Rac1 Protein Rescues Neurite Retraction Caused by G2019S Leucine-rich Repeat Kinase 2 (LRRK2) (original) (raw)
Abstract
Mutations in leucine-rich repeat kinase 2 (LRRK2) are currently the most common genetic cause of familial late-onset Parkinson disease, which is clinically indistinguishable from idiopathic disease. The most common pathological mutation in LRRK2, G2019S LRRK2, is known to cause neurite retraction. However, molecular mechanisms underlying regulation of neurite length by LRRK2 are unknown. Here, we demonstrate a novel interaction between LRRK2 and the Rho GTPase, Rac1, which plays a critical role in actin cytoskeleton remodeling necessary for the maintenance of neurite morphology. LRRK2 binds strongly to endogenous or expressed Rac1, while showing weak binding to Cdc42 and no binding to RhoA. Co-expression with LRRK2 increases Rac1 activity, as shown by increased binding to the p21-activated kinase, which modulates actin cytoskeletal dynamics. LRRK2 constructs carrying mutations that inactivate the kinase or GTPase activities do not activate Rac1. Interestingly, LRRK2 does not increase levels of membrane-bound Rac1 but dramatically changes the cellular localization of Rac1, causing polarization, which is augmented further when LRRK2 is co-expressed with constitutively active Rac1. Four different disease-related mutations in LRRK2 altered binding to Rac1, with the G2019S and R1441C LRRK2 mutations attenuating Rac1 binding and the Y1699C and I2020T LRRK2 mutations increasing binding. Co-expressing Rac1 in SH-SY5Y cells rescues the G2019S mutant phenotype of neurite retraction. We hypothesize that pathological mutations in LRRK2 attenuates activation of Rac1, causing disassembly of actin filaments, leading to neurite retraction. The interactions between LRRK2 and Rho GTPases provide a novel pathway through which LRRK2 might modulate cellular dynamics and contribute to the pathophysiology of Parkinson disease.
Keywords: Cellular Regulation, Cyclic GMP (cGMP), Cytoskeleton, Membrane Trafficking, Neurochemistry, Neurological Diseases, Parkinson Disease, Protein Kinases, Protein Translocation
Introduction
Parkinson disease (PD)2 is the most common neurodegenerative movement disorder affecting nearly 1% of elderly over 65 years old. Idiopathic PD may result from a combination of factors including age, genetic predisposition, environmental toxins, and neuroinflammation (1, 2). Mutations in LRRK2 are the most common genetic cause of PD and also contribute to many sporadic cases of PD (3, 4). At least 20 mutations identified to date in LRRK2 cause autosomal-dominant PD, accounting for ∼7% of all familial cases (4, 5). The missense mutation, G2019S, in the kinase domain of LRRK2 is by far the most common of the LRRK2 mutations associated with PD, and disease associated with the G2019S mutations is clinically indistinguishable from idiopathic disease (6).
The Lrrk2 gene of 51 exons encodes a large, multidomain protein that includes an ankyrin repeat and leucine-rich repeat regions (6). The kinase domain of LRRK2 shares homology with receptor-interacting protein kinases and mixed lineage kinases (3, 7). LRRK2 also contains a Ras-of-complex (ROC) domain that exhibits GTPase activity (8, 9). In between these two domains lies a C-terminal of Ras complex (COR) domain. Each of these domains is thought to be important for binding but have functions that are unclear at this time. The ROC-COR kinase domains appear to be key domains for function and disease. Most mutations linked to PD are found in the ROC-COR kinase region, and in cases where the binding sites of proteins have been identified, binding appears to occur in these domains (10–13).
Recent in vitro and in vivo studies reveal that the overexpression of pathological mutant G2019S LRRK2 causes neurite retraction (14–16). These studies also suggest that neurite pathology precedes dopaminergic neuronal death by apoptosis (14, 15). Although autophagy and increased Tau inclusions have been described in conjunction with neurite shortening, the only signaling pathway linked to the G2019S LRRK2-induced neurite pathology is the ERK pathway (10, 15, 17–20). However, no upstream triggers were identified. Pathological mutants of LRRK2 were shown to be more toxic than WT LRRK2 as well as LRRK1, a homologue of LRRK2 that does not segregate with disease (21). Although the biology of LRRK2 function is still unknown, the prima fascia link between neurite retraction and neurodegeneration provides a compelling avenue for further investigation.
The ROC domain in LRRK2 was shown to form homodimers, allowing this GTPase domain to regulate LRRK2 kinase activity (18, 22). The ROC domain of LRRK2 contributes to its homodimerization, although sequences outside of the ROC-COR bidomain are also required for dimer formation (18, 22, 23). The ability of the LRRK2 ROC GTPase domain to homodimerize with LRRK2 and heterodimerize with LRRK1 led us to hypothesize that this domain might also exhibit binding to other GTPases through a process analogous to heterodimerization.
Classical Rho GTPases such as Rac, Cdc42, and RhoA are GTP-binding proteins that have intrinsic GTPase activity (24). These Ras-related Rho p21 proteins act as molecular switches as they alternate between the active GTP-bound and the inactive GDP-bound forms. In their active forms, Rho GTPases stimulate downstream effectors to activate a diverse assortment of cellular mechanisms, including vesicular transport, microtubule dynamics, actin cytoskeleton remodeling, and cell cycle progression (25, 26). Rac and Cdc42 signal through the p21-activated kinase (PAK) family of serine/threonine kinases and other effectors to modulate actin cytoskeletal dynamics, which are critical for axonal outgrowth, maintenance of dendritic spine, and neurite morphology (27, 28). More specifically, PAK1 signals through ERK1/2 to regulate actin polymerization and lamellipodium stability (29). Activated Rac and Cdc42 also signal through PAK to activate LIM kinase, which inhibits downstream cofilin, thereby blocking actin depolymerization (30). Plowey et al. (15) found that an ERK inhibitor abolished the G2019S LRRK2-induced neurite retraction in their SH-SY5Y cellular model. PAK signaling through ERK is an interesting upstream trigger that might implicate Rho GTPases in the pathogenesis of neurite shortening.
