Anti-Aβ therapeutics in Alzheimer’s disease: The Need for a Paradigm Shift (original) (raw)
. Author manuscript; available in PMC: 2012 Jan 27.
Abstract
Most current Alzheimer’s disease (AD) therapies in advanced phases of development target amyloid beta-peptide (Aβ) aggregation and accumulation. Translational models suggest that anti-Aβ therapies may be highly effective if tested as agents to prevent or delay development of the disease or as therapies for asymptomatic patients with very early signs of AD pathology. However, anti-Aβ therapeutics are currently being tested in symptomatic patients where they are likely to be much less effective or ineffective. The lack of alignment between human clinical studies and preclinical studies, together with predictions about optimal trial design based on our understanding of the initiating role of Aβ aggregates in AD, have created a treatment versus prevention dilemma. In this perspective, we discuss why it is imperative to resolve this dilemma, and suggest ways for moving forward in the hopes of enhancing the development of truly effective AD therapeutics.
Introduction
Effective therapy for Alzheimer’s disease (AD) is a major unmet medical need. The major demographic risk for development of AD is age with risk doubling approximately every 5 years after age 65 such that by the age of 85 one’s chances of having dementia due to AD ranges from 25%–40+%. Therefore, the prevalence of AD is predicted to double every 20 years largely due to a predicted increase in the average expected life span. Based on estimates that ~35 million people worldwide have AD today, well over 100 million individuals are predicted to have AD in 2050 (Alzheimer's Association, 2010; Wimo et al., 2010). If nothing is done, the personal, economic and societal toll of the ongoing and growing AD epidemic will be immense.
Although key aspects of AD pathogenesis remain enigmatic, scientific advances over the last 25 years have provided sound rationale for the development of potentially disease-modifying AD therapies (Golde, 2005; Selkoe, 2001). These therapies primarily target the suspected trigger(s) of the disease. Therapies that have advanced the farthest have primarily been developed based on the proposed initiating role of amyloid β-protein (Aβ) aggregates (Golde et al., 2010). These therapeutic advances coupled with advances in early detection of AD-related pathology in non-demented individuals, suggest that concerted translational research efforts focusing on prevention or early intervention could dramatically reduce the incidence and prevalence of AD. However, current trial design involves treatment of symptomatic patients, a setting where failure to show efficacy may be even more likely given the disease progression. Misalignments of the rationale for the therapy, its preclinical testing, and the actual testing of the therapy in human AD clinical trials have resulted in barriers to effective drug development that we must recognize and will be very challenging to solve.
This perspective will focus on how this dilemma in AD translational research has evolved. We begin by summarizing both the current state of AD therapeutic development and the paradigm shift that is occurring with respect to being able to detect and track underlying AD-related pathologies in humans in the absence of significant cognitive impairment, as both of these issues are critical to how the dilemma has arisen and how we might solve it. We next focus on the core issue of the mismatch between the design of preclinical studies that evaluate potential AD therapies and the current translation of those therapies to human clinical trials. We conclude with a discussion of the main obstacles that must be overcome to solve this dilemma and create the desired paradigm shift in translational AD research..
The current state of AD therapeutics and therapeutic discovery
For the typical AD patient, current symptomatic therapies (acetylcholinesterase inhibitors and memantine), demonstrate only minimal to modest symptomatic benefit that is not sustained. Moreover, there is virtually no evidence that either of these types of treatments significantly alter disease progression (Schneider et al., 2011). Although there is renewed effort to develop novel cognitive enhancing agents that target different pathways, only one of these, a repurposed drug, dimebon (Doody et al., 2008), has entered phase III efficacy studies in humans. Results from the first phase III study designed to confirm promising phase II results, unfortunately, showed no evidence of efficacy (Jones, 2010).
A large percentage of current therapeutic development in AD is focused on therapies that target the Aβ peptide or Aβ aggregates (Golde et al., 2010). Accumulation of fibrillar Aβ aggregates in senile plaques within the brain parenchyma is one of the classic pathological hallmarks of AD. A detailed understanding of the proteolytic processing that releases Aβ from the amyloid precursor protein (APP) and its subsequent aggregation in the brain has provided a number of approaches to what may be generically referred to as anti-Aβ therapy. To date, four general categories of anti-Aβ therapy have been developed i) agents that decrease or modulate Aβ production in a manner that is designed to prevent or slow Aβ aggregation and accumulation ii) therapies that degrade or enhance clearance of Aβ aggregates iii) therapies designed to block Aβ aggregates and iv) therapies designed to neutralize toxic Aβ aggregates.
The rationale for these anti-Aβ therapies has been validated in preclinical models over the last 20 years and is rooted in the amyloid cascade hypothesis of AD (Hardy and Selkoe, 2002; Hardy and Higgins, 1992). This hypothesis posits that accumulation of Aβ aggregates in the brain triggers a complex neurodegenerative cascade, which results in progressive cognitive impairment and dementia. Though the original amyloid hypothesis has been expanded to encompass the likely possibility that multiple different types of Aβ aggregates (intracellular aggregates, oligomers, fibrils and protofibrils) contribute to the cascade (Walsh and Selkoe, 2004), the essential tenants of this hypothesis remain the same. The main support for the hypothesis has come from a convergence of genetic, cell biological, animal modeling, pathological and biophysical studies. Collectively, these studies demonstrate a primary effect of genetic alterations that cause familial forms of AD is to alter Aβ production or Aβ itself in a way that promotes its aggregation and accumulation in the brain (Selkoe, 2001). Additional indirect support for the hypothesis has come from studies of other CNS proteinopathies (Forman et al., 2004; Ross and Poirier, 2004). A common theme in many neurodegenerative diseases is that genetic mutations, overexpression (often due to gene duplication), ineffective removal or age-associated changes results in accumulation of alternatively folded protein aggregates that sequentially trigger a degenerative cascade, neuronal demise, and ultimately regional or widespread brain organ failure. In this regard, the British and Danish Familial Dementias are notable with respect to the parallels with AD, in both clinical and pathological features and hypothesized mechanism (Ghiso et al., 2006). The key difference between these two familial dementias and AD is that the trigger appears to be accumulation of different mutant amyloidogenic peptides derived from the BRI2 (ITM2B) protein versus the Aβ peptide.
