Synergistic Activity of Interleukin-17 and Tumor Necrosis Factor-α Enhances Oxidative Stress-Mediated Oligodendrocyte Apoptosis (original) (raw)
. Author manuscript; available in PMC: 2012 Feb 1.
Abstract
Th1 cytokine-induced loss of oligodendrocytes (OLs) is associated with axonal loss in CNS demyelinating diseases such as multiple sclerosis (MS), which contributes to neurological disabilities in affected individuals. Recent studies indicated that, in addition to Th1-phenotype cytokines including tumor necrosis factor (TNF)-α, Th17 phenotype cytokine, interleukin (IL)-17 also involved in the development of MS. Here, we investigated the direct effect of IL-17 on the survival of OLs in the presence of TNF-α and individually in vitro settings. Our findings suggest that IL-17 alone, however, was not able to affect the survival of OLs, but it exacerbates the TNF-α-induced OL apoptosis as compared to individual TNF-α treatment. This effect of cytokines was ascribed to an inhibition of cell survival mechanisms, co-localization of Bid/Bax proteins in the mitochondrial membrane and caspase 8 activation mediated release of apoptosis inducing factor from mitochondria in treated OLs. In addition, cytokine treatment disturbed the mitochondrial membrane potential in OLs with corresponding increase in the generation of reactive oxygen species, which were attenuated by _N_-acetyl cysteine treatment. In addition, combining of these cytokines induced cell cycle arrest at G1/S phases in OL-like cells and inhibited the maturation of OL progenitor cells (OPCs) that was attenuated by peroxisome proliferator activated receptor (PPAR)-γ/-β agonists. Collectively, these data provide initial evidence that IL-17 exacerbates TNF-α-induced OL loss and inhibits the differentiation of OPCs suggesting that antioxidant- or PPAR agonist-based therapies have potential to limit CNS demyelination in MS or other related demyelinating disorders.
Keywords: Tumor necrosis factor, Interleukin-17, oligodendrocyte, apoptosis, multiple sclerosis
Introduction
A typical feature of CNS demyelinating diseases such as multiple sclerosis (MS) is the recurrence of inflammatory attacks that lead to oligodendrocyte (OL) loss which causes axonal loss and subsequent neurological disabilities in affected individuals (Prineas et al. 1993). Functional OLs are essential for eradicating the early-stage lesions in MS brain by inducing the remyelination of regenerating or demyelinated neuronal axons, which subsequently prevents the progression of neurological disabilities in MS patients (Jeffery & Blakemore 1997, Prineas et al. 1993).
MS is an autoimmune disease initiated by the infiltration of autoreactive CD4+ T-helper 1 (Th1) cells into the CNS (Zamvil & Steinman 1990) with subsequent expression of pro-inflammatory cytokines including tumor necrosis factor (TNF)-α by activated resident glial cells leading to the formation of brain lesions (Sospedra & Martin 2005). Specifically, TNF-α has been shown to cause the degeneration of OLs in MS brain (Selmaj et al. 1991b, Ozawa et al. 1994, D'Souza et al. 1995). In fact, for some time, Th1 phenotype autoimmunity was believed to be responsible for the development of MS (Sospedra & Martin 2005). However, recent reports suggest that Th17 cells are critical for the development of MS (Park et al. 2005), and that CNS inflammation in the MS model is dependent on the proliferation of Th17 versus Th1 cells, which in turn triggers the activation of resident brain glial cells (Stromnes et al. 2008). IL-17 is a pro-inflammatory cytokine secreted by Th17 cells, which has been shown to play a critical role in autoimmune-mediated inflammation (Bettelli et al. 2007, McKenzie et al. 2006, Weaver et al. 2007, Park et al. 2005). Moreover, IL-17 has been reported to be a potent inducer of IL-1β and TNF-α in immune cells (Jovanovic et al. 1998) and implicated not only in MS model, but also in other autoimmune disease models such as rheumatoid arthritis (Nakae et al. 2003), inflammatory bowel disease (Hue et al. 2006), psoriasis (Zheng et al. 2007), and uveitis (Amadi-Obi et al. 2007).
Recent studies document that Th17 cells migrate preferentially across the blood brain barrier and that secreted IL-17 participates in inflammation and lesion formation in MS brain (Kebir et al. 2007). Moreover, IL-17-induced cellular signaling demonstrated additive or synergistic effects with other pro-inflammatory cytokines as described in rheumatoid arthritis (Chabaud et al. 2000). Therefore, elucidating the effect of IL-17 on the survival and maturation of OLs and investigating whether it synergizes with Th1 cytokine-mediated cytotoxicity in OLs are essential to improve our therapeutic strategies to limit CNS demyelination in MS brain. We hypothesized that IL-17 alone, or in the presence of TNF-α may affect the survival of OLs and the maturation of OL progenitor cells (OPCs) in MS brain. Therefore, we evaluated the direct effect of IL-17 on primary OLs and an OL-like B12 cell line in vitro individually and in the presence of TNF-α. Our findings suggest that IL-17 exacerbates TNF-α-induced OL apoptosis and that N-acetyl cysteine or peroxisome proliferator activated receptor (PPAR)-γ/-β agonists attenuate this cytokine-mediated toxicity in OLs thereby raising the possibility of the use of these agents in MS therapy.
Materials and Methods
Chemicals and reagents
Unless otherwise stated, all chemicals were purchased from Sigma (St. Louis, MO). _N_-acetyl cysteine (NAC), IETD-CHO peptide (caspase 8 inhibitor 1), 15d-PGJ2 (PPAR-γ agonist), and L165041 (PPAR-β agonist) were purchased from EMD Biosciences. Dulbecco's modified Eagle medium (DMEM; 4.5 g/L glucose), FBS, laminin-2 (meosin), mouse IgG and rabbit polyclonal IgG (control isotypes) and secondary antibodies such as Alexa Fluor conjugated goat anti-mouse IgG, and goat anti-rabbit IgG were purchased from Invitrogen (Carlsbad, CA). Antibodies against phospho-ERK1/2 (44/42), phospho-GSK-3β, apoptosis inducing factor (AIF), phosphor-c-Jun terminal kinase1/2 (JNK1/2), Bax, and pan-cadherin were purchased from cell signaling (Danvers, MA). Antibodies to Bid, p21Cip1, cyclin D1, cdk4 and A2B5 were purchased from Millipore. Antibodies to myelin basic protein (MBP), TNF-α receptor 1 (TNFR1), TNFR1-associated death domain (TRADD), IL-17R and TNFR1-associated factor 6 (TRAF6) were purchased from Santa Cruz biotechnology, Inc (Santa Cruz, CA). ECL-detecting reagents and nitrocellulose membranes were purchased from Amersham Biosciences (Arlington Heights, IL).
Cultures and treatments of OLs/OL-like B12 cells
Rat cortical glial cell cultures were generated from P1-P2 SD rat brains (Charles River, Wilmington, MA) as described earlier (Paintlia et al. 2005) using purification methods described by McCarthy and de Vellis (McCarthy & de Vellis 1980). Briefly, dissociated rat cortices were cultured on poly-lysine coated cultures flasks in DMEM containing 10% FBS and 4 mM L-glutamine. After 10 days, flasks were shaken (250 rev/min) on shaker incubator for 2 h to remove microglia. Then flasks were shaken again for another 8 h to dislodge OPCs from the astrocyte layer and cultured on laminin-2 coated culture dishes or glass cover slips in high-glucose, Sato-based medium containing 5 μg/ml insulin, 50 μg/ml human transferrin, 100 μg/ml BSA, 6.2 μg/ml progesterone, 16 μg/ml putrescine, 5 ng/ml sodium selenite, 4 mM L-glutamine and 2% FBS. Anti-A2B5 staining revealed that cultures consist of 98% pure OPCs (Paintlia et al. 2005).
OPCs (1 × 104 cells/ml) were plated in culture dishes or glass cover slips for 24 hr in proliferating Sato medium supplemented with PDGF-AA and fibroblast growth factor-2 (10 ng/ml each). After 24 hr, fresh Sato medium supplemented with thyroid hormone [tri-iodothyronine (T3), 30 ng/ml, Sigma] was added and cultured for another 96 hrs followed by treatment with cytokines as described for each experimental conditions. FACS analysis demonstrated that at DIV6 (Fig. 1D), OPCs were transformed into maturing OLs as indicative by mixed population of O1+/MBP+ cells (data not shown). Likewise, OL-like (B12) cells (kind gift from D. Schubert from the Salk Institute, La Jolla, CA) were plated at 1 × 105 cells/ml in DMEM containing 5% FBS and 25 μg/ml gentamycin in 25-cm2 flasks (Nunc, Roskilde, Denmark) and incubated at 37 °C in a humidified atmosphere of 95% air and 5% CO2 (Calderon et al. 1998). After 24 h, cells were transferred to reduced FBS (2%) medium and cultured for 48 h and these cells were GalC+ as marker of maturing OLs (Fig. 1E). For treatment, primary OLs/B12 cells were cultured in medium containing 2% of FBS.
