Physical Activity and Survival After Prostate Cancer Diagnosis in the Health Professionals Follow-Up Study (original) (raw)
Abstract
Purpose
To determine whether higher physical activity after prostate cancer (PCa) diagnosis decreases risk of overall and PCa-specific death.
Patients and Methods
We evaluated physical activity in relation to overall and PCa mortality among 2,705 men in the Health Professionals Follow-Up Study diagnosed with nonmetastatic PCa observed from 1990 to 2008. Proportional hazards models were used to evaluate physical activity and time to overall and PCa-specific death.
Results
Among men who lived at least 4 years after their postdiagnosis physical activity assessment, we documented 548 deaths, 20% of which were a result of PCa. In multivariable analysis, men who were physically active had lower risk of all-cause mortality (_P_trend < .001) and PCa mortality (_P_trend = .04). Both nonvigorous activity and vigorous activity were associated with significantly lower overall mortality. Those who walked ≥ 90 minutes per week at a normal to very brisk pace had a 46% lower risk of all-cause mortality (hazard ratio [HR] 0.54; 95% CI, 0.41 to 0.71) compared with shorter durations at an easy walking pace. Men with ≥ 3 hours per week of vigorous activity had a 49% lower risk of all-cause mortality (HR, 0.51; 95% CI, 0.36 to 0.72). For PCa-specific mortality, brisk walking at longer durations was suggestively inverse but not statistically significant. Men with ≥ 3 hours per week of vigorous activity had a 61% lower risk of PCa death (HR, 0.39, 95% CI, 0.18 to 0.84; P = .03) compared with men with less than 1 hour per week of vigorous activity. Men exercising vigorously before and after diagnosis had the lowest risk.
Conclusion
In men with PCa, physical activity was associated with lower overall mortality and PCa mortality. A modest amount of vigorous activity such as biking, tennis, jogging, or swimming for ≥ 3 hours a week may substantially improve PCa-specific survival.
INTRODUCTION
Prostate cancer (PCa) is the most frequently diagnosed cancer in men in the United States; however, more than 80% of patients are diagnosed with localized disease,1 with a relative 10-year survival rate of 93% for all stages combined.2 More than two million men in the United States and 16 million men worldwide are PCa survivors. Observational studies report that breast and colon cancer survivors who engage in regular activity have significantly lower overall mortality and cancer-specific mortality compared with survivors who are inactive,3–6 yet no studies have examined this association in PCa survivors.
We previously reported that vigorous activity was associated with reduced risk of incident advanced disease7 and therefore hypothesized that vigorous activity may reduce the risk of PCa-specific and overall mortality in PCa survivors. Because walking and walking pace were inversely associated with risk of cardiovascular disease and total mortality previously observed within this cohort,8,9 we also hypothesized that brisk walking may reduce the risk of PCa-specific and overall mortality. We prospectively assessed whether activity after diagnosis, specifically total, nonvigorous (including walking duration and pace), and vigorous activity, was inversely associated with these outcomes.
PATIENTS AND METHODS
Study Population
The Health Professionals Follow-Up Study is a prospective study of 51,529 US male health professionals who enrolled in 1986 by completing a mailed questionnaire. Participants provided information about medical history and risk factors for chronic diseases, including cancer. Participants complete biennial follow-up questionnaires to collect information on new medical diagnoses and to update information on lifestyle factors (response rate, 96%). This study was approved by the Institutional Review Board of the Harvard School of Public Health.
Assessment of Physical Activity
Leisure time activity was assessed every 2 years. Beginning in 1986, men reported the average time per week spent on the following activities during the previous year: walking to work or for exercise (including golf); jogging (> 10 min/mile); running (≤ 10 min/mile); bicycling (including stationary); lap swimming; tennis; squash or racquetball; calisthenics or rowing; and number of flights of stairs climbed daily. Data on heavy outdoor work and weight training were added in 1988 and 1990, respectively. Walking pace, categorized as easy (< 2 mph), normal (2 to 2.9 mph), brisk (3 to 3.9 mph), and very brisk (≥ 4 mph), was also recorded and updated every 2 to 4 years. The physical activity assessment was validated using four 1-week seasonal diaries.10 Each activity on the questionnaire was assigned a metabolic equivalent task (MET) value,11 which is the energy expended compared with sitting at rest. Nonvigorous activities were those with an MET value of less than 6, and vigorous activities were those with an MET value of ≥ 6. We chose categories for analysis of total MET-hours per week to correspond to the equivalent of less than 1, 1 to less than 3, 3 to less than 8, 8 to less than 16, and ≥ 16 hours per week of walking at an average pace.
Ascertainment of PCa Diagnosis and Death
After a participant reported a diagnosis of PCa, medical records and pathology reports were sought to confirm the diagnosis and obtain information on pathology, treatments, and prostate-specific antigen (PSA) values. Participants completed biennial follow-up questionnaires to update data on treatments, PSA, and clinical progression. The primary outcomes were death from any cause and fatal PCa. Using reports of deaths from families and the National Death Index for nonrespondents, we ascertained more than 98% of deaths.12 Causes of death were centrally adjudicated by study physicians who reviewed medical records and death certificates.