The Aβ aggregate hypothesis is reflected by biomarker studies in humans
Recent biomarker and imaging studies in living humans, along with classic postmortem studies from Braak and Braak, the Religious Order Study and other human studies (Bennett, 2006; Blennow, 2004; Braak and Braak, 1997; Jack et al., 2009; Morris and Price, 2001; Perrin et al., 2009; Schneider et al., 2009; Shaw et al., 2009), have begun to frame theoretical average timeline for the development of various pathological features that characterize AD and the relationship to initial diagnosis of dementia or prodromal dementia (i.e., mild cognitive impairment due to Alzheimer’s disease). Cross-sectional and ongoing longitudinal biomarker studies reveal that the diagnosis of AD occurs after a relatively long prodromal clinical phase prior to the diagnosis of AD itself, which by itself requires the presence of a dementia syndrome manifested by cognitive impairment that interferes with many aspects of daily function.
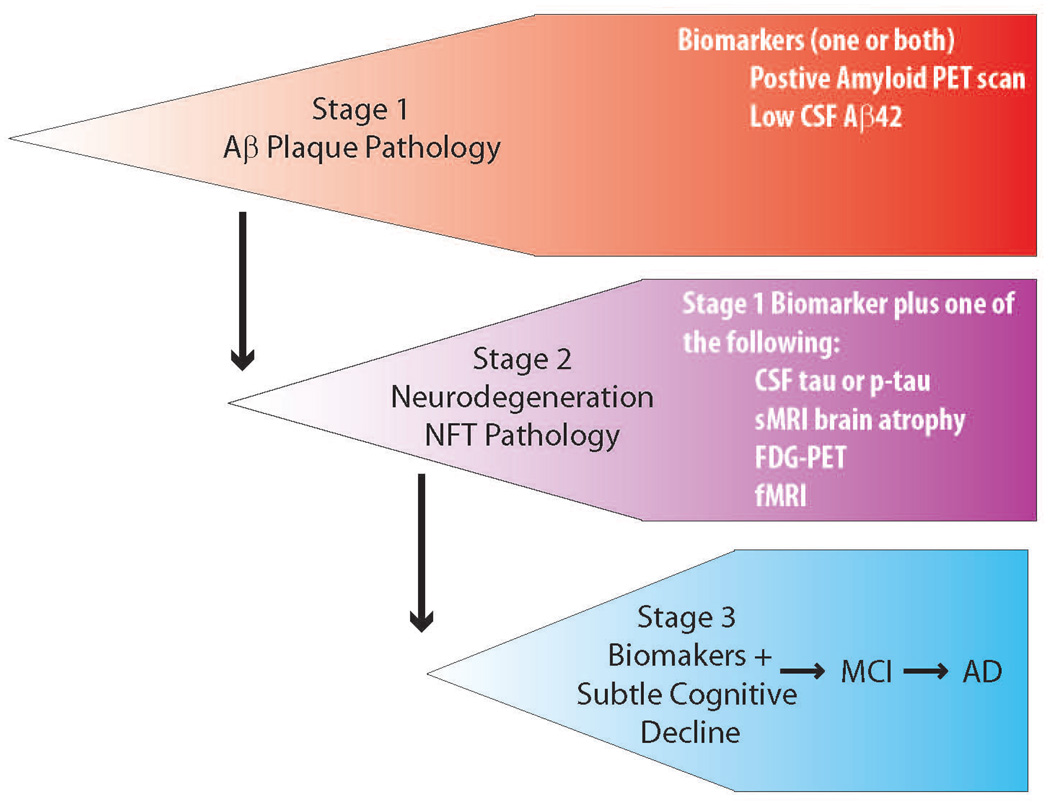
Although the human AD “biomarker and imaging cascade” is sure to be refined and advanced, the current data strongly support the following hypothetical scenario. First, in normal elderly individuals destined to develop AD, 10 or more years before the onset of dementia, Aβ aggregate accumulation begins in the brain as a result of reduced clearance/degradation or increased production. As the Aβ pathology progresses, clinical correlates of Aβ accumulation, such as amyloid plaques visualized by radioligand imaging and low cerebrospinal fluid (CSF) Aβ42 concentrations possibly due to sequestration in brain, can be detected. Within an estimated timeline of 10–15 years, Aβ accumulates to levels that are present in brains of AD individuals. Accumulation of Aβ may plateau, though these observations are based on cohort studies and ongoing longitudinal amyloid imaging studies will be needed to validate both the time course and the kinetics of accumulation. During this protracted phase of progressive Aβ accumulation in brain, a number of poorly understood cellular changes take place reflecting increasing neuronal injury. For example, there is an increase in CSF total tau and phosphorylated tau levels that likely reflects synaptic loss and neuronal demise in brain parenchyma. Coincident or shortly after tau CSF levels rise, structural MRI can reveal regional brain atrophy, and functional MRI can show evidence for altered network activity between brain regions. Cognitive function and instrumental activities of daily living may deteriorate but generally still fall within a normal range. More commonly, subtle memory impairments might be detected, with more severe cognitive changes and overt dementia occurring later. This concept that very early, prodromal AD, and mild cognitive impairment phases can be detected years before dementia becomes apparent has led to two workgroups proposing new guidelines that puts the clinical evolution of AD on a continuum that starts with a “preclinical” phase where the Aβ pathology of AD can be detected, followed by evidence of neurodegeneration, both without any clinical findings, followed by the earliest clinical signs (Dubois et al., 2010) (http://www.alz.org/research/diagnostic_criteria, Figure 1).
Figure 1. Preclinical AD.
The newly proposed staging of preclinical or prodromal AD reflects a cascade beginning with Aβ accumulation in the brain of clinically asymptomatic individuals and inexorably progressing to AD.
The remarkable parallels between the hypothesized cascade, experimental evidence from animal models, and measurable biological events occurring in humans, reinforce the rationale for anti-Aβ therapeutics. However, the cascade hypothesis only predicts that if Aβ accumulation in the brain is attenuated or prevented, then so too will be the subsequent development of AD. It remains an open question whether targeting Aβ aggregates at any stage in the pathological process will result in clinically effective therapeutics. For example, intervention with an anti-Aβ therapy in the disease state with longstanding amyloid deposited in plaques, substantial synaptic loss and neurodegeneration, and manifest clinical symptoms may be completely ineffective. Even fairly early intervention in non-demented individuals (Stage 2, Figure 1) in which the neurodegenerative disease process has started may be ineffective. It is possible the degenerative changes will continue regardless of whether the therapeutic agent decreases Aβ production or even clears Aβ deposits from brain. In general, given the reasonable possibility that some aspects of the neurodegenerative cascade may be self-reinforcing, become independent of the initial trigger, or are irreversible in nature, one might predict that anti-Aβ therapies will be increasingly ineffective in terms of attenuating the course of illness as the underlying pathology progresses and clinical symptoms emerge. Ultimately, the true test of the cascade hypothesis is to conduct a true primary prevention trial with anti-Aβ therapy in patients destined to develop AD but prior to the onset of measureable Aβ deposition. Alternatively, one might envision that the hypothesis could be reasonably well tested in secondary prevention (very early intervention) trial in subjects with Aβ pathology only, prior to the neurodegenerative phase (preclinical stage 1). In the later scenario, the test potentially would be more effective if the therapeutic modality enhanced clearance of Aβ.
