SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility (original) (raw)
Abstract
The transmembrane glycoprotein SHPS-1 binds the protein tyrosine phosphatase SHP-2 and serves as its substrate. Although SHPS-1 has been implicated in growth factor- and cell adhesion-induced signaling, its biological role has remained unknown. Fibroblasts homozygous for expression of an SHPS-1 mutant lacking most of the cytoplasmic region of this protein exhibited increased formation of actin stress fibers and focal adhesions. They spread more quickly on fibronectin than did wild-type cells, but they were defective in subsequent polarized extension and migration. The extent of adhesion-induced activation of Rho, but not that of Rac, was also markedly reduced in the mutant cells. Activation of the Ras–extracellular signal-regulated kinase signaling pathway and of c-Jun N-terminal kinases by growth factors was either unaffected or enhanced in the mutant fibroblasts. These results demonstrate that SHPS-1 plays crucial roles in integrin-mediated cytoskeletal reorganization, cell motility and the regulation of Rho, and that it also negatively modulates growth factor-induced activation of mitogen-activated protein kinases.
Keywords: cytoskeletal reorganization/integrin/mitogen-activated protein kinases/SHP-2/SHPS-1
Introduction
SHPS-1 (SHP substrate 1), also known as SIRPα1 (Kharitonenkov et al., 1997), BIT (Sano et al., 1997), MFR (Saginario et al., 1998) and p84 neural adhesion molecule (Comu et al., 1997), is a member of a recently identified family of signal-regulatory proteins (Fujioka et al., 1996; Yamao et al., 1997). It was detected initially as a tyrosine-phosphorylated transmembrane protein that binds SHP-1 and SHP-2, Src homology 2 (SH2) domain-containing protein tyrosine phosphatases (PTPs), and serves as their substrate (Fujioka et al., 1996; Noguchi et al., 1996). The putative extracellular region of SHPS-1 comprises three immunoglobulin-like domains with multiple _N_-linked glycosylation sites, whereas the cytoplasmic region of the protein contains four YXX(L/V/I) motifs, which are putative tyrosine phosphorylation and SH2 domain-binding sites (Fujioka et al., 1996; Kharitonenkov et al., 1997). SHPS-1 is abundant in certain neuronal and hematopoietic cells (Adams et al., 1998; Veillette et al., 1998; Seiffert et al., 1999), although other cell types, such as fibroblasts, also express this protein (Fujioka et al., 1996; Kharitonenkov et al., 1997). These observations suggest a role for SHPS-1 as a signal transducer for both protein tyrosine kinases and PTPs in various cell types.
Tyrosine phosphorylation of SHPS-1 is regulated by various soluble growth factors including insulin, epidermal growth factor (EGF), platelet-derived growth factor and lysophosphatidic acid (LPA) (Fujioka et al., 1996; Kharitonenkov et al., 1997; Ochi et al., 1997; Takeda et al., 1998). Phosphorylation of SHPS-1 in response to these growth factors induces its association with SHP-2, thereby recruiting and activating this PTP at a site near the cell membrane. This process might contribute to growth-promoting signal transduction, given that SHP-2 positively modulates growth factor-induced mitogenesis through the activation of Ras and extracellular signal-regulated kinases (ERKs) (Matozaki and Kasuga, 1996; Feng, 1999). Consistent with this notion, we have shown previously that overexpression of SHPS-1 in cultured cells promotes insulin- or LPA-induced activation of ERKs (Takada et al., 1998; Takeda et al., 1998). However, overexpression of SIRPα1, the human homolog of SHPS-1, inhibits insulin- or EGF-induced activation of ERKs and cell growth (Kharitonenkov et al., 1997). Thus, the role of SHPS-1 in growth factor-induced activation of ERKs and mitogenesis remains unclear.
Adhesion of cultured fibroblasts to various extracellular matrix (ECM) proteins also induces the tyrosine phosphorylation of SHPS-1 and its association with SHP-2 in a manner dependent on Src family kinases and focal adhesion kinase (FAK) (Tsuda et al., 1998; Oh et al., 1999). In addition, SHPS-1 undergoes tyrosine phosphorylation and binds SHP-1 on exposure of bone marrow-derived macrophages to fibronectin (Timms et al., 1999). Thus, tyrosine phosphorylation of SHPS-1 and its binding to SHPs are regulated by integrin-mediated cell adhesion. Studies with dominant-negative or loss-of-function mutants have demonstrated important roles for SHP-2 in the control of cell adhesion, cell migration and cytoskeletal architecture (Yu et al., 1998; Manes et al., 1999; Oh et al., 1999; Saxton and Pawson, 1999; Inagaki et al., 2000). A proportion of SHP-2 molecules localizes to focal adhesions (FAs) in cells adhered to the ECM (Miyamoto et al., 1995). These observations suggest that SHPS-1 might promote translocation of SHP-2 to FAs in response to integrin-mediated cell adhesion, and thereby facilitate regulation of cytoskeletal reorganization and cell migration by this PTP. However, the functional consequences of tyrosine phosphorylation of SHPS-1 and its association with SHP-2 in integrin-mediated signaling have remained unknown.
Recently, IAP (or CD47), an important component of signaling pathways triggered by integrins or cell–cell adhesion, was identified as a ligand for SHPS-1 (Jiang et al., 1999; Seiffert et al., 1999). SHPS-1 was also shown to provide a scaffold for the assembly of adhesion-regulated multiprotein complexes, which consist of SKAP55hom/R (Src-kinase-associated protein of 55 kDa homolog), FYB/SLAP-130 (Fyn-binding protein/SLP-76-associated protein of 130 kDa) and PYK2 (Timms et al., 1999). Thus, SHPS-1 appears to function in a variety of cellular signaling systems. To elucidate directly the biological roles of complex formation between SHPS-1 and its intracellular binding partners, we have generated mice that lack most of the cytoplasmic region of this protein. We now show that fibroblasts derived from these mice exhibit an increased number of actin stress fibers and FAs, as well as marked defects in polarized extension and migration on the ECM. Furthermore, integrin-mediated activation of Rho is markedly reduced in these fibroblasts. In addition, the SHPS-1 mutant cells appear hyper-responsive to growth factor stimulation in terms of activation of the Ras–ERK signaling pathway and the c-Jun N-terminal kinase (JNK) signaling pathway.
Results
Generation of SHPS-1 mutant cell lines
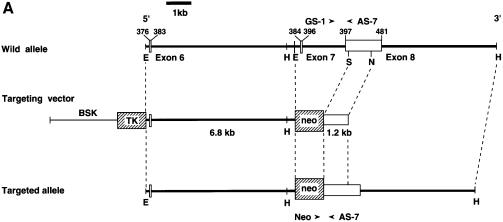
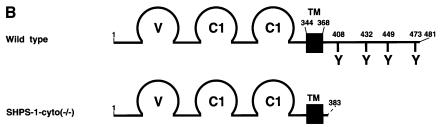
We generated mutant mice in which the SHPS-1 gene had been targeted by homologous recombination. A targeting vector was designed to replace a 2.5 kbp genomic fragment, which includes exons 7 and 8 (Sano et al., 1999), with a neomycin resistance cassette (Figure 1A). Thus, the insertion of a neomycin resistance cassette is expected to make an interruption or a frameshift mutation at the end of exon 6; it could yield a protein with a truncated cytoplasmic region, consisting of at least 15 residues (amino acids 369–383) instead of the normal 113 residues (amino acids 369–481) (Figure 1B). Progeny homozygous for the mutant SHPS-1 allele are born apparently healthy, survive to adulthood and are fertile; however, they exhibit mild thrombocytopenia as well as specific abnormalities in the distribution of B lymphocytes and megakaryocytes in spleen, which normally expresses SHPS-1 (unpublished data). A detailed phenotypic characterization of the mutant mice will be described elsewhere. To study the role of SHPS-1 in cell adhesion- or growth factor-induced signaling, we generated cell lines derived from embryonic fibroblasts of wild-type or homozygous mutant mice by spontaneous immortalization; these cell lines are referred to as wild-type and SHPS-1–cyto(–/–) cells, respectively. Genotyping of the established cell lines by PCR analysis confirmed the homozygous mutation in SHPS-1–cyto(–/–) cells (Figure 1C). We have established three independent SHPS-1–cyto(–/–) cell lines, all of which showed the same phenotype (data not shown).