Population for Analysis
We excluded men diagnosed before 1990 to allow for adjustment for prediagnosis physical activity ascertained 4 years before diagnosis. We included in our analyses men who initially were free of a cancer diagnosis (except nonmelanoma skin cancer) in 1990 and had provided activity data before and after diagnosis (n = 3,032). To reduce the impact of advanced disease on activity duration and intensity, we excluded men who died within 4 years of their first postdiagnostic activity assessment (n = 200), had metastatic disease at diagnosis (n = 107), or reported metastasis before their first postdiagnostic questionnaire (n = 7) or in the 2-year period after their first postdiagnostic questionnaire (n = 13), leaving 2,705 men for analysis.
Covariates
Our final model for PCa-specific mortality included age at diagnosis (5-year categories), clinical stage (T1, T2, or T3/4), clinical Gleason score (score of < 7, 7, or > 7), primary treatment (categorical), prediagnosis activity (same categories as postdiagnosis activity), and body mass index (BMI; < 25, 25 to < 30, or ≥ 30 kg/m2). We considered models that adjusted for PSA at diagnosis, race, height, family history of PCa, diabetes, smoking, and intakes of calcium, red meat, tomato sauce, fish, and α-linolenic acid, because these were previously associated with PCa incidence or progression in our study.13,14 There was little evidence of confounding by these factors, so they were not included in our final models. Total energy consumption may mediate the relation between physical activity and our outcome, so we excluded energy from the final models; however, including it made no difference in the estimates. Our final model for overall mortality additionally included parental history of myocardial infarction (MI) at age 60 years or younger, high blood pressure, elevated cholesterol, diabetes, smoking (categorical) from the postdiagnostic questionnaire, and comorbidity status (considered to be yes if participant reported any of the following: MI, coronary artery bypass or coronary angioplasty, stroke, Parkinson's disease, and emphysema or chronic bronchitis) updated over follow-up.
Statistical Analysis
We used Cox proportional hazards models to calculate hazard ratios (HRs) of death from any cause or death from PCa. We checked the proportionality assumption by introducing a cross-product term of each specific physical activity variable by a function of time into the model and testing for its statistical significance. No significant violation of the proportionality assumption was found. In the main analysis for PCa mortality, deaths from other causes were censored, and competing risk Cox survival analysis was used.15 We excluded participants who died within 4 years after the first assessment; therefore, person-years were calculated beginning 4 years from the date of the first postdiagnosis physical activity assessment until death or end of follow-up (January 1, 2008), whichever came first. Because of concerns of possible short-term effects of treatment on physical activity levels, if participants' first postdiagnosis physical activity questionnaires were within 6 months of primary treatment (n = 533), we did not use these data but entered these participants into the analysis upon the return of their next questionnaire. We performed a sensitivity analysis excluding these men completely from the study population, and the point estimates for physical activity did not change materially.
We updated activity every 2 years, maintaining a 4- to 6-year lag. For example, participants diagnosed with PCa between 1992 and 1994 entered the analysis in 1998. We applied their 1994 first postdiagnosis physical activity to deaths occurring between 1998 and 2000, their 1996 postdiagnosis physical activity to deaths occurring between 2000 and 2002, and so on. This approach allowed us to capture recent activity in relation to survival while minimizing reverse causation as a result of the effect of illness preceding death on physical activity.
Linear trends across categories were evaluated using the median of each category as a continuous variable.16 When examining nonvigorous and vigorous physical activity, we mutually adjusted for both. We assessed interactions between physical activity and potential effect modifiers by entering the cross products of activity with those variables in multivariate models. All P values were two sided. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
We documented 548 deaths, 112 (20%) as a result of PCa, among the 2,705 PCa survivors. The median time from diagnosis to the first physical activity assessment (not including questionnaires completed within 6 months of primary treatment) was 18 months. The median duration of follow-up time from the first postdiagnosis physical activity assessment to censoring (either until death or the end of follow-up in January 2008) was 9.7 years for survivors and 7.8 years for men who died. Age-standardized characteristics after diagnosis are listed in Table 1. Compared with participants in the lowest categories of total and vigorous activity, those in the top category were slightly younger, smoked less, consumed more alcohol, and had a lower BMI. Consumption of relevant foods, energy-adjusted nutrients, and prognostic risk were similar between the groups.
Table 1.
