ, et al. Diabetes. 2001;50:2472. doi: 10.2337/diabetes.50.11.2472. [DOI] [PubMed] [Google Scholar]
- 16.Shih DQ, Stoffel M. Proc Natl Acad Sci U S A. 2001;98:14189. doi: 10.1073/pnas.251558998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaret KS. Nature Rev Genet. 2002;3:499. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- 18.Jacquemin P, et al. Dev Biol. 2003;258:105. doi: 10.1016/s0012-1606(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 19.Fajans SS, et al. N Engl J Med. 2001;345:971. doi: 10.1056/NEJMra002168. [DOI] [PubMed] [Google Scholar]
- 20.Supporting data are available on Science Online Additional information is available at http://web.wi.mit.edu/young/pancregulators
- 21.Ng HH, Robert F, Young RA, Struhl K. Genes Dev. 2002;16:806. doi: 10.1101/gad.978902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bort R, Zaret K. Nature Genet. 2002;32:85. doi: 10.1038/ng0902-85. [DOI] [PubMed] [Google Scholar]
- 23.Boj SF, Parrizas M, Maestro MA, Ferrer J. Proc Natl Acad Sci U S A. 2001;98:14481. doi: 10.1073/pnas.241349398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas H, et al. Hum Mol Genet. 2001;10:2089. doi: 10.1093/hmg/10.19.2089. [DOI] [PubMed] [Google Scholar]
- 25.Milo R, et al. Science. 2002;298:824. doi: 10.1126/science.298.5594.824. [DOI] [PubMed] [Google Scholar]
- 26.Ferrer J. Diabetes. 2002;51:2355. doi: 10.2337/diabetes.51.8.2355. [DOI] [PubMed] [Google Scholar]
- 27.Barroso I, et al. PLoS Biol. 2003;1:41. doi: 10.1371/journal.pbio.0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Q, et al. Diabetologia. 2003;46:567. doi: 10.1007/s00125-003-1067-y. [DOI] [PubMed] [Google Scholar]
- 29.Love-Gregory L, et al. Diabetes. in press. [Google Scholar]
- 30.Silander K, et al. Diabetes. in press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data