Targeted Blockage of Signal Transducer and Activator of Transcription 5 Signaling Pathway with Decoy Oligodeoxynucleotides Suppresses Leukemic K562 Cell Growth (original) (raw)
Abstract
The protein signal transducer and activator of transcription 5 (STAT5) of the JAK/STAT pathway is constitutively activated because of its phosphorylation by tyrosine kinase activity of fusion protein BCR-ABL in chronic myelogenous leukemia (CML) cells. This study investigated the potential therapeutic effect of STAT5 decoy oligodeoxynucleotides (ODN) using leukemia K562 cells as a model. Our results showed that transfection of 21-mer-long STAT5 decoy ODN into K562 cells effectively inhibited cell proliferation and induced cell apoptosis. Further, STAT5 decoy ODN downregulated STAT5 targets bcl-xL, cyclinD1, and c-myc at both mRNA and protein levels in a sequence-specific manner. Collectively, these data demonstrate the therapeutic effect of blocking the STAT5 signal pathway by cis-element decoy for cancer characterized by constitutive STAT5 activation. Thus, our study provides support for STAT5 as a potential target downstream of BCR-ABL for CML treatment and helps establish the concept of targeting STAT5 by decoy ODN as a novel therapy approach for imatinib-resistant CML.
Introduction
Chronic myelogenous leukemia (CML) is a clonal stem cell disorder in which the reciprocal translocation of t(9;22) (q34;q11) generates two novel fusion genes: bcr-abl on the derivative 22q– (Philadelphia) chromosome and abl-bcr on chromosome 9q+. The derived fusion protein BCR-ABL has constitutive tyrosine kinase activity that dysregulates several signal transduction pathways, such as signal transducer and activator of transcription 5 (STAT5), phosphoinositide-3 kinase/AKT, and RAS-mitogen–activated protein kinase, leading to abnormal cell cycle progression, increased cell proliferation, and decreased apoptosis (Faderl et al., 1999). As a result, imatinib, an inhibitor of the tyrosine kinase activity of BCR-ABL, has been employed to treat CML. Although >90% of chronic-phase CML patients respond to imatinib, at least initially, imatinib resistance emerges as a serious problem for effective treatment of CML (Azam et al., 2003; Strout and Schatz, 2009).
It is thus conceivable that targeting the signaling downstream of BCR-ABL may contribute to control leukemic cell proliferation and overcome imatinib resistance. One of these signaling pathways is STAT5. The STAT5 protein plays a significant role in both gene transcription and signal transduction. Normally, STAT5 is activated by phosphorylation of a conserved tyrosine residue at the C-terminus. Tyrosine-phosphorylated STAT5 in the form of homodimers or heterodimers subsequently translocate to the nucleus and bind specific DNA elements, leading to transcriptional activation. In the CML condition, however, STAT5 is constitutively activated by the fusion protein BCR-ABL (Baśkiewicz-Masiuk and Machaliński, 2004). Different strategies, such as antisense RNAs, siRNAs, dominant-negative proteins, and inhibitors of STAT5 upstream kinase, have been employed to block STAT5 activation (Ilaria et al., 1999; Rascle and Lees, 2003; Xi et al., 2003; Nam et al., 2007). Recently, decoy oligodeoxynucleotides (ODN), a kind of short double-strand DNA serving as a cis-element competitor binding to the transcription factor, provides us an alternative approach to block STAT5 activity (Azuma et al., 2003; Chae et al., 2004; Xiuli et al., 2009; Zhang et al., 2010).
In this study, we hypothesized that targeted blockage of the STAT5 signaling pathway with the decoy ODN against STAT5 would suppress leukemic K562 cell growth. Therefore, the STAT5 decoy ODN targeting activated STAT5 was developed to investigate its effects on cell proliferation and apoptosis in K562 cells. Our results showed that the STAT5 decoy ODN inhibited cell proliferation, blocked cell cycle progression, induced apoptosis, and finally, attenuated the trans-activation potential of STAT5 on gene expression of bcl-xL, cyclinD1, and c-myc.
Materials and Methods
Cell culture
Both human erythromyeloblastoid leukemia cell lines BCR/ABL-positive K562 and promyelocytic leukemia cell lines BCR/ABL-negative HL-60 were purchased from the Cell Bank of Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). These cells were maintained in complete RPMI 1640 medium (Gibco) with 10% fetal calf serum (HyClone), 100 U/mL penicillin, and 100 mg/mL streptomycin in a 5% CO2 humidified incubator at 37°C.