Age-Standardized Baseline* Characteristics According to Physical Activity Category Among 2,705 Men With Prostate Cancer in the Health Professionals Follow-Up Study
| Characteristic | Total Activity | Vigorous Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| < 3 MET-h/wk | 3 to < 9 MET-h/wk | 9 to < 24 MET-h/wk | 24 to < 48 MET-h/wk | ≥ 48 MET-h/wk | < 1 h/wk | 1 to < 3 h/wk | ≥ 3 h/wk | |
| No. of participants | 302 | 373 | 710 | 660 | 660 | 1596 | 647 | 318 |
| Mean age at diagnosis, years† | 70.8 | 70.3 | 69.2 | 68.3 | 68.6 | 69.5 | 68.6 | 68.4 |
| Mean total activity, MET-h/wk | 0.9 | 5.8 | 16.3 | 34.4 | 87.3 | 23.4 | 34.5 | 75.1 |
| Mean vigorous activity, MET-h/wk | 0.05 | 1.0 | 4.5 | 11.1 | 30.3 | 0.5 | 12.0 | 49.1 |
| Stage category, % of participants | ||||||||
| T1 | 44.8 | 50.8 | 52.8 | 49.8 | 49.8 | 49.0 | 53.0 | 51.6 |
| T2 | 34.0 | 33.8 | 35.2 | 39.2 | 38.9 | 36.1 | 36.6 | 37.8 |
| T3 or T4 | 2.1 | 3.5 | 2.0 | 2.2 | 2.1 | 2.3 | 2.0 | 2.1 |
| Missing | 19.1 | 11.9 | 9.9 | 8.8 | 9.2 | 12.5 | 8.4 | 8.6 |
| Gleason score, % of participants | ||||||||
| < 7 | 50.8 | 51.5 | 55.9 | 59.5 | 53.8 | 53.2 | 58.1 | 57.3 |
| 7 | 15.3 | 19.8 | 18.6 | 15.7 | 19.3 | 18.2 | 17.3 | 17.1 |
| > 7 | 5.4 | 6.8 | 4.9 | 5.3 | 4.8 | 5.1 | 6.1 | 4.8 |
| Missing | 28.5 | 22.0 | 20.6 | 19.6 | 22.1 | 23.5 | 18.6 | 20.9 |
| Primary treatment, % of participants | ||||||||
| Radical prostatectomy | 36.8 | 45.1 | 45.5 | 51.5 | 47.3 | 45.7 | 46.5 | 47.4 |
| Radiation, seeds, or hormones | 34.6 | 36.2 | 34.3 | 31.6 | 34.5 | 32.8 | 35.3 | 36.2 |
| Watchful waiting | 7.5 | 5.1 | 7.7 | 6.7 | 5.9 | 6.8 | 7.2 | 5.5 |
| Other treatment | 1.4 | 2.6 | 2.2 | 1.3 | 1.5 | 1.9 | 1.9 | 1.2 |
| Missing treatment | 19.6 | 11.1 | 10.2 | 8.8 | 11.0 | 12.9 | 9.1 | 9.8 |
| Mean BMI, kg/m2 | 27.4 | 26.1 | 25.8 | 25.5 | 25.1 | 26.2 | 25.5 | 24.8 |
| Family history of prostate cancer, % of participants | 9.8 | 8.0 | 8.5 | 12.1 | 10.3 | 8.6 | 12.8 | 9.9 |
| Diabetes, % of participants | 12.5 | 10.1 | 6.7 | 8.3 | 6.0 | 8.7 | 8.6 | 5.6 |
| High blood pressure, % of participants | 50.3 | 48.1 | 41.8 | 43.7 | 39.4 | 45.6 | 42.0 | 39.6 |
| Elevated cholesterol, % of participants | 53.6 | 52.6 | 52.4 | 50.7 | 47.0 | 51.5 | 51.4 | 46.9 |
| Parental history of MI at age ≤ 60 years, % of participants | 9.6 | 11.8 | 12.0 | 11.7 | 10.4 | 11.0 | 11.3 | 11.9 |
| Smoking status, % of participants | ||||||||
| Current smoker | 5.8 | 5.9 | 4.8 | 3.3 | 2.7 | 5.1 | 3.7 | 1.5 |
| Past smoker | 52.7 | 52.0 | 50.1 | 51.5 | 49.2 | 51.2 | 50.8 | 49.1 |
| Never smoker | 41.5 | 42.0 | 45.1 | 45.2 | 48.1 | 43.7 | 45.5 | 49.3 |
| Mean intake | ||||||||
| Calories per day | 1,810 | 1,924 | 1,937 | 1,969 | 2,081 | 1,953 | 1,960 | 1,999 |
| Calcium, mg/d | 1,032 | 1,018 | 1,045 | 1,049 | 1,076 | 1,042 | 1,055 | 1,070 |
| Red meat, servings/d | 0.4 | 0.5 | 0.5 | 0.4 | 0.4 | 0.5 | 0.5 | 0.4 |
| Tomato sauce, servings/wk | 0.9 | 1.0 | 1.0 | 1.0 | 1.2 | 1.0 | 1.1 | 1.2 |
| Fish, servings/d | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 |
| Alcohol, % of participants | ||||||||
| Nondrinker | 36.2 | 23.9 | 23.9 | 20.5 | 23.9 | 26.9 | 22.8 | 18.4 |
| < 15 g/d | 44.7 | 53.0 | 52.0 | 51.5 | 46.7 | 47.8 | 51.4 | 55.2 |
| ≥ 15 g/d | 19.1 | 23.1 | 24.1 | 28.1 | 29.4 | 25.4 | 25.8 | 26.4 |
Walking comprised 36% of total MET-hours per week and 52% of total time spent on physical activity (Table 2). Other significant contributors to total MET-hours included heavy outdoor work (22%) and bicycling (10%). Vigorous activity comprised 37% of total MET-hours and 24% of total time spent on physical activity.