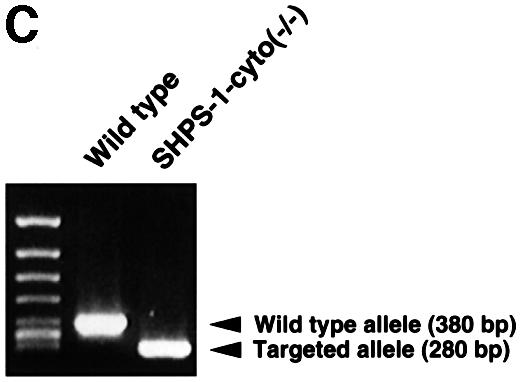
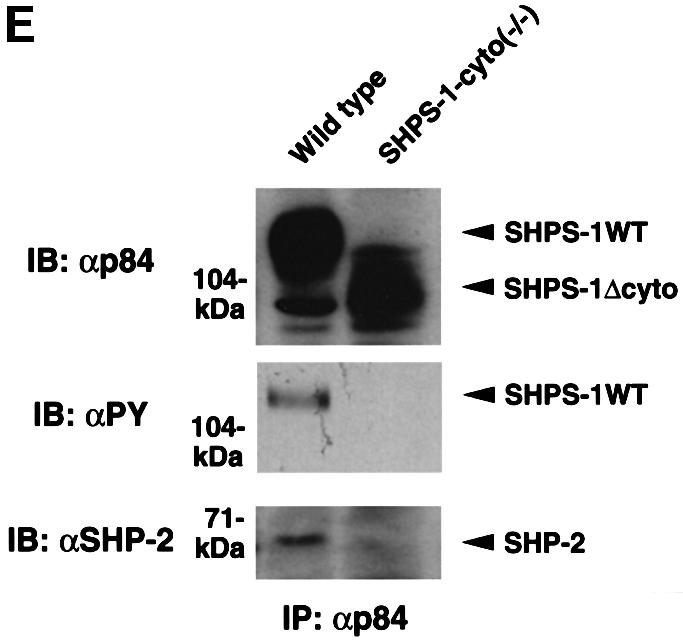
Fig. 1. Generation of cell lines expressing a truncation mutant of SHPS-1. (A) Construction of the targeting vector. A 2.5 kbp _Eco_RI–_Sma_I genomic fragment of the SHPS-1 locus including exons 7 and 8 was replaced with a neomycin resistance cassette (neo). Three exons (exons 6–8) of the wild-type murine SHPS-1 gene encoding the cytoplasmic region are indicated by open boxes. The numbers of mature mouse SHSP-1 amino acids encoded by each exon are indicated above each open box. These numbers of amino acids correspond to those described by Yamao et al. (1997). The PCR primers used to identify the homologous recombination are indicated by arrowheads. BSK, pBluescript; TK, thymidine kinase; E, _Eco_RI; H, _Hin_dIII; S, _Sma_I; N, _Nco_I. (B) Structure of wild-type and the mutant SHPS-1 proteins. The cytoplasmic region of the mutant SHPS-1 is expected to contain at least 15 residues (amino acids 369–383) instead of the normal 113 residues (amino acids 369–481), and thus lacks all of the four YXX(L/V/I) motifs. The transmembrane region (TM) is indicated by closed boxes. V, immunoglobulin V-like domain; C1, immunoglobulin C1-like domain; Y, tyrosine residue. (C) Genomic DNA prepared from wild-type or SHPS-1–cyto(–/–) cells was subjected to PCR analysis with primers designed to amplify the wild-type (GS-1 and AS-7) or mutant (Neo and AS-7) SHPS-1 alleles. The corresponding amplification products are indicated. The leftmost lane contains DNA molecular size standards (_Hin_cII digest of φX174). (D) Whole-cell lysates prepared from wild-type or SHPS-1–cyto(–/–) cells were subjected to immunoblot analysis (IB) with a mAb to p84 (SHPS-1) (αp84) (left panel) or with polyclonal antibodies raised against the cytoplasmic region of SHPS-1 (αSHPS-1) (right panel). (E) Lysates of adherent wild-type or the mutant cells were subjected to immunoprecipitation (IP) with a mAb to p84 and subsequent immunoblot analysis with the same antibody, horseradish peroxidase-conjugated mAb PY20 to phosphotyrosine (αPY) or polyclonal antibodies to SHP-2 (αSHP-2) as indicated. The positions of wild-type (SHPS-1WT) and mutant (SHPS-1Δcyto) SHPS-1 proteins, SHP-2 and molecular size standards, are indicated.
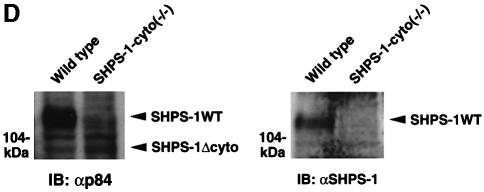
The production of a truncated protein from the mutant allele was confirmed by immunoprecipitation and immunoblot analysis of cell lysates. A monoclonal antibody (mAb) to the extracellular region of p84 (SHPS-1) (Chuang and Lagenaur, 1990) recognized an ∼100 kDa protein instead of the normal 115 kDa protein in SHPS-1–cyto(–/–) cells (Figure 1D). This molecular size is consistent with the calculated size of a truncated SHPS-1, of which 98 amino acid residues in the cytoplasmic region would be deleted. In contrast, polyclonal antibodies generated in response to the cytoplasmic region of SHPS-1 (Fujioka et al., 1996) did not react with the truncated protein (Figure 1D). The abundance of the mutant protein was substantially less than that of the wild-type protein (Figure 1D), possibly reflecting the instability of the mutant SHPS-1 mRNA or its protein product. These results indicate that SHPS-1–cyto(–/–) cells express the mutant SHPS-1 protein that lacks potential binding sites for SH2 domain-containing signaling molecules (Yamao et al., 1997). In fact, the mutant SHPS-1 protein neither underwent tyrosine phosphorylation nor associated with SHP-2 in response to exposure of SHPS-1–cyto(–/–) cells to cell adhesion (Figure 1E) or growth factor stimulation (data not shown). Thus, the mutant SHPS-1 lacks most of the cytoplasmic region, although we could not determine whether this truncated mutant contains exactly 15 residues (amino acids 369–383) in its cytoplasmic region.
Formation of stress fibers and FAs
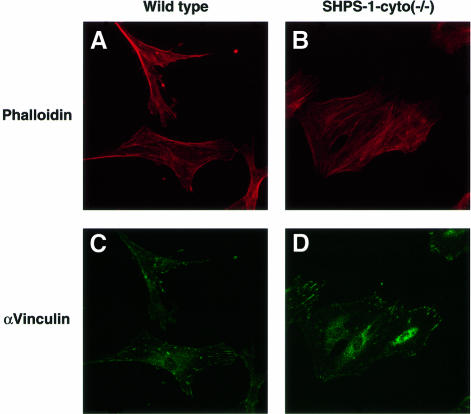
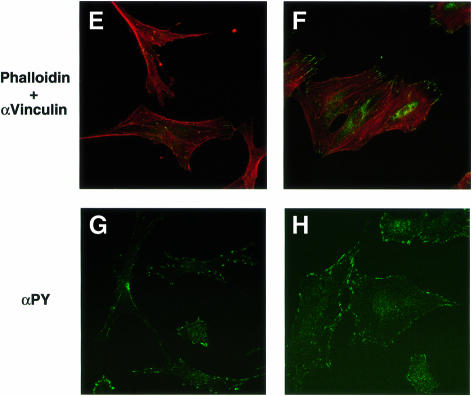
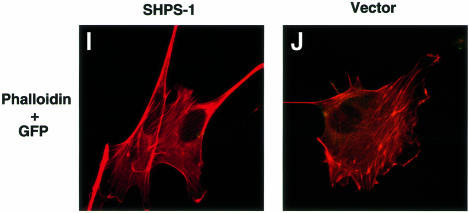
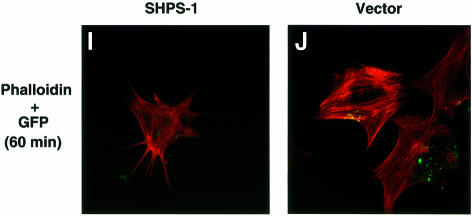
We performed immunofluorescence analysis with rhodamine-labeled phalloidin to examine filamentous actin (F-actin), as well as with mAbs to vinculin and phosphotyrosine in order to detect FAs. Whereas wild-type cells exhibited an elongated, spindle-shaped morphology typical of 3T3 fibroblasts, SHPS-1–cyto(–/–) cells displayed a polygonal, epithelial cell-like morphology (Figure 2A and B). The mutant cells also exhibited markedly greater numbers of both actin stress fibers that cross the cell body (Figure 2A and B) and vinculin-positive FAs (Figure 2C–F) than did wild-type cells. Immunostaining with a mAb to phosphotyrosine confirmed the increased number of FAs in the mutant cells (Figure 2G and H). The morphology of SHPS-1 mutant cells was restored to that of wild-type cells by transfection with SHPS-1 cDNA (Figure 2I and J), thus confirming that the morphological changes were indeed due to the SHPS-1 mutation. These results suggest that targeted deletion of the SHPS-1 cytoplasmic region results in a change from fibroblast-like to epithelial cell-like morphology that is accompanied by increased formation of both actin stress fibers and FAs.
