Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression (original) (raw)
Abstract
Complexes of D-type cyclins and cdk4 or 6 are thought to govern progression through the G1 phase of the cell cycle. In Drosophila, single genes for Cyclin D and Cdk4 have been identified, simplifying genetic analysis. Here, we show that Drosophila Cdk4 interacts with Cyclin D and the Rb homolog RBF as expected, but is not absolutely essential. Flies homozygous for null mutations develop to the adult stage and are fertile, although only to a very limited degree. Overexpression of inactive mutant Cdk4, which is able to bind Cyclin D, does not enhance the Cdk4 mutant phenotype, confirming the absence of additional Cyclin D-dependent cdks. Our results indicate, therefore, that progression into and through the cell cycle can occur in the absence of Cdk4. However, the growth of cells and of the organism is reduced in Cdk4 mutants, indicating a role of D-type cyclin-dependent protein kinases in the modulation of growth rates.
Keywords: Cdk4/cell growth/cell proliferation/cyclin D/Drosophila
Introduction
D-type cyclins, their kinase partners cdk4 and 6, the INK inhibitors and the kinase substrate retinoblastoma protein (Rb) are all known for their crucial importance in human tumorigenesis (Weinberg, 1995; Sherr, 1996; Sherr and Roberts, 1999). At the cellular level, Rb has been shown to regulate progression through the G1 phase of the mammalian cell cycle, predominantly by binding to E2F transcription factors, which control a large number of genes involved in cell proliferation and DNA replication (Dyson, 1998). Rb represses expression of E2F target genes by recruiting histone deacetylase activity and by inhibiting the E2F transcriptional activation domain (Harbour et al., 1999). The ability of Rb to block progression through G1 is abolished by Rb hyperphosphorylation, which is initiated by D-type cyclin-dependent kinases during G1 (Sherr, 1994; Sherr and Roberts, 1999). In mammalian cells, the synthesis of D-type cyclins is controlled by extracellular signals. Mitogens induce a rapid accumulation of D-type cyclins. Conversely, antimitogens or withdrawal of mitogens result in a rapid decline. D-type cyclins are therefore thought to function as a functional link between extracellular signals and the cell cycle machinery. Accordingly, D-type cyclin–cdk complexes might be predicted to play an important role in the regulation of cell proliferation during development.
In mice, a number of genes of the INK (Serrano et al., 1996; Franklin et al., 1998), cyclin D (Fantl et al., 1995; Sicinski et al., 1995, 1996), cdk4 (Rane et al., 1999; Tsutsui et al., 1999), Rb (Clarke et al., 1992; Jacks et al., 1992; Lee et al., 1992, 1996; Cobrinik et al., 1996), E2F (Field et al., 1996; Yamasaki et al., 1996) pathway have been knocked out. In general, cell proliferation during early embryonic development is normal in the absence of these genes. In addition, mouse embryo fibroblasts derived from homozygous mutants proliferate in cell culture. It is often not clear to what extent functional redundancies explain the absence of severe cell proliferation defects in these mutants, because multiple related pathway components encoded by small gene families are present in mammals. The three mammalian D-type cyclins, for instance, can bind to either cdk4 or cdk6 and the different complexes might have largely overlapping functions (Sherr, 1994). While genetic inactivation of either cyclin D1, D2 or cdk4 does not block cell cycle progression, overexpression of INK inhibitors (Guan et al., 1994; Koh et al., 1995; Lukas et al., 1995b; Medema et al., 1995) and microinjection of antibodies against D-type cyclins (Baldin et al., 1993; Quelle et al., 1993; Lukas et al., 1994, 1995a) have indicated that Rb+ cells fail to progress into S-phase when D-type cyclin–cdk complexes are inhibited.
In Drosophila, single genes for Cyclin D and its kinase partner Cdk4 (previously designated Cdk4/6) have been described (Finley et al., 1996; Sauer et al., 1996), as well as in the nematode Caenorhabditis elegans (Park and Krause, 1999). Extensive screening of genomic libraries at low stringency has not revealed additional cdk4-like genes in Drosophila (Sauer et al., 1996). Additional cdk4 homologs also cannot be identified in the large set of Drosophila EST sequences and in the published genome sequence (Adams et al., 2000). Therefore, the analysis of the Cdk4 mutant phenotype, which we describe here, can be expected to provide a definitive answer to the question of whether cdk4 activity represents an obligatory requirement for progression through the G1 phase. In addition, the cell proliferation program of wild-type Drosophila development is very well known and the effects of mutations can be studied at the cellular level within the organism.
Our results demonstrate that Cdk4 interacts genetically with the Drosophila Rb family member RBF, as expected from the analyses in mammals. However, Cdk4 is not essential for progression through the cell cycle or for development to the adult stage. Nevertheless, Cdk4 is clearly required for normal fertility and growth of cells and organism.
Results
Drosophila Cdk4 binds to Cyclin D in vivo
Analysis of full-length cDNAs indicated that the characteristic pRb-binding motif LXCXL, which is not encoded by the sequence described previously (Finley et al., 1996), is also present in Drosophila Cyclin D. Co-immunoprecipitation experiments confirmed that Drosophila Cyclin D associates with Cdk4 in vivo (Figure 1). Immunoblotting with a monoclonal antibody revealed the presence of Cyclin D in anti-myc immunoprecipitates isolated from extracts of Cdk4-myc-expressing embryos. In contrast, Cyclin D was not detected in immunoprecipitates from Cdk1-myc or Cdk2-myc extracts. Moreover, while Cyclins A, B, B3 and E were clearly present in either of these two latter immunoprecipitates, they were not detected in Cdk4-myc immunoprecipitates. Our results indicate therefore that Cyclin D binds specifically to Cdk4.
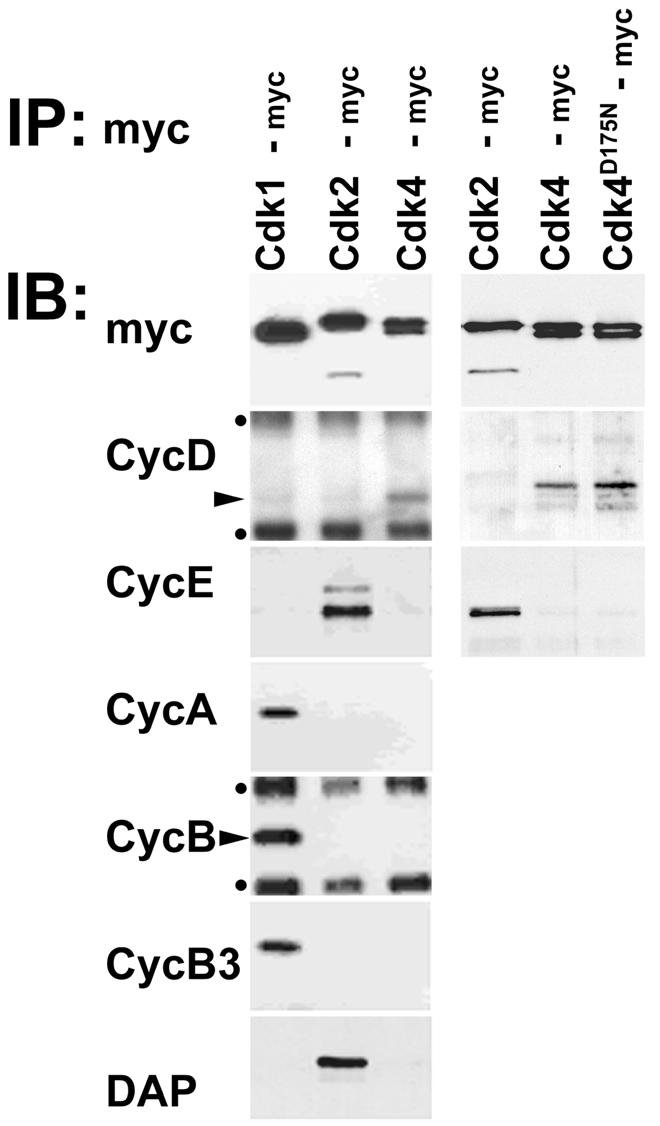
Fig. 1. Cyclin D but not Dacapo is bound to Drosophila Cdk4 in vivo. Immunoprecipitates (IP) were isolated with anti-myc antibodies from extracts of embryos expressing either myc-tagged Cdk1 (Cdk1-myc), Cdk2 (Cdk2-myc), Cdk4 (Cdk4-myc) or mutant Cdk4 (Cdk4D175N-myc) and analyzed in immunoblot experiments (IB) with antibodies against the myc epitope (myc), Cyclin D (CycD), Cyclin E (CycE), Cyclin A (CycA), Cyclin B (CycB), Cyclin B3 (CycB3) or Dacapo (DAP). Immunoblot signals resulting from the binding of secondary antibodies to mouse immunoglobulin used for immunoprecipitation are indicated by dots, while arrowheads mark signals reflecting the presence of Cyclin D and Cyclin B. Two independent experiments are shown in the panels on the right and left sides.
