Upregulation of the Catalytic Telomerase Subunit by the Transcription Factor ER81 and Oncogenic HER2/Neu, Ras, or Raf (original) (raw)
Abstract
One hallmark of tumor formation is the transcriptional upregulation of human telomerase reverse transcriptase, hTERT, and the resultant induction of telomerase activity. However, little is presently understood about how hTERT is differentially activated in tumor cells versus normal somatic cells. Specifically, it is unclear if oncoproteins can directly elicit hTERT expression. To this end, we now show that three oncoproteins, HER2/Neu, Ras, and Raf, stimulate hTERT promoter activity via the ETS transcription factor ER81 and ERK mitogen-activated protein (MAP) kinases. Mutating ER81 binding sites in the hTERT promoter or suppression of ERK MAP kinase-dependent phosphorylation of ER81 rendered the hTERT promoter unresponsive to HER2/Neu. Further, expression of dominant-negative ER81 or inhibition of HER2/Neu significantly attenuated telomerase activity in HER2/Neu-overexpressing SKBR3 breast cancer cells. Moreover, HER2/Neu, Ras, and Raf collaborated with ER81 to enhance endogenous hTERT gene transcription and telomerase activity in _hTERT_-negative, nonimmortalized BJ foreskin fibroblasts. Accordingly, hTERT expression was increased in HER2/Neu-positive breast tumors and breast tumor cell lines relative to their HER2/Neu-negative counterparts. Collectively, our data elucidated a mechanism whereby three prominent oncoproteins, HER2/Neu, Ras, and Raf, may facilitate tumor formation by inducing hTERT expression in nonimmortalized cells via the transcription factor ER81.
Telomeres are positioned at the ends of linear chromosomes, where they prevent chromosome ends from being recognized as double-strand breaks and preclude detrimental chromosomal recombination events from taking place (6, 28). In the absence of a mechanism to elongate telomeric DNA, telomeres shorten as cells proliferate, due to incomplete DNA replication at chromosomal ends. Critical shortening of one or more telomeres compromises cell survival and can trigger cellular senescence. As such, proliferating germ line, stem, and tumor cells often circumvent cell senescence by employing the enzyme telomerase to maintain telomere length.
Telomerase, a ribonucleoprotein complex that catalyzes the addition of hexameric repeats to telomeres, is comprised of an integral RNA moiety, a catalytic subunit with reverse transcriptase activity (human telomerase reverse transcriptase [hTERT]), and telomerase-associated proteins (53). Most normal somatic cells do not display telomerase activity, whereas telomerase activity is detected in the vast majority of tumor cells (31, 40), a difference largely attributed to the unique ability of tumor cells to upregulate hTERT transcription (2, 49). Unfortunately, little is known as to how tumor cells activate hTERT transcription. Indeed, even the role of a proto-oncoprotein implicated in the induction of hTERT expression, the E-box binding protein c-Myc (23, 56), has been called into question by recent reports demonstrating that c-Myc regulates hTERT transcription in a cell-type-specific manner (21), and E-box-dependent regulation of the hTERT promoter can occur independent of c-Myc (22, 34). Thus, there remains a tremendous need for further information pertaining to hTERT transcription. In particular, the role oncoproteins play in hTERT activation during cell transformation and immortalization merits special attention.
HER2/Neu is a highly characterized oncoprotein heavily implicated in tumorigenesis. As a receptor tyrosine kinase related to the epidermal growth factor receptor, HER2/Neu mediates tumor formation in the breast, ovary, lung, stomach, colon, kidney, bladder, and salivary gland. Significantly, HER2/Neu overexpression accounts for 20 to 30% of human breast tumors and adversely affects prognosis (36, 64). Additionally, mice engineered to express enhanced HER2/Neu levels in mammary tissue readily develop breast tumors (35). Accordingly, HER2/Neu has become a prominent target of drugs designed to combat breast cancer (66). Indeed, a humanized monoclonal anti-HER2/Neu antibody, trastuzumab (Herceptin), was recently approved for the treatment of HER2/Neu-overexpressing advanced breast cancers (47). Moreover, low-molecular-weight drugs that inhibit the enzymatic activity of HER2/Neu have shown promise as tumor therapeutics (51).
Ras, a downstream effector of HER2/Neu, is a prominent oncoprotein that is inappropriately active in excess of 30% of all human tumors (1, 20). Further, Raf, a downstream target of Ras, has recently been shown to play a significant role in human cancer formation (15). Therefore, like HER2/Neu, Ras and Raf currently represent attractive targets in cancer therapy (52).
Downstream of the HER2/Neu→Ras→Raf signaling cascade are the ERK mitogen-activated protein (MAP) kinases, which induce the phosphorylation and resultant activation of many transcription factors (13). Several reports have indicated that one such transcription factor, the ETS protein ER81, plays a critical role in HER2/Neu-mediated tumorigenesis. For instance, ER81 is activated by HER2/Neu, Ras, and Raf via MAP kinase pathways (8, 37). Furthermore, ER81 is readily expressed in human breast tumor specimens and a subset of breast cancer cell lines (3, 8), and transcription of the ER81 gene is enhanced in mammary tumors of HER2/Neu transgenic mice, where ER81 likely facilitates transcription of the HER2/Neu gene (9, 58). As such, we investigated the possibility that oncogenic HER2/Neu, Ras, and Raf induce hTERT transcription in concert with ER81.
MATERIALS AND METHODS
Transfection and luciferase assays.
Cells grown in 6-cm-diameter dishes were transiently transfected by the calcium phosphate coprecipitation method, except for BJ and SKBR3 cells, for which Lipofectamine 2000 reagent (Invitrogen) was used. At 36 h posttransfection, luciferase activity was determined as described previously (10). The amount of plasmid used in calcium phosphate transfections was as follows: HER2/Neu-V664E (250 ng), ER81 (100 ng), ER81334-477 (100 ng), Sap1a (100 ng), Elk1 (100 ng), Elf1 (100 ng), ER71 (100 ng), luciferase reporter (1 μg), BXB (250 ng), Ras-G12V (25 ng). Two micrograms of each expression plasmid (HER2/Neu, ER81, Ets1, Ets2, and ER81334-477) was utilized in lipofection of BJ and SKBR3 cells. Protein kinase inhibitors used were applied 24 and 12 h prior to the harvest of lysates at final concentrations of 10 μM (U0126) or 5 μM (SB202190 and AG825); in control experiments, an equal volume of the solvent dimethyl sulfoxide (DMSO) was added to the cell medium.
Protein isolation, Western blotting, and electrophoretic mobility shift assays.
Cell lysates were obtained from transiently transfected 293T cells as described elsewhere (55). Purified ER81249-477 was obtained with the IMPACT-CN system (New England Biolabs) as described previously (8). To detect expression of HER2/Neu, ER81, ER81334-477, Ets1, and Ets2, cell lysates were subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and subsequent Western blotting according to standard procedures. HER2/Neu was detected using the monoclonal mouse antibody PN2A (NeoMarkers), ER81 and ER81334-477 were detected using the goat polyclonal antibody sc-1953 (Santa Cruz Biotechnology), Ets1 was detected using the rabbit polyclonal antibody sc-111 (Santa Cruz Biotechnology), and Ets2 was detected using the rabbit polyclonal antibody sc-351 (Santa Cruz Biotechnology). For electrophoretic mobility shift assays, lysates containing approximately 2 μg of total protein or 1 μg of purified ER81249-477 were used as previously described (8).
Isolation of RNA and RT-PCR.
To isolate cytoplasmic RNA, BJ cells were incubated for 5 min in 375 μl of 50 mM Tris-HCl (pH 8), 100 mM NaCl, 5 mM MgCl2, 0.5% Nonidet-P40. Lysates were centrifuged for 2 min at 20,800 × g followed by the addition of 4 μl of 20% sodium dodecyl sulfate and 50 μg of proteinase K and incubation at 37°C for 15 min. This was followed by an extraction using phenol-chloroform-isoamyl alcohol, and RNA was precipitated with 40 μl of 3 M sodium acetate (pH 5.2) and 1 ml of ethanol. After washing in 75% ethanol and drying, the RNA was resuspended in 100 μl of water. RNA from breast tissue specimens frozen in OCT compound (Sakura) was isolated by employing the TRIzol (Gibco-BRL) method. Thereafter, 0.5 μg of RNA was used for reverse transcription-PCR (RT-PCR) analysis employing the Access RT-PCR system (Promega). The following primer pairs were employed: hTERT (457 bp), 5′-GCCTGAGCTGTACTTTGTCAA-3′ and 5′-CGCAAACAGCTTGTTCTCCATGTC-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 240 bp), 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ and 5′-TCCTTGGAGGCCATGTAGGCCAT-3′. The temperature program applied for hTERT (19) was 48°C for 45 min, 94°C for 2 min, and then 35 cycles at 94°C for 45 s, 55°C for 1 min, and 68°C for 2 min. Conditions for GAPDH were 48°C for 45 min, 94°C for 2 min, and then 25 cycles at 94°C for 45 s, 60°C for 1 min, and 68°C for 2 min. Reaction products were separated on a 1.5% agarose gel and detected by ethidium bromide staining. Agarose gel bands corresponding to hTERT and GAPDH cDNA were quantified using UV densitometry.
