Evasion of Cellular Antiviral Responses by Human Cytomegalovirus TRS1 and IRS1 (original) (raw)
Abstract
During infection with human cytomegalovirus (HCMV), cellular protein synthesis continues even as viral proteins are being synthesized in abundance. Thus, HCMV may have a mechanism for counteracting host cell antiviral pathways that act by shutting off translation. Consistent with this view, HCMV infection of human fibroblasts rescues the replication of a vaccinia virus mutant lacking the double-stranded RNA-binding protein gene E3L (VVΔE3L). HCMV also prevents the phosphorylation of the eukaryotic translation initiation factor eIF-2α, the activation of RNase L, and the shutoff of viral and cellular protein synthesis that otherwise result from VVΔE3L infection. To identify the HCMV gene(s) responsible for these effects, we prepared a library of VVΔE3L recombinants containing HCMV genomic fragments. By infecting nonpermissive cells with this library and screening for VV gene expression and replication, we isolated a virus containing a 2.8-kb HCMV fragment that rescues replication of VVΔE3L. The fragment comprises the 3′ end of the J1S open reading frame through the entire TRS1 gene. Analyses of additional VVΔE3L recombinants revealed that the protein encoded by TRS1, pTRS1, as well as the closely related IRS1 gene, rescues VVΔE3L replication and prevent the shutoff of protein synthesis, the phosphorylation of eIF-2α, and activation of RNase L. These results demonstrate that TRS1 and IRS1 are able to counteract critical host cell antiviral response pathways.
Among the cellular responses to viral infections are at least two interferon-induced, double-stranded RNA (dsRNA)-activated pathways that are designed to shut off protein synthesis and thereby limit viral replication (reviewed in references 30 and 35). Activation of the protein kinase R (PKR) results in phosphorylation of the eukaryotic translation initiation factor eIF-2α, which in turn inhibits translation initiation. Oligoadenylate synthetases (OAS) activate RNase L, resulting in degradation of rRNA and mRNAs and thereby limiting the synthesis of both viral and cellular proteins.
Because viral replication requires protein synthesis, many viruses contain genes that counteract these pathways. Some of these genes encode inhibitory RNAs, such as VA1 RNA of adenovirus, while others encode proteins that interfere with various steps in the PKR and OAS/RNase L pathways (30, 35). Several viruses contain two or more genes that prevent the shutoff of protein synthesis. For example, vaccinia virus (VV) produces a dsRNA-binding protein, pE3L, as well as a PKR pseudosubstrate, pK3L (19). Herpes simplex virus type 1 (HSV-1) contains US11, which encodes a dsRNA-binding protein as well as γ34.5, the product of which stimulates the dephosphorylation of eIF-2α phosphate (18, 27).
Preventing activation of these antiviral response pathways is critical for viral pathogenesis. VV lacking E3L (VVΔE3L) exhibits markedly reduced virulence compared to that of wild-type (WT) VV in animal models (5, 39). An HSV-1 mutant lacking the γ34.5 gene exhibits reduced virulence in WT mice, but it is virulent in PKR-null mice, highlighting the specificity of γ34.5 for countering the PKR-mediated response (26).
Previously, we reported that human cytomegalovirus (HCMV) infection rescues replication and late gene expression of VVΔE3L and prevents the shutoff of protein synthesis, the phosphorylation of eIF-2α, and the activation of RNase L that otherwise result from VVΔE3L infection of human fibroblasts (HF) (8). These observations suggested that HCMV has one or more genes that are able to block the PKR and OAS/RNase L pathways. Here we report that TRS1 and the closely related IRS1 gene can each rescue VVΔE3L replication, maintain active protein synthesis, reduce the phosphorylation of eIF-2α, and block OAS/RNase L activation. Thus, HCMV has at least two genes that function in blocking key interferon-induced dsRNA-activated antiviral pathways.
MATERIALS AND METHODS
Cells and virus.
HF and BHK cells were maintained at 37°C and in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium supplemented with 10% NuSerum (Collaborative Biomedical), penicillin-streptomycin (100 U/ml), and 2 mM l-glutamine. VV Copenhagen strain (VC2) (38) and VVΔE3L (2), both obtained from Bertram Jacobs (Arizona State University), were propagated and titered in BHK cells. Growth and titration of VV stocks were carried out in BHK cells essentially as has been described (13), except that virus stocks were partially purified after cell lysis by centrifugation through a 36% sucrose cushion prior to resuspension in 1 mM Tris-HCl (pH 9.0), aliquoting, and storage at −70°C. β-Galactosidase (β-Gal) activity in infected cells was measured by a fluorometric substrate cleavage assay as has been described (3).
Construction of recombination plasmids and HCMV(Towne) genomic libraries.
Our initial VV recombination vector was derived from pSC11 (6) by first removing the Escherichia coli lacZ gene by digesting with _Xho_I, blunting with Klenow fragment, and then digesting with _Cla_I. pEQ853 resulted from insertion into these sites of an _Afe_I-to-_Cla_I fragment containing the enhanced green fluorescent protein (EGFP)-Puro cassette from pEGFP-Puro (1) after eliminating the _Bam_HI site by _Bam_HI digestion, blunting, and religation. To remove an _Eco_RI site and an upstream AUG codon, the PCR product containing the 7.5K and 11K VV promoters, resulting from amplification of pEQ853 with primers ACCGGTAGCTCGAGGTATAGCACAGAAAAAAACAAAATG and GCCATACGCTCACAGAATTCCCGGGATCCGT, was digested with _Bam_HI and _Xho_I and then reinserted into the same sites of pEQ853. The resulting plasmid, pEQ854, contains unique _Bam_HI and _Eco_RI sites immediately downstream of the VV 7.5K promoter in opposite orientation to that of the VV 11K promoter-EGFP-Puro cassette, and both are flanked by VV thymidine kinase (TK) sequences to allow insertion into the viral genome (Fig. 1).
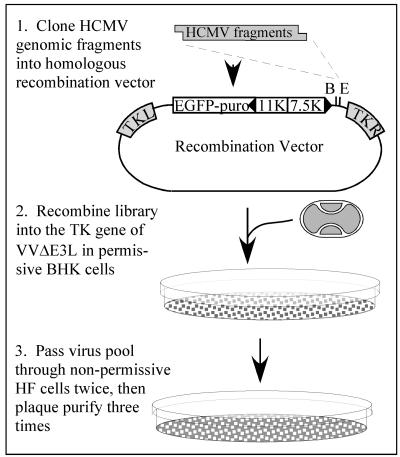
FIG. 1.
