Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor) (original) (raw)
Abstract
Global declines in amphibians likely have multiple causes, including widespread pesticide use. Our knowledge of pesticide effects on amphibians is largely limited to short-term (4-d) toxicity tests conducted under highly artificial conditions to determine lethal concentrations (LC50). We found that if we used slightly longer exposure times (10–16 d), low concentrations of the pesticide carbaryl (3–4% of LC504-d) killed 10–60% of gray treefrog (Hyla versicolor) tadpoles. If predatory cues also were present, the pesticide became 2–4 times more lethal, killing 60–98% of tadpoles. Thus, under more realistic conditions of increased exposure times and predatory stress, current application rates for carbaryl can potentially devastate gray treefrog populations. Further, because predator-induced stress is ubiquitous in animals and carbaryl's mode of action is common to many pesticides, these negative impacts may be widespread in nature.
Amphibian population declines around the world have been receiving increased attention, but the mechanisms responsible for many of these declines have remained elusive. Hypothesized mechanisms include natural population fluctuations, habitat destruction, introduced predators and pathogens, increased UV radiation, and environmental contaminants (1–7). Whereas evidence is accumulating for the first four hypotheses, little is known about the effects of environmental contaminants such as heavy metals and pesticides on amphibian populations. Given the pervasiveness of pesticide applications, negative effects of pesticides could have an impact on amphibians around the world. For example, in the United States alone, 2 billion kg of pesticides are used annually across many different habitats, including nearly 75% of all farms and homes. Worldwide use is nearly 5 times this amount (8).
Our knowledge of pesticide effects on amphibians comes primarily from acute toxicity tests that estimate LC50 (the concentration of a pesticide predicted to kill 50% of a test population within a given amount of time and under given conditions of exposure). These tests typically are conducted for only 1–4 d and they are conducted without consideration of many natural biotic and abiotic effects (9–11). LC50 estimates have been extremely useful in determining the relative lethality of different pesticides and the relative susceptibility of different organisms. However, the lethality of very low concentrations of pesticides (≪LC50) is often unknown (12, 13).
Abiotic and biotic stressors have the potential to interact with acute pesticide effects, and this has provoked a great deal of interest about the impact of multiple stressors. Abiotic factors such as pH, temperature, and light can synergistically affect mortality caused by pesticides (14–16), but we know little about the potential synergistic effects of biotic factors. For example, the fear of predation is a common stressor that causes most animals, including amphibians, to become less active and grow more slowly (17–22), but there appear to be no studies that have examined the interaction between predator-induced fear and pesticides as multiple stressors. In this study, we examined the impact of low concentrations of a pesticide and predator-induced stress on the behavior, growth, and survival of larval gray treefrogs (Hyla versicolor). The gray treefrog is a species common to eastern North America that breeds in the early summer throughout its range. Treefrogs lay their eggs in ponds, and the eggs hatch within a few days. The resulting tadpoles grow in the pond for 4–8 weeks and then metamorphose into terrestrial frogs.
For our experiments, we worked with carbaryl (1-naphthyl_N-_methylcarbamate; commercial name, Sevin), one of the world's most commonly used, broad-spectrum pesticides (an insecticide, acaricide, molluscicide, and ectoparasiticide). It acts by inhibiting acetylcholinesterase and has become popular throughout the world since 1959 because of its low toxicity to mammals and its relatively short lifetime in the environment. Whereas myriad tests of carbaryl toxicity have been conducted on birds, mammals, fish, and invertebrates, there are few published studies on amphibians. Past studies of amphibian responses to carbaryl have found that carbaryl reduces tadpole activity and growth, and LC50 estimates vary between 2.5 and 20.6 mg/liter (12, 13, 15, 23).
Carbaryl is applied to croplands (>100 crop species), rangelands, forests, wetlands, oceans, and sewage treatment plants to exterminate animal pests, and it is applied to domesticated animals to control lice, mites, ticks, and fleas (24). Ten to 15 million pounds of carbaryl are applied annually in the United States on 200 million acre-treatments (acres treated × number of treatments), including 28 million homes and 31 million gardens (25). Because carbaryl is widely used, it can enter amphibian-containing wetlands through direct aerial spraying, aerial drift, terrestrial runoff, or erosion (26, 27). While our study focused on just carbaryl, it is important to note that carbaryl represents only 1 of 21,000 chemical pesticides currently in use (8).