Table 2.
Types of Activity Reported on First Postdiagnosis Questionnaire Among 2,705 Men With Prostate Cancer in the Health Professionals Follow-Up Study
| Type of Activity | MET Value* | % of Total MET-Hours per Week† | % of Total Time Spent on Physical Activity |
|---|---|---|---|
| Walking to work or for exercise (including golf) | 3 (for average pace)‡ | 36.2 | 52.4 |
| Heavy outdoor work (eg, digging, chopping) | 5.5 | 21.6 | 19.2 |
| Bicycling (including stationary machine) | 7 | 10.0 | 7.0 |
| Tennis | 7 | 8.3 | 4.7 |
| Calisthenics, rowing, stair or ski machine, and so on | 6 | 7.3 | 6.0 |
| Weightlifting or weight machine | 4.5 | 4.0 | 4.3 |
| Running (10 min/mile or faster) | 12 | 3.7 | 1.5 |
| Jogging (slower than 10 min/mile) | 7 | 3.3 | 2.3 |
| Lap swimming | 7 | 2.8 | 1.9 |
| Squash or racquetball | 12 | 1.7 | 0.7 |
| Stair climbing | 0.11 | 1.1 | — |
Total Physical Activity
Each increasing category of total activity was associated with a decreased risk of all-cause mortality (_P_trend < .001; Table 3). HRs remained significant but were attenuated in multivariable analyses. Similar results were observed after further adjustment for prediagnosis physical activity. Men with ≥ 9 versus less than 9 MET-h/wk had a 33% reduction in all-cause mortality (HR, 0.67; 95% CI, 0.56 to 0.82; data not shown). We observed a significant trend with increasing MET-h/wk for PCa mortality (_P_trend = .04). Comparing ≥ 9 versus less than 9 MET-h/wk, the HR for PCa mortality was 0.65 (95% CI, 0.43 to 1.00).
Table 3.
Age- and Multivariable-Adjusted HRs According to Physical Activity Category After Prostate Cancer Diagnosis
| Measure | Total Activity | P for Trend | ||||
|---|---|---|---|---|---|---|
| < 3 MET-h/wk | 3 to < 9 MET-h/wk | 9 to < 24 MET-h/wk | 24 to < 48 MET-h/wk | ≥ 48 MET-h/wk | ||
| Median MET-hours per week on first postdiagnosis questionnaire | 0.6 | 5.7 | 16 | 33.4 | 71.0 | |
| All deaths (n = 548) | ||||||
| No. of deaths | 125 | 99 | 143 | 116 | 65 | |
| Age-adjusted HR | 1.00 | 0.79 | 0.63 | 0.57 | 0.33 | < .001 |
| 95% CI | 0.60 to 1.04 | 0.49 to 0.80 | 0.44 to 0.73 | 0.24 to 0.45 | ||
| Multivariable-adjusted HR* | 1.00 | 0.81 | 0.70 | 0.66 | 0.40 | < .001 |
| 95% CI | 0.61 to 1.07 | 0.54 to 0.90 | 0.51 to 0.87 | 0.29 to 0.54 | ||
| Multivariable-adjusted HR† | 1.00 | 0.80 | 0.69 | 0.65 | 0.38 | < .001 |
| 95% CI | 0.61 to 1.06 | 0.53 to 0.90 | 0.49 to 0.86 | 0.27 to 0.53 | ||
| Prostate cancer deaths (n = 112) | ||||||
| No. of prostate cancer deaths | 21 | 21 | 25 | 30 | 15 | |
| Age-adjusted HR | 1.00 | 0.90 | 0.61 | 0.85 | 0.41 | .02 |
| 95% CI | 0.49 to 1.67 | 0.34 to 1.10 | 0.48 to 1.50 | 0.21 to 0.80 | ||
| Multivariable-adjusted HR‡ | 1.00 | 0.96 | 0.65 | 0.93 | 0.46 | .04 |
| 95% CI | 0.51 to 1.80 | 0.36 to 1.20 | 0.51 to 1.68 | 0.23 to 0.92 | ||
| Multivariable-adjusted HR§ | 1.00 | 0.91 | 0.60 | 0.83 | 0.42 | .04 |
| 95% CI | 0.48 to 1.73 | 0.32 to 1.11 | 0.44 to 1.55 | 0.20 to 0.88 |
Nonvigorous Activity
We observed risk reductions for nonvigorous activity in relation to all-cause mortality starting at a modest level of 1 to less than 3 h/wk (Table 4). Compared with men with less than 1 h/wk of nonvigorous activity, men with 5 to less than 10 h/wk had a significant 28% reduction in total mortality, and men with ≥ 10 h/wk had a 51% risk reduction (_P_trend < .001).
Table 4.