The experiments described below investigate binding of LRRK2 to Rho family GTPases and whether this molecular pathway contributes to the mechanism through which LRRK2 regulates neurite extension and retraction. The current set of studies demonstrates that LRRK2 associates with Rac1, and that LRRK2 and Rac1 functionally interact to regulate neurite growth. Finally, we show that Rac1 can rescue the neurite retraction induced by G2019S LRRK2.
EXPERIMENTAL PROCEDURES
Constructs
WT and mutant LRRK2 V5-His-tagged vectors were generated as described by Greggio et al. (31). pRK5Myc Rac1 constructs including WT, Q61L (constitutively active), and T17N (dominant negative) mutants were obtained from Addgene and were constructed as described in Sabauste et al. (32).
Cell Culture
Human embryonic kidney 293 FT cells (Invitrogen) were maintained in DMEM supplemented with 10% FBS, 1× nonessential amino acids, and 1% penicillin/streptomycin (100 μg/ml). SH-SY5Y human neuroblastoma cells were maintained in DMEM supplemented with 10% FBS, 10 mm HEPES, and no antibiotics. SY5Y cells were plated on poly-l-lysine-coated glass coverslips. 10 μm retinoic acid was used to differentiate the SY5Y cells after 24 h in culture on the coverslips. After 72 h, the differentiated SY5Y cells were transfected with Lipofectamine 2000 (Invitrogen).
Flp-In Cells
The tetracycline-inducible HEK293 FT LRRK2 cell lines were generated using the Flp-In T-Rex system (Invitrogen). Flp-In T-Rex 293 host cells containing the tetracycline binding protein were purchased from Invitrogen and grown in medium containing DMEM, 10% (v/v) FBS, 2 mm l-glutamine, 1% (v/v) Pen-Strep mycin, 100 μg ml−1 Zeocin. To introduce LRRK2 (wild-type, G2019S, R1441C), we modified the pcDNA5/FRT/TO expression vector to accept Gateway cloning (Invitrogen) by ligating an attR lambda recombination signal using the topoisomerase cloning site and confirmed by sequencing. LRRK2 (wild-type, G2019S, R1441C) was then introduced into the vector using the Gateway clonase (Invitrogen). The resulting plasmids were co-transfected with a p0G44 plasmid carrying the Flp-recombinase into the Flp-In T-Rex 293 host cells, and stable integrants were selected by resistance to 200 μg ml−1 hygromycin and 15 μg ml−1 blasticidin. The cells were then maintained in medium containing 100 μg ml−1 hygromycin and 15 μg ml−1 blasticidin.
Co-immunoprecipitation
HEK293 FT cells were co-transfected with V5-His-tagged LRRK2 and Myc-tagged Rac1 constructs and harvested in immunoprecipitation buffer which consisted of 0.5% Triton-X, 1 mm EDTA, and 1× protease inhibitor mixture (Sigma). The lysates were rotated at 4 °C for 30 min followed by centrifugation at 13,000 × g for 10 min. The supernatant was precleared with protein G-Sepharose 4 Fast Flow (Amersham Biosciences) followed by addition of the appropriate antibody and rotated overnight at 4 °C. Protein G-Sepharose was added to pull down the antibody precomplex. Sepharose beads were washed stringently three times with immunoprecipitation buffer and once with 50 mm Tris (pH 8) wash buffer. The immunoprecipitation proteins were eluted in lithium dodecyl sulfate sample buffer (Invitrogen) by heating at 95 °C for 3 min. Immunoprecipitates were resolved by 3–8% NuPAGE Tris acetate or 4–20% Tris-glycine gels (Invitrogen) and analyzed by Western blot. Antibodies were purchased from Sigma (V5, FLAG, c-Myc, actin), Calbiochem (calnexin), and Biodesign (GAPDH).
Rac Activity Assay
Rac activation was assessed using the p21-binding domain of PAK-GST beads to immunoprecipitate GTP-bound fractions of Rac, according to the manufacturer's instructions (Millipore). Briefly, cells expressing LRRK2 were lysed in magnesium-containing lysis buffer. The PAK-GST beads were added to 600 μg of total protein and rotated at 4 °C for 1 h, after which the samples were washed and prepared for immunoblotting for endogenous Rac1 (Rac1 clone 23A8 antibody from Millipore).
Immunocytochemistry
HEK293 FT cells plated on glass coverslips were transfected with Lipofectamine 2000 at 50% confluency. After 48 h, the transfected cells were fixed with 4% paraformaldehyde in 1× PBS for 10 min at room temperature. The cells were washed three times with PBS at 5-min each and were then incubated with 0.3% Triton X-100 in PBS with 1% BSA for 1 h at room temperature. The cells were incubated with a 1:1000 dilution of anti-V5 and anti-myc antibodies overnight at room temperature. After washing the cells three times with PBS at 5-min each, the cells were incubated with a 1:750 dilution of FITC-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories) in 1× PBS. The washes were repeated with 1× PBS, and the cells were incubated with a 1:750 dilution of Texas Red-conjugated donkey anti-mouse antibody (Jackson ImmunoResearch Laboratories). After washing the cells three times with 1× PBS at 5-min each, they were embedded in Prolong Gold Antifade DAPI gel mounting medium (Invitrogen).
The cells were visualized by three-dimensional multiple wavelength fluorescence microscopy using the Zeiss LSM510 META confocal microscope for DAPI, FITC, and Texas Red fluorescence at room temperature using an oil immersion 63× Plan-Apochromate lens (aperture set at 1.40). The images were captured using HP CoolSNAP camera and examined as either single sections or projections of an entire stack of optical sections. All images were processed using Adobe Photoshop CS2.
Differentiated SH-SY5Y cells transfected with LRRK2 and Rac1 constructs were fixed and stained for filamentous actin (phalloidin), LRRK2, and Rac1 expression. Neurite processes from cells positive for LRRK2 expression were quantified using the NeuronJ macros for ImageJ software.
