Glutamate NMDA receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure (original) (raw)
. Author manuscript; available in PMC: 2012 Apr 15.
Abstract
Background
Despite widely reported clinical and preclinical studies of rapid antidepressant actions of glutamate N-methyl-D-aspartic acid (NMDA) receptor antagonists, there has been very little work examining the effects of these drugs in stress models of depression that require chronic administration of antidepressants, or the molecular mechanisms that could account for the rapid responses.
Methods
We used a rat 21-day chronic unpredictable stress (CUS) model to test the rapid actions of NMDA receptor antagonists on depressant-like behavior, neurochemistry, and spine density and synaptic function of prefrontal cortex (PFC) neurons.
Results
The results demonstrate that acute treatment with the non-competitive NMDA channel blocker ketamine or the selective NR2B antagonist Ro 25-6981 rapidly ameliorates CUS-induced anhedonia and anxiogenic behaviors. We also find that CUS exposure decreases the expression levels of synaptic proteins and spine number and the frequency/amplitude of synaptic currents (EPSCs) in layer V pyramidal neurons in the PFC, and that these deficits are rapidly reversed by ketamine. Blockade of the mammalian target of rapamycin (mTOR) protein synthesis cascade abolishes both the behavioral and biochemical effects of ketamine.
Conclusions
The results indicate that the structural and functional deficits resulting from long-term stress exposure, which could contribute to the pathophysiology of depression, are rapidly reversed by NMDA receptor antagonists in an mTOR-dependent manner.
Keywords: antidepressant, depression, ketamine, rapamycin, spines, synaptogenesis
Major depressive disorder (MDD) is a serious, debilitating and recurring psychiatric disorder that affects up to 17% of the American population (1). Despite a wide range of antidepressants available, only one third of the patients show significant mood improvement in response to an initial antidepressant treatment (2). Moreover, there is a time-lag of weeks to months with currently available medications, further highlighting a major unmet need for novel rapid-acting and more efficacious antidepressant agents. Recent studies with ketamine, a non-competitive glutamate NMDA receptor antagonist, may address this critical need. Ketamine produces rapid antidepressant responses (within hours) in treatment-resistant MDD patients (3–5). However, the widespread use of ketamine is limited by the potential for toxicity and abuse, and studies are being conducted in animal models to elucidate the mechanisms underlying the actions of ketamine and to develop safe, rapid-acting agents.
Preclinical studies have demonstrated antidepressant actions of ketamine in rodent behavioral despair models, including the forced swim test (FST) and learned helplessness (LH) paradigm (6–10). However, FST and LH are responsive to acute or sub-chronic antidepressant treatments, and do not provide a rigorous test of the rapid actions of ketamine (11). In the current study we utilized a chronic unpredictable stress (CUS) paradigm, which results in anhedonia, a core symptom of depression, that is responsive to chronic (3 weeks) but not acute or short-term antidepressant treatment (12). In addition, chronic stress exposure also causes atrophy of neurons in rodent prefrontal cortex and hippocampus (13–20), effects that could contribute to decreased volume of these regions reported in brain imaging studies of MDD patients (14,21–24). Another aim of the current study is to determine if ketamine can rapidly reverse the neuronal atrophy and functional deficits caused by CUS exposure.
We have recently reported that NMDA antagonists rapidly increase the density and function of spine-synapses in the PFC and that these effects, as well as the behavioral responses in the FST and LH, are mediated by activation of the mammalian target of rapamycin (mTOR) (25). The mTOR pathway has been implicated in activity-dependent synaptic plasticity and is localized in neuronal dendrites and spines where it controls the synthesis of proteins that are required for new synapse formation (26). NMDA antagonist-stimulation of mTOR-mediated synaptogenesis provides a mechanism for rapid reversal of stress- and/or depression-mediated deficits (25).
In the present study we report the ability of a single dose of NMDA antagonists to rapidly reverse the behavioral and synaptic deficits caused by long-term CUS exposure in an mTOR-dependent manner. These results highlight mTOR, and upstream signaling pathways as pivotal targets for development of novel rapid-acting and efficacious antidepressant agents.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats weighing 175–250 g were pair-housed and maintained in standard conditions with a 12-h light/dark cycle and ad libitum access to food and water. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committees.
CUS Procedure
Animals were exposed to a variable sequence of mild and unpredictable stressors for 21-day, a procedure which we have found produces depressive-like behavioral changes (27,28). A total of 10 different stressors were used (two stressors per day, see Figure 1A). The stressors included rotation on a shaker, placement in a 4°C ambient, lights off for 3h (10AM to 1PM), lights on overnight, strobe light overnight, aversive odor, 45° tilted cages, food and water deprivation, crowded housing and isolation housing.