Age-and Multivariable-Adjusted HRs According to Duration of Nonvigorous and Vigorous Physical Activity After Prostate Cancer Diagnosis
| Measure | Duration of Nonvigorous Activity | P for Trend | Duration of Vigorous Activity | P for Trend | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 1 h/wk | 1 to < 3 h/wk | 3 to < 5 h/wk | 5 to < 10 h/wk | ≥ 10 h/wk | < 1 h/wk | 1 to < 3 h/wk | ≥ 3 h/wk | |||
| Median duration per week on first postdiagnosis questionnaire, hours | 0 | 1.6 | 3.5 | 6.0 | 15.5 | 0 | 1.5 | 5 | ||
| All deaths (n = 536) | ||||||||||
| No. of deaths | 178 | 148 | 31 | 126 | 53 | 371 | 122 | 43 | ||
| Age-adjusted HR | 1.00 | 0.78 | 0.63 | 0.66 | 0.44 | < .001 | 1.00 | 0.90 | 0.47 | < .001 |
| 95% CI | 0.62 to 0.98 | 0.43 to 0.94 | 0.52 to 0.84 | 0.32 to 0.61 | 0.73 to 1.11 | 0.34 to 0.65 | ||||
| Multivariable-adjusted HR* | 1.00 | 0.80 | 0.73 | 0.74 | 0.52 | < .001 | 1.00 | 0.98 | 0.50 | < .001 |
| 95% CI | 0.64 to 1.01 | 0.49 to 1.08 | 0.58 to 0.95 | 0.37 to 0.71 | 0.79 to 1.22 | 0.36 to 0.70 | ||||
| Multivariable-adjusted HR† | 1.00 | 0.79 | 0.70 | 0.72 | 0.49 | < .001 | 1.00 | 1.00 | 0.51 | < .001 |
| 95% CI | 0.63 to 1.00 | 0.47 to 1.04 | 0.56 to 0.93 | 0.35 to 0.69 | 0.80 to 1.25 | 0.36 to 0.72 | ||||
| Prostate cancer deaths (n = 111) | ||||||||||
| No. of prostate cancer deaths | 26 | 33 | 11 | 29 | 12 | 71 | 31 | 9 | ||
| Age-adjusted HR | 1.00 | 1.03 | 1.30 | 1.05 | 0.65 | .21 | 1.00 | 1.10 | 0.33 | .04 |
| 95% CI | 0.61 to 1.74 | 0.63 to 2.70 | 0.61 to 1.80 | 0.32 to 1.32 | 0.71 to 1.70 | 0.08 to 1.37 | ||||
| Multivariable-adjusted HR‡ | 1.00 | 1.04 | 1.47 | 1.12 | 0.75 | .41 | 1.00 | 1.17 | 0.46 | .06 |
| 95% CI | 0.61 to 1.78 | 0.69 to 3.12 | 0.64 to 1.97 | 0.37 to 1.52 | 0.74 to 1.83 | 0.23 to 0.94 | ||||
| Multivariable-adjusted HR§ | 1.00 | 1.08 | 1.46 | 1.13 | 0.79 | .46 | 1.00 | 1.13 | 0.39 | .03 |
| 95% CI | 0.63 to 1.87 | 0.68 to 3.14 | 0.64 to 2.01 | 0.37 to 1.66 | 0.70 to 1.83 | 0.18 to 0.84 |
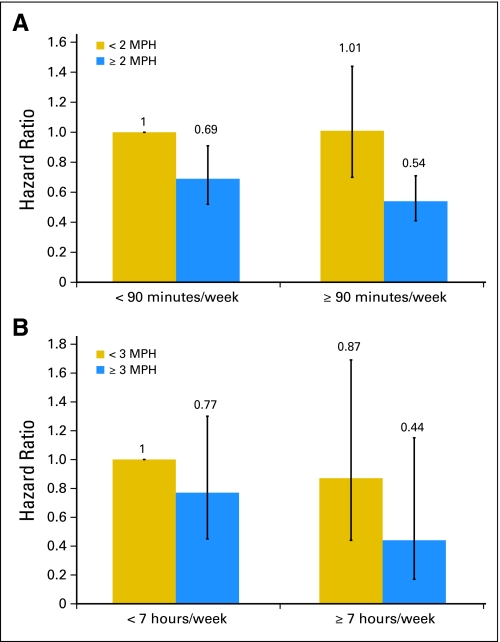
When evaluating walking separately, a significant benefit was observed at levels of ≥ 7 hours of walking per week (HR, 0.64; 95% CI, 0.47 to 0.86) versus < 20 minutes per week (_P_trend = .003). Compared with men with an easy walking pace, men with a normal pace had a 37% lower risk of all-cause mortality (HR, 0.63; 95% CI, 0.50 to 0.78), and men with a brisk or very brisk pace had a 48% lower risk of all-cause mortality (HR, 0.52; 95% CI, 0.39 to 0.70; _P_trend < .001, data not shown). An independent association of pace persisted after adjusting for walking duration. Compared with men who walked less than 90 minutes at an easy pace, those who walked ≥ 90 minutes at a normal to very brisk pace had a 46% lower risk of all-cause mortality (HR, 0.54, 95% CI, 0.41 to 0.71; Fig 1). No statistically significant inverse relation of nonvigorous activity (Table 4) or walking was observed for PCa mortality (≥ 7 hours of walking per week [HR, 0.75; 95% CI, 0.39 to 1.43] v < 20 minutes of walking per week, _P_trend = .53; brisk pace [HR, 0.66; 95% CI, 0.34 to 1.29] v easy pace, _P_trend = .14; data not shown). However, men walking ≥ 7 h/wk at a brisk pace had a trend toward lower risk of PCa mortality compared with shorter durations or slower pace (Fig 1). Compared with men who walked less than 7 hours at a nonbrisk pace, men who walked ≥ 7 hours at a brisk pace had an HR of 0.44 (95% CI, 0.17 to 1.15) for PCa mortality (Fig 1).