RNA Knockdown
HEK293 FT cells or differentiated SH-SY5Y cells were transfected with 30 pm negative control (Invitrogen catalog no. 4390843) or LRRK2 siRNA with a fluorescent tag (Invitrogen catalog nos. 272780 and 263837) with Lipofectamine 2000. HEK293 FT cells were lysed with co-immunoprecipitation buffer 3 days after transfection for Western blot analysis of endogenous LRRK2 levels. SH-SY5Y cells were fixed for immunocytochemistry, and cells were stained for filamentous actin (phalloidin) and DAPI. Neurite length was quantified only for cells containing fluorescent siRNA.
Statistical Analysis
All data were expressed as mean ± S.E. and graphed using Excel or Prism (GraphPad). One-way analysis of variance and Tukey's post hoc tests were performed to identify statistically significant differences using InStat (GraphPad).
RESULTS
LRRK2 Associates with Rac1 through ROC-COR Kinase Domains
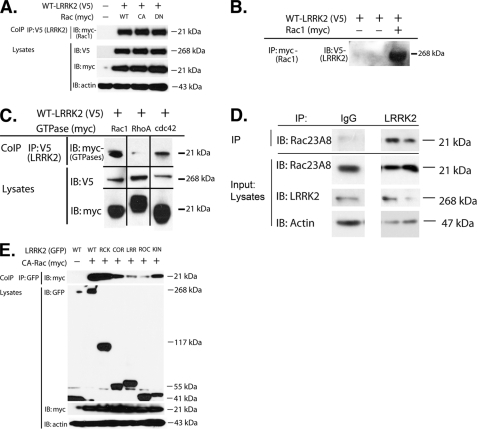
To determine whether LRRK2 interacts with classical Rho GTPases, co-immunoprecipitation studies were conducted in HEK293 FT cells transiently transfected with LRRK2 and Rho GTPases. Rac1 co-precipitated with LRRK2 following co-expression (Fig. 1A). The interaction between LRRK2 and Rac1 was robust, and LRRK2 bound each form of Rac1 that we examined, including WT, constitutively active (CA), and dominant negative (DN) forms of Rac1 (Fig. 1A), Association also was observed upon immunoprecipitation of Rac1 and probing for LRRK2 (Fig. 1B). We also examined binding between LRRK2 and two other Rho GTPases, Cdc42 and RhoA. LRRK2 showed moderate binding with Cdc42 and little binding with RhoA (Fig. 1C). Given that binding to Rac1 was stronger than binding to Cdc42 or RhoA, we proceeded to focus on the interaction between LRRK2 and Rac1.
FIGURE 1.
Co-immunoprecipitation of LRRK2 and Rac1. A, LRRK2 (V5, WT) was co-expressed with Rac1 (Myc, WT, CA, DN) in HEK293 FT cells, and LRRK2 was immunoprecipitated. Rac1 was readily detectable in complex. B, Co-association of LRRK2 and Rac1 was also readily apparent following overexpression of LRRK2 (WT) and Rac1 (CA) followed by immunoprecipitation (IP) of Rac1 and immunoblotting (IB) for LRRK2. C, association of LRRK2 with Rho GTPases appears to be strongest for Rac1. LRRK2 (V5, WT) was co-expressed with Rac1, RhoA, or Cdc42 (Myc, WT for each) in HEK293 cells, and the LRRK2 was immunoprecipitated. Rac1 and Cdc42 were detectable in the complexes following a rank order for detection of Rac1 > Cdc42 ≫ RhoA. D, LRRK2 was immunoprecipitated from human brain striatal lysate and then probed with anti-Rac1 antibody. A robust Rac1 signal was evident in the immunoprecipitate but absent when nonspecific IgG was substituted for the LRRK2 antibody. E, LRRK2 (GFP, WT) deletion constructs were expressed with Rac1 (Myc, CA) to identify which domains are most important for binding. The LRRK2 was immunoprecipitated and immunoblotted for Rac1. Rac1 binding was apparent with the ROC-COR kinase constructs, as well as with constructs carrying the subdomains of COR or kinase. KIN, kinase; RCK, ROC-COR-kinase domains; CoIP, co-immunoprecipitation.
We also observed association of endogenous LRRK2 and Rac1. LRRK2 was immunoprecipitated from human brain striatum and probed with anti-Rac1 antibody. Rac1 reactivity was readily evident in the LRRK2 immunoprecipitates but not observed when nonspecific IgG was substituted for LRRK2 antibody (Fig. 1D).
Next, we examined the domain requirements for association of LRRK2 and Rac1. Rac1 was co-expressed with LRRK2 constructs coding for known functional domains. Rac1 showed strong binding to LRRK2 constructs expressing the COR or kinase domains, as well as a construct expressing the ROC-COR kinase domains (Fig. 1E). Binding to the ROC-COR kinase construct was almost as strong as binding to WT LRRK2, suggesting that the presence of these domains was the predominant requirement for Rac1 binding.
Pathological Mutations in LRRK2 Affects Binding to Active Rac1
We examined whether disease-linked mutations in LRRK2 modified Rac1 activity. LRRK2 constructs (WT, G2019S, R1441C, Y1699C, I2020T) were co-transfected with Rac1 and binding to CA-Rac1 was examined. The G2019S decreased binding to Rac1 moderately, whereas the R1441C mutation strongly inhibited Rac1 binding (Fig. 2, A and B). Interestingly, Y1699C and I2020T constructs had the opposite effect and increased Rac1 activity (Fig. 2, A and B). We also examined the effects of a kinase-dead (KD) LRRK2 construct, which produced decreased Rac1 binding. Thus, mutations in LRRK2 modify binding to Rac1, but the direction of change is not consistent among different familial mutant constructs.
FIGURE 2.
Immunoprecipitation of LRRK2 constructs carrying disease-linked mutations. G2019S and R1441C exhibit weaker binding to CARac1, whereas Y1699C and I2020T exhibit increased binding. Shown is a representative immunoblot (IB). Shown is a quantification of three independent experiments (n = 3). *, p < 0.05; **, p < 0.001. CoIP, coimmunoprecipitation; KD, knockdown.