Mammalian cdk4 and cdk6 bind to INK- and CIP/KIP-type cdk inhibitors. While INK inhibitors have not been identified in Drosophila, the dacapo gene has been shown to encode a CIP/KIP-type inhibitor, which binds to Cyclin E–Cdk2 complexes (De Nooij et al., 1996; Lane et al., 1996). While Dacapo was readily observed in Cdk2-myc immunoprecipitates as expected, it could not be detected in Cdk4-myc immunoprecipitates (Figure 1).
Cdk4 is required for normal growth and fertility but is not essential for cell cycle progression
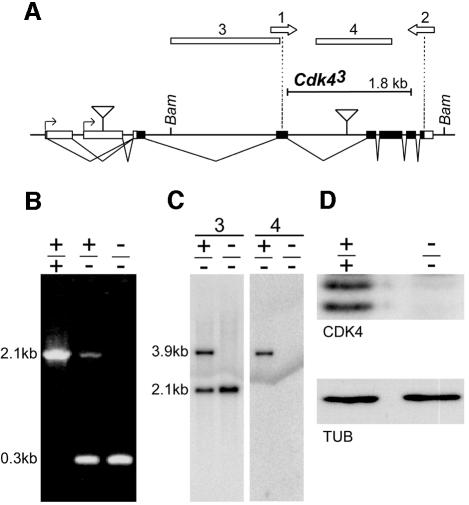
By mobilizing a P element [_l(2)s4639_] we isolated an intragenic deletion _Cdk4_3 eliminating essential kinase domains (Figure 2). Surprisingly, homozygous _Cdk4_3 progeny from heterozygous parents developed into adult flies which eclosed without a developmental delay. While the fertility of homozygous females was severely reduced (see Supplementary material available at The EMBO Journal Online for a more detailed description of the fertility defects), occasional progeny could be obtained even from homozygous parents demonstrating that Cdk4 is not absolutely essential for cell proliferation or development to the adult stage.
Fig. 2. Molecular characterization of wild-type and mutant Cdk4 alleles. (A) Cdk4 exon sequences are indicated by boxes. Filled boxes indicate translated regions. Putative transcriptional start sites are indicated by arrows and the structure of alternative transcripts is illustrated. Triangles indicate insertion sites of P elements. While the line EP(2)0844 carries a P element in the 5′ region, the following lines have insertions clustered around the position indicated by the second triangle: l(2)05428, l(2)s4639, l(2)k06503, EP(2)2192, EP(2)2358. The size and position of the intragenic deletion in _Cdk4_3 resulting from an imprecise excision of l(2)s4639 is indicated by the black bar. The positions of the primers 1 and 2 used for the PCR experiment (B) are indicated by the open arrows. The regions used as probes 3 and 4 for the Southern blot (C) are indicated by boxes and relevant _Bam_HI restriction sites are indicated. (B) Genomic DNA from wild-type (+/+), _Cdk4_3/CyO (+/–) and _Cdk4_3 (–/–) flies was analyzed by PCR for the presence of Cdk4 sequences. The primers 1 and 2 (A) result in the amplification of a 2.1 kb fragment from the wild-type allele and a 0.3 kb fragment from the _Cdk4_3 allele. (C) Genomic DNA from _Cdk4_3/CyO and _Cdk4_3 flies was digested with _Bam_HI and analyzed on Southern blots using Cdk4 fragments 3 and 4 (A) as probes. (D) 0–2 h embryo extracts from either wild type (+/+) or _Cdk4_3 (–/–) were analyzed for the presence of Cdk4 by immunoblotting with anti-Cdk4 antisera (CDK4). Immunoblotting with anti-tubulin (TUB) was used as a loading control.
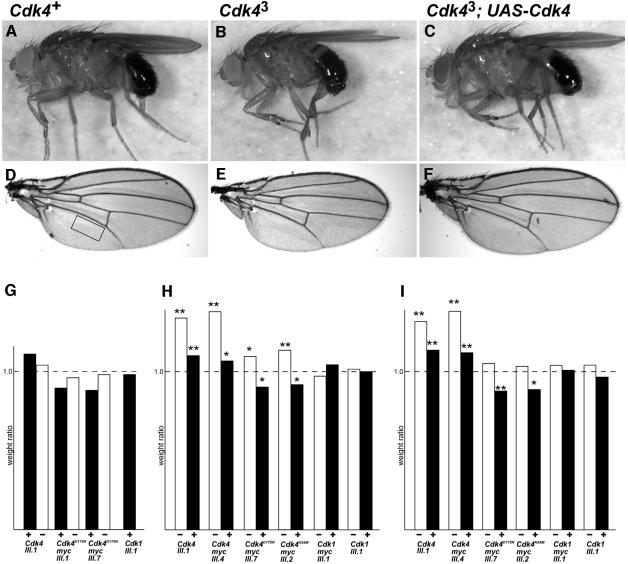
Interestingly, homozygous _Cdk4_3 flies were found to be significantly smaller than wild-type flies (Table I; Figure 3). Size reduction affected all aspects of the flies proportionally. The weight of _Cdk4_3 homozygotes was found to be ∼20% lower than that of heterozygotes (Table I). The wing area of _Cdk4_3 homozygotes was found to be ∼10% lower than that of heterozygotes. As each cell is known to secrete one hair during wing development, we determined the hair density in a defined region of the adult wings as a measure of cell size. The results indicated that the cells in wings of _Cdk4_3 homozygotes are slightly larger compared with heterozygotes (Table I). The values determined for the total wing size and for the cell size were used to extrapolate the total cell number present in the wing. These estimations indicated that the wings of _Cdk4_3 homozygotes contained fewer cells than those of heterozygotes (Table I).
Table I. The effect of Cdk4 on the size of cells, organs and organism.