Methods
Experiment 1.
In 1999, we conducted a pilot experiment to determine the chronic (longer-term) effect of carbaryl and predator stress on larval treefrog survival. We used eggs from 10 pairs of amplecting treefrogs collected from a pond in the Baskett Wildlife Area near Ashland, MO. We hatched the eggs in filtered tap water and then randomly assigned groups of 10 tadpoles (mean mass ± 1 SE in water = 56 ± 5 mg) to polyethylene tubs containing 10 liters of filtered tap water. Adsorption of carbaryl onto these plastic tubs has been found to be negligible (28). The tubs were placed on two shelves in two spatial blocks in a laboratory under a 15:9 h light:dark cycle.
Tubs were randomly assigned one of four chemical treatments and one of two predator treatments (all treatments replicated four times). The chemical treatments consisted of a negative control (water addition), a solvent control (acetone addition), and two levels of carbaryl addition. We made a stock solution of carbaryl by dissolving 501 mg of technical grade carbaryl (99.8% purity; Rhone-Poulenc, Research Triangle Park, NC) into 250 ml of acetone. The carbaryl concentration of the stock solution was 1.8 mg/ml, based on high-pressure liquid chromatography analyses by the Mississippi State Chemical Laboratory. Tubs assigned to the low and high carbaryl treatments received either 0.25 or 0.50 ml of stock solution for nominal carbaryl concentrations of 0.045 and 0.090 mg/liter, respectively. These compare with LC50 estimates of 12.9 mg/liter [a 2-d test (13)] and 2.5 mg/liter [a 4-d test (15)]. Solvent control tubs received 0.5 ml of acetone, whereas negative controls received 0.5 ml of water. Predator treatments consisted of either a caged larval salamander (Ambystoma maculatum) or an empty cage (250-ml plastic cups covered with fiberglass window screening). Caged predators emit chemical cues that induce antipredator responses in their prey without allowing the predators to kill the target animals (29–31).
During the 10-d experiment, tadpoles first were fed ground fish food at a rate of 18% of initial body mass per tadpole per day (an abundant food ration). Whereas shorter-term tests (1–4 d) are typically conducted in the absence of food, we added food because the tadpoles would not have survived the longer experiment without food and because foraging tadpoles reflect the more natural situation. Once we visually estimated that the tadpoles had doubled in mass across treatments, the food ration was doubled. Caged predators were fed five small tadpoles every other day to produce the chemical cue(s) and, if predators died, the predators were replaced. We changed the tub water on day 3 and day 7 (changes grouped by treatment), and the chemical treatments were reapplied after water changes. Each day, the number of surviving tadpoles was counted. On days requiring water changes, we quantified survival before changing the water. We did not monitor water temperature, but the laboratory was maintained at 24 ± 1°C.
The data were analyzed with an analysis of variance (ANOVA) using final survival as the response variable [transformed as log(survival + 0.1)]. A repeated-measures ANOVA was not possible because control treatments had no variance on several of the days sampled (100% ± 0% survival). Block effects never approached significance (P > 0.5) and were dropped from the analysis.
Experiments 2 and 3.
In 2000, we conducted two, more-extensive, experiments to determine the effects of carbaryl and predator stress on larval treefrog behavior, growth, and survival. For both experiments, we collected fertilized eggs from a different population (12 km south of the first population) and hatched them in filtered tap water. As in experiment 1, groups of 10 tadpoles were randomly assigned to 10-liter polyethylene tubs filled with filtered tap water. Tubs were placed on shelves in four spatial blocks in a laboratory under a 15:9 h light:dark ratio. The tadpoles in experiment 2 were a mixture of 21 sibships (mean mass ± 1 SE = 13 ± 1 mg), whereas tadpoles in experiment 3 were a mixture of 8 sibships (mean mass = 11 ± 1 mg).