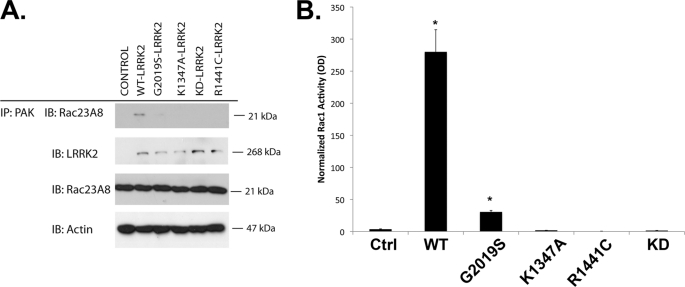
Overexpression of LRRK2 Increases Active GTP-bound Rac1
PAKs are activated by the GTP-bound forms of Rac1, and binding to PAKs are commonly used to monitor GTPase activity (33). To explore whether LRRK2 regulated Rac1 activity, we transfected HEK293 cells with LRRK2 and examined binding of endogenous Rac1 to PAK-GST beads by co-precipitation. Expression of WT LRRK2 increased the amount of active, endogenous GTP-bound Rac1, whereas the overall expression of Rac1 remained unchanged (Fig. 3, A and B). Interestingly, G2019S and R1441C LRRK2 (Fig. 3A) did not significantly increase Rac1 activity (Fig. 3B). Mutations inactivating LRRK2 kinase activity (K1906M, KD) or GTPase activity (K1347A) also prevented LRRK2 from activating Rac1 (Fig. 3, A and B). Similar results were observed in cells overexpressing both LRRK2 and Rac1 (data not shown).
FIGURE 3.
WT LRRK2 increases Rac1 activity. A, HEK293 cells were transfected with LRRK2 (WT, G2019S, R1441C, K1347A, K1906M, with the latter abbreviated as KD). Endogenous Rac1 was then precipitated using PAK-GST. Levels of Rac1 in the PAK complex and total lysates were immunoblotted (IB). The presence of WT LRRK2 increased the amount of Rac1 with PAK, but did not change total Rac1 levels. B, quantification of Rac1 in the PAK-GST precipitates (n = 4). *, p < 0.01. Ctrl, control; IP, immunoprecipitate.
Rac1 Increases LRRK2 Membrane Association
Activated Rac1 is known to associate with the plasma membrane, where it can activate downstream effectors in various signaling cascades that affect cytoskeletal dynamics and gene transcription, including PAKs (28). Because a significant proportion of LRRK2 is also known to be associated with cellular membranes under basal conditions, we hypothesized that LRRK2 might affect the membrane distribution of Rac1 or vice versa (34).
To explore the potential of whether LRRK2 or Rac1 modulate their respective cellular distribution, we investigated the distribution of Rac1 by immunofluorescence. HEK293 FT cells were transfected with LRRK2 and Rac1 (WT, CA, and DN), and localization of the two proteins were determined by immunofluorescence. LRRK2 exhibited a mixed cytoplasmic and membrane distribution when expressed alone, consistent with prior reports of its distribution (Fig. 4, a and c) (34). Rac1 exhibited strongest expression along the plasma membrane when expressed alone (Fig. 4, e and f). Co-expression of WT Rac1 and LRRK2 produced a striking redistribution of LRRK2 and Rac1, with Rac1 and LRRK2 becoming very polarized (Fig. 4, m–o). Co-transfection of CA-Rac1 accentuated the polarization of LRRK2 and Rac1, with Rac1 and LRRK2 expression being restricted to small foci near processes (Fig. 4, p–r), and also elicited a striking change in the morphology of the cells, consistent with an increased tendency for process extension (Fig. 4, p–r). Because R1441C LRRK2 does not bind or activate Rac1 (Figs. 2 and 3), we explored whether R1441C LRRK2 affected Rac1 localization. In contrast to the strong effect of WT LRRK2 on Rac1 localization, co-expressing R1441C LRRK2 with WT-, CA-, or DN-Rac1 had no effect on localization of Rac1 or LRRK2 (Fig. 5). These results are consistent with the known role of Rac1 in stimulating process outgrowth and suggest that LRRK2 might enhance this function of Rac1.
FIGURE 4.
Cellular distribution of LRRK2 and Rac1. Expression of WT LRRK2 alone produced a broad cellular distribution (a–c). In contrast, WT Rac1 yielded a distribution at or close to membranes (d–f), whereas the distribution of CA-Rac1 (g–i) and DN Rac1 (k–l) was less pronounced. Co-expression of LRRK2 with WT or CA-Rac1 produced striking polarization. WT LRRK2 plus WT-Rac1 yielded asymmetric polarization (m–o), whereas WT LRRK2 plus CA-Rac1 (q and r). However, DN-Rac1 did not elicit changes in LRRK2 distribution (s–u). Higher magnification figures of selected areas of putative co-localization are shown in the boxed insets.
FIGURE 5.
Rac1 does not affect localization of R1441C LRRK2 and vice versa. Expression of R1441C LRRK2 neither co-localized with Rac1 nor modified the distribution of Rac1. CA-Rac1 and DN-Rac1 also were not sensitive to R1441C LRRK2 and did not modify the distribution of R1441C LRRK2.
