Testosterone Replacement in Hypogonadal Men With Type 2 Diabetes and/or Metabolic Syndrome (the TIMES2 Study) (original) (raw)
Abstract
OBJECTIVE
This study evaluated the effects of testosterone replacement therapy (TRT) on insulin resistance, cardiovascular risk factors, and symptoms in hypogonadal men with type 2 diabetes and/or metabolic syndrome (MetS).
RESEARCH DESIGN AND METHODS
The efficacy, safety, and tolerability of a novel transdermal 2% testosterone gel was evaluated over 12 months in 220 hypogonadal men with type 2 diabetes and/or MetS in a multicenter, prospective, randomized, double-blind, placebo-controlled study. The primary outcome was mean change from baseline in homeostasis model assessment of insulin resistance (HOMA-IR). Secondary outcomes were measures of body composition, glycemic control, lipids, and sexual function. Efficacy results focused primarily on months 0−6 (phase 1; no changes in medication allowed). Medication changes were allowed in phase 2 (months 6−12).
RESULTS
TRT reduced HOMA-IR in the overall population by 15.2% at 6 months (P = 0.018) and 16.4% at 12 months (P = 0.006). In type 2 diabetic patients, glycemic control was significantly better in the TRT group than the placebo group at month 9 (HbA1c: treatment difference, −0.446%; P = 0.035). Improvements in total and LDL cholesterol, lipoprotein a (Lpa), body composition, libido, and sexual function occurred in selected patient groups. There were no significant differences between groups in the frequencies of adverse events (AEs) or serious AEs. The majority of AEs (>95%) were mild or moderate.
CONCLUSIONS
Over a 6-month period, transdermal TRT was associated with beneficial effects on insulin resistance, total and LDL-cholesterol, Lpa, and sexual health in hypogonadal men with type 2 diabetes and/or MetS.
Low serum testosterone is common in men with type 2 diabetes and/or metabolic syndrome (MetS) and numerous studies have reported an association between testosterone deficiency and visceral obesity, insulin resistance (IR) and dyslipidemia (1–4). In men with type 2 diabetes, low testosterone is associated with a high prevalence of symptomatic hypogonadism (3), frequently due to hypogonadotrophic hypogonadism (5).
Small studies have demonstrated that testosterone replacement therapy (TRT) in hypogonadal men with and without type 2 diabetes is associated with reductions in IR, waist circumference, cholesterol, glycated hemoglobin (HbA1c), and fasting plasma glucose (FPG) concentrations (6–9). Conversely, withdrawal of TRT in hypogonadal men leads to decreased insulin sensitivity (10). Furthermore, androgen suppression therapy for prostate cancer can result in alterations of individual cardiovascular risk factors and increases the occurrence of incident diabetes, myocardial infarction and sudden cardiac death (11,12). Epidemiologic studies have reported that low testosterone in men is associated with increased all-cause and cardiovascular mortality (13).
The TIMES2 (Testosterone replacement In hypogonadal men with either MEtabolic Syndrome or type 2 diabetes) study investigated the effects of transdermal TRT on IR, selected cardiovascular risk factors, and symptoms in hypogonadal men with MetS and/or type 2 diabetes. The safety and tolerability of a novel, metered-dose, transdermal 2% testosterone gel were also examined.
RESEARCH DESIGN AND METHODS
This 12-month, prospective, randomized, double-blind, placebo-controlled, multicenter study was conducted at 36 outpatient centers in Belgium, France, Germany, Italy, the Netherlands, Spain, Sweden, and the U.K. between February 2006 and March 2007. The study was conducted in accordance with the revised guidelines of the World Medical Association Declaration of Helsinki, the International Conference on Harmonization Standard Operating Procedures, and local laws and regulatory requirements. The study protocol was approved by independent ethics committees for each center. Participants provided written informed consent.
Men aged ≥40 years were eligible to enter the study if they had confirmed hypogonadism (early morning [0800−1000 h] total testosterone [TT] ≤11 nmol/L or free testosterone ≤255 pmol/L on two occasions ≥1 week apart), with at least two symptoms of hypogonadism (14) and fulfilled criteria for type 2 diabetes (15) and/or MetS (16) (Supplementary Data). Erectile dysfunction was not an inclusion criterion.
Exclusion criteria included TRT within 6 months of randomization, hormone-modulating therapies or topical/systemic glucocorticoids within 3 months of randomization, or insulin therapy; a history of/current prostate or breast cancer; abnormal digital rectal examination suggestive of prostate carcinoma; severe symptomatic benign prostatic hyperplasia or elevated age-specific prostate-specific antigen (PSA) (17). For PSA levels and other exclusion criteria see the Supplementary Data. Concomitant medications (oral antidiabetic drugs [OADs], lipid-lowering therapies, antihypertensives) were continued if doses had remained stable for ≥3 months before randomization. The first 6 months of the study, during which no therapy changes were permitted unless the investigator deemed it necessary for good clinical practice, was designated Phase 1. The second 6-month period, when changes in medication were allowed, was designated Phase 2.
Subjects were randomized (1:1) to receive either 3 g metered-dose 2% testosterone gel (60 mg testosterone, Tostran [also known as Fortigel, Tostrex, Itnogen, Foresta; ProStrakan, Galashiels, Scotland, U.K.]) or placebo gel once daily and stratified to treatment arms according to the presence of MetS only and type 2 diabetes with or without MetS. All individuals involved in monitoring, data management, or other study aspects were blinded to treatment.
Treatment was applied daily (0700−1000 h) to clean, dry, intact skin on the thighs or abdomen. Blood samples were taken 2 h after application. TT was measured at 2, 4, and 12 weeks with dose adjustments made as follows: TT >52 nmol/L, TRT dose reduced to 40 mg/day; TT <17 nmol/L, dose increased to 80 mg/day. Dummy dose changes were performed in the placebo group to maintain blinding.