Strategy for selection of HCMV fragments that rescue VVΔE3L replication. HCMV genomic DNA, digested with various enzyme combinations as described in Materials and Methods to yield fragments containing either _Bam_HI (B)- or _Eco_RI (E)-compatible ends, was cloned into either of these sites in a VV homologous recombination vector. The insert is under the transcriptional control of the VV 7.5K promoter and, along with a VV11K promoter/EGFP-puromycin gene, is flanked by portions of the VV TK gene (TK left and TK right). The libraries of HCMV fragments were recombined into the TK locus of VVΔE3L in BHK cells, and the resulting virus pools were passed through VVΔE3L-nonpermissive HF in order to select recombinants containing HCMV genes that rescue VVΔE3L replication.
A later version of our recombination vector, pEQ862, was generated by replacing the 11K VV promoter in pEQ854 with the HCMV immediate-early promoter. The HCMV promoter was derived from pEGFP-Puro by digestion with _Spe_I and _Eco_RI, treatment with mung bean nuclease, and religation. A 605-bp _Age_I-_Xba_I fragment from the resulting plasmid was then inserted into pEQ854 that had been digested with _Age_I and partially digested with _Xba_I to remove an ∼111-bp fragment containing the p11K promoter.
An HCMV(Towne) genomic library was constructed by digesting viral DNA with the restriction enzymes _Eco_RI, _Apo_I, and _Mfe_I, both individually and in each pairwise combination. The fragments were pooled and ligated into pEQ854 at the _Eco_RI site. The resulting library clones were pooled, and DNA was purified by using a Qiagen plasmid maxi kit. A second library was constructed by inserting HCMV genomic fragments digested with _Bam_HI, _Bcl_I, and _Bgl_II, both individually and in pairwise combination, into the _Bam_HI site of pEQ854.
pEQ855, which contains the VV E3L gene, was constructed by PCR amplification of VC2 DNA by using oligonucleotides AGTTCTCTACCACCATGGCTAAGATCTA and GATAACTAGAATCAGAATCT. The product was first cloned into the pcDNA3.1/V5-His TOPO TA expression vector (Invitrogen) and was then excised with _Bam_HI and _Eco_RV and ligated into pEQ854 that had been digested with _Eco_RI, blunted with Klenow fragment, and then digested with _Bam_HI.
A 10-kb _Hin_dIII fragment containing HCMV sequences in VVCL1 (see below) was gel isolated and cloned into pUC19 that had been digested with _Hin_dIII. The resulting plasmid was then digested with _Apo_I (which also cuts at _Eco_RI sites), and the resulting 2.8-kb fragment containing the entire HCMV insert in VVCL1 was introduced into recombination vector pEQ862 that had been cut with _Eco_RI, yielding pEQ880. Plasmid pEQ883, containing TRS1 and 278 bp of upstream sequences, was constructed by digesting pEQ880 with _Bam_HI and _Xho_I to eliminate 95 bp corresponding to J1S gene sequences, blunting with a Klenow fragment, and then religating. pEQ906, containing the J1S region and intergenic sequences upstream of the TRS1 open reading frame (ORF), was generated by cloning a 385-bp _Bam_HI to a blunted _Nco_I fragment from pEQ880 into pEQ862 that had been digested with _Eco_RI, blunted with Klenow fragment, and then cut with _Bam_HI. To make pEQ904, a vector expressing only the TRS1 ORF was PCR amplified from HCMV(Towne) DNA by using oligonucleotides GCCTCGACGTCGGATCCGTCCGGCGGCCATGGCC (number 356) and CACAGAATTCTCGTAAGCATGTTGACAACTG (number 357). The resulting product was cloned into pcDNA3.1/V5-His TOPO TA, removed by digestion with _Bam_HI and _Eco_RI, and cloned into the same sites of pEQ862. A frameshift mutant of TRS1, pEQ919, was generated by PCR amplifying HCMV(Towne) DNA with oligonucleotides GCCTCGACGTCGGATCCGTCCGGCGGCCATGGACCCAGCGCAACGGCA and number 357 (described above). The resulting PCR product was cloned into pcDNA3.1/V5-His TOPO TA, removed by digestion with _Bam_HI and _Eco_RI, and cloned into pEQ862. The HCMV IRS1 gene was PCR amplified from HCMV(Towne) DNA by using oligonucleotides number 356 (described above) and CAGAATTCTGGACTGGAGAGACTTT, cloned into pcDNA3.1/V5-His TOPO TA, removed by digestion with _Bam_HI and _Eco_RI, and inserted into the same sites in pEQ862 in order to generate pEQ905.
Selection of VVCL1 and construction of other VVΔE3L-based recombinants.
For recombination of each HCMV library into VVΔE3L, BHK cells in 60-mm-diameter dishes were infected with VVΔE3L (multiplicity of infection [MOI], 0.1), and at 4 h postinfection (hpi) the cells were transfected with 4 μg of the library DNA by using Lipofectamine Plus reagent (Invitrogen) as specified by the manufacturer. After 72 hpi, the cells were collected and freeze-thawed three times, and half of the lysate was used to infect a 150-mm-diameter dish of VVΔE3L-nonpermissive HF. After 72 hpi, these cells were harvested, and 1/5 of the resulting lysate was used to infect HF cells in a fresh 150-mm-diameter dish. Cells were again harvested at 72 hpi, and dilutions of the resulting lysate were plated on 100-mm dishes of HF. A single plaque arising from this passage was plaque purified three times in HF prior to further analyses. The resulting recombinant, VVCL1, was analyzed by Southern blotting and sequencing (34).
Other recombinant viruses were made by VVΔE3L infection, followed by plasmid transfection of BHK cells and then with selection in HF as described above. Subsequently, recombinant viruses were plaque purified three times in HF cells prior to further analysis. Recombinant VVs that did not replicate in HF were identified and plaque purified by passage of the infection-transfection progeny through BHK cells. One of two duplicate plaque lifts on nitrocellulose circles was hybridized with [32P]dCTP probes that were prepared by random priming of sequences purified from recombinant plasmids. Positive plaques were chosen from the unhybridized duplicate filter, used to infect BHK cells, and subjected to plaque lifts two more times prior to growth and analysis. Recombinants VVs were named according to the names of the pEQ vector used in their construction. For example, VVeq855 was made by the recombination of pEQ855 into VVΔE3L.