Critiques of the Aβ aggregate hypothesis
In order to conduct a primary prevention study with a therapeutic agent for any disease, one needs a strong scientific rationale. Although the Aβ aggregate/amyloid hypothesis is not without its critics, it has very strong scientific underpinnings. In the context of trying to move the field towards primary prevention studies with anti-Aβ therapies, it is important to consider the views of those who are skeptical about the role of Aβ in AD. One valid critique of the hypothesis is that most of the experimental evidence supporting the hypothesis comes from the study of the genetic alterations that cause familial autosomal dominant AD (Selkoe, 2001; Younkin, 1998). Thus, a key assumption is that pathological cascades in sporadic AD are the same as in familial AD. Given that the typical genetic and sporadic forms of AD are very similar with respect to postmortem findings, clinical course, and evidence from the emerging biomarker and imaging cascade in sporadic AD, this seems a reasonable assumption (Shepherd et al., 2009). Indeed, when examples from other human diseases with genetic and sporadic forms, such as hypercholesterolemia and prion diseases, are considered, this concept is quite tenable (Brown and Goldstein, 1986; Prusiner, 1998). Nevertheless, because of the late age of onset, sporadic AD is not a completely uniform clinical or pathological entity: it is complicated by other systemic or CNS illnesses and conditions, and the biological processes of aging. Thus, the underlying brain pathology that results in clinical dementia may represent the convergence of independent processes and not necessarily a single pathologic entity (Small and Duff, 2008), as thought to be the case in early onset familial AD. Indeed, in the over 80 year old population who have died with a diagnosis of clinical diagnosis of probable AD dementia, postmortem phenotypes often show not only plaques and tangles pathology characteristic of AD, but other proteinopathies and vascular insults as well (Dickson, 2001). However, even if this is the case, and multiple pathologies synergize in some subpopulations of those affected with what we define as AD, one would still predict that preventing Aβ deposition would have substantial, but perhaps not complete, efficacy.
Another critique of the Aβ cascade hypothesis arises from the imperfect correlation between cognitive status and Aβ deposits in brain, and the postmortem findings of significant Aβ accumulation in the brains of elderly who were cognitively intact shortly before death (Giannakopoulos et al., 2003; Naslund et al., 2000). The criticism that follows is Aβ deposition itself does not necessarily predict or cause clinical AD. Such observations, however, can be understood in several other ways. First, there may be a threshold effect that involves the density and duration, or even rate of Aβ accumulation that together with the age of onset of the pathological processes determines the onset of the clinical manifestations of AD. Second, as with other illnesses, there are almost certainly genetic, pathological, epigenetic, and environmental mediators that modulate progression, disease course, and manifestation of illness. For example, one proposed mediator involves the concept of ‘cognitive reserve’ that hypothesizes that factors that enhance neuroplasticity and synaptogenesis, may make an individual more resistant to the clinical manifestations of the underlying neuropathology, thereby delaying onset of the clinical expression of the illness (Cummings et al., 1998; Stern et al., 1999). Third, it is also possible that early subtle cognitive impairment of AD that we might now refer to as preclinical Stage 3 is often not recognized in elderly people who die and come to autopsy. Some evidence for this is from the Religious Order Study where those who died without cognitive impairment and who had intermediate or high likelihood of AD based on neuropathological examination scored 0.25 standard deviation lower on episodic memory tests than those without pathology (Bennett et al., 2006), worsening of episodic memory being the earliest and most characteristic cognitive phenotype for AD (Dubois and Albert, 2004).
Finally, it has become common to explain the results from failed AD therapeutic trials with presumptive anti-Aβ therapies as evidence that the hypothesis is wrong. This is clearly inaccurate, as to date, such trials were not definitive tests of the cascade hypothesis, but rather expedient ways to test potentially disease modifying AD therapeutics in the current clinical, regulatory, and fiscal environment. None of the putative anti-Aβ agents that have failed in pivotal phase III therapeutic trials were optimal or even optimized agents within their class of anti-Aβ therapeutics: Alzhemed™ (tramiprosate, homotaurine) was a weak aggregation inhibitor; Flurizan™ (tarenflurbil, R-flurbiprofen) was a γ-secretase modulator with low potency and poor brain penetration; and semagacestat, a non-selective γ-secretase inhibitor (GSI), had significant mechanism-based toxicity limiting its dosage and efficacy with respect to lowering Aβ production (Golde et al., 2010). None of these drugs showed efficacy against primary endpoints in phase II trials but were advanced to phase III nonetheless.
Other anti-Aβ therapies (e.g., Notch sparing GSIs, humanized anti-Aβ monoclonal antibodies and 2nd generation Aβ vaccines) currently in therapeutic trials for AD are more likely to have improved effects on their respective targets, but it is unclear whether this will translate to clinical efficacy. These ongoing and planned trials should provide an answer to the questions raised above as to whether targeting Aβ in symptomatic AD patients will have any efficacy at all. However, available Phase II data would suggest if these compounds are going to have disease modifying-effects and improve the course of cognitive decline in this patient population, the effect is going to be quite modest.
Other therapeutic approaches
Although most therapeutic activity in AD with respect to potentially disease modifying therapy has focused on anti-Aβ therapies designed to decrease Aβ production or aggregate formation or removal of preexisting aggregates, both tau targeted and more general neuroprotective agents among others are also being developed (Golde et al., 2010; Salloway et al., 2008). Development of anti-tau therapies has been hindered by a lack of clear insight into what is the optimal desired effect on tau (e.g., decreasing phosphorylation, blocking proteolysis, or preventing aggregation). Though animal modeling studies do provide evidence that Aβ aggregates promote some aspects of tau pathology (Gotz et al., 2001; Lewis et al., 2001), the precise mechanistic links between Aβ and tau pathologies have not been established, thus hindering not only our ability to appreciate the biologic connection but also to develop better animal models and identify druggable therapeutic targets.
Neuroprotective strategies are a rationale approach, but have generally not gained much traction with little progress in terms of new investigational drugs for AD (Salloway et al., 2008). The reasons for this may stem from i) a lack of understanding regarding the mechanisms of neural injury, ii) uncertainty regarding the best targets for neuroprotection in AD, iii) the inadequacy of current animal models as exemplified by the relative paucity of neurodegeneration in transgenic mice that are primarily models of amyloid deposition and do not exhibit the full spectrum of AD pathologies, or iv) the poor track record of successful translation of neuroprotective drugs from the preclinical to clinical phase in other neurological disease such as stroke or any neurodegenerative condition.