In each experiment, tubs were randomly assigned a factorial combination of two predator treatments and six chemical treatments (replicated four times). Predator treatments were identical to those in experiment 1, whereas the chemical treatments consisted of a negative control (water addition), a solvent control (acetone addition), and four concentrations of carbaryl. In experiment 2, we made a stock solution of carbaryl by dissolving 6,018 mg of technical grade carbaryl into 100 ml of acetone. The carbaryl concentration of the stock solution was 62.7 mg/ml (based on analyses by the Mississippi State Chemical Laboratory). Tubs assigned to the four carbaryl treatments received 1.33, 0.67, 0.33, or 0.17 ml of stock solution for nominal carbaryl concentrations 8.3, 4.2, 2.1, and 1.0 mg/liter, respectively. Solvent control tubs received 1.33 ml of acetone, whereas negative controls received 1.33 ml of water. In experiment 3, we made a stock solution of carbaryl by dissolving 501 mg of technical grade carbaryl into 250 ml of acetone. The carbaryl concentration of the stock solution was 2.7 mg/ml (based on analyses by the Mississippi State Chemical Laboratory). Tubs assigned to the four carbaryl treatments received 2.00, 1.00, 0.50, or 0.25 ml of stock solution for nominal carbaryl concentrations 0.54, 0.27, 0.14, and 0.07 mg/liter, respectively. Solvent control tubs received 2 ml of acetone, whereas negative controls received 2 ml of water.
During the 16-d experiments, tadpoles and predators were fed as in experiment 1. We changed the tub water every 4 days, and the chemical treatments were reapplied after water changes. We observed the activity of the tadpoles 10 times per day by slowly approaching each tub and counting the number of tadpoles alive in each tub and the proportion of live tadpoles that were active (moving) by using scan sampling (32). At the end of the experiment, the surviving tadpoles were counted and weighed. Because tadpole growth was based only on those tadpoles that survived, our estimates of growth could be upwardly biased if slower-growing tadpoles were more susceptible to the stresses of predators and carbaryl.
Midway through experiments 2 and 3, we quantified the oxygen, temperature, pH, and total ammonia in each tub. Oxygen and temperature were measured by using a Yellow Springs Instrument 55 dissolved oxygen meter (oxygen resolution = 0.01 mg/liter; temperature resolution = 0.1°C). Total ammonia and pH were measured using an Orion Expandable ionAnalyzer EA 940 (ammonia resolution = 0.001 mg/liter; pH resolution = 0.01 pH).
The activity, growth, and abiotic data were analyzed with standard ANOVA. The survivorship data did not meet the assumptions of standard ANOVA, so we conducted a nonparametric analysis on survivorship by first ranking the data and then conducting an ANOVA on the ranks. Block interactions never approached significance (P > 0.5) and were dropped from the analysis. For all of the experiments, animal care was in accordance with institutional guidelines.
Results
Experiment 1.
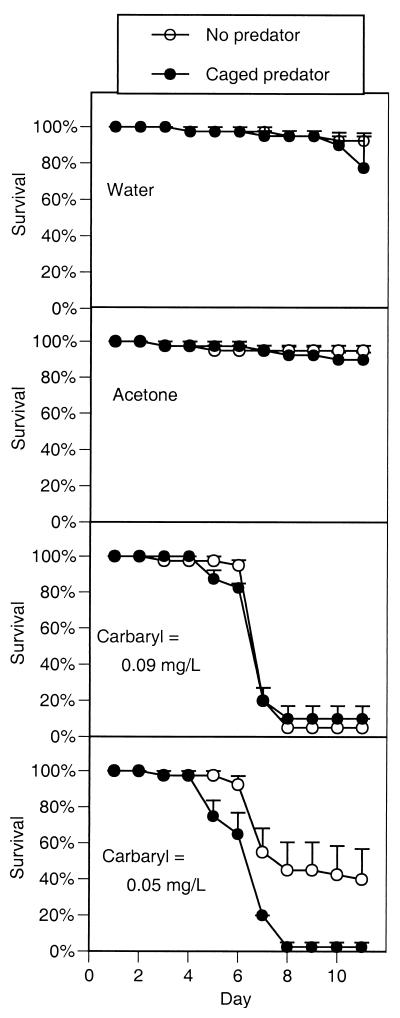
Survival remained high in the control chemical treatments, but the addition of carbaryl at all of the concentrations caused high mortality within 1 week (Fig. 1). Survival remained high in the presence of carbaryl through day 5 and then began a precipitous drop to a point that was significantly lower than the controls (_F_3,24 = 34.4,P < 0.0001). The chemical and predator treatments interacted with the predator treatments (_F_3,24 = 4.5, P = 0.012). When carbaryl was present at 0.090 mg/liter, survival declined to approximately 8% by day 8, regardless of predator treatment. When carbaryl was present at 0.05 mg/liter, tadpole survival declined to 40% with predators absent but declined to 3% with predators present.