Fig. 2. Cytoskeletal architecture of wild-type and SHPS-1 mutant cells. Wild-type (A, C, E and G), SHPS-1–cyto(–/–) (B, D, F and H) or SHPS-1–cyto(–/–) cells transiently transfected with pTracer CMV containing (SHPS-1) (I) or not containing (vector) (J) SHPS-1 cDNA were seeded on glass coverslips and subsequently fixed and stained with rhodamine-labeled phalloidin (A, B, E, F, I and J). Cells were also stained with a mAb to vinculin (αvinculin) (C–F), and the distribution of phosphotyrosyl proteins was examined with mAb PY20 to phospho tyrosine (αPY) (G and H). Transfected cells were also detected by autofluorescence of green fluorescent protein (GFP) encoded by the vector (I and J). Cells were examined with a confocal microscope (original magnification, 630×).
Cell spreading and extension on fibronectin
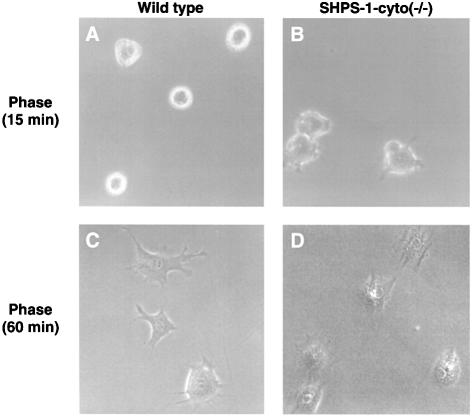
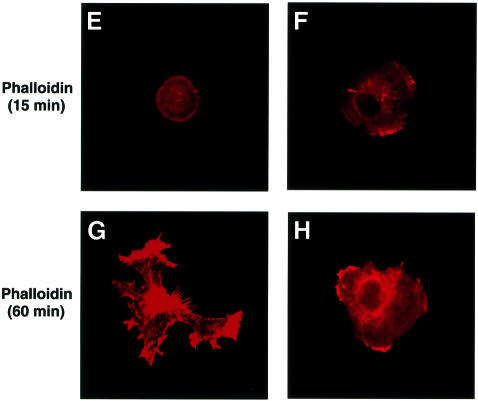
Tyrosine phosphorylation of SHPS-1 is regulated by cell adhesion to the ECM, suggesting that this protein functions in integrin-mediated signaling (Tsuda et al., 1998; Oh et al., 1999; Timms et al., 1999). We therefore investigated whether deletion of the cytoplasmic region of SHPS-1 affects cell spreading on the ECM, a process promoted by integrins. Wild-type and SHPS-1–cyto(–/–) cells were detached from their culture dishes and replated on fibronectin, and cell spreading was then examined by either phase-contrast microscopy or immunofluorescence analysis with rhodamine-labeled phalloidin. After 15 min, almost all wild-type cells still exhibited a phase-bright, rounded morphology with no sign of rearrangement of subcortical F-actin (Figure 3A and E). In contrast, a substantial proportion of the SHPS-1 mutant cells at this time had become phase-dark and exhibited well defined F-actin-containing structures at the cell periphery (Figure 3B and F), characteristics of the early stage of non-polar cell spreading. At 60 min, the numbers of spread cells were similar for the two cell lines (Figure 3C and D); however, whereas wild-type cells showed an elongated, highly polarized shape with numerous membranous ruffles and microspikes at the leading edge (Figure 3C and G), the SHPS-1 mutant cells continued to exhibit a symmetrical, less polarized shape (Figure 3D and H). Again, transfection of the mutant cells with SHPS-1 cDNA rendered their behavior and morphology in this assay similar to those of wild-type cells (Figure 3I and J). These results indicate that the early phase of non-polar cell spreading on fibronectin is accelerated in the absence of the SHPS-1 cytoplasmic region. However, this region of SHPS-1 may be required for the subsequent cytoskeletal rearrangement that results in establishment of the cellular asymmetry necessary for polarized extension on fibronectin.
Fig. 3. Effects of SHPS-1 truncation on cell spreading on fibronectin. Wild-type (A, C, E and G), SHPS-1–cyto(–/–) (B, D, F and H) or SHPS-1–cyto(–/–) cells transiently transfected with pTracer CMV containing (SHPS-1) (I) or not containing (vector) (J) SHPS-1 cDNA were detached from their culture dishes and replated on dishes or coverslips coated with fibronectin. After 15 (A, B, E and F) or 60 min (C, D and G–J), cells were either examined with a light microscope equipped with phase-contrast optics (phase) (original magnification, 400×) (A–D), or stained with rhodamine-labeled phalloidin and examined by confocal microscopy (original magnification, 630×) (E–J). Transfected cells were also detected by autofluorescence of green fluorescent protein (GFP) (I and J).
Cell migration on the ECM
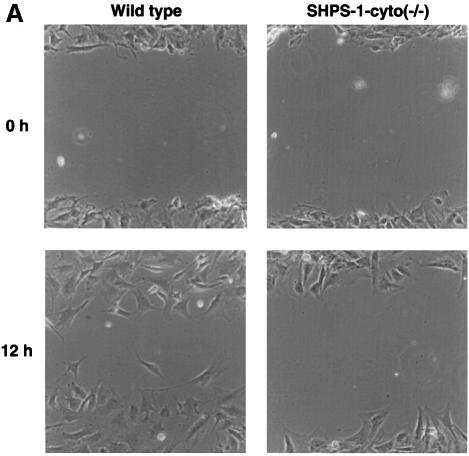
Although cell migration on the ECM is regulated by a complex mechanism, it appears to require traction forces and tension within the cell cortex generated by remodeling of FAs and the actin-based cytoskeleton (Lauffenburger and Horwitz, 1996; Mitchison and Cramer, 1996; Waterman-Storer and Salmon, 1999). Our observations that both these structures are altered by deletion of the SHPS-1 cytoplasmic region prompted us to examine whether this region might be required for cell migration on the ECM. We first examined cell migration with the use of an in vitro ‘wound healing’ assay, in which cells migrate unidirectionally from the rim of a scratch wound toward fibronectin. A substantial number of wild-type cells migrated in this assay; the migratory cells exhibited an elongated morphology, with the longitudinal axis oriented toward the wound, a characteristic of well ordered migration (Figure 4A). In contrast, the number of SHPS-1 mutant cells that migrated in this assay was markedly reduced, and the longitudinal axis of most of the migratory cells appeared to be oriented randomly.
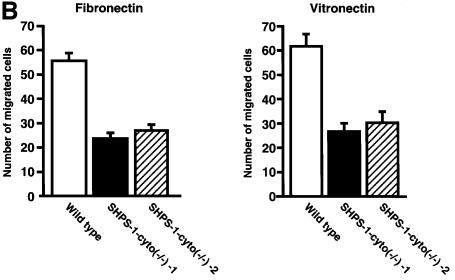
Fig. 4. Effects of SHPS-1 truncation on cell migration on the ECM. (A) Monolayers of wild-type or SHPS-1–cyto(–/–) cells grown on fibronectin-coated dishes were wounded and maintained in the absence of serum. Cell migration into the wound was examined at 0 and 12 h with a light microscope equipped with phase-contrast optics (original magnification, 200×). (B and C) Wild-type and SHPS-1–cyto(–/–) cells (1.5 × 105) were seeded on porous membranes coated with fibronectin or vitronectin in Boyden multiwell chambers without the addition of chemoattractant, and, after 3 h, cells that had migrated were stained with Giemsa solution, counted (B) and photographed (original magnification, 400×) (C). Data in (B) are means ± SD of triplicates from a representative experiment. Cells trapped by the pores of the fibronectin-coated membrane are indicated by arrowheads in (C).
The migration defect of the mutant cells was analyzed quantitatively with the use of a Boyden chamber assay, in which cells move through a porous membrane coated with ECM proteins. The number of cells that migrated through a membrane coated with either fibronectin or vitronectin was markedly greater for wild-type cells than for two independent SHPS-1–cyto(–/–) cell lines (Figure 4B). A substantially greater proportion of the mutant cells became trapped in the pores of the membrane (Figure 4C), suggesting a lack of traction forces and tension within these cells. Thus, the results from both migration assays indicate that the SHPS-1 cytoplasmic region is required for cell migration in response to the engagement of integrins by the ECM.
