Stress-relaxation behavior in gels with ionic and covalent crosslinks (original) (raw)
Abstract
Long-chained polymers in alginate hydrogels can form networks by either ionic or covalent crosslinks. This paper shows that the type of crosslinks can markedly affect the stress-relaxation behavior of the gels. In gels with only ionic crosslinks, stress relaxes mainly through breaking and subsequent reforming of the ionic crosslinks, and the time scale of the relaxation is independent of the size of the sample. By contrast, in gels with only covalent crosslinks, stress relaxes mainly through migration of water, and the relaxation slows down as the size of the sample increases. Implications of these observations are discussed.
INTRODUCTION
Hydrogels are materials of choice in diverse applications.1 In many applications, the mechanical behavior of hydrogels plays significant roles. As matrices to grow tissues in vivo, for example, hydrogels are subject to forces from bones, muscles, and blood vessels. In drug screening and apparatus for basic research in biology, hydrogels mimic the physical, chemical, and mechanical behaviors of the physiological environment.2, 3, 4 In food processing, polysacharide-based polymers, including pectins and alginates, are extensively used.5 Hydrogels have been developed in recent years as stimuli-responsive materials for biomimetic devices.6, 7 In all these applications, it is important to understand the mechanical behavior of the hydrogels.
Studies of the mechanical behavior of hydrogels have included swelling, deformation, and fracture, as well as instability of various forms.8, 9, 10, 11, 12, 13 This paper focuses on the stress-relaxation behavior.14 Hydrogels developed to repair articular cartilage, for example, may be more effective if their stress-relaxation behavior matches that of the native tissue, since such behavior affects transfer of loads and transport of nutrients.15, 16 As a second example, to upregulate the release of drugs from a hydrogel, one may apply a constant strain on the hydrogel for a period of time.3, 17 The profile of drug release would be greatly affected by how the stress evolves during this time.8 Finally, the texture and mouth feeling of many foods are strongly affected by the stress relaxation of polysacharide-based hydrogels in them.5
Previous studies on rheological properties of hydrogels focused on the effects of polymer type, polymer molecular weight, chain configuration, crosslink density, water concentration, and temperature.14, 18, 19, 20 However, less focus has been placed on the effect of specific crosslinking mechanism. Many long-chained polymers (e.g., alginate, chitosan) can form networks by either ionic or covalent crosslinks. In this paper, we show that the stress-relaxation behavior of hydrogels is strongly affected by how the polymers are crosslinked. In gels with ionic crosslinks, stress relaxes mainly through breaking and subsequent reforming of the ionic crosslinks. By contrast, in gels with covalent crosslinks, stress relaxes mainly through migration of water.
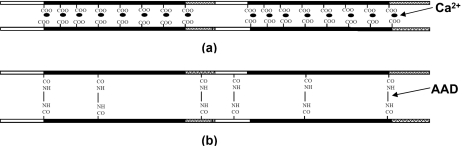
We chose alginate hydrogels as a model material. Alginate is one of the most attractive polymers to form hydrogels for biomedical applications.1 It is a naturally derived linear polysaccharide comprised of the β-D-mannuronic acid (M-unit) and the α-L-guluronic acid (G-unit), arranged in blocks rich in G units, blocks rich in M units, and blocks of alternating G and M units. The polymers of alginate can form a three dimensional network by crosslinks of two types, ionic or covalent (Fig. 1). Ionic crosslinks are formed by the binding of divalent cations (e.g., calcium) between G blocks on different alginate chains.21 Covalent crosslinks are typically formed by the reaction between carboxylic groups in alginate chains and a crosslinking molecule possessing primary diamines.22 We report the stress-relaxation behavior of the alginate hydrogels containing covalent crosslinks and ionic crosslinks. The density of the crosslinks is controlled such that both types of gels have a similar elastic modulus. We show that a gel with ionic crosslinks relaxes stress much more rapidly than a gel with covalent crosslinks. The time scale of the relaxation is unaffected by the size of the sample for the gels with ionic crosslinks, but increases with the size of the sample for the gels with covalent crosslinks.
Figure 1.