Fig 1.
Multivariable-adjusted hazard ratios for (A) all-cause mortality and (B) prostate cancer mortality according to categories of walking duration and pace after prostate cancer diagnosis. An easy pace is less than 2 mile per hour (MPH), a normal pace is 2 to 2.9 MPH, and a brisk pace is ≥ 3 MPH. See footnotes in Table 3 for variables included in the multivariable models for overall and prostate cancer mortality.
Vigorous Activity
Vigorous activity was inversely associated with total mortality in models that included both vigorous and nonvigorous activity, and the effect per hour was stronger than that for nonvigorous activity. Men engaging in ≥ 3 hours versus less than 1 hour per week of vigorous activity had a 49% reduction in all-cause mortality (HR 0.51; 95% CI, 0.36 to 0.72). We observed a significant risk reduction for PCa mortality with increasing vigorous activity (_P_trend = .03). Men with ≥ 3 h/wk of vigorous activity had a 61% lower risk of PCa-specific death (HR, 0.39; 95% CI, 0.18 to 0.84) compared with men with less than 1 h/wk of vigorous activity.
We evaluated the impact of change in vigorous activity, comparing participants' prediagnosis level to the postdiagnostic level immediately after diagnosis (excluding participants who completed the questionnaire within 6 months of their primary treatment). Compared with men with low levels (< 1 h/wk) of vigorous activity both before and after diagnosis (44.3%), men who reduced their activity from the highest category (≥ 3 h/wk) to a lower category (9.2%) had an HR of 1.08 (95% CI, 0.76 to 1.53) for total mortality. Men who increased their activity from a lower category to the highest category (7.6%) had an HR of 0.65 (95% CI, 0.44 to 0.97) for total mortality. Men in the highest category at both assessments (8.3%) had an HR of 0.61 (95% CI, 0.39 to 0.93) for total mortality. The corresponding estimates for PCa mortality were as follows: HR of 1.68 (95% CI, 0.91 to 3.11) for high to low; HR of 0.93 (95% CI, 0.43 to 1.99) for low to high; and HR of 0.40 (95% CI, 0.14 to 1.17) for staying in the highest category (data not shown). We found no significant interactions between activity and age at diagnosis, Gleason score, clinical stage, primary treatment, or BMI for all-cause or PCa-specific mortality.
DISCUSSION
In this population of men with PCa, men who exercised for ≥ 9 MET-h/wk had a 33% lower risk of death from any cause and a 35% lower risk of PCa-specific death, after adjustment for other risk factors for mortality and prediagnosis physical activity. Both nonvigorous activity and vigorous activity were associated with lower all-cause mortality. Only vigorous activity was associated with reduced PCa mortality, with a suggestion of a reduced risk for longer duration of brisk walking.
We considered the possibility that this association might be caused by undiagnosed metastatic cancer inducing a reduction in physical activity (reverse causation) and addressed this issue by excluding men with metastases at diagnosis and up to 2 years after their first postdiagnostic activity assessment and men who died within 4 years of this assessment. Additionally, we used activity information 4 to 6 years before death. The median time from metastasis to PCa death was 2.1 years, and the median time to death from other causes was also 2.1 years. This suggests that using activity information 4 to 6 years before death avoids much of the potential effect caused by reverse causation. Results were not materially different when using a longer lag time. On the basis of the analysis evaluating change in activity, the degree of reverse causation may not be severe because most of the reduction in risk was a result of men who were consistently high in activity or had moved from a lower category to the highest category and not mainly a result of an excess risk from men who reduced their activity. Additionally, in a sensitivity analysis in which we stopped updating activity before a diagnosis of metastasis, the results remained unchanged (data not shown).
Activity was self-reported and limited to a subset of common activities. However, this physical activity assessment has detected other well-established activity-disease relationships in cardiovascular disease8,9 and cancer.17,18 In addition, our population is homogenous by profession, so leisure time activity will capture most between-person variation in physical activity. Our physical activity assessment is a better measure of vigorous activity than nonvigorous activity10; nevertheless, we still observed a significant trend with increasing nonvigorous activity for all-cause mortality.
No prior studies have evaluated the relationship between physical activity after diagnosis and survival in men with PCa, but incidence studies suggest that vigorous activity could reduce risk for fatal disease. We previously reported a significant association between high levels of vigorous activity and reduced risk of advanced PCa in men age 65 years or older,7 and several recent cohort studies support an association of recreational or occupational activity with reduced risk of advanced and fatal disease.19–21 Patel et al19 reported a significant 31% reduction in risk of aggressive PCa among men engaged in more than 35 MET-h/wk of activity compared with men reporting no activity, whereas Johnson et al21 reported no association for leisure time activity but a significant inverse association with advanced disease for manual occupational activity in the European Prospective Investigation Into Cancer and Nutrition (EPIC) cohort. Levels of leisure activity were much higher in EPIC compared with our study, with half of the men having ≥ 43 MET-hours of leisure activity per week, reducing the exposure contrast compared with our population.