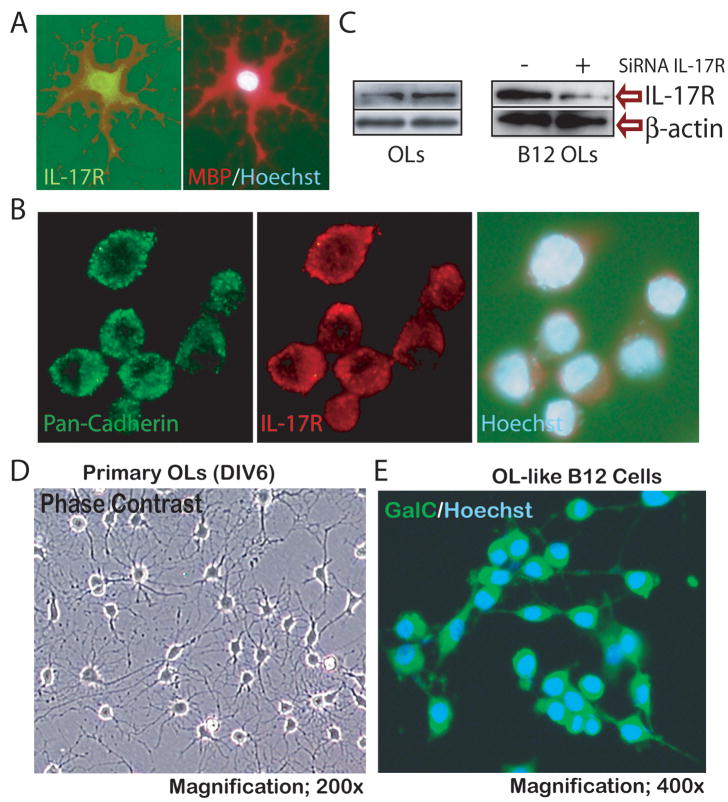
Figure 1. OL expresses IL-17R.
Primary OLs cultured on slides were labeled by double-immunocytochemistry using anti-MBP and IL-17R antibodies (A). Similarly, OL-like B12 cells were immunolabeled with anti-pan-cadherin and IL-17R antibodies (B). Western blot analysis depicts IL-17R protein level in OLs and B12 cells, including its reduction by knockdown of IL-17R gene expression by siRNA technique in B12 cells (C). Representative fields of slides depict maturing OLs at 6 days in vitro (DIV6) by phase contrast microscopy (D) and B12 cells expressing GalC by fluorescent microscopy (E) prior to treat with cytokines. Cells were counterstained with Hoechst dye for visualizing the nuclei and photographed at magnifications 400× (A) and 600× (B).
Immunocytochemistry
For single-labeling standard methodology was used. Briefly, slides were blocked with a serum-PBS solution and incubated with appropriately diluted primary antibody (1:100) at 4 °C overnight followed by washing and incubation with secondary antibodies (1:500). For double-labeling slides were incubated simultaneously with both types of primary antibodies after blocking with a serum-PBS solution at 4 °C overnight. Then, secondary antibodies for the appropriate marker either Alexa Fluor conjugated rabbit anti-IgG or mouse anti-IgG were used. Slides were also incubated with Alexa flour conjugated IgG without primary antibody as negative controls and an appropriate mouse IgG and rabbit polyclonal IgG as isotype controls. After thorough washings, slides were mounted with Fluoromount-G (Electron Microscopy Sciences) containing Hoechst dye. Slides were analyzed by immunofluorescence microscopy (Olympus BX-60) with an Olympus digital camera (Optronics, Goleta, CA) using a dual-band pass filter. Images were captured and processed using Adobe Photoshop 7.0 software. Total numbers of positive cells/field were determined by manual counting in 10 fields per slide from similarly treated slides (n= 5) in a blinded fashion.
Transient transfection with small interfering RNA (siRNA) oligonucleotides
Cells were transiently transfected with siRNA of IL-17R (Santa Cruz Biotech, SANTA CRUZ, CA) using transfection reagents as described previously (Paintlia et al. 2008b).
Detection of cytotoxicity
Cytotoxicity in treated cells was detected with an LDH release assay kit (Roche Diagnostics, Indianapolis, IN).
Analysis of cell death and cell cycle arrest by FACS
Cells were trypsinized and resuspended in 1 ml staining buffer (PBS/2% FBS) containing 200 ng propidium iodide (PI) and 200 ng RNase. After incubation at 37 °C for 20 min, cells were analyzed on a FACS Calibur using Cellquest software (BD Biosciences, San Jose, CA). Similarly, cellular DNA content was assessed by FACS as described previously (Rattan et al. 2005). Cells were acquired for determination of cell cycle arrest.
TUNEL assay
For staining of apoptotic cells TdT-mediated dUTP biotin nick end labeling (TUNEL) staining was performed on the cells using the ApopTag Fluorescein in situ Apoptosis Detection Kit (Millipore, Billerica, MA) according to the manufacturer's instructions. Briefly, cells were incubated with TdT for 60 min at 37 °C and were then labeled with anti-digoxigenin-fluorescein conjugate solution for 30 min at room temperature in the dark. Thus, DNA-breaks that are labeled by TdT with the digoxigenin-dUTP are tagged with the fluorescent antibody causing apoptotic cells to fluoresce green. Images were collected by immunofluorescence microscope as described above. Next, 5–10 fields/slide/treatment was counted in duplicate from three independent experiments to quantify apoptotic cells using ImagePro Plus version 5 Software.
DNA fragmentation assay
Harvested cells and their media were collected and processed for detection of DNA fragmentation as described previously (Haq et al. 2003).
Detection of caspase-8 activity
The fluorescent marker Red-IETD-FMK (Calbiochem Caspase-8 detection kit) was used to detect activated caspase-8 in the cells.
Proliferation assay
[3H]-thymidine DNA incorporation was measured in cells by a 1450 Microbeta Wallac Trilux Liquid Scintillation Counter (Perkin-Elmer Life Sciences) using procedures described previously (Nath et al. 2004).
Collection of cytosolic and mitochondrial fractions
Cells were washed with ice-cold PBS, then resuspended in ice-cold HMKEE buffer (20 mM HEPES-KOH, pH 7.0, 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin) containing 250 mM sucrose and they were allowed to swell on ice for 20 min. The cells were homogenized by passages through a 26-gauge needle and then centrifuged at 14,000 × g for 20 min at 4 °C. The cytosolic supernatant was removed and the pellet containing the mitochondria was resuspended in lysis buffer and stored at −70 °C or directly processed for western blot analysis. Immunoprecipitation of proteins in cell lysates was performed using standard methods.
Western blot analysis
Cells were lysed in ice-cold lysis buffer (RIPA buffer, Thermo Scientific) and processed for Western blot analysis as described previously (Paintlia et al. 2005). Autoradiographs were scanned and analyzed by using densitometry (Imaging GS800 Densitometer; Bio-Rad).
Electrophoretic mobility shift assay (EMSA) and pull down of NF-κB complex
Nuclear extracts from cells were prepared, and EMSA was performed as described previously (Giri et al. 2004, Prasad et al. 2006) with NF-κB consensus sequence (5-agttgaggggactttcccaggc-3′) that was end-labeled with [γ-32P] ATP. To pull down NF-κB complex, nuclear extracts were incubated with NF-κB gel shift oligonucleotide agarose conjugate (25 μL) for 30 min at 4 °C, as described before (Giri et al. 2004). To show specificity, mutant NF-κB consensus sequence (5-agttgaggcgactttcccaggc-3′) was used for gel shift assay. Agarose beads were washed three times with buffer (20 mM HEPES, pH 7.9; 10% (v/v) glycerol; 0.2 M NaCl; 1.5 mM MgCl2; 0.2 mM EDTA; and protease mixture) before addition of SDS loading buffer. In addition, super gel shift was observed by incubating sample with anti-p65 and – p50 antibodies.
Measurement of reactive oxygen species (ROS)
The generation of ROS was determined using the cell-permeable fluorescent dye 5-(and-6)-chloromethyl- 2′, 7′-dichlorodihydrofluorescein diacetate (CM-DCFDA; Invitrogen) in serum-free medium as described previously. The cultured cells, with or without treatment, were incubated with 5 μmol/L CM-DCFDA in PBS for 30 min at 37 °C. The change in fluorescence was determined at excitation/emission=485/530 nm using a Soft Max Pro spectrofluorometer (Molecular Devices, Sunnyvale, CA). Similarly, stained cells on slides were analyzed by immunofluorescence as described above.