Figure 1.
Survivorship of gray treefrog tadpoles reared in the presence or absence of predatory cues combined with the addition of either water (a negative control), acetone (a solvent control), or two concentrations of carbaryl (experiment 1). Data are means ± 1 SE.
Experiments 2 and 3.
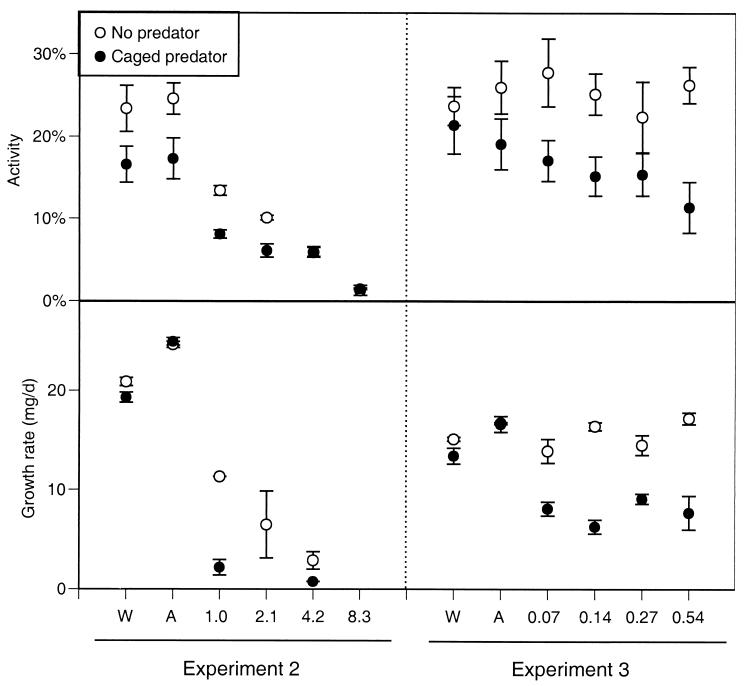
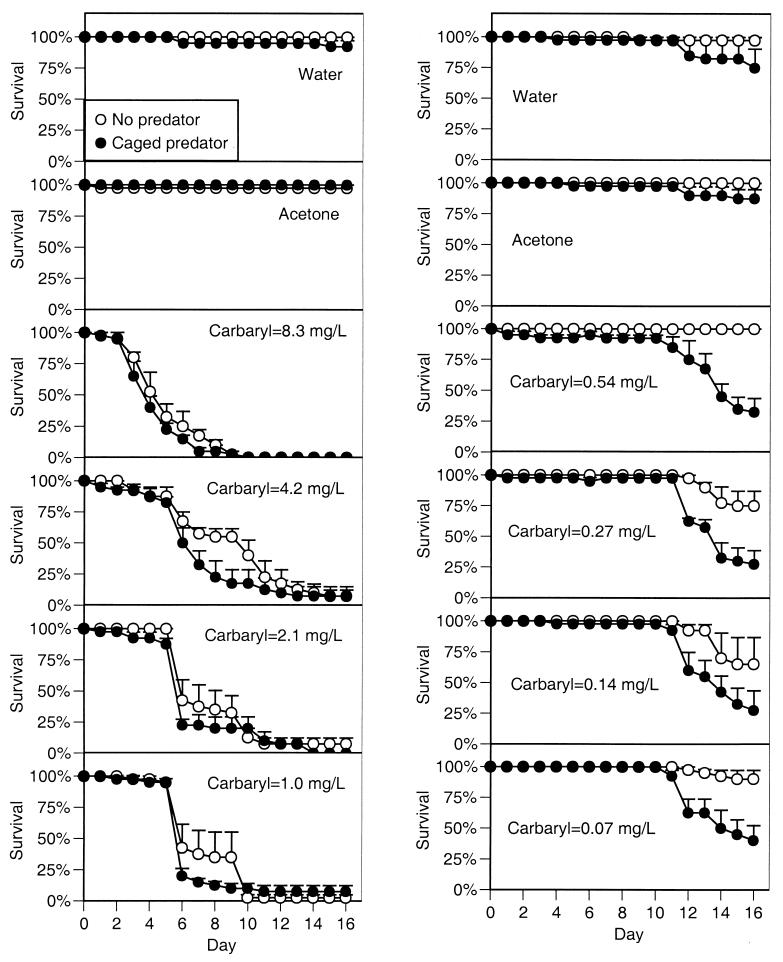
In the more extensive experiments conducted the following year, we found similar results (Figs. 2 and3). In experiment 2 (which contained the highest four carbaryl concentrations), survivorship was 98% with either control treatment (regardless of predator treatment). However, in the presence of carbaryl, survivorship dropped off precipitously beginning on day 3 at the highest concentration and day 6 at the lowest concentration. After 16 d, mean survival across the four carbaryl treatments was 4%, significantly lower than the control treatments (_F_5,36 = 35.0, P < 0.00001). Predators did not affect tadpole survivorship (_F_1,36 = 0.7, P < 0.407). Because of the widespread death, tadpole activity could be assessed only during the first 6 days. Carbaryl caused a reduction in activity, and this reduction was larger as carbaryl concentration increased (_F_5,33 = 60.7,P < 0.0001). Predators generally reduced activity across all chemical treatments (_F_1,33 = 22.0, P < 0.001), but the reduction was not significant under the highest two carbaryl concentrations in which the activity levels were already extremely low (activity = 1–6%). The widespread death among most of the tubs containing carbaryl precluded any analysis of growth rates (24 tubs had no tadpoles alive), but the few tadpoles that remained alive with carbaryl present experienced about 50% of the growth experienced with carbaryl absent.
Figure 2.
Activity and growth rate of gray treefrog tadpoles reared in the presence or absence of predatory cues combined with the addition of water (W; a negative control), acetone (A; a solvent control), or carbaryl (numbers along the x axis represent carbaryl concentrations in mg/liter). Data are means ± 1 SE (experiments 2 and 3).
Figure 3.
Survivorship of gray treefrog tadpoles reared in the presence or absence of predatory cues combined with the addition of water (a negative control), acetone (a solvent control), or carbaryl at eight concentrations (experiments 2 and 3). Data are means ± 1 SE. (Left) Experiment 2. (Right) Experiment 3.