Long-chained polymers of sodium alginate can form a network by (a) ionic crosslinks, Ca2+, or by (b) covalent crosslinks, adipic acid dihydrazide (AAD).
EXPERIMENTAL SECTION
Materials. Sodium alginate was purchased from Pronova Biopolymers Inc. (Porths-mouth, NH) and used without further purification. The sodium alginate had an overall guluronic acid (G-block) content of approximately 62% as previously verified with circular dichromism.11 Dulbecco’s phosphate buffered saline (PBS) was purchased from Invitrogen (Carlsbad, CA). Calcium sulfate (CaSO4), adipic acid dihydrazide (AAD), 1-ethyl-3-(dimethylaminopropyl) carbodiimide (EDC), 2-(N-morpholino)ethanesulfonic acid hydrate (MES), 1-hydroxybenzotriazole (HOBt), and ethylenediaminetetraacetic acid were purchased from Sigma-Aldrich (St. Louis, MO). All chemical reagents were of analytical grade and used as received.
Preparation of Hydrogels. To prepare alginate hydrogels with covalent crosslinks, 2% (w∕w) alginates solution in MES buffer (0.1M MES and 0.5M NaCl, pH 6.0) were sequentially mixed with HOBt, EDC, and AAD. The concentration of AAD was 5 mM. Alginate hydrogels with ionic crosslinks were formed by mixing 2% (w∕w) alginate aqueous solution with slurries of CaSO4, to a final concentration of 50 mM Ca2+. The mixtures of alginate and crosslinkers were immediately cast between two glass plates separated by a 4 mm spacer. After 2 h the gels were cut into disks with radii (R) of 5, 6, and 8 mm. The disks were stored at room temperature in PBS for 24 h before testing.
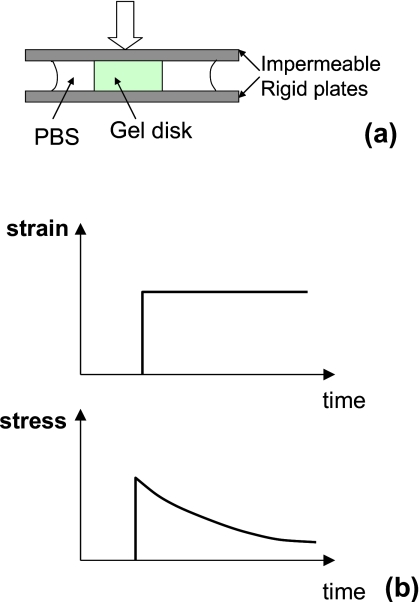
Mechanical Testing. The instantaneous elastic moduli (_E_0) and stress relaxation properties of gels were measured from compression tests of the disks performed with an Instron 3342 from Instron (Norwood, MA). [Fig. 2a] The gel disks were deformed with impermeable plates to a compressive strain of 15% with a deformation rate of 1 mm∕s, in order to approximate an instantaneous deformation. The alginate gel was slippery, so that the disk expanded freely when it was compressed. No bulging of the side faces was observed. Within 15% compression, the stresses versus strain relations of the gels are almost linear, and the slope of the stress versus strain curves gives _E_0. Subsequently, the strain was held constant, while the load was recorded as a function of time [Fig. 2b]. The gels were soaked in PBS during the stress-relaxation tests to prevent dehydration. In calculating the stress, we divided the force by the areas of the disks of the gel in the undeformed state.
Figure 2.
(a) Schematic of a stress-relaxation test. A disk of a gel is immersed in a PBS solvent and placed between two impermeable rigid plates. A strain is suddenly applied to the gel. (b) Subsequently, the strain is held constant, while the stress is recorded as a function of time.
Measurements of Weights. The weights of the disks of the gels were measured before and right after the stress relaxation tests. The disks of the gels were stored in PBS for another 24 h, and the weights of the disks were measured again.