Analysis of mitochondrial membrane potential (ΔΨm)
Cells cultured in 96-well black plates were treated with cytokines as described above and then incubated with JC-1, lipophilic cationic dye staining solution in 5% DMEM for 15–20 min at 37 °C to determine mitochondrial membrane potential (MMP) as described in the product manual (JC-1 MMP assay kit, Cayman Chemical Company, Ann Arbor, MI). Then cells were processed as per instructions in the assay kit and fluorescent intensity was measured with excitation/emission at 560/595 nm for J-aggregates as indicator of healthy cells and with excitation/emission at 485/535 nm for monomers as indicator of apoptotic or unhealthy cells. Ratio of J-aggregates versus monomers was computed for each analysis. For fluorescence microscopy, cells grown on glass cover-slips were treated with cytokines and incubated with JC-1 dye in medium for 15-20 min at 37 °C. Cells were then washed twice with PBS and mounted on glass slides with PBS/glycerol (50:50). Cells were photographed directly for J-aggregates at settings used for Texas red (excitation/emission=590/610 nm) and for monomers at settings used to detect FITC (excitation/emission=485/535 nm) using Fluorescence microscopy (Olympus BX-60).
RNA preparation, cDNA synthesis and quantitative RT-PCR (_QRT_-PCR) analysis
Cells were carefully processed for RNA isolation using TRIZOL reagent according to the manufacturer's protocol as described previously (Paintlia et al. 2005). Synthesis of single-stranded cDNA was carried out from total RNA using ‘iscript cDNA synthesis’ kit (Bio-Rad) and _QRT_-PCR was performed using iCycler iQ Real-Time PCR Detection System from BIO-RAD Laboratories. Primer sets used in the study were 18S rRNA; forward primer (FP): 5′-ccagagcgaaagcatttgccaaga-3′; reverse primer (RP): 5′-tcggcatcgtttatggtcggaact-3, platelet derived growth factor-α receptor (PDGF-αR); FP: 5′-cagacattgaccctgttccagagg-3′; RP: 5′-gaatctatgccaatatcatccatc-3′, MBP; FP: 5′-ctctggcaaggactcacacac-3′; RP: 5′-tctgctgagggacaggcctctc-3′, p21(Cip); FP: 5′-tggtcttctgcaagagaaagccct-3′; RP: 5′-atgaaggctaaggcagaagatggg-3′, cdk4; FP: 5′-gttgctggaaatgctgacctt-3′; RP: 5′-gtcactttcctccttgtgcaggta-3′ and Cyclin D1; FP: 5′tgctgcaaatggaactgcttctg-3′; RP: 5′-aaggtctgtgcatgtttgcggat-3′ were designed and purchased from Integrated DNA Technologies (Coralville, IA). IQ™ SYBR Green supermix was purchased from BIO-RAD (Hercules, CA). Thermal cycling conditions were as follows: activation of iTaq™ DNA polymerase at 95 °C for 10 min, followed by 35 cycles of amplification at 95 °C for 30 s and 58.5 °C for 1 min. The specificity and detection methods for data analysis are as described earlier (Paintlia et al. 2008a).
Statistical analysis
Using the Student's unpaired t-test and one-way multiple range ANOVA (Student-Newman-Keuls to compare all pairs of columns), p values were determined for the respective experiment from three identical experiments using GraphPad Prism 3.0 software (GraphPad Software Inc. San Diego, CA). The criterion for statistical significance was p<0.05.
Results
OL expresses IL-17R
Prior to investigating the direct effect of IL-17 on OL survival, we measured the expression of IL-17R in OLs. With immunocytochemistry, we found that IL-17R is expressed both in OLs (Fig. 1A) and B12 cells (Fig. 1B). Double-immunolabeling revealed that IL-17R is localized in the cell membrane of B12 cells as indicated by its co-localization with the membrane protein, pan-cadherin (Fig. 1B). Western blot analysis also demonstrated the expression of IL-17R in OLs and B12 cells, where its expression was reduced by knockdown of IL-17R expression by siRNA in B12 cells (Fig. 1C). These findings provide evidence that IL-17R is expressed constitutively in OLs, which may render them susceptible to IL-17-mediated cytotoxicity in MS brain lesions. Because both primary OLs and a fully characterized OL-like B12 cells (Roth et al. 2003) express IL-17R, we opted to use B12 cells in some experiments due to difficulties in generating the sufficient number of primary OPCs.
IL-17 and TNF-α exert cytotoxic effects in both OLs and B12 cells
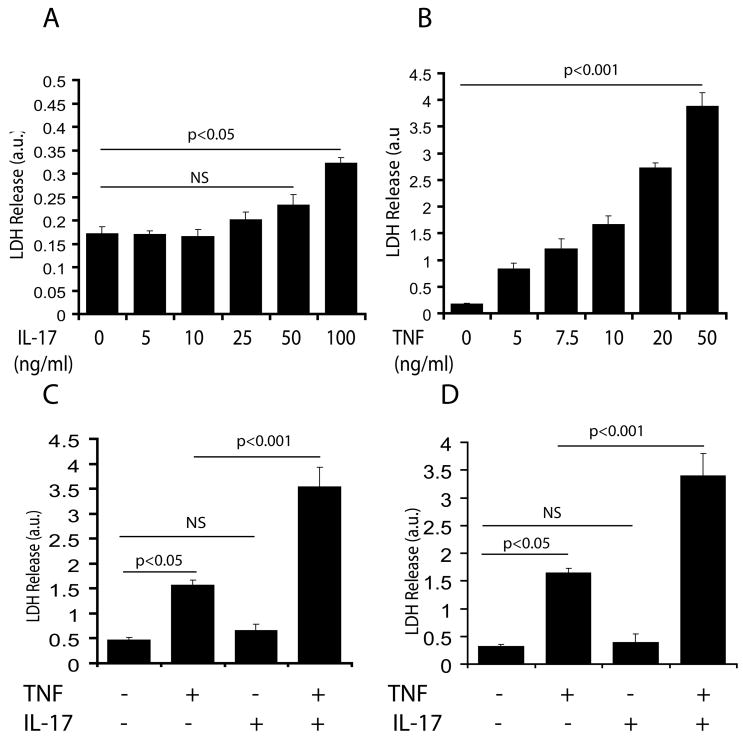
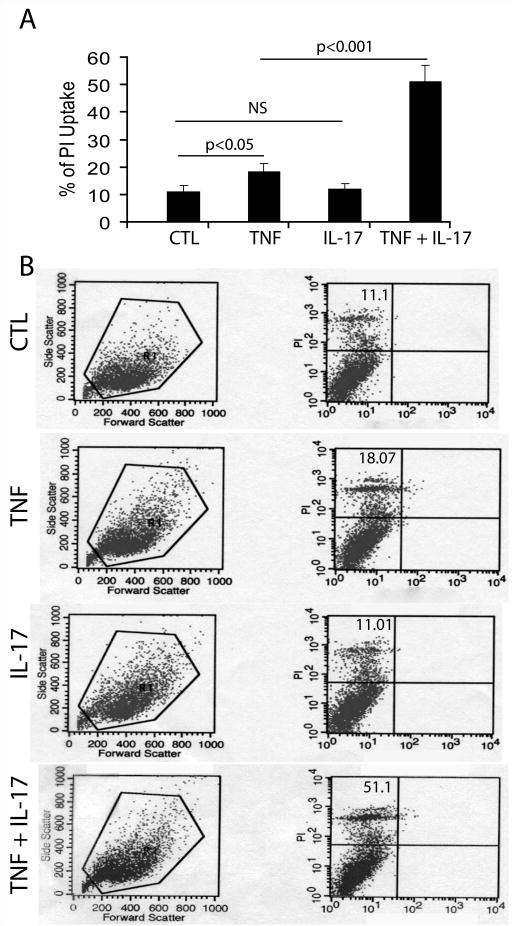
Next, we studied the effect of IL-17 and TNF-α on the survival of cultured OLs and B12 cells (Fig. 1D-E). Fig. 2A, demonstrates that IL-17 induced little or no cytotoxicity in OLs treated with its concentration ranging from 10–50 ng/ml, however, its concentration ≥100 ng/ml was significantly detrimental to OLs as compared to controls. In contrast, TNF-α alone was detrimental to OLs in a dose-dependent manner at concentrations ranging from 5–50 ng/ml (Fig. 2B). Interestingly, combining of these cytokines at their suboptimal concentrations i.e., TNF-α (10 ng/ml) and IL-17 (25 ng/ml) significantly increased cell death of both OLs and B12 cells compared to controls or individual cytokine treatments (Fig. 2C-D). FACS analysis revealed a significant increase (2-fold) in the percentage of propidium iodide (PI)+ve B12 cells upon treatment with TNF-α compared to controls, but this not happen upon treatment with IL-17 alone (Fig. 3A-B). Combining of both cytokines, however demonstrated a significant increase (3-4 fold) in the percentage of PI+ve B12 cells as compared to individual cytokine treatments (Fig. 3A-B). Trypan blue exclusion assay also confirmed these findings (data not shown). These data suggest that a synergy between IL-17 and TNF-α exacerbates the TNF-α induced OL apoptosis.