All laboratory assessments (Supplementary Data) were performed centrally by Pivotal Laboratories Ltd., York, U.K. Measurements were taken at baseline, then every 3 months for HbA1c, PSA, lipids (total cholesterol [TC], HDL cholesterol, LDL cholesterol, triglycerides, lipoprotein a [Lpa]), hematology, biochemistry, testosterone, FPG (no 9-month measurement) and insulin (mean of three samples at 5-min intervals, no 9-month measurement). Free testosterone was calculated from TT, albumin, and sex hormone binding globulin, using the Vermeulen equation (18). Waist circumference, BMI, body composition (TANITA BF-300 body fat analyzer; TANITA Corporation, Tokyo, Japan), Aging Males Symptoms (AMS) score (19), and International Index of Erectile Function (IIEF) (20) were assessed at baseline, 6, and 12 months. Adverse events (AEs) were elicited from the patient at each visit by a nonleading question.
Statistical analysis
The primary end point was the difference between treatment groups in homeostasis model assessment (HOMA)-IR from baseline to months 6 and 12. Secondary end points were changes from baseline in HbA1c, fasting insulin, FPG, lipid parameters, body composition, BMI, waist circumference, AMS and IIEF scores, AEs, and other safety parameters. HOMA of β-cell function (HOMA-B) was determined post hoc.
The safety population comprised all randomized patients who received ≥1 dose of study medication. The primary analysis of all end points was based on the intention-to-treat (ITT) population (all randomized patients). Two other populations were also studied: the per-protocol (PP) population (randomized patients who received ≥1 dose of study medication with no protocol violations during the first 6 months of the study) and the modified PP (mPP) population. This latter population was established post hoc because the PP population was substantially reduced in size after exclusion of patients who took unauthorized concomitant medications from the ITT population (Supplementary Data). Patients’ eligibility for inclusion in the mPP population was assessed on a case-by-case basis for each end point. Inclusion was allowed if there were no changes in drugs during the first 6 months that affected the end point in question. These were OADs and antiobesity drugs for HOMA-IR (n = 130); OADs, antiobesity drugs, β-blockers, and diuretics for body composition (n = 147); lipid-lowering drugs, β-blockers, and α-blockers for lipid profile (n = 139); OADs, antiobesity drugs, β-blockers, and diuretics for HbA1c and FPG (n = 140); phosphodiesterase-5 inhibitors and β-blockers for AMS (n = 152) and IIEF (n = 154); and antihypertensive drugs for blood pressure (n = 140).
The study was designed to have a power of 95% to detect a mean difference in HOMA-IR change from baseline between TRT and placebo of 0.82 (SD 1.7) at 6 months. After completion of an interim analysis in 63 patients, the study power was revised to 80%, giving a final target completer sample of 136 patients. All statistical analyses (Chiltern International Ltd; Quantics, Newtown St Boswells, U.K.) were conducted using SAS version 8.2 (SAS Institute, Cary, NC) or later. Descriptive statistics were calculated for continuous variables, with 95% CI, SD, and P values. For comparison of efficacy outcomes between treatment groups (treatment difference [TD]), an ANCOVA model was applied, with disease category as a covariate (MetS and type 2 diabetes; MetS only; type 2 diabetes only). P < 0.05 was considered statistically significant for all analyses. No adjustment was made for multiple testing as all secondary end points were regarded as exploratory. For the primary end point and HOMA-B, a log transformation was applied for the ANCOVA and a last observation carried forward approach was used. Unless otherwise stated, all efficacy data were analyzed in the ITT population, all safety data were analyzed in the safety population, and all results are quoted as mean ± SD.
RESULTS
Of 518 patients screened, 220 were randomized (testosterone n = 108; placebo n = 112) and received at least one dose of study drug (ITT and safety populations). Baseline demographic and clinical characteristics were comparable between groups (Table 1). Phase 1 was completed by 157 patients (71%), and Phases 1 and 2 were completed by 118 (54%) (Supplementary Fig. A1). Reasons for early withdrawal included protocol violation (n = 28), withdrawal of consent (n = 27), AEs (n = 26) and loss to follow-up (n = 9). Protocol was violated by 125 patients, giving a PP population of 95. Hypogonadotrophic or secondary hypogonadism, defined as low or normal luteinizing hormone, occurred in 82% of subjects whereas 18% demonstrated primary hypogonadism (Supplementary Table A1). The majority of subjects (80%) had MetS at baseline, and 62% had type 2 diabetes. MetS and type 2 diabetes were both present in 44 and 40% of the TRT and placebo groups, respectively. Baseline serum TT values were 9.2 ± 2.6 nmol/L (TRT) and 9.5 ± 3.3 nmol/L (placebo). The majority of subjects (62.6%) qualified for study entry, meeting both TT and free testosterone criteria. Of the subjects, 12.3 and 23.7% qualified on the basis of TT <11 nmol/L or free testosterone <255 pmol/L, respectively.
Table 1.