| Crossa | Genotype | Weightb (mg) | Wing areac (mm2) | Cell sized (µm2) | Cell numbere |
|---|---|---|---|---|---|
| 1 | _Cdk4_3/_Cdk4_3 | 0.69 | 1.19 | 137.9 | 8573 |
| 2 | _Cdk4_3/+ | 0.84 | 1.27 | 130.3 | 9687 |
| 3 | _Cdk4_3/_Cdk4_3; da-GAL4/+ | 0.68 | 1.14 | 146.5 | 7729 |
| _Cdk4_3/_Cdk4_3; da-GAL4/UAS-Cdk4 III.1 | 0.94* | 1.33** | 137.9*** | 9617 | |
| 4 | _Cdk4_3/_Cdk4_3; da-GAL4/+ | 0.73 | 1.16 | 147.5 | 7811 |
| _Cdk4_3/_Cdk4_3; da-GAL4/_UAS-Cdk4_D175N-mycIII.7 | 0.77 | 1.17 | 143.2 | 8114 | |
| 5 | _Cdk4_3/_Cdk4_3; da-GAL4/+ | 0.73 | 1.15 | 149.5 | 7641 |
| _Cdk4_3/_Cdk4_3; da-GAL4/_UAS-Cdk4_D175N-mycIII.4 | 0.77 | 1.22 | 145.6 | 8328 |
Fig. 3. Cdk4 is required for normal growth of organism and organs. (A–F) Adult male flies (A–C) and wings (D–F) are shown. The region boxed in (D) was used to determine cell densities (see Table I). Genotypes were +/_Cdk4_3 (Cdk4+), _Cdk4_3/_Cdk4_3 (_Cdk4_3) and _Cdk4_3/_Cdk4_3; da-GAL4, _UAS-Cdk4 III.1 (Cdk4_3; UAS-Cdk4). (G) Males heterozygous for a UAS transgene (UAS-Cdk4 III.1, _UAS-Cdk4_D175N-myc III.1, UAS-Cdk4_D175N-myc III.7 or UAS-Cdk1 III.1) were crossed with females homozygous for da-GAL4 (black bars) or with control females (white bars). The UAS transgene therefore was only present in 50% of the progeny from these crosses. The weight of progeny with or without UAS transgene was determined and the weight ratio (+UAS transgene/–_UAS transgene) was calculated. Black bars represent the weight ratio in flies carrying da-GAL4 (+); white bars indicate the weight ratio in flies lacking da-GAL4 (–). (H and I) _Cdk4_3/CyO; UAS transgene/+ males were crossed with _Cdk4_3/CyO; da-GAL4/da-GAL4 females. Thus, only 50% of the progeny expressed the UAS transgene (UAS-Cdk4 III.1, UAS-Cdk4-myc III.4, _UAS-Cdk4_D175N-myc III.7, UAS-Cdk4_K55M-myc III.2, UAS-Cdk1-myc III.1 or UAS-Cdk1 III.1). The weight of progeny that either expressed the UAS transgene or not was determined and the weight ratio (+UAS transgene/–_UAS transgene) was calculated. Weight ratios were determined for male (H) and female (I) progeny. White bars indicate the weight ratio in homozygous _Cdk4_3/_Cdk4_3 progeny (–); black bars in heterozygous _Cdk4_3/CyO siblings (+). The significant P values as determined by a _t_-test are indicated by asterisks (*P <0.05; **P <0.01).
To demonstrate that the decrease in weight, wing size and cell numbers observed in _Cdk4_3 homozygotes is caused by loss of Cdk4 function and not by potential second site mutations on the chromosome, we expressed UAS-Cdk4 ubiquitously in _Cdk4_3 homozygotes with the help of da-GAL4. This prevented the reduction in weight, wing size and cell numbers (Table I; Figure 3).
Analogous ubiquitous expression of mutant _Cdk4_D175N- myc had essentially no effect in _Cdk4_3 homozygotes (Table I). The D175N mutation affects an aspartate residue which is conserved in all protein kinases and known to be required for the phosphotransfer reaction (van den Heuvel and Harlow, 1993). Overexpression of cdk1 and cdk2 with analogous mutations results in dominant-negative inhibition of the corresponding endogenous cdks in mammalian cells. Mutant Cdk4D175N-myc protein binds to Cyclin D with the same efficiency as Cdk4-myc (Figure 1) and thus is expected to act in a dominant-negative manner. While _Cdk4_D175N-myc expression did not reduce the weight of _Cdk4_3 mutants, it resulted in a slight but significant weight decrease in Cdk4+ flies (Figure 3G). In additional experiments, we compared directly the effects of _Cdk4_D175N-myc expression on the weight of _Cdk4_3 homo- and heterozygotes that had developed in the same bottle (Figure 3H and I). These experiments clearly confirmed that _UAS-Cdk4_D175N-myc reduces fly weight when expressed in _Cdk4_3 heterozygotes, while it had at most the opposite effect in _Cdk4_3 homozygotes (Figure 3H and I). Moreover, this same differential effect on _Cdk4_3 homo- and heterozygotes was also observed with a _UAS-Cdk4_K55M-myc transgene (Figure 3H and I), which carried another mutation known to abolish kinase activity but not D-type cyclin binding, when introduced into mammalian cdk4 (Kato and Sherr, 1993). The finding that both _Cdk4_D175N-myc and _Cdk4_K55M-myc expression mimicked the _Cdk4_3 phenotype when expressed in Cdk4+ flies suggests that they act in a dominant-negative manner. The fact that they have no effect in _Cdk4_3 flies argues strongly that Cdk4 is the only Cyclin D-dependent cdk in Drosophila. We emphasize that this conclusion is complicated but not invalidated by the possibility that Cdk4 might not only act as a protein kinase but also by titrating putative INK inhibitors as described for mammalian cdk4/6. We point out that the Drosophila genome sequence (Adams et al., 2000) has not revealed INK orthologs.
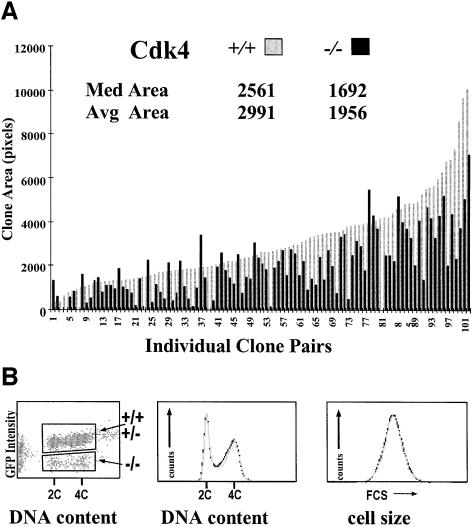
To analyze the effects of loss of Cdk4 function on cell growth and cell cycle progression, we compared the behavior of homozygous _Cdk4_3 and their wild-type sister clones induced by mitotic recombination (Figure 4). Sixty-seven hours after clone induction, the area covered by _Cdk4_3 clones in imaginal wing discs was found to be only about two-thirds of the area covered by sister clones (Figure 4A). Analysis by flow cytometry revealed a significant difference neither in cell size nor in the cell cycle profile (Figure 4B). In addition, we did not observe pyknotic nuclei in _Cdk4_3 mutant clones, suggesting that cell death did not play a significant role in the reduced growth of _Cdk4_3 mutant clones. These data indicate that _Cdk4_3 cells grow slowly and that their cell cycle is lengthened by a proportionate increase in the G1, S and G2 phases.
Fig. 4. Cdk4 is required for normal growth of imaginal disc cells. (A) Twin spots of homozygous _Cdk4_3 clones and homozygous Cdk4+ clones were induced in a _Cdk4_3/Cdk4+ background by mitotic recombination at 48 h AED. Wing imaginal discs were fixed at 115 h AED and the size of _Cdk4_3 (black) and Cdk4+ clones (gray) was determined. The sizes measured for clone pairs are indicated by bars ordered according to the size of the Cdk4+/Cdk4+ clones. Values for median and average clone area are indicated. (B) _Cdk4_3 clones lacking UAS-GFP were induced by mitotic recombination at 48 h AED. Wing imaginal discs were dissected and dissociated for FACS analysis at 96 h AED. Green fluorescent protein (GFP) fluorescence intensity and DNA content were analyzed as well as forward scatter (FCS) as a measure of cell size. Cells were gated as indicated on the left panel and gray traces reflect _Cdk4_3 mutant cells (GFP-negative) and black traces cells with either one or two Cdk4+ copies (GFP-positive) in the panels illustrating essentially indistinguishable cell cycle profiles and cell size distribution.
Cdk4 interacts with RBF and Cyclin E–Cdk2
In addition to D-type cyclin–cdk complexes, cyclin E–cdk2 has also been implicated in the regulation of cell cycle progression through the G1 phase. The presence of Cyclin E–Cdk2 might explain the relatively mild phenotype observed in flies lacking Cdk4 function. To evaluate this notion, we studied the effects of heterozygosity for Cyclin E and Cdk2 mutations in Cdk4 mutants. While heterozygosity for mutations in Cyclin E is readily tolerated in _Cdk4_3 heterozygotes, it resulted in complete lethality in _Cdk4_3 homozygotes. Similarly, heterozygosity for mutations in Cdk2 resulted in an almost complete lethality in _Cdk4_3 homozygotes, while it has no effect in _Cdk4_3 heterozygotes. In contrast, mutations in Cyclin A, Cyclin B, Cyclin B3 and Cdk1 had no effect on the survival of _Cdk4_3 homozygotes. These observations demonstrate that Cdk4 mutants are particularly sensitive to reduction in Cyclin E–Cdk2 levels.