Figure 2. Synergistic activity of IL-17 and TNF-α induces cell death in OLs/B12 cells.
Cells were cultured in 24-well plates and treated with IL-17 and TNF-α alone or in combination for 48 h as described under materials & methods. Plots depict LDH release in OLs treated with different concentrations of IL-17 (A) and TNF-α (B). Plots depict LDH release in OLs (C) and B12 cells (D) treated with IL-17 (25 ng/ml) and TNF-α (10 ng/ml) in combination or alone. Data in plots are expressed as mean ± SD in triplicate samples from 3 independent experiments. NS; non-significant.
Figure 3. Synergistic activity of IL-17 and TNF-α induces PI uptake.
B12 cells were cultured in 24-well plates and treated with IL-17 (25 ng/ml) and TNF-α (10 ng/ml) in combination or alone for 48 h followed by incubation with PI dye for another 6 h and FACS analysis. Plot depicts the percentage of PI+ve cells in cytokines treated/untreated B12 cells (A). Representative histograms demonstrate the distribution of PI+ve cells in cytokines treated/untreated B12 cells (B). Data in plot are expressed as mean ± SD in duplicate samples from 3 identical experiments. NS: non-significant; CTL: control.
Synergistic activity of IL-17 enhances TNF-α-mediated OL apoptosis
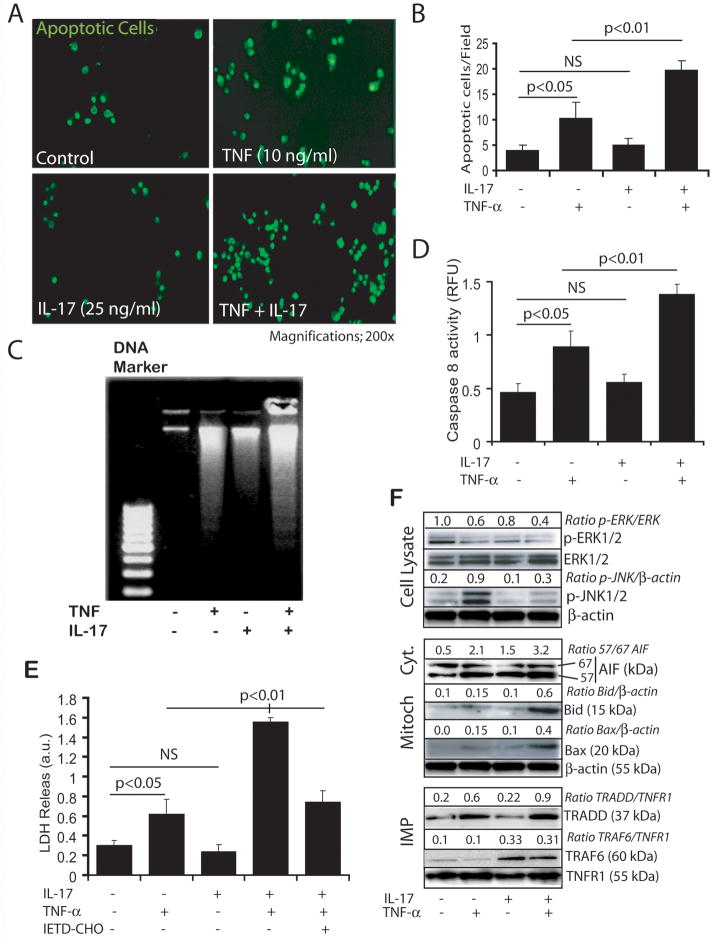
Because TNF-α is reported to induce apoptosis of OLs (Hisahara et al. 1997, Akassoglou et al. 1998, Jurewicz et al. 2003), we investigated whether its combination with IL-17 enhances the internucleosomal degradation of nuclear DNA (a biochemical marker of apoptosis used to quantify cell death) in OLs. As shown in Figs. 4A–B, TUNEL+ve cells were significantly increased in B12 cells upon treatment with TNF-α compared to controls. Moreover, the effect of TNF-α was enhanced (2–4-fold) significantly in the presence of IL-17 as compared to individual cytokine treatments (Fig. 4A-B). These results were confirmed by analysis of internucleosomal DNA cleavage via DNA agarose gel electrophoresis. However, the degradation of nuclear DNA was observed in OLs treated with TNF-α and IL-17 individually (Fig. 4C), but it was more pronounced in OLs treated with both cytokines (Fig. 4C). Accordingly, caspase 8 activity was also increased in dually treated OLs as compared to controls or individual cytokine treatments (Fig. 4D). OL cell death was greater with IL-17 and TNF-α treatment compared to controls or individual cytokine treatments, and this was reversed with simultaneous treatment with cell permeable specific inhibitor of caspase-8 (Fig. 4E), suggesting that caspase 8 activity participates in these cytokines induced OL apoptosis.
Figure 4. Synergistic activity of IL-17 and TNF-α induces OL apoptosis.
Cells were treated with IL-17 (25 ng/ml) and TNF-α (10 ng/ml) in combination or alone. Representative fields of the slides demonstrate TUNEL+ve cells (A) and subsequent plot depicts the count of TUNEL+ve cells (B) in cytokine treated/untreated B12 cells for 48 h. Agarose gel electrophoresis depicts the degradation of intranuclear chromosomal DNA (C) and plots depict caspase-8 activity (D) and cell death (E) in cytokine treated/untreated OLs for 48 h. Western blot analysis depicts change in the level of signaling proteins presented as ratio in cytokine treated OLs for different time points i.e., pERK1/2, ERK1/2, pJNK1/2, immunoprecipitated (IMP) TRADD and TRAF6 using anti-TNFR1 and IL-17R antibodies and TNFR1 in cell lysate at 20 min including AIF (67/57) and Bid/Bax in the cytosol and mitochondrial (mitoch) fractions, respectively, at 48 h (F). Data in plots are expressed as mean ± SD of triplicate samples from 3 independent experiments. NS: non-significant.
Western blot analysis revealed that cytokine-mediated cell death was associated with decreased activation of mitogen activated protein kinase (MAPK)s i.e., ERK1/2 as revealed by their dephosphorylation (∼2.5-fold) in OLs treated with combination of IL-17 and TNF-α as compared to controls or individual cytokine treatments (Fig. 4F). Likewise, the observed TNF-α-induced phosphorylation of JNK1/2 was also inhibited by IL-17 in OLs (Fig. 4F). This inhibition of MAPK activities was associated with mitochondrial dysfunction as indicated by increased AIF release (57 kDa) in the cytoplasm from mitochondria via co-localization of truncated Bid and pore-forming Bax proteins in the mitochondrial membrane (Fig. 4F).
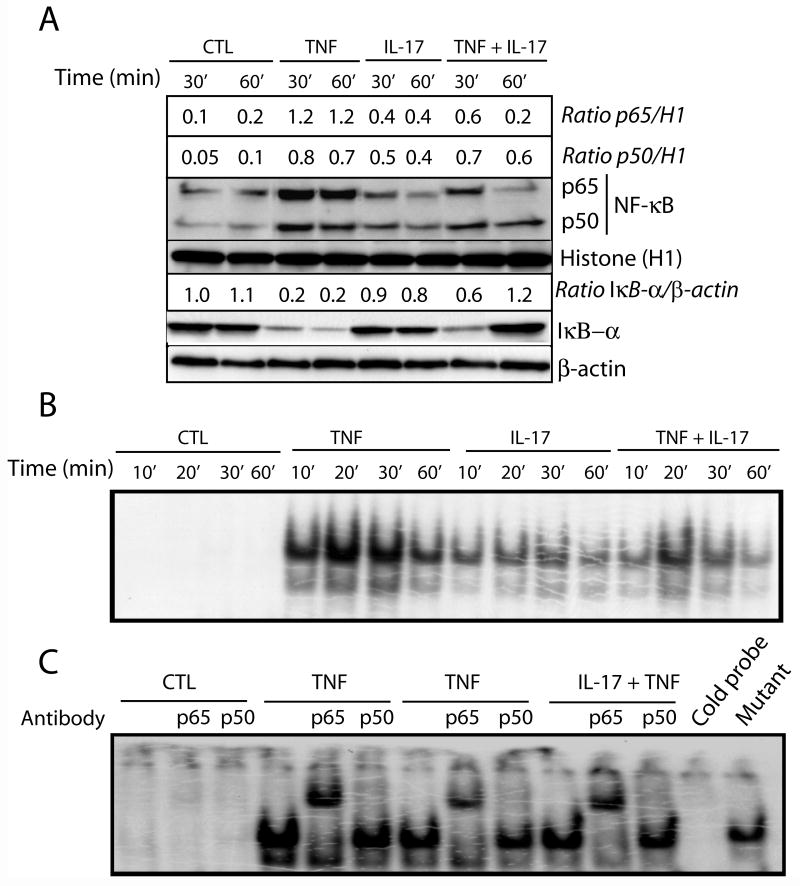
Moreover, TNF-α-induced NF-κB trans-activation in OLs was also decreased in the presence of IL-17, as shown by Western blot and EMSA analyses (Fig. 5). TNF-α-mediated nuclear translocation of NF-κB proteins (p50 and p65 subunits) were inhibited (2–6-fold) in the presence of IL-17 in a time-dependent manner (Fig. 5A). Accordingly, the level of IκB-α (an inhibitory protein of NF-κB trans-activation) was increased (3–6-fold) in treated OLs with both cytokines as compared to treatment with TNF-α alone (Fig. 5A). Of note, TNF-α-induced translocation of the NF-κB p65 subunit was specifically inhibited in OLs (detected 60 min post stimulation) in the presence of IL-17 with the corresponding increase in the level of IκB-α protein in treated OLs (Fig. 5A). Using EMSA, we confirmed these results: TNF-α-induced NF-κB trans-activation was inhibited in the presence of IL-17 in OLs (Fig. 5B) with specific inhibition of the NF-κB p65 subunit as shown by super shift assay (Fig. 5C). Importantly, the synergism of IL-17 and TNF-α in OLs was associated with an induction of a receptor-mediated downstream signaling cascade as revealed by immunoprecipitation of TRADD and TRAF6 proteins along with TNFR1 and IL-17R proteins (Fig. 4F). Collectively, these data suggest that an IL-17 and TNF-α-induced down-stream signaling cascade induces OL apoptosis via cell survival inhibition, caspase-8 activation, and mitochondrial dysfunction.