Baseline characteristics (safety population)
| Testosterone | Placebo | Total | |
|---|---|---|---|
| n | 108 | 112 | 220 |
| Age (years) | 59.9 ± 9.1 (37−77) | 59.9 ± 9.4 (39−88) | 59.9 ± 9.3 (37−88) |
| Race | |||
| Caucasian | 105 (97) | 110 (98) | 215 (98) |
| Asian | 3 (3) | 0 | 3 (1) |
| Afro-Caribbean | 0 | 1 (1) | 1 (0.5) |
| Other | 0 | 1 (1) | 1 (0.5) |
| Total serum testosterone (nmol/L) | 9.2 ± 2.6 (3.8−20.1) | 9.5 ± 3.3 (2.2−20.6) | 9.4 ± 3.0 (2.2−20.6) |
| Free testosterone (pmol) | 198.0 ± 49.3 (42−319) | 202.4 ± 62.1 (35−364) | 200.3 ± 56.1 (35−364) |
| Type 2 diabetes | 68 (6) | 69 (62) | 137 (62) |
| MetS | 88 (82) | 88 (79) | 176 (80) |
| Insulin resistance (HOMA-IR index) | 5.9 ± 3.8 (1−20) | 4.9 ± 3.3 (1−21) | 5.4 ± 3.6 (1−21) |
| Hematocrit (L/L) | 0.43 ± 0.04 (0.34−0.54) | 0.43 ± 0.04 (0.33−0.51) | 0.43 ± 0.04 (0.33−0.54) |
| Hemoglobin (g/dL) | 14.9 ± 1.5 (11.0−18.6) | 14.9 ± 1.3 (11.4−18.0) | 14.9 ± 1.4 (11.0−18.6) |
| PSA (μg/L) | 1.6 ± 1.8 (0.21−13.60) | 1.2 ± 1.2 (0.20−6.38) | 1.4 ± 1.5 (0.20−13.60) |
| FSH* (IU/L) | 12.3 ± 13.4 (1.2−63.6) | 12.5 ± 15.7 (1.2−78.9) | 12.4 ± 14.5 (1.2−78.9) |
| FSH* | |||
| Low | 0 | 0 | 0 |
| Normal | 77 (71) | 79 (71) | 156 (71) |
| High | 31 (29) | 33 (29) | 64 (29) |
| LH* (IU/L) | 5.7 ± 5.7 (0.9−34.3) | 5.3 ± 5.4 (0.6−36.1) | 5.5 ± 5.5 (0.6−36.1) |
| LH* | |||
| Low | 0 | 2 (2) | 2 (1) |
| Normal | 86 (80) | 92 (82) | 178 (81) |
| High | 22 (20) | 18 (16) | 40 (18) |
Efficacy: Phase 1
At the end of Phase 1 (6 months), serum TT values in the ITT population had increased by 19.0 ± 22.1 nmol/L with TRT versus 0.1 ± 2.9 nmol/L with placebo (P < 0.001).
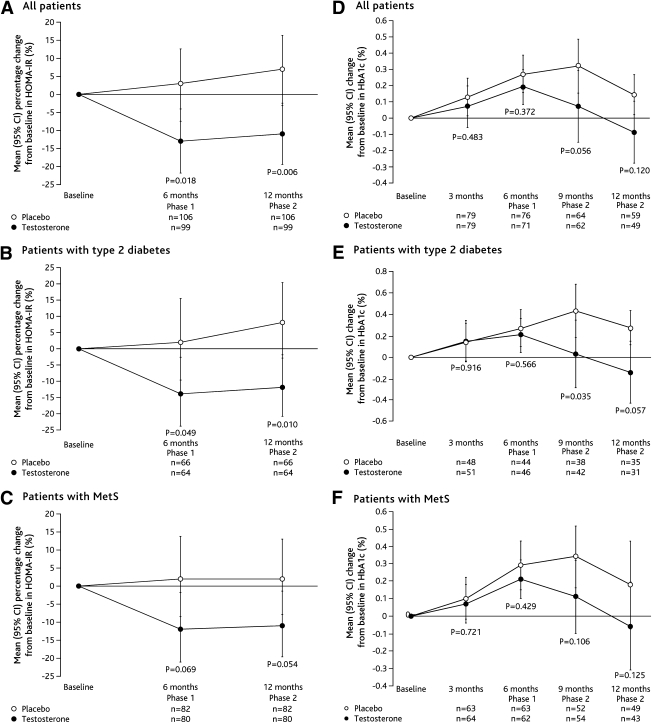
TRT reduced HOMA-IR by 15.2% (95% CI 3–26; P = 0.018) compared with placebo at 6 months (Fig. 1). Type 2 diabetic patients demonstrated mean reductions in HOMA-IR of 16.0% (0–29; P = 0.049) compared with placebo. The difference between treatments in the MetS patients approached significance (TD 13.3% [−1 to 26]; P = 0.069) (Fig. 1). Post hoc analyses of HOMA-IR in patients without type 2 diabetes only, showed no difference between treatment groups (Supplementary Table A2). There were no significant differences between treatments in the overall population or the disease subgroups in HbA1c (Fig. 1), fasting serum insulin, FPG, or HOMA-B (Table 2).
Figure 1.
Mean (95% CI) percentage change from baseline in HOMA-IR (ITT population, last observation carried forward) and change from baseline in HbA1c (ITT population, study completers) among all patients (A and D), patients with type 2 diabetes (B and E), and patients with MetS (C and F). P values reported for comparisons between groups.
Table 2.
Mean values for primary and secondary end points at baseline and months 6 and 12 (ITT population, last observation carried forward)
| All patients | MetS patients | Type 2 diabetic patients | ||||
|---|---|---|---|---|---|---|
| Testosterone | Placebo | Testosterone | Placebo | Testosterone | Placebo | |
| Glucose metabolism | ||||||
| Fasting serum insulin (pmol/L) | ||||||
| Baseline | 151.68 ± 153.26 | 137.65 ± 147.46 | 162.17 ± 166.29 | 153.76 ± 163.02 | 145.08 ± 158.55 | 126.19 ± 109.17 |
| n | 102 | 105 | 82 | 81 | 64 | 65 |
| Phase 1: Month 6 | 124.59 ± 98.65 | 128.20 ± 99.76 | 125.08 ± 86.89 | 135.50 ± 104.45 | 121.61 ± 102.01 | 136.40 ± 117.44 |
| n | 101 | 101 | 83 | 80 | 65 | 63 |
| Phase 2: Month 12 | 134.25 ± 108.57 | 126.89 ± 94.31 | 135.50 ± 100.92 | 131.33 ± 98.47 | 130.91 ± 113.99 | 131.96 ± 104.30 |
| n | 103 | 101 | 85 | 80 | 65 | 63 |
| FPG (mmol/L) | ||||||
| Baseline | 7.94 ± 2.99 | 7.59 ± 2.62 | 7.60 ± 2.41 | 7.60 ± 2.71 | 9.05 ± 3.18 | 8.49 ± 2.84 |
| n | 104 | 107 | 84 | 83 | 67 | 67 |
| Phase 1: Month 6 | 7.84 ± 3.03 | 7.99 ± 2.86 | 7.67 ± 2.82 | 7.88 ± 2.99 | 8.91 ± 3.31 | 9.09 ± 2.97 |
| n | 102 | 101 | 84 | 80 | 65 | 63 |