DNA analysis.
HCMV(Towne) cosmid DNA was isolated from E. coli (22). HCMV DNA was purified from infected HF lysates, and VV DNA was purified from cytoplasmic extracts (14). The DNAs were digested with the indicated restriction enzymes, separated on 0.7% agarose gels, blotted to nitrocellulose, and analyzed by Southern blotting. Probes consisted of VVCL1 genomic DNA or cloned, purified HCMV TRS1 or J1S fragments labeled with [32P]dCTP by random priming.
Immunoblot analysis.
At the indicated times postinfection, cells were washed with phosphate-buffered saline and then lysed with 2% sodium dodecyl sulfate (SDS) at 65°C. Cellular and viral DNA was sheared by three to six 30-s pulses in a Bransonic 3 bath sonicator. The protein concentration was determined by fluoraldehyde _o_-phthalaldehyde (Pierce) assay (16), equivalent amounts of the resulting cell lysates were denatured at 95°C for 5 min and separated on SDS-12% polyacrylamide gels, and the proteins were transferred to a polyvinylidene difluoride transfer membrane by electroblotting. Immunoblot analysis was carried out with the Western-Star chemiluminescent detection system (TROPIX, Inc.) according to the manufacturer's recommendations. Monoclonal antibodies specific for pTRS1 and pIRS1 were obtained from Thomas Shenk (Princeton University) (33). Anti-phospho-eIF-2α and anti-eIF-2α polyclonal antisera were purchased from Cell Signaling Technology, Inc., and used according to the manufacturer's recommendations.
Radiolabeling of virus-infected cells.
At 24 h post-VV infection, HF were pulse labeled with Tran[35S]label (50 μCi/ml; ICN) in medium lacking methionine and cysteine for 1 h. The cells were then washed and lysed, and genomic DNA was sheared as described above. The protein concentrations were determined, and equivalent amounts of each lysate were separated on SDS-12% polyacrylamide gels, also as described above. The gels were dried and visualized by autoradiography.
RNA analysis.
Whole-cell RNA was harvested by using TRIzol Reagent (Invitrogen) and resolved on 1% formaldehyde gels, followed by blotting to nitrocellulose and Northern blot hybridization with an 18S RNA-specific probe generated by PCR amplification of HF DNA with oligonucleotides TACCTGGTTGATCCTGCCAG and TAATGATCCTTCCGCAGGTTC and labeling with [32P]dCTP by random priming.
RESULTS
Isolation of VVCL1, a VVΔE3L recombinant that replicates in HF.
In order to identify the HCMV gene or genes that rescue VVΔE3L replication in HF, we prepared a library of VVΔE3L recombinants containing HCMV(Towne) genomic fragments (Fig. 1). We first digested viral DNA with combinations of restriction enzymes and inserted the resulting fragments into either the _Eco_RI or the _Bam_HI site of a recombination vector adjacent to a VV 7.5K late promoter. After transfection of the _Eco_RI site library into VVΔE3L-infected BHK cells and serial passage of the progeny virus through VVΔE3L-nonpermissive HF, we isolated a single recombinant virus, VVCL1. A similar protocol carried out using the _Bam_HI site library yielded no virus that was able to replicate in HF. For comparison in subsequent experiments, we also constructed a virus, designated VVeq855, that contained the E3L gene inserted into the TK locus of VVΔE3L. Both VVCL1 and VVeq855, like WT VV (VC2), formed plaques in HF, while VVΔE3L did not (Fig. 2).
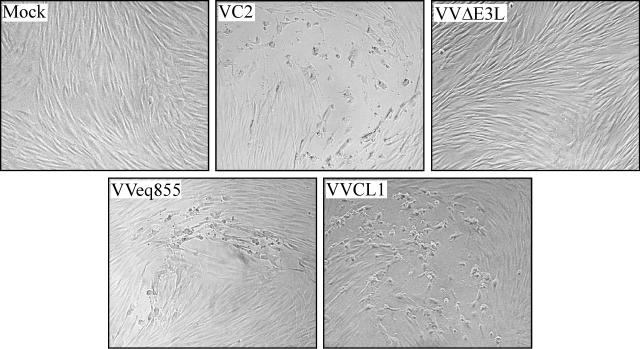
FIG. 2.
Plaque formation by VVCL1 in HF. HF were mock infected or infected with 10 to 100 PFU (as measured on BHK cells) of the indicated VV. Photomicrographs of plaques viewed 36 hpi by phase-contrast microscopy are shown. No plaques were detected in the mock-infected or VVΔE3L-infected wells (original magnification, ×200).
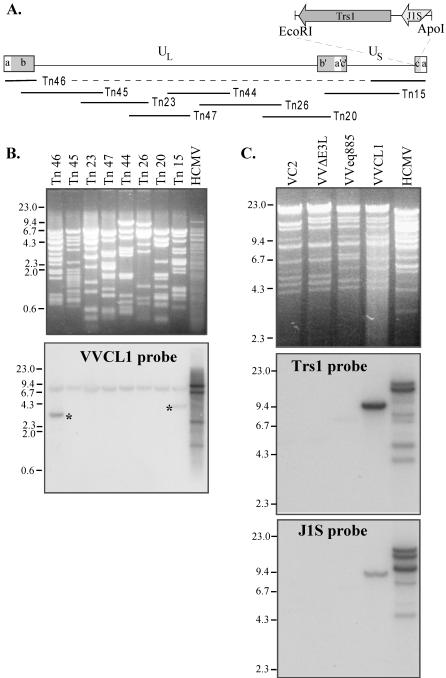
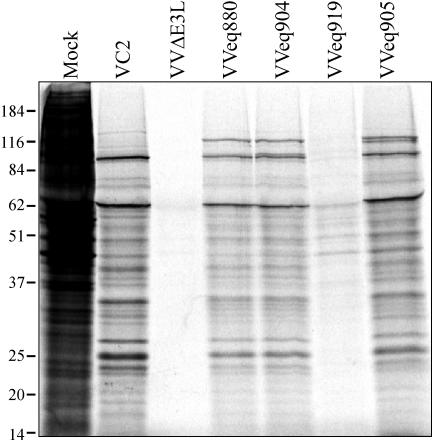
We next identified the HCMV fragment present in VVCL1. Southern blot analysis of a set of HCMV cosmids that was carried out by using VVCL1 genomic DNA as a probe revealed bands in lanes containing DNA from cosmids Tn_15_ and Tn_46_ (Fig. 3A and B). Shared sequences between these two cosmids include the entire a and c repeats and ∼13 kb from the middle of the unique short (US) region. The cross-hybridizing band (∼8 kb) present in all lanes corresponds to the cosmid backbone. After direct sequencing of VVCL1 DNA was carried out by using a TK right primer, which revealed sequences matching the 3′ end of the TRS1 gene (data not shown), we examined VV DNA by Southern blot hybridization using TRS1 and J1S probes (Fig. 3C). Both probes identified bands in VVCL1 but not in VC2, VVΔE3L, or VVeq855. HCMV lanes in these blots showed several bands, consistent with various terminal and junctional fragments expected to result from isomerization of the HCMV genome and amplification of the a sequence. Finally, we cloned a ∼10-kb _Hin_dIII fragment from VVCL1 containing the HCMV sequences along with the flanking EGFP-Puro cassette and sequenced the HCMV fragment present at the _Eco_RI site. This 2.8-kb fragment was 92% identical to nucleotides 227038 through 229768 of the HCMV-AD169 genome (GenBank accession number NC_001347) (29), including 160 bp that align with the 3′ end of the J1S ORF present in HCMV(AD169) (7) followed by 217 bp that included the putative TRS1 promoter, the entire 2.4-kb TRS1 ORF, and ∼42 bp downstream. The J1S sequence is poorly conserved among HCMV strains (7, 36), and a recent reevaluation suggests that it may not be a bona fide coding ORF (12).