TRAP assay.
The TRAPeze telomerase detection kit (Intergen) was utilized to perform telomeric repeat amplification protocol (TRAP) assays. Three micrograms of protein lysate derived from transiently transfected BJ cells was subjected to TRAP analysis. In contrast, semiquantitative TRAP assays of transiently transfected 293T and SKBR3 cells entailed the use of only 1 ng of protein lysate. Similarly, determination of differential telomerase activity in HER2/Neu-positive and HER2/Neu-negative breast cancer cell lines was assessed by using only 1 ng of protein lysate in TRAP assays. The following temperature program was employed in all TRAP analyses: 30°C for 30 min and then 33 cycles at 94°C for 30 s and 59°C for 30 s.
RESULTS
HER2/Neu status correlates with hTERT levels in breast tumor tissue specimens and breast cancer cell lines.
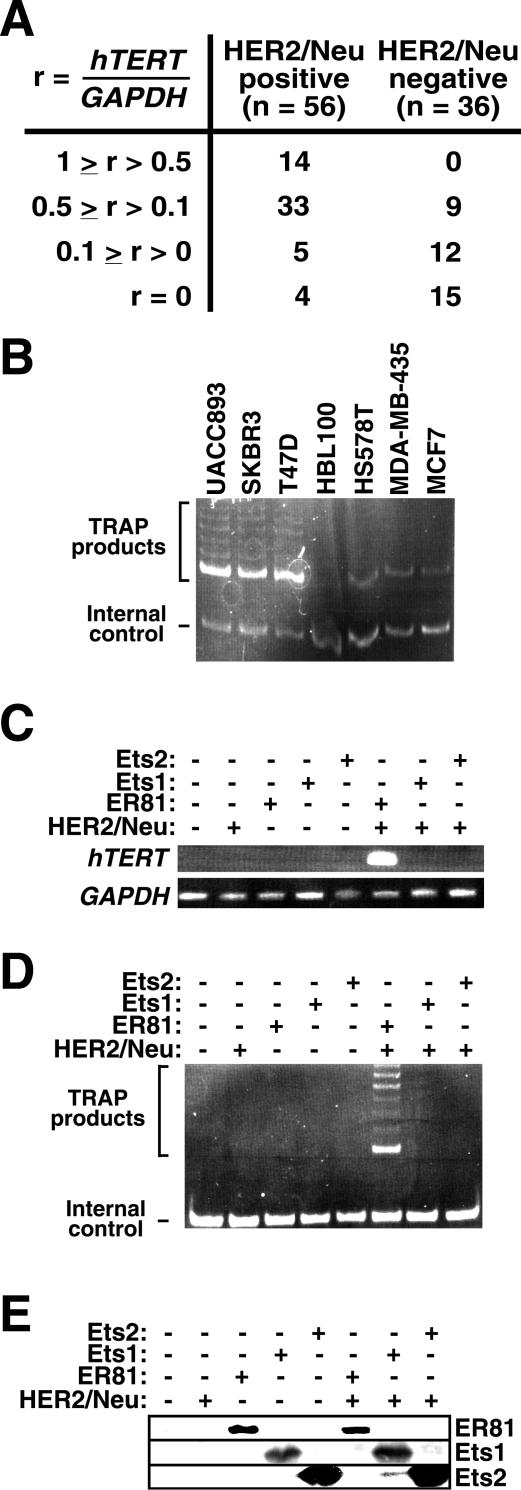
We began investigating the role of HER2/Neu on hTERT expression by measuring the levels of hTERT mRNA expressed in breast tumor specimens that were determined to be HER2/Neu positive or HER2/Neu negative by routine clinical immunohistochemistry. Using semiquantitative RT-PCR, where hTERT mRNA levels were normalized to GAPDH mRNA levels and the highest value observed was arbitrarily set to one, we found that only 4 out of 56 (7%) of the HER2/Neu-positive breast tumors tested negative for hTERT (Fig. 1A). In contrast, 15 out of 36 (42%) HER2/Neu-negative tumors did not express a detectable level of hTERT mRNA. Additionally, 25% (n = 14) of the HER2/Neu-positive tumor specimens had an hTERT expression level in excess of r = 0.5, whereas none of the HER2/Neu-negative tumor samples did. Collectively, these data indicate that HER2/Neu expression correlates with hTERT mRNA levels in breast tumors (P < 0.001; Pearson chi-square test).
FIG. 1.
(A) hTERT mRNA levels correlate with HER2/Neu expression. Relative hTERT mRNA levels in HER2/Neu-positive and -negative human breast tumor specimens were determined by semiquantitative RT-PCR. The highest _hTERT_-to-GAPDH signal ratio (r) detected was set to 1, and all other values were normalized accordingly. (B) Semiquantitative TRAP assay comparing telomerase activity in HER2/Neu-overexpressing breast cancer cell lines (UACC893, SKBR3, and T47D) versus breast cancer cell lines not overexpressing HER2/Neu (HBL100, HS578T, MDA-MB-435, and MCF7). The internal control band for PCR amplification is indicated. (C) Activation of endogenous hTERT transcription by HER2/Neu-V664E and ER81, but not Ets1 or Ets2, in transfected BJ foreskin fibroblasts. Expression of hTERT and, as a control, GAPDH was detected on agarose gels after RT-PCR. (D) Corresponding TRAP assay. (E) Western blot analysis revealing protein levels of ER81, Ets1, and Ets2 in transfected BJ cells.
hTERT mRNA levels have been shown to strongly correlate with telomerase activity in human breast tumor tissue (41, 63). Accordingly, we employed a semiquantitative TRAP assay to detect telomerase activity in human breast tumor cell lines (Fig. 1B) and found that the HER2/Neu-overexpressing cells, UACC893 (48), SKBR3 (57) and T47D (26), displayed higher telomerase activities than cells not overexpressing HER2/Neu, namely HBL100 (32), HS578T (44), MDA-MB-435 (67), and MCF7 (57). Altogether, these data suggest that HER2/Neu may be involved in hTERT upregulation in breast tumors.
ER81, but not Ets1 or Ets2, collaborates with HER2/Neu to induce hTERT transcription and telomerase activity in _hTERT_-negative cells.
To determine if HER2/Neu can directly activate hTERT expression, we assessed hTERT mRNA levels and telomerase activity by RT-PCR and TRAP assay, respectively, in telomerase-negative, nonimmortalized BJ foreskin fibroblasts transiently transfected with oncogenic HER2/Neu, the V664E mutant (4). We found that HER2/Neu alone did not induce hTERT expression or telomerase activity (Fig. 1C and D), possibly because essential effectors of HER2/Neu required to activate hTERT transcription are absent in BJ cells. Therefore, we expressed the ETS transcription factor ER81 in BJ cells. On its own, ER81 was also unable to activate hTERT transcription or telomerase activity, but joint expression of ER81 and HER2/Neu induced hTERT mRNA expression and telomerase activity (Fig. 1C and D); this was not due to altered ER81 protein levels upon HER2/Neu overexpression (Fig. 1E). Thus, stimulation of the transcription factor ER81 by oncogenic HER2/Neu may be the underlying cause of hTERT expression in many human breast tumors.
While we were conducting this study a report was published (45) indicating that epidermal growth factor receptor-mediated activation of another ETS transcription factor, Ets2, stimulates hTERT activity in _hTERT_-positive cells but not _hTERT_-negative cells. Accordingly, we demonstrated that Ets2 and its homolog Ets1 were unable to substitute for ER81 in HER2/Neu-mediated activation of hTERT expression in nonimmortalized, _hTERT_-negative BJ cells (Fig. 1C and D); this negative result was not due to lack of Ets1 and Ets2 protein expression (Fig. 1E). These data suggested that HER2/Neu has to specifically collaborate with the ETS transcription factor ER81 to upregulate hTERT transcription in cells that normally do not express hTERT.
HER2/Neu synergizes with ER81 to activate the hTERT promoter.