STAT5 decoy ODN
The ODNs were synthesized and purified by high-performance liquid chromatography (Sangon) with sequences as follows: the STAT5 decoy ODN, 5′-AGATTTCTAGGAATTCAAATC-3′ (the STAT5 consensus sequence is underlined); and mutant decoy ODN, 5′-AGATAGTAGTGTATTCAAATC-3′ (bases matching the STAT5 consensus sequence are underlined). ODNs were dissolved in a sterile annealing buffer (10 mM Tris [pH 8.0], 50 mM NaCl, 1 mM ethylenediamine tetraacetic acid) and then annealed by heating to 95°C, followed by cooling to 25°C at 5°C increments every 15 min in a polymerase chain reaction (PCR) machine (Bio-Rad). After that, the mixture was stored at −20°C. Fluorescent dye FAM-labeled ODN was prepared in the same way and was kept away from light.
Transfection
Twenty-four hours before transfection, the medium was replaced with fresh complete RPMI 1640 medium. Cells were washed twice with a serum-free RPMI 1640 medium and then transfected with ODN using cationic liposome lipofectin (Invitrogen) (molar ratio, DNA:lipid = 1:3) according to Invitrogen's instructions. The transfected cells were incubated at 37°C under 5% CO2 for 5 h. After addition of 4 mL complete RPMI 1640 medium containing 15% fetal calf serum, cells were maintained at 37°C in a 5% CO2 incubator for further study. The possible toxicity of ODN and cationic liposomes on cell viability was assessed by a trypan blue dye exclusion test. Subsequently, the transfection efficiency was evaluated by counting FAM-labeled ODN-positive cells under an inverted fluorescence microscope.
Cell growth curve
K562 and HL-60 cells, transfected with the STAT5 decoy ODN or mutant ODN, were seeded onto 24-well cell culture plates at 1 × 104 cells per well (K562 cells) or 2.5 × 104 cells per well (HL-60 cells). Here, HL-60 cells were set as a control to investigate cell specificity of the STAT5 decoy. Cell counts were performed with a hemocytometer at an indicated time point.
Analysis of cell cycle and apoptosis
Forty-eight hours after the ODN transfection, cells were washed and stained by Annexin-V and propidium iodide (PI) (Biovision) according to the manufacturer's instructions and analyzed by flow cytometry (FCM; Becton Dickinson; FACS Calibur). Also, cell cycle analysis was performed by FCM following routine procedure: fixation in ice-cold 70% ethanol, washing with phosphate-buffered saline (PBS), PI staining, and RNase treatment.
Transmission electron microscopy
Cells were fixed with 3% glutaraldehyde for 1 h, washed three times in PBS, then postfixed in 1% OsO4 in a cacodylate buffer for 1 h, and stained with 2% uranyl acetate for 15 min before being dehydrated in gradient ethanol and embedded in the water-soluble resin Durcupan (Fluka Chemie AG). Ultrathin sections (60–80 nm) were stained with uranyl acetate and lead citrate and examined under a transmission electron microscope (Philips 201).
Luciferase assay
The luciferase reporter assay was performed as described previously (Darwish et al., 2007). In brief, a 728-bp promoter fragment of the bcl-xL gene containing a STAT5 consensus sequence was obtained by PCR from K562 cells with primers containing a 5′-_Sac_I site (5′-AGACGAGCTCCGCATTTGTTGGGGGTCTCCG-3′) and a 5′-_Hin_dIII site (5′-AATCGAAAGCTTCTTCAGTGGACTCTGAATCTCC-3′) and cloned into the plasmid pGL3b-basic (a gift from Dr. Lin Zou) to construct the luciferase reporter vector pGL3b-bclxp, which was transiently cotransfected with the STAT5 decoy ODN or the mutant ODN using lipofectin into K562 cells. K562 cell lyses were collected at 48 h posttransfection, and luciferase activities were measured and normalized per μg protein by a standard luciferase assay (Promega).
Electrophoretic mobility shift assay
Nuclear proteins from the transfected and untransfected K562 cells were extracted as described previously (Tang et al., 2007). Protein concentration was determined using the Bradford method. The STAT5 decoy ODN and mutant ODN were labeled with gamma-32P-ATP (10 mCi/mL; Beijing FuRui Biomed) by using T4 polynucleotide kinase (Promega) and purified. 32P-labeled probe (0.5–1 ng, 10,000–15,000 cpm) and 1 μg polydeoxyinosinic-deoxycytidic acid (Sigma) were incubated with 10 μg nuclear extract in a 10 μL binding reaction buffer at room temperature for 30 min and then loaded onto 5% polyacrylamide gel. As controls, samples were incubated with an excess (× 100) of unlabeled ODN or an excess (× 100) of unlabeled mutant ODN. The gels were subjected to electrophoresis, dried, and analyzed by autoradiography. In addition, supershift assays were carried out with rabbit monoclonal anti-STAT5a antibody, anti-STAT5b antibody, or anti-STAT3 antibody (Santa Cruz Biotechnology). Each antibody was added to the samples after the initial binding reaction; the reaction lasted 30 min at room temperature and was subjected to electrophoresis as described above.
