Induction of colitis by a CD4+ T cell clone specific for a bacterial epitope (original) (raw)
Abstract
It is now well established that the intestinal flora plays an important role in the pathogenesis of inflammatory bowel disease (IBD). However, whether bacteria serve as the sole target of the immune response in this process or whether they act indirectly by triggering an anti-self response is still unclear. We have previously shown that specific pathogen-free IL-10-deficient (IL-10 KO) mice develop a T helper (Th1)-cytokine associated colitis after experimental infection with Helicobacter hepaticus. We here show that H. hepaticus Ag (SHelAg)-specific CD4+ Th1 clones transfer disease to _H. hepaticus_-infected T cell-deficient RAG KO hosts. Importantly, uninfected recipients of the SHelAg-specific clones did not develop intestinal inflammation, and a control _Schistosoma mansoni_-specific Th1 clone did not induce colitis upon transfer to infected RAG KO mice. The disease-inducing T cell clones recognized antigen(s) (Ag) specifically expressed by certain Helicobacter species as they responded when stimulated in vitro with H. hepaticus and Helicobacter typhlonius Ag, but not when cultured with Ag preparations from Helicobacter pylori, various non-helicobacter bacteria, or with cecal bacterial lysate from uninfected mice. Characterization of the Ag specificity of one of the clones showed that it reacts uniquely with a 15-mer peptide epitope on the flagellar hook protein (FlgE) of H. hepaticus presented by I-Ab. Together, our results demonstrate that colitis can be induced by clonal T cell populations that are highly specific for target Ag on intestinal bacteria, suggesting that an aberrant T cell response directed against gut flora is sufficient to trigger IBD.
Many inflammatory diseases are now thought to have a microbial etiology. For example, various bacteria and viruses have been implicated in the development of peptic ulcers, reactive arthritis, arteriosclerosis, type 1 diabetes, and multiple sclerosis. One of the best studied examples of the involvement of microbes in disease pathogenesis is inflammatory bowel disease (IBD). IBD is believed to be the result of an aberrant intestinal mucosal immune response (1-3), and several reports have implicated a role for gut flora in human IBD (3-5). Moreover, studies in experimental models have clearly demonstrated that intestinal flora play a crucial role in IBD pathogenesis because specific pathogen-free or germ-free animals develop minimal or no disease (6-9).
Among potential mechanisms by which microbes trigger inflammatory diseases are molecular mimicry, bystander activation or epitope spreading, and superantigen activation of T cells (10-12). A key question in the field of IBD is whether the tissue-damaging response is directed against the gut flora itself or whether it represents an autoimmune reaction triggered by microbial costimulation. Although data from experimental models suggest that bacterial antigen (Ag) are the initial trigger of T cell activation, it is not clear whether an antibacterial T cell response by itself is sufficient to induce IBD, thus leaving open the question of target Ag(s) and T cell fine specificity.
We have studied a model of IBD involving Helicobacter hepaticus infection of specific pathogen-free IL-10 knockout (KO) mice. Within weeks after inoculation with this bacterium, IL-10 KO animals, but not simultaneously infected WT mice, display marked inflammation in the cecum and colon (13). This colitis is associated with a T helper 1 (Th1)-type cytokine response to SHelAg, a soluble H. hepaticus Ag preparation, and treatment with anti-IL-12 mAb from the start of the infection or once disease is established reduces the inflammation as well as the SHelAg-induced IFN-γ production (13, 14). Together these studies indicate a strong correlation between the Th1-type response to the bacterium and disease severity.
In the present study we have investigated the role of the microbial-specific T cell response in the development of _H. hepaticus_-induced colitis. In particular, we have addressed the question of whether an aberrant T cell response directed exclusively against this bacterium is sufficient to trigger intestinal inflammation. By transferring SHelAg-specific T cell clones into T cell-deficient hosts, we demonstrate that colitis can develop as a result of an immune response directed solely against gut bacteria.
Materials and Methods
Experimental Animals and Infections. Six- to 8-wk-old female specific pathogen-free C57BL/10 WT, C57BL/10 IL-10 KO, C57BL/10 RAG-2 KO (Taconic Farms), and C57BL/6J SCID mice (The Jackson Laboratory) were used. The animals tested negative for Ab to specific murine viruses and were free of Helicobacter species as assessed by PCR. All animals were housed in sterile microisolator cages at the National Institute of Allergy and Infectious Diseases (NIAID) animal facility under a study proposal approved by the NIAID Animal Care and Use Committee.
Mice were inoculated intragastrically with 0.5 ml of an H. hepaticus suspension [“NCI-Frederick isolate 1A” (15) isolated from the same mouse colony as isolate Hh-1 (ATCC51449) (16)] prepared to a McFarland turbidity standard of 1.0 in PBS representing 2.45 × 109 colony-forming units (cfu)/ml.