| Phase 2: Month 12 | 7.97 ± 3.45 | 8.14 ± 3.19 | 7.74 ± 3.25 | 8.05 ± 3.41 | 9.18 ± 3.75 | 9.35 ± 3.46 |
| n | 102 | 101 | 84 | 80 | 65 | 63 |
| HOMA-B† | ||||||
| Baseline | 104.8 ± 77.5 | 99.9 ± 75.8 | 110.5 ± 78.3 | 104.7 ± 74.7 | 83.3 ± 67.0 | 84.1 ± 74.0 |
| n | 99 | 105 | 80 | 81 | 64 | 66 |
| Phase 1: Month 6 | 100.7 ± 78.9 | 98.3 ± 72.7 | 103.9 ± 79.4 | 106.0 ± 74.9 | 79.4 ± 66.0 | 75.3 ± 58.2 |
| n | 105 | 108 | 86 | 84 | 67 | 67 |
| Phase 2: Month 12 | 102.0 ± 79.5 | 99.4 ± 70.8 | 105.1 ± 80.1 | 105.4 ± 70.2 | 79.1 ± 65.4 | 79.3 ± 66.5 |
| n | 105 | 109 | 86 | 85 | 67 | 68 |
| Lipid parameters | ||||||
| Lpa (µmol/L) | ||||||
| Baseline | 1.44 ± 1.56 | 1.40 ± 1.17 | 1.50 ± 1.64 | 1.42 ± 1.21 | 1.36 ± 1.36 | 1.37 ± 1.28 |
| n | 98* | 102 | 81** | 79 | 62 | 64 |
| Phase 1: Month 6 | 1.22 ± 1.19 | 1.59 ± 1.80 | 1.22 ± 1.20 | 1.49 ± 1.65 | 1.20 ± 1.16 | 1.61 ± 2.15 |
| n | 96* | 98 | 80** | 79 | 62 | 60 |
| Phase 2: Month 9 | 1.39 ± 1.39 | 1.90 ± 1.94 | 1.37 ± 1.43 | 1.77 ± 1.58 | 1.51 ± 1.52 | 1.96 ± 2.34 |
| n | 51* | 56 | 43 | 44 | 35 | 35 |
| Phase 2: Month 12 | 1.26 ± 1.22 | 1.62 ± 1.82 | 1.26 ± 1.24 | 1.49 ± 1.38 | 1.29 ± 1.26 | 1.65 ± 2.19 |
| n | 99 | 100 | 83** | 80 | 63 | 61 |
| TC (mmol/L) | ||||||
| Baseline | 4.65 ± 1.18 | 4.87 ± 1.11 | 4.79 ± 1.22 | 4.98 ± 1.09 | 4.51 ± 1.17 | 4.55 ± 1.01 |
| n | 106 | 108 | 86 | 84 | 67 | 67 |
| Phase 1: Month 6 | 4.52 ± 1.11 | 4.89 ± 1.13 | 4.56 ± 1.11 | 5.05 ± 1.13 | 4.44 ± 1.12 | 4.57 ± 0.97 |
| n | 103 | 102 | 85** | 81 | 66 | 63 |
| Phase 2: Month 9 | 4.55 ± 1.24 | 4.80 ± 1.12 | 4.61 ± 1.24 | 4.95 ± 1.10 | 4.32 ± 1.14 | 4.59 ± 1.13 |
| n | 62 | 65 | 54 | 52 | 42 | 39 |
| Phase 2: Month 12 | 4.49 ± 1.16 | 4.77 ± 1.07 | 4.52 ± 1.17 | 4.88 ± 1.05 | 4.31 ± 1.05 | 4.52 ± 0.95 |
| n | 104 | 102 | 86 | 81 | 66 | 63 |
| LDL cholesterol (mmol/L) | ||||||
| Baseline | 2.78 ± 0.99 | 2.89 ± 0.96 | 2.89 ± 1.02 | 2.98 ± 0.95 | 2.61 ± 0.91 | 2.61 ± 0.82 |
| n | 106 | 108 | 86 | 84 | 67 | 67 |
| Phase 1: Month 6 | 2.65 ± 0.91 | 2.85 ± 0.91 | 2.70 ± 0.95 | 2.99 ± 0.93 | 2.60 ± 0.85 | 2.55 ± 0.70 |
| n | 103 | 102 | 85* | 81 | 66 | 63 |
| Phase 2: Month 9 | 2.67 ± 1.01 | 2.77 ± 0.88 | 2.70 ± 1.01 | 2.90 ± 0.90 | 2.53 ± 0.93 | 2.51 ± 0.77 |
| n | 62 | 65 | 54 | 52 | 42 | 39 |
| Phase 2: Month 12 | 2.59 ± 0.91 | 2.75 ± 0.89 | 2.62 ± 0.93 | 2.85 ± 0.91 | 2.49 ± 0.82 | 2.50 ± 0.70 |
| n | 104 | 102 | 86 | 81 | 66 | 63 |
| HDL cholesterol (mmol/L) | ||||||
| Baseline | 1.19 ± 0.30 | 1.23 ± 0.28 | 1.21 ± 0.31 | 1.21 ± 0.26 | 1.16 ± 0.31 | 1.23 ± 0.29 |
| n | 106 | 108 | 86 | 84 | 67 | 67 |
| Phase 1: Month 6 | 1.12 ± 0.26* | 1.21 ± 0.25 | 1.12 ± 0.24 | 1.20 ± 0.24 | 1.09 ± 0.27 | 1.21 ± 0.26 |
| n | 103 | 102 | 85* | 81 | 66* | 63 |
| Phase 2: Month 9 | 1.14 ± 0.31 | 1.20 ± 0.27 | 1.14 ± 0.28 | 1.18 ± 0.27 | 1.12 ± 0.33 | 1.20 ± 0.27 |
| n | 62 | 65 | 54* | 52 | 42 | 39 |
| Phase 2: Month 12 | 1.14 ± 0.30 | 1.21 ± 0.26 | 1.14 ± 0.28 | 1.20 ± 0.25 | 1.09 ± 0.30 | 1.21 ± 0.27 |
| n | 104 | 102 | 86 | 81 | 66 | 63 |
| Triglycerides (mmol/L) | ||||||
| Baseline | 1.92 ± 1.36 | 2.10 ± 1.44 | 1.94 ± 1.42 | 2.21 ± 1.52 | 2.06 ± 1.58 | 2.05 ± 1.30 |
| n | 106 | 108 | 86 | 84 | 67 | 67 |
| Phase 1: Month 6 | 1.93 ± 1.23 | 2.04 ± 1.28 | 1.92 ± 1.05 | 2.10 ± 1.34 | 1.98 ± 1.31 | 2.07 ± 1.30 |
| n | 103 | 102 | 85 | 81 | 66 | 63 |
| Phase 2: Month 9 | 2.00 ± 1.21 | 2.23 ± 1.71 | 2.07 ± 1.26 | 2.29 ± 1.76 | 1.91 ± 0.98 | 2.36 ± 1.99 |
| n | 62 | 65 | 54 | 52 | 42 | 39 |
| Phase 2: Month 12 | 1.90 ± 1.15 | 2.14 ± 1.49 | 1.91 ± 1.06 | 2.16 ± 1.54 | 1.90 ± 1.17 | 2.19 ± 1.49 |
| n | 104 | 102 | 86 | 81 | 66 | 63 |
| Abdominal obesity and body composition | ||||||
| Body fat (%) | ||||||
| Baseline | 33.60 ± 6.54 | 32.24 ± 6.33 | 33.99 ± 6.69 | 32.37 ± 6.17 | 33.58 ± 5.43 | 32.54 ± 6.55 |
| n | 108 | 112 | 88 | 88 | 68 | 69 |
| Phase 1: Month 6 | 33.01 ± 6.83 | 32.31 ± 5.77 | 33.31 ± 6.82 | 32.43 ± 5.59 | 32.86 ± 5.92 | 32.33 ± 5.88 |