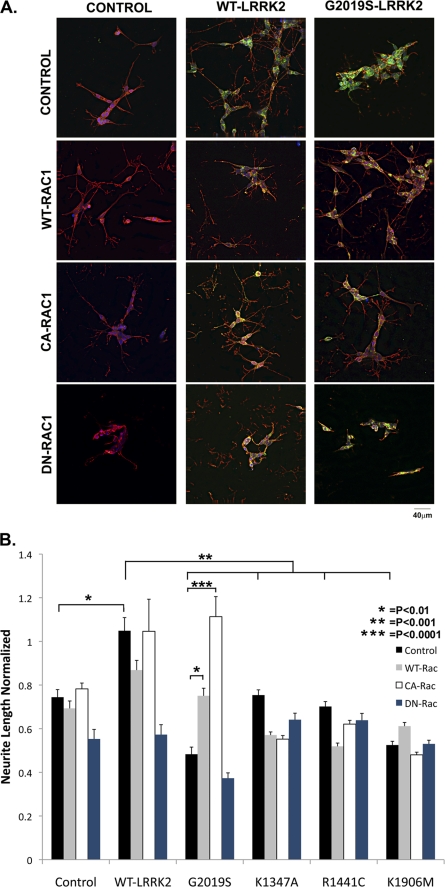
Rac1 Rescues Neurite Retraction Induced by G2019S in Differentiated SY5Y Cells
The striking interaction between LRRK2 and Rac1 related to membrane polarization prompted us to examine the interaction of the two proteins in neurons, which is a cell type that normally sends out processes. Previous studies indicate that G2019S LRRK2 causes striking neurite retraction when expressed in differentiated SY5Y cells (15). We used the same SY5Y neuroblastoma cellular model to investigate functional interactions between LRRK2 and Rac1. Under basal conditions, differentiated SY5Y cells show moderate process outgrowth (Fig. 6A). Expressing WT LRRK2 (Fig. 6A) or WT Rac1 (Fig. 6A) did not produce a statistically significant increase in the amount of outgrowth (Fig. 6B). In contrast, expressing G2019S LRRK2 strongly inhibited neurite outgrowth (Fig. 6, A and B).
FIGURE 6.
Rac1 rescues neurite retraction induced by G2019S LRRK2. A, immunocytochemical pictures showing the effects of LRRK2 (green) expression on process outgrowth in differentiated SH-SY5Y neurons, using phalloidin (red) to identify actin filaments. WT LRRK2 increased neurite length, whereas G2019S LRRK2 reduced neurite length. WT and CA-Rac1 rescued neuronal retraction induced by GG2019S LRRK2. DN-Rac1 blocked the effects of LRRK2, suggesting that the two proteins act in same pathway rather than through epistasis. B, quantification of changes in neurite outgrowth associated with LRRK2 and Rac1 expression. Lengths normalized to nontransfected differentiated SH-SY5Y neurons (n = 90 neurons). *, p < 0.01; **, p < 0.001; and ***, p < 0.0001.
Co-expressing WT LRRK2 with either WT or CA-Rac1 did not produce any more outgrowth than observed upon expression of any of the proteins alone (Fig. 6, A and B). However, co-expressing Rac1 with G2019S LRRK2 rescued the differentiated SY5Y neurite phenotype. Expressing WT Rac1 with G2019S LRRK2 restored neurite levels to that observed under basal conditions of differentiation (i.e. without transfected genes, Fig. 6, A and B). Co-expression of CA-Rac1 with G2019S LRRK2, however, increased neurite extension to that observed with co-expression of WT LRRK2 and CA-Rac1 (Fig. 6, A and B). These results show that Rac1 can compensate for functional deficits caused by G2019S expression. Other mutations did not affect neurite length, and Rac1 (even DN-Rac1) did not exert strong effects on neurite length in the presence of the mutant LRRK2 constructs (Fig. 6B).
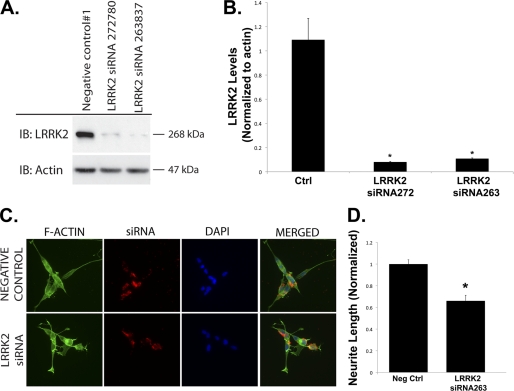
Knockdown of LRRK2 Reduces Neurite Length in Differentiated SY5Y Cells
We tested whether endogenous LRRK2 modulates neurite outgrowth by knocking down LRRK2 with siRNA (Fig. 7). Knockdown of LRRK2 was optimized in HEK293 cells where high transfection efficiency allowed immunoblotting and quantification of the LRRK2 protein levels (Fig. 7, A and B). Next, we examined the effects of siRNA knockdown in differentiated SH-SY5Y cells. Cells were transfected with fluorescence-tagged siRNA (LRRK2 or scrambled control). After 72 h, cells taking up siRNA were identified by fluorescence microscopy, and the neurite process length was quantified. siRNA for LRRK2, but not scrambled siRNA, significantly inhibited SH-SY5Y process length (Fig. 7, C and D). Thus, endogenous LRRK2 regulates neurite length.
FIGURE 7.
Knockdown of LRRK2 by siRNA reduces neurite process length. A and B, siRNA-mediated knockdown of LRRK2 reduced LRRK2 protein levels in HEK293 FT cells. C and D, fluorescent tagged siRNA was used to knockdown LRRK2 in differentiated SH-SY5Y cells. IP, immunoprecipitation; IB, immunoblot. Neg Ctrl, negative control.
DISCUSSION
The studies presented above indicate that LRRK2 binds the Rho-type GTPase Rac1 and that the two proteins functionally interact. We also investigated binding to RhoA and Cdc42 but observed that that the interactions were weaker. LRRK2 and Rac1 can be immunoprecipitated as a complex, they both partially co-localize at the membrane and exhibit mutual functional effects. Overexpressing Rac1 increases the amount of membrane-associated LRRK2. Overexpressing LRRK2 exerts a more subtle effect on Rac1. Biochemical fractionation does not show overt differences in Rac1 membrane translocation, possibly because Rac1 already is strongly membrane-associated, as suggested by the imaging studies. However, LRRK2 does cause a striking polarization phenotype, whereby the distribution of Rac1 (and LRRK2) becomes concentrated in one area of the plasma membrane. Use of CA-Rac1 accentuates this phenotype, localizing Rac1 and LRRK2 to budding processes and stimulating process outgrowth. We observe a corresponding increase in binding of Rac1 to PAK-GST, which is a classical indicator of increased activity. The functional interaction between Rac1 and LRRK2 appears to extend to the neurite retraction phenotype documented previously for G2019S LRRK2. We show that G2019S and R1441C LRRK2 both exhibit decreased binding to Rac1, but overexpressing WT or CA-Rac1 counteracts the effects of G2019S LRRK2 and restores normal neurite outgrowth in SY5Y neuroblastoma cells.
