Stimulus-induced S-Nitrosylation of Syntaxin 4 Impacts Insulin Granule Exocytosis (original) (raw)
Abstract
Glucose-stimulated insulin release from pancreatic islet β-cells involves increased levels of reactive oxygen and nitrogen species. Although this is normal, under pathophysiological conditions such as chronic hyperglycemia and inflammation, insulin exocytosis fails, and yet the mechanistic reason for failure is unclear. Hypothesizing that exocytotic proteins might be targets of _S_-nitrosylation, with their dysfunction under conditions of nitrosative stress serving as a mechanistic basis for insulin secretory dysfunction, we identified the t-SNARE protein Syntaxin 4 as a target of modification by _S_-nitrosylation. The cellular content of _S_-nitrosylated Syntaxin 4 peaked acutely, within 5 min of glucose stimulation in both human islets and MIN6 β-cells, corresponding to the time at which Syntaxin 4 activation was detectable. _S_-Nitrosylation was mapped to Syntaxin 4 residue Cys141, located within the Hc domain predicted to increase accessibility for v-SNARE interaction. A C141S-Syntaxin 4 mutant resisted _S_-nitrosylation induced in vitro by the nitric oxide donor compound _S_-nitroso-l-glutathione, failed to exhibit glucose-induced activation and VAMP2 binding, and failed to potentiate insulin release akin to that of wild-type Syntaxin 4. Strikingly, _S_-nitrosylation of Syntaxin 4 could be induced by acute treatment with inflammatory cytokines (TNFα, IL-1β, and IFNγ), coordinate with inappropriate Syntaxin 4 activation and insulin release in the absence of the glucose stimulus, consistent with nitrosative stress and dysfunctional exocytosis, preceding the cell dysfunction and death associated with more chronic stimulation (24 h). Taken together, these data indicate a significant role for reactive nitrogen species in the insulin exocytosis mechanism in β-cells and expose a potential pathophysiological exploitation of this mechanism to underlie dysfunctional exocytosis.
Keywords: Diabetes, Exocytosis, Insulin Secretion, Nitric Oxide, Pancreatic Islet, S-nitrosylation, Inflammatory Cytokines
Introduction
Regulated exocytosis encompasses a series of steps required for the transport of vesicles from the cell interior to the cell surface, a vital cellular function and essential for appropriate cellular and general physiologic homeostasis. Indeed, any loss of the tight control of these processes forms the molecular basis of disease and pathology ranging from neurological disorders to diabetes. Despite these divergent pathologies, the central SNARE2 hypothesis provides a unifying basic mechanism, centered on the interaction between SNARE proteins on the target membrane (t-SNARE) and those on the incoming vesicle (v-SNARE) in response to a stimulus, to mediate the essential vesicle exocytosis mechanism. Regulating the participation of the t-SNARE Syntaxin in the SNARE complex is the cognate regulatory Sec1/Munc18 (SM) protein (1). The fact that many of these SNARE and SM proteins are ubiquitously expressed but selectively mediate regulated exocytosis events in a cell-type and stimulus-specific manner suggests that layers of regulation must exist.
It is known that SNARE and SM proteins are post-translationally modified in a cell type- and stimulus-specific manner, which in turn impacts exocytosis. For example, the Syntaxin 1A-Munc18-1 complex is dissociated in response to the stimulus-induced serine/threonine phosphorylation of Munc18-1 in neuronal synaptic vesicle exocytosis (2, 3). Similarly, Syntaxin 4-Munc18c complexes in islet β-cells and 3T3-L1 adipocytes dissociate with the stimulus-induced tyrosine phosphorylation of Munc18c (4–7). Moreover, Munc18c undergoes _O_-linked glycosylation in 3T3-L1 adipocytes in response to insulin-resistant conditions, concomitant with reduced SNARE core complex formation and decreased GLUT4 vesicle exocytosis (8). Glucose-stimulated insulin secretion (GSIS) in pancreatic β-cells, like exocytotic processes in other cell types, is a highly regulated and nuanced homeostatic mechanism. Notably, GSIS utilizes t-SNARE and SM protein isoforms common to those of synaptic vesicle exocytosis; namely the t-SNAREs Syntaxin 1A and SNAP-25, the v-SNARE VAMP2/synaptobrevin, and the SM isoform nSec-1/Munc18-1 (9), as well as those used in GLUT4 vesicle exocytosis, Syntaxin 4, SNAP-23, and VAMP2 (10–12). Additional signaling mechanisms involving reactive oxygen and nitrogen species also have been implicated, including nitric oxide (NO)-mediated enhancement of SNARE core complex formation (specifically Syntaxin 1A, VAMP2, and SNAP-25) simultaneously with dissociation of nSec-1/Munc18-1 (13) and that Syntaxin 1A can undergo _S_-nitrosylation, which in turn dissociates it from Munc18-1 in PC12 cells (14).