Adhesion-induced tyrosine phosphorylation of FAK and p130Cas
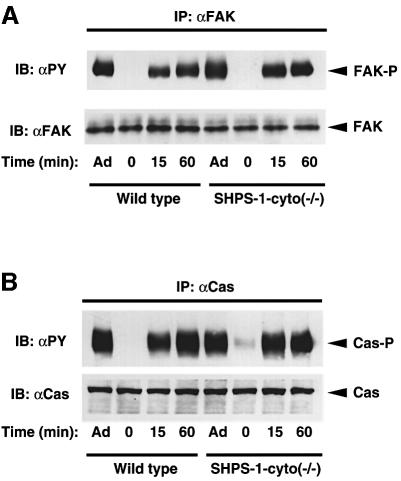
FA-associated proteins such as FAK and p130Cas play important roles in cell spreading and migration (Ilic et al., 1995; Honda et al., 1999). Regulation of tyrosine phosphorylation of these proteins by integrin-mediated cell adhesion is impaired in fibroblasts derived from SHP-2 mutant mice (Yu et al., 1998; Oh et al., 1999). We therefore examined the tyrosine phosphorylation status of FAK and p130Cas in SHPS-1–cyto(–/–) cells under various conditions. Cells were detached from culture dishes and replated on fibronectin-coated dishes, after which tyrosine phosphorylation of FAK and p130Cas was examined at various times by immunoblot analysis with a mAb to phosphotyrosine. Under basal conditions (adherence to the original culture dishes), the extent of tyrosine phosphorylation of FAK (Figure 5A) and p130Cas (Figure 5B) was similar in both wild-type and SHPS-1 mutant cells. Detachment from culture dishes induced a rapid decrease in the extent of tyrosine phosphorylation of both proteins to undetectable levels in both cell lines (Figure 5). Furthermore, subsequent attachment to fibronectin induced similar increases in the tyrosine phosphorylation of FAK and of p130Cas in both cell lines, although the extent of phosphorylation of each protein was slightly greater in the mutant cells at 15 min than in wild-type cells at this time (Figure 5). Thus, adhesion-regulated tyrosine phosphorylation of FAK and p130Cas appeared largely unaffected by the absence of the cytoplasmic region of SHPS-1.
Fig. 5. Adhesion-induced tyrosine phosphorylation of FAK (A) and p130Cas (B) in wild-type and SHPS-1 mutant cells. Wild-type and SHPS-1–cyto(–/–) cells were either maintained adherent (Ad) or detached from culture dishes, replated on dishes coated with fibronectin (10 µg/ml) and incubated for the indicated times at 37°C. Cell lysates were prepared and subjected to immunoprecipitation (IP) with mAbs to FAK (αFAK) (A) or p130Cas (αCas) (B). Both types of immuno precipitate were then subjected to immunoblot analysis (IB) with horseradish peroxidase-conjugated mAb PY20 to phosphotyrosine (αPY). Duplicate immunoprecipitates were probed with polyclonal antibodies to FAK (αFAK) (A) or p130Cas (αCas) (B), as indicated, to verify the presence of equal amounts of FAK or p130Cas in each sample. The positions of FAK, tyrosine-phosphorylated FAK (FAK-P), p130Cas (Cas) and tyrosine-phosphorylated p130Cas (Cas-P) are indicated.
Adhesion- or LPA-induced activation of Rho
Members of the Rho family of small GTPases, such as Rho and Rac, regulate cell spreading and migration on the ECM through rearrangement of the actin-based cytoskeleton (Nobes and Hall, 1995, 1999; Clark et al., 1998) and are activated by integrin-mediated cell adhesion (Price et al., 1998; Ren et al., 1999; del Pozo et al., 2000). To investigate further the molecular basis for the altered morphology and impaired migration of SHPS-1–cyto(–/–) cells, we examined whether adhesion-induced activation of Rho and Rac is affected in the mutant cells. The activity of these GTPases was monitored directly by precipitation of the GTP-bound (activated) forms of the proteins with GST fusion proteins containing either the Rho-binding domain of Rhotekin (Ren et al., 1999) or the CRIB domain of the Rac and Cdc42 effector protein p21PAKα (Glaven et al., 1999). Wild-type cells in suspension culture contained a substantial amount of activated Rho, which was increased further in response to cell adhesion to fibronectin (Figure 6A). In contrast, only a small amount of activated Rho was detected in suspended SHPS-1–cyto(–/–) cells, and this amount hardly increased in response to cell adhesion (Figure 6A). No marked difference in Rac activity was apparent between the two cell lines under adherent conditions, although the activity of this GTPase was slightly greater in suspended mutant cells than in suspended wild-type cells (Figure 6B). Furthermore, LPA, which induces SHPS-1–SHP-2 complex formation (Takeda et al., 1998) and is also a potent activator of Rho (Ridley and Hall, 1992), substantially increased Rho activity in wild-type cells, whereas it hardly did so in the mutant cells (Figure 6C). These results suggest that the SHPS-1 cytoplasmic region is required for the activation of Rho, but not for that of Rac, and that inactivation of Rho may account, at least in part, for the morphological changes and impaired migration apparent in the SHPS-1 mutant cells.
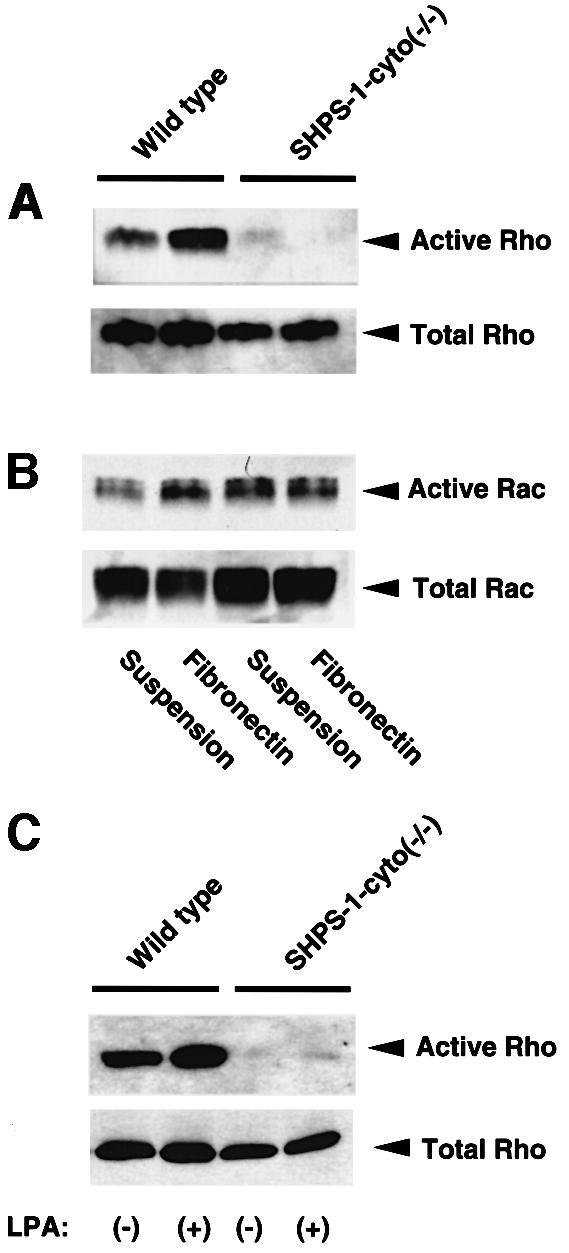
Fig. 6. Effects of SHPS-1 truncation on adhesion- or LPA-induced activation of Rho and Rac. (A and B) Wild-type and SHPS-1–cyto(–/–) cells were detached from culture dishes and then either maintained in suspension or replated on fibronectin-coated dishes. Cells were incubated for 60 (A) or 15 min (B) at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented (A) or not (B) with 1% fetal bovine serum (FBS). The active forms of the GTPases were then precipitated with GST fusion proteins containing either the Rho-binding domain of Rhotekin (A) or the CRIB domain of the Rac and Cdc42 effector protein p21PAKα (B); the resulting precipitates were subjected to immunoblot analysis with mAbs to RhoA (A) or Rac1 (B) (upper panels). Whole-cell lysates were also subjected directly to immunoblot analysis with the mAbs to RhoA or Rac1 to determine the total amount of each GTPase (lower panels). (C) Wild-type and SHPS-1–cyto(–/–) cells were deprived of serum for 12 h and then incubated for 1 min with (+) or without (–) 2 µM LPA (Sigma). The active form of Rho was precipitated as in (A).
Growth factor-induced activation of Ras, ERK and JNK
The complex formation of SHPS-1 and SHP-2 has been suggested to modulate growth factor-induced activation of the Ras–ERK signaling pathway (Kharitonenkov et al., 1997; Takada et al., 1998; Takeda et al., 1998). This pathway, besides Rho, also regulates cell motility (Klemke et al., 1997; Nobes and Hall, 1999). We therefore examined the effects of various growth factors on Ras activity in SHPS-1–cyto(–/–) cells. The active form of Ras was precipitated from cell lysates with a GST fusion protein containing the Ras-binding domain of c-Raf1 (Taylor and Shalloway, 1996). EGF and basic fibroblast growth factor (bFGF) each induced similar increases in the amount of active Ras in wild-type and the mutant cells (Figure 7). In contrast, the extent of Ras activation induced by insulin-like growth factor-1 (IGF-1) was substantially greater in the SHPS-1 mutant cells than in wild-type cells (Figure 7). The extents of agonist-induced tyrosine phosphorylation of IGF-1 receptor and its downstream adaptors such as insulin receptor substrate (IRS)-1 and Shc appeared similar between wild-type and the mutant cell lines, suggesting that the kinase activity of the IGF-1 receptor is not altered in the mutant cells (data not shown).