Figure 1.
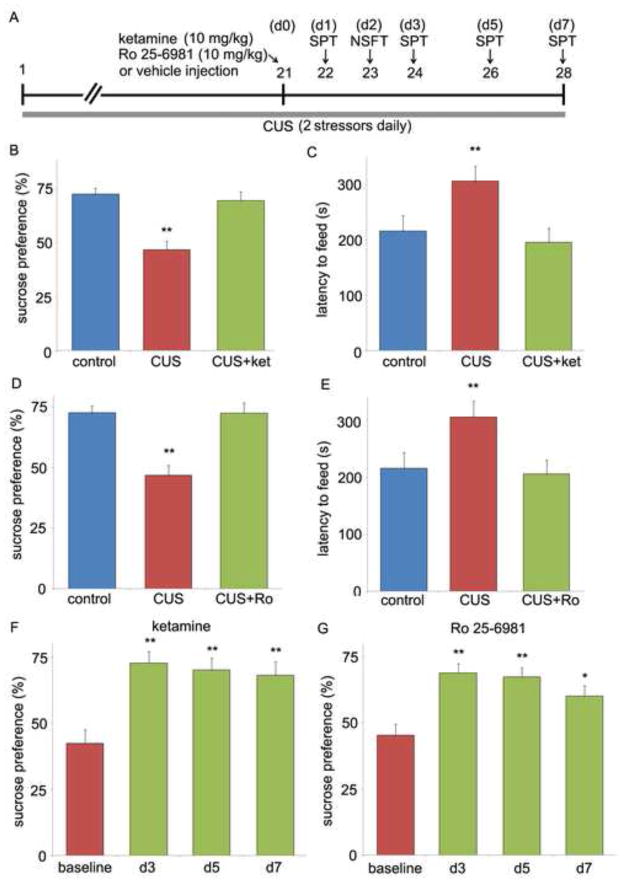
NMDA receptor antagonists produce rapid antidepressant responses in a CUS paradigm. (A) Schematic demonstrating the time line for CUS exposure, drug administration, and behavioral testing. Numbers in parentheses represents days after drug administration. Rats were exposed to CUS and administered ketamine or Ro 25-6981 (both at 10 mg/kg, i.p) on day 21. The SPT was conducted 1 day later (B, D) and NSFT 2 day after drug treatment (C, E). Ketamine and Ro 25-6981 administration in CUS rats reversed the decreased sucrose preference and increased latency to feed to the level of non-stressed control rats. The SPT was also conducted at 3, 5, and 7 days after ketamine or Ro 25-6981 (F,G). Baseline was measured on day 21 before drug injections. Values represent mean ± SEM [n = 6 per group. **P < 0.01, analysis of variance (ANOVA)].
Drug Administration and Surgical Procedure
Animals received a single acute intraperitoneal (i.p.) injection of vehicle, ketamine, or Ro 25-6981 on day 21 of CUS treatment. Based on previous studies (25), the dose used for both drugs was 10 mg/kg. Tissue was collected for molecular assays or animals were tested in behavioral paradigms as described below. For experiments involving central administration of inhibitors, rats were implanted with guide cannulae (22GA) into the lateral ventricles [coordinates from bregma: −0.9 anterior/posterior (AP), −1.5 medial/lateral (ML), −3.3 dorsal/ventral (DV) from dura]. The surgical procedures were carried out under Nembutal anesthesia (i.p. 55mg/kg). Postoperative care consisted of peri-surgical administration of carprofen (5 mg/kg) and topical triple antibiotic ointment. During recovery, animals carried a dummy cannula. After a 7-day recovery period, rapamycin (0.2 nmol in 2 μl), or a vehicle (DMSO) was delivered at the rate of 0.25 μl/min with a injection cannula (26GA) protruding 0.5mm beyond the guide cannula 30 minutes before drug injections. These doses were chosen based on previous reports demonstrating effective and selective inhibition of the respective targets (25,29). The injection cannula stayed in the guide cannula for 1 minute after infusions.
Behavioral Tests
Sucrose Preference Test (SPT)
For the SPT, rats were exposed to a palatable sucrose solution (1%; Sigma, St Louis, MO, USA) for 48 h, followed by 4 h of water deprivation and a 1 h exposure to two identical bottles, one filled with sucrose solution and the other with water. This procedure was adapted from previous studies and has been used previously in our lab (30,31). Sucrose and water consumption were determined by measuring the change in the volume of fluid consumed. Sucrose preference was defined as the ratio of the volume of sucrose versus total volume of sucrose and water consumed during the 1-h test.