When we monitored the chemical conditions in the water midway through the experiment, we found that carbaryl had no effect on water temperature (mean = 23.0°C, P = 0.089) and only minor effects on oxygen (P ≤ 0.001, range of means = 6.6–7.3 mg/liter), and pH (P = 0.048, range of means = 8.5–8.6) that were not related to carbaryl concentration. Increased carbaryl was associated with increased ammonia levels (P < 0.0001, range of means = 0.21–0.99 mg/liter), but this effect was likely due to the presence of dead tadpoles and an excess of unconsumed food (regression of survival against ammonia, _P_ = 0.001,_R_2 = 0.395). Predators had no effect on ammonia or temperature (_P_ > 0.1) and only small effects on oxygen and pH (a 9% decrease in oxygen, P < 0.0001; a 0.5% decrease in pH, P = 0.019, range of means = 8.59–8.63).
In experiment 3 (which included the lowest four carbaryl concentrations), survivorship was again very high in both control treatments, regardless of predator presence (mean = 90%). However, in the presence of carbaryl, survivorship declined beginning on days 10–11, and the final survivorship with carbaryl present was significantly reduced (mean across carbaryl treatments = 83%;_F_5,36 = 8.3, P = 0.00003). Predator cues made the pesticide 4 times more lethal (_F_1,36 = 48.1, P < 0.00001); final survivorship across the four carbaryl treatments with caged predators averaged 32%. Over the duration of the experiment, there were small differences in activity among the control and carbaryl treatments (_F_5,33 = 3.5,P = 0.011), but activity with carbaryl was similar to activity with the solvent control (P ≥ 0.05). Predators did not affect tadpole activity when carbaryl was absent but significantly reduced activity when carbaryl was present (chemical × predator interaction: _F_5,33 = 4.8,P = 0.002). Predators and carbaryl also had an interactive effect on growth rate (_F_5,29 = 9.7, P = 0.00002); predators did not affect growth in the absence of carbaryl but reduced tadpole growth by 50% in the presence of carbaryl.
When we monitored the chemical conditions in the water midway through experiment 3, we found that carbaryl treatments and the solvent control had 6% lower oxygen concentrations than the negative control (P ≤ 0.002, range of means = 3.8–4.9 mg/liter), but there were no differences in pH (mean = 8.5), temperature (mean = 22.9°C), or ammonia (mean = 0.22 mg/liter). Predators had no effect on oxygen, pH, temperature, or ammonia (P > 0.1).