RESULTS
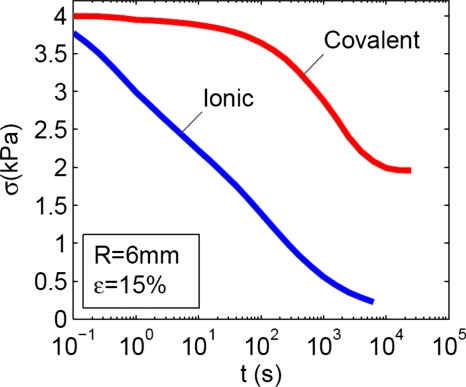
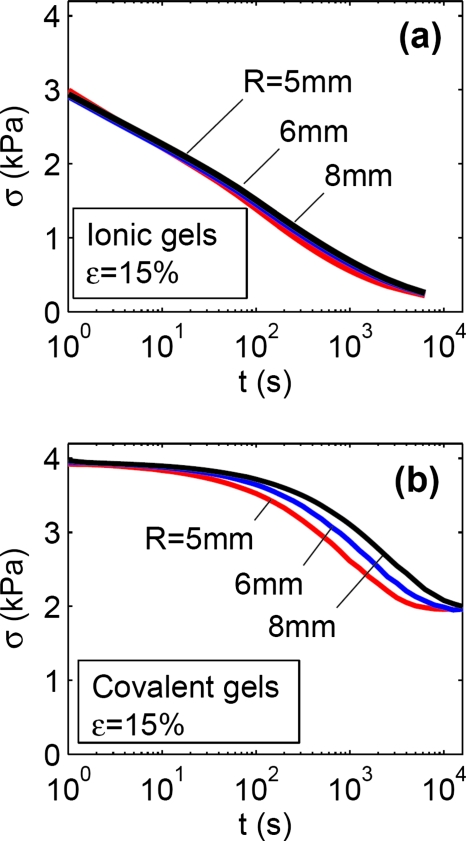
Stress relaxation in gels with ionic and covalent crosslinks. The hydrogels formed from 2% alginate solution crosslinked using either 50 mM of calcium or 5 mM of AAD gave almost the same instantaneous elastic modulus, ∼26 kPa, and swelling ratio, ∼49. The two types of hydrogels, however, exhibited greatly different stress-relaxation behaviors (Fig. 3). For disks of gels of 6 mm radius, it took ∼20 s for the gel with ionic crosslinks to relax half of the stress, but it took ∼3600 s for the gel of covalent crosslinks to relax half of the stress. The stress in the gel with ionic crosslinks kept decaying to approximately zero, while the stress in the gel with covalent crosslinks reaches a plateau at ∼46% of the maximum stress and showed no further decrease.
Figure 3.
The stress-relaxation behavior of a gel with ionic crosslinks, and of a gel with covalent crosslinks. The two gels have a similar elastic modulus, but very different relaxation times.
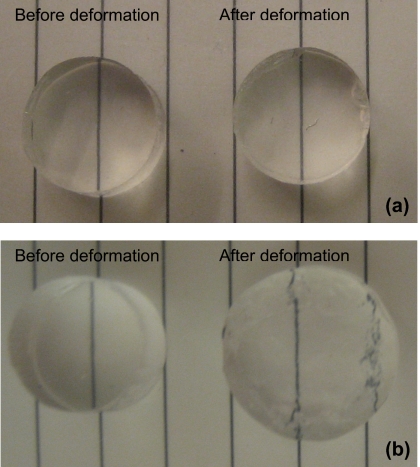
Plastic and elastic deformation. After the stress-relaxation tests, the forces were removed, and the disks of the gels were stored in PBS for another 24 h. The photographs of the disks before and after deformation are compared in Fig. 4. After soaking in PBS, the covalent gel recovered to its original size [Fig. 4a], but the ionic gel exhibited permanent deformation [Fig. 4b].
Figure 4.
Photographs of gels before and after deformation. The gels were subjected to the stress relaxation test. After the test, the load was removed to allow the gels to recover. (a) For a gel with covalent crosslinks, deformation fully recovers after the load is removed. (b) For a gel with ionic crosslinks, part of the deformation is permanent after the load is removed.