Antigens. SHelAg was prepared as described (13) and, in certain experiments, boiled for 5 min (17). Following the same protocol, Ag was prepared from the following bacteria from the American Type Culture Collection: Escherichia coli (ATCC25922), Klebsiella pneumoniae (ATCC13883), Pseudomonas aeruginosa (ATCC27853), Proteus vulgaris (ATCC13315), Staphylococcus epidermidis (ATCC12228), Staphylococcus aureus (ATCC25923), and Helicobacter muridarum (ATCC51212), or from Helicobacter typhlonius (18), and Helicobacter pylori (strain G27, cagA+) (19). Cecal bacterial lysate (CBL) and Schistosoma mansoni soluble worm antigen preparation were prepared as described (20, 21). Synthetic 15-mer peptides (96-98% pure) were purchased from Multiple Peptide Systems (San Diego). The sequence of the peptide with stimulatory activity was confirmed by Edman degradation.
Cloning and Expression of H. hepaticus Flagellar Hook Protein (FlgE). The flgE gene was amplified from H. hepaticus genomic DNA by using gene-specific primers (Table 1, which is published as supporting information on the PNAS web site) designed based on the H. hepaticus genome sequence (22). PCR products were cloned into the pCR 4-TOPO vector (Invitrogen). Miniprep DNA was cut with _Nde_I and _Xho_I, and inserts were ligated into the expression vector pET17b (Novagen). Recombinant proteins were expressed in E. coli strain BL21(DE3) pLys S (Invitrogen). Isolated inclusion bodies were solubilized (20 mM Tris·HCl, pH 8/10 mM DTT/6 M guanidinium hydrochloride) and proteins purified by gel filtration (Superdex 200, 20 mM Tris·HCl, pH 8/0.1 M NaCl/3 M urea). Purified proteins were dialyzed against Tris-buffered saline followed by distilled water. The purity of recombinant proteins was verified by SDS/PAGE.
Generation of T Cell Clones. T cell lines were established from 4-wk _H. hepaticus_-infected anti-CD8-treated IL-10 KO mice (1 mg of mAb 2.43 i.p. per week). Briefly, mesenteric lymph node cells (3 × 106 per well) were cultured in 24-well plates with 50 ng/ml SHelAg in complete medium (17). Six days later, CD4+ cells were purified by cell sorting, cultured in 5 units/ml human recombinant IL-2 (Chiron) for 2 days, and then twice restimulated (2.5 × 105 per well) in 48-well plates with splenocytes (3 × 106 per well) pulsed with 0.5 μg/ml SHelAg for 3.5 h at 37°C followed by irradiation (3,000 rad) and washing before addition to wells. After 48-72 h, IL-2 (5 units/ml) was added. After 5-21 days in IL-2, this T cell line was injected i.v. into severe combined immunodeficient (SCID) mice (1-2 × 106 cells per mouse) inoculated with H. hepaticus 1-3 days earlier to allow selection and expansion of bacterium-specific cells in vivo. Development of colitis was followed by weekly body weight measurements, and SHelAg-reactive T cell clones were generated from the spleen of one mouse from five of six recipients displaying pronounced weight loss 11 wk after cell transfer. Briefly, splenocytes were stained with phycoerythrin anti-CD4 (BD Biosciences), and CD4+ T cells were cloned at 1 cell per well into round-bottomed 96-well plates containing 3 × 105 irradiated splenocytes and 0.3 μg/ml boiled SHelAg in 0.1 ml per well by using a FACStar sorter (Becton Dickinson). One day later, 0.1 ml per well of 20 units/ml recombinant IL-2 was added. After 10 days, fresh medium with antigen-presenting cells (APC) and Ag was added, followed by recombinant IL-2 48 h later. After another round of stimulation, wells containing proliferating cells were identified visually, and cells were expanded.
The generation of S. mansoni calpain-specific CD4+ Th1 clone B has been described (21).
Cell Cultures and Cytokine Assays. T cell clones were maintained by restimulation every 3-5 wk in 24-well plates (3 × 105 per well) with irradiated splenocytes (3 × 106 per well) and boiled SHelAg (0.3-5 μg/ml) for _H. hepaticus_-specific clones B1 and B2, and soluble worm antigen preparation (50 μg/ml) for _S. mansoni_-specific clone B, followed by addition of recombinant IL-2 (5 units/ml) 48 h later.
