Oxygen sensing and signaling: impact on the regulation of physiologically important genes (original) (raw)
. Author manuscript; available in PMC: 2011 Feb 26.
Abstract
A growing number of physiologically relevant genes are regulated in response to changes in intracellular oxygen tension. It is likely that cells from a wide variety of tissues share a common mechanism of oxygen sensing and signal transduction leading to the activation of the transcription factor hypoxia-inducible factor 1 (HIF-1). Besides hypoxia, transition metals (Co2+, Ni2+ and Mn2+) and iron chelation also promote activation of HIF-1. Induction of HIF-1 by hypoxia is blocked by the heme ligands carbon monoxide and nitric oxide. There is growing, albeit indirect, evidence that the oxygen sensor is a flavoheme protein and that the signal transduction pathway involves changes in the level of intracellular reactive oxygen intermediates. The activation of HIF-1 by hypoxia depends upon signaling-dependent rescue of its α-subunit from oxygen-dependent degradation in the proteasome, allowing it to form a heterodimer with HIF-1β (ARNT), which then translocates to the nucleus and impacts on the transcription of genes whose cis-acting elements contain cognate hypoxia response elements.
Keywords: Gene regulation, oxygen sensing, Hypoxia, hypoxia-inducible factor 1, cellular sensor, signal transduction, Oxygen, sensing, genes
1. Introduction
Man and other mammals adapt to hypoxia by a number of physiologically appropriate responses, such as increased production of erythropoietin (Epo) which augments the red cell mass, induction of tyrosine hydroxylase (TH) which facilitates the control of ventilation via the carotid body, and stimulation of new blood vessels by up-regulation of vascular endothelial growth factor (VEGF) (Bunn and Poyton, 1996). The regulation of the genes encoding these proteins depends upon accurate sensing of pO2 and transduction of a signal that activates HIF-1, a heterodimeric transcription factor that enables enhanced transcription (Guillemin and Krasnow, 1997). It is likely that the mechanism for the sensing of oxygen and its subsequent signaling departs from well established systems of receptor binding. Due to oxygen’s peculiar chemical properties, there is a limited repertoire of molecules to which it can combine. Oxygen is known to bind to and react with heme proteins (and, in certain invertebrates, hemerythrin, hemocyanin and chlorocruorin). Heme proteins play a critical role in oxygen sensing by bacteria and yeast (Bunn and Poyton, 1996). Considering that higher eukaryotes are exposed to less varied environmental stimuli but must respond to more diverse and complex internal stimuli, it is likely that they have become endowed with more elaborate and delicate mechanisms for oxygen sensing and signal transduction. In all vertebrates and many non-vertebrates, oxygen transport depends on the circulation of erythrocytes which enable oxygen unloading to tissues at relatively high O2 tension. Therefore in order to monitor perturbations in oxygen transport there is need for sensors with relatively low oxygen affinity.
Initial work on oxygen sensing and signal transduction began with studies on neural transmission in the carotid body and erythropoietin production in hepatic cell lines. The subsequent discovery of HIF-1 (Wang et al., 1995) and the realization that the sensing and signaling process is probably shared among many if not all types of cells (Maxwell et al., 1993) have prompted experiments in a variety of other experimental systems. HIF-1 is a heterodimeric protein composed of HIF-1α and HIF-1β (ARNT) subunits, both of which belong to the basic helix–loop–helix PAS family (Wang and Semenza, 1993b; Wang et al., 1995). At the mRNA level, both HIF-1α and ARNT genes are constitutively expressed and not significantly up-regulated by hypoxia (Gradin et al., 1996; Huang et al., 1996; Wood et al., 1996; Kallio et al., 1997). Whereas changes in oxygen tension fails to affect ARNT protein abundance, hypoxia markedly increases levels of HIF-1α (Wang et al., 1995; Huang et al., 1996; Kallio et al., 1997), rescuing the subunit from oxygen-dependent degradation in the proteasome (Huang et al., 1998). Thus, hypoxia-induced activation of HIF-1 depends in part on post-translational stabilization of HIF-1α.