Vertebrate D-type cyclin–cdk complexes inhibit Rb function. The defects observed in Cdk4 mutants, therefore, might reflect increased Rb function. To evaluate this notion, we studied the effects of heterozygosity for mutations in the gene encoding the Drosophila Rb family member (RBF) on the fertility and size of Cdk4 mutant females. For these experiments we used two putative null alleles, _RBF_11 and _RBF_14. We developed a PCR assay to monitor the presence of the _RBF_11 allele (Figure 5A). Cdk4 mutant females heterozygous for _RBF_11 (_RBF_11/+; _Cdk4_3/_Cdk4_3) were found to be significantly more fertile than sibling females without _RBF_11 (+/+; _Cdk4_3/_Cdk4_3) (Figure 5, compare B with C). In the experiment with _RBF_14, we were unable to monitor the presence of this mutation. Based on our crossing scheme, however, one half of the Cdk4 mutant females was expected to be heterozygous for _RBF_14 (_RBF_14/+; _Cdk4_3/_Cdk4_3), while the other half was expected to have two functional RBF gene copies (+/+; _Cdk4_3/_Cdk4_3). As in the _RBF_11 experiment, we also observed an increased fertility in ∼50% of the Cdk4 mutant females in the _RBF_14 experiment (Figure 5D). These 50% females with increased fertility, therefore, presumably correspond to the _RBF_14 heterozygotes. We conclude that the fertility of Cdk4 mutant females is increased by a reduction in the copy number of functional RBF genes from two to one. Similarly, heterozygosity for mutations in RBF was found to increase the weight of Cdk4 mutant females (data not shown). The antagonistic activities of Cdk4 and RBF could also be demonstrated in experiments involving overexpression using the UAS-GAL4 system (Datar et al., 2000).
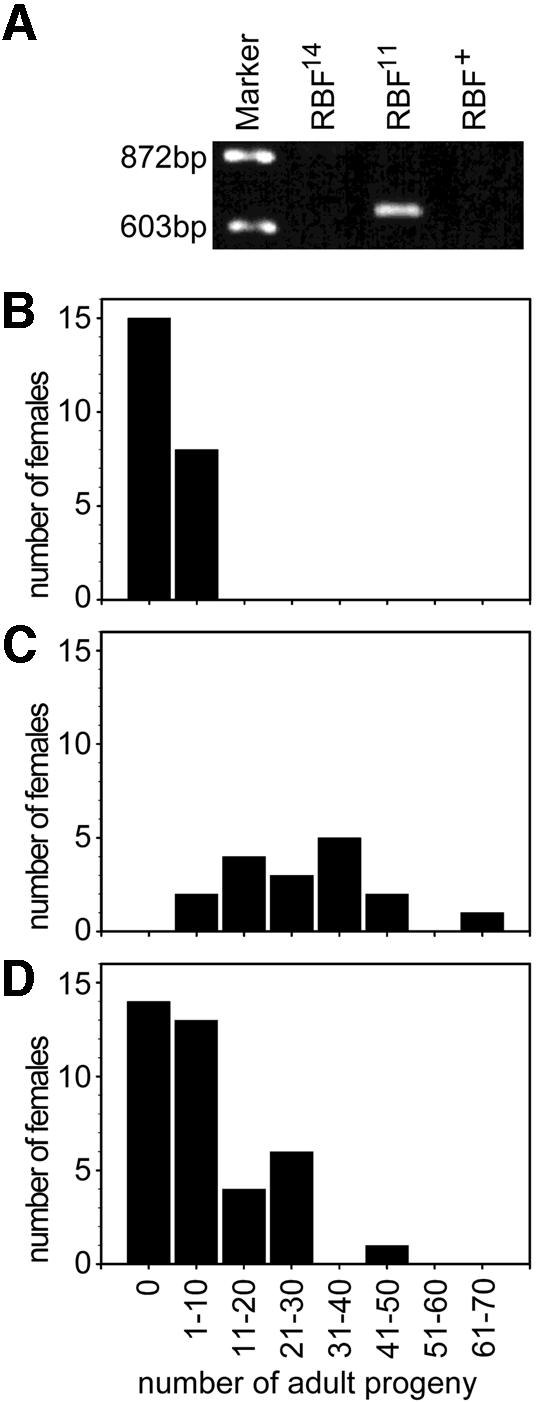
Fig. 5. Reduction of RBF gene dose suppresses fertility defects of mutant Cdk4 females. (A) The presence of the _RBF_11 allele was monitored by a PCR assay. A 650 bp fragment is amplified exclusively when _RBF_11 is present. (B and C) Sibling _Cdk4_3 mutant females, which were either +/+ (B) or _RBF_11/+ (C), were crossed individually to wild-type males and the number of progeny developing to the adult stage was determined. (D) Sibling _Cdk4_3 mutant females, which were either +/+ or _RBF_14/+, were crossed individually to wild-type males and the number of progeny developing to the adult stage was determined. The presence of _RBF_14 could not be monitored. However, ∼50% of the females were found to have significantly higher numbers of adult progeny than _Cdk4_3 mutant females and are therefore presumably _RBF_14/+.
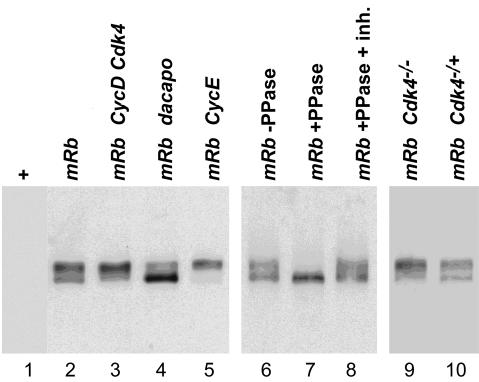
D-type cyclin complexes are thought to be the major Rb kinase in mammalian cells (Geng et al., 1999). Therefore it was of interest to compare Rb kinase activity in wild-type and Cdk4 mutant embryos. Unfortunately, our antibodies failed to precipitate Rb kinase activity even from wild-type embryo extracts. Moreover, in contrast to vertebrate Rb, Drosophila RBF does not change its apparent electrophoretic mobility upon phosphorylation (Du et al., 1996). As also observed with mammalian Rb (Khandjian and Tremblay, 1992), we were unable to focus Drosophila RBF on two-dimensional gels. Thus, using da-GAL4 we expressed mouse Rb in Drosophila embryos from a UAS transgene (UAS-mRb). Immunoblotting with an antibody against Rb clearly revealed two forms with different electrophoretic mobility (Figure 6A, lane 2). Phosphatase treatment converted the lower into the higher mobility form (Figure 6, lane 7). Conversely, co-expression of UAS-Cdk4 and UAS-Cyclin D resulted in a relative increase in the lower mobility form (Figure 6, lane 3). These observations suggested that Cyclin D–Cdk4 might in principle function as Rb kinase. However, we failed to detect a decrease in the abundance of the low mobility form in extracts from embryos lacking both maternal and zygotic Cdk4 function (Figure 6, lane 9). It appears therefore that kinases other than Cyclin D–Cdk4 can phosphorylate mRb. In fact, experiments involving expression of either UAS-Cyclin E or UAS-dacapo suggested that Cyclin E–Cdk2 is a major Rb kinase in Drosophila embryos. UAS-Cyclin E resulted in a strong enrichment of the lower mobility form (Figure 6, lane 5). In contrast, UAS-dacapo resulted in a severe reduction of the lower mobility form (Figure 6, lane 4).
Fig. 6. Rb kinase in Cdk4 mutants. Extracts from embryos with UAS-mRb (lane 1), da-GAL4 and UAS-mRb (lane 2), da-GAL4, UAS-mRb, UAS-Cyclin D and UAS-Cdk4 (lane 3), da-GAL4, UAS-mRb, UAS-dacapo (lane 4) and da-GAL4, UAS-mRb and UAS-Cyclin E (lane 5), were analyzed by immunoblotting with anti-Rb. Alternatively, mRb was immunoprecipitated from da-GAL4, UAS-mRb embryo extracts and incubated without phosphatase (lane 6) or with phosphatase either in the absence (lane 7) or presence (lane 8) of phosphatase inhibitors before immunoblotting with anti-Rb. To analyze the effect of Cdk4 on mRB phosphorylation, we analyzed extracts from embryos collected from a cross of _Cdk4_3; UAS-mRb females with either _Cdk4_3; da-GAL4 males (lane 9) or da-GAL4 males (lane 10) by immunoblotting with anti-Rb.