SHelAg-reactive T cell clones (5 × 104 per well) were cultured (0.2 ml per well) in 96-well flat-bottomed plates with irradiated splenocytes (7.5 × 105 per well) in the presence of medium, SHelAg (1-10 μg/ml), various bacterial Ag preparations, CBL, recombinant H. hepaticus proteins, or synthetic peptides. In some experiments, splenocytes were cultured overnight (107 per ml) in medium, SHelAg (10 μg/ml), or CBL (200 μg/ml), irradiated, and washed before addition to wells. When indicated, 10 μg/ml anti-CD4 (GK1.5), anti-CD8 (2.43), or anti-class II MHC mAb (M5/114) were added to cultures. Supernatants were collected after 48-72 h, and IFN-γ was measured by ELISA using mAb from BD Biosciences.
Flow Cytometric Analysis. T cell clones were analyzed for T cell receptor (TCR) Vβ expression by using mAb from BD Biosciences: phycoerythrin anti-CD4 (RM4-5), Cy-chrome anti-TCR Vβ (H57-597), FITC anti-Vβ2, Vβ3, Vβ4, Vβ5.1/5.2, Vβ6, Vβ7, Vβ8.1/8.2, Vβ8.3, Vβ9, Vβ10b, Vβ11, Vβ12, Vβ13, Vβ14, or Vβ17a.
Transfer of T Cell Clones into RAG KO Mice. Twelve to 25 days after Ag stimulation, T cell clones (4 × 107 per mouse) were transferred i.v. into uninfected or 2-day-infected RAG KO animals. Seven to eight wk later, mice were killed and intestinal tissues were collected for histology.
Pathology and Immunohistochemistry. Intestinal tissues were processed and inflammation was scored in a blinded fashion by the same pathologist (A.W.C.) on a scale from 0 to 20 as described (17). Staining for CD3+ T cells was performed as described (13, 14).
Statistical Analysis. Colitis scores were compared by using non-parametric Mann-Whitney U test, and cytokine data were analyzed by Student's two-tailed t test. Differences were considered statistically significant with P < 0.05.
Results and Discussion
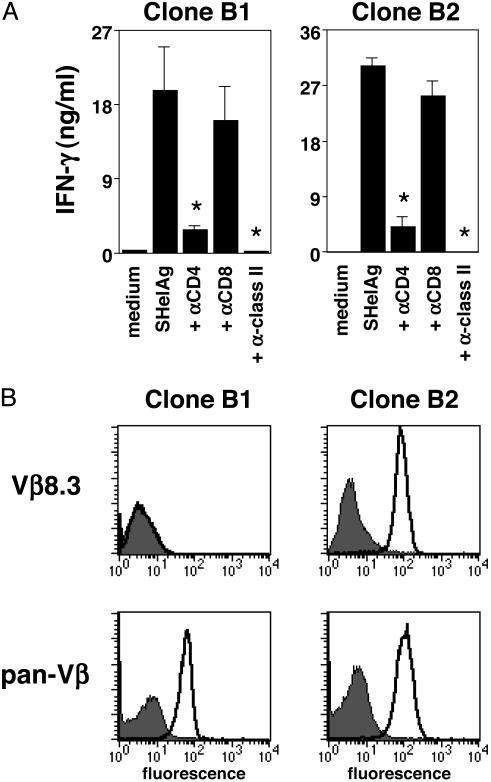
Generation of T Cell Clones Recognizing H. hepaticus. CD4+ T cells were cloned from the spleen of a SCID mouse receiving a T cell line from _H. hepaticus_-infected IL-10 KO mice, and several Th1 clones reactive with SHelAg were obtained (Fig. 1_A_ shows clones B1 and B2). Proteinase K treatment abrogated the stimulatory activity of SHelAg, indicating that the Ag(s) recognized were proteins (data not shown). Moreover, T cell activation depended on CD4 and MHC class II (Fig. 1 A), supporting a conventional TCR/MHC-peptide interaction.
Fig. 1.
Characterization of SHelAg-reactive T cell clones. (A) T cell clones B1 and B2 were cultured with APC and medium or boiled SHelAg in the absence or presence of anti-CD4, anti-CD8, or anti-class II mAb. IFN-γ was measured after 48 h. *, P < 0.05 compared with cells stimulated with SHelAg alone. No IL-4 or IL-5 was detected in cultures stimulated with SHelAg; however, both clones produced tumor necrosis factor α in response to this Ag (not shown). (B) TCR Vβ expression was analyzed by flow cytometry using anti-Vβ chain-specific or anti-pan-Vβ mAb (thick line) or isotype control (filled histograms). Histograms shown were gated on CD4+ cells. By PCR, clone B1 tested positive for Vβ15 (not shown).