Pancreatic islet β-cells are exquisitely sensitive to reactive oxygen stress due in part to lack of compensatory mechanisms to alleviate reactive oxygen and are highly susceptible to cytokine-induced insulin exocytosis aberrations. Combined with their ability to selectively utilize both Syntaxin 1A-Munc18-1 and Syntaxin 4-Munc18c-based SNARE complexes to evoke the two distinct phases of insulin secretion, β-cells are a unique model system for examination of SNARE/SM regulation by _S_-nitrosylation. Although the mechanistic basis for this early cytokine-induced aberration remains unresolved, glucose stimulation of the β-cell induces oxidative phosphorylation to produce ATP, with additional signaling mechanisms involving reactive oxygen and nitrogen species (15–17). Post-translational modification by _S_-nitrosylation entails the modification of proteins on the sulfhydryl side chain of cysteine residues with NO. This modification process is believed to be dynamic, like that of phosphorylation, though occurring in the absence of a protein catalyst, unlike that of most other characterized protein modification mechanisms. Nitric-oxide synthase (NOS) enzymes can synthesize NO endogenously from l-arginine, oxygen, and NADPH. Remarkably, while in β-cells, cytokine-induced nitrosative stress is a major cause of β-cell failure, increasing evidence suggests that redox-mediated processes may also contribute in a positive manner to the normal milieu (18), suggestive that low level or transient NO-related molecular events facilitate normal exocytotic mechanisms of insulin secretion.
Here, we report that in human islets and MIN6 pancreatic β-cells, _S_-nitrosylation of Syntaxin 4 occurred in response to an acute (5 min) glucose stimulation. Furthermore, the _S_-nitrosylation modification mapped specifically to Cys141 and served to facilitate activation of Syntaxin 4 as measured by association with its cognate v-SNARE, VAMP2. Interestingly, MIN6 β-cells stimulated acutely (2 h) with inflammatory cytokines TNF-α, IL-1β, and IFN-γ, showed a similar induction of Syntaxin 4 _S_-nitrosylation and stimulation of insulin secretion in the absence of the glucose stimulus. Given that NO is implicated as a modulator of insulin secretion in pancreatic β-cells, and islets are generally considered antioxidant-deficient and are particularly vulnerable to elevated oxidative stress conditions (19), an acute direct NO-mediated process could provide a mechanistic explanation for the early onset of secretory dysregulation in pancreatic β-cells subjected to inflammatory stress and/or chronic stimulation resulting in stress.
EXPERIMENTAL PROCEDURES
Materials
l-NG-monomethyl arginine citrate (l-NMMA) was purchased from Cayman Chemical (Ann Arbor, MI), and _S_-nitroso-l-glutathione (GSNO) was purchased from Thermo Fisher Scientific (Waltham, MA). Cytokine mixture containing recombinant mouse TNFα, IL-1β, and IFN-γ was purchased from R&D Systems (Minneapolis, MN). Protein G plus-agarose was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The MIN6 β-cells were a kind gift from Dr. John Hutton (University of Colorado Health Sciences Center). Goat anti-mouse and anti-rabbit horseradish peroxidase secondary antibodies and Transfectin lipid reagent were acquired from Bio-Rad. Monoclonal FLAG antibody, donkey serum, radioimmunoassay grade bovine serum albumin, and d-glucose were purchased from Sigma. Anti-mouse FITC was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Enhanced chemiluminescence (ECL) and SuperSignal West Femto reagents were obtained from Amersham Biosciences and Pierce, respectively. Vectashield mounting medium was purchased from Vector Laboratories (Burlingame, CA). The antibodies used in this study included: rabbit anti-actin (Sigma), rabbit anti-caspase-3 (Cell Signaling Technology, Beverly, MA), mouse anti-Cdc42 and SNAP-25 (BD Transduction Laboratories, San Jose, CA), mouse anti-FLAG (Sigma), rabbit anti-GST and SNAP-23 (Affinity Bioreagents, Golden, CO), mouse anti-Munc18-1 and anti-VAMP2 (Synaptic Systems, Göttingen, Germany), anti-Syntaxin 1A (Upstate Biotechnology/Millipore, Billerica, MA, and Synaptic Systems). Munc18c antibody was generated in our laboratory as described previously (20). Rabbit anti-Syntaxin 4 antibody was produced using the mouse 2–23 residue antigenic peptide and affinity purified for our laboratory (Invitrogen).