In any case, if one assumes the temporal sequencing in the Aβ aggregate cascade is correct, then one would predict that anti-tau therapy will be most effective in the very early pathological phases of the disease, and not after the stage when robust Aβ deposition, synaptic loss, and neurofibrillary changes have begun. In contrast, general or focused neuroprotective strategies might be efficacious even in such later stages, as there is evidence for continued neuronal demise as the clinical dementia worsens.
The Treatment versus Prevention Dilemma
The preceding overview of the status of AD therapeutics highlights what can be referred to succinctly as the treatment versus prevention dilemma. It is cogent to state that anti-Aβ therapy is most likely to show efficacy in a primary prevention setting. Certain therapeutic modalities that can result in Aβ clearance could also show efficacy in Aβ deposition stages of AD. However, current clinical trials designs have involved treatment of patients with AD dementia or mild cognitive impairment over generally 18-month to 4-year intervals (Schneider and Sano, 2009). In these instances, one might predict that the likely outcome of anti-Aβ therapy will be no clinically observable beneficial effect. Testing anti-Aβ therapy in patients with clinically diagnosed AD may be analogous to treating a patient with atherosclerosis, myocardial infarctions and heart failure with a cholesterol lowering agent and expecting his current cardiac function and subsequent clinical course would noticeably improve. In such a setting, targeting the trigger of disease, or the pathophysiologic process that is protracted, is not likely to demonstrate efficacy. Indeed, one can speculate that if the early trial designs for the testing of statins were in patients in complete cardiac failure and used morbidity and mortality endpoints (as opposed to plasma cholesterol lowering), it is likely that statins would have failed to show efficacy. Though statins clearly lower cholesterol, even today it is challenging to demonstrate that statin treatment has significant impact on cardiovascular morbidity and mortality in non-selected patient populations.
Not only does the amyloid cascade hypothesis provide support for initiation of primary prevention or possibly very early intervention (‘secondary prevention’ trials) with anti-Aβ therapy, but preclinical studies in Aβ depositing transgenic mouse models do as well (Ashe and Zahs, 2010). The vast majority of preclinical studies in APP transgenic mice that show efficacy are equivalent to primary or secondary prevention, as treatment is typically begun either before the onset of amyloid pathology or in mice with very modest Aβ loads. Even very old APP/Aβ mouse models only appear to be good models of preclinical AD as they show amyloid deposition, amyloid-associated neuritic dystrophy, and plaque associated micro- and astrogliosis (Price and Sisodia, 1998) but typically no neurodegenerative phenotypes and minimal tau pathology. Thus, any study at any age in these mice mainly reflects likely outcomes in early preclinical stages of AD. Although many models do show cognitive impairments that can be interpreted to be reminiscent of AD in that they involve memory systems, the cognitive changes and their relationship to other pathological features vary from model to model, have inconsistent relationships with amyloid and other pathologies, and can often be rapidly reversed (Ashe, 2001). Lastly, treatments given to APP transgenic mice when amyloid deposition and associated pathology are established are generally less effective (Abramowski et al., 2008; Das et al., 2001). Thus, it is uncertain whether efficacy with respect to alleviating the behavioral changes observed in such mouse models, especially performed in pre-plaque stages, would be able to predict efficacy with respect to behavioral alterations in humans with symptomatic AD.
Recognizing and overcoming the medical and scientific obstacles to solving the treatment versus prevention dilemma
There are two straightforward ways to solve the dilemma through medical and scientific progress. First, with anti-Aβ therapies and perhaps anti-tau therapies, we must conduct primary prevention or intervention trials in minimally affected individuals (secondary prevention in stage 1/2) A second, alternative, strategy would be to develop therapies more likely to work in symptomatic patients (i.e., in a preclinical stage 3 or prodromal AD).
When considering primary prevention or very early intervention in asymptomatic subjects, the key scientific issue will be whether a therapy can be developed that hits the target sufficiently to have a very good chance for disease modification, and is sufficiently safe for use in people included in the trial but not destined to develop AD, or likely to develop the clinically symptomatic illness only after several years of good health. Whether a candidate drug can be considered sufficiently safe will depend on i) the underlying biology of the target (mechanism based toxicity), ii) the ability to avoid off-target effects, and iii) an empirically determined assessment of benefit versus liabilities. Whether a therapy is safe enough will also be influenced by the conditions of use, whether one is considering a true primary prevention trial, a trial in preclinical AD, or a trial in established symptomatic AD, as the risk to benefit profile will shift toward tolerating greater risk with advancing clinical disease. In the later populations, the bar for ‘safe enough’ is lower than in the primary/secondary prevention setting given the evidence for irreversible though protracted progression.
Many current anti-Aβ therapeutic modalities fail the safe enough test even in symptomatic patients, especially given the long term treatment that is necessary in this chronic condition. However, a number of modalities, such as selective γ-secretase inhibitors, γ-secretase modulators, and 2nd or 3rd generation vaccines, theoretically, hold some promise for meeting the safe enough requirement for testing as prophylactic agent (Golde et al., 2010).
From a medical perspective, a key issue will be whether the community will accept the concept of presymptomatic AD, which, to reiterate, is the presence of Aβ aggregate accumulation with or without some evidence for neurodegeneration in the absence of detectible cognitive symptoms. This diagnostic construct is invaluable when considering moving towards primary prevention or early intervention trials as it potentially identifies the earliest manifestation of the disease. As noted previously, preclinical AD may exist for a decade or more before what has been referred to as prodromal AD or mild cognitive impairment due to AD, providing a window of opportunity for intervention. Preclinical AD also represents the boundary condition between two important therapeutic approaches: the true primary prevention of illness and the so-called ‘secondary prevention’ or treatment of the very earliest manifestations of illness. Future trials will need to account for and distinguish between the truly asymptomatic and preclinical AD.
Although we have seen remarkable and rapid advances in the ability to diagnose preclinical AD (Weiner et al., 2010), in order to move towards primary prevention we need to advance our ability to predict who is at very high risk for AD and in what time frame they might develop observable pathology, and subsequently clinical symptoms. Based on current data we know that APOE ε4 genotype, low CSF Aβ42, and increased PET amyloid tracer binding in brain, all confer substantially increased risk for the progression of preclinical AD to MCI and MCI to AD (Blennow, 2004; De Meyer et al., 2010; Romas et al., 1999; Storandt et al., 2009). But these markers do not provide information regarding onset of pathology. Even presence of an APOE ε4 genotype only indicates increased risk or earlier age-of-onset but fails to provide precise information with respect to timing of disease onset. Identification of additional factors that predict more precisely the risk for development of AD, what are generically referred to as premorbid biomarkers, could be very useful in identifying an at risk population for a primary prevention study. Again, if we make an analogy to atherosclerotic disease, plasma cholesterol testing serves as such as premorbid biomarker.