Physical activity may affect cancer progression and mortality through the insulin/insulin-like growth factor (IGF) axis. The binding of IGFs and insulin to their receptors can influence cell proliferation, differentiation, apoptosis, adhesion, migration, and angiogenesis.22 Physical activity increases insulin sensitivity and may affect IGF-1 bioactivity. Ma et al23 reported that men in the highest quartile of prediagnostic plasma C-peptide (a marker of insulin production) had a 2.4-fold higher risk of dying from PCa compared with men in the lowest quartile. Laboratory studies have reported that exercise resulted in lower serum insulin and IGF-1 and higher IGF binding protein-1 compared with controls, and the serum from men engaged in regular aerobic exercise reduced cell growth, induced apoptosis, and increased p53 protein content in serum-stimulated LNCaP cells in vitro.24,25
Physical activity lowers inflammatory factors, increases anti-inflammatory cytokines, and inhibits the production of proinflammatory cytokines.26–29 In a 12-month randomized controlled trial of a physical activity intervention among elderly persons, Nicklas et al30 reported significantly lower circulating levels of inflammatory cytokine interleukin-6. Strong evidence supports a role of chronic inflammation in prostate carcinogenesis,31 and the degree of inflammation in prostate tumors32 and specific inflammatory markers33 are associated with progression and can improve prediction for biochemical progression.34 Stark et al35 reported that in men with a BMI less than 25 kg/m2, those with the highest level of IL-6 had an HR of 1.73 (95% CI, 0.86 to 3.51; _P_trend = .02) for increased risk of lethal PCa compared with men with the lowest IL-6 level. Physical activity also increases adiponectin levels, which has anti-inflammatory and mitogenic actions,36 and men with the highest compared with lowest quintile of adiponectin concentration had a 61% lower risk of PCa mortality (HR, 0.39; 95% CI, 0.17 to 0.85; _P_trend < .02).37
Physical activity also affects the innate immune system.38 Exercise in patients with breast cancer was associated with improved natural killer cell cytolytic activity,39 monocyte function,40 and proportion of circulating granulocytes.40 Physical activity may also affect tumor angiogenesis.41
Although previous studies have focused on physical activity and improvement in fatigue, physical functioning, and quality of life,42 we focused on physical activity after PCa diagnosis in relation to overall and PCa-specific mortality. The findings are based on prospective data, with activity data collected every 2 years, before and after diagnosis. Lastly, we had an adequate number of PCa deaths to evaluate this outcome after excluding participants who died within 4 years of diagnosis.
In conclusion, our results suggest that among men with PCa, moderate physical activity may improve overall survival, whereas a greater amount of activity is necessary to improve PCa-specific survival. A modest amount of vigorous activity such as biking, tennis, jogging, or swimming at levels of ≥ 3 h/wk may substantially improve PCa-specific survival. Mechanistic studies and randomized trials of physical activity interventions are needed in PCa survivors to determine whether physical activity reduces PCa progression and what regimens are optimal.
Acknowledgment
We thank the participants and staff of the Health Professionals Follow-Up Study for their valuable contributions. We thank the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, Wyoming.
Footnotes
Supported by Grant No. P01CA055075 from the National Institutes of Health (NIH)/National Cancer Institute; Grants No. CA133891, CA141298, and T32CA009001 from the NIH; the Charles A. King Trust Research Fellowship Award; and the Prostate Cancer Foundation.
Presented in part at the 8th Annual American Association for Cancer Research International Conference on Frontiers in Cancer Prevention Research, December 6-9, 2009, Houston, TX; the 16th Annual Prostate Cancer Foundation Scientific Retreat, September 23-25, 2009, Incline Village, NV; and the 2010 American Society of Clinical Oncology Genitourinary Cancers Symposium, March 5-7, 2010, San Francisco, CA.