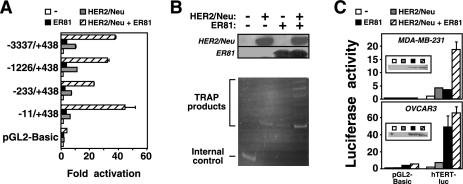
To confirm that HER2/Neu and ER81 cooperate to activate the hTERT promoter, we utilized a luciferase reporter construct driven by the hTERT promoter (nucleotides −3337 to +438) in transient transfections of 293T cells. As shown in Fig. 2A, oncogenic HER2/Neu and ER81 when expressed alone activated the hTERT promoter 9.4-fold and 3.4-fold, respectively. In contrast, joint expression of oncogenic HER2/Neu and ER81 synergistically stimulated the hTERT reporter plasmid 37-fold, whereas they had a minimal effect on the parental luciferase expression vector, pGL2-Basic. Control experiments revealed that HER2/Neu and ER81 protein levels were not altered upon coexpression compared to expression of each protein alone in 293T cells (Fig. 2B, upper panel). Furthermore, using a semiquantitative TRAP assay we found that HER2/Neu and ER81 cooperatively induced telomerase activity in transiently transfected 293T cells (Fig. 2B, lower panel). Please note that we used very low amounts of protein extract for this semiquantitative TRAP assay, which is why no telomerase activity was observable in the _hTERT_- and telomerase-positive 293T cells in the absence of ER81 and HER2/Neu.
FIG. 2.
(A) The hTERT promoter is synergistically activated by HER2/Neu and ER81. Full-length (−3337/+438) or truncated hTERT promoter luciferase constructs or the parental vector pGL2-Basic were cotransfected with HER2/Neu-V664E and ER81 into 293T cells as indicated. Activation of luciferase activity by HER2/Neu and ER81 is depicted. (B) HER2/Neu and ER81 synergize to enhance telomerase activity in 293T cells. Protein levels for HER2/Neu and ER81 are depicted in the upper panel, and the corresponding TRAP assay is shown in the lower panel. (C) Response of the −11/+431 hTERT promoter to HER2/Neu-V664E and ER81 in MDA-MB-231 and OVCAR3 cells. Inserts show anti-ER81 Western blots.
To delineate the region of the hTERT promoter responsive to HER2/Neu and ER81, we subcloned and tested progressively shorter fragments of the hTERT promoter upstream of the luciferase gene in 293T cells (Fig. 2A). None of the shorter promoter constructs lost its responsiveness to HER2/Neu and ER81. The shortest promoter fragment tested, −11 to +438, was the most responsive one, probably due to the absence of repressive promoter elements that are present 200 bp upstream of the hTERT transcription start site (23, 56).
We then wished to confirm that HER2/Neu and ER81 can also activate the hTERT promoter in cell lines other than 293T. Indeed, HER2/Neu and ER81 synergistically induced a slightly shorter version (nucleotides −11 to +431) of the above utilized −11 to +438 hTERT promoter in MDA-MB-231 breast tumor cells (Fig. 2C, top panel). To the contrary, ER81 stimulated the hTERT promoter nearly maximally in OVCAR3 ovarian cancer cells in the absence of exogenous HER2/Neu (Fig. 2C, bottom panel). This is likely due to the fact that OVCAR3 cells express a much higher level of endogenous HER2/Neu relative to MDA-MB-231 cells (30, 60). In conclusion, our data indicate that ER81, when stimulated by HER2/Neu, efficiently activates the hTERT promoter.
Identification of ER81 binding elements in the hTERT promoter.
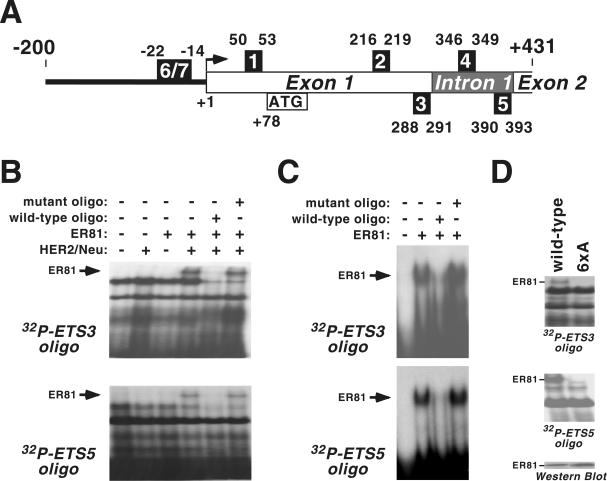
The smallest hTERT promoter used (−11 to +431) contains five putative ER81 binding sites (Fig. 3A) characterized by a core ETS sequence of GGAA/T (27). To identify which of these sites bind to ER81, we synthesized double-stranded oligonucleotides corresponding to each of the five ETS sites and used them in electrophoretic mobility shift assays with lysates derived from transiently transfected 293T cells. As shown in Fig. 3B (top panel), full-length ER81 alone did not bind to ETS site 3, whereas unidentified endogenous proteins present in the cell lysate interacted with the ETS3 oligonucleotide. However, when ER81 was coexpressed with HER2/Neu-V664E, an additional DNA-protein complex was observed. This additional complex formation was inhibited in the presence of an excess of the respective wild-type nonradiolabeled oligonucleotide, whereas an excess of the mutant oligonucleotide (GGAA core sequence of the ETS site mutated to CCAA) had no effect. As similar results were obtained with ETS site 5 (Fig. 3B, bottom panel), but binding of ER81 to ETS sites 1, 2, and 4 was never observed (data not shown), it appears that ER81, only when stimulated by HER2/Neu, binds exclusively to ETS sites 3 and 5 of the hTERT promoter.
FIG. 3.
(A) Scheme of the hTERT promoter from −200 to +431. The five intragenic ETS core sites of the promoter are designated as numbered boxes 1 through 5. Two upstream ETS sites are marked as numbers 6 and 7. The transcription start site (59) is marked by a black arrow, and the start methionine (ATG) is indicated. (B) Electrophoretic mobility shift assays with 32P-labeled oligonucleotides corresponding to ETS site 3 or 5 and full-length ER81 expressed in 293T cells in the presence and absence of HER2/Neu-V664E. Where indicated, a 20-fold excess of the respective nonlabeled wild-type or mutated (ETS core, GGAA→CCAA) oligonucleotide was added. (C) Analogous to the above results, binding of purified ER81249-477 to ETS sites 3 and 5. (D) Similar to the above results, DNA binding of wild-type ER81 and the 6xA mutant that were expressed in 293T cells in the presence of HER2/Neu-V664E. Protein levels are shown in the bottom panel.
In contrast to full-length ER81, we found that a bacterially expressed, purified C-terminal fragment of ER81 (ER81249-477), encompassing its ETS DNA binding domain but lacking all MAP kinase-dependent phosphorylation sites (8, 37), readily bound to ETS sites 3 and 5 in the absence of HER2/Neu (Fig. 3C). This suggests that the N terminus of ER81 precludes binding to the hTERT promoter and that the previously reported HER2/Neu-induced N-terminal phosphorylation of ER81 unmasks its DNA binding domain, thereby enabling it to bind to the hTERT promoter.
To further explore this hypothesis, we mutated in the N-terminal region of ER81 the four MAP kinase phosphorylation sites (8) as well as the two phosphorylation sites that are targeted by MAP kinase-activated protein (MAPKAP) kinases (38, 39, 61). This 6xA mutant of ER81, unlike wild-type ER81, failed to bind to ETS sites 3 and 5 of the hTERT promoter in the presence of HER2/Neu despite equal expression of both proteins (Fig. 3D). Thus, HER2/Neu-triggered phosphorylation of ER81 within its N terminus appears to be required for binding to the hTERT promoter.
Functional characterization of ER81 binding sites in the hTERT promoter.