1.1. Carotid body
Ventilation in mammals, birds and perhaps fish, is regulated in part by the carotid body (Dejours, 1981; Schmidt-Nielson, 1990). This very small and highly vascular organ, located in man at the bifurcation of the carotid artery, is composed of glomus type I chemoreceptor cells which, upon challenge with hypoxia, release neurotransmitters that set the level of electrical activity in the afferent fibers of the carotid sinus nerve. Type I cells have voltage dependent K+, Na+ and Ca+ + channels (López-Barneo et al., 1988). Patch clamp studies on isolated plasma membranes from type I cells have shown that a single K+ channel type is down-regulated by lowering oxygen tension (Ganfornina and López-Barneo, 1991). The rapid response indicates that the oxygen sensing mechanism is independent of transcription and translation. Moreover, this physiologically relevant oxygen sensor appears to be localized in the plasma membrane as opposed to somewhere in the cell interior. The addition of relatively low levels of carbon monoxide to the hypoxic gas mixture substantially reverses the inhibition of K+ currents (López-López and González, 1992) and the chemosensory nerve discharge (Lahiri et al., 1993). In excitable O2 sensitive cells, intracellular free Ca2+ may be a primary mechanism for gene regulation (Raymond and Millhorn, 1997).
1.2. Erythropoietin production
Erythropoietin is a glycoprotein hormone that regulates proliferation and differentiation of erythroid cells (Jelkmann, 1992; Porter and Goldberg, 1993). A large number of classic physiologic studies demonstrated that Epo production is markedly enhanced by hypoxia. The only other known stimulus to Epo production in vivo is the administration of certain transition metals. Experiments with intact animals (Goldwasser et al., 1958) as well as perfused kidneys (Fisher and Langston, 1968) demonstrated that cobaltous chloride stimulates erythropoiesis by increasing the production of Epo. Intrarenal injections of nickel have also been shown to induce erythrocytosis (Jasmin and Solymoss, 1975; Morse et al., 1977). When human hepatoma cells (Hep3B or HepG2) were incubated for 24 h in the presence of increasing amounts of CoCl2 or NiCl2 there was a dose-dependent enhancement of Epo mRNA expression (Goldberg et al., 1988; Fandrey and Bunn, 1993) and protein production (Goldberg et al., 1988) similar to that observed with increasing degrees of hypoxia. When other transition metals (manganese, zinc, iron, cadmium, and tin) were tested, only manganese induced measurable Epo protein, but less than that obtained by either cobalt or nickel. Hypoxic induction of erythropoietin protein (Goldberg et al., 1988) and mRNA (Huang et al., 1999) in Hep3B cells was markedly inhibited by the presence of carbon monoxide (CO). In contrast to its effect in hypoxic cells, CO did not inhibit the induction by cobalt or nickel of Epo protein (Goldberg et al., 1988) or mRNA (Huang et al., 1999).
2. Evidence that the sensor is a heme protein
The effect of carbon monoxide on oxygen sensing signaling extends beyond the carotid body and the production of erythropoietin in Hep3B cells. CO has also been shown to offset hypoxia’s effect on the expression of VEGF (Goldberg and Schneider, 1994), platelet derived growth factor (Morita and Kourembanas, 1995), endothelin-1 (Morita and Kourembanas, 1995) and phosphoenolpyruvate carboxykinase (Kietzmann et al., 1993). More recently, Liu et al. (1998) and our laboratory (Huang et al., 1999) have shown that CO suppresses the hypoxic activation of HIF-1. Carbon monoxide has remarkable specificity in biological systems, binding non-covalently to ferrous heme groups in hemoglobin, myoglobin, certain cytochromes and other heme proteins. Thus these experiments strongly support the hypothesis that the oxygen sensor is a heme protein. The results with CO indicate that the affinity of this ligand for the heme group in the sensor is low, compared to hemoglobin which binds CO over 200-fold more tightly than oxygen. It is likely that the sensor’s low affinity for CO has adaptive significance. The primary toxicity of CO in higher organisms is due to its high affinity binding to hemoglobin, locking the tetramer in the ‘oxy’ comformation and thereby increasing oxygen affinity and decreasing oxygen unloading to tissues. When subjected to CO-induced hypoxic stress, the organism needs intact oxygen sensors. These sensors would be unresponsive if they had high affinity for CO.
The induction of Epo by the transition metals Co2+, Ni2+ and Mn2+ led to experiments which support the hypothesis that cobalt, nickel and manganese atoms substitute for the iron atom in the heme moiety of the oxygen sensor (Goldberg et al., 1988). Cobalt has been shown to be a substrate for ferrochelatase, the enzyme responsible for the incorporation of iron into protoporphyrin IX to make heme (Labbe and Hubbard, 1961). Radiolabeling studies both in vivo (Sinclair et al., 1979) and in cultured cells (Sinclair et al., 1982) have demonstrated the incorporation of transition metals including cobalt and nickel into heme. If the effect of cobalt and nickel depends on incorporation into the heme moiety, increased levels of iron should competitively inhibit the stimulatory effects of these metals on Epo gene expression. We have provided experimental support for this prediction (Ho and Bunn, 1996). The response to cobalt is not specific for the Epo gene or for Epo producing cells. For example, cobalt has been shown to mimic hypoxia in stimulating the expression of VEGF (Goldberg and Schneider, 1994; Ladoux and Frelin, 1994; Minchenko et al., 1994) and glycolytic enzymes (Firth et al., 1994; Semenza et al., 1994; Ebert et al., 1996) in a number of different types of cells Moreover, reporter gene experiments show that Epo’s 3′ enhancer (discussed in detail below) is responsive to both hypoxia and cobalt in a variety of cell lines from different tissues (Maxwell et al., 1993). The fact that CO does not block the induction of Epo by cobalt or nickel (Goldberg et al., 1988; Huang et al., 1999) is consistent with the inability of cobalt and nickel substituted hemes to bind to CO.