Reverse transcription–PCR analysis
Cells were transiently transfected with the STAT5 decoy ODN or mutant ODN using lipofectin. Forty-eight hours later, semiquantitative reverse transcription (RT)-PCR was performed to detect the expression level of bcl-xL, c-myc, and cyclinD1 transcripts. Primer sequences were as follows: bcl-xL, 5′-GAGGCAGGCGACGAGTTTGAA-3′ and 5′-TGGGAGGTAGAGTGGATGGT-3′ (Chalfant et al., 2002); c-myc, 5′-CCAGCAGCGACTCTGAGG-3′ and 5′-CCAAGACGTTGTGTGTTC-3′ (Kiaris and Schally, 1999); and cyclinD1, 5′-CTGGCCATGAACTACCTGGA-3′ and 5′-GTCACACTTGATCACTCTGG-3′ (Wandji et al., 2000). The variation in RT-PCR efficiency was evaluated with the GAPDH gene as an internal control, using the sense primer 5′-CCATGGAGAAGGCTGGGG-3′ and the antisense primer 5′-CAAAGTTGTCATGGATGACC-3′ (Cho et al., 2000).
Western blotting analysis
K562 cells and HL-60 cells were treated as earlier. Forty-eight hours later, about 10 × 106 cells were washed twice with cold PBS and lysed with the lysis buffer 5 mM ethylenediamine tetraacetic acid, 300 mM NaCl, 0.1% Igepal CA-630, 0.5 mM NaF, 0.5 mM Na3VO4, 0.5 mM phenylmethyl-sulfonyl fluoride, and 10 μg/mL each of aprotinin, pepstatin, and leupeptin (Sigma). Protein concentrations were measured by the Bradford method. Equal amounts of lysate were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Gibco BRL). Immunoblotting was performed using the following antibodies: anti-bcl-xL (Neomarkers); anti-c-myc, anti-cyclin D1, and anti-β-actin (Santa Cruz). Peroxidase-conjugated anti-mouse IgG antibody (Sigma) was used as a secondary antibody. Finally, after washing with PBS containing 0.1% Tween 20 three times, the membranes were developed with 3,3-diaminobenzidine. The immune complex was visualized using the Bio-Rad blot detection system (Gel Doc 1000), and the optical densities of each band on the polyvinylidene fluoride membrane were determined by computerized image analysis.
Statistical analysis
All experiments were performed at least three times. The experimental data were expressed as mean ± standard deviation. Statistical significance was determined by the Student's _t_-test, and p < 0.05 was considered significant.
Results
Incorporation of STAT5 decoy ODN into K562 cells
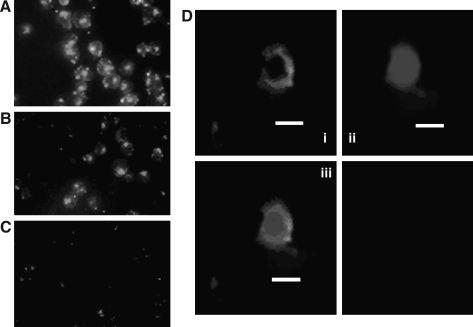
To determine the efficiency and the stability of the STAT5 decoy ODN's incorporation into K562 cells, an FAM-labeled STAT5 decoy ODN was added to K562 cells, followed by the examination under an inverted fluorescence microscope. As shown in Figure 1, the incorporation rate of the STAT5 decoy ODN was very high on day 1 and 3 (95.2% ± 4.6% [Fig. 1A], 76.0% ± 6.7% [Fig. 1B], respectively), and the positive percentage was maintained at 43.5 ± 2.9 (Fig. 1C) on day 5. Similar results were obtained for the mutant control decoy (data not shown). Confocal laser scanning microscopy confirmed that STAT5 decoy ODN was effectively transported into the cytoplasm of K562 cells (Fig. 1D).
FIG. 1.
Incorporation and subcellular localization of FAM-labeled ODN in K562 cells. Dynamic change of intracellular FAM-labeled ODN in K562 cells on day 1 (A), day 3 (B), and day 5 (C) after transfection (original × 400). (D) Confocal laser scanning microscopy demonstrating internalization of the STAT5 decoy ODN in K562 cells. Internalized ODN (light gray, i) and nuclei (dark gray, ii) were overlaid with the corresponding differential interface contrast image iii. Intracellular distribution of FAM-labeled ODN was acquired by confocal laser scanning microscopy (i, FAM-labeled ODN, light gray; ii, nuclei, dark gray; iii, color overlay). The scale bar is 20 μm. STAT5, signal transducer and activator of transcription 5; ODN, oligodeoxynucleotides.