Given this reality, there is substantial interest in the field to test preventive agents in genetic forms of AD where large kindreds, such as one in Antioquia, Colombia with a deterministic early-onset presenilin 1 mutation (www.dian-info.org), or in individuals who are homozygous for the ApoE ε4 allele (Reiman et al., 2010; Strittmatter and Roses, 1995). Though laudable and perhaps the only way forward at the present time, these studies have some limitations. Even in large kindreds with deterministic AD causing mutations, the number of asymptomatic mutation carriers who might be predicted to develop or have preclinical AD within a reasonable time frame is relatively small. Thus, the number of different therapies that might be tested in such a setting will likely be very limited, and because of variance in the age of onset, it is unclear how long such studies would need to extend in order to convincingly demonstrate efficacy. Further, it has been shown that some anti-Aβ treatments may have altered efficacy in presenilin mutation carriers (Weggen et al., 2003).
The 1–2% of the population that is homozygous for the ApoE ε4 allele represents another at-risk or preclinical sample for clinical trials (Reiman et al., 2010), and being homozygote increases risk for AD by 15 times AD compared to APOE ε3 or ε2 carriers (Farrer et al., 1997; Reiman et al., 2010). Simple genetic screening could theoretically provide cohorts for selection of trial participants with this geneotype. However, the low frequency of homozygous ApoE ε4 carriers severely limit their recruitment and possibly even generalizability to the population as a whole.
Choosing such samples as familial early onset AD or people who are homozygous APOE ε4 carriers as the intent to treat population also raises another issue which is whether the expected action of the drug is influenced by the genetic makeup of the individuals. For example, presenilin-linked AD is associated with altered Aβ42 production (Selkoe, 2001), while APOE ε4 is associated with decreased clearance of Aβ from the brain (Holtzman et al., 2000).
APOE ε4 heterozygotes are another at-risk potential sample for prevention trials: these individuals constitute approximately 24–30% of the population, have three times the risk for AD, about a 10- year lower age-of-onset compared to APOE ε3 or ε2 carriers (Farrer et al., 1997)and represent ~ 50% of AD cases (Roses, 1997). Although they have one-fifth the risk for AD compared to ε4 homozygotes, they are more than 8 times easier to recruit by virtue of their prevalence. Thus, a prevention trial with heterozygotes may be carried out efficiently and be more generalizable to the majority of AD patients.
In many scenarios, a 15–20 year timeline would be the minimum time to test, possibly retest, and widely deploy an effective true primary prevention therapy or a therapy for the clearly asymptomatic preclinical stages of AD. In the interval, millions of people will continue to develop AD. So what do we do for them? First, we can simply hope that the predictions of the cascade hypothesis are wrong and that trigger-targeting therapy will show better efficacy in current trials than might be predicted. Evidence for efficacy and safety would likely mean much more rapid approval for symptomatic use. Second, we can renew our efforts to identify novel downstream targets and develop novel neuroprotective or regenerative therapies that may be more efficacious than targeting upstream pathways in true treatment trials. These studies would be greatly facilitated by the development of animal models that recapitulate the full disease phenotype.
An important area where the medical and scientific field can improve in order to overcome the treatment versus prevention dilemma is to better align the design of preclinical studies with subsequent clinical trial designs. This means that the usual chronic dosing studies in pre- or early-amyloid depositing APP transgenic mice with anti-Aβ therapy must be accompanied or replaced by studies in which the mice have AD-like Aβ loads at the time the treatment is initiated. Given that in practice we have not had animal models that recapitulate the entire AD degenerative cascade (Ashe and Zahs, 2010), there are some practical limitations as to how far one can take this realignment. Thus, we cannot truly evaluate the potential of anti-Aβ or neuroprotection therapies to halt neuronal loss, because there is such limited neuronal loss in current APP mouse models. Nevertheless, we can at least attempt to be more rigorous and self-critical with respect to the potential clinical translation of pre-clinical data.
Recognizing and overcoming the other obstacles to solving the treatment versus prevention dilemma
There are many non-scientific and non-medical challenges to implementing primary prevention or early intervention in AD. Some of the most challenging aspects are financial in nature; others are regulatory barriers.
Financial obstacles
Phase 2 and 3 clinical trials in the pharmaceutical industry overall are inherently complicated, resource intensive endeavors with high probabilities for failure. Together, phase 2 and 3 programs consume 48% of the costs for each drug launched and may cost on average 185millionand185 million and 185millionand235 million, respectively (Paul et al., 2010). Commercially sponsored AD therapeutic trials and most prevention trials are typically more expensive. It is difficult to source the costs of an AD prevention trial for industry as only one such trial has been sponsored: a Ginkgo biloba extract study in France involving about 2800 patients over 5 years (Vellas et al., 2006b).
NIH has funded several “prevention” trials including WHIMS, ADAPT, GEM and Pre-Advise (Craig et al., 2005; Kryscio et al., 2004; Martin et al., 2002; Snitz et al., 2009). These trials were designed in a manner that cost significantly less than current industry-funded treatment trials (Table 1). For example, some of the studies enhanced the likelihood for AD by choosing participants who were at higher risk or who already had MCI, outcomes were onset of AD or MCI, they had relatively short follow up periods of 4 to 7 years, and they did not incorporate the comprehensive biomarker or imaging assessments that are available today. This enabled recruitment of 2500 to 4500 participants. Based on publicly listed sources (http://www.projectreporter.nih.gov/reporter.cfm) the comparably large ADAPT (ADAPT Research Group, 2007) and GEM Ginkgo biloba extract study (DeKosky et al., 2008) studies have received approximately 44millionand44 million and 44millionand28 million, respectively, in direct and indirect funding. Total costs for these studies are likely higher as they typically leverage infrastructure within the NIH and participating academic institutions. Taken together, it is reasonable to estimate that a federally-sponsored prevention trial would cost around US$80–100M for a 5-year US study. Given this fiscal reality, we must explore ways to run well powered primary prevention or early intervention study that do not cost substantially more or even cost less. However, this will be challenging as biomarkers, imaging, or both will likely be needed to both select and stratify the sample population.
Table 1.