The funding sources had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the article.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Stacey A. Kenfield, Meir J. Stampfer, Edward Giovannucci, June M. Chan
Financial support: Stacey A. Kenfield, Meir J. Stampfer, Edward Giovannucci, June M. Chan
Administrative support: Stacey A. Kenfield
Provision of study materials or patients: Meir J. Stampfer, Edward Giovannucci
Collection and assembly of data: Stacey A. Kenfield, Meir J. Stampfer, Edward Giovannucci, June M. Chan
Data analysis and interpretation: Stacey A. Kenfield, Meir J. Stampfer, Edward Giovannucci, June M. Chan
Manuscript writing: Stacey A. Kenfield, Meir J. Stampfer, Edward Giovannucci, June M. Chan
Final approval of manuscript: Stacey A. Kenfield, Meir J. Stampfer, Edward Giovannucci, June M. Chan
REFERENCES
- 1.American Cancer Society. Atlanta, GA: American Cancer Society; 2010. Cancer Facts and Figures 2010. [Google Scholar]
- 2.American Cancer Society. Atlanta, GA: American Cancer Society; 2009. Cancer Facts and Figures 2009. [Google Scholar]
- 3.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 4.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 5.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Giovannucci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci EL, Liu Y, Leitzmann MF, et al. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165:1005–1010. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]
- 8.Tanasescu M, Leitzmann MF, Rimm EB, et al. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 9.Tanasescu M, Leitzmann MF, Rimm EB, et al. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107:2435–2439. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 10.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Liu Y, Platz EA, et al. Risk factors for prostate cancer incidence and progression in the Health Professionals Follow-Up Study. Int J Cancer. 2007;121:1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States) Cancer Causes Control. 2006;17:199–208. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 15.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 16.Rosner B. Fundamentals of Biostatistics (ed 5) Florence, KY: Duxbury Press; 2000. [Google Scholar]
- 17.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 19.Patel AV, Rodriguez C, Jacobs EJ, et al. Recreational physical activity and risk of prostate cancer in a large cohort of U.S. men. Cancer Epidemiol Biomarkers Prev. 2005;14:275–279. [PubMed] [Google Scholar]
- 20.Nilsen TI, Romundstad PR, Vatten LJ. Recreational physical activity and risk of prostate cancer: A prospective population-based study in Norway (the HUNT study) Int J Cancer. 2006;119:2943–2947. doi: 10.1002/ijc.22184. [DOI] [PubMed] [Google Scholar]
- 21.Johnsen NF, Tjønneland A, Thomsen BL, et al. Physical activity and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer. 2009;125:902–908. doi: 10.1002/ijc.24326. [DOI] [PubMed] [Google Scholar]
- 22.Werner H, Bruchim I. The insulin-like growth factor-I receptor as an oncogene. Arch Physiol Biochem. 2009;115:58–71. doi: 10.1080/13813450902783106. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: A long-term survival analysis. Lancet Oncol. 2008;9:1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnard RJ, Ngo TH, Leung PS, et al. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate. 2003;56:201–206. doi: 10.1002/pros.10251. [DOI] [PubMed] [Google Scholar]
- 25.Leung PS, Aronson WJ, Ngo TH, et al. Exercise alters the IGF axis in vivo and increases p53 protein in prostate tumor cells in vitro. J Appl Physiol. 2004;96:450–454. doi: 10.1152/japplphysiol.00871.2003. [DOI] [PubMed] [Google Scholar]
- 26.Geffken DF, Cushman M, Burke GL, et al. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 27.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 28.Wannamethee SG, Lowe GD, Whincup PH, et al. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–1790. doi: 10.1161/hc1502.107117. [DOI] [PubMed] [Google Scholar]
- 29.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 30.Nicklas BJ, Hsu FC, Brinkley TJ, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J Urol. 2004;171(suppl):S36–S40. doi: 10.1097/01.ju.0000108131.43160.77. [DOI] [PubMed] [Google Scholar]
- 32.Irani J, Goujon JM, Ragni E, et al. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy: Pathologist Multi Center Study Group. Urology. 1999;54:467–472. doi: 10.1016/s0090-4295(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 33.Shariat SF, Kattan MW, Traxel E, et al. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin Cancer Res. 2004;10:1992–1999. doi: 10.1158/1078-0432.ccr-0768-03. [DOI] [PubMed] [Google Scholar]
- 34.Kattan MW, Shariat SF, Andrews B, et al. The addition of interleukin-6 soluble receptor and transforming growth factor beta1 improves a preoperative nomogram for predicting biochemical progression in patients with clinically localized prostate cancer. J Clin Oncol. 2003;21:3573–3579. doi: 10.1200/JCO.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Stark JR, Li H, Kraft P, et al. Circulating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortality. Int J Cancer. 2009;124:2683–2689. doi: 10.1002/ijc.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You T, Nicklas BJ. Effects of exercise on adipokines and the metabolic syndrome. Curr Diab Rep. 2008;8:7–11. doi: 10.1007/s11892-008-0003-4. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Stampfer MJ, Mucci L, et al. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 2010;56:34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods JA, Davis JM, Smith JA, et al. Exercise and cellular innate immune function. Med Sci Sports Exerc. 1999;31:57–66. doi: 10.1097/00005768-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Peters C, Lötzerich H, Niemeier B, et al. Influence of a moderate exercise training on natural killer cytotoxicity and personality traits in cancer patients. Anticancer Res. 1994;14:1033–1036. [PubMed] [Google Scholar]
- 40.Peters C, Lötzerich H, Niemeir B, et al. Exercise, cancer and the immune response of monocytes. Anticancer Res. 1995;15:175–179. [PubMed] [Google Scholar]
- 41.Jones LW, Eves ND, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 42.Thorsen L, Courneya KS, Stevinson C, et al. A systematic review of physical activity in prostate cancer survivors: Outcomes, prevalence, and determinants. Support Care Cancer. 2008;16:987–997. doi: 10.1007/s00520-008-0411-7. [DOI] [PubMed] [Google Scholar]