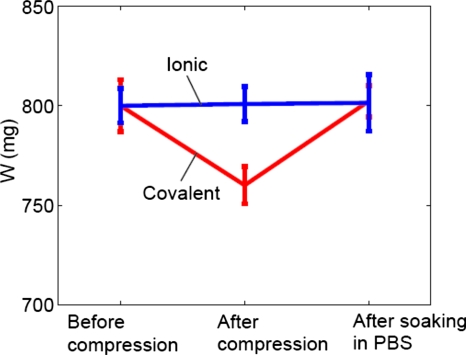
Weight variations of gels. As shown in Fig. 5, the weights of ionic gel disks were kept at a constant level before the stress-relaxation test, right after the test, and after storing the tested disks in PBS for 24 h. On the other hand, stress relaxation tests decreased the weights of covalent gel disks ∼6%. Furthermore, the compressed covalent gel disks recovered their weights after storing in PBS for 24 h (Fig. 5).
Figure 5.
The weights of ionic and covalent gel disks before the stress-relaxation test, right after the test, and after storing in PBS for 24 h.
Effect of sample size on stress relaxation. For the gel of ionic crosslinks, the radius of the disk did not affect the stress-relaxation behavior [Fig. 6a]. For the gel of covalent crosslinks, however, the stress relaxed slower when the radius of the disk was larger [Fig. 6b]. For the covalent gel, the radii of the disks did not affect the level of the stress at either very short times or very long times.
Figure 6.
(a) For gels with ionic crosslinks, the stress-relaxation behavior is independent of the radius of the disk. (b) For gels with covalent crosslinks, the relaxation slows down as the radius of the disk increases. The radius of the disk, however, does not affect the levels of the stress at short and long times.
DISCUSSIONS
The deformation is plastic in the gel with ionic crosslinks, but is elastic in the gel with covalent crosslinks (Fig. 4). Stress relaxation tests decrease the weights of covalent gel disks but do not change the weights of ionic gel disks (Fig. 5). The time scale of the stress relaxation is independent of the size of the gel with ionic crosslinks, but increases with the size of the gel with covalent crosslinks (Fig. 6). These behaviors are consistent with the following mechanisms of deformation: the gel with ionic crosslinks relaxes stress by breaking and reforming ionic crosslinks,23 while the gel with covalent crosslinks relaxes stress by migration of water.24
Mechanism for stress relaxation in gels with covalent crosslinks. To ascertain the mechanism of water migration in the gel with covalent crosslinks, we relate the time of relaxation and the radius of disk. Because the top and the bottom plates compressing the gel are impermeable, water can only migrate out of the disk from the edges. Consequently, the radius of the disk is the only relevant length scale for the migration of water. If the gel relaxes stress primarily by the migration of water, the stress should take the following functional form:25
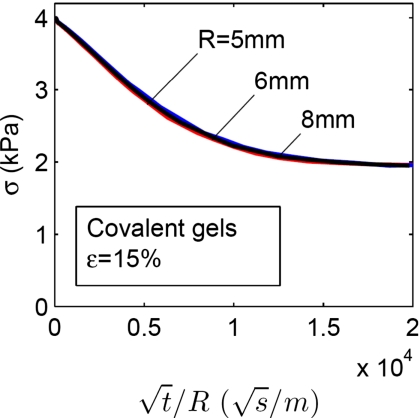
where σ is the stress, t is the time, and R is the radius of the disk. We now replot the data in Fig. 6b to show the effect of the radius of the disk on the stress-relaxation behavior of the gel with covalent crosslinks. When the stress is plotted as a function of t∕R (Fig. 7), the curves for the disks of different radii collapse into a single curve. The relation between the time of relaxation and the radius of disk strongly suggests that the covalent gel relaxes stress by the migration of water. Figure 7 also provides an estimate of the coefficient of diffusion, D. Let D_∼_R_2∕_t and using 3600 s for t and 6 mm for R, we obtain that _D_∼10−8 m2∕s. This order of magnitude is similar to those reported in the literature.26
Figure 7.
The effect of the radius R of the disk on the stress-relaxation behavior of a gel with covalent crosslinks. When the of stress is plotted as a function of t∕R, the curves for the disks of different radii collapse into a single curve.
