Telomerase RNA Accumulates in Cajal Bodies in Human Cancer Cells (original) (raw)
Abstract
Telomerase synthesizes telomeric DNA repeats at the ends of eukaryotic chromosomes. The RNA component of the enzyme (hTR) provides the template for telomere synthesis, which is catalyzed by telomerase reverse transcriptase (hTERT). Little is known regarding the subcellular localization of hTR and hTERT and the pathway by which telomerase is assembled. Here we report the first glimpse of the detailed subcellular localization of endogenous hTR in human cells, which we obtained by fluorescence in situ hybridization (FISH). Our studies have revealed a distinctive hTR localization pattern in cancer cells. We have found that hTR accumulates within intranuclear foci called Cajal bodies in all typical tumor-derived cell lines examined (in which telomerase is active), but not in primary or ALT cells (where little or no hTERT is present). Accumulation of hTR in the Cajal bodies of primary cells is induced when hTERT is ectopically expressed. Moreover, we report that hTERT is also found in Cajal bodies. Our data suggest that Cajal bodies are involved in the assembly and/or function of human telomerase.
INTRODUCTION
Telomeres are nucleoprotein structures that cap the ends of linear eukaryotic chromosomes. In all vertebrates, telomeres consist of tandem TTAGGG DNA repeats and associated proteins (Moyzis et al., 1988; Meyne et al., 1989; de Lange, 2002). The function of telomeres is to protect chromosomes from degradation, recombination, and end-to-end fusion (McEachern et al., 2000; Bertuch, 2002; Harrington and Robinson, 2002). Maintenance of telomere length is critical for genomic stability. Telomeres are synthesized by telomerase, a unique DNA polymerase that minimally consists of telomerase RNA and reverse transcriptase subunits (hTR and hTERT respectively in humans; Feng et al., 1995; Meyerson et al., 1997; Nakamura et al., 1997; Nakamura and Cech, 1998). Telomerase uses a short sequence within its integral RNA as a template for the synthesis of telomeric DNA by the hTERT subunit.
In humans, activation of telomerase appears to be an essential, limiting step in cellular immortalization and tumor progression. Although telomerase activity is not detected in most adult somatic tissues, telomerase activity is present in greater than 90% of >2000 malignant tumors examined (Shay and Bacchetti, 1997). hTERT is normally the limiting determinant of telomerase activity. hTR is present in both normal and cancer cells (Feng et al., 1995; Avilion et al., 1996; Yi et al., 1999). In contrast, hTERT is undetectable in most normal adult cells and tissues, but is readily detected in most cancer cells and primary tumors (Meyerson et al., 1997; Nakamura et al., 1997; Kolquist et al., 1998). Recently, trace levels of hTERT and telomerase activity have been detected in cultured human fibroblasts; however, the amount of telomerase present is insufficient to prevent steady erosion of telomere length during cell division (Masutomi et al., 2003). Most cancer cells maintain telomeres, and the ability to divide indefinitely, by upregulating hTERT expression and telomerase activity (Kim et al., 1994; Shay and Bacchetti, 1997; Collins and Mitchell, 2002; Cong et al., 2002; Masutomi and Hahn, 2003). Moreover, ectopic expression of hTERT in a number of primary cells reinstates telomerase activity and immortalizes the cells (Bodnar et al., 1998; Nakayama et al., 1998; Vaziri and Benchimol, 1998; Hahn et al., 1999; Morales et al., 1999).
Despite the importance of telomerase to chromosome maintenance, cellular immortalization, and oncogenesis, little is known about the essential steps in the generation of the enzyme from individual RNA and protein components including how or where within the cell hTR and hTERT assemble to form telomerase. The intracellular localization of YFP- or GFP-hTERT fusion proteins in human cell lines has recently been reported. These studies indicate that hTERT is found throughout the nucleoplasm and is concentrated in nucleoli (Etheridge et al., 2002; Wong et al., 2002; Yang et al., 2002). Interestingly, exogenous hTERT undergoes changes in distribution between nucleoli and the nucleoplasm in response to cell cycle phase, expression of viral oncogenes (cellular transformation), and double-stranded DNA breaks (Wong et al., 2002).
The existing information about the trafficking and subcellular localization of vertebrate telomerase RNA within the nucleus is primarily from microinjection studies in Xenopus oocytes that addressed the early trafficking patterns of telomerase RNA. This work indicates that telomerase RNA localizes to Cajal bodies and nucleoli at various times (Narayanan et al., 1999; Lukowiak et al., 2001). The nucleolus is well known as the site of assembly of ribosomes (Olson et al., 2002). Cajal bodies are dynamic, spherical structures present in plant and animal cell nuclei (Matera, 1999; Gall, 2000; Carmo-Fonseca, 2002; Ogg and Lamond, 2002) that have recently been implicated as sites of posttranscriptional RNA modification (Darzacq et al., 2002; Jady and Kiss, 2001; Verheggen et al., 2002; Verheggen et al., 2001; Narayanan et al., 1999) and assembly of various RNPs (Terns and Terns, 2001). The microinjection studies revealed that the box H/ACA motif found in hTR and all other vertebrate telomerase RNAs (Mitchell et al., 1999a; Chen et al., 2000) plays a role in the subcellular localization of the RNA. The H/ACA motif is characteristic of the H/ACA RNAs, small nucleolar (sno)RNAs and small Cajal body (sca)RNAs that guide nucleotide modification (pseudouridylation) of pre-rRNA within the nucleolus, or of snRNAs in the Cajal body, respectively (Kiss, 2002; Terns and Terns, 2002). Like snoRNAs, telomerase RNA is not exported to the cytoplasm but is retained within the nucleus (Lukowiak et al., 2001). The H/ACA domain of hTR has been shown to be responsible for retention of the RNA within the nucleus (the cellular compartment where telomere synthesis occurs) and localization to the nucleolus (Lukowiak et al., 2001). In addition to its roles in localization, the H/ACA domain mediates the binding of four H/ACA snoRNA-binding proteins to hTR (dyskerin, GAR1, NHP2, and NOP10; Mitchell et al., 1999b; Dragon et al., 2000; Pogacic et al., 2000; Dez et al., 2001) and is important for the metabolic stability of the RNA (Mitchell et al., 1999a; Lukowiak et al., 2001; Fu and Collins, 2003).
The detailed subcellular localization of endogenous hTR in human cells has not been reported. The presence of hTR in a variety of cancerous, precancerous, and normal cells has been demonstrated by low-resolution in situ hybridization primarily of histological tissue sections (reviewed in Dhaene et al., 2000). These studies indicate that hTR is primarily or exclusively located in the nucleus. Subcellular fractionation of HeLa cells has also shown that the majority of hTR is in the nucleus and that a small percentage (∼7%) is found in a biochemical fraction enriched in nucleoli (Mitchell et al., 1999a).
In this work, we have developed a specific and sensitive fluorescence in situ hybridization (FISH) assay and have performed the first detailed analysis of the subcellular localization of hTR in normal and cancer human cell lines. We have found that hTR accumulates in Cajal bodies in telomerase-positive cancer cell lines but not in primary cell lines, which lack hTERT and telomerase activity, or in U2OS cells, an atypical cancer line that expresses hTR but not hTERT and maintains telomeres by an alternative mechanism (i.e., an ALT [alternative lengthening of telomeres] cell line; Henson et al., 2002). The accumulation and detection of hTR in nuclear foci including Cajal bodies appears to be dependent on hTERT expression and was detected in hTERT-transfected fibroblasts but not untransfected fibroblasts. We did not detect accumulation of hTR at PML bodies, gems, or telomeres. Finally, we have found that YFP-tagged hTERT also specifically localizes to Cajal bodies. These findings implicate the Cajal body as a site of telomerase biogenesis and/or function.