Alzheimer’s Disease Prevention Studies
| Study | InclusionCriteria | Age, yr | Samplesize | Length | Outcomes | Status |
|---|---|---|---|---|---|---|
| ADAPT/ naproxen, celecoxib (Meinert et al., 2009) | First degree relative with AD | ≥ 70 | 2528 | 5–7 | AD, cognitive decline | Early termination |
| GEM/ ginkgo biloba (Snitz et al., 2009) | Asymptomatic 60%, MCI 40% | ≥ 75 | 3072 | 5 | AD, cognitive decline, cardiovascular | No significant effects |
| GUIDAGE/ ginkgo biloba (Vellas et al., 2006a) | Memory complaints | > 70 | 2600 | 4 | AD | No significant effects |
| Physicians Health Study-II/ vitamin E, folate, β- carotene (Christen et al., 2000) | Asymptomatic | > 65 | 10,000 | 9 | Telephone cognitive testing | Ongoing |
| Heart Protection Study/ vitamin E, C, β-carotene, simvastatin (Group, H.P.S.C. 2002a, b) | Asymptomatic with cardiovascular risk factors | 40–80 | 20,536 | 5 | AD, telephone interview for cognitive status (TICS) | No differences |
| PreADVISE/ selenium, vitamin E (Kryscio et al., 2004) | Asymptomatic, males only | ≥ 60 | 10,400 | 9–12 | Dementia onset, cognitive tests | Terminated |
| HERS/estrogen medroxyprogesterone (MPA) (Grady et al., 2002) | Asymptomatic, females | mean = 67 | 1060 | 4.2 | Cognitive tests | Improvement on one test |
| WHI-MS/estrogen + MPA (Craig et al., 2005) | Asymptomatic, female | 65–80 | 4532 | 4–5 | AD and MCI, cognitive scores (add on) | Increased risk for MCI /AD, worse scores with HRT) |
| WHI-MS/estrogen alone (Craig et al., 2005) | Asymptomatic, female | 65–80 | 2497 | 4–5 | AD and MCI, cognitive scores (add on) | Increased risk for MCI /AD, worse scores with HRT) |
In reality, these aforementioned trials were not exclusively primary prevention trials, with varying mixtures of enrolled participants ranging from subject without AD pathology, to others with varying degrees of preclinical AD pathology, and others with MCI. Given that these trials largely lacked ancillary biomarker and imaging studies, one can speculate that the trial participants who developed significant symptoms of AD in the first few years of such trials probably had significant AD pathology at the time of enrollment. Without a biomarker and imaging based stratification, mixed disease status at enrollment will complicate trial design by creating uncertainty regarding group size and length of trial, and also potentially confounding results.
Biomarkers and imaging, as discussed more extensively below, are likely to be essential for future prevention trials, perhaps to either exclude (primary prevention) patients with prodromal AD or select (secondary prevention) participants at risk for progression to symptomatic AD, or for use as a surrogate outcome instead of assessing clinical status, and the costs and complexity will rise. Further, the duration of a primary or secondary prevention trial is far longer than what the commercial sector is generally willing to entertain. Thus, the need for novel prevention trials and cost-sharing models need to be explored that involve public and private sector partnership with shared risks and shared rewards. On a much smaller financial scale, such a cost-sharing model has been successfully implemented with the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the returns have exceeded most investigators’ initial hopes, although ADNI is not a clinical trial (http://www.adni-info.org/).
Other financial considerations deal with patents and exclusivity of marketing a new therapy for AD. As noted above, the financial resources required to conduct primary prevention or early intervention trials in AD are going to be substantial and they are certain to take many years to reach a meaningful result. If patents expire during or shortly after clinical testing, a high likelihood if conducting primary or secondary prevention studies, and exclusivity is limited or non-existent, then private sector developers of AD therapies will be reluctant to conduct primary or secondary prevention trials in AD as the return on investment would be limited and not justify the risks. The sufficient return on investment issue is a sensitive one. In our current drug development environment, we need to revisit the legal policies that would discourage investment in primary prevention studies. Such policies need to transparently balance public health needs with private sector marketplace driven incentives. These issues are of course not restricted to AD, but germane to our broader efforts to move away from a health care system that is designed to treat the sick, to one that tries to maintain our wellness.
Regulatory considerations
Coupled to these financial obstacles are regulatory and methodological obstacles that deal with acceptable trial design. Current Food and Drug Administration (FDA) regulatory guidelines for AD require that a drug show benefit for patients with AD dementia on cognition and that clinical benefit be demonstrated either by global or staging assessment or in activities of daily living (ADL). For example, nearly all 18-month long trials for mild or for mild to moderate AD used the ADAS-cog as the cognitive measure and the clinical dementia rating (CDR) or an ADL scale for the test for clinical significance (Schneider and Sano, 2009). The trials that tested cholinesterase inhibitors for mild cognitive impairment used the onset of AD dementia as the primary outcome supported by required improvement on a cognitive test (Raschetti et al., 2007). If such cognitive and functional measures are required in the design of primary or secondary prevention trials, they will likely require nearly 10 or more years to conduct. To adequately power such a study will be challenging and add significant and possibly insurmountable costs.
From a regulatory point of view, a primary prevention trial would be done to support an indication or “label” such as, “drug X is indicated for the treatment of patients at risk for AD (or have preclinical AD), to delay the onset of prodromal AD, or attenuate the course of cognitive impairment.” Such an indication would break new ground as it would define a particular at-risk state defined by biomarkers and outcomes that fall short of currently recognized clinical states or diagnoses such as AD dementia. This requires, however, that the at-risk state or marker be recognized as defining the target population for the drug and that the outcomes of cognitive change or onset of a subtle clinical state such as mild cognitive impairment be accepted as legitimate outcomes by regulators.
For early phase prevention trials, changes in the nonclinical endpoints can be reasonably accepted if the change is predictive of a clinical outcome. For AD, however, this would require demonstration that, for example, reductions in brain amyloid tracer due to an experimental drug are associated with subsequent attenuation of cognitive decline or improved quality of life. At present, it is uncertain whether current AD biomarkers can be used as surrogates for clinical measures. Indeed, at this point the field is trapped by a conundrum, we would like to use biomarkers as surrogates in clinical trials, but we have yet to definitely establish the link between the surrogate marker with cognitive or functional endpoints.
Next Steps: How do we collectively insure that novel therapies are tested in the appropriate populations?
A largely unspoken, concern among many is that AD therapies in development will demonstrate efficacy against the target or biomarker (e.g., lower Aβ levels, clear Aβ deposits from the brain), meet the “safe enough” requirement, yet fail to show clinical efficacy in clinical trials. Because of economic and regulatory forces, such clinical failures will result in the termination of development of the drug. But, if tested in a primary prevention or early intervention trial, the therapy might show remarkable efficacy. Clearly, no one wants this very plausible hypothetical situation to become reality. In order to prevent this from happening we outline some of the key next steps.