Binding between LRRK2 and Rac1 appears to require the ROC-COR and kinase domains, with the COR and kinase domains being sufficient for some binding. This pattern of binding is consistent with the patterns observed for other LRRK2 binding proteins, including mitogen activated kinase kinases (MKKs) and c-Jun N-terminal Kinase-interacting proteins (JIP) (10, 11); the binding sites for other putative binding proteins such as Fas-associated protein with death domain (FADD), moesin, Rab5, 14-3-3, ArhGef7, and 4E-BP are not known (16, 35–39) (40, 41). LRRK2 also binds to itself and to LRRK1 as homo- and heterodimers (22, 23, 31, 42). These protein crystallization studies examining dimerization were conducted using LRRK2 constructs containing only the ROC-COR kinase domains, which were similar to the domains analyzed in our study (22). Thus, binding of LRRK2 to Rac1 might be very similar to homo- and heterodimerization of LRRK2 (18, 22). Our studies indicate that LRRK2 binds Rac1 via the ROC-COR kinase domains, supporting the hypothesis that LRRK2 is forming a heterodimer with Rac1 through the ROC-COR kinase domain.
Pathological mutations in LRRK2 modulate binding to Rac1, but the relevance to PD is currently unclear because the effects vary among different mutations. We examined four different disease-linked mutations in LRRK2, G2019S, R1441C, Y1699C, and I2020T. Each of the mutations showed binding to Rac1 that differed from WT, but the direction of change varied among the mutations. The G2019S and R1441C mutations showed decreased binding, whereas the Y1699C and I2020T mutations showed increased binding. The observation that all four mutations affected binding to Rac1 is interesting because only two other LRRK2 binding proteins, FADD and the 14-3-3 family, have been shown to be affected by four different disease-linked mutations; however, for 14-3-3, G2019S does not inhibit binding (35, 40). Under the strictest criteria of disease linkage, every disease-linked mutation tested should modify binding activity and should do so in a consistent manner. LRRK2 kinase activity also does not meet these strict criteria because only the G2019S LRRK2 mutation has been consistently shown to increase kinase activity (36, 42, 43). Binding of LRRK2 to proteins such as 4E-BP either is not affected by disease-linked mutations or has not been investigated (37). FADD is the only LRRK2 binding protein that show consistent changes in binding (increased) with a panel of multiple disease-linked LRRK2 mutations (35). The consistency of this effect is striking and suggests that the link between FADD and LRRK2-mediated disease is tighter than for Rac1, where disease-linked mutations do not exert consistent effects. However, further studies are needed to clarify this point.
Our study adds to the increasing literature suggesting a role for LRRK2 in regulating actin and microtubule dynamics. LRRK2 has been shown to modulate neurite length in cell lines (SY5Y), in vivo after gene transfer by electroporation and in transgenic and knock-out mice (14–16). Overexpression of LRRK2 increases neurite outgrowth, whereas G2019S LRRK2 and LRRK2 knock-out decrease neurite outgrowth (14–16). Our data agree with these studies, in that we observed that G2019S LRRK2 and LRRK2 knockdown decreased neurite outgrowth. The mechanism underlying these changes has been unclear previously. Parisiadou et al. (16, 36) show that LRRK2 regulates phosphorylation of the actin binding proteins ezrin and moesin; previous studies suggest that LRRK2 binds to moesin. Our study integrates with the putative role of LRRK2 in actin biology because Rac1 binds PAK, which is part of a complex that regulates actin filament formation and includes the proteins ezrin, moesin and radixin (44), (45–46). The cellular locations where actin filaments form are critical to cellular function. The ability of LRRK2 to bind reversibly to membranes might allow it to act as a scaffold able to translocate to the membrane to promote formation of Rac1/PAK/actin complexes. These Rac1/PAK/actin complexes regulate membrane-associated functions such as neurite outgrowth, endocytosis (suggested by the interaction of LRRK2 with Rab5) and apoptosis (suggested by the interaction of LRRK2 with FADD) (16, 35–39).
The role of LRRK2 in regulating Rac1 differs from many classic activators because LRRK2 appears to play a larger role in regulating the localization of Rac1 than in regulating its overt activity. Co-expressing LRRK2 and Rac1 increases the GTPase activity of Rac1, as shown by the PAK-GST assay. LRRK2 appears to direct the location of active, membrane-bound Rac1 but does not increase the total amount of membrane bound Rac1. Interestingly, R1441C LRRK2, which does not bind Rac1, does not modulate Rac1 localization; this provides a cogent negative control for the expression studies. Cellular polarization plays a critical role in the biology of Rac1 because polarization is an essential step in growth of axons and dendrites, and multiple studies demonstrate that Rac1 is required for formation of axons and dendrites (24). In this context, the effects of LRRK2 on Rac1 are striking because co-expression induces dramatic polarization of Rac1 and LRRK2. Co-expression of WT LRRK2 and CA-Rac1 produces a phenotype that is even more pronounced, with consolidation of the LRRK2/Rac1 complex at focal points in the cell and formation of processes. Dominant negative Rac1 has the opposite affect as CA-Rac1 and prevents neurite outgrowth. Interestingly, G2019S LRRK2 has the same effect and also prevents neurite outgrowth. Other mutations in LRRK2 were unable to stimulate neurite outgrowth, unlike WT LRRK2, including R1441C, a pathological mutation, R1347A, a GTPase-dead mutation and K1906M, a kinase-dead mutation. The loss of function associated with G2019S LRRK2 can be complemented by WT or CA-Rac1 (Fig. 5). This complementation suggests that Rac1 acts downstream from LRRK2. Complementation due to epistasis appears to be unlikely because WT Rac1 has little effect on its own and only shows enhanced neurite outgrowth when LRRK2 is present; thus, the argument of additive effects (which is central to epistasis) cannot be invoked. The cumulative data suggest that LRRK2 binds Rac1 and assists Rac1 in stimulating its downstream targets, which includes PAK.