Since nitric oxide (NO), like CO is a gaseous ligand that binds to ferrous atoms in heme proteins, it is of interest to determine its impact on oxygen sensing and signaling. Several laboratories have shown that NO from nitroprusside or other donors suppresses hypoxic activation of HIF-1 DNA binding, the stabililzation of HIF-1α protein and the induction of reporter genes containing HIF-1 response elements (Liu et al., 1998; Sogawa et al., 1998; Huang et al., 1999).
The best studied system of oxygen sensing and signal transduction has been in nitrogen-fixing bacteria, Rhizobium, whose oxygen sensor has been shown to be an oxygen-binding heme protein containing a protein kinase domain (Gilles-Gonzalez et al., 1991, 1994; Rodgers et al., 1996; Lukat-Rodgers and Rodgers, 1997).
The evidence summarized above for a central role of heme protein(s) has led to the proposal of two clearly distinct sites and mechanisms for oxygen sensing and signaling:1 (a) a cytochrome _b_-like NAD(P)H oxidase on the plasma membrane; and (b) mitochondrial complex IV cytochrome oxidase
3. Cytochrome b-like NAD(P)H oxidase
3.1. Role of peroxide in signaling
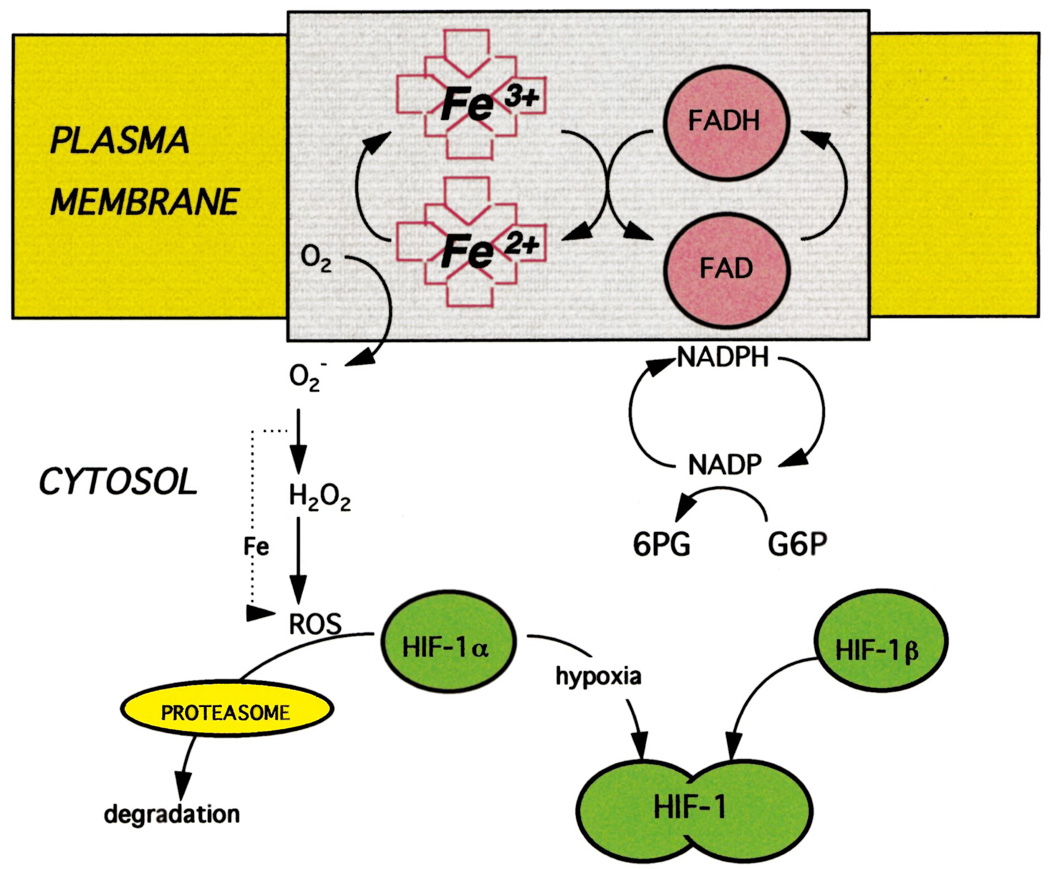
Fandrey et al. (1994) showed that the induction of Epo production, following exposure of Hep3B cells to hypoxia, could be aborted by the addition of either hydrogen peroxide, menadione, an agent which increases intracellular peroxide production, or aminotriazole, an inhibitor of catalase. As mentioned above, treatment of Hep3B cells with cobalt induces Epo production. HepG2 cells exposed to cobalt undergo a significant decrease in production of hydrogen peroxide (Görlach et al., 1994). Taken together, as shown in Fig. 1, these experiments suggest that molecular oxygen is chemically reduced, presumably by the sensing apparatus, to superoxide and peroxide, thereby providing a plausible chemical signal that could impact on the activity of HIF-1, which in turn regulates oxygen responsive genes.