First, we must continue the funding of studies that are needed to prove biomarkers can be used as endpoints and represent truly valid clinical surrogate endpoints. Given the cost and risks associated with developing drugs for prevention of AD, it is likely that the development process will need to be staged and all phases of the approach linked to biomarkers. In the first stage, premorbid biomarkers for the pathology of AD would be used to select patients or enrich a sample for likelihood of progression to AD in a reasonable time-frame. Examples of premorbid biomarkers for primary prevention studies might be those based on ApoE genotype alone or more extended genotypes that might emerge from ongoing GWAS studies. For secondary prevention studies, one might consider diagnostic biomarkers such as CSF Aβ, tau, or both, or imaging studies such as FDG-PET profile, brain amyloid load, or hippocampal or medial temporal lobe volume. In either case, more than one biomarker may be needed to better indentify an asymptomatic risk state or pre-clinical AD that is currently defined explicitly as a biomarker positive risk state. In the second stage, biomarkers will be needed to demonstrate that the therapy is appropriately modifying the target. For example, with an anti-Aβ antibody based therapy, a decrease in brain amyloid tracer retention with an Aβ antibody therapy would indicate target engagement and thereby justify further trials. In the third stage, biomarkers might be used as surrogate endpoints. In a primary prevention trial the endpoint might be time to conversion to a stage 1 biomarker or for a secondary prevention trial time to conversion of a stage 2 biomarker. Although biomarker based trials can add substantial costs on a per subject basis to the trial, these costs might be offset partially or wholly by possible reductions in length of trial, reductions in sample size needed, or both. Development of plasma based biomarkers that predict preclinical stages of AD could considerably reduce the cost of a biomarker based prevention trial, making it much more feasible from an economic point of view. However, despite intensive efforts, even state dependent diagnostic plasma biomarkers that reliably distinguish AD patients from controls have yet to be developed. In any case, the knowledge-based, regulatory and legal issues involved in using both validated and novel surrogate biomarkers for Alzheimer disease trials are substantial and require detailed consideration (Katz, 2004).
A biomarker based approach to AD prevention or early intervention trials will likely increase the costs associated with the trial and is not without inherent risk. Even if the regulatory agencies give initial approval of a therapy based on biomarker outcomes, there would have to be long-term post approval efficacy studies (phase IV) that track whether the biomarker effect led to delay of cognitive impairment and dementia. Only this type of morbidity data will provide the evidence base for continued use of the therapy.
Second, we must find ways to insure that the commercial sector will invest in prevention trials even if they take 10 or more years to complete. With huge investments already made by the commercial sector in novel AD therapeutics, it will not take too many additional negative trials for the pharmaceutical industry to significantly reduce their investment in novel AD therapeutics. To ensure that we have the best possible therapies moving forward, we cannot afford to have the commercial sector largely abandon their efforts to develop novel AD therapeutics. The recent history of stroke therapeutics is highly informative in this regard. As highlighted in a recent review (O'Collins et al., 2006), out of 114 novel treatments tested in humans for stroke, only tissue plasminogen activator demonstrated sufficient efficacy and safety in human studies to be approved by the FDA. Because of this poor record of translation, efforts to develop novel stroke therapies have been severely curtailed in the commercial sector. The net effect of these negative trials is that the chances of developing novel breakthrough stroke therapies in the foreseeable future have been significantly reduced. The authors of that review on stroke therapeutics make several conclusions that are highly relevant to the AD field regarding alignment of preclinical studies and human clinical trials design. They suggest that some of the underlying factors that may have led to the high failure rate of stroke drugs are i) limited preclinical assessment of many stroke therapies prior to human testing, ii) lack of alignment between the preclinical studies and the human trials and iii) overall lack of concordance between efficacy observed in preclinical models and clinical trial outcomes.
As compared to stroke where defining a homogenous intent to treat population is extremely challenging, in AD we may have the tools to identify a well-defined population with respect to AD-related pathology or, lack thereof, and also the capability to design preclinical studies that might more closely match the pathological state of those enrolled in the trial, at least with respect to amyloid burdens for anti-Aβ therapies. Thus, a third key step moving forward is to insure that these kinds of alignments, when feasible, occur for investigative new drug approvals. By insisting that preclinical data and clinical trial design are aligned, the likelihood of translational success in novel AD therapeutics might be increased.
Given the recent advances in the Alzheimer’s field, it is reasonable to believe that the many of medical and scientific advances necessary to conduct prevention studies will be overcome. The non-medical challenges that may prove more difficult to overcome are those regarding the financial underpinnings of prevention or early intervention trials. At present there is no clear road map regarding how such trials might be financially underwritten and who receives the financial rewards if a therapy is shown to have benefit. Moreover, if the scientific and medical advances result in trial designs that are substantially more expensive, rather than less expensive, then the financial obstacles will become greater. Because there is no clear path forward at this time, a fourth step is to make certain the issues of, “Who pays? and “Who gets rewarded?” are openly discussed. Indeed, all the stake holders need to recognize that this may be a critical issue to address, not only for AD prevention trials but prevention trials for many neurodegenerative conditions. Ultimately, addressing this obstacle may require revisiting patent law and laws or guidelines regarding market exclusivity.