Novelty-Suppressed Feeding Test (NSFT)
The NSFT was performed as previously described (31). Before testing rats were food-deprived overnight. Rats were placed in an open field (76.5 cm * 76.5 cm * 40 cm, Plexiglas) with a small amount of food in the center. Animals were allowed to explore the open field for 8 min. The latency to feed, specifically, the time it took for the animal to approach and take the first bite of the food, was recorded by a stopwatch. Home cage food intake was also measured right away after the test as a control.
Immunoblotting
Prefrontal cortex synaptoneurosomes were prepared as previously reported (25) and sonicated in protein lysis buffer. Protein concentration was determined by BCA protein assay. For western blotting, equal amount of proteins (10–20 μg) for each sample were loaded into 10–15% SDS PAGE gel for electrophoresis. Polyvinylidene difluoride (PVDF) membranes with transferred proteins were blocked with 2% BSA in PBST (PBS + 0.1% Tween-20) for 1 h and kept with primary antibodies overnight at 4ºC. The following primary antibodies were used: Synapsin I (from BD Biosciences), PSD95 (from Invitrogen), GluR1(from Abcam). The next day, blots were washed three times in PBST and incubated with horseradish peroxidase conjugated anti-mouse or anti-rabbit secondary antibody (1:5000 to 1:10000) for 1 h. After three final washes with PBST, bands were detected using enhanced chemiluminescence (ECL). The blots were then incubated in stripping buffer for 30 min at 37ºC followed by three washes with PBST. The stripped blots were blocked for 1 h and incubated with primary antibody directed against glyceraldehyde 3-phosphate dehydrogenase (GAPDH, from Advanced Immunochemical) for loading control. Densitometric analysis of immunoreactivity for each protein was conducted using NIH Image J software. Immunoreactivity was normalized to the control group for each protein.
Brain Slice Preparation and Electrophysiological Recordings
Brain slices were prepared as previously described (13,25). Briefly, one day after drug treatments, rats were anesthetized (chloral hydrate, 400 mg/kg, i.p.) and brains removed and placed in ice-cold (4°C) artificial cerebrospinal fluid (ACSF) in which sucrose (252 mM) was substituted for NaCl (sucrose-ACSF). A block of tissue containing prefrontal cortex and coronal slices (400 μm) were cut in sucrose-ACSF with an oscillating-blade tissue slicer. Slices were placed in a submerged recording chamber; bath temperature was then raised slowly to 32 °C. Known concentrations of drugs in ACSF were applied through a stopcock arrangement (~4 ml/min) to reach the slice within 7–10 s. The standard ACSF (pH 7.35), equilibrated with 95% O2/5% CO2, contained 128 mM NaCl, 3 mM KCl, 2 mM CaCl2, 2 mM MgSO4, 24 mM NaHCO3, 1.25 mM NaH2PO4, and 10 mM, d-glucose. There was recovery period of 1–2 hr before recording.
Pyramidal neurons in layer V were visualized by videomicroscopy using a microscope (60x IR lens) with infrared differential interference contrast (IR/DIC). Patch pipettes (3–5 MΩ) were pulled from glass tubing by using a Flaming-Brown Horizontal Puller. The pipette solution contained the following: 115 mM K gluconate, 5 mM KCl, 2 mM MgCl2, 2 mM Mg-ATP, 2 mM Na2ATP, 10 mM Na2-phosphocreatine, 0.4 mM Na2GTP, and 10 mM Hepes, pH 7.33. Neurobiotin (0.3%) was added to the pipette solution to mark cells for later processing and imaging.
Whole-cell recordings were with an Axoclamp-2B amplifier. The output signal was low-pass-filtered at 3 KHz and digitized at 15 kHz; data were acquired by pClamp 9.2/Digidata 1320 software. Series resistance, which was monitored throughout the experiment, was usually between 4 and 8 MΩ. To minimize series resistance errors, cells were discarded if series resistance rose above 10Ω. Postsynaptic currents were studied in the continuous single-electrode voltage-clamp mode (3000 Hz low-pass filter) clamped near resting potential (75 mV ± 5 mV).