Discussion
Our results demonstrate that very low concentrations of carbaryl can have dramatic effects on amphibian behavior, growth, and survival. As in past studies, carbaryl reduced tadpole activity and growth (12,15, 22). Estimates of carbaryl LC50 in treefrog tadpoles have ranged from 12.9 mg/liter [a 2-d test (13)] to 2.5 mg/liter [a 4-d test (15)], similar to LC50 estimates in other larval anurans (12, 23). Our lowest pesticide concentrations in the two years were 2.8–3.8% of the LC504-d and 0.4–0.5% of the LC502-d, suggesting that our concentrations should have little short-term effect on tadpole survival in any of the experiments. Indeed, after 4 d, our pesticide concentrations had no negative effect on survival in any of the experiments. However, by the end of the experiments, up to 97% of the tadpoles died. Thus, very low concentrations of carbaryl can still cause widespread amphibian death, it just takes a few more days to observe the effect.
Our results also demonstrated that predatory cues can interact with carbaryl to cause substantial tadpole mortality. Predator-induced stress alone generally reduced tadpole activity, but it never reduced tadpole growth or survival. Similarly, when carbaryl concentrations were low, carbaryl alone had small impacts on tadpole survival. However, when both stressors were present, tadpole mortality increased by 2–4 times. When carbaryl concentrations were high, carbaryl-induced stress dominated, causing rapid mortality regardless of whether the predator-induced stress was present. The carbaryl concentration at which predators played a synergistic role differed between the two years; this difference may be attributable to either different initial sizes or genetic differences between the two source populations.
We are only beginning to appreciate how abiotic and biotic stressors can interact with pesticides. Researchers have found that changes in abiotic factors such as temperature, pH, and UV-B radiation can synergistically affect the lethality of pesticides (14–16). Our study is unique in that the synergism was caused by a biotic factor (predatory cues) that is extremely common in aquatic systems (the majority of ponds inhabited by treefrogs also are inhabited by aquatic predators; E. E. Werner, R.A.R., D. K. Skelly, and K. L. Yurewicz, unpublished data). Given the ubiquity of predator-induced stress in a wide variety of animals (17, 19), similar interactions among predator stress and similarly acting, widely used insecticides may be common. Further, it seems likely that other biotic stressors (e.g., competition, parasites) also could have interactive effects with pesticides. However, given the preliminary nature of our knowledge, it is important to note that this interaction between carbaryl and predator-induced stress is known only to occur in gray treefrogs. Further research will be necessary to determine whether the phenomenon occurs in other amphibian species.
The mechanism underlying the pesticide–predator interaction is currently unknown, but there are several possibilities. Predators produce chemical cues that induce prey fear (28, 29) and this fear may simply be an additional stressor on the amphibian's physiology that, when combined with the stress of the pesticide, causes a high rate of mortality. The predator-induced reduction of activity and growth provides evidence that predators do indeed pose a stressful environment to tadpoles. Alternatively, predators may alter the abiotic conditions, including the production of nitrogenous wastes that can be toxic to fish and amphibians (33, 34). Our monitoring of the abiotic conditions in experiments 2 and 3 demonstrated that while predator cues reduced survival at low carbaryl concentrations, predators did not affect the temperature, pH, dissolved oxygen, or ammonia concentrations in the water.
A potential concern in this study is the realistic nature of the concentrations that were used in our experiments and the rate of carbaryl breakdown. There is little information available on typical carbaryl concentrations in natural ponds, but carbaryl can be as high as 4.8 mg/liter (35, 36). The low concentrations used in experiments 1 and 3 were only 1–11% of the highest concentrations detected in ponds under field conditions. Carbaryl also breaks down in natural ponds and is typically considered to have a short lifetime (24). The rate of hydrolytic breakdown depends on pH, which typically varies from 5 to 8 in natural ponds (37). When pH ≥ 7, carbaryl is relatively short-lived [at pH = 9, half-life = 0.1 d; at pH = 8, half-life = 1 d; at pH = 7, half-life = 10 d (37, 38)]. Thus, in our experiment, the mean concentrations of carbaryl between water changes were probably substantially lower than the initial concentrations added (approximately one-third as concentrated). In more acidic ponds (pH = 5–6.5), breakdown is negligible and the half-life of carbaryl is nearly 4 years (37, 39,40), providing plenty of time to cause amphibian mortality, even at low concentrations. Photolytic breakdown can also be relatively rapid. In full sunlight, photolysis can cause a 4- to 7-d half-life. Under natural conditions (e.g., shaded ponds and cloudy water) and in different seasons (e.g., lower temperature and light intensities), this half-life is expected to vary (22, 41). In either case, the half-life is sufficiently long to maintain carbaryl concentrations that can cause severe tadpole mortality. Finally, whereas some pesticides can be biologically decomposed, biological breakdown of carbaryl is negligible (41).