| n | 94 | 98 | 78 | 78 | 61 | 62 |
| Phase 2: Month 12 | 32.81 ± 7.08 | 32.63 ± 5.57 | 33.08 ± 7.18 | 32.66 ± 5.51 | 32.69 ± 6.44 | 32.68 ± 5.71 |
| n | 100 | 98 | 83 | 78 | 63 | 62 |
| BMI (kg/m2) | ||||||
| Baseline | 32.87 ± 6.58 | 31.29 ± 5.44 | 33.27 ± 6.80 | 31.63 ± 5.07 | 32.76 ± 6.12 | 31.56 ± 5.87 |
| n | 108 | 112 | 88 | 88 | 68 | 69 |
| Phase 1: Month 6 | 33.07 ± 6.96 | 31.45 ± 5.26 | 33.28 ± 7.28 | 31.67 ± 5.03 | 33.16 ± 6.35 | 31.70 ± 5.55 |
| n | 97 | 99 | 80 | 79 | 62 | 62 |
| Phase 2: Month 12 | 32.90 ± 7.01 | 31.43 ± 5.27 | 33.17 ± 7.35 | 31.53 ± 5.07 | 32.92 ± 6.52 | 31.73 ± 5.57 |
| n | 102 | 99 | 84 | 79 | 64 | 62 |
| Waist circumference (cm) | ||||||
| Baseline | 112.7 ± 13.22 | 110.1 ± 13.77 | 113.3 ± 13.43 | 110.8 ± 12.65 | 112.7 ± 13.35 | 111.7 ± 15.23 |
| n | 108 | 112 | 88 | 88 | 68 | 69 |
| Phase 1: Month 6 | 111.9 ± 13.64 | 110.2 ± 13.36 | 112.0 ± 13.62 | 110.3 ± 12.02 | 113.1 ± 13.57 | 111.8 ± 14.46 |
| n | 96 | 99 | 80 | 79 | 61 | 62 |
| Phase 2: Month 12 | 111.6 ± 13.89 | 110.6 ± 13.96 | 112.0 ± 13.92 | 110.8 ± 12.52 | 111.9 ± 13.79 | 112.4 ± 15.52 |
| n | 100 | 99 | 83 | 79 | 63* | 62 |
| Blood pressure | ||||||
| Systolic blood pressure (mmHg) | ||||||
| Baseline | 138.6 ± 17.30 | 136.7 ± 17.12 | — | — | — | — |
| n | 108 | 112 | ||||
| Phase 2: Month 12 | 138.7 ± 15.15 | 134.9 ± 16.49 | — | — | — | — |
| n | 50 | 58 | ||||
| Diastolic blood pressure (mmHg) | ||||||
| Baseline | 82.5 ± 10.23 | 81.6 ± 9.50 | ||||
| n | 108 | 112 | ||||
| Phase 2: Month 12 | 82.8 ± 9.72 | 80.3 ± 9.80 | — | — | — | — |
| n | 50 | 58 | ||||
| Sexual dysfunction | ||||||
| IIEF total score | ||||||
| Baseline | 32.4 ± 18.47 | 32.8 ± 21.24 | — | — | — | — |
| n | 94 | 98 | ||||
| Phase 1: Month 6 | 41.3 ± 19.97 | 39.3 ± 22.12 | — | — | — | — |
| n | 82* | 81 | ||||
| Phase 2: Month 12 | 40.9 ± 20.34 | 37.3 ± 22.08 | — | — | — | — |
| n | 88* | 88 | ||||
| Erectile function domain score | ||||||
| Baseline | 12.0 ± 9.07 | 11.9 ± 10.02 | ||||
| n | 101 | 103 | ||||
| Phase 1: Month 6 | 15.6 ± 9.53 | 15.1 ± 10.49 | — | — | — | — |
| n | 84 | 89 | ||||
| Phase 2: Month 12 | 15.2 ± 9.71 | 14.4 ± 10.42 | — | — | — | — |
| n | 90 | 93 | ||||
| Orgasmic function domain score | ||||||
| Baseline | 4.6 ± 3.80 | 4.7 ± 4.20 | — | — | — | |
| n | 105 | 107 | ||||
| Phase 1: Month 6 | 5.9 ± 3.89 | 5.8 ± 4.18 | — | — | — | — |
| n | 88 | 90 | ||||
| Phase 2: Month 12 | 5.5 ± 3.91 | 5.3 ± 4.23 | — | — | — | — |
| n | 95 | 93 | ||||
| Sexual desire domain score | ||||||
| Baseline | 5.3 ± 2.13 | 5.6 ± 2.47 | — | — | — | |
| n | 105 | 106 | ||||
| Phase 1: Month 6 | 6.5 ± 2.27 | 5.9 ± 2.38 | — | — | — | — |
| n | 89** | 91 | ||||
| Phase 2: Month 12 | 6.3 ± 2.21 | 5.8 ± 2.55 | — | — | — | — |
| n | 94** | 95 | ||||
| Intercourse satisfaction domain score | ||||||
| Baseline | 4.7 ± 4.58 | 4.7 ± 4.84 | — | — | — | — |
| n | 106 | 108 | ||||
| Phase 1: Month 6 | 6.2 ± 4.95 | 6.3 ± 4.99 | — | — | — | — |
| n | 88 | 91 | ||||
| Phase 2: Month 12 | 6.4 ± 5.01 | 5.7 ± 4.89 | — | — | — | — |
| n | 94* | 92 | ||||
| Overall sexual satisfaction domain score | ||||||
| Baseline | 4.7 ± 2.46 | 4.8 ± 2.68 | — | — | — | — |
| n | 96 | 99 | ||||
| Phase 1: Month 6 | 6.0 ± 2.62 | 5.7 ± 2.81 | — | — | — | — |
| n | 85 | 89 | ||||
| Phase 2: Month 12 | 5.9 ± 2.70 | 5.5 ± 2.73 | — | — | — | — |
| n | 91 | 94 | ||||
| AMS total score | ||||||
| Baseline | 40.6 ± 11.44 | 39.9 ± 11.46 | — | — | — | — |
| n | 88 | 96 | ||||
| Phase 1: Month 6 | 37.1 ± 11.72 | 36.1 ± 10.60 | — | — | — | — |
| n | 80 | 86 | ||||
| Phase 2: Month 12 | 36.4 ± 11.16 | 37.8 ± 11.57 | — | — | — | — |
| n | 89 | 92 |