Discussion
D-type cyclin–cdk complexes are thought to control specifically progression through G1 in response to extracellular growth factors. Surprisingly, we find that Drosophila Cdk4, which encodes the kinase partner of Drosophila Cyclin D, is not an absolutely essential gene. Cdk4 mutant flies are smaller than normal and almost infertile. However, some progeny can develop into adults in the complete absence of both maternal and zygotic Cdk4 function. Moreover, we demonstrate that imaginal disc cells lacking Cdk4 function are characterized by a reduced cellular growth rate, an increased cell cycle duration with a proportional extension during G1, S and G2 and a normal cell size. Rather than promoting the progression specifically through G1 in response to cellular growth, therefore, D-type cyclin–cdk complexes appear primarily to stimulate cellular growth in Drosophila. This notion is further supported by our data from overexpression analyses (Datar et al., 2000). The discussion here is focused on Cdk4 loss-of-function mutations and their genetic interactions, while the growth-regulatory role of Cyclin D–Cdk4 is discussed in detail in Datar et al. (2000).
Progression through the cell division cycle in the absence of Cdk4
It is not yet clear to what extent the presence of the highly related cdk6 gene explains the fact that cdk4 is not required for development to the adult stage in mice (Rane et al., 1999; Tsutsui et al., 1999). In contrast, Drosophila does not appear to have a Cdk6 gene (Sauer et al., 1996; Adams et al., 2000). Moreover, our experiments involving overexpression of dominant-negative Cdk4 proteins (Cdk4D175N, Cdk4K55M) argue strongly against the idea that Cdk4 null mutants are rescued by functionally redundant D-type cyclin–cdk complexes. Overexpression of these mutant kinases in Cdk4+ flies mimics a Cdk4 loss-of-function phenotype, while it does not at all enhance the mutant phenotype in Cdk4 null mutants.
Just like Cdk4, Drosophila Cyclin D should not be an essential gene, if Cyclin D functions exclusively in a complex with Cdk4. Indeed, near-saturation mutagenesis for recessive lethal mutations uncovered by a deficiency removing Cyclin D has not led to the identification of Cyclin D alleles (B.A.Edgar and A.Katzen, personal communication), supporting the idea that cyclin D might not be essential. In contrast to complexes of Cdk1 and Cdk2, which are clearly encoded by essential genes and provide functions indispensable for progression through the cell cycle, Cyclin D–Cdk4 complexes, therefore, do not appear to be basic components of the cell cycle machinery.
Like Drosophila, C.elegans appears to have single genes for cyclin D and cdk4 (Park and Krause, 1999). C.elegans cdk4 has been demonstrated to be an essential gene. Mutant analysis and RNA interference experiments have indicated that cyclin D and cdk4 are required for cell cycle progression during the larval stages. Cell proliferation during these larval stages is known to be dependent on nutrition, and thus the cyclin D–cdk4 might be required to regulate the resulting growth. However, consistent with our findings in Drosophila, cyclin D and cdk4 do not appear to be necessary during embryonic proliferation in C.elegans. Thus these results also suggest that cell cycle progression is not necessarily dependent on the presence of D-type cyclin-dependent kinase activity.
Antagonistic activities of Cdk4 and RBF
Experiments in vertebrate cells have suggested that D-type cyclin–cdk activity is required for progression through G1 in Rb+ cells and is dispensable in Rb– cells (Lukas et al., 1994, 1995a,b). RBF are also expressed in Drosophila. In addition to the previously characterized RBF gene (Du et al., 1996; Du and Dyson, 1999), the genome sequence has uncovered a second family member. The genetic interactions described here indicate that Cyclin D–Cdk4 and RBF act antagonistically. We find that the fertility defect of Cdk4 mutant females is suppressed by a reduction in the dose of RBF+ copies. Additional evidence demonstrating this antagonistic relationship is described by Datar et al. (2000).
Hierarchy and functional specificity of Cdk4 and Cdk2
Drosophila Cdk4 is not essential even though RBF is known to be expressed and to inhibit cell proliferation (Du et al., 1996; Neufeld et al., 1998; Du and Dyson, 1999; Datar et al., 2000). RBF therefore might either not be sufficient for a complete inhibition of cell proliferation in Drosophila or it might be inactivated by a redundant pathway. In vertebrate cells, Rb is thought to be phosphorylated by both D-type–cdk complexes and cyclin E–cdk2. Recent evidence emphasized that these kinases act sequentially to inactivate separate functions of Rb. Initial phosphorylation by cyclin D-dependent kinase activity blocks the binding of histone deacetylase to Rb and disrupts Rb structure so that cyclin E-dependent kinase activity gains access to additional phosphorylation sites. The subsequent phosphorylation of these sites dissociates Rb from E2F (Harbour et al., 1999). Similarly, a ‘knock-in’ of the cyclin E gene into the cyclin D1 locus in mice results in a strong reduction of Rb hyperphosphorylation. Interestingly, this expression of cyclin E instead of cyclin D1 does not result in the characteristic cell proliferation defects associated with a cyclin D1 ‘knock-out’, even though Rb hyperphosphorylation is not restored (Geng et al., 1999). These findings suggest, therefore, that cyclin D1 complexes represent the major Rb kinase in the tissues analyzed and that they are primarily important for the induction of cyclin E expression.
Our results suggest that Cyclin D–Cdk4 and Cyclin E–Cdk2 might act as independent RBF kinases in Drosophila. Overexpression of either cyclin D–Cdk4 or Cyclin E resulted in an increase of hyperphosphorylated Rb. The specific Cyclin E–Cdk2 inhibitor Dacapo severely inhibited Rb hyperphosphorylation. Thus, Drosophila Cyclin E–Cdk2, which is known to phosphorylate RBF efficiently in vitro, might also phosphorylate Rb in Cdk4 mutants, thereby explaining why a decrease in Rb phosphorylation cannot be observed in Cdk4 mutants. For technical reasons, however, we have analyzed the phosphorylation of mouse Rb expressed in Drosophila embryos instead of investigating RBF phosphorylation directly. Our suggestion that Cyclin D–Cdk4 and Cyclin E–Cdk2 might act as functionally overlapping RBF kinases must therefore remain tentative, since mouse Rb might not faithfully report RBF kinase activity. Our finding that Cdk4 mutants are extremely sensitive to a reduction of the Cyclin E+ and Cdk2+ gene dose further stresses the intimate functional relationship between Cyclin D–Cdk4 and Cyclin E–Cdk2 but does not address their interaction hierarchy. Even though we favor the idea that Cyclin D–Cdk4 and Cyclin E–Cdk2 might act as overlapping RBF kinases in Drosophila, we emphasize that a number of phenotypic differences resulting from both loss- and gain-of-function mutations in the genes encoding the subunits of these different kinase complexes clearly demonstrate that they must also have distinct functions.
Cdk4 stimulates growth
Drosophila Cdk4 is clearly required for normal growth of cells and the organism. Size regulation at the level of cells, as well as at the level of organs and organism is an important but poorly understood process. A number of observations in yeast and mammalian cells have indicated that cell cycle progression is dependent on a critical cell size (for a recent review see Polymenis and Schmidt, 1999). Many cell types delay the G1–S transition (at a point called START in yeast and restriction point in mammalian cells) until a critical size has been reached. Slow growth due to nutrient or growth factor limitations therefore results primarily in an extension of the G1 phase. In budding yeast, the Cln cyclins, and in particular Cln3p, have been implicated in this coupling of cell growth and cell cycle progression. Cln3p is a very unstable protein which might only accumulate to an effective concentration that triggers progression through START when ribosome numbers have increased sufficiently (Polymenis and Schmidt, 1997). Experimental uncoupling of growth rates and the rates of Cln3p accumulation affects primarily cell division size without a comparable change of cell cycle duration. D-type cyclin–cdk complexes have been proposed to regulate progression through the restriction point in response to growth factors and mitogens in a comparable manner. However, proliferating Cdk4 mutant cells in wing imaginal discs are of wild-type size and progress more slowly through the cell cycle with a proportionate extension of G1, S and G2 phases. This phenotype is incompatible with an exclusive role of Cdk4 in the coupling of cell size and progression through the restriction point. Cdk4 appears to regulate, rather than simply sense growth. This notion is further supported by Datar et al. (2000).