FIG. 3.
Southern blot analyses of VVCL1. (A) The HCMV genome contains unique long (UL) and short (US) segments flanked by inverted repeats (ab, b′a′c′, ca). The locations of the HCMV cosmid inserts and of the _Eco_RI/_Apo_I fragment present in VVCL1 are shown. (B) DNAs from HCMV and from HCMV cosmids were digested with Eco_RI and Xho_I, separated electrophoretically, stained with ethidium bromide (top), and then hybridized with 32P-labeled VVCL1 DNA. Autoradiography (bottom) identified specific bands in Tn_46 and Tn_15 (*). (C) DNA from HCMV or from the indicated VV was digested with _Hin_dIII and then viewed by ethidium bromide staining (top) and hybridized with TRS1 (middle) and J1S (bottom) probes. VCL1 and HCMV, but none of the other VVs, contained TRS1 and IRS1 sequences. Molecular size markers (in kilobases) are indicated.
The protein encoded by TRS1 rescues VVΔE3L replication.
Additional experiments verified that VVCL1 replicated much more efficiently than did VVΔE3L. In single-step growth experiments, VVCL1 produced ∼60-fold more virus than did VVΔE3L (Fig. 4B). Titers of VVCL1 were 100-fold lower than those of VC2 but were only fivefold lower than those of VVeq855. Since VVΔE3L and its derivatives contain a lacZ gene that is under the control of a late VV promoter, monitoring β-Gal provides a measure of VV late gene expression (8). VVCL1 and VVeq855 expressed 50- to 100-fold-higher levels of β-Gal than did VVΔE3L (Fig. 4C). VC2 does not have the lacZ cassette and so could not be compared to the VVΔE3L-derived recombinants by using this assay. Surprisingly, neither VVCL1 nor VVeq855 expressed detectable levels of EGFP, even though they contained an EGFP-Puro cassette. We used a slightly modified recombination vector, pEQ862, to construct the viruses described below. Despite confirmation of the EGFP-Puro cassette structure and observations of high levels of EGFP expression from both recombination vectors (pEQ854 and pEQ862) in transient-infection-transfection experiments, none of our recombinant viruses expressed EGFP at a level detectable by fluorescence microscopy (data not shown). Although we cannot explain the lack of EGFP expression, we were able to construct and plaque purify the viruses by selection in HF and by plaque hybridization (as described in Materials and Methods), and we were able to verify their genomic structures by Southern blot hybridizations (data not shown).
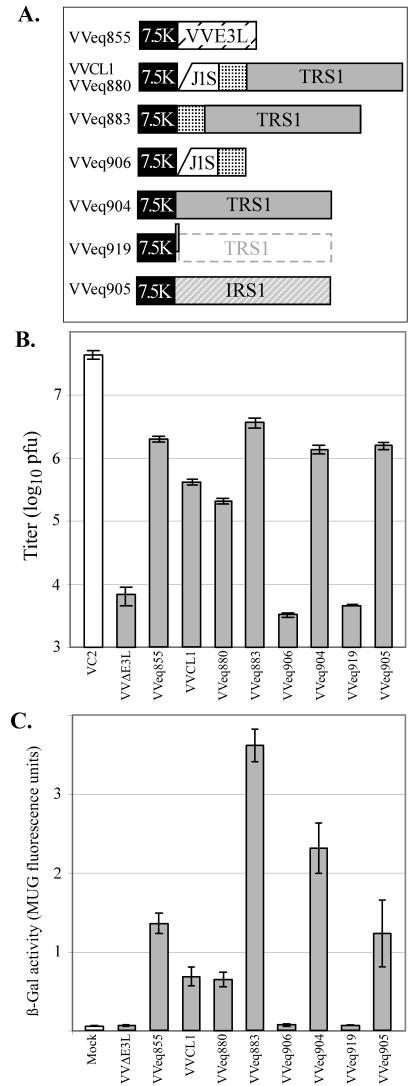
FIG.4.
Replication of VV in HF. (A) Recombinant VV viruses containing the 7.5K promoter driving expression of the E3L gene or of the indicated fragments from HCMV, including a portion of the J1S ORF, an intercistronic region (stippled), and the TRS1 or IRS1 ORF were constructed as described in Materials and Methods. VVeq880 contains the same HCMV insert as VVCL1, but they were made independently and used different recombination plasmids. Compared to VVeq904, VVeq919 contains a single-nucleotide insertion at the start of the TRS1 ORF. (B and C) HF were mock infected or infected with the indicated VV (MOI, 3). At 24 hpi (panel B), viral titers of freeze-thaw lysates were measured by plaque assays on BHK cells, and β-Gal activities (panel C) were measured as described in Materials and Methods. Means ± standard deviations of results for triplicate samples are shown.