STAT5 decoy ODN inhibits cell proliferation
Because STAT5 contributes to malignant transformation partially by promoting cell-cycle progression (de Groot et al., 2000), a cell growth curve was drawn to determine the effect of the STAT5 decoy ODN on cell proliferation. Our results showed that the STAT5 decoy ODN inhibited K562 cell proliferation in a dose-dependent manner, with maximum inhibitory effect at 25 μM (Fig. 2A). In contrast, the mutant ODN had no detected effect on the proliferation of K562 cells (Fig. 2B). To address the potential toxicity of STAT5 decoy-mediated STAT5 abrogation on STAT5-negative cells, HL-60 cells were employed (Fig. 2C). The results demonstrated that proliferation of HL-60 cells showed no significant difference upon treatment with either the STAT5 decoy ODN or mutant ODN.
FIG. 2.
Effect of the STAT5 decoy ODN on K562 cell proliferation. K562 cells treated with 0–25 μM STAT5 decoy ODN demonstrated a dose-dependent cell proliferation inhibition (A). K562 cells (B) and HL-60 cells (C) were treated with the STAT5 decoy ODN or mutant ODN at a concentration of 25 μM. Viable cells were counted by trypan blue dye exclusion at indicated time points after treatment.
STAT5 decoy ODN regulates cell cycle and induces apoptosis
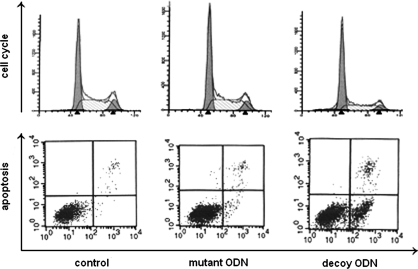
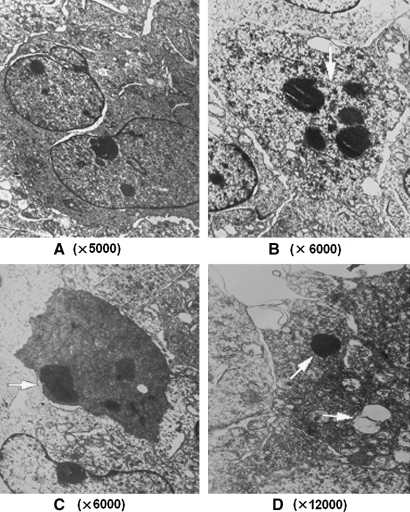
To explore the possible mechanisms of STAT5 decoy ODN-mediated inhibition of K562 cell growth, cell cycle and apoptosis were assessed. As shown in Figure 3 (upper panel), the STAT5 decoy ODN led to cell arrest at the G0/G1 phase and decreased cells in the S phase. Also, annexin-V/PI double staining revealed that cells treated with the STAT5 decoy ODN exhibited the highest cell count in the lower right quadrant compared with that of mutant ODN-treated or untreated cells (p < 0.05). In addition, 48 h after decoy ODN treatment, typical apoptotic morphology changes were observed by transmission electron microscopy, including condensed nuclear chromatin, karyopyknosis and nuclear fragmentation, vacuolated cytoplasm, and apoptotic body (Fig. 4).
FIG. 3.
Effect of STAT5 decoy ODN on cell cycle and apoptosis of K562 cells. Cell cycle was assayed by the PI staining method (upper panel), and cell apoptosis was examined by the Annexin-V/PI double staining method (lower panel) as described in the Materials and Methods section. The numbers indicate the percentage of cells in each quadrant (lower left: FITC−/PI−, intact cells; lower right: FITC+/PI−, apoptotic cells; upper left: FITC−/PI+, necrotic cells; upper right: FITC+/PI+, late apoptotic or necrotic cells). PI, propidium iodide.
FIG. 4.
Ultrastructural change of K562 cells at 48 h after transfection with STAT5 decoy ODN. Untreated K562 cells (A) exhibited “normal” morphology, whereas ODN-treated cells (B–D) exhibited morphologic changes of apoptosis (indicated by white arrow), such as karyopyknosis and nuclear fragmentation (B), increased electron density (C), and apoptotic body (D).