Mechanism for stress relaxation in gels with ionic crosslinks. In a gel with ionic crosslinks, both mechanisms of breaking∕reforming of ionic crosslinks and migration of water are expected to be concurrent. Our experimental data [Fig. 6a], however, show that the relaxation time is independent of the radii of the disks. This observation is understood as follows. To break and reform an ionic crosslink, calcium ions detach from the anions on the alginate chains, migrate over a distance about the size of the molecular units, and then reattach to the anions on the alginate chains. The time τ needed for this process is independent of the size of the disk of the gel.11 This characteristic time is the one relevant to the stress relaxation if the gel relaxes stress only by the reformation of ionic crosslinks. A combination of τ and D defines a characteristic length:
If this characteristic length is much smaller than the radius of the disk of the ionic gel, Λ⪡R, the ionic crosslinks reform much faster than water migrates out. Consequently, when a large gel disk with only ionic crosslinks is subject to a constant strain, the stress in the gel is relaxed before any appreciable water migration, so the stress-relaxation behavior of the gel is independent of the size of the gel. By contrast, if Λ⪢R, both the migration of water and the reformation of ionic crosslinks are concurrent. That is, over the time needed for the reformation of ionic crosslinks, water redistributes rapidly such that the chemical potential of water everywhere in the gel equalizes the chemical potential of water in the external solvent. An inspection of Fig. 3 gives an estimate τ∼10 s. Similar time scales were reported for creep tests of ionically crosslinked alginate hydrogels.14 The time scale was observed to depend on the calcium ion concentration and the distance between crosslinks.14 Together with the estimate _D_∼10−8 m2∕s, we obtain a value for the characteristic length, Λ∼300 μm. The size of the gel in our tests is ∼6 mm, which is much greater than Λ. Consequently, the reformation of ionic crosslinks is the dominant mechanism of deformation of the ionic gels in our tests. This conclusion is also supported by the observation that the weights of ionic gel disks were unchanged before and after the stress-relaxation tests (Fig. 5).
CONCLUDING REMARKS
When a gel is subject to a constant strain, the stress in the gel relaxes by different mechanisms, depending on the type of crosslinks. For a gel with ionic crosslinks, the stress relaxes as the crosslinks dissociate and reform elsewhere, so that the network undergoes plastic deformation. For a gel with covalent crosslinks, the stress relaxes as water migrates out of the gel, so that the network undergoes elastic deformation. The time scale of the relaxation is unaffected by the size of the sample for the gels with ionic crosslinks, but increases with the size of the sample for the gels with covalent crosslinks. We estimate a size scale, below which migration of water becomes an operative mechanism to relax stress in gels of ionic crosslinks.
ACKNOWLEDGMENTS
X.H.Z. and Z.G.S. thank the National Science Foundation for the financial support through a program on Large Deformation and Instability of Soft Active Materials (0800161). N.H. and D.J.M. acknowledge funding from the NIH∕NIDCR (R37DE013033). X.H.Z. and D.J.M. acknowledge funding from the Materials Research Science and Engineering Center (MRSEC) at Harvard University. X.H.Z. acknowledges the support of the Founder’s Prize, of the American Academy of Mechanics, sponsored by the Robert M. and Mary Haythornthwaite Foundation. N.H. was supported by a National Science Foundation Graduate Research Fellowship. The authors thank Hyun Joon Kong (University of Illinois at Urbana-Champaign) for providing the schematic in Fig. 1.