MATERIALS AND METHODS
Cell Lines
Table 1 shows the cell lines and media used in these experiments. All cells were cultured at 37°C under 5% CO2.
Table 1.
Human cell lines used in this study
| Cell line | Description | Medium |
|---|---|---|
| HeLa (S3, PV, KN) | Cervix adenocarcinoma | Dulbecco's modified Eagle's medium (Sigma) |
| MCF7 | Mammary gland adenocarcinoma | Minimum essential medium Eagle's with 0.01 mg/ml bovine insulin (ATCC) |
| H1299 | Non-small cell lung cancer (ATCC No. CRL-5803) | RPMI 1640 (ATCC) |
| A549 | Lung carcinoma | Ham's F12K medium (ATCC) |
| PC-3 | Prostate adenocarcinoma | Ham's F12K medium (ATCC) |
| DU145 | Prostate carcinoma | Minimum essential medium Eagle's (ATCC) |
| JEG-3 | Choriocarcinoma | Minimum essential medium Eagle's (ATCC) |
| BJ | Normal foreskin fibroblast (ATCC No. CRL-2522) | Minimum essential medium Eagle's (ATCC) |
| BJ-hTERT | BJ with hTERT stable transfection and SV40 transformation | α-modification of Eagle's medium (Mediatech) |
| 2yo | Normal smooth muscle cell line | Endothelial cell basal medium-2 with supplements and growth factors (Clonetics) |
| 2yo-hTERT | 2yo with hTERT stable transfection and SV40 transformation | Endothelial cell basal medium-2 with supplements and growth factors (Clonetics) |
| IMR-90 | Normal lung fibroblast (ATCC No. CCL-186) | Minimum essential medium Eagle's (ATCC) |
| VA13 | SV40 transformed lung fibroblast cell line WI38 subline 2RA (ATCC No. CCL-75.1) | Dulbecco's modified Eagle's medium (Sigma) |
| U2OS | Osteosarcoma | Dulbecco's modified Eagle's medium (Sigma) |
hTR FISH
All probes were aminoallyl-T-modified deoxyoligonucleotides synthesized by OPERON (Operon/Qiagen, Valencia, CA). The T* in the probe sequences indicates aminoallyl-thymine. Antisense hTR probe 1 (5′-T * G C G C G C G G G G A G C A A A A G C A C G G C G C C T * A C G C C C T T C T C A G T T * A G G G T T A G A C A-3′) is complementary to hTR nt 43–96 with an additional T at the 5′ end. Antisense hTR probe 2 (5′-GC T * G A C A T T T T T * T G T T T G C T C T * A G A A T G A A C G G T * G G A A G G C G G C A G G C C G A G G C T * T-3′) is complementary to hTR nt 128–183. hTR sense probe (5′-G A G T * C A A T C C C A A T * C T G T T T T T T * A C C G G T G GTGGGGAGGGT*CCGGGTGGGAGGCGT*TG)is the same as nt 3–60 of hTR. The aminoallyl-modified dTs in the probes were chemically conjugated with Cy3 fluorophore (Cy3 monofunctional reactive dye; Amersham Pharmacia, Piscataway, NJ). FISH was performed essentially as described by Robert Singer (http://www.singerlab.org/protocols). Briefly, cells were grown on coverslips for 18–24 h, rinsed once with 1× PBS, and fixed with 4% formaldehyde (37% liquid stock from Electron Microscope Sciences, Fort Washington, PA), 10% acetic acid, 1× PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4) for 10 min at room temperature. After two washes with PBS, cells were permeablized by treatment with 70% ethanol overnight at 4°C. Cells were rehydrated for 5 min at room temperature in 2× SSC and 50% formamide and prehybridized with a solution of 10% dextran sulfate (Sigma, St. Louis, MO), 2 mM vanadyl ribonucleotide complex (GIBCO BRL, Gaithersburg, MD), 0.02% RNase-free BSA (Sigma), 40 μg Escherichia coli tRNA (Roche, Nutley, NJ), 2× SSC, and 50% formamide for 1 h at 37°C. Cells were then hybridized for 4 h at 37°C in 40 μl of the prehybridization solution described above containing a total of 80 ng of fluorescently labeled DNA probe. After hybridization, cells were washed twice with 2× SSC, and 50% formamide for 30 min at 37°C. Slides were mounted in 90% glycerol, 1× PBS, 1 mg/ml _p_-phenylediamine, and 0.1 μg/ml DAPI.
RNase Treatment
After permeabilization in 70% ethanol overnight, cells were rehydrated in 1× PBS containing 1.5 mM MgCl2 at room temperature for 5 min. The cells were incubated with RNase A (0.2 mg/ml) at 37°C for 2 h. hTR FISH was performed after the cells were washed two times with 2× SSC, 50% formamide for 10 min at 37°C.
Indirect Immunofluorescence
Following hTR FISH, cells were washed again with PBS at room temperature twice for 10 min per wash. Cells were incubated with one or two of the following antibodies at the indicated dilution for 1 h at room temperature: R288, rabbit anti-p80 coilin (1:1000; Andrade et al., 1991) or mouse anti-p80 coilin (1:100; Almeida et al., 1998); 5E10, mouse anti-PML (1:10; Stuurman et al., 1992); mouse anti-SMN (1:1000; BD); rabbit anti-TRF2 (1:100; Karlseder et al., 1999); rabbit anti-TRF1 (1:100; Smith and de Lange, 1999). Cells were washed with PBS three times for 10 min at room temperature followed by incubation with 1:100 Cy2-conjugated goat anti-rabbit or anti-mouse IgG (Jackson ImmnoResearch, West Grove, PA), 1:50 Alexa Fluor 350–conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR), or 1:100 Texas Red–conjugated goat anti-mouse IgG (Jackson ImmnoResearch) for 1 h at room temperature. All antibodies were diluted in PBST (0.05% Tween 20 in PBS). After three 10-min washes in PBS, slides were mounted as described above.
Transfection and Visualization of YFP-hTERT
HeLa cells were grown on coverslips for 18 h and then transiently transfected with 1 μg of either plasmid pYFP-hTERT (Etheridge et al., 2002) or control plasmid pEGFP-N1 (Clontech, Palo Alto, CA) using LipofectAMINE 2000 (Invitrogen) reagent according to the manufacturer's instruction. Twenty-four hours after transfection, cells were extracted in 0.5% Triton X-100 in CSK (10 mM PIPES, pH 6.8, 300 mM sucrose, 100 mM NaCl, 1 mM EGTA, 3 mM MgCl2) for 1 min at 4°C. Coverslips were washed three times in 1× PBS. Cells were then fixed in 4% formaldehyde in PBS for 10 min at room temperature and washed twice with 1× PBS, and indirect immunofluorescence with anti-p80 coilin antibodies was performed as described above.
Microscopy
Analysis was performed on a Zeiss Axiovert S100 inverted fluorescence microscope (Thornwood, NY). All images were acquired at 63× magnification using a cooled charge-coupled device camera (Quantix-Photometrics, Roper Scientific, Duluth, GA) and IP Lab Spectrum software (Scanalytics, Fairfax, VA).
RESULTS
Detection of the Subcellular Localization of Human Telomerase RNA
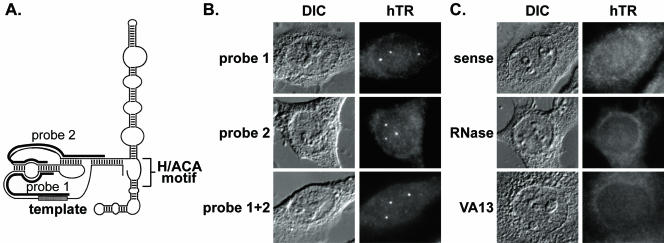
We have established a FISH technique to determine the subcellular localization of hTR in human cells. We designed two probes complementary to different regions in the 5′ domain of hTR (Figure 1A and see MATERIALS AND METHODS). On hybridization with either probe, 1–5 bright foci were observed in the nuclei of HeLa cells. Maximal hTR signal was observed when both probes were used in combination (Figure 1B). A series of controls indicate further that the FISH signals observed in our experiments are specific to telomerase RNA. No foci were apparent in HeLa cells upon hybridization with a sense probe or after treatment of HeLa cells with RNase A, showing that telomerase RNA, rather than the telomerase RNA gene, is being detected (Figure 1C). Furthermore, signals were not observed in VA13 cells, an unusual ALT cell line that lacks hTR transcripts (as well as hTERT; Bryan et al., 1997b; Guiducci et al., 2001; Figure 1C).