In order to confirm that the 2.8-kb insert rather than some adventitious second site mutation arising during the selection of VVCL1 was responsible for the rescue of VVΔE3L, we introduced the entire 2.8-kb fragment present in VVCL1 back into VVΔE3L. The resulting virus, VVeq880, recapitulated the phenotype of VVCL1 in that it replicated more efficiently and produced more β-Gal than did VVΔE3L (Fig. 4). Thus, the 2.8-kb HCMV segment in VVCL1 most likely contains a gene or genes that can substitute for E3L during VV replication in HF.
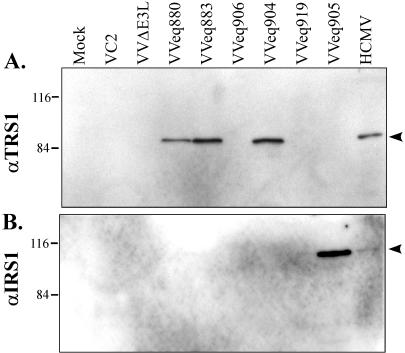
We next constructed additional VVΔE3L-based recombinants in order to determine which genetic element within the 2.8-kb insert in VVCL1 was responsible for rescuing VVΔE3L replication. VVeq883 contains the TRS1 promoter region and ORF but lacks the J1S segment, while VVeq906 contains the J1S segment and the TRS1 promoter region but not the TRS1 ORF (Fig. 4A). Immunoblot assays confirmed that VVeq883 expressed the _TRS1_-encoded protein, pTRS1, while VVeq906 did not (Fig. 5). VVeq883, but not VVeq906, replicated efficiently and produced high levels of β-Gal (Fig. 4), indicating that the TRS1 gene rather than the J1S segment contained the sequences required for rescue of VVΔE3L. The ∼10-fold higher titers and β-Gal expression resulting from VVeq883 compared to that of VVCL1 and VVeq880 might be due to an inhibitory effect of the J1S fragment on expression of pTRS1. Consistent with this possibility, VVeq883 appeared to express more pTRS1 than did VVeq880 (Fig. 5).
FIG. 5.
Identification of protein products expressed by VVΔE3L recombinant viruses. BHK cells were infected with the different recombinant viruses as indicated in Materials and Methods. Cell lysates were prepared 24 h later and, along with lysates of HCMV-infected HF, were separated by SDS-PAGE and analyzed by immunoblot assay using antibody specific for the TRS1 protein (A) and the IRS1 protein (B). Molecular size markers (in kilodaltons) are shown to the left.
We next constructed the virus VVeq904, which contained only the TRS1 ORF without the intercistronic and TRS1 promoter sequences. In this virus, the TRS1 ORF is expected to be under the transcriptional control of only the VV 7.5K promoter. VVeq904 replicated efficiently and expressed high levels of β-Gal, similar to VVeq883 (Fig. 4). Thus, the TRS1 ORF, but neither the J1S segment nor the intercistronic region between the J1S and TRS1 ORFs, was necessary to rescue VVΔE3L replication and late gene expression.
In other viral systems, RNAs and proteins have been identified that block the PKR pathway. To assess whether rescue of VVΔE3L required pTRS1 or only the TRS1 transcript, we constructed another recombinant, VVeq919, that contained the TRS1 fragment but with a single extra nucleotide inserted into the second codon of the TRS1 ORF. This insertion was designed to eliminate production of pTRS1 with minimal alteration of the mRNA structure. Instead of pTRS1, translation of this mRNA would be expected to produce a 72-amino-acid peptide from an alternative reading frame. We detected no full-length or truncated pTRS1 in cells infected with VVeq919 by immunoblot analysis (Fig. 5A). VVeq919 replicated to a very limited extent and produced low levels of β-Gal in HF, as was true for VVΔE3L (Fig. 4). This result strongly suggests that the protein product of TRS1, not its transcript, is responsible for the rescue of VVΔE3L.
IRS1 also complements VVΔE3L replication.
Because TRS1 is partially encoded within the c repeats that flank the US region of the HCMV genome (Fig. 3), a second viral gene product, pIRS1, is highly related. In HCMV(AD169), the TRS1 and IRS1 ORFs are identical over the 5′-proximal 549 codons, corresponding to ∼2/3 of the corresponding proteins, except for a single nucleotide difference reported to be at codon 190 (7). To investigate whether IRS1 can rescue VVΔE3L replication, we constructed a VVΔE3L-based recombinant virus containing the entire IRS1 ORF. We confirmed by immunoblot assay (Fig. 5B) that this virus, VVeq905, expressed pIRS1. In a single-step growth experiment, VVeq905 replicated to approximately the same level and expressed similar levels of β-Gal as did the recombinants that express pTRS1 (Fig. 4). These results identify IRS1 as a second HCMV gene that is able to complement the replication defect of VVΔE3L.
TRS1 and IRS1 prevent translational shutoff as well as PKR and RNase L activation by VVΔE3L.
VVΔE3L infection of most cell types shuts off overall protein synthesis, at least in part, as a result of activation of the PKR and OAS/RNase L pathways (19). In HF, prior infection with HCMV blocks each of these effects of VVΔE3L (8). To determine whether the TRS1 and IRS1 genes inhibit these translational effects of VVΔE3L infection, we monitored overall protein synthesis as well as the PKR and OAS/RNase L pathways in cells that were infected with various VV recombinants.
To assess protein synthesis, we metabolically labeled proteins at 24 hpi. SDS-polyacrylamide gel electrophoresis (PAGE) and autoradiography revealed the expected marked reduction in protein synthesis in cells infected with VVΔE3L compared to those infected with VC2 (Fig. 6). Cells infected with VVeq880 and VVeq904 (expressing pTRS1) and with VVeq905 (expressing pIRS1) synthesized proteins nearly as efficiently as those infected with VC2. The observation that VVeq904 and VVeq905 expressed two- to fourfold more β-Gal than did VVeq880 (Fig. 4C), despite similarities in overall protein synthesis, may be due to the fact that the β-Gal assay measures accumulation over 24 h rather than measuring synthesis during a 1-h pulse at 24 hpi. Regardless, protein synthesis was greatly inhibited in cells infected with the TRS1 frameshift mutant VVeq919, similar to results with VVΔE3L, indicating that pTRS1 is required to rescue the protein synthetic defect of VVΔE3L.
FIG. 6.
Protein synthesis in HF infected with various VVΔE3L recombinant viruses. HF were mock infected or infected with the indicated virus (MOI, 3) and labeled 24 h later for 1 h with [35S]methionine. Cell extracts were then prepared, and 20 μg of each was analyzed by SDS-PAGE and autoradiography. Molecular size markers (in kilodaltons) are indicated on the left.