TRT had a number of beneficial effects on lipid profile. In the MetS subgroup, TRT was associated with significantly greater reductions from baseline in plasma levels of Lpa (TD −0.31 µmol/L [95% CI −0.543 to −0.082]; P = 0.008), TC (TD −0.336 mmol/L [−0.558 to −0.114]; P = 0.003) and LDL cholesterol (TD −0.210 mmol/L [95−0.374 to −0.047]; P = 0.012) than placebo (Table 2). TRT also reduced Lpa in the overall ITT population (TD −0.235 µmol/L [−0.431 to −0.039]; P = 0.019). HDL cholesterol decreased from baseline significantly more with TRT than placebo in all three analysis groups (total population [TD −0.049 mmol/L (−0.094 to −0.004); P = 0.032], MetS [TD −0.058 mmol/L (−0.105 to −0.011); P = 0.016], type 2 diabetes [TD −0.062 mmol/L (−0.123 to −0.002); P = 0.043]). There were no significant effects of TRT on triglycerides, abdominal obesity, percentage body fat, BMI, or waist circumference.
In the overall ITT population, TRT was associated with significantly greater increases from baseline in total IIEF score (TD 4.868 [95% CI 0.644–9.092]; P = 0.024) and in the sexual desire domain (TD 0.800 [0.271–1.329]; P = 0.003) (Table 2). Treatment had no significant effect on erectile function, orgasmic function, or overall sexual satisfaction, although these three measures did show a consistent trend for greater improvement with TRT versus placebo. Change in AMS score was similar in both treatment groups.
Data for secondary end points in the mPP and PP populations are shown in Supplementary Tables A3 and A4, respectively. The small size of the PP population negated statistical power and the difference between groups in HOMA-IR reduction was not statistically significant (TRT −0.92 ± 2.454, placebo −0.06 ± 2.451; P = 0.17). However, reductions from baseline in Lpa, TC, LDL cholesterol, and HDL cholesterol were significantly greater in the TRT group than in the placebo group (overall PP cohort). In the exploratory mPP population, TRT was associated with significant reductions from baseline in percentage body fat, Lpa, TC, and LDL cholesterol in all patients and in the MetS subgroup (Supplementary Table A3). The type 2 diabetes subgroup showed significant reductions in Lpa and percentage body fat with TRT (Supplementary Table A3). There were no significant between-group differences in HDL cholesterol in the mPP population (Supplementary Table A3). No other end points were significantly different between treatment groups in the PP or mPP populations.
Efficacy: Phase 2
At the end of Phase 2 (12 months), serum TT values in the ITT population were significantly higher following TRT (19.5 ± 17.0 nmol/L) versus placebo (−0.6 ± 3.3 nmol/L) (P < 0.001).
Changes in concomitant medications were allowed in Phase 2. Efficacy results are therefore not presented for this phase except for HOMA-IR (the primary end point) and sexual function, a measure of hypogonadism. In the total ITT population, HOMA-IR was significantly improved with TRT versus placebo at 12 months (TD 16.4% [95% CI 5–26]; P = 0.006).
Exploratory analysis of the type 2 diabetes mPP subgroup showed HbA1c decreased slightly by month 9 with TRT but increased with placebo, creating a difference of −0.58% that was significant (P = 0.005). This trend continued nonsignificantly at 12 months (−0.49%, P = 0.066).
There was a significant improvement in IIEF total score and sexual desire and intercourse satisfaction domain scores in all patients at 12 months in the ITT population (Table 2). The positive effect of TRT on the erectile function domain in the ITT population (Table 2) approached significance in the mPP exploratory analysis (TD 3.225 [95% CI −0.454 to 6.904]; P = 0.089).
Safety: Phases 1 and 2
In the safety population, 137 patients (62%) experienced 502 AEs with 224 (45%) considered to be related to study medication (TRT, 116; placebo, 108). The majority of AEs were classified as mild or moderate (TRT, 98%; placebo, 97%) with no significant differences between treatments. The most common AEs in patients experiencing ≥1 AE were erythema (TRT, 4%; placebo, 5%), pruritis (TRT, 4%; placebo, 4%), and nasopharyngitis (TRT, 3%; placebo 4%) (Supplementary Table A5).