Figure 1.
Human telomerase RNA is present in intranuclear foci. (A) The predicted secondary structure of hTR is shown (adapted from Chen et al., 2000). Black bars denote regions of complementarity for each hTR probe. Probe 1 is complementary to hTR nts 43–96; probe 2 is complementary to nts 128–183. (B) Fluorescence in situ hybridization (FISH) of HeLa cells was performed with probe 1 (top), probe 2 (middle), or both (bottom). The combination of probes 1 and 2 is used in all subsequent figures except where noted. Fluorescence (hTR) and differential interference contrast (DIC) microscopy images are shown in this and subsequent figures. (C) Top panel shows FISH of HeLa cells performed with a sense probe. hTR FISH signal is lost upon treatment of HeLa cells with RNase A before FISH (middle). There is no detectable accumulation of hTR in VA13 cells, which do not express hTR (bottom).
In the course of these studies, we found that hTR probes spanning nucleotides 37–44 gave false-positive FISH signals (i.e., occurring in hTR-negative VA13 cells). As previously noted (Lukowiak et al., 2001), nts 37–44 of hTR (AUUUUUUUG) match a consensus Sm protein binding site found in ubiquitously expressed and relatively abundant spliceosomal snRNAs. The false-positive signals observed with probes that included this region colocalized with Sm proteins, indicating that those probes recognized snRNAs. These results suggest caution with the use of probes against nts 37–44 of hTR and reevaluation of previous in situ experiments employing full-length hTR probes (reviewed in Dhaene et al., 2000).
hTR Accumulates in Intranuclear Foci in Telomerase-positive Cancer Cells, But Not Telomerase-negative Primary Cells
Telomerase activity is readily detected in the majority of human cancer cells, but not in the majority of normal cells. Although hTERT is selectively expressed in most cancer cells, hTR is expressed in both normal and cancer cells (Feng et al., 1995; Avilion et al., 1996; Yi et al., 1999). With this in mind, we investigated whether the localization of hTR differed between cancer and normal cell lines.
First, to determine the generality of the pattern of hTR localization observed in HeLa (cervical adenocarcinoma) cells (Figure 1), we performed hTR FISH on MCF7 (breast carcinoma), H1299 (nonsmall cell lung carcinoma), A549 (lung carcinoma), PC-3 (prostate adenocarcinoma), JEG-3 (choriocarcinoma), and DU145 (prostate carcinoma) cancer cells (Figure 2A and unpublished data). As with HeLa cells, 1–5 hTR-containing foci were present in the nuclei of all the cancer cells examined.
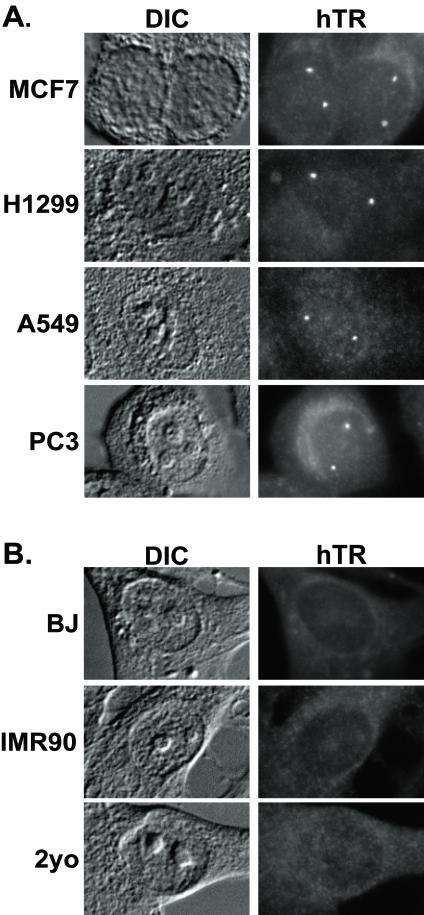
Figure 2.
hTR accumulates in intranuclear foci in telomerase-positive cancer cells, but not telomerase-negative primary cells. (A) FISH analysis of hTR localization in the following telomerase-positive cancer cells is shown: MCF7 (breast carcinoma), H1299 (non-small cell lung carcinoma), A549 (lung carcinoma), and PC3 (prostate carcinoma). (B) FISH analysis of hTR localization in the following telomerase-negative primary cells is shown: BJ and IMR90 fibroblasts and 2yo smooth muscle cells.
To test whether similar hTR foci are also found in normal cell lines, hTR FISH was performed on primary somatic cell lines. Interestingly, no accumulation of hTR at nuclear foci was observed in the normal primary fibroblast cell lines BJ and IMR-90 or the smooth muscle cell line 2yo (Figure 2B). hTR levels are generally lower in normal cells than cancer cells (Yi et al., 1999, 2001); however, the difference in localization pattern is not likely due to a simple difference in hTR levels. The amount of hTR present in particular primary cells that we examined (e.g., BJ and IMR90 fibroblasts) is statistically equivalent to the amount measured for DU145 cancer cells (Yi et al., 2001), where localization to foci is observed (unpublished data and see Figure 4). Thus, the total amount of hTR present in normal cells is not below the level that can be detected by our FISH procedure when localized as in cancer cells. The above data indicate that the distribution of hTR differs in normal and cancer cells; hTR accumulates within intranuclear foci in cancer cells (where telomerase assembly is presumably ongoing) but not in normal cell lines (where little or no telomerase is made).
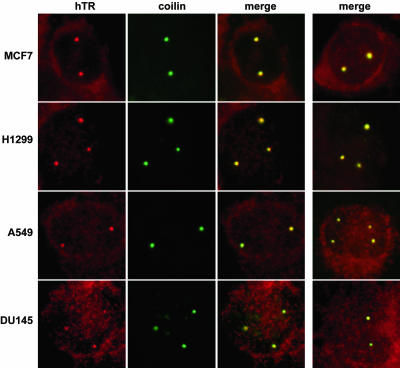
Figure 4.
hTR is found in Cajal bodies in all telomerase-positive cancer cell lines examined. hTR FISH (red) was combined with IF with anticoilin antibodies (green) in MCF7, H1299, A549, and DU145 cells. Merged data from an additional cell is shown in the last column.
hTR Accumulates in Cajal Bodies in Cancer Cells
We next sought to determine the identity of the spherical nuclear foci that accumulated hTR in the tumor-derived cells. These foci are similar in size and shape to several nuclear bodies, including PML (promyelocytic leukemia) bodies and Cajal bodies. We performed double-labeling experiments to simultaneously evaluate the localization of hTR (via FISH) and these nuclear bodies (via immunofluorescence using antibodies against specific marker proteins).