To confirm clonality, T cell clones were analyzed for TCR Vβ usage by flow cytometry. Clone B2 expressed Vβ8.3, whereas clone B1 was negative for all 15 Vβ for which mAb are available (Fig. 1_B_ and data not shown). However, clone B1 stained positive with a pan-Vβ mAb (Fig. 1_B_), and by PCR this clone was positive for Vβ15 and Vα1, whereas clone B2 was positive for Vα3 (data not shown).
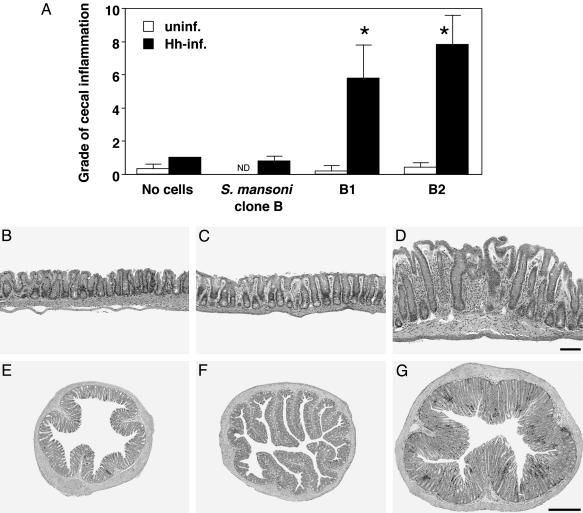
SHelAg-Reactive Clones Induce Colitis Upon Transfer to H. hepaticus-Infected RAG KO Mice. To analyze whether the SHelAg-reactive Th1 clones induce colitis, cells were transferred to RAG KO animals inoculated 2 days earlier with H. hepaticus. Infected RAG KO mice receiving clones B1 or B2 displayed cecal and colonic inflammation and epithelial hyperplasia 7-8 wk after cell transfer (Fig. 2 A and D). Importantly, minimal inflammation was observed in uninfected recipients of the SHelAg-reactive clones (Fig. 2 A and B) and in infected mice receiving a control Th1 clone specific for S. mansoni (clone B; ref. 21) (Fig. 2 A and C). These results demonstrate that SHelAg-reactive T cells and H. hepaticus are both required for induction of colitis.
Fig. 2.
SHelAg-specific T cell clones induce colitis when transferred to _H. hepaticus_-infected RAG KO mice. (A) Uninfected (open bars) or infected (filled bars) RAG KO mice were reconstituted with SHelAg-specific clones B1 or B2, or with _S. mansoni_-specific clone B as indicated. Shown is cecal inflammation (typhlitis) 7 wk after cell transfer. Bars represent mean histology scores ± SD of three to four mice per group from one representative experiment of three performed. Similar results were observed for the colon (data not shown). *, P < 0.05 compared with infected mice receiving no cells or S. mansoni clone B. (_B_-G) Histology of representative cecum and colon sections from mice in the experiment shown in A. (B and E) Uninfected RAG KO plus clone B1. (C and F) Infected RAG KO plus S. mansoni clone B. (D and G) Infected RAG KO plus clone B1. (_B_-D) Hematoxylin/eosin-stained cecal sections. (Bar = 100 μm.) (_E_-G) Immunohistochemical staining for CD3+ cells in ascending colon (≈1 cm from the cecum). (Bar = 500 μm.)
Flow cytometric analysis of T cells in spleens of infected RAG KO mice that received the pathogenic T cell clones revealed expression of integrin α4 and β7, but not integrin αIEL (data not shown). Moreover, when cecal and colonic sections from the same mice were stained with anti-CD3, many positive T cells were found in the lamina propria, supporting the finding that these cells express receptors allowing homing to the intestine. A colon section from an infected recipient of clone B1 is shown in Fig. 2_G_. No or very few T cells were found in uninfected RAG KO mice given SHelAg-reactive clones or in infected recipients of the _S. mansoni_-specific clone (Fig. 2 E and F).