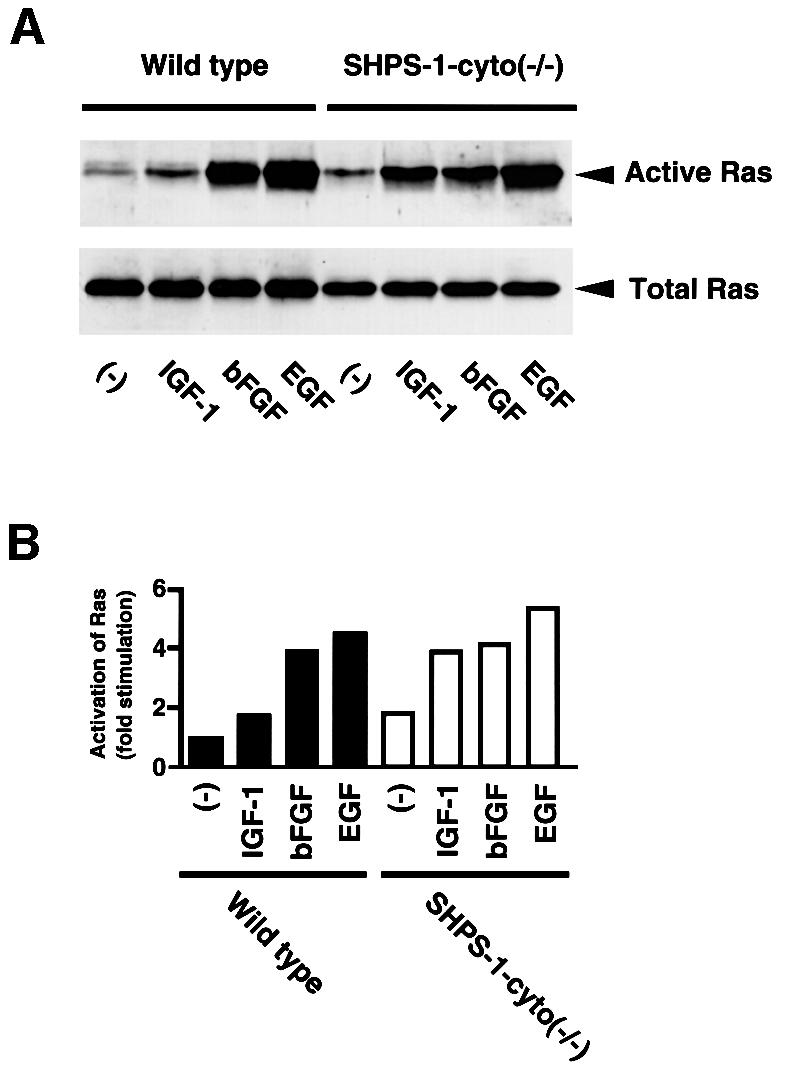
Fig. 7. Effects of SHPS-1 truncation on growth factor-induced activation of Ras. (A) Wild-type and SHPS-1–cyto(–/–) cells were deprived of serum for 12 h and then incubated in the absence (–) or presence of IGF-1 (100 ng/ml), bFGF (20 ng/ml) or EGF (100 ng/ml) for 2 min. The active form of Ras was precipitated with a GST fusion protein containing the Ras-binding domain of c-Raf-1 and subjected to immunoblot analysis with a mAb to H-Ras (upper panel). Whole-cell lysates were also subjected directly to immunoblot analysis with the mAb to H-Ras to determine the total amount of Ras (lower panel). (B) The activity of Ras in (A) was quantified by scanning densitometry with the NIH Image program.
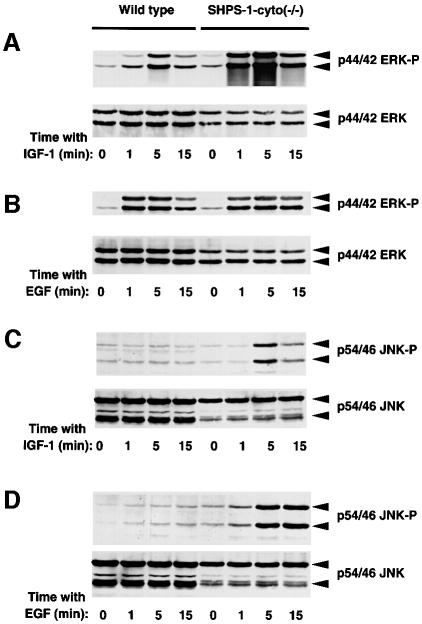
We next examined the effects of IGF-1 and EGF on the activity of ERK, a downstream effector of Ras. Both growth factors induced marked activation of p44 and p42 isoforms of ERK in wild-type cells in a time-dependent manner (Figure 8A and B). Whereas the extent of EGF-induced ERK activation in SHPS-1–cyto(–/–) cells was similar to that in wild-type cells, the effect of IGF-1 on ERK activity was substantially greater in the mutant cells than in wild-type cells at all time points examined (Figure 8A and B). These results are consistent with the notion that the cytoplasmic region of SHPS-1 is not required for growth factor-induced activation of the Ras–ERK signaling pathway. Rather, this region of SHPS-1 appears to downregulate the activation of this signaling pathway by some growth factors. However, we cannot exclude the possibility that this region of SHPS-1 also positively regulates growth factor-induced ERK activation under certain circumstances, as suggested previously (Takada et al., 1998; Takeda et al., 1998).
Fig. 8. Effects of SHPS-1 truncation on growth factor-induced activation of ERKs (A and B) and JNKs (C and D). Wild-type and SHPS-1–cyto(–/–) cells were deprived of serum for 18 h and then incubated in the presence of IGF-1 (100 ng/ml) (A and C) or EGF (100 ng/ml) (B and D) for the indicated times. Cell lysates were subjected to immunoblot analysis with antibodies specific for the activated (phosphorylated) forms either of p44 and p42 ERK (A and B, upper panels) or of p54 and p46 JNK (C and D, upper panels). Duplicate samples were also probed with antibodies to total ERK protein (A and B, lower panels) or to total JNK protein (C and D, lower panels). The positions of ERKs and JNKs, as well as of their phosphorylated forms, are indicated.
Finally, we examined growth factor-induced activation of JNK, another member of the family of mitogen-activated protein kinases whose activity is thought to be regulated by a mechanism distinct from that for ERK (Coso et al., 1995; Minden et al., 1995). Whereas IGF-1 had little effect on the activity of p54 and p46 isoforms of JNK in wild-type cells, it markedly activated JNKs in the mutant cells; this activation was transient, peaking at 5 min after stimulation (Figure 8C). EGF also induced only slight activation of JNKs in wild-type cells, but it markedly activated these enzymes in the mutant cells (Figure 8D). There was no significant difference between the two cell lines in the extents of tyrosine phosphorylation of cellular proteins in response to EGF (data not shown). Thus, JNK activation induced by IGF-1 or EGF was greatly enhanced in the absence of the cytoplasmic region of SHPS-1, suggesting that this region normally inhibits the activation of these enzymes.
Discussion
In the present study, we generated 3T3 fibroblasts that express an SHPS-1 mutant that lacks most of the cytoplasmic region of the wild-type protein. The truncated SHPS-1 appears to be functionally impaired, given that it does not form a complex with SHP-2; it presumably is also not able to function as a molecular scaffold (Timms et al., 1999). Characterization of the mutant cells has provided two important insights into the biological roles of SHPS-1: (i) the cytoplasmic region of SHPS-1 is essential for cytoskeletal reorganization and cell motility; and (ii) this region of SHPS-1 is dispensable for, or, in some instances, inhibits growth factor-induced activation of the mitogen-activated protein kinase cascades.
The SHPS-1 mutant cells exhibited increased numbers of both actin stress fibers and FAs. In addition, these cells were not able to achieve cellular asymmetry when plated on ECM proteins. Such defects might inhibit the generation of traction forces and tension within the cell cortex, resulting in impaired polarized extension and cell migration on the ECM. The cytoplasmic region of SHPS-1 may thus be required for the remodeling of actin stress fibers and FAs in response to integrin engagement by the ECM.
The mechanism by which SHPS-1 regulates cytoskeletal reorganization is not yet fully understood. However, the morphological characteristics of the SHPS-1 mutant cells are similar to those of cells either lacking functional SHP-2 (Yu et al., 1998) or overexpressing a dominant-negative mutant of this PTP (Inagaki et al., 2000). This similarity suggests that the inability of the truncated form of SHPS-1 to recruit and activate SHP-2 might account, at least in part, for the phenotype of the mutant cells. Cell adhesion-dependent regulation of tyrosine phosphorylation of FAK and p130Cas is affected by inactivation of SHP-2 (Yu et al., 1998; Oh et al., 1999); however, such regulation appeared almost intact in the SHPS-1 mutant cells. In addition, whereas the total amount of cellular phosphotyrosyl proteins was reduced in cells lacking SHP-2 (Oh et al., 1999), the SHPS-1 mutant cells contained almost the same amount of phosphotyrosine as did wild-type cells (data not shown). These results suggest that FAK and p130Cas are not major contributors to SHPS-1-mediated cytoskeletal reorganization, and that SHPS-1 may also affect the cytoskeleton through intracellular binding partners other than SHP-2.