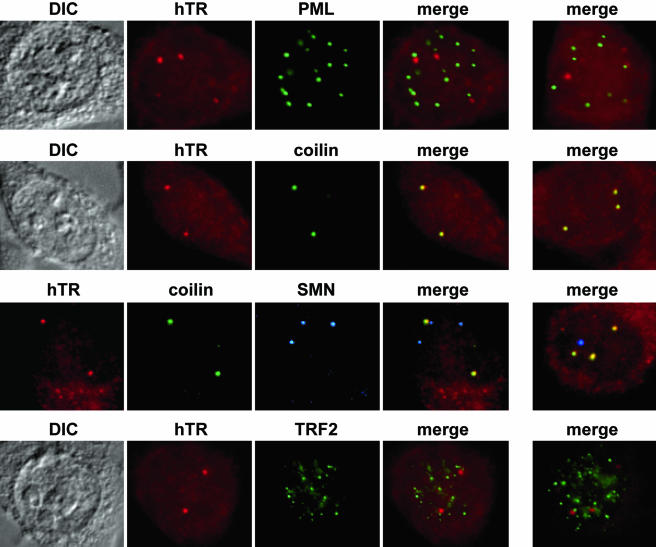
The PML (promyelocytic leukemia) protein is present in PML bodies and has been implicated in the processes of cell proliferation, apoptosis, and senescence (reviewed in Maul et al., 2000; Zhong et al., 2000; Borden, 2002; Salomoni and Pandolfi, 2002). Furthermore, specialized PML bodies, APBs (ALT-associated PML bodies), are found in telomerase-negative ALT cells (Yeager et al., 1999; Henson et al., 2002). However, as can be observed in Figure 3 (top panel), no colocalization of hTR and the PML protein was observed in HeLa cells, indicating that hTR does not accumulate in PML bodies.
Figure 3.
hTR localizes to Cajal bodies. hTR FISH (red) was combined with indirect immunofluorescence (IF) using antibodies against the marker proteins for PML bodies (first row, PML, green) or Cajal bodies (second row, coilin, green) in HeLa cells. Merge panels show merged FISH and IF data; yellow indicates overlap of red and green signals. Merged data from an additional cell is shown in the last column. In the third row, hTR FISH (red) was combined with IF with both anticoilin (green) and anti-SMN antibodies (blue) to visualize Cajal bodies and gems in HeLa-PV cells. Second merge shows the analysis of HeLa-KN cells. In the fourth row, hTR FISH (red) was combined with IF with anti-TRF2 antibodies (green) to visualize telomeres in HeLa cells.
Previously, we had shown that telomerase RNA associates with Cajal bodies in Xenopus oocytes (Lukowiak et al., 2001). Cajal bodies are thought to serve as centers of biogenesis for several nuclear RNPs (reviewed in Gall, 2000; Terns and Terns, 2001; Carmo-Fonseca, 2002; Ogg and Lamond, 2002). It is currently hypothesized that the SMN complex (SMN and six other proteins of the gemin family) mediates the assembly of RNPs including spliceosomes, snoRNPs, and transcriptosomes within Cajal bodies (Liu and Dreyfuss, 1996; Terns and Terns, 2001; Meister et al., 2002; Paushkin et al., 2002). SMN is the protein implicated in the fatal neuromuscular disease, spinal muscular atrophy (SMA; Lefebvre et al., 1995). Recent reports indicate a physical association of telomerase and SMN (Bachand et al., 2002; Whitehead et al., 2002). In HeLa cells, there was a precise colocalization between hTR and the Cajal body marker protein, coilin (Figure 3, second panel), indicating that the hTR-containing foci are Cajal bodies.
We also investigated whether hTR is present within gems in related cell lines where these structures are observed. Gems are nuclear bodies that also contain the SMN complex (Liu and Dreyfuss, 1996; Meister et al., 2002; Paushkin et al., 2002). The name gems (for gemini or twin) was assigned based on the initial observation that these nuclear bodies were found to be immediately adjacent to or coincident with Cajal bodies (Liu and Dreyfuss, 1996). Subsequent localization studies found that gems and Cajal bodies are merged, indistinguishable structures that contain coilin and SMN in most human cell lines (including those used in this study except where noted; Matera and Frey, 1998; Carvalho et al., 1999; Young et al., 2002). We have examined hTR localization in two special HeLa cell lines, in which gems are distinct structures that are enriched in SMN and coilin methylation is reduced (Hebert et al., 2002). Specifically, we performed triple labeling of hTR, coilin, and SMN in HeLa-PV and HeLa-KN cells. In all cases, hTR accumulated in Cajal bodies, but never in gems (Figure 3, third panel), suggesting that hTR does not simply colocalize with SMN and that coilin methylation and SMN and are not likely required for recruitment of hTR to Cajal bodies.
We also tested whether any of the hTR-containing foci were associated with telomeres, the site where telomerase functions. Using antibodies against telomere-binding proteins TRF1 and TRF2, we found that hTR signals do not typically overlap with telomeres (Figure 3, lower panel and unpublished data). In a small fraction of cells, at a few telomeres, we did observe colocalization of hTR (within Cajal bodies; unpublished data). However, the low frequency of the colocalization made it difficult to distinguish from random colocalization of Cajal bodies with the numerous telomeres. We cannot exclude the possibility that low levels of hTR are present at telomeres (or other sites) due to detection limits of the FISH approach. Our data suggest that hTR is present in Cajal bodies and that the Cajal bodies are not usually found at telomeres.
We then tested for localization of hTR to Cajal bodies (by hTR FISH and coilin immunofluorescence) in other telomerase-positive cancer cell lines derived from various tissue sources (Figure 4 and unpublished data). In all cancer cells investigated, each of the hTR foci corresponded to a Cajal body, consistent with what was observed in HeLa cells. Thus, accumulation of hTR in Cajal bodies appears to be hallmark of a variety of cancer cell types.
hTERT Expression in Normal Cells Induces Accumulation of hTR in Foci Including Cajal Bodies
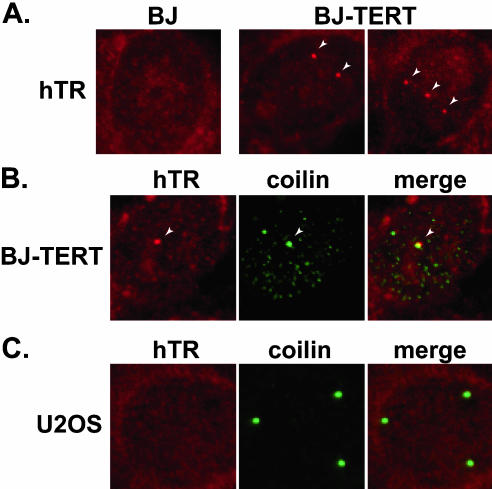
Cancer cells express readily detectable levels of hTERT (and telomerase activity), but primary cells do not. To assess whether the difference in distribution of hTR in normal and cancer cells could be attributed to hTERT expression, we compared hTR localization in primary BJ fibroblasts and BJ cells that stably express ectopic hTERT protein (Figure 5A). hTR was found in nuclear foci in BJ-hTERT cells. At the same time, no foci were observed in primary BJ cells, indicating that hTERT expression can induce the accumulation of hTR in foci within the nucleus. Similar results were obtained with 2yo smooth muscle cells and hTERT-expressing 2yo cells (unpublished data). The expression of hTERT in primary cells likely increases the level of hTR (perhaps about twofold; Yi et al., 2001), and thus the appearance of hTR in foci could reflect increases in hTR levels as well as redistribution of the RNA. The expression of hTERT in these cells also initiates the assembly of telomerase, which may account for the redistribution of hTR.
Figure 5.
Ectopic expression of hTERT induces accumulation of hTR in nuclear foci including Cajal bodies. (A) hTR was examined by FISH (red) in BJ and BJ-TERT (BJ cells that stably expresses hTERT) cells. Arrows indicate obvious foci of hTR accumulation. (B) hTR FISH (red) was combined with IF with anticoilin antibodies (green) in BJ-TERT cells. Merge panel shows merged FISH and IF data. Arrow indicates a prominent Cajal body in all three panels. (C) hTR FISH (red) was combined with IF with anticoilin antibodies (green) in hTERT- and telomerase-negative U2OS cells. Merge panel shows merged FISH and IF data.