Fig. 1.
Proposed model of oxygen sensing and signaling. In oxygenated cells, a flavo-heme protein in the plasma membrane functions as an NADPH oxidase, transferring electrons through the flavin and heme to molecular oxygen, generating superoxide O2− which in the presence of iron is converted to OH· and other reactive oxygen species. As a result HIF-1α is oxidatively modified so that it is recognized by the proteasome and rapidly degraded. At low oxygen tension, HIF-1α is stable and can form a heterodimer with constitutively expressed HIF-1β, thereby activating HIF-1 which translocates to the nucleus and binds to response elements in hypoxia inducible genes.
In cells containing hydrogen peroxide, highly reactive oxygen compounds such as hydroxyl radical and singlet oxygen can be formed. The generation of these reactive oxygen species (ROS) is catalyzed by free iron via the Fenton reaction. Virtually all genes that are inducible by hypoxia are also up-regulated by desferrioxamine and other strong chelators of iron (Wang and Semenza, 1993a; Semenza et al., 1994; Gleadle et al., 1995a). As depicted in Fig. 1, it is likely that drastic reduction in intracellular free iron lowers the level of ROS, thereby mimicking a hypoxic environment.
3.2. Spectral analyses
Support for the central role of an oxidase has been provided by Acker (1994a,b) who have made direct spectral measurements of cells in which oxygen sensing plays a critical role. They have obtained difference spectra in the visible range in the presence and absence of inhibitors of respiratory cytochromes in both Type I carotid body cells (Cross et al., 1990) and in HepG2 cells (Görlach et al., 1993). In each case, their spectral data could be deconvoluted to suggest the presence of a _b_-like cytochrome. This heme protein was estimated to comprise ~6% of the total cytochrome b in the cell. It appeared to bind oxygen with relatively low affinity and also carbon monoxide (Görlach et al., 1993). Moreover, cobalt treatment of HepG2 cells abolished the response of this b-like cytochrome to hypoxia whereas the redox states of mitochondrial cytochromes c and aa3 were unaffected (Görlach et al., 1994). Although these absorbance measurements are potentially of considerable importance and value, they are inherently difficult to execute and to interpret, owing to a low ratio of signal to noise.
3.3. Tissue localization
The spectral evidence that the oxygen sensor is a b cytochrome led Acker et al. to focus on neutrophil-macrophage cytochrome b558, which has similar spectral properties and, importantly, functions as a NAD(P)H oxidase, converting oxygen to superoxide, in keeping with the postulated role of reactive oxygen compounds in signaling. They demonstrated by Western blot analysis that both type 1 cells of the carotid body (Kummer and Acker, 1995) and HepG2 cells (Görlach et al., 1993) contain at least two of the subunits (p22phox and p47phox) of the oxidase. However, the specific subunits that compose the NADPH oxidase in neutrophils and macrophages are unlikely to play important roles in oxygen sensing since patients with genetic subtypes of chronic granulomatous disease, characterized by absence or abnormality of these subunits, have a very restricted clinical phenotype with no apparent evidence of disordered oxygen sensing. Moreover, lymphoid cell lines from patients deficient in gp91phox, p22phox, when transfected with an oxygen sensitive reporter genes, showed normal responses to hypoxia (Wenger et al., 1996).