The results of this study illustrate the dangers of extrapolating short-term toxicity results to long-term population effects on nontarget organisms. Such extrapolations are conducted because the U.S. Environmental Protection Agency has received the daunting mandate, from the Toxic Substances Control Act, to determine the effects of >50,000 chemicals on our natural flora and fauna. While the importance of long-term toxicity tests has been appreciated for some time, the only practical approach to meet this mandate has been to conduct acute (1- to 4-d) toxicity tests on a subset of chemicals and taxa and extrapolate safe chemical levels from these limited test results (42). Using extrapolations has been viewed as a necessary compromise, but the results of our study underscore the fact that such extrapolations may be without a strong foundation. For amphibian populations, low concentrations of carbaryl (<3% of LC50 levels) can kill up to 97% of treefrog tadpoles within a week. As an acetylcholinesterase inhibitor, carbaryl shares its mode of action with many other insecticides. Thus, the impacts of longer exposure time and predator-induced stress may be relevant to a wide variety of insecticides and numerous organisms. At the very least, our data call for extending these tests by only a few days and conducting these tests under more realistic ecological conditions.
The dramatic changes in amphibian populations observed throughout the world likely have multiple causes, and all hypothesized mechanisms deserve our attention. We have information on the concentrations of commonly used pesticides in the environment, but we have little appreciation of the impact that this contamination could be having on amphibians. We have shown that very low concentrations of just one pesticide can cause high rates of mortality in gray treefrogs. However, there are far too few studies to assess the potential that such contamination could be having on amphibians. It is imperative that investigators continue to incorporate more natural experimental conditions to understand the full impact that pesticides may be having on our amphibian fauna.
Acknowledgments
We thank C. Glaude, J. Hoverman, M. Seaborn, and L. Winter for assistance in conducting the experiments, R. D. Semlitsch for the use of laboratory facilities, and M. D. Boone, C. M. Bridges, W. Carson, R. D. Semlitsch, and E. E. Werner for their reviews of this manuscript.
Abbreviations
LC50
the concentration of a pesticide that is predicted to kill 50% of a test population within a given amount of time under given exposure conditions
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Blaustein A R, Wake D B. Trends Ecol Evol. 1990;5:203–204. [Google Scholar]
- 2.Wake D B. Science. 1991;253:860. doi: 10.1126/science.253.5022.860. (lett.). [DOI] [PubMed] [Google Scholar]
- 3.Pechmann J H K, Scott D E, Semlitsch R D, Caldwell J P, Vitt L J, Gibbons J W. Science. 1991;253:892–895. doi: 10.1126/science.253.5022.892. [DOI] [PubMed] [Google Scholar]
- 4.Blaustein A R. Herpetologica. 1994;50:85–97. [Google Scholar]
- 5.Blaustein A R, Wake D B, Sousa W P. Cons Biol. 1994;8:60–71. [Google Scholar]
- 6.Pechmann J H K, Wilbur H M. Herpetologica. 1994;50:65–84. [Google Scholar]
- 7.Wake D B. Trends Ecol Evol. 1998;13:379–380. doi: 10.1016/s0169-5347(98)01428-1. [DOI] [PubMed] [Google Scholar]
- 8.Aspelin A L. Pesticides Industry Sales and Usage. Washington, DC: Environ. Protection Agency; 1997. , U.S. EPA Report No. 733-R-97-002. [Google Scholar]
- 9.Anonymous. Methods for Acute Toxicity Tests with Fish, Macroinvertebrates, and Amphibians. Washington, DC: Environ. Protection Agency; 1975. , U.S. EPA Report No. EPA-660/3-75-009. [Google Scholar]
- 10.Rand G M, Wells P G, McCarty L S. In: Fundamentals of Aquatic Toxicology. Rand G M, editor. London: Taylor and Francis; 1995. pp. 3–67. [Google Scholar]
- 11.(1996) Annual Book of ASTM Standards (American Society for Testing and Materials, Philadelphia, PA), p. E729–E796.