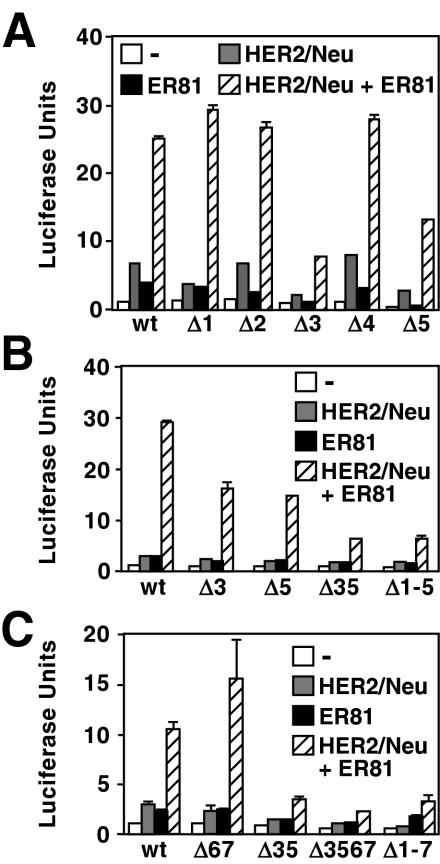
To assess the functional significance of each of the aforementioned five ETS sites in mediating the effects of oncogenic HER2/Neu and ER81 on hTERT promoter activity, we generated reporter constructs in which the luciferase gene was placed downstream of the −11/+431 hTERT promoter containing mutations (GGAA to CCAA) in each one of the ETS sites. Consistent with our DNA binding analyses, mutation of ETS sites 3 and 5 attenuated the ability of HER2/Neu and ER81 to activate the hTERT promoter, whereas mutation of the other ETS sites had little effect (Fig. 4A). A double mutant promoter (Fig. 4B, Δ35) was less responsive to HER2/Neu and ER81 than any of the single mutants, revealing that both ETS sites 3 and 5 are involved in hTERT activation. Moreover, mutation of all five ETS sites (Δ1-5) did not result in reduced promoter activity compared to the Δ35 mutant.
FIG. 4.
(A and B) Functional effect of mutating from GGAA to CCAA the indicated ETS core sites (indicated by the prefix Δ) of the −11/+431 hTERT luciferase construct in 293T cells. (C) Similarly, analysis of the larger −200/+431 hTERT promoter fused to luciferase cDNA.
To ensure that we did not exclude important basal promoter elements upstream of −11 in our analysis, we assessed the importance of the aforementioned ETS sites in the context of the −200/+431 hTERT core promoter shown to play a critical role in hTERT regulation (14, 33, 59). Mutation of ETS sites 3 and 5 in the core promoter significantly reduced its response to HER2/Neu and ER81 (Fig. 4C). In contrast, mutation of an upstream promoter sequence containing two ETS sites (sites 6 and 7 at −14 to −22 [Fig. 3A]) considered important in Ets2 regulation of the hTERT promoter (45) had no effect on the ability of ER81 and HER2/Neu to induce the hTERT promoter (Fig. 4C, Δ67). In addition, mutation of these two ETS sites did not significantly alter the phenotype of the Δ35 and Δ1-5 mutants (Δ3567 and Δ1-7, respectively). Accordingly, we were unable to detect binding of full-length, HER2/Neu-stimulated ER81 to a radiolabeled probe corresponding to the −14 to −22 ETS sites in electrophoretic mobility shift assays (data not shown). In conclusion, ETS sites 3 and 5 mediate the response of the hTERT promoter to HER2/Neu and ER81. Given that ETS site 3 and ETS site 5 are located within exon 1 and intron 1 of the hTERT gene, respectively, our results further demonstrate that regulation of the TATA box-devoid hTERT gene is critically dependent on intragenic promoter elements.
HER2/Neu-triggered hTERT transcription is dependent on ER81 or related ETS proteins.
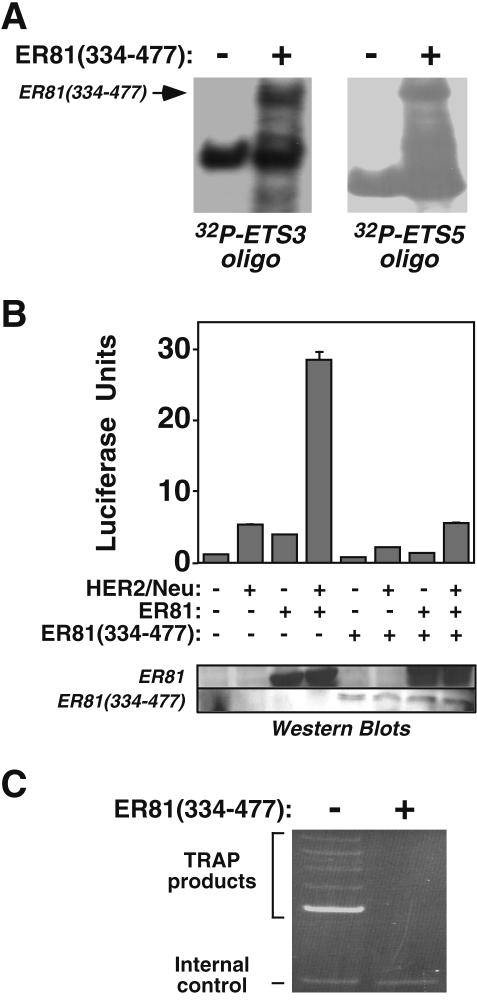
Since HER2/Neu alone modestly activated the hTERT promoter in 293T cells (Fig. 2A), we determined if HER2/Neu-mediated induction of hTERT expression is dependent on endogenous ER81. To this end, we employed a transcriptionally inactive ER81 molecule (ER81334-477) corresponding to the ER81 ETS binding domain that competes with full-length ER81 for DNA binding (8, 37). Accordingly, and similar to the bacterially expressed ER81249-477 molecule shown above (Fig. 3C), ER81334-477 derived from transiently transfected 293T cells readily bound ETS sites 3 and 5 of hTERT (Fig. 5A). Further, ER81334-477 significantly reduced HER2/Neu-mediated activation of the hTERT promoter (Fig. 5B). In addition, ER81334-477 effectively competed with wild-type ER81 for hTERT binding, as HER2/Neu- and ER81-mediated activation of the hTERT promoter was greatly reduced in the presence of ER81334-477. This may seem surprising, since protein levels for ER81334-477 were lower than for full-length ER81 (Fig. 5B, lower panel); however, ER81334-477 does not contain the ER81 N-terminal amino acids that inhibit DNA binding and as such is expected to bind much more avidly to DNA than full-length ER81, as attested by the fact that ER81334-477 bound to ETS sites 3 and 5 of the hTERT promoter even in the absence of HER2/Neu stimulation (Fig. 5A). Importantly, expression of ER81334-477 completely abolished telomerase activity in HER2/Neu-overexpressing SKBR3 breast tumor cells (Fig. 5C), further indicating that HER2/Neu-mediated induction of the hTERT promoter is dependent on endogenous ER81 or related ETS protein family members.
FIG. 5.
(A) Electrophoretic mobility shift assays with 32P-labeled oligonucleotides corresponding to ETS site 3 or 5 incubated with lysates of 293T cells that were or were not transfected with ER81334-477 expression plasmid. (B) Effect of dominant-negative ER81334-477 on HER2/Neu- and ER81-mediated −11/+431 hTERT promoter activation in 293T cells. Protein levels corresponding to wild-type ER81 and ER81334-477 are depicted in the lower panel. (C) Telomerase activity measured by TRAP assay in HER2/Neu-overexpressing SKBR3 cells transfected with or without ER81334-477.
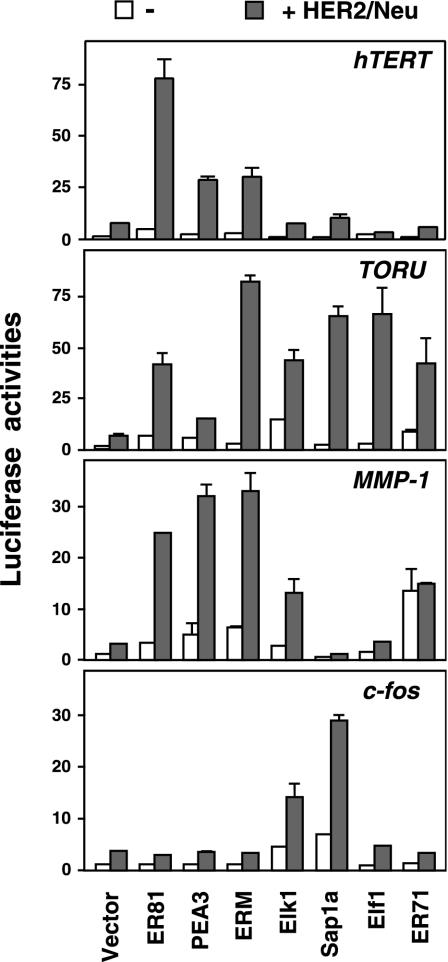
ER81, together with PEA3 and ERM, belongs to a subfamily of ETS proteins that show significant structural and functional homology (27). Accordingly, PEA3 and ERM also synergized with HER2/Neu to activate the hTERT promoter (Fig. 6, upper panel), albeit to a lesser extent than ER81. In contrast, four transcription factors (Elk1, Sap1a, Elf1, and ER71) belonging to other ETS protein subfamilies only marginally, if at all, induced the hTERT promoter (Fig. 6, upper panel). Most notably, Elk1 and Sap1a, prominent downstream effectors of HER2/Neu-activated MAP kinases (12, 65), did not enhance hTERT promoter activity over the vector control in the presence of HER2/Neu. Therefore, we concluded that HER2/Neu induction of the hTERT promoter is dependent on the subfamily of ETS transcription factors comprised of ER81, PEA3, and ERM.