References
- Lee K. Y. and Mooney D. J., Chem. Rev. (Washington, D.C.) 101, 1869 (2001). 10.1021/cr000108x [DOI] [PubMed] [Google Scholar]
- Kong H. J. and Mooney D. J., Nat. Rev. Drug Discovery 6, 455 (2007). 10.1038/nrd2309 [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Peters M. C., Anderson K. W., and Mooney D. J., Nature (London) 408, 998 (2000). 10.1038/35050141 [DOI] [PubMed] [Google Scholar]
- Kong H. J., Polte T. R., Alsberg E., and Mooney D. J., Proc. Natl. Acad. Sci. U.S.A. 102, 4300 (2005). 10.1073/pnas.0405873102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilnik W. and Rombouts F. M., Carbohydr. Res. 142, 93 (1985). 10.1016/S0008-6215(00)90736-5 [DOI] [PubMed] [Google Scholar]
- Westbrook K. K. and Qi H. J., J. Intell. Mater. Syst. Struct. 19, 597 (2008). 10.1177/1045389X07077856 [DOI] [Google Scholar]
- Calvert P., Adv. Mater. (Weinheim, Ger.) 21, 743 (2009). 10.1002/adma.200800534 [DOI] [Google Scholar]
- Lee K. Y., Rowley J. A., Eiselt P., Moy E. M., Bouhadir K. H., and Mooney D. J., Macromolecules 33, 4291 (2000). 10.1021/ma9921347 [DOI] [Google Scholar]
- Gong J. P., Katsuyama Y., Kurokawa T., and Osada Y., Adv. Mater. (Weinheim, Ger.) 15, 1155 (2003). 10.1002/adma.200304907 [DOI] [Google Scholar]
- Baumberger T., Caroli C., and Martina D., Nature Mater. 5, 552 (2006). 10.1038/nmat1666 [DOI] [PubMed] [Google Scholar]
- Trujillo V., Kim J., and Hayward R. C., Soft Matter 4, 564 (2008). 10.1039/b713263h [DOI] [PubMed] [Google Scholar]
- Zhang Y., Matsumoto E. A., Peter A., Lin P. C., Kamien R. D., and Yang S., Nano Lett. 8, 1192 (2008). 10.1021/nl0801531 [DOI] [PubMed] [Google Scholar]
- Kundu S. and Crosby A. J., Soft Matter 5, 3963 (2009). 10.1039/b909237d [DOI] [Google Scholar]
- Mitchell J. R., J. Texture Stud. 11, 315 (1980). 10.1111/j.1745-4603.1980.tb01312.x [DOI] [Google Scholar]
- Mow V. C., Holmes M. H., and Lai W. M., J. Biomech. 17, 377 (1984). 10.1016/0021-9290(84)90031-9 [DOI] [PubMed] [Google Scholar]
- Marijnissen W., van Osch G., Aigner J., van der Veen S. W., Hollander A. P., Verwoerd-Verhoef H. L., and Verhaar J. A. N., Biomaterials 23, 1511 (2002). 10.1016/S0142-9612(01)00281-2 [DOI] [PubMed] [Google Scholar]
- Kim B. S., Nikolovski J., Bonadio J., and Mooney D. J., Nat. Biotechnol. 17, 979 (1999). 10.1038/13671 [DOI] [PubMed] [Google Scholar]
- Mancini M., Moresi M., and Rancini R., J. Food Eng. 39, 369 (1999). 10.1016/S0260-8774(99)00022-9 [DOI] [Google Scholar]
- Kapnistos M., Lang M., Vlassopoulos D., Pyckhout-Hintzen W., Richter D., Cho D., Chang T., and Rubinstein M., Nature Mater. 7, 997 (2008). 10.1038/nmat2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Tung M. A., and Zeng Y., J. Food Eng. 38, 279 (1998). 10.1016/S0260-8774(98)00114-9 [DOI] [Google Scholar]
- Grant G. T., Morris E. R., Rees D. A., Smith P. J. C., and Thom D., FEBS Lett. 32, 195 (1973). 10.1016/0014-5793(73)80770-7 [DOI] [Google Scholar]
- Kong H. J., Wong E., and Mooney D. J., Macromolecules 36, 4582 (2003). 10.1021/ma034137w [DOI] [Google Scholar]
- Pines E. and Prins W., Macromolecules 6, 888 (1973). 10.1021/ma60036a020 [DOI] [Google Scholar]
- Peleg M. and Pollak N., J. Texture Stud. 13, 1 (1982). 10.1111/j.1745-4603.1982.tb00873.x [DOI] [Google Scholar]
- Hong W., Zhao X., Zhou J., and Suo Z., J. Mech. Phys. Solids 56, 1779 (2008). 10.1016/j.jmps.2007.11.010 [DOI] [Google Scholar]
- Oyaas J., Storro I., Svendsen H., and Levine D. W., Biotechnol. Bioeng. 47, 492 (1995). 10.1002/bit.260470411 [DOI] [PubMed] [Google Scholar]