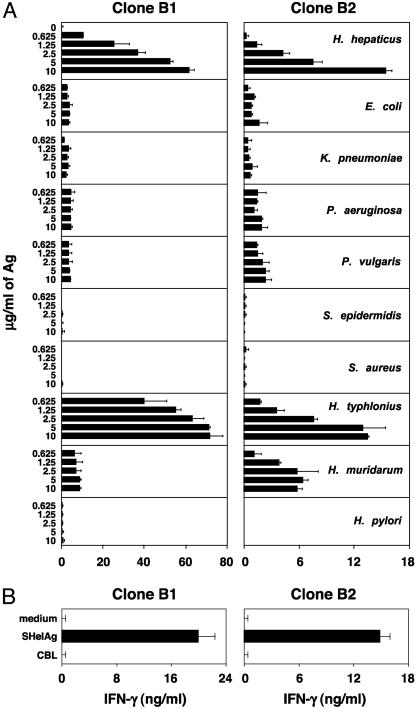
Bacterial Specificity of Disease-Inducing T Cell Clones. Although the SHelAg-reactive clones induced colitis in infected RAG KO mice, it was still possible that bacteria other than H. hepaticus activated these clones in vivo and that H. hepaticus merely provides a favorable milieu for such a process. To test this hypothesis we prepared various bacterial extracts and assessed their ability to stimulate the clones. As shown in Fig. 3_A_, none of the non-helicobacter Ag preparations stimulated an IFN-γ response from the SHelAg-reactive T cell clones. Moreover, CBL prepared from cecal contents of uninfected mice did not activate the clones, either when used to pulse APC overnight (Fig. 3_B_) or when added as soluble Ag (data not shown).
Fig. 3.
SHelAg-specific T cell clones fail to recognize Ag from non-helicobacter bacteria, but respond to other murine helicobacters. Clones B1 and B2 were cultured with APC in medium or 0.625-10 μg/ml soluble Ag from the bacteria indicated (A) or APC pulsed overnight with medium, SHelAg, or CBL (B). IFN-γ was measured after 72 h. Bars represent means ± SD of culture duplicates.
We next examined whether the Ag(s) recognized by the SHelAg-reactive T cell clones are conserved between Helicobacter species. Interestingly, neither B1 nor B2 recognized Ag from the human pathogen H. pylori, but both clones responded to Ag from the murine organism H. typhlonius and B2 responded weakly to H. muridarum Ag (Fig. 3_A_). Because H. typhlonius is a urease-negative organism, these results indicate that B1 and B2 do not recognize urease.
Clone B2 Recognizes a Defined Peptide Epitope on H. hepaticus FlgE. The Ag(s) recognized by clones B1 and B2 were shown to be associated with bacterial membranes because the majority of the stimulatory activity resided in the pellet after centrifugation (100,000 × g) of SHelAg. When outer membrane proteins (OMP) were isolated (23) and tested in culture, both clones responded, with B2 displaying enhanced responses to OMP relative to SHelAg, suggesting that the Ag recognized by this clone is enriched in the OMP. By electroeluting OMP bands from gels (24), the stimulatory activity for both clones was located in the high molecular mass region (64-80 kDa, data not shown).
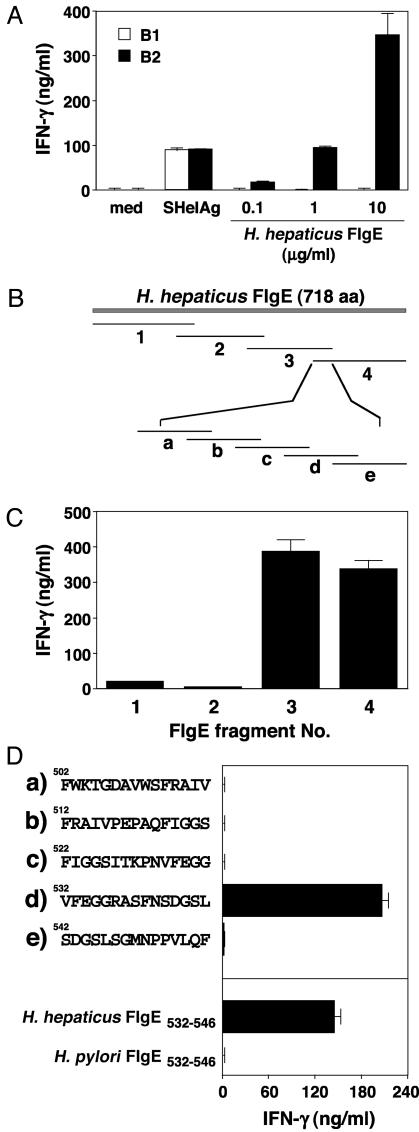
To identify the Ag(s) containing stimulatory activity, OMP were applied to SDS/PAGE and transferred to polyvinylidene difluoride (PVDF) membranes, and protein bands were excised and N-terminal sequenced. The amino acid sequence for one of the major bands in the 64-80 kDa region corresponded to the flagellar hook protein (FlgE). To assess whether the T cell clones recognize this protein, the flgE gene was PCR amplified and cloned, the Ag was expressed, and recombinant FlgE was purified. As shown in Fig. 4_A_, SHelAg-specific clone B2, but not B1, responds to recombinant H. hepaticus FlgE.
Fig. 4.