A total of 17 serious AEs were experienced by 13 patients. Of these, 3 in the placebo group were considered related to treatment. Thirteen resolved without sequelae. One patient (placebo) died as the result of myocardial infarction. A total of 30 patients withdrew from the study because of AEs (TRT, 18; placebo, 12). The most common AEs leading to discontinuation were skin and subcutaneous tissue disorders (21 AEs; testosterone, 11; placebo, 10). Cardiovascular events occurred more commonly with placebo (10.7 vs. 4.6%; P = 0.095).
No clinically relevant mean changes in laboratory parameters were noted. After 12 months, hematocrit had increased by 0.03 ± 0.04 L/L for TRT versus a decrease of −0.01 ± 0.02 L/L for placebo, and hemoglobin levels had increased by 1.42 ± 1.55 g/dL from baseline (TRT) versus 0.06 ± 0.7 g/dL (placebo). There was no significant increase in age-adjusted PSA values. The PSA level exceeded the upper limit of age-adjusted normal values in three subjects at baseline (testosterone, 2; placebo, 1). This violation of the entry criteria would not have affected the primary efficacy end point, and because the patients had been randomized and were satisfactorily proceeding in the study they were not withdrawn. None of these patients underwent prostate biopsy during the study. PSA levels exceeded normal limits in four subjects at 12 months (TRT, 3; placebo, 1).
CONCLUSIONS
This study demonstrates that transdermal TRT in hypogonadal men with type 2 diabetes and/or MetS improves IR, the central biochemical defect associated with these conditions. This result is of added interest, as many study participants were already taking OADs (mainly metformin), which affect insulin sensitivity. Although an improvement in IR could be expected to result in better glycemic control, no reduction in HbA1c was demonstrated after 6 months of treatment. Previous studies have reported that TRT does improve glycemic control (6,9); however, the current study was not powered to detect changes in glycemic control and included patients with controlled diabetes (HbA1c <6.5%). A significant difference in HbA1c between the TRT and placebo groups was observed at 9 months, but this potential treatment effect was confounded by the permitted changes in diabetes-related medications for ethical reasons. Therefore, no clear conclusion can be made. A larger definitive study with HbA1c as a primary end point in hypogonadal men with uncontrolled diabetes is required to investigate this further.
The majority of cross-sectional studies have shown that hypogonadism is associated with dyslipidemia, albeit with conflicting results (4). There are only limited data supporting the effect of testosterone gel formulations on lipids. Two studies reported a small rise in HDL cholesterol but no effect on TC or LDL cholesterol in hypogonadal men (9,21). In the current study, Lpa was significantly reduced compared with placebo in the overall population after 6 months of therapy. Moreover, the subgroup of patients with MetS at baseline showed significant reductions from baseline with TRT versus placebo, in Lpa, TC, and LDL cholesterol after 6 months, even though the majority of these patients were taking statins and had mean baseline TC concentrations below 5 mmol/L. A reduction in HDL cholesterol has been reported in studies using intramuscular testosterone ester therapy (4).
Lpa is a strong independent risk factor for premature coronary heart disease (22). Surgical orchidectomy for prostate cancer increases Lpa concentrations, whereas administration of testosterone in healthy men reduces Lpa levels (23). In the current study, reductions from baseline in serum Lpa concentration were significantly greater in TRT-treated patients at month 6 in both the overall population and the MetS subgroup. The long-term cardiovascular benefits of reducing Lpa are unknown.
The safety and tolerability profile of TRT was comparable to placebo with no significant differences in cardiovascular events, including blood pressure. Caution has been urged following a recent study that reported an increase in cardiovascular events in testosterone-treated, frail, elderly men (24). However, cardiovascular events were not a planned outcome in this study, and verification of events varied among subjects. Moreover, >70% of the men in that trial received higher doses of testosterone (100 or 150 mg/day) than any of the men in the current study (40−80 mg/day). The current study showed a trend for cardiovascular events to occur more frequently in the placebo group than in the TRT group. A similar frailty study that used standard doses (testosterone gel 50 mg/day) in clinical practice found no increase in cardiovascular events (25).
The frequency of application site reactions in this study was higher than has been reported in other trials involving transdermal TRT. However, in TIMES2 mandatory application site inspections were performed at each study visit, and the investigators were trained to identify and document skin-related reactions. This is in contrast to other studies that relied on patients reporting a dermatological AE.
The beneficial effects of TRT on overall sexual function and, importantly, libido (a key symptom of testosterone deficiency) support the assessment and treatment of hypogonadism in men with type 2 diabetes and/or MetS who do not present specifically with erectile dysfunction.
Results from the mPP population should be regarded as exploratory only, since this population was created as part of a post hoc analysis. However, these results have been included because they allow assessment of the impact of testosterone on individual parameters without the potential confounding influence of changes in concomitant medication. Although this information is typically gained from the PP population, the small size of the PP population here limited the power of any analyses and precluded analysis of disease subgroup data (MetS and type 2 diabetes). The mPP data provide supportive evidence that TRT improves glycemic control, lipid profile, central obesity and body composition in hypogonadal men with type 2 diabetes and/or MetS. In this population, the loss of statistical significance for some end points between 6 and 12 months is likely to be due to the smaller size of the population at month 12 and the allowed use of concomitant medications after 6 months (Supplementary Table A3). The delayed effect of TRT on HbA1c could be explained by the cumulative effect on reduction in body fat over several months.
The high dropout rate is a limitation of this study. However, there was no difference between treatment groups in the number or characteristics of the patients who failed to complete the study.
This study has shown that TRT in hypogonadal men with type 2 diabetes and/or MetS improves several cardiovascular risk factors, most notably IR, Lpa, TC, and LDL cholesterol. These findings and the therapeutic benefit on sexual function strongly support a role for TRT in these clinical conditions.