A recent report estimated that the current annual worldwide costs of care for those with AD is approximately 1% of the world’s GDP (~US $600 billion/yr) http://www.alz.org/documents/national/World_Alzheimer_Report_2010_Summary(1).pdf. Given the enormous economic burden, there is an overriding imperative to transcend the obstacles to conducting the most appropriate trials that will have the greatest potential impact on the disease for any given novel therapeutic. If we can gain the scientifically-based consensus among the many stakeholders, then we can collectively develop a road map that addresses the obstacles highlighted in this review that block conducting the necessary preventative studies. This road map will be complex in its formulation as it will need to not only involve physicians, researchers, and patients and their caregivers, but also the commercial sector, foundations, drug approval agencies, legislators and governments, and expensive to implement. However, it is a challenge that we must face and overcome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowski D, Wiederhold KH, Furrer U, Jaton AL, Neuenschwander A, Runser MJ, Danner S, Reichwald J, Ammaturo D, Staab D, et al. Dynamics of Abeta turnover and deposition in different beta-amyloid precursor protein transgenic mouse models following gamma-secretase inhibition. J Pharmacol Exp Ther. 2008;327:411–424. doi: 10.1124/jpet.108.140327. [DOI] [PubMed] [Google Scholar]
- ADAPT Research Group. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- Ashe KH. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn Mem. 2001;8:301–308. doi: 10.1101/lm.43701. [DOI] [PubMed] [Google Scholar]
- Ashe KH, Zahs KR. Probing the biology of Alzheimer's disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association. 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006;20:S63–S68. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837, 1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Christen WG, Gaziano JM, Hennekens CH. Design of Physicians' Health Study II--a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DG. The Women's Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol. 2005;4:190–194. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. Alzheimer's disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 1998;51:S2–S17. doi: 10.1212/wnl.51.1_suppl_1.s2. discussion S65-17. [DOI] [PubMed] [Google Scholar]
- Das P, Murphy MP, Younkin LH, Younkin SG, Golde TE. Reduced effectiveness of Abeta1-42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging. 2001;22:721–727. doi: 10.1016/s0197-4580(01)00245-7. [DOI] [PubMed] [Google Scholar]
- De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, Lopez OL, Burke G, Carlson MC, Fried LP, et al. Ginkgo biloba for Prevention of Dementia: A Randomized Controlled Trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Neuropathology of Alzheimer's disease and other dementias. Clin Geriatr Med. 2001;17:209–228. doi: 10.1016/s0749-0690(05)70066-5. [DOI] [PubMed] [Google Scholar]
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer's disease? The Lancet Neurology. 2004;3:246–248. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurology. 2010;9:xxx–xxx. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of Age, Sex, and Ethnicity on the Association Between Apolipoprotein E Genotype and Alzheimer Disease: A Meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- Ghiso J, Rostagno A, Tomidokoro Y, Lashley T, Bojsen-Moller M, Braendgaard H, Plant G, Holton J, Lal R, Revesz T, et al. Genetic alterations of the BRI2 gene: familial British and Danish dementias. Brain Pathol. 2006;16:71–79. doi: 10.1111/j.1750-3639.2006.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Golde TE. The Abeta hypothesis: leading us to rationally-designed therapeutic strategies for the treatment or prevention of Alzheimer disease. Brain Pathol. 2005;15:84–87. doi: 10.1111/j.1750-3639.2005.tb00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Petrucelli L, Lewis J. Targeting Abeta and tau in Alzheimer's disease, an early interim report. Exp Neurol. 2010;223:252–266. doi: 10.1016/j.expneurol.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med. 2002;113:543–548. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- Group, H.P.S.C. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002a;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- Group, H.P.S.C. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002b;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009 doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RW. Dimebon disappointment. Alzheimers Res Ther. 2010;2:25. doi: 10.1186/alzrt49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. Biomarkers and Surrogate Markers: An FDA Perspective. NeuroRx. 2004;1:189–195. doi: 10.1602/neurorx.1.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryscio RJ, Mendiondo MS, Schmitt FA, Markesbery WR. Designing a large prevention trial: statistical issues. Stat Med. 2004;23:285–296. doi: 10.1002/sim.1716. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Martin BK, Meinert CL, Breitner JC. Double placebo design in a prevention trial for Alzheimer's disease. Control Clin Trials. 2002;23:93–99. doi: 10.1016/s0197-2456(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Meinert CL, McCaffrey LD, Breitner JC. Alzheimer's Disease Anti-inflammatory Prevention Trial: design, methods, and baseline results. Alzheimers Dement. 2009;5:93–104. doi: 10.1016/j.jalz.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. Jama. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DL, Sisodia SS. Mutant genes in familial Alzheimer's disease and transgenic models. Annual Review of Neuroscience. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase Inhibitors in Mild Cognitive Impairment: A Systematic Review of Randomised Trials. PLoS Med. 2007;4:e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Langbaum JB, Tariot PN. Alzheimer's prevention initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomark Med. 2010;4:3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romas SN, Tang MX, Berglund L, Mayeux R. APOE genotype, plasma lipids, lipoproteins, and AD in community elderly. Neurology. 1999;53:517–521. doi: 10.1212/wnl.53.3.517. [DOI] [PubMed] [Google Scholar]
- Roses AD. Genetic testing for Alzheimer disease. Practical and ethical issues. Archives of Neurology. 1997;54:1226–1229. doi: 10.1001/archneur.1997.00550220036011. [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10 Suppl:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Salloway S, Mintzer J, Weiner MF, Cummings JL. Disease-modifying therapies in Alzheimer's disease. Alzheimers Dement. 2008;4:65–79. doi: 10.1016/j.jalz.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Insel P, Weiner MW. Cholinesterase inhibitors and memantine use by patients in the Alzheimer Disease Neuroimaging Initiative. Archives of Neurology. 2011 doi: 10.1001/archneurol.2010.343. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Sano M. Current Alzheimer's disease clinical trials: Methods and placebo outcomes. Alzheimer's and Dementia. 2009;5:388–397. doi: 10.1016/j.jalz.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd C, McCann H, Halliday GM. Variations in the neuropathology of familial Alzheimer's disease. Acta Neuropathol. 2009;118:37–52. doi: 10.1007/s00401-009-0521-4. [DOI] [PubMed] [Google Scholar]
- Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, O'Meara ES, Carlson MC, Arnold AM, Ives DG, Rapp SR, Saxton J, Lopez OL, Dunn LO, Sink KM, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302:2663–2670. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer disease. [Review] Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4725–4727. doi: 10.1073/pnas.92.11.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellas B, Andrieu S, Ousset PJ, Ouzid M, Mathiex-Fortunet H. The GuidAge study: methodological issues. A 5-year double-blind randomized trial of the efficacy of EGb 761 for prevention of Alzheimer disease in patients over 70 with a memory complaint. Neurology. 2006a;67:S6–S11. doi: 10.1212/wnl.67.9_suppl_3.s6. [DOI] [PubMed] [Google Scholar]
- Vellas B, Andrieu S, Ousset PJ, Ouzid M, Mathiex-Fortunet H and for the GuidAge Study, G. The GuidAge study: Methodological issues. A 5-year double-blind randomized trial of the efficacy of EGb 761(R) for prevention of Alzheimer disease in patients over 70 with a memory complaint. Neurology. 2006b;67:S6–S11. doi: 10.1212/wnl.67.9_suppl_3.s6. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, Golde TE, Koo EH. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity. J Biol Chem. 2003;278:31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- Weiner MW, Aisen PS, Jack CR, Jr, Jagust WJ, Trojanowski JQ, Shaw L, Saykin AJ, Morris JC, Cairns N, Beckett LA, et al. The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimers Dement. 2010;6:202–211. doi: 10.1016/j.jalz.2010.03.007. e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimo A, Winblad B, Jonsson L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimers Dement. 2010;6:98–103. doi: 10.1016/j.jalz.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Younkin SG. The role of A beta 42 in Alzheimer's disease. Journal of Physiology, Paris. 1998;92:289–292. doi: 10.1016/s0928-4257(98)80035-1. [DOI] [PubMed] [Google Scholar]