Clone B2 recognizes a defined peptide epitope on H. hepaticus FlgE. (A) Clones B1 and B2 were cultured with APC in medium or 10 μg/ml SHelAg or 0.1-10 μg/ml recombinant H. hepaticus FlgE. (B) Schematic map of H. hepaticus FlgE with fragments 1-4 and peptides a-e. (C and D) Clone B2 was cultured with APC and 1 μg/ml recombinant FlgE fragments (C) or 1 μg/ml synthetic peptides as indicated (D). IFN-γ was measured after 48 h. Bars represent means ± SD of culture duplicates.
The flgE gene of H. hepaticus encodes a 718-aa protein with a predicted molecular mass of 77.1 kDa (Fig. 5). To localize the epitope recognized by clone B2, we cloned and expressed FlgE in four overlapping fragments, each ≈200 aa in length (Figs. 4_B_ and 5). When tested in culture, fragments 3 and 4 were both highly stimulatory for clone B2 (Fig. 4_C_), suggesting that the antigenic epitope is located within the 44-aa overlap between these two fragments. To confirm the localization and identify the relevant epitope, 15-mer overlapping peptides were synthesized (Fig. 4_B_). As shown in Fig. 4_D_, one of these peptides (H. hepaticus FlgE532-546, VFEGGRASFNSDGSL) induced a strong IFN-γ response from clone B2, identifying that sequence as the epitope recognized by this disease-inducing clone.
Fig. 5.
Amino acid sequence of H. hepaticus FlgE. The flgE gene was cloned from H. hepaticus NCI-Frederick isolate 1A and sequenced (GenBank accession no. AJ583505). Shown is the amino acid sequence in one-letter code aligned to FlgE of H. pylori strain 26695 (GenBank accession no. NP 207664, H. pylori 26695 database accession no. HP0870). The first 20 underlined amino acids represent the sequence from the initial N-terminal sequencing of OMP from SHelAg. Recombinant FlgE fragments 1-4 correspond to amino acids 1-233, 194-393, 355-550, and 507-718, respectively. The boxed sequence represents peptide FlgE532-546 that has stimulatory activity for T cell clone B2.
Database searches revealed no homology between H. hepaticus FlgE532-546 and existing mammalian protein entries (data not shown), supporting the concept that clone B2 recognizes a bacterial-derived peptide and not a cross-reactive host molecule. The closest sequence match was a 15-aa stretch in FlgE of H. pylori that showed 80% identity to H. hepaticus FlgE532-546 (12 of 15 aa identities). When synthesized and tested, this H. pylori peptide had no stimulatory activity for Clone B2 (Fig. 4_D_). Therefore, the three amino acid differences between H. hepaticus and H. pylori FlgE532-546 (VFEGGRASFNSDGSL versus VFEGGRLHFNNDGSL; Fig. 5) may explain the differential responsiveness of clone B2 to Ag from these two helicobacters (Fig. 3_A_) and further identify some or all of these three amino acids as critical for binding to either I-Ab or the TCR of clone B2.
The flagellar hook protein exists as a polymer (the hook) on the cell surface of Gram-negative bacteria, where it connects the flagellar filament, consisting of flagellin, to the basal body, a multiprotein complex spanning both the outer and cytoplasmic membrane. However, we do not believe that this protein is the only target Ag for a disease-inducing immune response. On the contrary, our studies identify three different SHelAg-specific pathogenic T cells clones (B1, B2, and a third clone, B7, not shown) based on distinct TCR Vβ expression that appear to possess three different Ag specificities, as evidenced by their differential responsiveness to soluble versus membranous fractions of SHelAg as well as to other Helicobacter species (Figs. 1, 2, 3 and data not shown). Nonetheless, recent experiments indicate that _H. hepaticus_-exposed IL-10 KO as well as WT animals develop Ab responses against recombinant FlgE as detected by Western blotting (not shown). In addition, infected IL-10 KO mice mount a significant Th1 response to the protein. Nevertheless, whereas 7.5 μg/ml recombinant FlgE induced 30 ± 4 ng/ml IFN-γ from mesenteric lymph node of these animals, an equivalent amount of SHelAg stimulated 232 ± 10 ng/ml of the cytokine. The latter finding suggests that, although FlgE is recognized during infection, the response to the protein is minor relative to the total Th1 activity triggered by the whole bacterium.