Unlike cancer cells, primary human cells do not frequently display prominent Cajal bodies (Spector et al., 1992; Carmo-Fonseca et al., 1993) and hTR-containing foci observed in the BJ-hTERT and 2yo-hTERT cells generally did not colocalize with coilin, SMN, or PML proteins (unpublished data). Thus, in contrast to the foci observed in cancer cells, most of the hTR-containing foci in normal cells ectopically expressing hTERT did not correspond to Cajal bodies and currently remain unidentified. However, hTR colocalized with the infrequent Cajal bodies observed in BJ-hTERT and 2yo-hTERT cells, but not with those found in BJ or 2yo cells (Figure 5B and unpublished data). These results suggest that accumulation of hTR in Cajal bodies is dependent on hTERT expression. Finally, we also examined hTR localization in cancer cells that contain numerous Cajal bodies and express hTR but not hTERT. U2OS is an osteosarcoma-derived ALT line (Bryan et al., 1997a). We were unable to detect hTR in foci within the nuclei of these cells, despite the presence of numerous Cajal bodies (Figure 5C). Taken together, the results indicate the importance of hTERT expression on hTR accumulation in Cajal bodies.
hTERT Also Localizes to Cajal Bodies
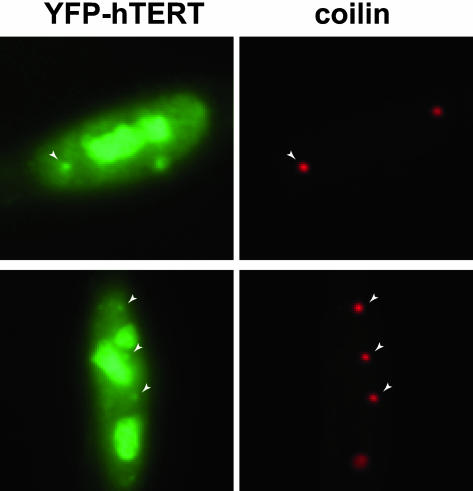
Given that hTR is targeted to Cajal bodies in cancer cells where telomerase is active and hTERT is expressed, we asked if hTERT also localizes to these structures. Previous studies have shown that hTERT is present in nucleoli and is also distributed throughout the nucleoplasm (Etheridge et al., 2002; Wong et al., 2002; Yang et al., 2002). The high levels of YFP-TERT protein present in the nucleoplasm of transfected cells in our earlier analysis (Etheridge et al., 2002) obscured our ability to determine if hTERT was present in Cajal bodies. The use of mild preextraction conditions, which remove the soluble pool of nucleoplasmic YFP-TERT, revealed a clear association of hTERT with Cajal bodies in HeLa cells (in addition to nucleoli as previously reported; Figure 6). Transfection of GFP alone results in no accumulation in any nuclear structure after preextraction (unpublished data), demonstrating that hTERT is also present in Cajal bodies.
Figure 6.
hTERT is present in Cajal bodies. YFP-TERT was transiently expressed in HeLa cells. Before fixation, cells were preextracted to remove soluble nucleoplasmic YFP-TERT. YFP-TERT (green) localizes to nucleoli and Cajal bodies (red, coilin, indicated in both panels by arrows).
DISCUSSION
Formation of the telomerase enzyme depends on the intracellular interaction of hTR and hTERT. To understand the biogenesis and function of telomerase, knowledge of the subcellular localization and trafficking of these key telomerase components is required. hTERT is expressed in most cancer cells, but little or no hTERT is present in most somatic cells. In contrast, hTR is expressed in both primary and cancer cells. Here we describe a FISH procedure that, for the first time, has enabled determination of the detailed subcellular localization of endogenous telomerase RNA in human cells. A major finding is that hTR localizes to intranuclear foci called Cajal bodies in telomerase-positive cancer cells, but not in normal cells. The difference in hTR localization may reflect active production of telomerase in cancer cells.
Our results establish the Cajal body as a site on the pathway traveled by telomerase in human cells—a potential site of telomerase biogenesis or function. Here we consider the significance of the localization of telomerase to Cajal bodies in the context of our current knowledge.
Cajal Bodies as Sites of Telomerase Biogenesis
We consider it likely that the Cajal body is the site where hTR and hTERT interact to form an active enzyme. Our results show that both of the key components of telomerase, hTR and hTERT, are found in Cajal bodies (Figures 3 and 6). Moreover, we find that hTR accumulates in Cajal bodies, specifically in cells where telomerase assembly is presumably ongoing (Figures 1, 2, 3, 4), consistent with the notion that the Cajal body is the site of assembly. No accumulation was observed in either tumor-derived (ALT) or normal cells in which hTERT is reduced or absent and telomerase is not readily detectable (Figures 2 and 5). Furthermore, ectopic expression of hTERT (and telomerase activity) in primary fibroblasts or smooth muscle cells resulted in accumulation of hTR in Cajal bodies (Figure 5). The hypothesis that telomerase assembly takes place within Cajal bodies is also based on considerable evidence implicating the Cajal body as the site of assembly of a variety of cellular RNPs (reviewed in Matera, 1999; Gall, 2000; Terns and Terns, 2001; Carmo-Fonseca, 2002; Ogg and Lamond, 2002). An implication of our findings is that Cajal body localization of hTR could serve as a marker for telomerase-positive cells.
Within Cajal bodies, the assembly of hTR and hTERT into active RNP complexes may be mediated the SMN (survival of motor neuron) complex. This macromolecular complex consists of the SMN protein (the spinal muscular atrophy disease protein) and six additional protein components called gemins 2–7 and is present in Cajal bodies of most cells (Liu and Dreyfuss, 1996; Matera and Frey, 1998; Carvalho et al., 1999; Young et al., 2002). Growing evidence indicates that SMN chaperones the assembly of several cellular RNPs, including both snRNPs and snoRNPs (reviewed in Terns and Terns, 2001; Meister et al., 2002; Paushkin et al., 2002). Recent data suggest SMN may also play a role in telomerase biogenesis, because SMN directly interacts with the telomerase-associated protein GAR1 (Whitehead et al., 2002), and antibodies against SMN immunoprecipitate catalytically active telomerase (Bachand et al., 2002).
Additional steps in telomerase biogenesis may also occur in the Cajal body. For example, hTR is associated with the H/ACA snoRNA-binding proteins dyskerin (Mitchell et al., 1999a), GAR1 (Dragon et al., 2000), NHP2, and NOP10 (Pogacic et al., 2000; Dez et al., 2001) in vivo. The H/ACA snoRNP proteins are found in Cajal bodies (Gall, 2000; Pogacic et al., 2000; Terns and Terns, 2002) and may assemble with telomerase here. Recent studies also suggest that the Cajal body is the site of posttranscriptional modification (ribose methylation and pseudouridylation) of some RNAs by the guide RNAs that reside in this structure (scaRNAs, the small Cajal body RNAs; Darzacq et al., 2002; Kiss et al., 2002). Like most other stable cellular RNAs, hTR likely undergoes posttranscriptional nucleotide modifications that influence the stability and function of the RNA. Conceivably, hTR modifications occur within Cajal bodies.
Studies using the Xenopus oocyte system provided the first evidence that vertebrate telomerase RNA localizes to Cajal bodies. Fluorescently labeled human or Xenopus telomerase RNA is specifically targeted to Cajal bodies (and nucleoli) after microinjection of the RNA into Xenopus oocyte nuclei (Narayanan et al., 1999; Lukowiak et al., 2001). Taken together with the findings of this study (that hTR localizes to Cajal bodies in a variety of human cell lines), the evidence indicates that hTR localization to Cajal bodies is a common step in the biogenesis of vertebrate telomerase. Interestingly, ciliate telomerase RNA is also predominantly localized to spherical intranuclear foci, some of which may be Cajal bodies (Fang and Cech, 1995). The hTR-containing foci in ciliates contain hypermethylated RNA, a hallmark feature of Cajal bodies found in other eukaryotes (Fang and Cech, 1995). Thus, Cajal bodies appear to be a component of the telomerase biogenesis pathway for a variety of diverse eukaryotes.