Figure 5. Synergistic activity of IL-17 and TNF-α inhibits NF-κB trans-activation in OLs.
Cells were treated with IL-17 (25 ng/ml) and TNF-α (10 ng/ml) in combination or alone at different time points. Western blot analysis depicts the change in the level of NF-κB complex (p65/p50) proteins presented as ratio with nuclear histone H1 or cytoplasmic β-actin proteins in cytokine treated OLs (A). EMSA demonstrates the interaction of NF-κB complex proteins with its DNA binding sequence probe in cytokine treated OLs (B). EMSA demonstrates the super shift of NF-κB complex proteins by their interaction with anti-p50 and -p65 antibodies (C). Cold DNA and mutant DNA probes were included as controls. CTL: control.
IL-17 and TNF-α induce reactive oxygen species (ROS) generation thus affecting the mitochondrial membrane potential (ΔΨm) in B12 cells
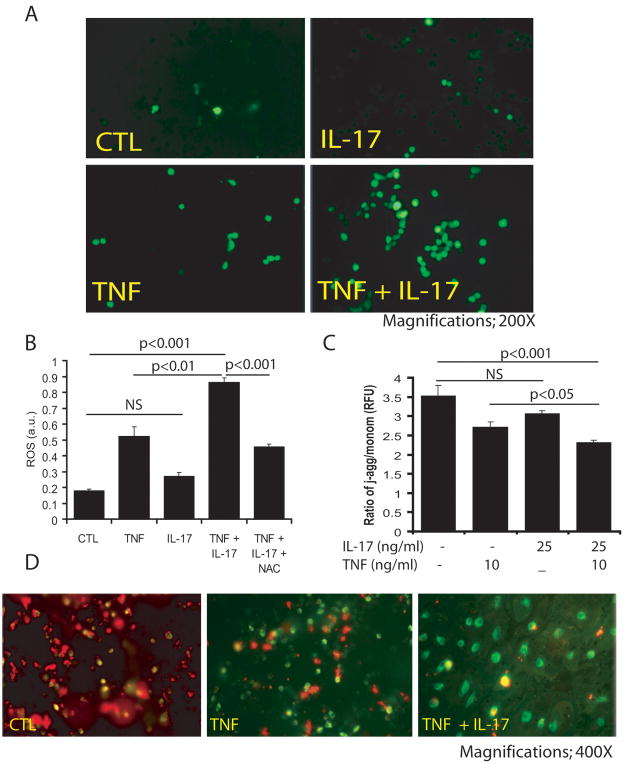
Because OL degeneration is reported to be associated with oxidative stress (Casaccia-Bonnefil 2000, Jana & Pahan 2005), we next investigated whether ROS (oxidative stress) generation is involved in IL-17 and TNF-α-induced OL apoptosis. Cytokine-treated B12 cells were incubated with the fluorescent dye CM-DCFDA, which is oxidized in the presence of cellular oxidative stress. TNF-α treatment produced more CM-DCFDA-labeled cells compared to controls or cells treated with IL-17 alone (Fig. 6A), and the number of labeled cells increased remarkably with concomitant treatment of IL-17 (Fig. 6A). Also, dual treatment was associated with a significant increase in ROS in B12 cells compared to controls or individual cytokine treatments (Fig. 6B). This cytokine-induced ROS generation in B12 cells was blocked with NAC treatment (Fig. 6B), suggesting that oxidative stress is a significant participant in cytokine-mediated OL apoptosis.
Figure 6. Synergistic activity of IL-17 and TNF-α affects MMP thus induces ROS generation in B12 cells.
Cells were treated with IL-17 (25 ng/ml) and TNF-α (10 ng/ml) in combination or alone for 48 h. Representative fields of the slides demonstrate stained cells with fluorescence dye, CM-DCFDA as described under materials & methods (A). Plot depicts the level of ROS in cytokine treated/untreated cells including the presence of 10 mM of NAC (B). Plot depicts the ratio of relative fluorescent units (RFU) of J-aggregates/monomer an indicator of change in mitochondrial membrane potential (C) and representative images of the slides demonstrate J-aggregate (red; healthy cells) and monomer (dead cells) (D) in cytokine treated/untreated cells analyzed by JC-1 dye. NS: non-significant; CTL: control.
To investigate whether cytokine-mediated ROS generation affects the mitochondrial membrane potential (MMP) in OLs, we labeled B12 cells with JC-1 dye (lipophilic cation), because JC-1 dye uptake by mitochondria is in proportion to the MMP. Corresponding to the observed loss of B12 cells (Fig. 2D), IL-17 and TNF-α treatment significantly reduced the MMP in treated cells compared to controls or individual cytokine treatments as revealed by a decrease in the ratio of j-aggregates and monomers (Fig. 6C). Similarly, JC-1 dye staining revealed fewer cells and less red J-aggregate fluorescence in treated B12 cells (Fig. 6D), suggesting that cytokine treatment generates ROS which impairs MMP in OLs, leading to their apoptotic cell death.
IL-17 and TNF-α induce cell cycle arrest in proliferating B12 cells
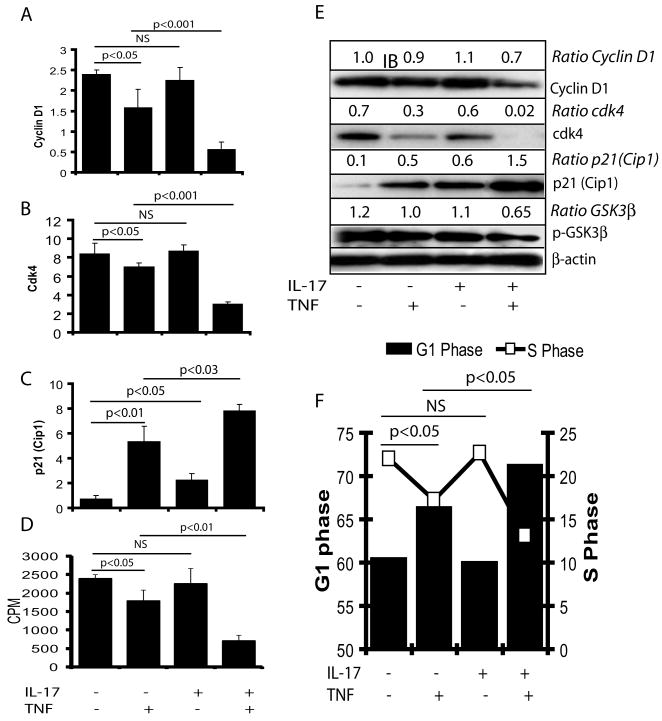
Because TNF-α is known to inhibit the expression of cell survival proteins and induce apoptosis in tumor cells (Takada et al. 2004), we next explored whether the synergistic activity of IL-17 and TNF-α can affect OPC cell cycle progression. Thus, we measured the expression of cyclin D1 and cell cycle regulatory proteins in cytokine treated proliferating B12 cells. Interestingly, IL-17 plus TNF-α significantly inhibited the expression of cyclin D1 and cdk4 proteins in B12 cells compared with controls or individual cytokine treatments (Fig. 7A-B). Reciprocally, p21Cip1 expression was increased significantly in B12 cells treated with both IL-17 and TNF-α compared to controls or individual cytokine treatments (Fig. 7C). Densitometeric analysis of Western blots corroborated these cytokine-induced changes in the levels of cyclin D1, cdk4, and p21Cip1 proteins in treated B12 cells (Fig. 7D). In addition, glycogen synthase kinase (GSK)-3β phosphorylation was also reduced with the corresponding decrease in (3H) thymidine incorporation in DNA of B12 cells treated with IL-17 and TNF-α compared to controls or individual cytokine treatments (Fig. 7D-E). FACS analysis revealed a significant decrease in the percentage of cells at G1/S phases in B12 cells treated with IL-17 plus TNF-α compared to controls or individual cytokine treatments (Fig. 7E). Of note, TNF-α alone significantly increased cell cycle proteins in treated B12 cells compared to controls or those treated with IL-17 alone, but cell cycle proteins were even greater with combined treatment of cytokines (Fig. 7E). Together, these data suggest that synergistic activities of IL-17 and TNF-α may affect the maturation of OPCs via inhibition of cell cycle progression.