The observation that cells are slightly larger in adult wings of Cdk4 mutants does not fit readily with the suggestion that Cdk4 promotes growth. We emphasize, however, that the effect on cell size was small and obvious only in differentiated adult cells and not in proliferating imaginal wing cells. Adult Cdk4 mutant wings contain fewer cells. The slight increase in adult wing size might reflect a partial compensation for decreased cell numbers during wing differentiation late in development.
More prominent than the slight cell size increase observed in wing cells is, however, the reduced size of Cdk4 mutant flies. A number of mutations have been shown to result in size reduction. The Drosophila myc homolog (Gallant et al., 1996; Johnston et al., 1999) and several genes of the insulin signaling pathway have been shown to be specifically required for growth to normal size (Leevers et al., 1996; Böhni et al., 1999; Huang et al., 1999; Montagne et al., 1999; Weinkove et al., 1999). Interestingly, while all these mutations result in developmental delays, we find that Cdk4 mutants eclose on time. The size reduction observed in Cdk4 mutants, however, is less pronounced than in most of these other mutants.
Reduced growth has also been reported to result from inactivation of mouse cdk4 function (Rane et al., 1999; Tsutsui et al., 1999). Intriguingly, the phenotypic similarities observed in cdk4 mutant mice and flies extend further. Mutant mice are also infertile particularly when female. In these females, infertility was shown to be caused by deficits in the hypothalamic pituitary axis rather than by developmental abnormalities in reproductive organs (Rane et al., 1999). In addition, cdk4 mutant mice developed diabetes within 2 months after birth due to a loss of the insulin-expressing β-islet cells (Rane et al., 1999). In Drosophila, we note a striking similarity of the phenotypic consequences of mutations in Cdk4 and in genes of the insulin signaling pathway. A future analysis of the relationship of Cdk4 and insulin signaling in Drosophila, therefore, might reveal a striking evolutionary conservation.
Apart from emphasizing functional homology, our analysis also reveals an apparent clear difference between the regulatory role of cyclin D–Cdk4 in Drosophila and in mammalian cells. In mammalian cells, D-type cyclin complexes do not only function catalytically as protein kinases. They also act stoichiometrically by titrating the cdk inhibitors p21CIP1 and p27KIP1. Thereby, they contribute to the activation of cyclin E–Cdk2 complexes. In contrast, we have been unable to detect the Drosophila cdk inhibitor Dacapo (the only detectable CIP/KIP family member in the known genome sequence) in Cyclin D–Cdk4 complexes. This apparent inability of Drosophila Cyclin D–Cdk4 to titrate Dacapo, might explain why, to some extent, mammalian D-type cyclin complexes behave in a functionally distinct manner.
In summary, our observations clearly implicate Cdk4 in the control of growth rates at the cellular and organismal level. Moreover, while our results confirm an involvement of Cdk4 in the regulation of cell proliferation, they also emphasize the importance of alternative pathways. In Drosophila, the developmental program of cell proliferation is surprisingly normal in the absence of Cdk4. The fact that the cyclin D–Cdk4 pathway is of paramount importance for tumorigenesis, therefore, should probably not lead to the view that it is the only pathway controlling entry into and exit from the cell cycle.
Materials and methods
Fly stocks
The P element lines l(2)s4639 and l(2)05428 (Spradling et al., 1995) with insertions in the chromosomal region 53C containing Cdk4 (Sauer et al., 1996) were characterized by PCR using a P element primer (5′-GCA GGTAGCACCTTATGTTATTTCATCATG-3′) in combination with _Cdk4_-specific primers (primer 1: 5′-GAGAACGGTGTGCCAATG-3′, primer 2: 5′-GCAAGATCTAGGGGTTCCTGCTGAAAG-3′). Imprecise excisions were generated from l(2)s4639 and intragenic deletions were identified by PCR. A PCR fragment spanning the intragenic deletion in _Cdk4_3 was cloned and sequenced. Breakpoints were found at positions corresponding to base pairs 325 and 855 of the Cdk4 cDNA (Sauer et al., 1996). This intragenic deletion also results in a frame shift. PCR with primers 1 and 2 were used to detect the _Cdk4_3 allele.
To monitor the presence of _RBF_11 in single _Cdk4_3 mutant females, we devised a PCR assay. _RBF_11 was isolated as an imprecise excision of a P element insertion within the 5′ untranslated region of RBF (Du and Dyson, 1999). A molecular analysis of _RBF_11 revealed the presence of residual P element sequences allowing an enzymatic amplification of a 650 bp fragment specifically from this allele with the help of the P element primer and a RBF primer (5′-CACATGCTATTCCAC CTCCTG-3′).
Lines with UAS transgenes (UAS-Cdk1-myc, UAS-Cdk2-myc, UAS-Cdk4-myc) allowing the expression of Cdk1, Cdk2 or Cdk4 as fusion proteins with an extension of six myc epitope copies at the C-terminus were produced with the help of pUAST constructs (Brand and Perrimon, 1993). In addition, we generated lines (_UAS-Cdk4_D175N-myc, _UAS-Cdk4_K55M-myc) allowing the expression of mutant Cdk4 proteins. A Sculptor in vitro mutagenesis kit (Amersham) was used to introduce the corresponding changes. We also generated UAS-Cdk4 lines allowing the expression of Cdk4 without a C-terminal extension.
For the production of lines with UAS-Cyclin D transgenes, we used a pUAST construct containing a fragment of the Drosophila Cyclin D cDNA clone DNB15. This and seven additional cDNA clones were isolated from an embryo cDNA library (Brown and Kafatos, 1988) using a probe generated from a partial cDNA clone (Sauer et al., 1996). The cDNA DNB15 was sequenced (DDBJ/EMBL/GenBank accession No. AF260583) and found to have stop codons upstream of the open reading frame, suggesting that it contained the complete coding sequence.
For expression of mouse Rb in Drosophila embryos, a fragment from pECE-ΔB/X-HA (Hamel et al., 1992) was inserted into a modified pUAST vector. Molecular details of the resulting UAS-mRb and the other transgenes described here are provided upon request. In comparison with _da-GAL4_-driven expression of UAS-RBF, which results in small flies with defects such as rough eyes, wing and tergite defects, analogous expression of UAS-mRb appeared to have little effect in Drosophila.
For a detailed description of the additional Drosophila strains used in this study see the Supplementary material available at The EMBO Journal Online.
Weight and size measurements
For the measurements presented in Table I, we analyzed progeny from the following crosses: w*; Cdk4_3/CyO males with w*; Cdk4_3/CyO females (cross 1); w males with _w*; Cdk4_3/CyO females (cross 2), _w*; Cdk4_3/CyO; UAS-Cdk4 III.1/+ males with w*; Cdk4_3/CyO; da-GAL4 females (cross 3), w*; Cdk4_3/CyO; _UAS-Cdk4_D175N-myc III.7/+ males with _w*; Cdk4_3/CyO; da-GAL4 females (cross 4), _w*; Cdk4_3/CyO; UAS-Cdk4_D175N-myc III.4/+ males with w*; Cdk4_3/CyO; da-GAL4 females (cross 5). Progeny were collected for 24 h, aged for 3 days after eclosion, sorted according to gender and genotype as indicated by visible markers (Cy, mini w+ dose), counted and stored at –70°C. Collections were repeated on four consecutive days. Subsequently, pools of known numbers of flies (in general ∼30) were weighed on a Mettler AE50 balance and the average fly weight was calculated. The weight of male flies averaged from the four consecutive collections is given in Table I. The weight of female flies is strongly influenced by the weight of the ovaries. To minimize fertility effects on fly weight measurements, we analyzed male flies.