LRRK2 appears to exhibit a broad range of functions. LRRK2 has been shown to regulate vesicular endocytosis through the Rab5 protein (39). This is a function that is shared in common with α-synuclein (47). LRRK2 and α-synuclein also appear to share roles in regulating autophagy (15, 48). The interaction of LRRK2 with FADD suggests a role in apoptosis induced by external ligands, such as Fas or tumor necrosis factor (35). Our study adds to several studies showing a role for LRRK2 in regulating actin dynamics through interaction with proteins such as Rac1 and moesin (16, 36). A key question is whether these disparate activities represent functions that are independent or related. One of the most consistent observations related to PD is the involvement of mitochondria. Most of the genes linked to PD appear to affect mitochondrial function, and our recent study using Caenorhabditis elegans lines expressing LRRK2 show that LRRK2 modulates neuronal vulnerability to mitochondrial toxins much like mutations in parkin, PINK1, DJ-1, or α-synuclein (2, 49–53). It is possible that the role of LRRK2 in actin dynamics, autophagy, and cell death all connect to PD by functional convergence on mitochondria because mitochondria are trafficked by cytoskeletal elements, including actin filaments and microtubules, mitochondria turnover is mediated by autophagy, and apoptosis is also mediated by mitochondria.
Acknowledgments
We thank M. R. Cookson and E. Greggio for input into this project.
*
This work was supported, in whole or in part, by National Institutes of Health Grants NIEHS ES15567 and NINDS NS41786 and 066108 (to B. W.) and NINDS NS066658 (to D. C.). This work was also supported by the Alzheimer Association and Michael J. Fox Foundation (to B. W.).
2
The abbreviations used are:
PD
Parkinson disease
LRRK2
leucine-rich repeat kinase 2
PAK
p21-activated kinase
ROC
Ras-of-complex
COR
C-terminal of Ras complex
CA
constitutively active
DN
dominant negative.
REFERENCES
- 1.Dauer W., Przedborski S. (2003) Neuron 39, 889–909 [DOI] [PubMed] [Google Scholar]
- 2.Dawson T. M., Moore D. J., West A. B. (2009) Biochim. Biophys. Acta 1792, 585–586 [DOI] [PubMed] [Google Scholar]
- 3.Greggio E., Cookson M. R. (2009) ASN Neuro. 1, e00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biskup S., Gerlach M., Kupsch A., Reichmann H., Riederer P., Vieregge P., Wüllner U., Gasser T. (2008) J. Neurol. 255, 8–17 [DOI] [PubMed] [Google Scholar]
- 5.Taylor J. P., Mata I. F., Farrer M. J. (2006) Trends Mol. Med. 12, 76–82 [DOI] [PubMed] [Google Scholar]
- 6.Mata I. F., Wedemeyer W. J., Farrer M. J., Taylor J. P., Gallo K. A. (2006) Trends Neurosci. 29, 286–293 [DOI] [PubMed] [Google Scholar]
- 7.Festjens N., Vanden Berghe T., Cornelis S., Vandenabeele P. (2007) Cell Death Differ. 14, 400–410 [DOI] [PubMed] [Google Scholar]
- 8.Lewis P. A., Greggio E., Beilina A., Jain S., Baker A., Cookson M. R. (2007) Biochem. Biophys. Res. Commun. 357, 668–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Tan Y. C., Poulose S., Olanow C. W., Huang X. Y., Yue Z. (2007) J. Neurochem. 103, 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu C. H., Chan D., Wolozin B. (2010) Neurodegener. Dis. 7, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu C. H., Chan D., Greggio E., Saha S., Guillily M. D., Ferree A., Raghavan K., Shen G. C., Segal L., Ryu H., Cookson M. R., Wolozin B. (2010) J. Neurochem. 112, 1593–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith W. W., Pei Z., Jiang H., Moore D. J., Liang Y., West A. B., Dawson V. L., Dawson T. M., Ross C. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18676–18681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko H. S., Bailey R., Smith W. W., Liu Z., Shin J. H., Lee Y. I., Zhang Y. J., Jiang H., Ross C. A., Moore D. J., Patterson C., Petrucelli L., Dawson T. M., Dawson V. L. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 2897–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLeod D., Dowman J., Hammond R., Leete T., Inoue K., Abeliovich A. (2006) Neuron 52, 587–593 [DOI] [PubMed] [Google Scholar]
- 15.Plowey E. D., Cherra S. J., 3rd, Liu Y. J., Chu C. T. (2008) J. Neurochem. 105, 1048–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parisiadou L., Xie C., Cho H. J., Lin X., Gu X. L., Long C. X., Lobbestael E., Baekelandt V., Taymans J. M., Sun L., Cai H. (2009) J. Neurosci. 29, 13971–13980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carballo-Carbajal I., Weber-Endress S., Rovelli G., Chan D., Wolozin B., Klein C. L., Patenge N., Gasser T., Kahle P. J. (2010) Cell Signal 22, 821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gloeckner C. J., Schumacher A., Boldt K., Ueffing M. (2009) J. Neurochem. 109, 959–968 [DOI] [PubMed] [Google Scholar]
- 19.Liou A. K., Leak R. K., Li L., Zigmond M. J. (2008) Neurobiol. Dis. 32, 116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White L. R., Toft M., Kvam S. N., Farrer M. J., Aasly J. O. (2007) J. Neurosci. Res. 85, 1288–1294 [DOI] [PubMed] [Google Scholar]
- 21.Greggio E., Lewis P. A., van der Brug M. P., Ahmad R., Kaganovich A., Ding J., Beilina A., Baker A. K., Cookson M. R. (2007) J. Neurochem. 102, 93–102 [DOI] [PubMed] [Google Scholar]