Instead, our observations suggest that SHPS-1 participates in cytoskeletal reorganization and cell motility, at least in part, through regulation of Rho. Consistent with this notion, it has been shown recently that Rho acts downstream of SHP-2 in a signaling pathway that leads to elongation of Xenopus animal caps (O’Reilly et al., 2000). Thus, failure of SHP-2-mediated positive regulation might account for the defect in Rho activation in the SHPS-1 mutant cells. The low level of Rho activity in these cells is consistent with the observed defects in polarized extension and migration on ECM proteins, cellular functions that depend on Rho (Clark et al., 1998; Nobes and Hall, 1999). Thus, it is possible that failure of SHPS-1–SHP-2 complex formation in the mutant cells results in aberrant regulation of Rho activity and reduced cell motility in response to integrin-mediated cell adhesion. The cytoplasmic region of SHPS-1 might bind a certain type of the GDP/GTP exchange protein that activates Rho. Vav-2, a member of the Vav family proteins, is a likely candidate, given that this protein is ubiquitously expressed and acts as a GDP/GTP exchange protein for Rho (Schuebel et al., 1998). In support of this idea, SHPS-1 recently has been shown to form a complex with FYB/SLAP-130, and thus, possibly, SLP-76, a binding partner for Vav-1, which catalyzes the GDP/GTP exchange of another Rho family member, Rac (Timms et al., 1999). Since Vav-2 is structurally similar to Vav-1, it might associate with the cytoplasmic region of SHPS-1 in a manner analogous to Vav-1.
The low level of Rho activity in the SHPS-1 mutant cells may appear inconsistent with the increased number of actin stress fibers and FAs observed in these cells, given that the formation of such structures depends on Rho (Ridley and Hall, 1992). However, the residual Rho activity in the mutant cells might be sufficient to trigger the formation of these structures. In addition, the uncovered mechanism, which does not involve Rho, may stabilize actin stress fibers and FAs in the mutant cells. An adhesion-dependent negative feedback regulation of Rho activity in response to FA formation has been described (Ren et al., 1999). The increased formation of FAs in the SHPS-1 mutant cells might therefore result in the activation of this negative feedback loop, and thereby downregulate Rho activity. However, suspension of the mutant cells did not restore Rho activity, suggesting that this may not be the case. In any case, further efforts clearly are required to elucidate the molecular mechanism for the regulation of Rho by SHPS-1.
The Ras–ERK signaling pathway initiated by growth factors or integrin-mediated cell adhesion has also been implicated in cytoskeletal reorganization and cell motility (Klemke et al., 1997; Nobes and Hall, 1999). However, this pathway was not impaired in cells expressing the truncated SHPS-1 protein. Growth factors promote the association of SHP-2 with members of another class of docking proteins with overall structural similarity to Dos, the product of the Drosophila daughter of sevenless gene (Herbst et al., 1996). These proteins include IRS proteins (White et al., 1998), Gab-1 (Holgado-Madruga et al., 1996), Gab-2 (Gu et al., 1998) and FGF receptor substrate-2 (or SNT) (Kouhara et al., 1997). Thus, the results of previous studies based on the overexpression of SHPS-1 are difficult to interpret with regard to the physiological roles of this protein (Kharitonenkov et al., 1997; Takada et al., 1998; Takeda et al., 1998), given that such an experimental approach might result in the sequestration of SHP-2 and consequently impair its function. If the requirement for SHP-2 in activation of the Ras–ERK signaling pathway depends on SHPS-1, then this pathway would be expected to be affected by the absence of the SHPS-1 cytoplasmic region. Our data therefore indicate that the role of SHP-2 in this pathway might be mediated by a docking protein (or proteins) other than SHPS-1. Consistent with this notion, EGF-induced ERK activation recently was proposed to be mediated by Gab-1, SHP-2 and an unidentified SHP-2 target (Shi et al., 2000).
We have also shown that, in certain instances, growth factor-induced activation of the Ras–ERK pathway as well as that of JNKs is enhanced in the SHPS-1 mutant cells. This appears to be consistent with the previous observation that overexpression of SHPS-1/SIRPα1 inhibits growth factor-induced ERK activation (Kharitonenkov et al., 1997); in contrast, it may be inconsistent with the notion that the SHPS-1–SHP-2 complex positively regulates such a signaling event (Takada et al., 1998; Takeda et al., 1998). The mechanism of this effect of SHPS-1 is unknown; however, it is now clear that the cytoplasmic region of SHPS-1 is crucial for proper regulation of the Ras–ERK pathway or the JNK pathway by some growth factors. It is possible that the SHPS-1–SHP-2 complex may directly regulate the upstream element required for these pathways. In contrast, two studies suggest that the increased formation of FAs and actin stress fibers in these cells might be responsible. Thus, IGF-1-induced activation of ERKs is also enhanced in FAK-deficient fibroblasts (Arbet-Engels et al., 1999), which also exhibit an increased number of FAs. In addition, the protein kinase MEKK1, which is activated by EGF and acts as an upstream activator of JNKs (Fanger et al., 1997), localizes to stress fibers and FAs (Christerson et al., 1999). These observations indicate that growth factor-induced activation of ERKs and JNKs might be promoted through an indirect mechanism by adhesion-regulated formation of actin stress fibers or FAs. Thus, SHPS-1 probably functions, in concert with SHP-2, to limit the number of these cellular structures, thereby preventing aberrant activation of mitogenic signals by growth factors.
In conclusion, our results show that SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility and that these effects of SHPS-1 may be mediated, at least in part, through regulation of Rho. Furthermore, SHPS-1 may down-modulate growth factor-induced activation of the Ras–ERK and JNK pathways.
Materials and methods
Generation of SHPS-1 mutant mice and cell lines
The murine SHPS-1 genomic clone was screened from the C57/Black 6 genomic library (Stratagene). A targeting vector was designed to replace a 2.5 kbp _Eco_RI–_Sma_I fragment including the last two exons, exons 7 and 8, of the SHPS-1 gene with a neomycin resistance cassette from pKJ 2 (Gibco-BRL). A herpes simplex virus-thymidine kinase cassette was inserted into the 5′ end of the vector. The targeting vector was electroporated into E14.1 embryonic stem cells; three clones were selected for microinjection into blastocytes. One of these clones gave germline transmission, and was interbred to generate mice homozygous for the mutant SHPS-1 allele.
Primary embryonic fibroblasts were prepared from 13.5 day post-coitum embryos of wild-type mice or mice homozygous for the SHPS-1 mutation according to the established 3T3 protocol. After removal of the head and organs, embryos were rinsed with phosphate-buffered saline (PBS), minced and digested for 10 min at 37°C with a solution containing 0.05% trypsin (Sigma) and 0.53 mM EDTA. Trypsin was then inactivated by the addition of FBS (Gibco-BRL). Cells from one embryo were plated on four 100 mm culture dishes in DMEM supplemented with 10% FBS, 0.1 mM minimal essential medium (MEM) non-essential amino acids, 55 µM 2-mercaptoethanol and gentamycin (10 µg/ml) (Sigma), and were incubated at 37°C in a humidified incubator containing 5% CO2. The cells were detached from each dish with trypsin and reseeded at a density of 7.5 × 105 cells per 100 mm dish or 2.7 × 105 cells per 60 mm dish every 3 days until crisis was overcome.
Genotyping of the established cell lines was performed by PCR analysis with genomic DNA as a template as well as Neo or GS-1 sense and AS-7 antisense primers. Neo (5′-TCGTGCTTTACGGTATCGCCGCTCCCGAAT) and AS-7 (5′-TAGTACAGACCACAGCCCCATTCACTTCCT) primers amplify a 280 bp fragment corresponding to the mutant allele; GS-1 (5′-ATACCATTGGCTGAGCCCACTGGGAAAGAA) and AS-7 primers amplify a 380 bp fragment corresponding to the wild-type allele.
The expression of SHPS-1 protein was evaluated by immunoprecipitation and immunoblot analysis with antibodies specific to SHPS-1 as described below. All experiments described in the present study were performed with at least three independent established cell lines.
Expression vector, transfection and antibodies
Full-length wild-type mouse SHPS-1 cDNA was inserted into the _Eco_RI and _Not_I sites of pTracer CMV (Invitrogen). For reintroduction of SHPS-1, cells (∼2 × 105 per 60 mm dish) were transiently transfected with 20 µg of SHPS-1 cDNA with the use of a Superfect Transfection Kit (Qiagen). Transfected cells were detected by fluorescence of the vector-encoded green fluorescent protein marker.