Figure 7. Synergistic activity of IL-17 and TNF-α inhibits cell cycle progression in B12 cells.
Cells were cultured in 24-well plates for 24 hr followed by treatment with IL-17 (25 ng/ml) and TNF-α (10 ng/ml) in combination or alone. QRT-PCR analysis demonstrates the levels of cyclin D1 (A), cdk4 (B) and p21Cip1 (C) transcripts in cytokine treated/untreated cells for 12 h. Plot depicts the incorporation of (3H) thymidine in DNA of similarly treated cells for 24 h (D). Western blot analysis depicts change in the level of cell cycle proteins presented as ratio with β-actin in treated cells for 48 h (E). FACS analysis depicts the percentage of cells at G1/S phases in cytokine treated/untreated cells for 48 h (F). Data in plot are expressed as mean ± SD of triplicate samples from three independent experiments. NS: non-significant.
PPAR agonists protect OPCs against IL-17 and TNF-α-induced cytotoxicity
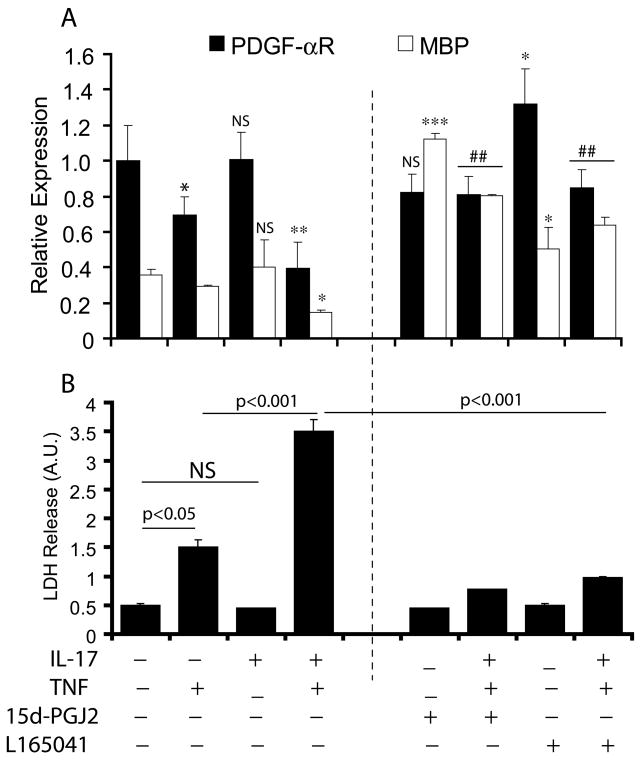
Our data provide evidence that synergistic activities of IL-17 and TNF-α induce ROS generation which subsequently affects MMP in OLs leading to apoptosis and cell cycle arrest in proliferating B12 cells. Previously, agonists of PPAR isotypes i.e., PPAR-γ/-β, have been reported to induce anti-oxidant mechanisms in OLs (Cimini et al. 2003, Bernardo et al. 2009). Therefore, we next examined the potential of PPAR agonists to prevent IL-17 and TNF-α-induced cytotoxicity in OLs and OPCs. As expected, TNF-α decreased the levels of PDGF-αR and MBP transcripts in treated OPCs indicating a decrease in the number of proliferating OPCs and maturing OLs (Fig. 8A). This effect of TNF-α was significant (2-fold) in the presence of IL-17 in treated OPCs compared to individual cytokine treatments (Fig. 8A). Conversely, co-treatment of OPCs with 15d-PGJ2 (PPAR-γ agonist) and L165041 (PPAR-δ agonist) reversed these cytokine induced effects and significantly increased the levels of MBP transcripts compared to untreated OPCs (Fig. 8A). Correspondingly, cytokine-induced cell death in OPCs was also attenuated by co-treatment with agonists of PPAR-γ/-δ as revealed by LDH release assay (Fig. 8B). As expected, OPC cultures treated individually with 15d-PGJ2 and L165041 demonstrated increased levels of MBP transcripts compared to controls, suggesting that PPAR agonists promote the maturation of OPCs into myelin-forming OLs (Fig. 8A). Together, these data imply that PPAR-γ/-δ agonists can protect OLs/OPCs against this cytokine-mediated cytotoxicity.
Figure 8. Agonists of PPAR (γ/δ) attenuate the effect of IL-17 and TNF-α in maturing OPCs.
OPCs were cultured in 24-well plates for 48 hr and then treated with IL-17 (25 ng/ml) and TNF-α (10 ng/ml) in combination or alone in the presence of PPAR-γ agonist, 15d-PGJ2 (5 μM) and PPAR-δ agonist, L145641 (2.5 μM) for another 72 h. Plot depicts the levels of PDGF-αR and MBP transcripts in treated/untreated OPC cultures (A). Plot depicts the release of LDH in the supernatant of treated/untreated OPC cultures (B). Data in plots are expressed as mean ± SD of triplicate samples from 3 independent experiments. Statistical significance was set at *p<0.05 and **p<0.01 versus CTL (A).
Discussion
CNS demyelinating diseases such as MS are initiated by the infiltration of autoreactive Th1 cells into the CNS, activation of resident glial cells, and secretion of pro-inflammatory cytokines such as IFN-γ, IL-1β, and TNF-α. These cytokines cause the loss of OLs and neuronal axons which give rise to subsequent neurological disabilities in affected individuals (Prineas et al. 1993). Recently, Th17 phenotype cytokines (i.e., IL-17) have been implicated in the development of MS (Bettelli et al. 2007, McKenzie et al. 2006, Weaver et al. 2007). Therefore, we investigated the effect of IL-17 alone on OLs as well its contribution to TNF-α-mediated death of OLs. Our findings demonstrate that IL-17-mediated cellular signaling synergizes with TNF-α-induced cell death signaling cascades in OLs (Fig. 9) suggesting that Th1 and Th17 cytokines participate in MS pathogenesis. Our conclusions are based on the observed increase in cytokine-mediated OL cell death as revealed by an increase in PI+ve cells, TUNEL+ve nuclei, DNA laddering, AIF release and the activation of caspase-8 in cytokine treated OLs. In addition, we noted cytokine-mediated i) inhibition of the activities of NF-κB and MAPKs i.e., ERK1/2 and JNK1/2 including dephosphorylation of GSK-3β, ii) induction of oxidative stress that was attenuated by NAC and mitochondrial dysfunction due to change in MMP in OLs, and iii) inhibition of cell cycle progression in OLs and the maturation of OPCs, which was blocked by treatment with PPAR-γ/-β agonists.
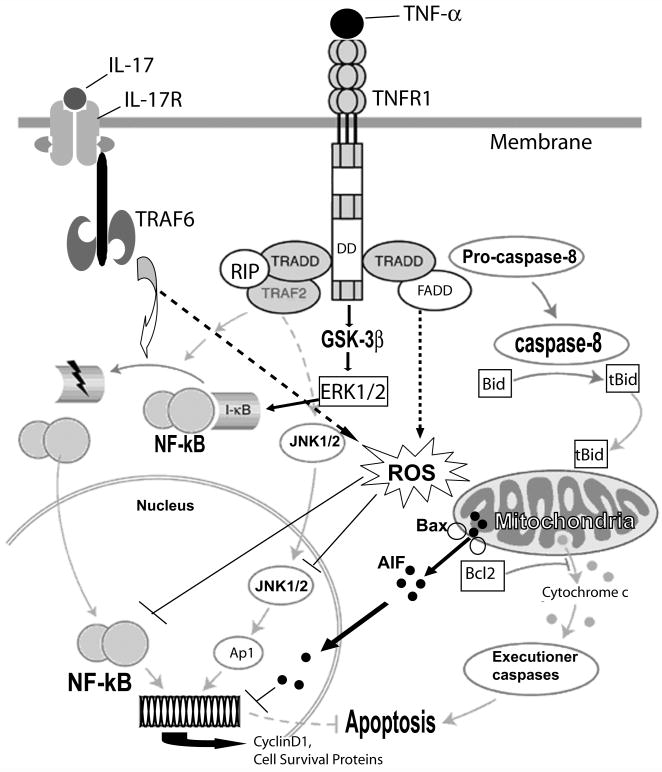
Figure 9. Schematic diagram illustrates IL-17 and TNF-α induced cell death mechanism in OLs.