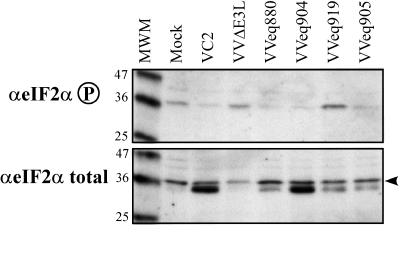
We examined the PKR pathway by immunoblot assays of total and phosphorylated eIF-2α. Cells infected with VC2 and with each recombinant that expressed pTRS1 or pIRS1 contained a lower level of phosphorylated eIF-2α (Fig. 7, top) than did mock-infected HF. Cells infected with VVΔE3L or VVeq919 contained at least as much phosphorylated eIF-2α as did mock-infected cells. The pattern of total eIF-2α was somewhat surprising in that VV-infected cells contained an additional immunoreactive band having slightly faster mobility than did the major form of eIF-2α present in mock-infected cells (Fig. 7, bottom). This band did not appear consistently in other experiments. Regardless of whether this band represents a modified form of eIF-2α or a cross-reactive protein, the increased level of phosphorylated eIF-2α seen in cells infected with VVΔE3L and VVeq919 was not simply due to there being more total eIF-2α in these cells. Even if only the upper band in the eIF-2α total blot is considered, the relative amount of phosphorylated eIF-2α is considerably greater in cells infected with VVΔE3L or VVeq919 than it is in cells infected with viruses that express TRS1, IRS1, or E3L. If the lower band is also a form of eIF-2α, then the differences in relative phosphorylation of eIF-2α would be even greater. Therefore, we conclude that TRS1 and IRS1 are able to reduce the level of phosphorylated eIF-2α in infected cells.
FIG. 7.
Phosphorylation of eIF-2α after infection with the VVΔE3L recombinant viruses. HF were mock infected or infected with the indicated virus (MOI, 3). Cell lysates were prepared after 24 h, separated by SDS-PAGE, and analyzed by immunoblot assay using antibody specific for phosphorylated eIF-2α or total eIF-2α. Molecular size markers (in kilodaltons) are indicated to the left of each panel.
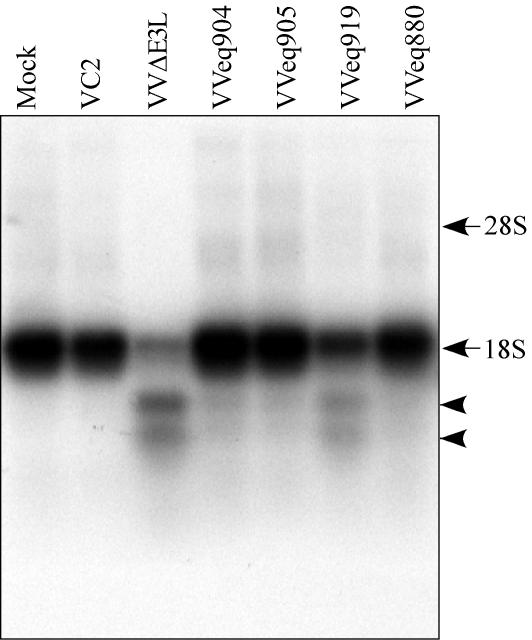
Finally, we examined the integrity of rRNA in infected cells to assess the OAS/RNase L pathway. Activation of RNase L results in degradation of rRNA as was detected here by Northern blot hybridization of whole-cell RNA using an 18S rRNA probe (Fig. 8). In mock-infected cells and cells infected with VC2, VVeq904, VVeq905, and VVeq880, the 18S rRNA was largely intact. Cells infected with the TRS1 frameshift mutant VVeq919 contained some degraded rRNA, although the extent of degradation appeared to be less than that seen after VVΔE3L infection. Thus, expression of pTRS1 and pIRS1 reduces RNase L activity during infection by VVΔE3L.
FIG. 8.
RNase L activity in cells infected with the VVΔE3L recombinant viruses. HF were mock infected or infected with the viruses indicated (MOI, 3). At 24 hpi, RNA was harvested, and 2 μg of each sample was analyzed by formaldehyde agarose gel electrophoresis and by Northern blot hybridization using a 32P-labeled 18S rRNA-specific probe. Characteristic 18S rRNA RNase L cleavage products (arrowheads) and the positions of intact rRNA bands made visible by ethidium bromide staining (arrows) are indicated.
DISCUSSION
Many viruses, including members of the α and γ subfamilies of the Herpesviridae, contain genes designed to prevent the shutoff of protein synthesis that otherwise results from activation of the PKR and OAS/RNase L pathways (30, 31, 35). Little is known about whether members of the betaherpesvirus subfamily also interfere with these pathways. The finding that HCMV infection complements VVΔE3L replication and that it prevents eIF-2α phosphorylation, RNase L activation, and the shutoff of protein synthesis in cells coinfected with VVΔE3L suggested that HCMV has one or more genes capable of interfering with these cellular antiviral responses (8). Here we report the identification of TRS1 and IRS1 as two such HCMV genes.
Our previous observation that HCMV, but not UV-inactivated HCMV, rescued VVΔE3L replication indicated that complementation requires de novo protein synthesis after HCMV infection (8). Since ganciclovir treatment did not abolish the effect, we hypothesized that the responsible gene was most likely an immediate-early or early gene. pTRS1 and pIRS1 are in fact synthesized from immediate-early times onward throughout infection. However, they are also present in virions (32). A possible explanation for the failure of UV-inactivated HCMV to rescue VVΔE3L in our previous studies is that too little TRS1 and IRS1 protein might be delivered to cells when an MOI of 3 PFU/cell was used, or perhaps they persist for too short a time to rescue VVΔE3L. Rather, de novo synthesis of these proteins may be necessary to prevent shutoff of protein synthesis during the time period from 24 to 48 h post-HCMV infection, during which we monitored VVΔE3L replication (8).