FIG. 6.
Induction of the −11/+431 hTERT promoter, the TORU promoter, the human −525/+15 MMP-1 promoter, or the human −711/+39 c-fos promoter by HER2/Neu-V664E in the presence of various ETS proteins in 293T cells.
An alternative explanation for the inability of Elk1, Sap1a, Elf1, and ER71 to activate the hTERT promoter might be that they are not as well expressed as ER81, PEA3, and ERM. However, we believe that this is not the case, since we utilized the same, identical amounts of ETS protein expression vectors as before in order to activate the ER81-regulated TORU and MMP-1 promoters (8, 50) and observed different results (Fig. 6). For instance, Elf1 and ER71 efficiently activated the TORU promoter in conjunction with HER2/Neu, and the level of activation was comparable to that with ER81 and even higher than that observed with PEA3. Moreover, ER71, unlike the case of the hTERT promoter, greatly stimulated the MMP-1 promoter as reported before (17), but this was independent of the presence of HER2/Neu. Also, Elk1 was able to activate the TORU promoter as efficiently as ER81 upon HER2/Neu stimulation. Furthermore, ER81, PEA3, and ERM differed in their abilities to activate the various promoters. Whereas ER81 was clearly the most efficient ETS protein tested to activate the hTERT promoter upon coexpression of HER2/Neu, ERM was the most effective molecule in the case of the TORU promoter and no great difference was observed among ER81, PEA3, and ERM in the case of the MMP-1 promoter (Fig. 6). Finally, we also assessed the activity of the various ETS proteins on the c-fos promoter. Here, only Elk1 and Sap1a were able to greatly induce promoter activity upon HER2/Neu coexpression, whereas ER81, PEA3, ERM, Elf1, and ER71 were unable to do so (Fig. 6). Thus, the four gene promoters tested are differently regulated by different ETS proteins, and the hTERT promoter is in particular activated by ER81 upon HER2/Neu stimulation.
HER2/Neu signaling is required for stimulation of the hTERT promoter and telomerase activity.
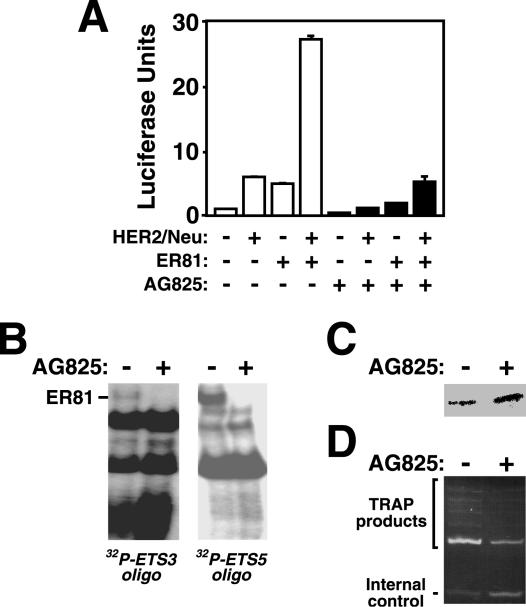
To ensure that HER2/Neu signaling, as opposed to simple overexpression, is required for the induction of hTERT expression we employed a HER2/Neu kinase inhibitor, AG825 (54). Indeed, AG825 significantly reduced the response of the hTERT promoter to HER2/Neu and ER81 (Fig. 7A); the slight repression observed in the absence of ectopic HER2/Neu was due to the inhibition of endogenous HER2/Neu by AG825. In addition, binding of full-length ER81 to the hTERT promoter in the presence of HER2/Neu was abolished by AG825 (Fig. 7B); a control Western blot revealed that ER81 was comparably expressed in the presence and absence of AG825 (Fig. 7C). Finally, incubation of HER2/Neu-overexpressing SKBR3 breast tumor cells with AG825 resulted in a significant decline in endogenous telomerase activity (Fig. 7D), further solidifying the role of HER2/Neu signaling in hTERT regulation.
FIG. 7.
(A) Effect of the HER2/Neu inhibitor AG825 on the ability of HER2/Neu-V664E and ER81 to activate the −11/+431 hTERT promoter in 293T cells. (B) ER81 and HER2/Neu-V664E were coexpressed in 293T cells treated with or without AG825. Protein lysates were prepared and utilized in electrophoretic mobility shift assays with radioactively labeled ETS3 and ETS5 oligonucleotides. (C) Respective Western blot showing comparable expression of ER81. (D) TRAP assay demonstrating the effect of AG825 on telomerase activity in HER2/Neu-overexpressing SKBR3 cells.
HER2/Neu-mediated activation of hTERT transcription is dependent on the ERK MAP kinase pathway.
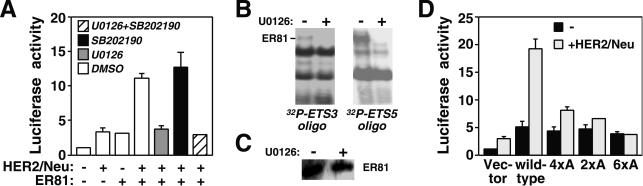
Previously, our investigators have shown that HER2/Neu-triggered activation and phosphorylation of ER81 can proceed via both the ERK and p38 MAP kinase pathways (8). To elucidate which of these signaling pathways are involved in HER2/Neu-dependent hTERT transcription, two kinase inhibitors were utilized: U0126, which blocks activation of ERK MAP kinases (24), and SB202190, which inhibits p38 MAP kinases (42). As shown in Fig. 8A, HER2/Neu-dependent activation of ER81 was completely abolished by the addition of U0126, whereas SB202190 had no effect, thereby illustrating that activation of hTERT transcription by HER2/Neu and ER81 is dependent on the ERK, but not the p38, MAP kinase pathway. Consistently, U0126 inhibited the ability of ER81 to bind to ETS sites 3 and 5 of the hTERT promoter upon stimulation with HER2/Neu (Fig. 8B); a control Western blot revealed that comparable amounts of ER81 were expressed in the presence and absence of U0126 (Fig. 8C).
FIG. 8.
(A) Determination of MAP kinase pathways involved in HER2/Neu induction of the −11/+431 hTERT promoter. 293T cells were transfected as indicated and treated with the ERK MAP kinase pathway inhibitor U0126, the p38 MAP kinase inhibitor SB202190, or the vehicle DMSO. Due to the adverse effect of DMSO on cell growth, absolute luciferase activities were lower than observed before. (B) Electrophoretic mobility shift assays with 32P-labeled oligonucleotides corresponding to ETS site 3 or 5 incubated with lysates from 293T cells transfected with both ER81 and HER2/Neu-V664 and treated with or without U0126. (C) Corresponding anti-ER81 Western blot. (D) Phosphorylation dependence of ER81 activation of the −11/+438 hTERT promoter. Previously identified phosphorylation sites in ER81 were mutated, and the ability of ER81 to stimulate the hTERT promoter was assessed in transfected 293T cells. The 4xA mutant of ER81 corresponds to an ER81 protein where all MAP kinase phosphorylation sites (S94, T139, T143, and S146) have been mutated to alanine, whereas the 2xA mutant has alanine at the MAP kinase-activated protein kinase phosphorylation sites (S191 and S216) and the 6xA mutant has alanine at all six aforementioned phosphorylation sites.
To further demonstrate the importance of ERK MAP kinases in hTERT regulation, we mutated the four ERK MAP kinase phosphorylation sites in ER81 (4xA mutant) (8) and/or the two MAPKAP kinase phosphorylation sites (2xA mutant) (61). The 4xA and 2xA mutants as well as a combination mutant (6xA) were severely compromised in their abilities to activate the hTERT promoter in the presence of HER2/Neu in 293T cells (Fig. 8D). Thus, phosphorylation of ER81 mediated by both ERK MAP kinases and MAPKAP kinases appears to be required for the stimulation of hTERT expression by oncogenic HER2/Neu.
Ras and Raf synergize with ER81 to induce hTERT mRNA expression and telomerase activity.