Additional support for the role of an NAD(P)H oxidase in oxygen sensing has come from experiments utilizing iodonium compounds (Goldwasser et al., 1995; Gleadle et al., 1995b) which inhibit this type of enzyme along with other flavoproteins (Stuehr et al., 1991). Diphenylene iodonium (DPI) transiently increased the spontaneous neural discharge in isolated perfused carotid body preparations and blocked the hypoxia-induced increase in discharge (Cross et al., 1990). The same result was observed with pulmonary neuroepithelial cells (Youngson et al., 1993). However, interpretation of these results is confounded by the observation that DPI is a non-specific inhibitor of ion channels (Wyatt et al., 1994).
Despite major gaps in our understanding, it seems likely that most cells share a common oxygen sensing apparatus which is depicted schematically in Fig. 1. The sensor is likely to be a cytosolic, membrane bound, multisubunit b-like cytochrome which binds oxygen and reduces it to superoxide, thereby generating ROS which serve as chemical signals that impact on the transcription factor HIF-1 that regulates oxygen responsive genes. This general model accommodates a considerable body of physiologic, biochemical and genetic evidence described above. In its simplest form this scheme would provide a continuous monitor of intracellular oxygen tension over a wide range.
4. Mitochondrial complex IV heme protein
Since the mitochondrion is the primary site of oxygen metabolism, this organelle might seem to be a logical site for sensing and initiation of signal transduction. As mentioned above, patch clamp experiments indicate that the oxygen sensor in carotid body type I cells resides in the plasma membrane (Ganfornina and López-Barneo, 1991). Nevertheless, several reports have suggested that mitochondria may play a critical role in oxygen sensing by the carotid body (Mulligan et al., 1981; Obeso et al., 1985; Duchen and Biscoe, 1992; Wilson et al., 1994; Lahiri et al., 1995). In view of the extraordinarily rich vascularity and high oxygen consumption of the carotid body, there may be a unique functional role for a mitochondrial oxygen sensor.
Recently, Schumacker et al. (Chandel et al., 1997, 1999; Duranteau et al., 1998) have presented evidence that mitochondrial cytochrome oxidase (Complex IV) serves as an oxygen sensor in other cell types: hepatocytes and cardiac myocytes. These investigators observed that when cells are exposed to moderate degrees of hypoxia (20 torr=3% O2), oxygen uptake decreased significantly, owing to a significant reduction in the Vmax of cytochrome oxidase. This effect was noted after a latency of ~2 h and was fully reversible. Measurements of mitochondrial membrane potential indicated that the decrease in respiration following hypoxia was due solely to a direct inhibition of mitochondrial proton pumping and not to decreased ATP utilization. Mitochondria are a major source of superoxide (O2−) owing to inefficient transfer of electrons in the respiratory chain. Accordingly, Schumacker’s group performed a set of experiments to test whether superoxide produced proximal to mitochondrial Complex III serves as a signalling molecule. They showed that graded decreases in pO2 from 35 to 7 torr (5–1% O2) resulted in a progressive increase in ROS, as measured by the fluorescent probe dichloro-fluoriscein. The addition of inhibitors supported the mitochondria as a source of the induced ROS. Rotenone and TTFA (inhibitors of Complexes I and II, respectively) suppressed formation of ROS, whereas antimycin A and azide (inhibitors of Complex III and IV, respectively) caused an increase in ROS. These investigators then showed that HIF-1 activation and Epo gene expression in Hep3B cells correlated directly with changes in ROS, induced either by mitochondrial inhibitors or by reducing agents (Chandel et al., 1999). Moreover, in ρo Hep3B cells, whose mitochondria have been destroyed by ethidium bromide, hypoxia failed to induce either HIF-1 activity or Epo gene expression. In contrast, when ρo Hep3B cells are exposed to cobalt, HIF-1 is activated and Epo mRNA is up-regulated. The model for oxygen sensing suggested by these findings is in direct conflict with the one described above, in which a cytochrome b-like oxidase generates decreased levels of ROS during hypoxia.
Considerably more experimental work is needed in order to establish whether either this mitochondrial model or the NADPH oxidase model of oxygen sensing and signaling is correct.
Footnotes
1
Recently, Srinivas et al. (1998) have proposed yet another model of oxygen sensing. They expressed the α-subunit of the heterodimeric transcription HIF-1 as a GST-fusion protein in E. coli and reported that it contains up to 2 moles of nonheme iron per mole protein. They suggested that the iron atoms on this protein could bind O2, thereby serving as a non-heme sensing mechanism that would impact directly on HIF-1 activation. However, the authors have subsequently retracted this report [J. Biol. Chem. 74, 1180].