STAT5 decoy ODN specifically binds to STAT5
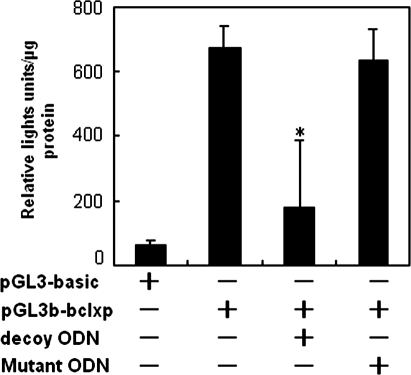
Next, we applied promoter analysis to evaluate the STAT5 binding ability of decoy ODN in K562 cells and found that the STAT5 decoy ODN decreased the luciferase activity significantly (Fig. 5, decoy ODN-treated group vs. mutant ODN-treated group, p < 0.05), suggesting that the decoy ODN indeed attenuated the STAT5-mediated gene expression by promoter competition in K562 cells. Further, to test the hypothesis that the STAT5 decoy would interfere with the binding of STAT5 to STAT5-specific DNA response elements, nucleus protein was extracted from K562 cells and evaluated by electrophoretic mobility shift assay using the STAT5 ODN sequence as a probe. The 100-fold excess unlabeled probe but not the mutant probe could markedly decrease the formation of the ODN–protein complex in the gel shift assay, which indicated that the STAT5 decoy ODN sequence firmly bound to the nucleus protein. A supershift experiment showed that the STAT5a and STAT5b, but not STAT3, monoclonal antibody had a supershift band (Fig. 6), indicating that the protein binding to the decoy ODN is STAT5.
FIG. 5.
Effect of STAT5 decoy ODN on STAT5 promoter activity. K562 cells were transiently cotransfected with luciferase reporter plasmid and STAT5 decoy or mutant ODNs. Cells were harvested and luciferase activity was determined at 48 h after transfection. Luciferase activity was represented as arbitrary units and as mean ± standard deviation from three independent experiments. There was significant difference between the decoy ODN-treated group, the mutant ODN-treated group, and untreated groups; *p < 0.05.
FIG. 6.
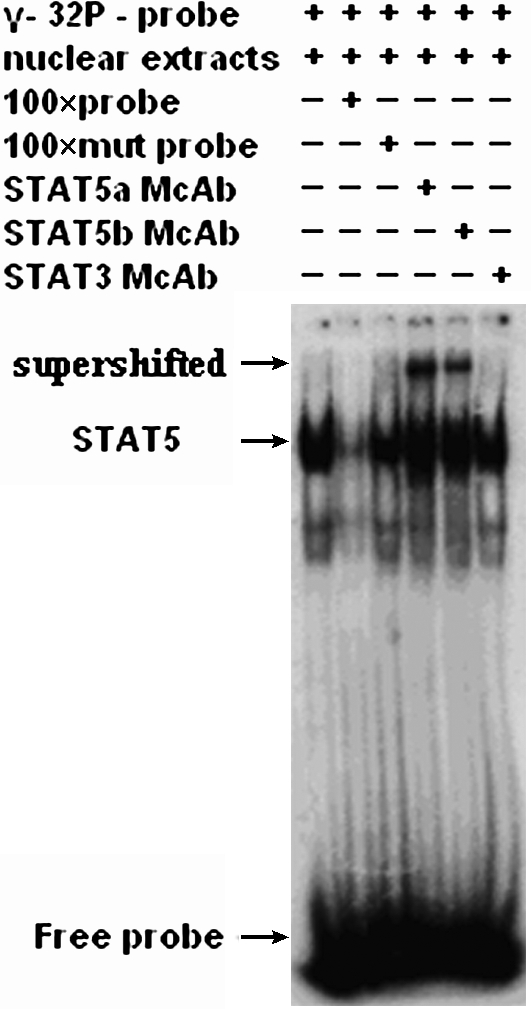
Specific binding of STAT5 to STAT5 decoy ODN. Electrophoretic mobility shift assay was performed using the STAT5 binding sequences and nuclear protein extracts of K562 cells. Competitions were performed with a 100-fold molar excess of unlabeled probe or a 100-fold molar excess of mutated probe. For supershift experiments, nuclear protein extracts were preincubated with STAT5a, STAT5b, or STAT3 monoclonal antibody, respectively. Arrows indicate migrational location of supershift band, STAT5-DNA complex, or free probe.
STAT5 decoy ODN modulates STAT5-mediated gene expression
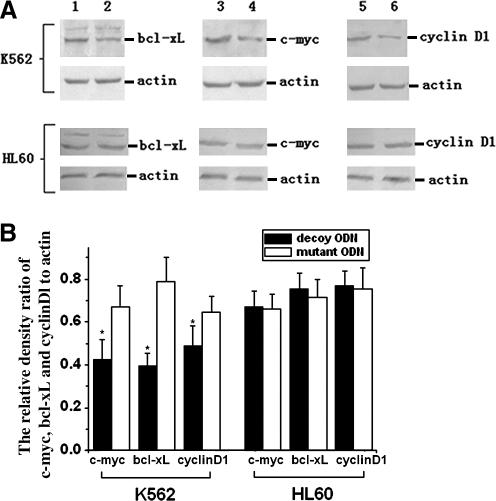
The biologic effects of activated STAT5 are potentially mediated through regulation of STAT5 target genes. To examine the consequence of the STAT5 decoy on STAT5-regulated gene expression, semiquantitative RT-PCR was performed. As shown in Figure 7, there was a 46% decrease in c-myc, a 59% decrease in bcl-xL, and a 48% decrease in cyclinD1 at the mRNA level after treatment with the STAT5 decoy, but the mRNA levels of these genes remained unchanged in HL-60 cells under the same treatment. Moreover, Western blotting analysis showed that the STAT5 decoy ODN could downregulate bcl-xL, c-myc, and cyclinD1 by 36.58%, 49.70%, and 31.04% at the protein level, respectively (Fig. 8).