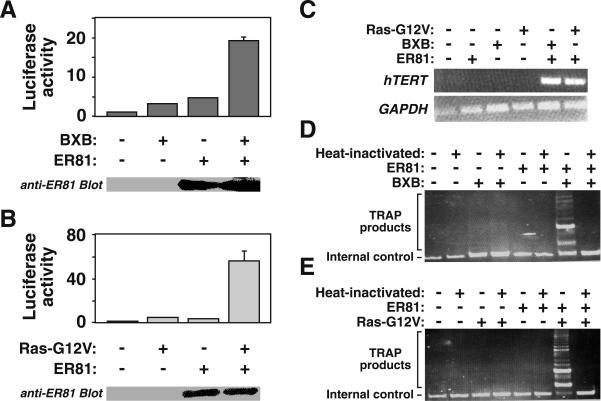
Since HER2/Neu activates hTERT expression via ER81 and ERK MAP kinases, we postulated that Ras and Raf, two upstream components of the ERK MAP kinase pathway (13), also stimulate hTERT transcription. Accordingly, we found that a constitutively active Raf-1 protein, BXB (11), and ER81 synergistically activated the hTERT promoter (Fig. 9A). Similarly, an oncogenic Ras mutant, Ras-G12V (7), greatly enhanced hTERT promoter activity in conjunction with ER81 (Fig. 9B). Cotransfection of _hTERT_-negative BJ foreskin fibroblasts with ER81 and either BXB or Ras-G12V revealed that oncogenic Raf and Ras, as does HER2/Neu, collaborated with ER81 to induce hTERT mRNA expression (Fig. 9C) and telomerase activity (Fig. 9D and E). To the contrary, Ras-G12V and BXB failed to elicit hTERT transcription and induce telomerase activity in the absence of ER81, thereby indicating that oncogenic Ras and Raf require the presence of ER81 to stimulate the hTERT promoter.
FIG. 9.
(A) Constitutively active Raf-1 (BXB) enhances the ability of ER81 to activate the −11/+431 hTERT luciferase reporter in 293T cells. The bottom panel shows an anti-ER81 Western blot confirming that comparable amounts of ER81 were expressed in the presence and absence of BXB. (B) Analogous results to those described above, with oncogenic Ras-G12V. (C) RT-PCR illustrating the collaboration of BXB and Ras-G12V with ER81 to stimulate hTERT transcription in nonimmortalized BJ cells. (D and E) Corresponding TRAP assays for ER81 and either BXB or Ras-G12V, respectively.
DISCUSSION
Although hTERT upregulation is widely regarded as a crucial facet of tumorigenesis (29), little is known about how hTERT expression is activated in tumor cells. In particular, aside from the controversial involvement of Myc, the role of oncoproteins in hTERT induction has not been elucidated. Here, we show that oncogenic HER2/Neu, Ras, and Raf collaborate with a common downstream target of all three proteins, the ETS transcription factor ER81, to induce hTERT mRNA expression and telomerase activity.
Induction of hTERT transcription by HER2/Neu is mediated by the ERK MAP kinase pathway, as an inhibitor of this signaling pathway, U0126, completely abolished ER81 activation and binding of the hTERT promoter in the presence of HER2/Neu. Consistently, oncogenic Ras and Raf, downstream signaling molecules in the HER2/Neu→Ras→Raf→ERK MAP kinase cascade (65), substituted for HER2/Neu in activating ER81-dependent hTERT transcription. Furthermore, mutation of either ERK MAP kinase sites (4xA mutant of ER81) or MAPKAP kinase sites (2xA mutant of ER81) attenuated ER81-mediated activation of the hTERT promoter, and mutation of all six in vivo ER81 phosphorylation sites resulted in a loss of binding and activation of the hTERT promoter upon stimulation with HER2/Neu. Thus, HER2/Neu-mediated activation of the hTERT promoter appears to depend on ERK MAP kinases as well as p90RSK or MSK1, two MAPKAP kinases downstream of ERK MAP kinase capable of phosphorylating ER81 in vivo (16, 25, 39, 61).
The ability of HER2/Neu to stimulate the hTERT promoter appears to be uniquely dependent on the subfamily of ETS transcription factors comprised of ER81, PEA3, and ERM. All three of these proteins, which share >95% identity within their DNA binding domains and an overall similarity in excess of 50% (18), synergized with HER2/Neu to activate the hTERT promoter, whereas other unrelated ETS transcription factors did not. In particular, Elk1 and the homologous Sap1a protein, both of which are prominent targets of ERK MAP kinase (12), were barely, if at all, able to activate hTERT transcription. Similarly, in contrast to ER81, Ets1 and its homolog Ets2, both of which are phosphorylated and activated by MAP kinases (46, 62), were unable to activate hTERT mRNA expression and telomerase activity in _hTERT_-negative BJ cells in the presence of HER2/Neu. Furthermore, as the dominant-negative ER81334-477 molecule rendered the hTERT promoter unresponsive to HER2/Neu and inhibited telomerase activity in HER2/Neu-overexpressing SKBR3 cells, it appears that ER81 or related ETS proteins are indispensable in HER2/Neu-mediated hTERT upregulation.
A recent study has shown that expression of a dominant-negative PEA3 molecule that abolishes the activity of PEA3, ER81, and ERM significantly retarded mammary tumor development in HER2/Neu transgenic mice. In addition, expression of ER81, ERM, and PEA3 was greatly increased in breast cells engineered to overexpress HER2/Neu, whereas expression of other ETS proteins was reduced or unaffected (58). Accordingly, ER81, PEA3, or ERM are overexpressed in many human breast tumor cell lines, and PEA3 has been shown to be coordinately overexpressed with HER2/Neu in human breast tumor specimens (3, 5). Moreover, HER2/Neu and ER81, or PEA3, collaborate to enhance the expression of HER2/Neu itself (5, 9). As such, our discovery that HER2/Neu requires an ER81-related protein to activate hTERT expression further supports the notion that ER81, ERM, and PEA3 are essential in HER2/Neu-mediated tumor formation.
Our finding that HER2/Neu expression highly correlates with hTERT levels in human breast tumor specimens and telomerase activity in breast tumor cell lines suggests a physiologically relevant relationship between HER2/Neu and hTERT expression. Indeed, the HER2/Neu kinase inhibitor AG825 significantly reduced telomerase activity in HER2/Neu-overexpressing SKBR3 breast tumor cells. Therefore, we speculate that the enhanced frequency and higher levels of hTERT mRNA expression and telomerase activity may account, in part, for the aggressive nature of tumors overexpressing HER2/Neu (64), as numerous studies have shown that the level of hTERT expression correlates with enhanced malignancy and poor prognosis (31). At worst, as overexpression or mutations in Ras, Raf, and HER2/Neu collectively contribute to the formation of approximately half of all human tumors (1, 15, 36), these oncoproteins may enable a large proportion of all tumor cells to circumvent cell senescence by inducing hTERT expression, one hallmark of cancer (29).
hTERT transcription appears to be actively repressed in normal somatic cells (23). Factors suggested to contribute to this repression include Mad1, p53, and the Wilms' tumor suppressor gene product 1 (56). Recently, another tumor suppressor, Menin, has been shown to associate with the hTERT promoter, and knocking down its expression resulted in hTERT expression in _hTERT_-negative BJ cells (43). Thus, inactivating mutations in tumor suppressors may lead to the derepression of the hTERT gene and consequently to telomerase activity and immortalization. However, our study suggests that hTERT gene expression can also be enforced by activating mutations of oncogenes such as HER2/Neu, Ras, and Raf. Thus, two different mechanisms, the inactivation of tumor suppressors and/or the activation of oncoproteins, may account for immortalization of tumor cells through the induction of hTERT transcription.
It has recently been reported that the epidermal growth factor receptor collaborates with Ets2 to enhance hTERT expression in immortalized, _hTERT_-positive cells but not in nonimmortalized cells that are representative of normal somatic cells prior to oncogenic transformation (45). However, direct binding of Ets2 to the hTERT promoter has not been shown. To this end, we noted that Ets1 and Ets2 were incapable of binding to any of the ETS sites of the −200/+431 hTERT core promoter in electrophoretic mobility shift assays (data not shown). Thus, Ets1 and Ets2, although capable of indirectly enhancing hTERT transcription in previously transformed, _hTERT_-positive cells, are unable to induce hTERT activation in nonimmortalized, _hTERT_-negative cells. Therein lies the critical importance of our study, since it reveals that three prominent human oncoproteins (HER2/Neu, Ras, and Raf) can transform a telomerase-negative cell to a telomerase-positive cell by activating ER81 and consequently upregulating hTERT expression, as occurs in ∼90% of all human tumor cells (31, 40, 49). Therefore, our elucidation of a mechanism whereby oncogenic HER2/Neu, Ras, and Raf induce hTERT transcription via the ERK MAP kinase pathway and ER81 may prove instrumental in the development of cancer therapies designed to downregulate telomerase expression.