Interaction between IL-17/IL-17R activates TRAF6, which in turn induces NF-κB trans-activation to induce survival mechanisms in OLs. Likewise, TNF-α/TNFR1 interactions trigger TRADD mediated activation of JNK1/2 and NF-κB trans-activation to induce survival mechanisms in OLs. In contrast, TNF-α induced TRADD/FADD signaling activates caspase 8 via localization of truncated Bid (t-Bid) and pore forming Bax in the mitochondrial membrane, which leads to the release of AIF from mitochondria and subsequent degradation of nuclear DNA in apoptotic OLs. Synergism between TNF-α and IL-17 induces mitochondrial dysfunction and apoptotic cell death of OLs ascribed to the generation of ROS, inhibition of cell survival mechanisms (activation of MAPKs and NF-kB) and cell cycle progression. DD: death domain; ROS: reactive oxygen species.
Up-regulated expression of TNF-α and TNFR1 has been documented in active MS lesions (Hofman et al. 1989, Selmaj et al. 1991a, Bonetti & Raine 1997) and the presence of TNF-α in the cerebrospinal fluid of MS patients is reported to correlate with disease severity (Sharief & Hentges 1991). In vitro studies established that TNF-α/TNFR1 signaling mechanisms are involved in OL apoptosis (Selmaj & Raine 1988, Hisahara et al. 1997, Akassoglou et al. 1998). As described in Fig. 9, the outcome of TNF-α/TNFR-1 interaction depends on the activation of different signaling transduction pathways i.e., the caspase cascade, NF-kB trans-activation, and MAPKs activation. TNFR1 activation results in the recruitment of TRADD (Hsu et al. 1995), which subsequently interacts with TRAF2 or with Fas-associated death domain protein (FADD) (Hsu et al. 1996). TRAF2 mediates NF-κB trans-activation, while FADD interacts with procaspase 8 and produce activated caspase 8 (Boldin et al. 1996). Similarly, IL-17/IL-17R interactions employ TRAF6 to transduce its signal in immune cells (Chang & Dong 2009). TNF-α-mediated activation of JNK1/2 has been implicated in cell death signaling and studies suggest that NF-κB activation protects cells from apoptosis via inhibition of JNK1/2 (De Smaele et al. 2001, Tang et al. 2001). Moreover, IL-17 has been reported to transduce its signaling in immune cells via activation of JNK1/2 (Iyoda et al. 2010). Consistent with these reports, the observed increase in apoptosis of OLs with TNF-α and IL-17 combination treatment favors ligand/receptor interactions i.e., TNF-α/TNFR1 and IL-17/IL-17R as demonstrated by recruitment of TRADD with TNFR1 and TRAF6 with IL-17R in OLs (Fig. 4F). Moreover, IL-17R is expressed constitutively in OLs (Fig. 1), providing evidence that synergy between IL-17 and TNF-α signaling participates in OL apoptosis, which is associated with ligand/receptor interaction-mediated activation of the down-stream signaling cascades. TNF-α/TNFR1-induced NF-κB trans-activation is reported to induce survival pathways in cancer cells (Jackson-Bernitsas et al. 2007). In addition, TNF-α has been reported to induce cell survival mechanisms via GSK-3β (a ubiquitously active kinase involved in the cell survival)-mediated activation of ERK1/2 with the induction of NF-κB trans-activation and expression of cyclin D1 in tumor cells (Takada et al. 2004). Moreover, IL-17-mediated activation of NF-κB in T cells is reported to be involved in the pathogenesis of autoimmune diseases including MS (Brown et al. 2008). Interestingly, the observed synergy between IL-17/TNF-α signaling inhibited NF-κB trans-activation and ERK1/2 activation indicating the inhibition of cell survival mechanisms in OLs. Importantly, it was associated with TNFR1/FADD-mediated caspase 8 activation and mitochondrial dysfunction (Fig. 4). In addition, TNF-α-mediated activation of JNK1/2 favoring cell death was inhibited in OLs in the presence of IL-17, suggesting that synergistic effects of TNF-α/IL-17 in OLs are independent of JNK1/2 activation. Moreover, the role of JNK1/2 in TNF-α-induced cell death is still obscure; earlier, the activation of JNK3 has been demonstrated to be involved in TNF-α-induced OL cell death, but not the activation of JNK1/2 (Jurewicz et al. 2003).
Our findings demonstrated that TNF-α and IL-17 induced synergistic activities cause ROS generation and impair MMP in OLs, which was attenuated by treatment with NAC (Fig. 6B) suggesting that cytokine-induced oxidative-stress participates in OL apoptosis. Pro-inflammatory cytokines are reported to transduce cellular signaling through ROS (as second messenger) under disease conditions (Koch et al. 2006) and ROS play a major role in MS pathogenesis as mediators of CNS demyelination and axonal damage (Gilgun-Sherki et al. 2004). Moreover, ROS are potentially toxic to neurons and OLs as they are known to damage lipids, proteins, and nucleic acids. Particularly, OLs are more sensitive to oxidative stress than other brain cells, possibly due to a diminished capacity for antioxidant defense, and the presence of raised risk factors i.e., high iron content (Smith et al. 1999). In addition, IL-1β and TNF-α-induced ROS generation is reported to down-regulate the expression of myelin proteins in primary OLs (Jana & Pahan 2005). IL-17-induced ROS generation in endothelial cells have been implicated in the compromised blood brain barrier integrity in MS model (Huppert et al. 2009). ROS are reported to induce apoptosis or permanent growth arrest in normal cells ascribed to the loss of MMP, DNA damage (Qanungo et al. 2005) and the accumulation of p21Cip1 protein with subsequent cell cycle arrest at the G1 and S phases (Kopnin et al. 2004). Accordingly, we observed IL-17 and TNF-α-mediated apoptosis of B12 cells, which was accompanied with accumulation of p21Cip1 with corresponding reduction of cyclin D1 and cdk4 proteins (Fig. 7). This was associated with AIF release from mitochondria via co-localization of truncated Bid and pore-forming Bax proteins in the mitochondrial membrane of OLs (Fig. 4F). These findings provide evidence that IL-17 and TNF-α-induced oxidative stress inhibits the cell survival mechanisms and cell cycle progression in OPCs/OLs leading to their apoptotic cell death.
PPARs are a group of nuclear receptor proteins that function as transcription factor to regulate the expression of genes essential to cellular differentiation, development, and metabolism. Previously, we documented that IL-4-mediated activation of PPAR-γ in glial cells protects OLs against inflammatory insult (Paintlia et al. 2006). Also, agonists of PPAR-γ/-β are reported to enhance the maturation of OLs/ B12 cells in vitro (Roth et al. 2003, Saluja et al. 2001). In addition, we recently documented that activation of PPAR-γ participates in the differentiation of OPCs into myelin-forming OLs treated with lovastatin (Paintlia et al. 2010). In contrast, TNF-α has been reported to down-regulate the expression of PPAR-β in OLs (Cimini et al. 2003). Likewise, IL-17 is reported to inhibit PPAR-γ expression in non-neural cell types (Afif et al. 2007). These findings indicate that IL-17 plus TNF-α mediated down-regulation of PPAR-γ/-β in OLs may be involved in their apoptotic cell death. In support to these, we observed that both agonists of PPAR-γ/-β attenuated the observed IL-17 and TNF-α induced loss of OPCs/OLs in OPC cultures (Fig. 8). These protective effects of PPAR-γ/-β agonists in OPCs could be ascribed to their enhanced antioxidant defenses and maintenance of PPAR homeostasis in OLs as demonstrated by enhanced antioxidant defenses in PPAR agonist-treated OLs via maintenance of glutathione homeostasis (Bernardo et al. 2009). Moreover, the observed protection of OLs against cytotoxic effects of IL-17/TNF-α by NAC and agonists of PPAR-β/-γ are consistent with previous studies demonstrating the therapeutic efficacies of NAC in periventricular leukomalacia model (Paintlia et al. 2004) and PPAR agonists in MS model (Diab et al. 2002, Polak et al. 2005).
In summary, this is first report to document that synergy between IL-17 and TNF-α induces OL apoptosis via generation of ROS, mitochondrial dysfunction and inhibition of cell cycle progression. The observed protection of OLs/OPCs against this cytokine-mediated cytotoxicity by NAC and PPAR-γ/-β agonists suggesting that, in addition to their immunomodulatory activities in MS models (Diab et al. 2002, Polak et al. 2005, Stanislaus et al. 2005), these agents could possibly limit CNS demyelination and reduce neurological disabilities in MS patients.