TRS1 and IRS1 counteract all the effects of VVΔE3L that we examined. The frameshift mutant of TRS1 behaved most similarly to VVΔE3L, supporting the conclusion that pTRS1 (or pIRS1), rather than just the transcript, is responsible for complementing the deletion of E3L. However, we did detect minor but reproducible differences in protein synthesis and RNase L activity in cells that were infected with the TRS1 frameshift recombinant compared to those observed for VVΔE3L (Fig. 6 and 8). Ribosomal frameshifting, leaky scanning, or reinitiation could result in expression of a low level of full-length or truncated but active pTRS1. Studies of HSV-1 revealed that a γ34.5 gene nonsense mutant, unlike a deletion mutant, rescued protein synthesis in some cells, suggesting that a low but functionally significant level of full-length or truncated protein was being produced (9). Thus, although we did not detect any TRS1 protein by immunoblot assay (Fig. 5), even a low level of pTRS1 expression might account for the observed slight rescue of protein synthesis and inhibition of RNase L by VVeq919.
The rescue of VVΔE3L replication by TRS1 and IRS1 likely depends at least in part on their ability to prevent activation of the PKR and OAS/RNase L pathways, thereby enabling continued protein synthesis. VVΔE3L grows better in PKR-null, RNase L-null mouse fibroblasts than in WT fibroblasts, supporting the conclusion that the PKR and OAS/RNase L pathways are key E3L targets (39). However, VVΔE3L does not replicate as efficiently as WT VV in PKR-null, RNase L-null cells. One possible explanation for these observations is that other effects of E3L, such as blocking activation of interferon regulatory factor 3, may also be important for VV replication (39). The fact that recombinants containing TRS1 or IRS1 replicate as well as does VVeq855, in which E3L is also expressed from the TK locus, suggests that TRS1 and IRS1 have the same activities necessary for VV replication in HF as does E3L. However, elucidation of the biochemical mechanism by which TRS1 and IRS1 function will be needed to clarify whether TRS1 and IRS1 affect all or only some of the same cellular pathways as does E3L.
The effects of pTRS1 and pIRS1 reported here suggest that they have direct effects on the host cell translational machinery. Both proteins localize primarily to the cytoplasm, at least at late times of infection (33). Their ability to substitute for E3L protein (a known dsRNA-binding protein) in preventing eIF-2α phosphorylation and RNase L activation would be explained by dsRNA-binding activity, although they do not contain any known dsRNA-binding motifs (15, 24). Other studies have suggested that pTRS1 and pIRS1 act as transcriptional regulators of gene expression. They stimulate reporter gene expression in transient-transfection assays, and an alternative IRS1 transcript produces a truncated protein that acts as a repressor in transfection assays (23, 33, 37). Although it is possible that pTRS1 and pIRS1 rescue VVΔE3L indirectly by activating transcription of a cellular gene that in turn blocks the PKR and OAS/RNase L pathways, VV replication shuts off expression of most cellular genes (17). Moreover, the effects of pTRS1 and pIRS1 in transient-transfection studies do not exclude the possibility that they act at the translational level. Their stimulatory effects on reporter gene expression might be due to enhanced translation of the reporter gene mRNA itself, as has been reported for the adenovirus VAI RNA and VV E3L and K3L genes (10, 11, 21). It is also possible that TRS1 and IRS participate in both transcriptional and translational control of gene expression.
Since both TRS1 and IRS1 complement VVΔE3L, the identical amino-terminal 2/3 of these genes most likely contains the domain responsible for these effects. If so, then the deletion of both genes may be necessary to detect inhibition of protein synthesis in HCMV-infected cells. HCMV mutants have been constructed that contain single deletions of all of IRS1 or of the carboxy terminus of TRS1 or of IRS1 (4, 20). Unlike the IRS1 mutants, which have no replication defect in HF, the TRS1 C-terminal mutant produces ∼200-fold less virus than does WT HCMV, apparently due to a packaging defect. Since this phenotype occurs in a virus that expresses pIRS1 and may also express the amino-terminal 2/3 of pTRS1, we suspect that the packaging defect is unrelated to the ability of pTRS1 to complement VVΔE3L. These observations support the hypothesis that pTRS1 has multiple distinct functions.
Our strategy for identifying HCMV genes that can block the PKR and OAS pathways took advantage of the well-characterized VV mutant VVΔE3L, a virus that is interferon sensitive and avirulent and that replicates poorly in most cultured cells (5, 8, 19). Previous studies showing that genes from other viruses, including the NSP4 gene from group C rotavirus and the S4 gene from reovirus, rescue VVΔE3L replication suggested that this approach might be feasible (2, 25). However, we isolated only one recombinant, VVCL1, containing TRS1 and did not identify the IRS1 gene which, as we discovered later, also complements VVΔE3L. Therefore, technical limitations in this method may have limited our ability to detect other HCMV genes that rescue VVΔE3L. For example, TRS1 and IRS1 may be members of a gene family (7, 28), other members of which might also participate in the preservation of protein synthetic capacity during infection. Given the importance of genes that counteract the cellular PKR and OAS/RNase L pathways in other viral systems, TRS1, IRS1, and possibly other HCMV genes that counter these host cell responses are likely to be critical determinants of HCMV pathogenesis.
Acknowledgments
We thank Bertram Jacobs (Arizona State University) for VC2 and VVΔE3L, George Kemble (MedImmune, Inc.) for HCMV(Towne) cosmids, Michael McVoy (Medical College of Virginia) for pEGFP-Puro, Tom Shenk (Princeton) for antibodies to TRS1 and IRS1, and Victoria Harper and the Fred Hutchinson Cancer Research Center Biotechnology and Image Analysis Resources for technical assistance.
This work was supported by Public Health Service grant number AI26672.