Acknowledgments
We thank Andrea Mariani for help with statistics and Fergus Couch for sharing breast cancer cell lines, as well as Craig Hauser and Eiji Hara for providing Ets1 and Ets2 expression plasmids, respectively.
This work was supported by the Mayo Foundation, a grant from the National Cancer Institute (CA085257), a scholarship (to R.J.) from the Sidney Kimmel Foundation for Cancer Research, and the Fraternal Order of Eagles Cancer Fund.
REFERENCES
- 1.Adjei, A. A. 2001. Blocking oncogenic Ras signaling for cancer therapy. J. Natl. Cancer Inst. 93**:**1062-1074. [DOI] [PubMed] [Google Scholar]
- 2.Aisner, D. L., W. E. Wright, and J. W. Shay. 2002. Telomerase regulation: not just flipping the switch. Curr. Opin. Genet. Dev. 12**:**80-85. [DOI] [PubMed] [Google Scholar]
- 3.Baert, J. L., D. Monte, E. A. Musgrove, O. Albagli, R. L. Sutherland, and Y. de Launoit. 1997. Expression of the PEA3 group of ETS-related transcription factors in human breast-cancer cells. Int. J. Cancer 70**:**590-597. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Levy, R., H. F. Paterson, C. J. Marshall, and Y. Yarden. 1994. A single autophosphorylation site confers oncogenicity to the Neu/ErbB-2 receptor and enables coupling to the MAP kinase pathway. EMBO J. 13**:**3302-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benz, C. C., R. C. O'Hagan, B. Richter, G. K. Scott, C. H. Chang, X. Xiong, K. Chew, B. M. Ljung, S. Edgerton, A. Thor, and J. A. Hassell. 1997. HER2/Neu and the Ets transcription activator PEA3 are coordinately upregulated in human breast cancer. Oncogene 15**:**1513-1525. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn, E. H. 2001. Switching and signaling at the telomere. Cell 106**:**661-673. [DOI] [PubMed] [Google Scholar]
- 7.Block, C., R. Janknecht, C. Herrmann, N. Nassar, and A. Wittinghofer. 1996. Quantitative structure-activity analysis correlating Ras/Raf interaction in vitro to Raf activation in vivo. Nat. Struct. Biol. 3**:**244-251. [DOI] [PubMed] [Google Scholar]
- 8.Bosc, D. G., B. S. Goueli, and R. Janknecht. 2001. HER2/Neu-mediated activation of the ETS transcription factor ER81 and its target gene MMP-1. Oncogene 20**:**6215-6224. [DOI] [PubMed] [Google Scholar]
- 9.Bosc, D. G., and R. Janknecht. 2002. Regulation of Her2/neu promoter activity by the ETS transcription factor, ER81. J. Cell. Biochem. 86**:**174-183. [DOI] [PubMed] [Google Scholar]
- 10.Bredemeier-Ernst, I., A. Nordheim, and R. Janknecht. 1997. Transcriptional activity and constitutive nuclear localization of the ETS protein Elf-1. FEBS Lett. 408**:**47-51. [DOI] [PubMed] [Google Scholar]
- 11.Bruder, J. T., G. Heidecker, and U. R. Rapp. 1992. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 6**:**545-556. [DOI] [PubMed] [Google Scholar]
- 12.Cahill, M. A., R. Janknecht, and A. Nordheim. 1996. Signalling pathways: jack of all cascades. Curr. Biol. 6**:**16-19. [DOI] [PubMed] [Google Scholar]
- 13.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410**:**37-40. [DOI] [PubMed] [Google Scholar]
- 14.Cong, Y. S., J. Wen, and S. Bacchetti. 1999. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum. Mol. Genet. 8**:**137-142. [DOI] [PubMed] [Google Scholar]
- 15.Davies, H., G. R. Bignell, C. Cox, P. Stephens, S. Edkins, S. Clegg, J. Teague, H. Woffendin, M. J. Garnett, W. Bottomley, N. Davis, E. Dicks, R. Ewing, Y. Floyd, K. Gray, S. Hall, R. Hawes, J. Hughes, V. Kosmidou, A. Menzies, C. Mould, A. Parker, C. Stevens, S. Watt, S. Hooper, R. Wilson, H. Jayatilake, B. A. Gusterson, C. Cooper, J. Shipley, D. Hargrave, K. Pritchard-Jones, N. Maitland, G. Chenevix-Trench, G. J. Riggins, D. D. Bigner, G. Palmieri, A. Cossu, A. Flanagan, A. Nicholson, J. W. Ho, S. Y. Leung, S. T. Yuen, B. L. Weber, H. F. Seigler, T. L. Darrow, H. Paterson, R. Marais, C. J. Marshall, R. Wooster, M. R. Stratton, and P. A. Futreal. 2002. Mutations of the BRAF gene in human cancer. Nature 417**:**949-954. [DOI] [PubMed] [Google Scholar]
- 16.Deak, M., A. D. Clifton, L. M. Lucocq, and D. R. Alessi. 1998. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17**:**4426-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Haro, L., and R. Janknecht. 2002. Functional analysis of the transcription factor ER71 and its activation of the matrix metalloproteinase-1 promoter. Nucleic Acids Res. 30**:**2972-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Launoit, Y., J. L. Baert, A. Chotteau, D. Monte, P. A. Defossez, L. Coutte, H. Pelczar, and F. Leenders. 1997. Structure-function relationships of the PEA3 group of Ets-related transcription factors. Biochem. Mol. Med. 61**:**127-135. [DOI] [PubMed] [Google Scholar]
- 19.Dhaene, K., J. Wauters, B. Weyn, J. P. Timmermans, and E. van Marck. 2000. Expression profile of telomerase subunits in human pleural mesothelioma. J. Pathol. 190**:**80-85. [DOI] [PubMed] [Google Scholar]
- 20.Downward, J. 1998. Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 8**:**49-54. [DOI] [PubMed] [Google Scholar]
- 21.Drissi, R., F. Zindy, M. F. Roussel, and J. L. Cleveland. 2001. c-Myc-mediated regulation of telomerase activity is disabled in immortalized cells. J. Biol. Chem. 276**:**29994-30001. [DOI] [PubMed] [Google Scholar]
- 22.Ducrest, A. L., M. Amacker, Y. D. Mathieu, A. P. Cuthbert, D. A. Trott, R. F. Newbold, M. Nabholz, and J. Lingner. 2001. Regulation of human telomerase activity: repression by normal chromosome 3 abolishes nuclear telomerase reverse transcriptase transcripts but does not affect c-Myc activity. Cancer Res. 61**:**7594-7602. [PubMed] [Google Scholar]
- 23.Ducrest, A. L., H. Szutorisz, J. Lingner, and M. Nabholz. 2002. Regulation of the human telomerase reverse transcriptase gene. Oncogene 21**:**541-552. [DOI] [PubMed] [Google Scholar]
- 24.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273**:**18623-18632. [DOI] [PubMed] [Google Scholar]
- 25.Frödin, M., and S. Gammeltoft. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 151**:**65-77. [DOI] [PubMed] [Google Scholar]
- 26.Graus-Porta, D., R. R. Beerli, and N. E. Hynes. 1995. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol. Cell. Biol. 15**:**1182-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graves, B. J., and J. M. Petersen. 1998. Specificity within the ets family of transcription factors. Adv. Cancer Res. 75**:**1-55. [DOI] [PubMed] [Google Scholar]
- 28.Hackett, J. A., D. M. Feldser, and C. W. Greider. 2001. Telomere dysfunction increases mutation rate and genomic instability. Cell 106**:**275-286. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100**:**57-70. [DOI] [PubMed] [Google Scholar]
- 30.Hellstrom, I., G. Goodman, J. Pullman, Y. Yang, and K. E. Hellstrom. 2001. Overexpression of HER-2 in ovarian carcinomas. Cancer Res. 61**:**2420-2423. [PubMed] [Google Scholar]
- 31.Hiyama, E., and K. Hiyama. 2002. Clinical utility of telomerase in cancer. Oncogene 21**:**643-649. [DOI] [PubMed] [Google Scholar]
- 32.Hong, R. L., W. H. Spohn, and M. C. Hung. 1999. Curcumin inhibits tyrosine kinase activity of p185neu and also depletes p185neu. Clin. Cancer Res. 5**:**1884-1891. [PubMed] [Google Scholar]
- 33.Horikawa, I., P. L. Cable, C. Afshari, and J. C. Barrett. 1999. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 59**:**826-830. [PubMed] [Google Scholar]