Supplementary Material
Supplementary Data
Acknowledgments
This study was sponsored and funded by ProStrakan. Investigators collected study data and the sponsor monitored the study conduct. Data collection and analysis was performed by Chiltern International Ltd., U.K.; post-hoc analysis was performed by AY (Quantics Consulting Limited, U.K.). T.H.J. has served as a consultant to ProStrakan and on advisory boards and as a speaker for ProStrakan, Bayer-Schering Pharma, and Ipsen. K.S.C. has served as a consultant to ProStrakan. S.A. has received lecture fees and served as a consultant to ProStrakan and Bayer, and has received research grants from Bayer. H.M.B. has received honoraria for lectures or has served on the advisory boards of Bayer, Ferring Pharmaceuticals, ProStrakan, and Solvay Pharmaceuticals and has received research grants from Acrux, Bayer, ProStrakan, and Solvay Pharmaceuticals. J.B. has recently been, or is currently a consultant and/or speaker or investigator for Bayer-Schering Pharma, Boehringer Ingelheim, Eli Lilly and Co., Johnson & Johnson, Janssen-Cilag, and ProStrakan. I.M. has served on the advisory boards of Janssen-Cilag, Eli Lilly and Co., Bayer-Schering, and ProStrakan; as a speaker for Janssen-Cilag, Eli Lilly and Co., and Bayer-Schering; and as a clinical investigator for Eli Lilly and Co.; and has received research grants from Pfizer. A.M.M. has served on the advisory board of ProStrakan. A.Y. has served as a retained consultant for ProStrakan. J.D.H. is an employee of ProStrakan. No other potential conflicts of interest relevant to this article were reported.
T.H.J. and K.S.C. conceived and designed the study protocol and produced the initial draft of the manuscript. T.H.J., K.S.C., S.A., H.M.B., J.B., E.M., I.M., A.M.M., M.V., A.Y., and J.D.H. contributed to data analysis and interpretation and to the writing and editing of the manuscript. All authors had access to all data and had final responsibility for the manuscript content; T.H.J. had final responsibility for the decision to submit for publication.
The authors thank the TIMES2 investigators and their staff for the conduct of this study and Polly Winter, Euro RSCG Life Medicom (supported by ProStrakan) for additional writing support and assistance with the collation of author comments.
Footnotes
Clinical trial reg. no. ISRCTN457417, isrctn.org.
References
- 1.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288–1299 10.1001/jama.295.11.1288 [DOI] [PubMed] [Google Scholar]
- 2.Guay A, Jacobson J. The relationship between testosterone levels, the metabolic syndrome (by two criteria), and insulin resistance in a population of men with organic erectile dysfunction. J Sex Med 2007;4:1046–1055 10.1111/j.1743-6109.2007.00530.x [DOI] [PubMed] [Google Scholar]
- 3.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007;30:911–917 10.2337/dc06-1426 [DOI] [PubMed] [Google Scholar]
- 4.Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis 2009;207:318–327 10.1016/j.atherosclerosis.2009.04.016 [DOI] [PubMed] [Google Scholar]
- 5.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004;89:5462–5468 10.1210/jc.2004-0804 [DOI] [PubMed] [Google Scholar]
- 6.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006;154:899–906 10.1530/eje.1.02166 [DOI] [PubMed] [Google Scholar]
- 7.Naharci MI, Pinar M, Bolu E, Olgun A. Effect of testosterone on insulin sensitivity in men with idiopathic hypogonadotropic hypogonadism. Endocr Pract 2007;13:629–635 [DOI] [PubMed] [Google Scholar]
- 8.Pitteloud N, Mootha VK, Dwyer AA, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005;28:1636–1642 10.2337/diacare.28.7.1636 [DOI] [PubMed] [Google Scholar]
- 9.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 2009;30:726–733 10.2164/jandrol.108.007005 [DOI] [PubMed] [Google Scholar]
- 10.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 2007;92:4254–4259 10.1210/jc.2007-0454 [DOI] [PubMed] [Google Scholar]
- 11.Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 2009;27:3452–3458 10.1200/JCO.2008.20.0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448–4456 10.1200/JCO.2006.06.2497 [DOI] [PubMed] [Google Scholar]
- 13.Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol Metab 2010;21:496–503 10.1016/j.tem.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 14.Nieschlag E, Swerdloff R, Behre HM, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, and EAU recommendations. J Androl 2006;27:135–137 10.2164/jandrol.05047 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization, Department of Noncommunicable Disease Surveillance. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part 1: Diagnosis and classification of diabetes. Geneva, Switzerland. World Health Org., 1999 [Google Scholar]
- 16.International Diabetes Federation. (2006) The IDF consensus worldwide definition of the metabolic syndrome. Brussels: International Diabetes Federation. Available from http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf Accessed 25 March 2010
- 17.Richardson TD, Oesterling JE. Age-specific reference ranges for serum prostate-specific antigen. Urol Clin North Am 1997;24:339–351 10.1016/S0094-0143(05)70381-5 [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen A, Kaufman JM. Diagnosis of hypogonadism in the aging male. Aging Male 2002;5:170–176 [PubMed] [Google Scholar]
- 19.Heinemann LAJ, Zimmermann T, Vermeulen A, Thiel C, Hummel W. A new ‘Aging male’s symptoms’ (AMS) rating scale. Aging Male 1999;2:105–114 10.3109/13685539909003173 [DOI] [Google Scholar]
- 20.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822–830 10.1016/S0090-4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 2004;89:2085–2098 10.1210/jc.2003-032006 [DOI] [PubMed] [Google Scholar]
- 22.Rosengren A, Wilhelmsen L, Eriksson E, Risberg B, Wedel H. Lipoprotein (a) and coronary heart disease: a prospective case-control study in a general population sample of middle aged men. BMJ 1990;301:1248–1251 10.1136/bmj.301.6763.1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zmuda JM, Thompson PD, Dickenson R, Bausserman LL. Testosterone decreases lipoprotein(a) in men. Am J Cardiol 1996;77:1244–1247 10.1210/jc.81.7.2633 [DOI] [PubMed] [Google Scholar]
- 24.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010;363:109–122 10.1056/NEJMoa1000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2010;95:639–650 10.1210/jc.2009-1251 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data