References
- Acker H. Cellular oxygen sensors. Ann. N.Y. Acad. Sci. 1994a;718:3–10. doi: 10.1111/j.1749-6632.1994.tb55698.x. [DOI] [PubMed] [Google Scholar]
- Acker H. Mechanisms and meaning of cellular oxygen sensing in the organism. Respir. Physiol. 1994b;95:1–10. doi: 10.1016/0034-5687(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Budinger GRS, Choe SH, Schumacker PT. Cellular respiration during hypoxia: role of cytochrome oxidase as the oxygen sensor in hepatocytes. J. Biol. Chem. 1997;272:18808–18816. doi: 10.1074/jbc.272.30.18808. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA. 1999;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AR, Henderson L, Jones OTG, Delpiano MA, Hentschel J, Acker H. Involvement of an NAD(P)H oxidase as PO2 sensor protein in the rat carotid body. Biochem. J. 1990;272:743–747. doi: 10.1042/bj2720743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejours P. Comparative Respiratory Physiology. 2nd ed. Amsterdam: Elsevier; 1981. [Google Scholar]
- Duchen MR, Biscoe TJ. Relative mitochondrial membrane potential and [Ca2+] in type I cells isolated from the rabbit carotid body. J. Physiol. Lond. 1992;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranteau J, Chandel NS, Kuliscz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J. Biol. Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- Ebert BL, Gleadle JM, O’Rourke JF, Bartlett SM, Poulton J, Ratcliffe PJ. Isoenzyme-specific regulation of genes involved in energy metabolism by hypoxia: similarities with the regulation of erythropoietin. Biochem. J. 1996;313:809–814. doi: 10.1042/bj3130809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandrey J, Bunn HF. In vivo and in vitro regulation of erythropoietin mRNA: measurement by competitive polymerase chain reaction. Blood. 1993;81:617–623. [PubMed] [Google Scholar]
- Fandrey J, Frede S, Jelkmann W. Role of hydrogen peroxide in hypoxia-induced erythropoietin production. Biochem. J. 1994;303:507–510. doi: 10.1042/bj3030507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: Similarities with the erythropoietin 3′ enhancer. Proc. Natl. Acad. Sci. USA. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JW, Langston JW. Effects of testosterone, cobalt, and hypoxia on erythropoietin production in the isolated perfused dog kidney. Ann. NY Acad. Sci. 1968;149:75–87. doi: 10.1111/j.1749-6632.1968.tb15139.x. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, López-Barneo J. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc. Natl. Acad. Sci. USA. 1991;88:2927–2930. doi: 10.1073/pnas.88.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles-Gonzalez MA, Ditta GS, Helinski DR. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature. 1991;350:170–172. doi: 10.1038/350170a0. [DOI] [PubMed] [Google Scholar]
- Gilles-Gonzalez MA, Gonzalez G, Perutz MF, Kiger L, Marden MC, Poyart C. Heme-based sensors exemplified by the kinase FixL, are a new class of heme protein with distinctive ligand binding and autoxidation. Biochemistry. 1994;33:8067–8073. doi: 10.1021/bi00192a011. [DOI] [PubMed] [Google Scholar]
- Gleadle JM, Ebert BL, Firth JD, Ratcliffe PJ. Regulation of angiogenic growth factor expression by hypoxia, transition metals and chelating agents. Am. J. Physiol. 1995a;268:C1362–C1368. doi: 10.1152/ajpcell.1995.268.6.C1362. [DOI] [PubMed] [Google Scholar]
- Gleadle JM, Ebert BL, Ratcliffe PJ. Diphenylene iodonium inhibits the induction of erythropoietin and other mammalian genes by hypoxia: implications for the mechanism of oxygen sensing. Eur. J. Biochem. 1995b;234:92–99. doi: 10.1111/j.1432-1033.1995.092_c.x. [DOI] [PubMed] [Google Scholar]
- Goldberg MA, Dunning SP, Bunn HF. Regulation of the erythopoietin gene: Evidence that the oxygen sensor is a heme protein. Science. 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- Goldberg MA, Schneider TJ. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J. Biol. Chem. 1994;269:4355–4359. [PubMed] [Google Scholar]
- Goldwasser E, Jacobson LO, Fried W, Plzak LF. The effect of cobalt on the production of erythropoietin. Stud. Erythrop. V. 1958;13:55–60. [PubMed] [Google Scholar]
- Goldwasser E, Alibali P, Gardner A. Differential inhibition by iodonium compounds of induced erythropoietin expression. J. Biol. Chem. 1995;270:2628–2629. doi: 10.1074/jbc.270.6.2628. [DOI] [PubMed] [Google Scholar]