Spine Density Analysis
After completion of recording, slices were transferred to 4% paraformaldehyde (0.1 M phosphate buffer) and stored overnight at 4°C. Slices were then processed with streptavidin conjugated to Alexa 594 (1:1000) for visualization of labeled cells. Labeled neurons within layer V of anterior cingulate and prelimbic mPFC were imaged with a dual-photon Ti:sapphire laser scanning system (810 nM; Mai Tai) coupled to direct detection Radiance 2000 BioRad laser scanner mounted on a Olympus BX50WI microscope, using a 60x (0.9 N.A.) water-immersion objective. For spine density analysis, Z-stacks usually consisted of 2–5 scans at high zoom at 1-μm steps in the z axis. Spine density was sampled in two zones: (i) tips of tuft branches as they approach the pial membrane and (ii) proximal tuft dendrites just distal to the bifurcation of the apical shaft; previous studies had shown that, in contrast to basilar dendrites, the distal tuft in layer V pyramidal cells is especially sensitive to chronic stress (13). Results were expressed in terms of spine density/10μ.
RESULTS
NMDA receptor antagonists rapidly reverse the behavioral deficits caused by CUS: Requirement for mTOR signaling
The development of depressive behaviors, notably anhedonia, with CUS exposure and requirement for chronic antidepressant treatment to reverse these effects, make CUS one of the most valid models of depression, although stress and behavioral testing variables also make it one of the most difficult to establish (12). We have successfully established and utilized a CUS model in our laboratory (27,31). In the present study, CUS exposed rats, when compared to unstressed controls, exhibited a reduction in sucrose preference (SPT), an indication of anhedonia behavior (Figure 1B). In addition, CUS exposure increased the latency to feed in a novel environment (NSFT), an indication of increased anxiety levels (Figure 1C). Administration of single dose of ketamine rapidly reversed the CUS-induced behavioral deficits in both the SPT and NSFT (Figure 1B,C). Acute ketamine injection did not affect sucrose preference in non-stressed control rats (Figure S1 in the Supplement). In addition, there was no difference in home cage feeding conducted immediately following the NSFT (data not shown), indicating that the effects of ketamine were not due to general increase in feeding. In contrast to the rapid actions of ketamine, chronic (21 d) administration of typical antidepressants is required to produce similar behavioral actions in these paradigms (11). The SPT was also conducted at additional time points, with continued exposure to CUS, to determine if the actions of ketamine are transient or sustained. The results demonstrate that a single dose of ketamine produces a long-lasting (up to 7 days) increase in sucrose preference relative to CUS exposed animals (Figure 1F), a time frame comparable to that reported in clinical studies (4). The performance of CUS and control animals remained consistent across different time points (Table S1 in the Supplement), indicating that the animals did not habituate to SPT after repeated testing.
We next determined if a selective glutamate NR2B receptor antagonist, Ro 25-6981, produces similar effects in the CUS paradigm. Previous studies have reported that Ro 25-6981 has antidepressant actions in the forced swim and learned helplessness paradigms (7,25), and we have reported that the behavioral and synaptic actions of Ro 25-6981 are dependent on mTOR signaling. In the present study we show that administration of Ro 25-6981 also rapidly reversed the behavior deficits caused by CUS exposure in both SPT and NSFT (Figure 1D,E). In addition, the actions of Ro 25-6981 in the SPT were long-lasting (up to 7 days) (Figure 1G), similar to the actions of ketamine. A previous study has reported that the behavioral actions of Ro 25-6981 in the FST do not persist as long as ketamine (7). In the present study the actions of Ro 25-6981 were long-lasting, although there was some reduction at the 7 day time point.
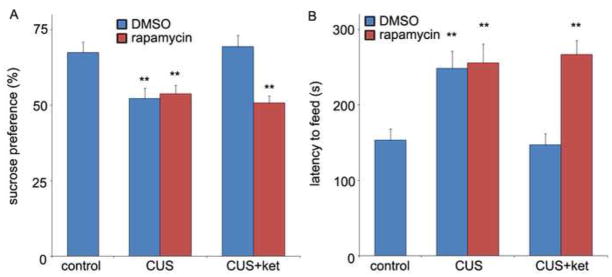
The requirement for activation of mTOR signaling in the actions of ketamine was determined using rapamycin, a selective inhibitor of mTOR. Rapamycin was administered (ICV infusion) 30 min prior to ketamine injection and behavioral testing conducted the following day. Once again, ketamine completely reversed the behavioral deficits in the SPT and NSFT resulting from exposure to CUS (Figure 2A,B), and these effects were completely blocked by rapamycin pretreatment (Figure 2A,B). A single dose of rapamycin infusion alone, in the absence of ketamine and in stressed rats, had no effect in either test (Figure 2A,B).
Figure 2.