- 22.Deng J., Lewis P. A., Greggio E., Sluch E., Beilina A., Cookson M. R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1499–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein C. L., Rovelli G., Springer W., Schall C., Gasser T., Kahle P. J. (2009) J. Neurochem. 111, 703–715 [DOI] [PubMed] [Google Scholar]
- 24.Watabe-Uchida M., Govek E. E., Van Aelst L. (2006) J. Neurosci. 26, 10633–10635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffe A. B., Hall A. (2005) Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 26.Heasman S. J., Ridley A. J. (2008) Nat. Rev. Mol. Cell Biol. 9, 690–701 [DOI] [PubMed] [Google Scholar]
- 27.Linseman D. A., Loucks F. A. (2008) Front Biosci. 13, 657–676 [DOI] [PubMed] [Google Scholar]
- 28.Kreis P., Barnier J. V. (2009) Cell Signal 21, 384–393 [DOI] [PubMed] [Google Scholar]
- 29.Smith S. D., Jaffer Z. M., Chernoff J., Ridley A. J. (2008) J. Cell Sci. 121, 3729–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards D. C., Sanders L. C., Bokoch G. M., Gill G. N. (1999) Nat. Cell Biol. 1, 253–259 [DOI] [PubMed] [Google Scholar]
- 31.Greggio E., Zambrano I., Kaganovich A., Beilina A., Taymans J. M., Daniëls V., Lewis P., Jain S., Ding J., Syed A., Thomas K. J., Baekelandt V., Cookson M. R. (2008) J. Biol. Chem. 283, 16906–16914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subauste M. C., Von Herrath M., Benard V., Chamberlain C. E., Chuang T. H., Chu K., Bokoch G. M., Hahn K. M. (2000) J. Biol. Chem. 275, 9725–9733 [DOI] [PubMed] [Google Scholar]
- 33.Ogita H., Takai Y. (2006) Methods Enzymol. 406, 415–424 [DOI] [PubMed] [Google Scholar]
- 34.Biskup S., Moore D. J., Celsi F., Higashi S., West A. B., Andrabi S. A., Kurkinen K., Yu S. W., Savitt J. M., Waldvogel H. J., Faull R. L., Emson P. C., Torp R., Ottersen O. P., Dawson T. M., Dawson V. L. (2006) Ann. Neurol. 60, 557–569 [DOI] [PubMed] [Google Scholar]
- 35.Ho C. C., Rideout H. J., Ribe E., Troy C. M., Dauer W. T. (2009) J. Neurosci. 29, 1011–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaleel M., Nichols R. J., Deak M., Campbell D. G., Gillardon F., Knebel A., Alessi D. R. (2007) Biochem. J. 405, 307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai Y., Gehrke S., Wang H. Q., Takahashi R., Hasegawa K., Oota E., Lu B. (2008) EMBO J. 27, 2432–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A., Greggio E., Beilina A., Kaganovich A., Chan D., Taymans J. M., Wolozin B., Cookson M. R. (2010) PLoS One 5, e8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin N., Jeong H., Kwon J., Heo H. Y., Kwon J. J., Yun H. J., Kim C. H., Han B. S., Tong Y., Shen J., Hatano T., Hattori N., Kim K. S., Chang S., Seol W. (2008) Exp. Cell Res. 314, 2055–2065 [DOI] [PubMed] [Google Scholar]
- 40.Nichols R. J., Dzamko N., Morrice N. A., Campbell D. G., Deak M., Ordureau A., Macartney T., Tong Y., Shen J., Prescott A. R., Alessi D. R. (2010) Biochem. J. 430, 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haebig K., Gloeckner C. J., Miralles M. G., Gillardon F., Schulte C., Riess O., Ueffing M., Biskup S., Bonin M. (2010) PLoS One 5, e13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sen S., Webber P. J., West A. B. (2009) J. Biol. Chem. 284, 36346–36356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anand V. S., Reichling L. J., Lipinski K., Stochaj W., Duan W., Kelleher K., Pungaliya P., Brown E. L., Reinhart P. H., Somberg R., Hirst W. D., Riddle S. M., Braithwaite S. P. (2009) FEBS J. 276, 466–478 [DOI] [PubMed] [Google Scholar]
- 44.Guo F., Debidda M., Yang L., Williams D. A., Zheng Y. (2006) J. Biol. Chem. 281, 18652–18659 [DOI] [PubMed] [Google Scholar]
- 45.Niggli V., Rossy J. (2008) Int. J. Biochem. Cell Biol. 40, 344–349 [DOI] [PubMed] [Google Scholar]
- 46.Hughes S. C., Fehon R. G. (2007) Curr. Opin. Cell Biol. 19, 51–56 [DOI] [PubMed] [Google Scholar]
- 47.Nemani V. M., Lu W., Berge V., Nakamura K., Onoa B., Lee M. K., Chaudhry F. A., Nicoll R. A., Edwards R. H. (2010) Neuron 65, 66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuervo A. M., Stefanis L., Fredenburg R., Lansbury P. T., Sulzer D. (2004) Science 305, 1292–1295 [DOI] [PubMed] [Google Scholar]
- 49.Saha S., Guillily M. D., Ferree A., Lanceta J., Chan D., Ghosh J., Hsu C. H., Segal L., Raghavan K., Matsumoto K., Hisamoto N., Kuwahara T., Iwatsubo T., Moore L., Goldstein L., Cookson M., Wolozin B. (2009) J. Neurosci. 29, 9210–9218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ved R., Saha S., Westlund B., Perier C., Burnam L., Sluder A., Hoener M., Rodrigues C. M., Alfonso A., Steer C., Liu L., Przedborski S., Wolozin B. (2005) J. Biol. Chem. 280, 42655–42668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vives-Bauza C., Zhou C., Huang Y., Cui M., de Vries R. L., Kim J., May J., Tocilescu M. A., Liu W., Ko H. S., Magrané J., Moore D. J., Dawson V. L., Grailhe R., Dawson T. M., Li C., Tieu K., Przedborski S. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Exner N., Treske B., Paquet D., Holmström K., Schiesling C., Gispert S., Carballo-Carbajal I., Berg D., Hoepken H. H., Gasser T., Krüger R., Winklhofer K. F., Vogel F., Reichert A. S., Auburger G., Kahle P. J., Schmid B., Haass C. (2007) J. Neurosci. 27, 12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schapira A. H. (2008) The Lancet Neurology 7, 97–109 [DOI] [PubMed] [Google Scholar]