A rat mAb to p84 (SHPS-1) was kindly provided by C.Lagenaur. Rabbit polyclonal antibodies to SHPS-1 or to SHP-2 were described previously (Fujioka et al., 1996). A mouse mAb to vinculin was obtained from Sigma; mouse mAbs to p130Cas, H-Ras and Rac1 were from Transduction Laboratories; a mouse mAb to FAK was from Upstate Biotechnology; horseradish peroxidase-conjugated mouse mAb PY20 to phosphotyrosine, a mouse mAb to RhoA, and rabbit polyclonal antibodies to FAK and p130Cas were from Santa Cruz Biotechnology; rabbit polyclonal antibodies that react with tyrosine-phosphorylated forms of ERK or JNK, or with total ERK or JNK proteins, were from New England BioLabs; fluorescein isothiocyanate (FITC)-conjugated mouse mAb PY20 to phosphotyrosine was from Calbiochem; and FITC-conjugated sheep polyclonal antibodies to mouse immunoglobulin were from Amersham Pharmacia Biotech.
Immunofluorescence
Cells seeded on glass coverslips were washed with PBS, fixed with 3.7% formaldehyde in PBS for 20 min, permeabilized with 0.5% Triton X-100 in PBS for 5 min and incubated for 2 h in TBS-T [20 mM Tris–HCl (pH 7.6), 150 mM NaCl, 0.05% Tween-20] containing 5% non-fat dry milk, 10% FBS and 1% bovine serum albumin. The cells were then incubated for 1 h at room temperature with a mAb to vinculin (20 µg/ml) or FITC-conjugated mAb PY20 to phosphotyrosine (0.5 µg/ml), together with rhodamine-labeled phalloidin (0.1 µg/ml) (Sigma). For vinculin staining, cells were then washed twice with PBS and incubated with FITC-conjugated secondary antibodies for 30 min. After three washes with PBS, the cells were examined with a laser-scanning confocal microscope (Bio-Rad model MRC-1024) and built-up images were constructed.
Cell spreading assays
Cells were detached from culture dishes by treatment with 0.025% trypsin, collected by centrifugation, washed once with PBS and transferred at a density of 1 × 105 cells/ml to 60 mm culture dishes or glass coverslips coated with fibronectin (10 µg/ml) (Sigma). After incubation in serum-free DMEM for 15–60 min at 37°C in a humidified incubator containing 5% CO2, the cells were examined with a light microscope equipped with phase-contrast optics (model IX 70; Olympus) and random fields were photographed. The cells were also stained with rhodamine-labeled phalloidin and examined by confocal microscopy as described above.
Cell migration assays
For the wound-healing assay, cells were grown in six-well culture plates coated with fibronectin (10 µg/ml) until a confluent monolayer was formed. After wounding of the monolayer by scoring with a 200 µl sterile plastic micropipet tip, the cells were washed with PBS, incubated in serum-free DMEM for 12 h at 37°C in a humidified incubator containing 5% CO2, and examined with a light microscope equipped with phase-contrast optics. Random fields were photographed.
Boyden chamber assays of cell migration were performed as described previously (Inagaki et al., 2000). In brief, 8 µm polyvinylpyrrolidine-free polycarbonate filters (Neuroprobe) coated with fibronectin (10 µg/ml) or vitronectin (10 µg/ml) (Sigma) were placed over the lower wells of a Boyden multiwell chemotactic chamber that had been filled with serum-free DMEM. The gasket and upper part of the chamber were then assembled, and 1.5 × 105 cells in 0.2 ml of serum-free DMEM were added to each of the upper wells. The chamber was placed in a humidified incubator containing 5% CO2 and incubated for 3 h at 37°C. Cells that had migrated were fixed in methanol, washed with PBS, and exposed to Giemsa stain for 15 s (Nakarai Tesque). The number of cells that had migrated in at least five fields was counted under a microscope fitted with a grid eyepiece at a total magnification of 200×.
Immunoprecipitation and immunoblot analysis
Cells were washed with ice-cold PBS and then lysed on ice in 1 ml of lysis buffer (20 mM Tris–HCl pH 7.6, 140 mM NaCl, 1 mM EDTA, 1% NP-40) containing 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), aprotinin (10 µg/ml) and 1 mM sodium vanadate. The lysates were centrifuged at 10 000 g for 15 min at 4°C, and the resulting supernatants were subjected to immunoprecipitation and immunoblot analysis. For immunoprecipitation, the supernatants were incubated for 3 h at 4°C with antibody-coupled protein G–Sepharose beads (20 µl of beads) (Amersham Pharmacia Biotech). The beads were then washed three times with 1 ml of WG buffer (50 mM HEPES–NaOH pH 7.6, 150 mM NaCl, 0.1% Triton X-100), suspended in Laemmli sample buffer, boiled for 5 min and subjected to SDS–PAGE and immunoblot analysis with various antibodies and the ECL detection system (Amersham Pharmacia Biotech).
Assay of GTPase activation
GST fusion proteins containing either the Rho-binding domain (residues 7–89) of mouse Rhotekin, the CRIB domain (residues 70–106) of rat p21PAKα or the Ras-binding domain (residues 1–149) of human c-Raf-1 were expressed in bacteria and bound to glutathione–Sepharose beads (20 µg of protein per 15 µl of packed beads) (Amersham Pharmacia Biotech). For Rho and Rac activity assays, cells in one 100 mm dish were lysed in 600 µl of a solution containing 25 mM HEPES–NaOH pH 7.4, 100 mM NaCl, 0.5% NP-40, 10 mM MgCl2, 10 mM β-glycerophosphate, 10% glycerol, 1 mM PMSF, leupeptin (10 µg/ml) and aprotinin (10 µg/ml). For assay of Ras activity, cells were lysed in the same volume of a solution containing 25 mM HEPES–NaOH pH 7.5, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM EDTA, 1 mM PMSF, aprotinin (10 µg/ml) and 1 mM sodium vanadate. Cell lysates were incubated at 4°C with the GST fusion protein-coupled beads for 30 (Ras assay), 45 (Rho assay) or 60 min (Rac assay). Proteins that bound to the beads were resolved on a 12.5% polyacrylamide gel and subjected to immunoblot analysis with antibodies specific for the respective GTPase. The total abundance of each GTPase was determined by immunoblot analysis of cell lysates. Activated Ras was quantified by scanning densitometry with the NIH Image program.
Assay of ERK and JNK activation
Cells in one 60 mm dish were treated for 1–15 min with IGF-1 (100 ng/ml) (Boehringer Mannheim) or EGF (100 ng/ml) (Calbiochem), and then lysed in 400 µl of a solution containing 50 mM HEPES–NaOH pH 7.8, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 0.1% Triton X-100, 20 mM β-glycerophosphate, 100 mM NaF, 10 mM sodium pyrophosphate, 1 mM PMSF, aprotinin (10 µg/ml) and 1 mM sodium vanadate. Lysates (20 µg of protein) were resolved on 10% polyacrylamide gels, and the extent of ERK or JNK activation was assessed by immunoblot analysis with antibodies specific for activated forms of each protein. The overall abundance of ERKs and JNKs was also determined by immunoblot analysis with antibodies to total ERK or JNK protein.
Acknowledgments
Acknowledgements
We thank C.Lagenaur for providing the rat mAb to p84 (SHPS-1), E.Manser for the cDNA encoding the CRIB domain of p21PAKα, N.Homma for technical assistance, and Y.Fujioka for helpful discussion. This work was supported by a grant-in-aid for cancer research and a grant-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan, and by a grant-in-aid from the Research for the Future Program of the Japan Society for the Promotion of Science.