Rapid behavioral actions of ketamine in the CUS model require mTOR signaling. On day 21, rapamycin (0.2 nmol, ICV) or DMSO was infused 30 min before ketamine (10 mg/kg, i.p.) or vehicle treatment. The SPT was conducted 1 day (A) and the NSFT 2 days after drug administration. Pre-treatment with rapamycin completely abolished the behavioral actions of ketamine in both SPT (A) and NSFT (B). Values represent mean ± SEM (n = 6 per group; **P < 0.01, ANOVA).
Ketamine rapidly reverses the deficit in synaptic proteins resulting from CUS: Requirement for mTOR signaling
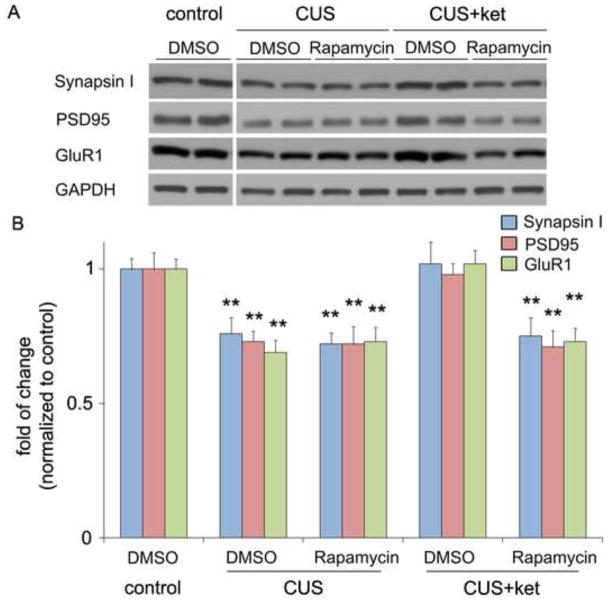
Chronic stress paradigms have been demonstrated to profoundly alter brain structure and function in rodents, causing atrophy of pyramidal neurons in the PFC and the hippocampus (12,13,15-18,20,32). Studies were conducted to determine if our CUS paradigm results in alterations of synapse-associated proteins, as well as the number and function of spine synapses, and if ketamine can reverse these effects. CUS exposure (21 d) decreased levels of several well-characterized synaptic proteins in synaptoneurosome preparations of PFC (Figure 3). This included decreased levels of the presynaptic protein synapsin I and the postsynaptic proteins GluR1 subunit and PSD95. Conversely, a single dose of ketamine administered to animals after 21 d of CUS exposure completely reversed the deficit of these synaptic proteins (Figure 3). Moreover, the ability of ketamine to reverse the deficit in synapsin I, GluR1 and PSD95 was blocked by infusion (ICV) of rapamycin (Figure 3). Further analysis in another cohort shows that 7 days after drug treatments, ketamine still reversed CUS-induced deficits of synaptic protein expression (Figure S2 in the Supplement), consistent with the behavioral actions of ketamine in the SPT (Figure 1).
Figure 3.
CUS exposure decreases synaptic proteins: rapid reversal by ketamine. Rats were exposed to CUS and on day 21 were infused with vehicle or rapamycin (0.2 nmol, ICV) 30 minutes before ketamine (10 mg/kg, i.p.) or vehicle treatment. Levels of synaptic proteins in PFC synaptoneurosome were determined by western blot 2 days later. (A) Representative western blot images of synapsin I, GluR1, and PSD95 are shown, and (B) Results were quantified and are the mean ± SEM, percent of control (n = 6 animals; **P < 0.01, ANOVA). Levels of GAPDH were determined to control for differences in amounts of protein loading. CUS decreased expression of synapsin I, PSD95 and GluR1, and this deficit was completely reversed by a single dose of ketamine. Pre-treatment with rapamycin completely abolished the effects of ketamine, but rapamycin infusion alone did not affect synaptic protein levels.