FIG. 7.
Effect of STAT5 decoy ODN on transcription of c-myc, bcl-xL, and cyclinD1. (A) Semiquantitative reverse transcription (RT)–polymerase chain reaction results for c-myc, bcl-xL, and cyclinD1 in K562 and HL-60 cells at 48 h after STAT5 decoy or mutant ODN treatment. M: DNA marker; lanes 1, 2: c-myc; lanes 3, 4: GAPDH; lanes 5, 6: bcl-xL; lanes 7, 8: cyclinD1; lanes 1, 3, 5, 7: mutant ODN group; lanes 2, 4, 6, 8: decoy ODN group. (B) Gel intensity was analyzed using the Bio-Rad software (Gel Doc 1000). Values are represented as mean ± standard deviation of three independent experiments. Statistical significance was determined as *p < 0.05 compared with the mutant ODN control.
FIG. 8.
Effect of STAT5 decoy ODN on protein expression of c-myc, bcl-xL, and cyclinD1. Lanes 1, 3, 5: mutant ODN group; lanes 2, 4, 6: decoy ODN group. (A) Western blot analysis of c-myc, bcl-xL, and cyclinD1 in lysates of K562 and HL-60 cells at 48 h after STAT5 decoy or mutant ODN treatment. β-Actin was used as a loading control. (B) Figures were representative of three independent experiments with similar results. Statistical significance was determined as *p < 0.05 compared with the mutant ODN control.
Discussion
Cumulative evidence unveils a correlation between aberrant STAT5 activation and tumor progression (Nosaka et al., 1999; Welte et al., 1999; Birkenkamp et al., 2001; Baśkiewicz-Masiuk and Machaliński, 2004; Ye et al., 2006). CML is characterized by the activation of signaling pathways including STAT5 downstream of the fusion protein BCR-ABL, and STAT5 also contributes to the deregulation of cell growth and the antiapoptotic mechanism in CML. Therefore, STAT5 emerges as a potential molecular target for anticancer therapy, and several approaches, other than decoy ODN, have been reported to target STAT5 (Ilaria et al., 1999; Rascle and Lees, 2003; Xi et al., 2003; Nam et al., 2007).
Decoy ODN has been well established as an efficient approach to block gene transcription by competing with the binding of the transcription factor to the DNA cis-element (Azuma et al., 2003; Chae et al., 2004; Darwish et al., 2007; Xiuli et al., 2009; Zhang et al., 2010). Therefore, here we introduced the STAT5 decoy ODN into human leukemia K562 cells to examine its antagonistic effects on the STAT5 pathway and cell proliferation. Considering the potential instability of foreign nucleic acid, we adopted whole phosphorothioate-modified ODN to overcome the instability of ODN. Further, we found that cationic liposome lipofectin could highly enhance the incorporation rate of FAM-labeled ODN, and phosphorothioate modification did not affect the kinetics of uptake or degree of incorporation. The STAT5 decoy ODN could be maintained in K562 cells for at least 5 days in our experiment.
FCM analysis showed the enhanced G0/G1 arrest and apoptosis in STAT5 decoy ODN-treated K562 cells. Further, apoptosis of K562 cells was confirmed by ultrastructural examination. Therefore, our results demonstrate that the STAT5 decoy ODN could not only inhibit K562 cell growth but also induce apoptosis.
The basic theory of the ODN decoy approach is to abrogate the gene transcription function of the transcription factor, that is, to block the recruitment of the transcription factor to its promoter element. The consensus sequence and its flank sequence determine ODN binding intensity to the transcription factor (Soldaini et al., 2000). Thus, we used several approaches to examine the specificity of the STAT5 decoy ODN. First, we designed the mutant ODN as a control, which had the same GC content as the STAT5 decoy ODN and had the same chemical modification. It was proved that mutant ODN had no significant effects on cell proliferation and apoptosis in all experiments. Next, we checked the effect of the STAT5 decoy ODN on promoter activity by employing an artificial luciferase report system. Because bcl-xL was reported as one target of STAT5 and played an antiapoptosis role in CML (de Groot et al., 2000), we amplified its promoter region and inserted it into a luciferase report plasmid. The results showed that the STAT5 decoy ODN could specifically inhibit luciferase expression. Moreover, the electrophoretic mobility shift assay proved that the STAT5 decoy ODN could bind to STAT5 specially, but not to STAT3, which has a consensus sequence similar to STAT5 and is activated in CML as well.