References
- Adams S., van der Laan,L.J., Vernon-Wilson,E., de Lavalette,C.R., Dopp,E.A., Dijkstra,C.D., Simmons,D.L. and van den Berg,T.K. (1998) Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol., 161, 1853–1859. [PubMed] [Google Scholar]
- Arbet-Engels C., Janknecht,R. and Eckhart,W. (1999) Role of focal adhesion kinase in MAP kinase activation by insulin-like growth factor-1 or insulin. FEBS Lett., 454, 252–256. [DOI] [PubMed] [Google Scholar]
- Christerson L.B., Vanderbilt,C.A. and Cobb,M.H. (1999) MEKK1 interacts with α-actinin and localizes to stress fibers and focal adhesions. Cell Motil. Cytoskeleton, 43, 186–198. [DOI] [PubMed] [Google Scholar]
- Chuang W. and Lagenaur,C.F. (1990) Central nervous system antigen P84 can serve as a substrate for neurite outgrowth. Dev. Biol., 137, 219–232. [DOI] [PubMed] [Google Scholar]
- Clark E.A., King,W.G., Brugge,J.S., Symons,M. and Hynes,R.O. (1998) Integrin-mediated signals regulated by members of the Rho family of GTPases. J. Cell Biol., 142, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comu S., Weng,W., Olinsky,S., Ishwad,P., Mi,Z., Hempel,J., Watkins,S., Lagenaur,C.F. and Narayanan,V. (1997) The murine P84 neural adhesion molecule is SHPS-1, a member of the phosphatase-binding protein family. J. Neurosci., 17, 8702–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso O.A., Chiariello,M., Yu,J.C., Teramoto,H., Crespo,P., Xu,N., Miki,T. and Gutkind,J.S. (1995) The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell, 81, 1137–1146. [DOI] [PubMed] [Google Scholar]
- del Pozo M.A., Price,L.S., Alderson,N.B., Ren,X.D. and Schwartz,M.A. (2000) Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J., 19, 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger G.R., Johnson,N.L. and Johnson,G.L. (1997) MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J., 16, 4961–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G.S. (1999) Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res., 253, 47–54. [DOI] [PubMed] [Google Scholar]
- Fujioka Y., Matozaki,T., Noguchi,T., Iwamatsu,A., Yamao,T., Takahashi,N., Tsuda,M., Takada,T. and Kasuga,M. (1996) A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol., 16, 6887–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaven J.A., Whitehead,I., Bagrodia,S., Kay,R. and Cerione,R.A. (1999) The Dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J. Biol. Chem., 274, 2279–2285. [DOI] [PubMed] [Google Scholar]
- Gu H., Pratt,J.C., Burakoff,S.J. and Neel,B.G. (1998) Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell, 2, 729–740. [DOI] [PubMed] [Google Scholar]
- Herbst R., Carroll,P.M., Allard,J.D., Schilling,J., Raabe,T. and Simon,M.A. (1996) Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell, 85, 899–909. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M., Emlet,D.R., Moscatello,D.K., Godwin,A.K. and Wong,A.J. (1996) A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature, 379, 560–564. [DOI] [PubMed] [Google Scholar]
- Honda H., Nakamoto,T., Sakai,R. and Hirai,H. (1999) p130(Cas), an assembling molecule of actin filaments, promotes cell movement, cell migration, and cell spreading in fibroblasts. Biochem. Biophys. Res. Commun., 262, 25–30. [DOI] [PubMed] [Google Scholar]
- Ilic D. et al. (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature, 377, 539–544. [DOI] [PubMed] [Google Scholar]
- Inagaki K., Noguchi,T., Matozaki,T., Horikawa,T., Fukunaga,K., Tsuda,M., Ichihashi,M. and Kasuga,M. (2000) Roles for the protein tyrosine phosphatase SHP-2 in cytoskeletal organization, cell adhesion and cell migration revealed by overexpression of a dominant negative mutant. Oncogene, 19, 75–84. [DOI] [PubMed] [Google Scholar]
- Jiang P., Lagenaur,C.F. and Narayanan,V. (1999) Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem., 274, 559–562. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A., Chen,Z., Sures,I., Wang,H., Schilling,J. and Ullrich,A. (1997) A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature, 386, 181–186. [DOI] [PubMed] [Google Scholar]
- Klemke R.L., Cai,S., Giannini,A.L., Gallagher,P.J., de Lanerolle,P. and Cheresh,D.A. (1997) Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol., 137, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhara H., Hadari,Y.R., Spivak-Kroizman,T., Schilling,J., Bar-Sagi,D., Lax,I. and Schlessinger,J. (1997) A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell, 89, 693–702. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D.A. and Horwitz,A.F. (1996) Cell migration: a physically integrated molecular process. Cell, 84, 359–369. [DOI] [PubMed] [Google Scholar]
- Manes S., Mira,E., Gomez-Mouton,C., Zhao,Z.J., Lacalle,R.A. and Martinez,A.C. (1999) Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell. Biol., 19, 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T. and Kasuga,M. (1996) Roles of protein-tyrosine phosphatases in growth factor signalling. Cell Signal., 8, 13–19. [DOI] [PubMed] [Google Scholar]
- Minden A., Lin,A., Claret,F.X., Abo,A. and Karin,M. (1995) Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell, 81, 1147–1157. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Teramoto,H., Coso,O.A., Gutkind,J.S., Burbelo,P.D., Akiyama,S.K. and Yamada,K.M. (1995) Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol., 131, 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T.J. and Cramer,L.P. (1996) Actin-based cell motility and cell locomotion. Cell, 84, 371–379. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1995) Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1999) Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol., 144, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Matozaki,T., Fujioka,Y., Yamao,T., Tsuda,M., Takada,T. and Kasuga,M. (1996) Characterization of a 115-kDa protein that binds to SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in Chinese hamster ovary cells. J. Biol. Chem., 271, 27652–27658. [DOI] [PubMed] [Google Scholar]
- Ochi F. et al. (1997) Epidermal growth factor stimulates the tyrosine phosphorylation of SHPS-1 and association of SHPS-1 with SHP-2, a SH2 domain-containing protein tyrosine phosphatase. Biochem. Biophys. Res. Commun., 239, 483–487. [DOI] [PubMed] [Google Scholar]
- Oh E.S. et al. (1999) Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol. Cell. Biol., 19, 3205–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly A.M., Pluskey,S., Shoelson,S.E. and Neel,B.G. (2000) Activated mutants of SHP-2 preferentially induce elongation of Xenopus animal caps. Mol. Cell. Biol., 20, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price L.S., Leng,J., Schwartz,M.A. and Bokoch,G.M. (1998) Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell, 9, 1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X.D., Kiosses,W.B. and Schwartz,M.A. (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J., 18, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J. and Hall,A. (1992) The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell, 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Saginario C., Sterling,H., Beckers,C., Kobayashi,R., Solimena,M., Ullu,E. and Vignery,A. (1998) MFR, a putative receptor mediating the fusion of macrophages. Mol. Cell. Biol., 18, 6213–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S., Ohnishi,H., Omori,A., Hasegawa,J. and Kubota,M. (1997) BIT, an immune antigen receptor-like molecule in the brain. FEBS Lett., 411, 327–334. [DOI] [PubMed] [Google Scholar]
- Sano S., Ohnishi,H. and Kubota,M. (1999) Gene structure of mouse BIT/SHPS-1. Biochem. J., 344, 667–675. [PMC free article] [PubMed] [Google Scholar]
- Saxton T.M. and Pawson,T. (1999) Morphogenetic movements at gastrulation require the SH2 tyrosine phosphatase Shp2. Proc. Natl Acad. Sci. USA, 96, 3790–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuebel K.E., Movilla,N., Rosa,J.L. and Bustelo,X.R. (1998) Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J., 17, 6608–6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert M., Cant,C., Chen,Z., Rappold,I., Brugger,W., Kanz,L., Brown,E.J., Ullrich,A. and Buhring,H.J. (1999) Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood, 94, 3633–3643. [PubMed] [Google Scholar]
- Shi Z.Q., Yu,D.H., Park,M., Marshall,M. and Feng,G.S. (2000) Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell. Biol., 20, 1526–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada T. et al. (1998) Roles of the complex formation of SHPS-1 with SHP-2 in insulin-stimulated mitogen-activated protein kinase activation. J. Biol. Chem., 273, 9234–9242. [DOI] [PubMed] [Google Scholar]
- Takeda H. et al. (1998) Lysophosphatidic acid-induced association of SHP-2 with SHPS-1: roles of RHO, FAK, and a SRC family kinase. Oncogene, 16, 3019–3027. [DOI] [PubMed] [Google Scholar]
- Taylor S.J. and Shalloway,D. (1996) Cell cycle-dependent activation of Ras. Curr. Biol., 6, 1621–1627. [DOI] [PubMed] [Google Scholar]
- Timms J.F., Swanson,K.D., Marie-Cardine,A., Raab,M., Rudd,C.E., Schraven,B. and Neel,B.G. (1999) SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr. Biol., 9, 927–930. [DOI] [PubMed] [Google Scholar]
- Tsuda M. et al. (1998) Integrin-mediated tyrosine phosphorylation of SHPS-1 and its association with SHP-2. Roles of Fak and Src family kinases. J. Biol. Chem., 273, 13223–13229. [DOI] [PubMed] [Google Scholar]
- Veillette A., Thibaudeau,E. and Latour,S. (1998) High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem., 273, 22719–22728. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C.M. and Salmon,E.D. (1999) Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr. Opin. Cell Biol., 11, 61–67. [DOI] [PubMed] [Google Scholar]
- White M.F. (1998) The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell. Biochem., 182, 3–11. [PubMed] [Google Scholar]
- Yamao T., Matozaki,T., Amano,K., Matsuda,Y., Takahashi,N., Ochi,F., Fujioka,Y. and Kasuga,M. (1997) Mouse and human SHPS-1: molecular cloning of cDNAs and chromosomal localization of genes. Biochem. Biophys. Res. Commun., 231, 61–67. [DOI] [PubMed] [Google Scholar]
- Yu D.H., Qu,C.K., Henegariu,O., Lu,X. and Feng,G.S. (1998) Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem., 273, 21125–21131. [DOI] [PubMed] [Google Scholar]