- 12.Marian M P, Arul V, Pandian T J. Arch Environ Contam Toxicol. 1983;12:271–275. doi: 10.1007/BF01059402. [DOI] [PubMed] [Google Scholar]
- 13.Bridges C M. Environ Toxicol Chem. 1997;16:1935–1939. [Google Scholar]
- 14.Lohner T W, Fisher S W. Aquatic Toxicol. 1990;16:335–354. [Google Scholar]
- 15.Zaga A, Little E E, Raben C F, Ellersieck M R. Environ Toxicol Chem. 1998;17:2543–2553. [Google Scholar]
- 16.Boone M D, Bridges C M. Environ Toxicol Chem. 1999;18:1482–1484. [Google Scholar]
- 17.Lima S L, Dill L M. Can J Zool. 1990;68:619–640. [Google Scholar]
- 18.Skelly D K. Ecology. 1992;73:704–708. [Google Scholar]
- 19.Kats L B, Dill L M. Ecoscience. 1998;5:361–394. [Google Scholar]
- 20.Relyea R A, Werner E E. Ecology. 1999;80:2117–2124. [Google Scholar]
- 21.Relyea, R. A. (2001) Ecology82, in press.
- 22.Bridges C M. J Herpetol. 1999;33:303–306. [Google Scholar]
- 23.Marchal-Segault D. Bull Ecol. 1976;7:411–416. [Google Scholar]
- 24.(1989) Insecticide Background Statement (U.S. Dept. of Agriculture, Beltsville, MD) U.S. Dept. of Agriculture Handbook No. 685.
- 25.(1992) Pesticide Industry Sales and Usage: 1990 and 1991 Market Estimates (Environ. Protection Agency, Washington, DC), U.S. EPA Report, Pesticides and Toxic Substances.
- 26.Edwards C A. Environmental Pollution by Pesticides. New York: Plenum; 1973. [Google Scholar]
- 27.Currier W W, MacCollom G B, Baumann G L. J Econ Entomol. 1982;75:1062–1068. doi: 10.1093/jee/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 28.Bridges C M. Arch Environ Contam Toxicol. 2000;39:91–96. doi: 10.1007/s002440010084. [DOI] [PubMed] [Google Scholar]
- 29.Petranka J W, Kats L B, Sih A. Anim Behav. 1987;35:420–425. [Google Scholar]
- 30.Kats L B, Petranka J W, Sih A. Ecology. 1988;69:1865–1870. [Google Scholar]
- 31.McCollum S A, Leimberger J D. Oecologia. 1997;109:615–621. doi: 10.1007/s004420050124. [DOI] [PubMed] [Google Scholar]
- 32.Altmann J. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 33.Rubin A J, Elmaraghy G A. Water Res. 1977;14:927–935. [Google Scholar]
- 34.Hecnar S J. Environ Toxicol Chem. 1995;14:2131–2137. [Google Scholar]
- 35.Norris L A, Lorz H W, Gregory S Z. General Technical Report PNW-149. Portland, OR: U.S. Dept. of Agriculture Forest Service; 1983. [Google Scholar]
- 36.Peterson H G, Boutin C, Martin P A, Freemark K E, Ruecker N J, Moody M J. Aquatic Toxicol. 1994;28:275–292. [Google Scholar]
- 37.Aly O M, El-Dib M A. Water Res. 1971;5:1191–1205. [Google Scholar]
- 38.Sharom M S, Miles J R W, Harris C R, McEwen F L. Water Res. 1980;14:1089–1093. [Google Scholar]
- 39.Wauchope R D, Haque R. Bull Environ Contam Toxicol. 1973;9:257–260. doi: 10.1007/BF01684779. [DOI] [PubMed] [Google Scholar]
- 40.Sikka H C, Miyazaki S, Lynch R S. Bull Environ Contam Toxicol. 1975;13:666–672. doi: 10.1007/BF01721933. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe N L, Zepp R G, Paris D F. Water Res. 1978;12:565–571. [Google Scholar]
- 42.Cooney J D. In: Fundamentals of Aquatic Toxicology. Rand G M, editor. London: Taylor and Francis; 1995. pp. 71–102. [Google Scholar]