Acknowledgments
We thank Dr. Jennifer G. Schnellmann for critical reading of this manuscript and Ms. Joyce Bryan and Ms. Carrie Barnes for their technical assistance. This study was supported by grants from the NIH (NS-22576, NS-34741, NS-37766, C06-RR015455, and C06-RR018823).
References
- Afif H, Benderdour M, Mfuna-Endam L, Martel-Pelletier J, Pelletier JP, Duval N, Fahmi H. Peroxisome proliferator-activated receptor gamma1 expression is diminished in human osteoarthritic cartilage and is downregulated by interleukin-1beta in articular chondrocytes. Arthritis Res Ther. 2007;9:R31. doi: 10.1186/ar2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998;153:801–813. doi: 10.1016/S0002-9440(10)65622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Bianchi D, Magnaghi V, Minghetti L. Peroxisome proliferator-activated receptor-gamma agonists promote differentiation and antioxidant defenses of oligodendrocyte progenitor cells. J Neuropathol Exp Neurol. 2009;68:797–808. doi: 10.1097/NEN.0b013e3181aba2c1. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Bonetti B, Raine CS. Multiple sclerosis: oligodendrocytes display cell death-related molecules in situ but do not undergo apoptosis. Ann Neurol. 1997;42:74–84. doi: 10.1002/ana.410420113. [DOI] [PubMed] [Google Scholar]
- Brown KD, Claudio E, Siebenlist U. The roles of the classical and alternative nuclear factor-kappaB pathways: potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res Ther. 2008;10:212. doi: 10.1186/ar2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon FH, von Bernhardi R, De Ferrari G, Luza S, Aldunate R, Inestrosa NC. Toxic effects of acetylcholinesterase on neuronal and glial-like cells in vitro. Mol Psychiatry. 1998;3:247–255. doi: 10.1038/sj.mp.4000383. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P. Cell death in the oligodendrocyte lineage: a molecular perspective of life/death decisions in development and disease. Glia. 2000;29:124–135. doi: 10.1002/(sici)1098-1136(20000115)29:2<124::aid-glia5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine. 2009;46:7–11. doi: 10.1016/j.cyto.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini A, Bernardo A, Cifone MG, Di Marzio L, Di Loreto S. TNFalpha downregulates PPARdelta expression in oligodendrocyte progenitor cells: implications for demyelinating diseases. Glia. 2003;41:3–14. doi: 10.1002/glia.10143. [DOI] [PubMed] [Google Scholar]
- D'Souza S, Alinauskas K, McCrea E, Goodyer C, Antel JP. Differential susceptibility of human CNS-derived cell populations to TNF-dependent and independent immune-mediated injury. J Neurosci. 1995;15:7293–7300. doi: 10.1523/JNEUROSCI.15-11-07293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251:261–268. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24:479–487. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq E, Giri S, Singh I, Singh AK. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J Neurochem. 2003;86:1428–1440. doi: 10.1046/j.1471-4159.2003.01941.x. [DOI] [PubMed] [Google Scholar]
- Hisahara S, Shoji S, Okano H, Miura M. ICE/CED-3 family executes oligodendrocyte apoptosis by tumor necrosis factor. J Neurochem. 1997;69:10–20. doi: 10.1046/j.1471-4159.1997.69010010.x. [DOI] [PubMed] [Google Scholar]
- Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170:607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert J, Closhen D, Croxford A, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2009 doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- Iyoda M, Shibata T, Kawaguchi M, Hizawa N, Yamaoka T, Kokubu F, Akizawa T. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-alpha and IL-1beta. Am J Physiol Renal Physiol. 2010;298:F779–787. doi: 10.1152/ajprenal.00198.2009. [DOI] [PubMed] [Google Scholar]
- Jackson-Bernitsas DG, Ichikawa H, Takada Y, Myers JN, Lin XL, Darnay BG, Chaturvedi MM, Aggarwal BB. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-kappaB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene. 2007;26:1385–1397. doi: 10.1038/sj.onc.1209945. [DOI] [PubMed] [Google Scholar]
- Jana M, Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol Med. 2005;39:823–831. doi: 10.1016/j.freeradbiomed.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery ND, Blakemore WF. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain. 1997;120(Pt 1):27–37. doi: 10.1093/brain/120.1.27. [DOI] [PubMed] [Google Scholar]
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Tybor K, Selmaj K. TNF-induced death of adult human oligodendrocytes is mediated by c-jun NH2-terminal kinase-3. Brain. 2003;126:1358–1370. doi: 10.1093/brain/awg146. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Ramsaransing GS, Arutjunyan AV, Stepanov M, Teelken A, Heersema DJ, De Keyser J. Oxidative stress in serum and peripheral blood leukocytes in patients with different disease courses of multiple sclerosis. J Neurol. 2006;253:483–487. doi: 10.1007/s00415-005-0037-3. [DOI] [PubMed] [Google Scholar]
- Kopnin PB, Kravchenko IV, Furalyov VA, Pylev LN, Kopnin BP. Cell type-specific effects of asbestos on intracellular ROS levels, DNA oxidation and G1 cell cycle checkpoint. Oncogene. 2004;23:8834–8840. doi: 10.1038/sj.onc.1208108. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- Nath N, Giri S, Prasad R, Singh AK, Singh I. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol. 2004;172:1273–1286. doi: 10.4049/jimmunol.172.2.1273. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, Lassmann H. Patterns of oligodendroglia pathology in multiple sclerosis. Brain. 1994;117(Pt 6):1311–1322. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- Paintlia AS, Paintlia MK, Khan M, Vollmer T, Singh AK, Singh I. HMG-CoA reductase inhibitor augments survival and differentiation of oligodendrocyte progenitors in animal model of multiple sclerosis. Faseb J. 2005;19:1407–1421. doi: 10.1096/fj.05-3861com. [DOI] [PubMed] [Google Scholar]
- Paintlia AS, Paintlia MK, Singh AK, Orak JK, Singh I. Activation of PPAR-gamma and PTEN cascade participates in lovastatin-mediated accelerated differentiation of oligodendrocyte progenitor cells. Glia. 2010;58:1669–1685. doi: 10.1002/glia.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintlia AS, Paintlia MK, Singh AK, Singh I. Inhibition of rho family functions by lovastatin promotes myelin repair in ameliorating experimental autoimmune encephalomyelitis. Mol Pharmacol. 2008a;73:1381–1393. doi: 10.1124/mol.107.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintlia AS, Paintlia MK, Singh I, Singh AK. IL-4-induced peroxisome proliferator-activated receptor gamma activation inhibits NF-kappaB trans activation in central nervous system (CNS) glial cells and protects oligodendrocyte progenitors under neuroinflammatory disease conditions: implication for CNS-demyelinating diseases. J Immunol. 2006;176:4385–4398. doi: 10.4049/jimmunol.176.7.4385. [DOI] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK. N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res. 2004;78:347–361. doi: 10.1002/jnr.20261. [DOI] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Khan M, Singh I, Singh AK. Modulation of peroxisome proliferator-activated receptor-alpha activity by N-acetyl cysteine attenuates inhibition of oligodendrocyte development in lipopolysaccharide stimulated mixed glial cultures. J Neurochem. 2008b;105:956–970. doi: 10.1111/j.1471-4159.2007.05199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak PE, Kalinin S, Dello Russo C, et al. Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;168:65–75. doi: 10.1016/j.jneuroim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Prasad R, Giri S, Nath N, Singh I, Singh AK. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside attenuates experimental autoimmune encephalomyelitis via modulation of endothelial-monocyte interaction. J Neurosci Res. 2006;84:614–625. doi: 10.1002/jnr.20953. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26:958–967. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280:39582–39593. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- Roth AD, Leisewitz AV, Jung JE, Cassina P, Barbeito L, Inestrosa NC, Bronfman M. PPAR gamma activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J Neurosci Res. 2003;72:425–435. doi: 10.1002/jnr.10596. [DOI] [PubMed] [Google Scholar]
- Saluja I, Granneman JG, Skoff RP. PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia. 2001;33:191–204. [PubMed] [Google Scholar]
- Selmaj K, Raine CS, Cannella B, Brosnan CF. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991a;87:949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K, Raine CS, Farooq M, Norton WT, Brosnan CF. Cytokine cytotoxicity against oligodendrocytes. Apoptosis induced by lymphotoxin. J Immunol. 1991b;147:1522–1529. [PubMed] [Google Scholar]
- Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Stanislaus R, Gilg AG, Singh AK, Singh I. N-acetyl-L-cysteine ameliorates the inflammatory disease process in experimental autoimmune encephalomyelitis in Lewis rats. J Autoimmune Dis. 2005;2:4. doi: 10.1186/1740-2557-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]