Cajal Bodies and Telomerase Function
In telomerase-positive cells, active telomerase RNPs ultimately encounter telomeres to carry out telomere replication. In this study, we were generally unable to detect hTR at the telomeres. This may reflect the detection limit of the FISH procedure given that only a few molecules of hTR may function at each telomere. In addition, hTR may only interact with these structures transiently. For example, hTR may localize to telomeres only during S phase when telomeres are synthesized (Ten Hagen et al., 1990; Wright et al., 1999). Evidence for this notion comes from important work done in ciliates that showed that a small fraction of telomerase RNA is mobilized during S phase to the replication band, the site of telomere synthesis (Fang and Cech, 1995). At all stages of the cell cycle (including S phase) the majority of detectable ciliate telomerase RNA is present at intranuclear foci (that may include Cajal bodies; Fang and Cech, 1995).
In both cilates (Fang and Cech, 1995) and human cells (this study), much of the telomerase RNA appears to be in intranuclear structures and sequestered away from telomeres. Regulated movement of hTR (or assembled telomerase) out of Cajal bodies during S phase may be necessary for telomere synthesis. Several properties of Cajal bodies suggest that they may deliver telomerase to telomeres. Cajal bodies undergo cell cycle–related structural changes (Andrade et al., 1993; Carmo-Fonseca et al., 1993; Chan et al., 1994; Boudonck et al., 1998; Liu et al., 2000), are highly motile structures (Boudonck et al., 1999; Platani et al., 2000; Platani et al., 2002), and are known to associate with specific chromosomal loci (reviewed in Matera, 1998; Gall, 2000).
Telomerase and Nucleoli
Previous studies have proposed that the association of hTR and hTERT takes place within the nucleolus. A fraction of both hTERT (Etheridge et al., 2002; Wong et al., 2002; Yang et al., 2002) and hTR (Mitchell et al., 1999a; Narayanan et al., 1999; Lukowiak et al., 2001) localizes to nucleoli (as well as Cajal bodies). Furthermore, the nucleolus is well known as the site of assembly of the ribosome and perhaps other small RNPs (Pederson, 1998; Olson et al., 2002; Gerbi et al., 2003). Although we did not detect hTR in nucleoli in this study, it is possible that hTR transiently localizes to nucleoli where assembly occurs.
A recent study indicates that the intranuclear distribution of ectopically expressed hTERT may shift between nucleoli and the nucleoplasm in response to a variety of stimuli (Wong et al., 2002). The authors propose that nucleolar localization provides a means to sequester active telomerase away from nucleoplasmic telomeres. The model is based on the assumption that the nucleolar hTERT is part of active telomerase complexes (and not unassembled hTERT molecules). However, nucleolar targeting of hTERT occurs in human cells that do not express hTR (Etheridge et al., 2002), indicating that unassembled hTERT accumulates in nucleoli. In addition, the fraction of hTR that may be in nucleoli is very small; it is undetectable by FISH (this study) and was estimated as ∼7% of total hTR by biochemical fractionation analysis (Mitchell et al., 1999a). Thus, it seems possible that the hTERT observed in nucleoli does not represent active telomerase.
It will be important to precisely elucidate the steps along the telomerase biogenesis pathway and to understand how each step is coordinated in the cell both temporally and spatially to control the availability of functional telomerase.
Acknowledgments
We are grateful to the following people for providing cell lines: Christopher Counter, Duke University (BJ-hTERT, 2yo, 2yo-hTERT, DU145 and U2OS); Gregory Matera, Case Western Reserve University (HeLa lines); J. David Puett, University of Georgia (A549, PC-3, JEG-3); and Michael Pierce, University of Georgia (MCF7). We also thank the following people for providing antibodies: Edward Chan and Eng Tan, The Scripps Research Institute (rabbit anti-p80 coilin, R288); Gregory Matera, Case Western Reserve University (mouse anti-p80 coilin mAb); Roel van Driel, University of Amsterdam (mouse anti-PML mAb 5E10); Dominique Broccoli, Fox Chase Cancer Center (rabbit anti-TRF2); and Susan Smith, Skirball Institute of Biomolecular Medicine (rabbit anti-TRF1). This work is supported by an American Cancer Society grant to M.P.T. and R.M.T, and R.L.T is supported by an National Institutes of Health training grant to the Department of Genetics at the University of Georgia.
References
- Almeida, F., Saffrich, R., Ansorge, W., and Carmo-Fonseca, M. (1998). Microinjection of anti-coilin antibodies affects the structure of coiled bodies. J. Cell Biol. 142, 899–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade, L.E., Chan, E.K., Raska, I., Peebles, C.L., Roos, G., and Tan, E.M. (1991). Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J. Exp. Med. 173, 1407–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade, L.E., Tan, E.M., and Chan, E.K. (1993). Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc. Natl. Acad. Sci. USA 90, 1947–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilion, A.A., Piatyszek, M.A., Gupta, J., Shay, J.W., Bacchetti, S., and Greider, C.W. (1996). Human telomerase RNA and telomerase activity in immortal cell lines and tumor tissues. Cancer Res. 56, 645–650 [PubMed] [Google Scholar]
- Bachand, F., Boisvert, F.M., Cote, J., Richard, S., and Autexier, C. (2002). The product of the survival of motor neuron (SMN) gene is a human telomerase-associated protein. Mol. Biol. Cell 13, 3192–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuch, A.A. (2002). Telomeres: the molecular events driving end-to-end fusions. Curr. Biol. 12, R738–R740. [DOI] [PubMed] [Google Scholar]
- Bodnar, A.G. et al. (1998). Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 [DOI] [PubMed] [Google Scholar]
- Borden, K.L. (2002). Pondering the promyelocytic leukemia protein (PML) puzzle: possible functions for PML nuclear bodies. Mol. Cell Biol. 22, 5259–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudonck, K., Dolan, L., and Shaw, P.J. (1998). Coiled body numbers in the Arabidopsis root epidermis are regulated by cell type, developmental stage and cell cycle parameters. J. Cell Sci. 111(Pt 24), 3687–3694 [DOI] [PubMed] [Google Scholar]
- Boudonck, K., Dolan, L., and Shaw, P.J. (1999). The movement of coiled bodies visualized in living plant cells by the green fluorescent protein. Mol. Biol. Cell 10, 2297–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan, T.M., Englezou, A., Dalla-Pozza, L., Dunham, M.A., and Reddel, R.R. (1997a). Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 3, 1271–1274 [DOI] [PubMed] [Google Scholar]