The ubiquitous expression of STAT5 raises the possibility that blocking STAT5 may be harmful to normal cells. Therefore, the nonspecific effects of the STAT5 decoy ODN was considered in our study, and we did not observe any other effects of STAT5 decoy ODN in HL-60 cells.
The function of STAT5 depends mainly on its regulation of gene transcription. Several STAT5 target genes have been reported, including bcl-xL, cyclinD1, and c-myc (Lord et al., 2000; Xie et al., 2002). Therefore, we wonder whether the inhibitory effects of the STAT5 decoy ODN on K562 cell proliferation is mediated by regulating its targets bcl-xL, cyclinD1, and c-myc. By RT-PCR and western blotting, we did find that the STAT5 decoy ODN led to the downregulation of its targets bcl-xL, cyclinD1, and c-myc at both mRNA and protein levels. CyclinD1 is a typical marker around the G1/S check point of the cell cycle, and its downregulation was consistent with our results of the cell cycle profile examined by FCM. In addition, bcl-xL, which plays a key antiapoptosis role in CML, was downregulated in STAT5 decoy ODN-treated cells. Further, the downregulation of c-myc in STAT5 decoy ODN-treated cells was also in agreement with the well-established role of c-myc in cell cycle progression, apoptosis, and cellular transformation. Taken together, our results suggest that the STAT5 decoy ODN especially blocks the STAT5 signaling pathway and downstream target genes to suppress leukemic K562 cell growth.
Conclusions
In this study, we showed for the first time that targeted blockage of STAT5 by decoy ODN could inhibit the proliferation of K562 cells, which is consistent with decreased expression of antiapoptosis and cell cycle–related genes. The data obtained in this study support STAT5 as a potential target downstream of BCR-ABL for CML treatment. Given that imatinib resistance emerges as a challenging clinical problem and the underlying mechanism remains elusive, our results may help establish the concept of targeting STAT5 by decoy ODN as a novel therapeutic approach for imatinib-resistant CML.
Acknowledgments
The authors thank Dr. Lin Zhou (Chongqing Medical University, Chongqing 400016, China) for the gift of plasmid. This study was supported by a grant from the National Science Foundation of China (No. 30270574) to W.F.
Disclosure Statement
The authors declare that they have no competing interests.
References
- Azam M. Latek R.R. Daley G.Q. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- Azuma H. Tomita N. Kaneda Y. Koike H. Ogihara T. Katsuoka Y. Mor-ishita R. Transfection of NFkappaB-decoy oligodeoxynucleotides using efficient ultrasound-mediated gene transfer into donor kidneys prolonged survival of rat renal allografts. Gene Ther. 2003;10:415–425. doi: 10.1038/sj.gt.3301882. [DOI] [PubMed] [Google Scholar]
- Baśkiewicz-Masiuk M. Machaliński B. The role of the STAT5 proteins in the proliferation and apoptosis of the CML and AML cells. Eur J Haematol. 2004;72:420–429. doi: 10.1111/j.1600-0609.2004.00242.x. [DOI] [PubMed] [Google Scholar]
- Birkenkamp K.U. Geugien M. Lemmink H.H. Kruijer W. Vellenga E. Regulation of constitutive STAT5 phosphorylation in acute myeloid leukemia blasts. Leukemia. 2001;15:1923–1931. doi: 10.1038/sj.leu.2402317. [DOI] [PubMed] [Google Scholar]
- Chae Y.M. Park K.K. Magae J. Lee I.S. Kim C.H. Kim H.C. Hong S. Chang Y.C. Sp1-decoy oligodeoxynucleotide inhibits high glucose-induced mesangial cell proliferation. Biochem Biophys Res Commun. 2004;319:550–555. doi: 10.1016/j.bbrc.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Chalfant C.E. Rathman K. Pinkerman R.L. Wood R.E. Obeid L.M. Ogretmen B. Hannun Y.A. De Novo ceramide regulates the alternative splicing of caspase 9 and bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- Cho J.M. Song D.J. Bergeron J. Benlimame N. Wold M.S. Alaoui-Jamali M.A. RBT1, a novel transcriptional co-activator, binds the second subunit of replication protein A. Nucl Acids Res. 2000;28:3478–3485. doi: 10.1093/nar/28.18.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish H. Cho J.M. Loignon M. Alaoui-Jamali M.A. Overexpression of SERTAD3, a putative oncogene located within the 19q13 amplicon, induces E2F activity and promotes tumor growth. Oncogene. 2007;26:4319–4328. doi: 10.1038/sj.onc.1210195. [DOI] [PubMed] [Google Scholar]