- 34.Horikawa, I., P. L. Cable, S. J. Mazur, E. Appella, C. A. Afshari, and J. C. Barrett. 2002. Downstream E-box-mediated regulation of the human telomerase reverse transcriptase (hTERT) gene transcription: evidence for an endogenous mechanism of transcriptional repression. Mol. Biol. Cell 13**:**2585-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchinson, J. N., and W. J. Muller. 2000. Transgenic mouse models of human breast cancer. Oncogene 19**:**6130-6137. [DOI] [PubMed] [Google Scholar]
- 36.Hynes, N. E., and D. F. Stern. 1994. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim. Biophys. Acta 1198**:**165-184. [DOI] [PubMed] [Google Scholar]
- 37.Janknecht, R. 1996. Analysis of the ERK-stimulated ETS transcription factor ER81. Mol. Cell. Biol. 16**:**1550-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janknecht, R. 2001. Cell type-specific inhibition of the ETS transcription factor ER81 by mitogen-activated protein kinase-activated protein kinase 2. J. Biol. Chem. 276**:**41856-41861. [DOI] [PubMed] [Google Scholar]
- 39.Janknecht, R. 2003. Regulation of the ER81 transcription factor and its coactivators by mitogen- and stress-activated protein kinase 1 (MSK1). Oncogene 22**:**746-755. [DOI] [PubMed] [Google Scholar]
- 40.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266**:**2011-2015. [DOI] [PubMed] [Google Scholar]
- 41.Kirkpatrick, K. L., G. Clark, M. Ghilchick, R. F. Newbold, and K. Mokbel. 2003. hTERT mRNA expression correlates with telomerase activity in human breast cancer. Eur. J. Surg. Oncol. 29**:**321-326. [DOI] [PubMed] [Google Scholar]
- 42.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, J. E. Strickler, M. M. McLaughlin, I. R. Siemens, S. M. Fisher, G. P. Livi, J. R. White, J. L. Adams, and P. R. Young. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372**:**739-746. [DOI] [PubMed] [Google Scholar]
- 43.Lin, S. Y., and S. J. Elledge. 2003. Multiple tumor suppressor pathways negatively regulate telomerase. Cell 113**:**881-889. [DOI] [PubMed] [Google Scholar]
- 44.Littlewood-Evans, A. J., G. Bilbe, W. B. Bowler, D. Farley, B. Wlodarski, T. Kokubo, T. Inaoka, J. Sloane, D. B. Evans, and J. A. Gallagher. 1997. The osteoclast-associated protease cathepsin K is expressed in human breast carcinoma. Cancer Res. 57**:**5386-5390. [PubMed] [Google Scholar]
- 45.Maida, Y., S. Kyo, T. Kanaya, Z. Wang, N. Yatabe, M. Tanaka, M. Nakamura, M. Ohmichi, N. Gotoh, S. Murakami, and M. Inoue. 2002. Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via MAP kinase signaling pathway. Oncogene 21**:**4071-4079. [DOI] [PubMed] [Google Scholar]
- 46.McCarthy, S. A., D. Chen, B. S. Yang, J. J. Garcia Ramirez, H. Cherwinski, X. R. Chen, M. Klagsbrun, C. A. Hauser, M. C. Ostrowski, and M. McMahon. 1997. Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol. Cell. Biol. 17**:**2401-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKeage, K., and C. M. Perry. 2002. Trastuzumab: a review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs 62**:**209-243. [DOI] [PubMed] [Google Scholar]
- 48.Meltzer, P., A. Leibovitz, W. Dalton, H. Villar, T. Kute, J. Davis, R. Nagle, and J. Trent. 1991. Establishment of two new cell lines derived from human breast carcinomas with HER-2/neu amplification. Br. J. Cancer 63**:**727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyerson, M., C. M. Counter, E. N. Eaton, L. W. Ellisen, P. Steiner, S. D. Caddle, L. Ziaugra, R. L. Beijersbergen, M. J. Davidoff, Q. Liu, S. Bacchetti, D. A. Haber, and R. A. Weinberg. 1997. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90**:**785-795. [DOI] [PubMed] [Google Scholar]
- 50.Monte, D., L. Coutte, J. L. Baert, I. Angeli, D. Stehelin, and Y. de Launoit. 1995. Molecular characterization of the ets-related human transcription factor ER81. Oncogene 11**:**771-779. [PubMed] [Google Scholar]
- 51.Noonberg, S. B., and C. C. Benz. 2000. Tyrosine kinase inhibitors targeted to the epidermal growth factor receptor subfamily: role as anticancer agents. Drugs 59**:**753-767. [DOI] [PubMed] [Google Scholar]
- 52.Nottage, M., and L. L. Siu. 2002. Rationale for Ras and raf-kinase as a target for cancer therapeutics. Curr. Pharm. Des. 8**:**2231-2242. [DOI] [PubMed] [Google Scholar]
- 53.Nugent, C. I., and V. Lundblad. 1998. The telomerase reverse transcriptase: components and regulation. Genes Dev. 12**:**1073-1085. [DOI] [PubMed] [Google Scholar]
- 54.Osherov, N., A. Gazit, C. Gilon, and A. Levitzki. 1993. Selective inhibition of the epidermal growth factor and HER2/neu receptors by tyrphostins. J. Biol. Chem. 268**:**11134-11142. [PubMed] [Google Scholar]
- 55.Pearson, K. L., T. Hunter, and R. Janknecht. 1999. Activation of Smad1-mediated transcription by p300/CBP. Biochim. Biophys. Acta 1489**:**354-364. [DOI] [PubMed] [Google Scholar]
- 56.Poole, J. C., L. G. Andrews, and T. O. Tollefsbol. 2001. Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT). Gene 269**:**1-12. [DOI] [PubMed] [Google Scholar]
- 57.Scott, G. K., C. Marden, F. Xu, L. Kirk, and C. C. Benz. 2002. Transcriptional repression of ErbB2 by histone deacetylase inhibitors detected by a genomically integrated ErbB2 promoter-reporting cell screen. Mol. Cancer Ther. 1**:**385-392. [PubMed] [Google Scholar]
- 58.Shepherd, T. G., L. Kockeritz, M. R. Szrajber, W. J. Muller, and J. A. Hassell. 2001. The pea3 subfamily ets genes are required for HER2/Neu-mediated mammary oncogenesis. Curr. Biol. 11**:**1739-1748. [DOI] [PubMed] [Google Scholar]
- 59.Takakura, M., S. Kyo, T. Kanaya, H. Hirano, J. Takeda, M. Yutsudo, and M. Inoue. 1999. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 59**:**551-557. [PubMed] [Google Scholar]
- 60.Wosikowski, K., D. Schuurhuis, K. Johnson, K. D. Paull, T. G. Myers, J. N. Weinstein, and S. E. Bates. 1997. Identification of epidermal growth factor receptor and c-erbB2 pathway inhibitors by correlation with gene expression patterns. J. Natl. Cancer Inst. 89**:**1505-1515. [DOI] [PubMed] [Google Scholar]
- 61.Wu, J., and R. Janknecht. 2002. Regulation of the ETS transcription factor ER81 by the 90-kDa ribosomal S6 kinase 1 and protein kinase A. J. Biol. Chem. 277**:**42669-42679. [DOI] [PubMed] [Google Scholar]
- 62.Yang, B. S., C. A. Hauser, G. Henkel, M. S. Colman, C. Van Beveren, K. J. Stacey, D. A. Hume, R. A. Maki, and M. C. Ostrowski. 1996. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol. Cell. Biol. 16**:**538-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yano, Y., K. Yoshida, A. Osaki, T. Toge, H. Tahara, T. Ide, and W. Yasui. 2002. Expression and distribution of human telomerase catalytic component, hTERT, in human breast tissues. Anticancer Res. 22**:**4101-4107. [PubMed] [Google Scholar]
- 64.Yarden, Y. 2001. Biology of HER2 and its importance in breast cancer. Oncology 61**:**1-13. [DOI] [PubMed] [Google Scholar]
- 65.Yarden, Y., and M. X. Sliwkowski. 2001. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell. Biol. 2**:**127-137. [DOI] [PubMed] [Google Scholar]
- 66.Yu, D., and M. C. Hung. 2000. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 19**:**6115-6121. [DOI] [PubMed] [Google Scholar]
- 67.Yu, D., B. Liu, M. Tan, J. Li, S. S. Wang, and M. C. Hung. 1996. Overexpression of c-erbB-2/neu in breast cancer cells confers increased resistance to Taxol via mdr-1-independent mechanisms. Oncogene 13**:**1359-1365. [PubMed] [Google Scholar]