Our earlier studies in the H. hepaticus IBD model demonstrated a correlation between intestinal inflammation and the Th1 response to the bacterium in susceptible IL-10 KO animals (13, 14). Similarly, T cell reactivity to bacterial sonicates and fecal extracts has been observed in other models of colitis (20, 25, 26) as well as in humans with active IBD (27). Moreover, Cong et al. (20, 28) have shown that polyclonal T cell populations activated in vitro with bacterial Ag can trigger IBD. That a T cell response to a single bacterially associated Ag may trigger colitis was initially suggested by Iqbal and colleagues (29), who showed that Th1/Th2-polarized ovalbumin (OVA)-specific CD4+ T cell populations from DO11.10 αβ TCR transgenic mice can drive the progression of colitis in RAG KO recipients carrying OVA-expressing E. coli, a bacterium that by itself does not trigger colitis. To our knowledge, the results reported here represent the first example in which the target Ag and T cell fine specificity of a pathogenic T cell response to a bacterium known in itself to induce colitis has been identified and also demonstrate for the first time that such a T cell response is sufficient to trigger disease.
Whereas our experiments and previous reports (20, 28, 29) in which colitis was induced by T cell transfer used recipients that were themselves T lymphocyte deficient, one would predict that the same Th1 populations would not trigger disease in T cell- and IL-10-sufficient hosts because of suppression by T regulatory (Treg) lymphocytes present in such mice (17, 30, 31). In this regard, we have previously demonstrated that CD4+ CD45RBlow cells from _H. hepaticus_-infected, but not uninfected WT mice, completely inhibit the colitis induced in RAG KO hosts by Helicobacter infection plus CD4+ T cells from IL-10 KO animals (17). These results suggest the induction of _H. hepaticus_-reactive Treg cells in WT mice after infection (17). Thus, intestinal bacteria likely induce both pathogenic effector and disease-suppressive Treg cells, with the balance between these two populations determining the disease outcome (17, 31).
The identification of both the target Ag and the fine specificity of pathogenic T cell clone B2 should open new directions of research leading to novel information on the pathogenesis of IBD. Our work now provides a monoclonal system with a defined T cell specificity to induce colitis and to study in detail the mechanisms in situ underlying disease induction. Toward this end, we have now constructed a transgenic mouse expressing the TCR of clone B2 to track the function of these T cells in vivo and to determine whether, under the appropriate circumstances, they can express Treg as well as effector activity.
Supplementary Material
Supporting Table
Acknowledgments
We thank Susan Barbieri for cell sorting, Barbara Kasprzak for immunohistochemical stainings, Ricardo Dreyfuss for help with photomicrographs, and Mark Garfield for Edman degradation analysis. We also thank Drs. Jose Ribeiro, Warren Strober, David Margulies, and Kannan Natarajan for support and critical reading of the manuscript. S.S. was supported by Deutsche Forschungsgemeinschaft Grant SFB479/A5, and J.G.F. was supported by National Institutes of Health Grant R01-CA67529.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ag, antigen; FlgE, flagellar hook protein; IBD, inflammatory bowel disease; OMP, outer membrane protein; SHelAg, soluble Helicobacter hepaticus Ag; Treg, T regulatory cell; KO, knockout; Th1, T helper 1; TCR, T cell receptor; CBL, cecal bacterial lysate.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ583505).
References
- 1.Strober, W. & Ehrhardt, R. O. (1993) Cell 75**,** 203-205. [DOI] [PubMed] [Google Scholar]
- 2.Powrie, F. (1995) Immunity 3**,** 171-174. [DOI] [PubMed] [Google Scholar]
- 3.Sartor, R. B. (1997) Am. J. Gastroenterol. 92**,** 5S-11S. [PubMed] [Google Scholar]
- 4.Gionchetti, P., Rizzello, F., Venturi, A., Ugolini, F., Rossi, M., Brigidi, P., Johansson, R., Ferrieri, A., Poggioli, G. & Campieri, M. (1999) Eur. Rev. Med. Pharmacol. Sci. 3**,** 27-30. [PubMed] [Google Scholar]
- 5.Campieri, M. & Gionchetti, P. (1999) Gastroenterology 116**,** 1246-1249. [DOI] [PubMed] [Google Scholar]