Ketamine rapidly reverses the deficit in spine density resulting from CUS
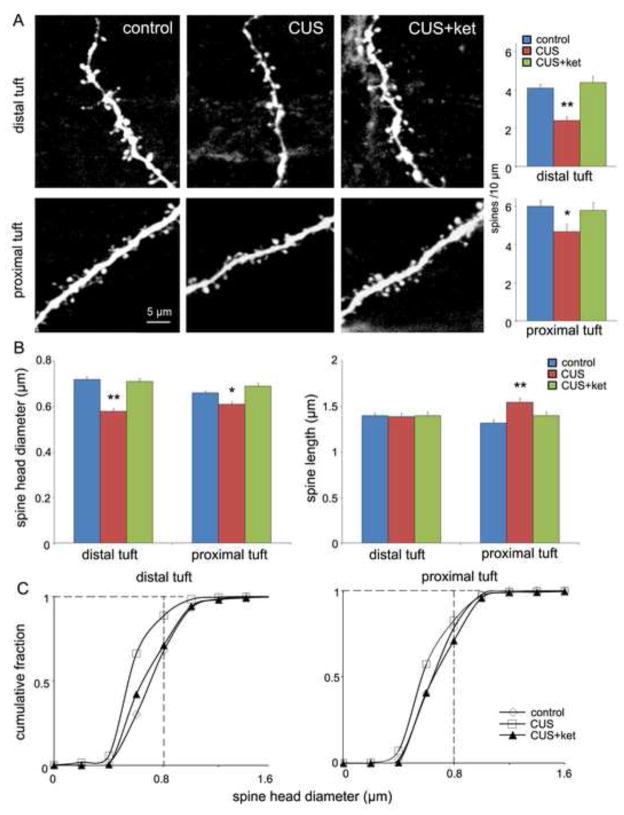
A decrease in synapse-associated proteins indicates that CUS impairs synapse/spine formation and function, and that ketamine reverses this deficit. To directly test this possibility, we examined the influence of CUS on spine number by two-photon laser imaging of the apical tuft of prelabeled layer V mPFC pyramidal neurons. The results demonstrate that CUS exposure significantly decreases spine density in both distal and proximal segments of the apical tuft 24 hrs after the last stressor (Figure 4A). Administration of ketamine to CUS exposed animals completely reversed the spine deficit on both the proximal and distal apical tuft dendrites (Figure 4A). Further analysis of spine morphology revealed that CUS decreased the population of mature mushroom-like (large-diameter, short-length) spines, indicating a loss of mature spines and synaptic connections. All of these deficits in spine density and morphology resulting from 21 d CUS exposure were reversed rapidly and completely by a single dose of ketamine (Figure 4B, C).
Figure 4.
CUS exposure decreases spine density in PFC layer V pyramidal cells: rapid reversal by ketamine. Animals were exposed to CUS for 21 d and then received ketamine injections (10 mg/kg, i.p.). Twenty-four hours later slices of PFC were prepared for whole-cell recordings followed by neurobiotin labeling, and post hoc two-photon microscopy image of the neurobiotin-labeled layer V pyramidal cells. (A) Representative images are shown of high magnification Z-stack projections of distal and proximal segments of the layer V pyramidal cell apical tuft dendrites (Scale: 5 μm). The density of spines was analyzed using Neurolucida Explorer (version 9) and the results are the mean ± SEM (~12 cells from 4 rats in each group; *P < 0.05; **P < 0.01, ANOVA). CUS decreased spine density of both distal and proximal segments of the apical tuft, and this deficit was completely reversed by ketamine treatment (bar graphs to right of images). (B) Quantification of distal and proximal spine head diameter and spine length. (C) Cumulative fraction curves for distal and proximal tuft spine diameter; note large decrease in population of large diameter, mushroom spines (>0.8 μm; vertical dashed line) in the CUS group as compared to other groups in the distal tuft; stress-induced changes are less pronounced in the proximal tuft, consistent with previous studies (13).
Ketamine rapidly reverses the deficit in synaptic functions resulting from CUS
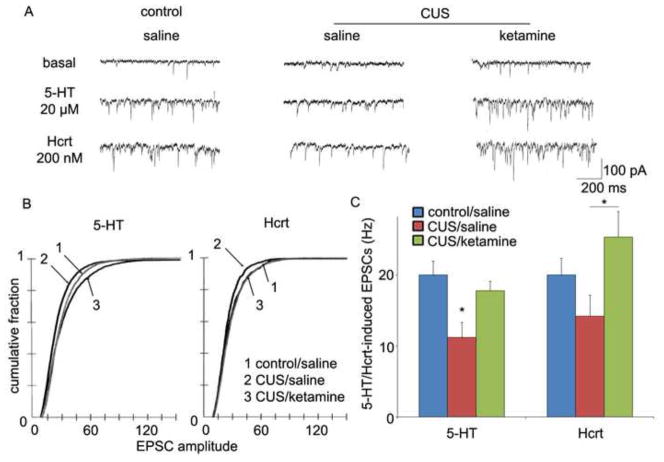
Electrophysiological analysis of the same cells demonstrates that the frequency/amplitude of excitatory postsynaptic currents (EPSCs) induced by 5-HT and/or hypocretin/orexin are decreased by CUS (Figure 5). These functional deficits are consistent with the drastic reduction in large diameter (>0.8 μm) mushroom spines caused by CUS exposure. Once again, a single dose of ketamine completely reversed the deficit of both the 5-HT and hypocretin-induced EPSCs caused by 21 d CUS exposure (Figure 5).