- Bryan, T.M., Marusic, L., Bacchetti, S., Namba, M., and Reddel, R.R. (1997b). The telomere lengthening mechanism in telomerase-negative immortal human cells does not involve the telomerase RNA subunit. Hum. Mol. Genet. 6, 921–926 [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca, M. (2002). New clues to the function of the Cajal body. EMBO Rep. 3, 726–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca, M., Ferreira, J., and Lamond, A.I. (1993). Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis— evidence that the coiled body is a kinetic nuclear structure. J. Cell Biol. 120, 841–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, T., Almeida, F., Calapez, A., Lafarga, M., Berciano, M.T., and Carmo-Fonseca, M. (1999). The spinal muscular atrophy disease gene product, SMN: a link between snRNP biogenesis and the Cajal (coiled) body. J. Cell Biol. 147, 715–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, E.K., Takano, S., Andrade, L.E., Hamel, J.C., and Matera, A.G. (1994). Structure, expression and chromosomal localization of human p80-coilin gene. Nucleic Acids Res. 22, 4462–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J.L., Blasco, M.A., and Greider, C.W. (2000). Secondary structure of vertebrate telomerase RNA. Cell 100, 503–514 [DOI] [PubMed] [Google Scholar]
- Collins, K., and Mitchell, J.R. (2002). Telomerase in the human organism. Oncogene 21, 564–579 [DOI] [PubMed] [Google Scholar]
- Cong, Y.S., Wright, W.E., and Shay, J.W. (2002). Human telomerase and its regulation. Microbiol. Mol. Biol. Rev. 66, 407–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq, X., Jady, B.E., Verheggen, C., Kiss, A.M., Bertrand, E., and Kiss, T. (2002). Cajal body-specific small nuclear RNAs: a novel class of 2′-O-methylation and pseudouridylation guide RNAs. EMBO J. 21, 2746–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange, T. (2002). Protection of mammalian telomeres. Oncogene 21, 532–540 [DOI] [PubMed] [Google Scholar]
- Dez, C., Henras, A., Faucon, B., Lafontaine, D., Caizergues-Ferrer, M., and Henry, Y. (2001). Stable expression in yeast of the mature form of human telomerase RNA depends on its association with the box H/ACA small nucleolar RNP proteins Cbf5p, Nhp2p and Nop10p. Nucleic Acids Res. 29, 598–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaene, K., Van Marck, E., and Parwaresch, R. (2000). Telomeres, telomerase and cancer: an up-date. Virchows Arch. 437, 1–16 [DOI] [PubMed] [Google Scholar]
- Dragon, F., Pogacic, V., and Filipowicz, W. (2000). In vitro assembly of human H/ACA small nucleolar RNPs reveals unique features of U17 and telomerase RNAs. Mol. Cell Biol. 20, 3037–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge, K.T., Banik, S.S., Armbruster, B.N., Zhu, Y., Terns, R.M., Terns, M.P., and Counter, C.M. (2002). The nucleolar localization domain of the catalytic subunit of human telomerase. J. Biol. Chem. 277, 24764–24770 [DOI] [PubMed] [Google Scholar]
- Fang, G., and Cech, T.R. (1995). Telomerase RNA localized in the replication band and spherical subnuclear organelles in hypotrichous ciliates. J. Cell Biol. 130, 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, J. et al. (1995). The RNA component of human telomerase. Science 269, 1236–1241 [DOI] [PubMed] [Google Scholar]
- Fu, D., and Collins, K. (2003). Distinct biogenesis pathways for human telomerase RNA and H/ACA small nucleolar RNAs. Mol. Cell 11, 1361–1372 [DOI] [PubMed] [Google Scholar]
- Gall, J.G. (2000). Cajal bodies: the first 100 years. Annu. Rev. Cell Dev. Biol. 16, 273–300 [DOI] [PubMed] [Google Scholar]
- Gerbi, S.A., Borovjagin, A.V., and Lange, T.S. (2003). The nucleolus: a site of ribonucleoprotein maturation. Curr. Opin. Cell Biol. 15, 318–325 [DOI] [PubMed] [Google Scholar]
- Guiducci, C., Cerone, M.A., and Bacchetti, S. (2001). Expression of mutant telomerase in immortal telomerase-negative human cells results in cell cycle deregulation, nuclear and chromosomal abnormalities and rapid loss of viability. Oncogene 20, 714–725 [DOI] [PubMed] [Google Scholar]
- Hahn, W.C., Counter, C.M., Lundberg, A.S., Beijersbergen, R.L., Brooks, M.W., and Weinberg, R.A. (1999). Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 [DOI] [PubMed] [Google Scholar]
- Harrington, L., and Robinson, M.O. (2002). Telomere dysfunction: multiple paths to the same end. Oncogene 21, 592–597 [DOI] [PubMed] [Google Scholar]
- Hebert, M.D., Shpargel, K.B., Ospina, J.K., Tucker, K.E., and Matera, A.G. (2002). Coilin methylation regulates nuclear body formation. Dev. Cell 3, 329–337 [DOI] [PubMed] [Google Scholar]
- Henson, J.D., Neumann, A.A., Yeager, T.R., and Reddel, R.R. (2002). Alternative lengthening of telomeres in mammalian cells. Oncogene 21, 598–610 [DOI] [PubMed] [Google Scholar]
- Jady, B.E., and Kiss, T. (2001). A small nucleolar guide RNA functions both in 2′-O-ribose methylation and pseudouridylation of the U5 spliceosomal RNA. EMBO J. 20, 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlseder, J., Broccoli, D., Dai, Y., Hardy, S., and de Lange, T. (1999). p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283, 1321–1325 [DOI] [PubMed] [Google Scholar]
- Kim, N.W. et al. (1994). Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 [DOI] [PubMed] [Google Scholar]
- Kiss, A.M., Jady, B.E., Darzacq, X., Verheggen, C., Bertrand, E., and Kiss, T. (2002). A Cajal body-specific pseudouridylation guide RNA is composed of two box H/ACA snoRNA-like domains. Nucleic Acids Res. 30, 4643–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, T. (2002). Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell 109, 145–148 [DOI] [PubMed] [Google Scholar]
- Kolquist, K.A., Ellisen, L.W., Counter, C.M., Meyerson, M., Tan, L.K., Weinberg, R.A., Haber, D.A., and Gerald, W.L. (1998). Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat. Genet. 19, 182–186 [DOI] [PubMed] [Google Scholar]
- Lefebvre, S. et al. (1995). Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80, 155–165 [DOI] [PubMed] [Google Scholar]
- Liu, J., Hebert, M.D., Ye, Y., Templeton, D.J., Kung, H., and Matera, A.G. (2000). Cell cycle-dependent localization of the CDK2-cyclin E complex in Cajal (coiled) bodies. J. Cell Sci. 113(Pt 9), 1543–1552 [DOI] [PubMed] [Google Scholar]
- Liu, Q., and Dreyfuss, G. (1996). A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 15, 3555–3565 [PMC free article] [PubMed] [Google Scholar]
- Lukowiak, A.A., Narayanan, A., Li, Z.H., Terns, R.M., and Terns, M.P. (2001). The snoRNA domain of vertebrate telomerase RNA functions to localize the RNA within the nucleus. RNA 7, 1833–1844 [PMC free article] [PubMed] [Google Scholar]
- Masutomi, K., and Hahn, W.C. (2003). Telomerase and tumorigenesis. Cancer Lett. 194, 163–172 [DOI] [PubMed] [Google Scholar]
- Masutomi, K. et al. (2003). Telomerase maintains telomere structure in normal human cells. Cell 114, 241–253 [DOI] [PubMed] [Google Scholar]
- Matera, A.G. (1998). Of coiled bodies, gems, and salmon. J. Cell Biochem. 70, 181–192 [PubMed] [Google Scholar]
- Matera, A.G. (1999). Nuclear bodies: multifaceted subdomains of the inter-chromatin space. Trends Cell Biol. 9, 302–309 [DOI] [PubMed] [Google Scholar]
- Matera, A.G., and Frey, M.R. (1998). Coiled bodies and gems: Janus or gemini? Am. J. Hum. Genet. 63, 317–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul, G.G., Negorev, D., Bell, P., and Ishov, A.M. (2000). Review: properties and assembly mechanisms of ND10, PML bodies, or PODs. J. Struct. Biol. 129, 278–287 [DOI] [PubMed] [Google Scholar]
- McEachern, M.J., Krauskopf, A., and Blackburn, E.H. (2000). Telomeres and their control. Annu. Rev. Genet. 34, 331–358 [DOI] [PubMed] [Google Scholar]
- Meister, G., Eggert, C., and Fischer, U. (2002). SMN-mediated assembly of RNPs: a complex story. Trends Cell Biol. 12, 472–478 [DOI] [PubMed] [Google Scholar]