For the data shown in Figure 3G, we weighed pools of 2- to 3-day-old males. Three different measurements were averaged. For the data shown in Figure 3H and I, we weighed at least 20 flies of each genotype individually and calculated the average.
For the analysis of the size of adult wings and of cells in these wings, dissected wings were deposited on a glass slide and covered with a coverslip. To flatten the preparations, a weight was placed on top of the coverslip and nail polish was used to fix the coverslip to the slide. Microscopic images of wings were captured using a CCD camera. IPLab software was used to determine total wing area and to count wing hairs in a defined wing area of constant size.
Immunoprecipitation, immunoblotting and immunolabeling
For Cdk co-immunoprecipitation experiments, virgin females homozygous for arm-GAL4 were crossed to males homozygous for either UAS-Cdk1-myc II.1, UAS-Cdk2-myc II.2, UAS-Cdk4-myc II.1 or _UAS-Cdk4_D175N-myc II.1. Eggs were collected from these crosses for a 3 h period and aged for 4 h at 25°C.
To monitor Rb phosphorylation, we expressed UAS-mRb using da-GAL4. The mouse monoclonal antibody G3-245 (PharMingen) was used for immunoblot analysis. The rabbit polyclonal antibodies Rb(M153) (Santa Cruz Biotechnology Inc.) were used for immunoprecipitation from extracts prepared from 3–6 h embryos.
To monitor mRB phosphorylation in Cdk4 mutants, we crossed w*; Cdk4_3; UAS-mRb virgin females with w*; Cdk4_3; da-GAL4 males and analyzed progeny lacking both maternal and zygotic Cdk4 function 3–6 h after egg deposition (AED) by immunoblotting with anti-Rb. For control, progeny from w*; Cdk4_3; UAS-mRb_ females, which had been crossed to w*; da-GAL4 males, were analyzed in parallel. While these control embryos expressed zygotic Cdk4 function from the paternally contributed endogenous Cdk4 gene, they were also lacking maternal Cdk4 function. All the different embryo collections analyzed in these experiments, therefore, were affected to the same extent by maternal effect abnormalities. Moreover, UAS-mRb expression occurred only in those embryos that developed beyond the stage where zygotic expression starts.
Embryos were fixed and immunolabeled as described previously (Knoblich et al., 1994). Pulse labeling with BrdU was performed for 20 min, also as described previously (Knoblich et al., 1994).
Information on additional antibodies and methodological details is provided in the Supplementary material available at The EMBO Journal Online.
Clonal analysis and flow cytometry
Mitotic clones were induced using the FLP/FRT technique (Xu and Rubin, 1993). Larvae of the genotype y, w, Hs-FLP; FRT(42D)/FRT(42D) π_Myc, Cdk4_3 were given a heat shock for 30 min at 37°C at 48 h AED. π_Myc_ expression was induced by a heat shock for 1.5 h at 37°C at 112 h AED, and discs were allowed to recover for 1.5 h at 23°C before fixation and anti-myc immunolabeling.
Fluorescence activated cell sorting (FACS) was performed as described by Neufeld et al. (1998). For analysis of Cdk4_3 cells, Ub-GFPS65T#73 (generously provided by W.Bender) was recombined onto FRT(42D). Mitotic clones were induced in larvae of the genotype y, w, Hs-FLP; FRT(42D) Ub-GFPS65T#73/FRT(42D) π_Myc, _Cdk4_3 by administering a heat shock for 1.5 h at 37°C at 48 h AED. Discs were dissociated for >2 h at 96 h AED and analyzed in a Becton Dickinson FACS Vantage or LSR. Cell quest (Becton Dickinson) software was used for data analysis.
Supplementary material
Supplementary material to this paper is available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank J.Campos-Ortega for providing fly stocks, J.Lukas for the mouse Rb plasmid, D.Heidmann, A.Herr and E.Keidel for help during the early stages of the work. This work was supported by the Deutsche Forschungsgemeinschaft (DFG Le 987/1-2 and 1-3, DFG Le 987/2-1).
References
- Adams M.D. et al. (2000) The genome sequence of Drosophila melanogaster. Science, 287, 2185–2195. [DOI] [PubMed] [Google Scholar]
- Baldin V., Lukas,J., Marcote,M.J., Pagano,M. and Draetta,G. (1993) Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev., 7, 812–821. [DOI] [PubMed] [Google Scholar]
- Böhni R., Riesgo-Escovar,J., Oldham,S., Brogiolo,W., Stocker,H., Andruss,B.F., Beckingham,K. and Hafen,E. (1999) Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell, 97, 865–875. [DOI] [PubMed] [Google Scholar]
- Brand A.H. and Perrimon,N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- Brown N.H. and Kafatos,F.C. (1988) Functional cDNA libraries from Drosophila embryos. J. Mol. Biol., 203, 425–437. [DOI] [PubMed] [Google Scholar]
- Clarke A.R., Maandag,E.R., van Roon,M., van der Lugt,N.M., van der Valk,M., Hooper,M.L., Berns,A. and te Riele,H. (1992) Requirement for a functional Rb-1 gene in murine development. Nature, 359, 328–330. [DOI] [PubMed] [Google Scholar]
- Cobrinik D. et al. (1996) Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev., 10, 1633–1644. [DOI] [PubMed] [Google Scholar]
- Datar S.A., Jacobs,H.W., Flor de la Cruz,A., Lehner,C.F. and Edgar,B.A. (2000) The Drosophila Cyclin D–Cdk4 complex promotes cellular growth. EMBO J., 19, 4543–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nooij J.C., Letendre,M.A. and Hariharan,I.K. (1996) A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell, 87, 1237–1247. [DOI] [PubMed] [Google Scholar]
- Du W. and Dyson,N. (1999) The role of RBF in the introduction of G1 regulation during Drosophila embryogenesis. EMBO J., 18, 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Vidal,M., Xie,J.E. and Dyson,N. (1996) RBF, a novel RB-related gene that regulates E2F activity and interacts with cyclin E in Drosophila. Genes Dev., 10, 1206–1218. [DOI] [PubMed] [Google Scholar]
- Dyson N. (1998) The regulation of E2F by pRB-family proteins. Genes Dev., 12, 2245–2262. [DOI] [PubMed] [Google Scholar]
- Fantl V., Stamp,G., Andrews,A., Rosewell,I. and Dickson,C. (1995) Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev., 9, 2364–2372. [DOI] [PubMed] [Google Scholar]
- Field S.J., Tsai,F.Y., Kuo,F., Zubiaga,A.M., Kaelin,W.G.,Jr, Livingston,D.M., Orkin,S.H. and Greenberg,M.E. (1996) E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell, 85, 549–561. [DOI] [PubMed] [Google Scholar]
- Finley R.L. Jr, Thomas,B.J., Zipursky,S.L. and Brent,R. (1996) Isolation of Drosophila cyclin D, a protein expressed in the morphogenetic furrow before entry into S phase. Proc. Natl Acad. Sci. USA, 93, 3011–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin D.S., Godfrey,V.L., Lee,H., Kovalev,G.I., Schoonhoven,R., Chen-Kiang,S., Su,L. and Xiong,Y. (1998) CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev., 12, 2899–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P., Shiio,Y., Cheng,P.F., Parkhurst,S.M. and Eisenman,R.N. (1996) Myc and max homologs in Drosophila. Science, 274, 1523–1527. [DOI] [PubMed] [Google Scholar]
- Geng Y., Whoriskey,W., Park,M.Y., Bronson,R.T., Medema,R.H., Li,T., Weinberg,R.A. and Sicinski,P. (1999) Rescue of cyclin D1 deficiency by knockin cyclin E. Cell, 97, 767–777. [DOI] [PubMed] [Google Scholar]
- Guan K.L., Jenkins,C.W., Li,Y., Nichols,M.A., Wu,X.Y., Okeefe,C.L., Matera,A.G. and Xiong,Y. (1994) Growth suppression by p18, a p16(ink4/mts1)-related and p14(ink4b/mts2)-related cdk6 inhibitor, correlates with wild-type pRb function. Genes Dev., 8, 2939–2952. [DOI] [PubMed] [Google Scholar]