- 6.Sadlack, B., Merz, H., Schorle, H., Schimpl, A., Feller, A. C. & Horak, I. (1993) Cell 75**,** 253-261. [DOI] [PubMed] [Google Scholar]
- 7.Taurog, J. D., Richardson, J. A., Croft, J. T., Simmons, W. A., Zhou, M., Fernandez-Sueiro, J. L., Balish, E. & Hammer, R. E. (1994) J. Exp. Med. 180**,** 2359-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dianda, L., Hanby, A. M., Wright, N. A., Sebesteny, A., Hayday, A. C. & Owen, M. J. (1997) Am. J. Pathol. 150**,** 91-97. [PMC free article] [PubMed] [Google Scholar]
- 9.Powrie, F., Mauze, S. & Coffman, R. L. (1997) Res. Immunol. 148**,** 576-581. [DOI] [PubMed] [Google Scholar]
- 10.Benoist, C. & Mathis, D. (2001) Nat. Immunol. 2**,** 797-801. [DOI] [PubMed] [Google Scholar]
- 11.Panoutsakopoulou, V. & Cantor, H. (2001) J. Autoimmun. 16**,** 341-345. [DOI] [PubMed] [Google Scholar]
- 12.Dalwadi, H., Wei, B., Kronenberg, M., Sutton, C. L. & Braun, J. (2001) Immunity 15**,** 149-158. [DOI] [PubMed] [Google Scholar]
- 13.Kullberg, M. C., Ward, J. M., Gorelick, P. L., Caspar, P., Hieny, S., Cheever, A., Jankovic, D. & Sher, A. (1998) Infect. Immun. 66**,** 5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kullberg, M. C., Rothfuchs, A. G., Jankovic, D., Caspar, P., Wynn, T. A., Gorelick, P. L., Cheever, A. W. & Sher, A. (2001) Infect. Immun. 69**,** 4232-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward, J. M., Fox, J. G., Anver, M. R., Haines, D. C., George, C. V., Collins, M. J., Jr., Gorelick, P. L., Nagashima, K., Gonda, M. A., Gilden, R. V., et al. (1994) J. Natl. Cancer Inst. 86**,** 1222-1227. [DOI] [PubMed] [Google Scholar]
- 16.Fox, J. G., Dewhirst, F. E., Tully, J. G., Paster, B. J., Yan, L., Taylor, N. S., Collins, M. J., Jr., Gorelick, P. L. & Ward, J. M. (1994) J. Clin. Microbiol. 32**,** 1238-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kullberg, M. C., Jankovic, D., Gorelick, P. L., Caspar, P., Letterio, J. J., Cheever, A. W. & Sher, A. (2002) J. Exp. Med. 196**,** 505-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin, C. L., Gorelick, P. L., Riley, L. K., Dewhirst, F. E., Livingston, R. S., Ward, J. M., Beckwith, C. S. & Fox, J. G. (2001) J. Clin. Microbiol. 39**,** 3920-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang, Z., Censini, S., Bayeli, P. F., Telford, J. L., Figura, N., Rappuoli, R. & Covacci, A. (1995) Infect. Immun. 63**,** 94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cong, Y., Brandwein, S. L., McCabe, R. P., Lazenby, A., Birkenmeier, E. H., Sundberg, J. P. & Elson, C. O. (1998) J. Exp. Med. 187**,** 855-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jankovic, D., Åslund, L., Oswald, I. P., Caspar, P., Champion, C., Pearce, E., Coligan, J. E., Strand, M., Sher, A. & James, S. L. (1996) J. Immunol. 157**,** 806-814. [PubMed] [Google Scholar]
- 22.Suerbaum, S., Josenhans, C., Sterzenbach, T., Drescher, B., Brandt, P., Bell, M., Droge, M., Fartmann, B., Fischer, H. P., Ge, Z., et al. (2003) Proc. Natl. Acad. Sci. USA 100**,** 7901-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doig, P. & Trust, T. J. (1994) Infect. Immun. 62**,** 4526-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhaskar, S., Dutt, S. & Mukherjee, R. (2000) J. Immunoassay 21**,** 355-375. [DOI] [PubMed] [Google Scholar]
- 25.Duchmann, R., Schmitt, E., Knolle, P., Meyer zum Buschenfelde, K. H. & Neurath, M. (1996) Eur. J. Immunol. 26**,** 934-938. [DOI] [PubMed] [Google Scholar]
- 26.Brimnes, J., Reimann, J., Nissen, M. & Claesson, M. (2001) Eur. J. Immunol. 31**,** 23-31. [DOI] [PubMed] [Google Scholar]
- 27.Duchmann, R., Kaiser, I., Hermann, E., Mayet, W., Ewe, K. & Meyer zum Buschenfelde, K. H. (1995) Clin. Exp. Immunol. 102**,** 448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cong, Y., Weaver, C. T., Lazenby, A. & Elson, C. O. (2000) J. Immunol. 165**,** 2173-2182. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal, N., Oliver, J. R., Wagner, F. H., Lazenby, A. S., Elson, C. O. & Weaver, C. T. (2002) J. Exp. Med. 195**,** 71-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh, B., Read, S., Asseman, C., Malmström, V., Mottet, C., Stephens, L. A., Stepankova, R., Tlaskalova, H. & Powrie, F. (2001) Immunol. Rev. 182**,** 190-200. [DOI] [PubMed] [Google Scholar]
- 31.Asseman, C., Read, S. & Powrie, F. (2003) J. Immunol. 171**,** 971-978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table