- Görlach A, Holtermann G, Jelkmann W, Hancock JT, Jones SA, Jones OTG, Acker H. Photometric characteristics of haem proteins in erythropoietin-producing hepatoma cells (HepG2) Biochem. J. 1993;290:771–776. doi: 10.1042/bj2900771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlach A, Fandrey J, Holtermann G, Acker H. Effects of cobalt on haem proteins of erythropoietin-producing HepG2 cells in multicellular spheroid culture. FEBS Lett. 1994;348:216–218. doi: 10.1016/0014-5793(94)00607-5. [DOI] [PubMed] [Google Scholar]
- Gradin K, McGuire J, Wenger RH, Kvietikova I, Whitelaw M, Toftgard R, Tora L, Gassman M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the ARNT transcription factor. Mol. Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Ho VT, Bunn HF. Effects of transition metals on the expression of the erythropoietin gene: further evidence that the oxygen sensor is a heme protein. Biochem. Biophys. Res. Commun. 1996;223:175–180. doi: 10.1006/bbrc.1996.0865. [DOI] [PubMed] [Google Scholar]
- Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its α-subunit. J. Biol. Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by its oxygen-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LE, Willmore W, Gu J, Goldberg MA, Bunn HF. Inhibition of HIF-1 activation by carbon monoxide and nitric oxide: implications for oxygen sensing and signaling. J. Biol. Chem. 1999;274:9038–9044. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- Jasmin G, Solymoss B. Polycythemia induced in rats by intrarenal injection of nickel sulfide, Ni3S2. Proc. Soc. Exp. Biol. Med. 1975;148:774–776. doi: 10.3181/00379727-148-38628. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol. Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1a: posttranslational regulation and conformational change by recruitment of the ARNT transcription factor. Proc. Natl. Acad. Sci. USA. 1997;94:5667–5672. doi: 10.1073/pnas.94.11.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzmann T, Schmidt H, Unthan-Fechner K, Probst I, Jungermann K. A ferro-heme protein senses oxygen levels, which modulate the glucagon dependent activation of the phosphoenolpyruvate carboxykinase in rat hepatocyte cultures. Biochem. Biophys. Res. Comm. 1993;195:792–798. doi: 10.1006/bbrc.1993.2115. [DOI] [PubMed] [Google Scholar]
- Kummer W, Acker H. Immunohistochemical demonstration of four subunits of neutrophil NAD(P)H oxidsase in type I cells of the carotid body. J. Appl. Physiol. 1995;78:1904–1909. doi: 10.1152/jappl.1995.78.5.1904. [DOI] [PubMed] [Google Scholar]
- Labbe RF, Hubbard N. Metal specificity of the iron-protoporphyrin chelating enzyme from rat liver. Biochim. Biophys. Acta. 1961;52:130–135. doi: 10.1016/0006-3002(61)90910-6. [DOI] [PubMed] [Google Scholar]
- Ladoux A, Frelin C. Cobalt stimulates the expression of vascular endothelial growth factor mRNA in rat cardiac cells. Biochem. Biophys. Res. Commun. 1994;204:794–798. doi: 10.1006/bbrc.1994.2529. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Iturriaga R, Mokashi A, Ray DK, Chugh D. CO reveals dual mechanisms of O2 chemoreception in the cat carotid body. Respir. Physiol. 1993;94:227–240. doi: 10.1016/0034-5687(93)90050-k. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Buerk DG, Chugh D, Osanai S, Mokashi A. Reciprocal photolabile O2 consumption and chemoreceptor excitation by carbon monoxide in the cat carotid body: evidence for cytochrome a3 as the primary O2 sensor. Brain Res. 1995;684:194–200. doi: 10.1016/0006-8993(95)00420-u. [DOI] [PubMed] [Google Scholar]
- Liu Y, Christou H, Morita T, Laughner E, Semenza GL, Kourembanas S. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor via the 5′ enhancer. J. Biol. Chem. 1998;273:15257–15262. doi: 10.1074/jbc.273.24.15257. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, López-Ló pez JR, Urena J, González C. Chemotransduction in the carotid body: K+ current modulated by pO2 in the type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- López-Ló pez JR, González C. Time course of K+ current inhibition by low oxygen in chemoreceptor cells of adult rabbit carotid body. FEBS Lett. 1992;299:251–254. doi: 10.1016/0014-5793(92)80126-2. [DOI] [PubMed] [Google Scholar]
- Lukat-Rodgers GS, Rodgers KR. Characterization of ferrous FixL-nitric oxide adducts by resonance Raman spectroscopy. Biochemistry. 1997;36:4178–4187. doi: 10.1021/bi9628230. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Pugh CW, Ratcliffe PJ. Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: Evidence for a widespread oxygen-sensing mechanism. Proc. Natl. Acad. Sci. USA. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchenko A, Bauer T, Salceda S, Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab. Invest. 1994;71:374–379. [PubMed] [Google Scholar]
- Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J. Clin. Invest. 1995;96:2676–2682. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse EE, Lee T-Y, Reiss RF, Sunderman FW. Dose-response and time-response study of erythrocytosis in rats after intrarenal injection of nickel subsulfide. Ann. Clin. Lab. Sci. 1977;7:17–24. [PubMed] [Google Scholar]
- Mulligan E, Lahiri S, Storey BT. Carotid body O2 chemoreception and mitochondrial oxidative phosphorylation. J. Appl. Physiol. 1981;51:438–446. doi: 10.1152/jappl.1981.51.2.438. [DOI] [PubMed] [Google Scholar]
- Obeso A, Almaraz L, Gonzalez C. Correlation between adenosine triphosphate levels, dopamine release and electrical activity in the carotid body: support for the metabolic hypothesis of chemoreception. Brain Res. 1985;348:64–68. doi: 10.1016/0006-8993(85)90360-9. [DOI] [PubMed] [Google Scholar]
- Porter DL, Goldberg MA. Regulation of erythropoietin production. Exp. Hematol. 1993;21:399–404. [PubMed] [Google Scholar]
- Raymond R, Millhorn DE. Regulation of tyrosine hydroxylase gene expression during hypoxia: Role of Ca2+ and PKC. Kidney Intl. 1997;51:536–541. doi: 10.1038/ki.1997.74. [DOI] [PubMed] [Google Scholar]
- Rodgers KR, Lukat-Rodgers GS, Barron JA. Structural basis for ligand discrimination and response initiation in the heme-based oxygen sensor FixL. Biochemistry. 1996;35:9539–9548. doi: 10.1021/bi9530853. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielson K. Animal Physiology. 4th ed. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Semenza GL, Roth PH, Fang H-M, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Sinclair P, Gibbs AH, Sinclair JF, Matteis FD. Formation of cobalt protoporphyrin in the liver of rats: a mechanism for the inhibition of liver haem biosynthesis by inorganic cobalt. Biochem. J. 1979;178:529–538. doi: 10.1042/bj1780529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair PR, Sinclair JF, Bonkowsky HL, Gibbs AH, Matteis FD. Formation of cobalt protoporphyrin by chicken hepatocytes in culture: relationship to decrease of 5-aminolaevulinate synthase caused by cobalt. Biochem. Pharmacol. 1982;31:993–999. doi: 10.1016/0006-2952(82)90333-1. [DOI] [PubMed] [Google Scholar]
- Sogawa K, Numayama-Tsuruta K, Ema M, Abe M, Abe H, Fujii-Kuriyama Y. Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia. Proc. Natl. Acad. Sci. USA. 1998;95:7368–7373. doi: 10.1073/pnas.95.13.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas V, Zhu X, Salceda S, Nakamura R, Caro J. Hypoxia-inducible factor 1a (HIF-1a) is a non-heme iron protein. J. Biol. Chem. 1998;273:18019–18022. doi: 10.1074/jbc.273.29.18019. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Fasehun OA, Kwon NS, Gross SS, Gonzalez JA, Levi R, Nathan CF. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991;5:98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993a;82:3610–3615. [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA. 1993b;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang B-H, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic helix–loop–helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger RH, Marti HH, Schuerer-Maly CC, Kvietikova I, Bauer C, Gassman M, Maly FE. Hypoxic induction of gene expression in chronic granulomatous disease-derived B-cell lines: oxygen sensing is independent of the cytochrome b558-containing nicotinamide adenine dinucleotide phosphate oxidase. Blood. 1996;87:756–761. [PubMed] [Google Scholar]
- Wilson DF, Mokashi A, Chugh D, Vinogradov S, Osanai S, Lahiri S. The primary oxygen sensor of the cat carotid body is cytochrome a3 of the mitochondrial respiratory chain. Fed. Eur. Biochem. Soc. 1994;351:370–374. doi: 10.1016/0014-5793(94)00887-6. [DOI] [PubMed] [Google Scholar]
- Wood SM, Gleadle JM, Pugh CW, Hankinson O, Ratcliffe PJ. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. J. Biol. Chem. 1996;269:15117–15123. doi: 10.1074/jbc.271.25.15117. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Weir EK, Peers C. Diphenylene iodonium blocks K+ and Ca2+ currents in type I cells isolated from the neonatal rat carotid body. Neurosci. Lett. 1994;172:63–66. doi: 10.1016/0304-3940(94)90663-7. [DOI] [PubMed] [Google Scholar]
- Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993;365:153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]