- Meyerson, M. et al. (1997). hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785–795 [DOI] [PubMed] [Google Scholar]
- Meyne, J., Ratliff, R.L., and Moyzis, R.K. (1989). Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl. Acad. Sci. USA 86, 7049–7053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, J.R., Cheng, J., and Collins, K. (1999a). A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol. Cell Biol. 19, 567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, J.R., Wood, E., and Collins, K. (1999b). A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 [DOI] [PubMed] [Google Scholar]
- Morales, C.P., Holt, S.E., Ouellette, M., Kaur, K.J., Yan, Y., Wilson, K.S., White, M.A., Wright, W.E., and Shay, J.W. (1999). Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 21, 115–118 [DOI] [PubMed] [Google Scholar]
- Moyzis, R.K., Buckingham, J.M., Cram, L.S., Dani, M., Deaven, L.L., Jones, M.D., Meyne, J., Ratliff, R.L., and Wu, J.R. (1988). A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 85, 6622–6626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, T.M., and Cech, T.R. (1998). Reversing time: origin of telomerase. Cell 92, 587–590 [DOI] [PubMed] [Google Scholar]
- Nakamura, T.M., Morin, G.B., Chapman, K.B., Weinrich, S.L., Andrews, W.H., Lingner, J., Harley, C.B., and Cech, T.R. (1997). Telomerase catalytic subunit homologs from fission yeast and human. Science 277, 955–959 [DOI] [PubMed] [Google Scholar]
- Nakayama, J., Tahara, H., Tahara, E., Saito, M., Ito, K., Nakamura, H., Nakanishi, T., Ide, T., and Ishikawa, F. (1998). Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat. Genet. 18, 65–68 [DOI] [PubMed] [Google Scholar]
- Narayanan, A., Lukowiak, A., Jady, B.E., Dragon, F., Kiss, T., Terns, R.M., and Terns, M.P. (1999). Nucleolar localization signals of box H/ACA small nucleolar RNAs. EMBO J. 18, 5120–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg, S.C., and Lamond, A.I. (2002). Cajal bodies and coilin—moving towards function. J. Cell Biol. 159, 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, M.O., Hingorani, K., and Szebeni, A. (2002). Conventional and non-conventional roles of the nucleolus. Int. Rev. Cytol. 219, 199–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paushkin, S., Gubitz, A.K., Massenet, S., and Dreyfuss, G. (2002). The SMN complex, an assemblyosome of ribonucleoproteins. Curr. Opin. Cell Biol. 14, 305–312 [DOI] [PubMed] [Google Scholar]
- Pederson, T. (1998). The plurifunctional nucleolus. Nucleic Acids Res. 26, 3871–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platani, M., Goldberg, I., Lamond, A.I., and Swedlow, J.R. (2002). Cajal body dynamics and association with chromatin are ATP-dependent. Nat. Cell Biol. 4, 502–508 [DOI] [PubMed] [Google Scholar]
- Platani, M., Goldberg, I., Swedlow, J.R., and Lamond, A.I. (2000). In vivo analysis of Cajal body movement, separation, and joining in live human cells. J. Cell Biol. 151, 1561–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogacic, V., Dragon, F., and Filipowicz, W. (2000). Human H/ACA small nucleolar RNPs and telomerase share evolutionarily conserved proteins NHP2 and NOP10. Mol. Cell Biol. 20, 9028–9040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni, P., and Pandolfi, P.P. (2002). The role of PML in tumor suppression. Cell 108, 165–170 [DOI] [PubMed] [Google Scholar]
- Shay, J.W., and Bacchetti, S. (1997). A survey of telomerase activity in human cancer. Eur. J. Cancer 33, 787–791. [DOI] [PubMed] [Google Scholar]
- Smith, S., and de Lange, T. (1999). Cell cycle dependent localization of the telomeric PARP, tankyrase, to nuclear pore complexes and centrosomes. J. Cell Sci. 112, 3649–3656 [DOI] [PubMed] [Google Scholar]
- Spector, D.L., Lark, G., and Huang, S. (1992). Differences in snRNP localization between transformed and nontransformed cells. Mol. Biol. Cell 3, 555–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuurman, N., de Graaf, A., Floore, A., Josso, A., Humbel, B., de Jong, L., and van Driel, R. (1992). A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J. Cell Sci. 101, 773–784 [DOI] [PubMed] [Google Scholar]
- Ten Hagen, K.G., Gilbert, D.M., Willard, H.F., and Cohen, S.N. (1990). Replication timing of DNA sequences associated with human centromeres and telomeres. Mol. Cell Biol. 10, 6348–6355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terns, M.P., and Terns, R.M. (2001). Macromolecular complexes: SMN—the master assembler. Curr. Biol. 11, R862–R864. [DOI] [PubMed] [Google Scholar]
- Terns, M.P., and Terns, R.M. (2002). Small nucleolar RNAs: versatile transacting molecules of ancient evolutionary origin. Gene Expr. 10, 17–39 [PMC free article] [PubMed] [Google Scholar]
- Vaziri, H., and Benchimol, S. (1998). Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8, 279–282 [DOI] [PubMed] [Google Scholar]
- Verheggen, C., Lafontaine, D.L., Samarsky, D., Mouaikel, J., Blanchard, J.M., Bordonne, R., and Bertrand, E. (2002). Mammalian and yeast U3 snoRNPs are matured in specific and related nuclear compartments. EMBO J. 21, 2736–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen, C., Mouaikel, J., Thiry, M., Blanchard, J.M., Tollervey, D., Bordonne, R., Lafontaine, D.L., and Bertrand, E. (2001). Box C/D small nucleolar RNA trafficking involves small nucleolar RNP proteins, nucleolar factors and a novel nuclear domain. EMBO J. 20, 5480–5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead, S.E., Jones, K.W., Zhang, X., Cheng, X., Terns, R.M., and Terns, M.P. (2002). Determinants of the interaction of the spinal muscular atrophy disease protein SMN with the dimethylarginine-modified box H/ACA small nucleolar ribonucleoprotein GAR1. J. Biol. Chem. 277, 48087–48093 [DOI] [PubMed] [Google Scholar]
- Wong, J.M., Kusdra, L., and Collins, K. (2002). Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat. Cell Biol. 4, 731–736 [DOI] [PubMed] [Google Scholar]
- Wright, W.E., Tesmer, V.M., Liao, M.L., and Shay, J.W. (1999). Normal human telomeres are not late replicating. Exp. Cell Res. 251, 492–499 [DOI] [PubMed] [Google Scholar]
- Yang, Y., Chen, Y., Zhang, C., Huang, H., and Weissman, S.M. (2002). Nucleolar localization of hTERT protein is associated with telomerase function. Exp. Cell Res. 277, 201–209 [DOI] [PubMed] [Google Scholar]
- Yeager, T.R., Neumann, A.A., Englezou, A., Huschtscha, L.I., Noble, J.R., and Reddel, R.R. (1999). Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 59, 4175–4179 [PubMed] [Google Scholar]
- Yi, X., Shay, J.W., and Wright, W.E. (2001). Quantitation of telomerase components and hTERT mRNA splicing patterns in immortal human cells. Nucleic Acids Res. 29, 4818–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, X., Tesmer, V.M., Savre-Train, I., Shay, J.W., and Wright, W.E. (1999). Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol. Cell Biol. 19, 3989–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, P.J., Le, T.T., thi Man, N., Burghes, A.H., and Morris, G.E. (2002). The relationship between SMN, the spinal muscular atrophy protein, and nuclear coiled bodies in differentiated tissues and cultured cells. Exp. Cell Res. 256, 365–374 [DOI] [PubMed] [Google Scholar]
- Zhong, S., Salomoni, P., and Pandolfi, P.P. (2000). The transcriptional role of PML and the nuclear body. Nat. Cell Biol. 2, E85–E90. [DOI] [PubMed] [Google Scholar]