Figure 5.
CUS exposure decreases EPSC responses in PFC layer V pyramidal cells: rapid reversal by ketamine. (A) Sample whole cell voltage-clamp traces of 5-HT and hypocretin-induced EPSCs in slices from non-stressed control, CUS, or CUS + ketamine rats are shown (24 hr post drug treatment). (B) Frequency of 5-HT- and hypocretin-induced EPSCs and (C) Cumulative probability distributions showing that CUS exposure causes decreases in amplitude (P < 0.0001 for 5-HT, KS-z value = 3.89; p < 0.05 for Hcrt, KS-z value = 1.92) (n = 12 neurons/group; *P < 0.05, ANOVA). These deficits were completely reversed by a single dose of ketamine (B, C).
DISCUSSION
Glutamatergic NMDA receptor antagonists, notably ketamine, have emerged as promising candidates for next-generation fast-acting antidepressants (33). The results of the present study demonstrate that a single dose of ketamine completely reverses the behavioral deficits caused by long-term exposure to CUS. Similar effects were observed with a selective NR2B antagonist, Ro 25-6981, which also rapidly reversed the anhedonic and anxiogenic behaviors resulting from CUS. This is consistent with a recent report that administration of a selective NR2B receptor antagonist, CP-101,606, produces rapid antidepressant effects in depressed patients (34). Reversal of the CUS effects by ketamine and Ro 25-6981 are sustained for up to 7 days, similar to the time course for the therapeutic actions of ketamine (3,35,36). Although previous studies have reported antidepressant actions of ketamine in models that are responsive to acute or sub-chronic administration of typical antidepressant agents (7,8,10), the current study provides a rigorous test of the rapid actions of NMDA antagonists in a CUS paradigm that requires chronic (21d) antidepressant treatment (11,37). These findings differ from a recent negative CUS study (38), although the latter measured consumption instead of preference, which is generally considered a better measure of anhedonic behavior.
We recently reported that ketamine increases synaptic protein levels and produces antidepressant behavioral actions in non-stressed naïve animals, and that these effects are blocked by rapamycin, a selective inhibitor of mTOR (25). Similarly, we show here that ketamine-reversal of the behavioral deficits caused by CUS is blocked by rapamycin, indicating that the effects of ketamine are mTOR-dependent alterations in spine/synapse. In support of this hypothesis, we found that CUS exposure decreased levels of synapsin I, GluR1 and PSD95, effects that were rapidly reversed by ketamine in a rapamycin-dependent manner. The requirement for mTOR signaling is consistent with our recent findings that ketamine directly activates several components of the mTOR pathway (25). Another study has reported that rapamycin paradoxically produces an antidepressant response (39), although rapamycin was administered systemically, which could influence mTOR signaling and other kinases in peripheral tissues that could indirectly impact behavior. In addition, the latter paper only used the forced swim and tail suspension, which are typically used as rapid drug screens and have limited validity as measures of depressive behavior.
We examined the possibility that mTOR inhibition underlies the decrease in synaptic proteins caused by CUS, but found no significant differences in levels of phosphorylated/activated mTOR, 4EB-P1, and p70S6 kinase (Figure S3 in the Supplement). It is possible that there is a complex time course for stress regulation of mTOR signaling (e.g., S6 kinase is up-regulated by exposure to acute restraint stress) (40). Alternatively, the decrease in synaptic proteins could be mediated by other mechanisms, including increased degradation or other via other mTOR signaling pathways (e.g., neurotrophic factor stimulated kinases are decreased by exposure to repeated stress or corticosterone) (41–45). Further studies of these and other pathways are currently being conducted to characterize the mechanisms underlying the neuronal atrophy caused by chronic stress exposure.
Decreased levels of synaptic proteins resulting from CUS is consistent with previous studies demonstrating that chronic stress causes dendritic atrophy of neurons in limbic brain regions, including the PFC (14–19). This possibility is confirmed in the present study, which shows that CUS decreases the density of apical spines in layer V pyramidal neurons of the PFC. Notably, CUS caused a marked reduction in the population of large-diameter mushroom spines, which correlates with a significant reduction in the amplitude as well as frequency of both 5-HT- and hypocretin-induced EPSCs. These transmitter induced synaptic currents involve both cortical-cortical (5-HT) and thalamocortical (hypocretin) synaptic inputs (13), indicating that diverse pathways are affected. Administration of ketamine rapidly and completely reversed these deficits in spine density and function, indicating a causal relationship between the morphological and physiological responses. However, it is possible that the 5-HT and hypocretin-induced EPSPs reflect only a functional change in synaptic efficacy and further studies of mini EPSPs, measured under depolarization blockade, may be useful to directly test this causal interaction.