- de Groot R.P. Raaijmakers J.A. Lammers J.W. Koenderman L. STAT5-Dependent CyclinD1 and Bcl-xL expression in Bcr-Abl-transformed cells. Mol Cell Biol Res Commun. 2000;3:299–305. doi: 10.1006/mcbr.2000.0231. [DOI] [PubMed] [Google Scholar]
- Faderl S. Talpaz M. Estrov Z. O'Brien S. Kurzrock R. Kantarjian H.M. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- Ilaria R.L. Hawley R.G. Van Etten R.A. Dominant negative mutants implicate STAT5 in myeloid cell proliferation and neutrophil differentiation. Blood. 1999;93:4154–4166. [PubMed] [Google Scholar]
- Kiaris H. Schally A.V. Decrease in telomerase activity in U-87MG human glioblastomas after treatment with an antagonist of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1999;96:226–231. doi: 10.1073/pnas.96.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J.D. McIntosh B.C. Greenberg P.D. Nelson B.H. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J Immunol. 2000;164:2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- Nam S. Williams A. Vultur A. List A. Bhalla K. Smith D. Lee F.Y. Jove R. Dasatinib (BMS-354825) inhibits Stat5 signaling associated with apoptosis in chronic myelogenous leukemia cells. Mol Cancer Ther. 2007;6:1400–1405. doi: 10.1158/1535-7163.MCT-06-0446. [DOI] [PubMed] [Google Scholar]
- Nosaka T. Kawashima T. Misawa K. Ikuta K. Mui A.L. Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascle A. Lees E. Chromatin acetylation and remodeling at the Cis promoter during STAT5-induced transcription. Nucleic Acids Res. 2003;31:6882–6890. doi: 10.1093/nar/gkg907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldaini E. John S. Moro S. Bollenbacher J. Schindler U. Leonard W.J. DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol Cell Biol. 2000;20:389–401. doi: 10.1128/mcb.20.1.389-401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strout M.P. Schatz D.G. Imatinib resistance and progression of CML to blast crisis: somatic hypermutation AIDing the way. Cancer Cell. 2009;16:174–176. doi: 10.1016/j.ccr.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Tang D. Kang R. Xiao W. Jiang L. Liu M. Shi Y. Wang K. Wang H. Xiao X. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol. 2007;178:7376–7384. doi: 10.4049/jimmunol.178.11.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandji S.A. Gadsby J.E. Simmen F.A. Barber J.A. Hammond J.M. Porcine ovarian cells express messenger ribonucleic acids for the acid-labile subunit and insulin-like growth factor binding protein-3 during follicular and luteal phases of the estrous cycle. Endocrinology. 2000;141:2638–2647. doi: 10.1210/endo.141.7.7563. [DOI] [PubMed] [Google Scholar]
- Welte T. Leitenberg D. Dittel B.N. al-Ramadi B.K. Xie B. Chin Y.E. Janeway C.A., Jr. Bothwell A.L. Bottomly K. Fu X.Y. STAT5 interaction with the T cell receptor complex and stimulation of T cell proliferation. Science. 1999;283:222–225. doi: 10.1126/science.283.5399.222. [DOI] [PubMed] [Google Scholar]
- Xi S. Zhang Q. Gooding W.E. Smithgall T.E. Grandis J.R. Constitutive activation of STAT5b contributes to carcinogenesis in vivo. Cancer Res. 2003;63:6763–6771. [PubMed] [Google Scholar]
- Xie S. Lin H. Sun T. Arlinghaus R.B. Jak2 is involved in c-Myc induction by Bcr-Abl. Oncogene. 2002;21:7137–7146. doi: 10.1038/sj.onc.1205942. [DOI] [PubMed] [Google Scholar]
- Xiuli W. Su-ping H. Hui-hua D. Zhi-xue Y. Shi-long F. Pin-hong L. NF-kappaB decoy oligonucleotides suppress RANTES expression and monocyte chemotactic activity via NF-kappaB inactivation in stromal cells of ectopic endometrium. J Clin Immunol. 2009;29:387–395. doi: 10.1007/s10875-009-9274-z. [DOI] [PubMed] [Google Scholar]
- Ye D. Wolff N. Li L. Zhang S. Ilaria R.L., Jr STAT5 signaling is required for the efficient induction and maintenance of CML in mice. Blood. 2006;107:4917–4925. doi: 10.1182/blood-2005-10-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Liu P. Zhang B. Wang A. Yang M. Role of STAT3 decoy oligodeoxynucleotides on cell invasion and chemosensitivity in human epithelial ovarian cancer cells. Cancer Genet Cytogenet. 2010;197:46–53. doi: 10.1016/j.cancergencyto.2009.10.004. [DOI] [PubMed] [Google Scholar]