- Hamel P.A., Gill,M.R., Phillips,R.A. and Gallie,B.L. (1992) Regions controlling hyperphosphorylation and conformation of the retinoblastoma gene product are independent of domains required for transcriptional repression. Oncogene, 7, 693–701. [PubMed] [Google Scholar]
- Harbour J.W., Luo,R.X., Dei Santi,A., Postigo,A.A. and Dean,D.C. (1999) Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell, 98, 859–869. [DOI] [PubMed] [Google Scholar]
- Huang H., Potter,C.J., Tao,W., Li,D., Brogiolo,W., Hafen,E., Sun,H. and Xu,T. (1999) PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development, 126, 5365–5372. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli,A., Schmitt,E.M., Bronson,R.T., Goodell,M.A. and Weinberg,R.A. (1992) Effects of an Rb mutation in the mouse. Nature, 359, 295–300. [DOI] [PubMed] [Google Scholar]
- Johnston L.A., Prober,D.A., Edgar,B.A., Eisenman,R.N. and Gallant,P. (1999) Drosophila myc regulates cellular growth during development. Cell, 98, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J.-Y. and Sherr C.J. (1993) Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc. Natl Acad. Sci. USA, 90, 11513–11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E.W. and Tremblay,S. (1992) Phosphorylation of the retinoblastoma protein is modulated in mouse kidney cells infected with polyomavirus. Oncogene, 7, 909–917. [PubMed] [Google Scholar]
- Knoblich J.A., Sauer,K., Jones,L., Richardson,H., Saint,R. and Lehner,C.F. (1994) Cyclin E controls S phase progression and its downregulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell, 77, 107–120. [DOI] [PubMed] [Google Scholar]
- Koh J., Enders,G.H., Dynlacht,B.D. and Harlow,E. (1995) Tumor-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature, 375, 506–510. [DOI] [PubMed] [Google Scholar]
- Lane M.E., Sauer,K., Wallace,K., Jan,Y.N., Lehner,C.F. and Vaessin,H. (1996) Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell, 87, 1225–1236. [DOI] [PubMed] [Google Scholar]
- Lee E.Y., Chang,C.Y., Hu,N., Wang,Y.C., Lai,C.C., Herrup,K., Lee,W.H. and Bradley,A. (1992) Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature, 359, 288–294. [DOI] [PubMed] [Google Scholar]
- Lee M.H., Williams,B.O., Mulligan,G., Mukai,S., Bronson,R.T., Dyson,N., Harlow,E. and Jacks,T. (1996) Targeted disruption of p107: functional overlap between p107 and Rb. Genes Dev., 10, 1621–1632. [DOI] [PubMed] [Google Scholar]
- Leevers S.J., Weinkove,D., Macdougall,L.K., Hafen,E. and Waterfield,M.D. (1996) The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J., 15, 6584–6594. [PMC free article] [PubMed] [Google Scholar]
- Lukas J., Müller,H., Bartkova,J., Spitkovsky,D., Kjerulff,A., Jansen-Dürr,P., Strauss,M. and Bartek,J. (1994) DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell’s requirement of cyclin D1 function in G1. J. Cell Biol., 125, 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J., Bartkova,J., Rohde,M., Strauss,M. and Bartek,J. (1995a) Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells, independent of CDK4 activity. Mol. Cell. Biol., 15, 2600–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J., Parry,D., Aagaard,L., Mann,D.J., Bartkova,J., Strauss,M., Peters,G. and Bartek,J. (1995b) Retinoblastoma-protein-dependent cell-cycle inhibition by the tumor-suppressor p16. Nature, 375, 503–506. [DOI] [PubMed] [Google Scholar]
- Medema R.H., Herrera,R.E., Lam,F. and Weinberg,R.A. (1995) Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc. Natl Acad. Sci. USA, 92, 6289–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne J., Stewart,M.J., Stocker,H., Hafen,E., Kozma,S.C. and Thomas,G. (1999) Drosophila S6 kinase: a regulator of cell size. Science, 285, 2126–2129. [DOI] [PubMed] [Google Scholar]
- Neufeld T.P., de la Cruz,A.F., Johnston,L.A. and Edgar,B.A. (1998) Coordination of growth and cell division in the Drosophila wing. Cell, 93, 1183–1193. [DOI] [PubMed] [Google Scholar]
- Park M. and Krause,M.W. (1999) Regulation of postembryonic G1 cell cycle progression in Caenorhabditis elegans by a cyclin D/CDK-like complex. Development, 126, 4849–4860. [DOI] [PubMed] [Google Scholar]
- Polymenis M. and Schmidt,E.V. (1997) Coupling of cell division to cell growth by translational control of the G1 cyclin CLN3 in yeast. Genes Dev., 11, 2522–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenis M. and Schmidt,E.V. (1999) Coordination of cell growth with cell division. Curr. Opin. Genet. Dev., 9, 76–80. [DOI] [PubMed] [Google Scholar]
- Quelle D.E., Ashmun,R.A., Shurtleff,S.A., Kato,J.Y., Barsagi,D., Roussel,M.F. and Sherr,C.J. (1993) Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev., 7, 1559–1571. [DOI] [PubMed] [Google Scholar]
- Rane S.G., Dubus,P., Mettus,R.V., Galbreath,E.J., Boden,G., Reddy,E.P. and Barbacid,M. (1999) Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nature Genet., 22, 44–52. [DOI] [PubMed] [Google Scholar]
- Sauer K., Weigmann,K., Sigrist,S. and Lehner,C.F. (1996) Novel members of the cdc2-related kinase family in Drosophila: cdk4/6, cdk5, PFTAIRE and PITSLRE kinase. Mol. Biol. Cell, 7, 1759–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Lee,H.W., Chin,L., Cordoncardo,C., Beach,D. and DePinho,R.A. (1996) Role of the ink4a locus in tumor suppression and cell mortality. Cell, 85, 27–37. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. (1994) G1 phase progression—cycling on cue. Cell, 79, 551–555. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. (1996) Cancer cell cycles. Science, 274, 1672–1677. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Donaher,J.L., Parker,S.B., Li,T.S., Gardner,H., Haslam,S.Z., Bronson,R.T., Elledge,S.J. and Weinberg,R.A. (1995) Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell, 82, 621–630. [DOI] [PubMed] [Google Scholar]
- Sicinski P. et al. (1996) Cyclin D2 is an FSH responsive gene involved in gonadal cell proliferation and oncogenesis. Nature, 384, 470–474. [DOI] [PubMed] [Google Scholar]
- Spradling A.C., Stern,D.M., Kiss,I., Roote,J., Laverty,T. and Rubin,G.M. (1995) Gene disruptions using P transposable elements: an integral component of the Drosophila genome project. Proc. Natl Acad. Sci. USA, 92, 10824–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui T., Hesabi,B., Moons,D.S., Pandolfi,P.P., Hansel,K.S., Koff,A. and Kiyokawa,H. (1999) Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol. Cell. Biol., 19, 7011–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel S. and Harlow,E. (1993) Distinct roles for cyclin-dependent kinases in cell cycle control. Science, 262, 2050–2054. [DOI] [PubMed] [Google Scholar]
- Weinberg R.A. (1995) The retinoblastoma protein and cell-cycle control. Cell, 81, 323–330. [DOI] [PubMed] [Google Scholar]
- Weinkove D., Neufeld,T.P., Twardzik,T., Waterfield,M.D. and Leevers,S.J. (1999) Regulation of imaginal disc cell size, cell number and organ size by Drosophila class I(A) phosphoinositide 3-kinase and its adaptor. Curr. Biol., 9, 1019–1029. [DOI] [PubMed] [Google Scholar]
- Xu T. and Rubin,G.M. (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development, 117, 1223–1237. [DOI] [PubMed] [Google Scholar]
- Yamasaki L., Jacks,T., Bronson,R., Goillot,E., Harlow,E. and Dyson,N.J. (1996) Tumor induction and tissue atrophy in mice lacking E2F-1. Cell, 85, 537–548. [DOI] [PubMed] [Google Scholar]