Dendritic atrophy of PFC neurons in response to chronic CUS is consistent with previous reports with other paradigms such as chronic restraint stress (15–19), and provides a cellular mechanism that could explain the decreased PFC volume reported in depressed patients (20-23). Neuronal atrophy and loss of spines/synapses in the PFC could underlie some of the behavioral deficits observed in depressed patients (46), including reduced cognitive functions and loss of inhibitory control of emotions, perhaps mediated by amygdala circuits (47,48).
The ability of ketamine to increase synaptogenesis represents a fundamental, conceptual frame shift in our understanding of synaptic plasticity and the treatment of depression. First, the results demonstrate a basic characteristic of brain plasticity, the ability to rapidly stimulate the formation and function of new spines/synapses. Second, the findings show that the dendritic atrophy resulting from long-term stress exposure is reversible. This also implies that the structural deficits caused by, and implicated in the pathophysiology of mood disorders, including treatment resistant depression, are reversible and that the rapid actions of ketamine are mediated by increased synaptogenesis. Brain imaging studies that directly or indirectly measure synaptogenesis and/or glutamate transmission will be required to directly test this hypothesis in depressed patients.
In summary, the results provide direct evidence that NMDA receptor antagonists rapidly reverse the behavioral, morphological, and physiological deficits resulting from chronic stress via a rapamycin-sensitive, mTOR pathway. These findings, together with earlier studies, provide promising targets for development of next-generation fast-acting antidepressant medications that are safer than ketamine. This possibility is supported by the finding that a NR2B selective antagonist also produces rapid antidepressant actions in the CUS model (present study), as well as other paradigms (7,25), and also increases mTOR-dependent synaptogenesis (25). Studies are currently underway to test additional targets in the glutamate-mTOR signaling pathway that could also be developed as rapid acting antidepressants.
Supplementary Material
01
Acknowledgments
This work is supported by United States Public Health Service Grants MH45481 (RSD) and 2 P01 MH25642 (RSD), the Connecticut Mental Health Center (RSD), Korea Ministry of Science and Technology Brain Research Center 21st Century Frontier Research Program Grant M103KV010008-06K2201-00810 (HS).
Footnotes
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 4.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 5.Liebrenz M, Borgeat A, Leisinger R, Stohler R. Intravenous ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Wkly. 2007;137:234–236. doi: 10.4414/smw.2007.11852. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–344. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]
- 7.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Popik P, Kos T, Sowa-Kucma M, Nowak G. Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacology (Berl) 2008;198:421–430. doi: 10.1007/s00213-008-1158-z. [DOI] [PubMed] [Google Scholar]
- 10.Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 12.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 13.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 15.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 19.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 23.Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 24.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 30.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 31.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- 33.Machado-Vieira R, Salvadore G, Ibrahim LA, Diaz-Granados N, Zarate CA., Jr Targeting glutamatergic signaling for the development of novel therapeutics for mood disorders. Curr Pharm Des. 2009;15:1595–1611. doi: 10.2174/138161209788168010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 35.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 36.Krystal JH. Ketamine and the potential role for rapid-acting antidepressant medications. Swiss Med Wkly. 2007;137:215–216. doi: 10.4414/smw.2007.11932. [DOI] [PubMed] [Google Scholar]
- 37.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Cleary C, Linde JA, Hiscock KM, Hadas I, Belmaker RH, Agam G, et al. Antidepressive-like effects of rapamycin in animal models: Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res Bull. 2008;76:469–473. doi: 10.1016/j.brainresbull.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Yang PC, Yang CH, Huang CC, Hsu KS. Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. J Biol Chem. 2008;283:2631–2643. doi: 10.1074/jbc.M706954200. [DOI] [PubMed] [Google Scholar]
- 41.Gourley SL, Wu FJ, Taylor JR. Corticosterone regulates pERK1/2 map kinase in a chronic depression model. Ann N Y Acad Sci. 2008;1148:509–514. doi: 10.1196/annals.1410.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi X, Lin W, Li J, Li H, Wang W, Wang D, et al. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008;31:278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Qi X, Lin W, Li J, Pan Y, Wang W. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behav Brain Res. 2006;175:233–240. doi: 10.1016/j.bbr.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 45.Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soetanto A, Wilson RS, Talbot K, Un A, Schneider JA, Sobiesk M, et al. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry. 2010;67:448–457. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 48.Duman RS. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues Clin Neurosci. 2009;11:239–255. doi: 10.31887/DCNS.2009.11.3/rsduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01