REFERENCES
- 1.Abbate, J., J. C. Lacayo, M. Prichard, G. Pari, and M. A. McVoy. 2001. Bifunctional protein conferring enhanced green fluorescence and puromycin resistance. BioTechniques 31**:**336-340. [DOI] [PubMed] [Google Scholar]
- 2.Beattie, E., K. L. Denzler, J. Tartaglia, M. E. Perkus, E. Paoletti, and B. L. Jacobs. 1995. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J. Virol. 69**:**499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biegalke, B. J., and A. P. Geballe. 1990. Translational inhibition by cytomegalovirus transcript leaders. Virology 177**:**657-667. [DOI] [PubMed] [Google Scholar]
- 4.Blankenship, C. A., and T. Shenk. 2002. Mutant human cytomegalovirus lacking the immediate-early TRS1 coding region exhibits a late defect. J. Virol. 76**:**12290-12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75**:**850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5**:**3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchison III, T. Kouzarides, J. A. Martignetti, E. Preddie, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169, p. 125-169. In J. K. McDougall (ed.), Cytomegaloviruses, vol. 154. Springer-Verlag, New York, N.Y. [DOI] [PubMed]
- 8.Child, S. J., S. Jarrahian, V. M. Harper, and A. P. Geballe. 2002. Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus. J. Virol. 76**:**4912-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, J., A. P. Poon, J. Johnson, and B. Roizman. 1994. Differential response of human cells to deletions and stop codons in the γ134.5 gene of herpes simplex virus. J. Virol. 68**:**8304-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, M. V., H. W. Chang, B. L. Jacobs, and R. J. Kaufman. 1993. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J. Virol. 67**:**1688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, M. V., O. Elroy-Stein, R. Jagus, B. Moss, and R. J. Kaufman. 1992. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J. Virol. 66**:**1943-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davison, A. J., A. Dolan, P. Akter, C. Addison, D. J. Dargan, D. J. Alcendor, D. J. McGeoch, and G. S. Hayward. 2003. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 84**:**17-28. [DOI] [PubMed] [Google Scholar]
- 13.Earl, P. L., N. Cooper, L. S. Wyatt, B. Moss, and M. W. Carroll. 2003. Preparation of cell cultures and vaccinia virus stocks, unit 16.16. In F. M. Ausubel, R. Brent, R. E. Kingston, D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology online. John Wiley and Sons, New York, N.Y.
- 14.Earl, P. L., B. Moss, L. S. Wyatt, and M. W. Carroll. 2003. Generation of recombinant vaccinia viruses, unit 16.17. In F. M. Ausubel, R. Brent, R. E. Kingston, D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology online. John Wiley and Sons, New York, N.Y.
- 15.Fierro-Monti, I., and M. B. Mathews. 2000. Proteins binding to duplexed RNA: one motif, multiple functions. Trends Biochem. Sci. 25**:**241-246. [DOI] [PubMed] [Google Scholar]
- 16.Geballe, A. P., and E. S. Mocarski. 1988. Translational control of cytomegalovirus gene expression is mediated by upstream AUG codons. J. Virol. 62**:**3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerra, S., L. A. Lopez-Fernandez, A. Pascual-Montano, M. Munoz, K. Harshman, and M. Esteban. 2003. Cellular gene expression survey of vaccinia virus infection of human HeLa cells. J. Virol. 77**:**6493-6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He, B., M. Gross, and B. Roizman. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1[alpha] to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94**:**843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs, B. L. 2000. Translational control in poxvirus-infected cells, p. 951-971. In W. B. Hershey, M. B. Matthews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Jones, T. R., and V. P. Muzithras. 1992. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J. Virol. 66**:**2541-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman, R. J., M. V. Davies, V. K. Pathak, and J. W. Hershey. 1989. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol. Cell. Biol. 9**:**946-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemble, G., G. Duke, R. Winter, and R. Spaete. 1996. Defined large-scale alterations of the human cytomegalovirus genome constructed by cotransfection of overlapping cosmids. J. Virol. 70**:**2044-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerry, J. A., M. A. Priddy, T. Y. Jervey, C. P. Kohler, T. L. Staley, C. D. Vanson, T. R. Jones, A. C. Iskenderian, D. G. Anders, and R. M. Stenberg. 1996. Multiple regulatory events influence human cytomegalovirus DNA polymerase (UL54) expression during viral infection. J. Virol. 70**:**373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoo, D., C. Perez, and I. Mohr. 2002. Characterization of RNA determinants recognized by the arginine- and proline-rich region of Us11, a herpes simplex virus type 1-encoded double-stranded RNA binding protein that prevents PKR activation. J. Virol. 76**:**11971-11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langland, J. O., S. Pettiford, B. Jiang, and B. L. Jacobs. 1994. Products of the porcine group C rotavirus NSP3 gene bind specifically to double-stranded RNA and inhibit activation of the interferon-induced protein kinase PKR. J. Virol. 68**:**3821-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97**:**6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulvey, M., J. Poppers, A. Ladd, and I. Mohr. 1999. A herpesvirus ribosome-associated, RNA-binding protein confers a growth advantage upon mutants deficient in a GADD34-related function. J. Virol. 73**:**3375-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novotny, J., I. Rigoutsos, D. Coleman, and T. Shenk. 2001. In silico structural and functional analysis of the human cytomegalovirus (HHV5) genome. J. Mol. Biol. 310**:**1151-1166. [DOI] [PubMed] [Google Scholar]
- 29.Pearson, W. R., T. Wood, Z. Zhang, and W. Miller. 1997. Comparison of DNA sequences with protein sequences. Genomics 46**:**24-36. [DOI] [PubMed] [Google Scholar]
- 30.Pe'ery, T., and M. B. Mathews. 2000. Viral translational strategies and host defense mechanisms, p. 371-424. In W. B. Hershey, M. B. Matthews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Poppers, J., M. Mulvey, C. Perez, D. Khoo, and I. Mohr. 2003. Identification of a lytic-cycle Epstein-Barr virus gene product that can regulate PKR activation. J. Virol. 77**:**228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romanowski, M. J., E. Garrido-Guerrero, and T. Shenk. 1997. pIRS1 and pTRS1 are present in human cytomegalovirus virions. J. Virol. 71**:**5703-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romanowski, M. J., and T. Shenk. 1997. Characterization of the human cytomegalovirus irs1 and trs1 genes: a second immediate-early transcription unit within irs1 whose product antagonizes transcriptional activation. J. Virol. 71**:**1485-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 35.Schneider, R. J., and I. Mohr. 2003. Translation initiation and viral tricks. Trends Biochem. Sci. 28**:**130-136. [DOI] [PubMed] [Google Scholar]
- 36.Spaete, R. R., and E. S. Mocarski. 1985. The a sequence of the cytomegalovirus genome functions as a cleavage/packaging signal for herpes simplex virus defective genomes. J. Virol. 54**:**817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stasiak, P. C., and E. S. Mocarski. 1992. Transactivation of the cytomegalovirus ICP36 gene promoter requires the α gene product TRS1 in addition to IE1 and IE2. J. Virol. 66**:**1050-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tartaglia, J., M. E. Perkus, J. Taylor, E. K. Norton, J. C. Audonnet, W. I. Cox, S. W. Davis, J. van der Hoeven, B. Meignier, M. Riviere, et al. 1992. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188**:**217-232. [DOI] [PubMed] [Google Scholar]
- 39.Xiang, Y., R. C. Condit, S. Vijaysri, B. Jacobs, B. R. Williams, and R. H. Silverman. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 76**:**5251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]