Plasmids
The generation of pGEX-4T1-Syntaxin 4ΔTM (TM domain deleted to enhance solubility) plasmid has been described previously (21). To this construct, site-directed mutagenesis was performed to convert the Cys141 to _S_-nitrosylation-deficient serine using the QuikChange II site-directed mutagenesis kit (Stratagene) and the manufacturer's recommended protocol. pGEX fusion constructs were transformed into Escherichia coli for expression of all GST fusion proteins and purified by glutathione-agarose affinity chromatography as described previously (22). The mammalian expression plasmid pCMV2-FLAG-Syntaxin 4-WT (full-length, includes TM) was generated by subcloning the PCR-amplified full-length rat Syntaxin 4 (residues 1–298) into the 5′ HindIII and 3′ SalI restriction sites of the pCMV2-FLAG vector, followed by site-directed mutagenesis to generate the C141S variant. All constructs were verified by DNA sequencing analysis.
Cell Culture, Transient Transfection, and Secretion Assays
MIN6 β-cells were cultured in DMEM (with 25 mm glucose) supplemented with 15% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 292 μg/ml l-glutamine, and 50 μm β-mercaptoethanol as described previously (23). MIN6 β-cells at 50–60% confluence were transfected with 40 μg of plasmid DNA per 10-cm2 dish using Transfectin (Bio-Rad) to obtain ∼50% transfection efficiency. After 48 h of incubation, cells were washed twice with freshly prepared modified Krebs-Ringer bicarbonate buffer (MKRBB; 5 mm KCl, 120 mm NaCl, 15 mm Hepes, pH 7.4, 24 mm NaHCO3, 1 mm MgCl2, 2 mm CaCl2, and 1 mg/ml radioimmunoassay grade bovine serum albumin). Following a 2-h incubation in MKRBB, cells were stimulated with 20 mm glucose for the times indicated in the figures.
Human Islet Culture
Isolated human pancreatic islets from independent cadaver donors were obtained from the Islet Cell Resource Centers. Human islets were cultured in fresh basal islet medium CMRL (Invitrogen, catalog no. 11530-037) medium for 2 h in a 37 °C, 5% CO2 cell incubator and hand-selected with a microscope to remove extraneous acinar tissue or dead islet tissue. Selected islets were washed twice with Kreb's ringer buffer (119 mm NaCl, 4.6 mm KCl, 1 mm MgSO4, 0.15 mm Na2HPO4, 0.4 mm KH2PO4, 25 mm NaHCO3, 2 mm CaCl2, 20 mm HEPES, pH 7.4, 0.05% BSA and then incubated for 2 h at 37 °C in low glucose conditions (2.8 mm) prior to experimental stimulation and harvest.
At time of harvest, supernatant was collected to assay for insulin release using 125I-insulin radioimmunoassay (Millipore, Bellerica, MA). Cells were subsequently lysed in Nonidet P-40 lysis buffer (25 mm Tris, pH 7.4, 1% Nonidet P-40, 10% glycerol, 50 mm sodium fluoride, 10 mm sodium pyrophosphate, 137 mm sodium chloride, 1 mm sodium vanadate, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml pepstatin, and 5 μg/ml leupeptin), and lysates were cleared by microcentrifugation for 10 min at 4 °C for subsequent use in interaction assays and co-immunoprecipitation experiments.
Biotin-Switch Assay
Lysates from either MIN6 mouse pancreatic β-cells or isolated human cadaveric donor islets were prepared as described above, with the exception that vanadate was excluded from the lysis buffer to avoid a potential metal-derived signal artifact. In addition, due to the limited amount of protein obtained from human islets, an additional 1 mg of purified radioimmunoassay-grade BSA was added to the lysate to obtain a sufficient protein concentration. All samples were then subjected to the biotin-switch protocol as described previously (24). Briefly, 1.5–2.5 mg of lysate-derived protein was incubated at 50 °C in the presence of methyl methanethiosulfonate (Sigma) to methylate any cysteine residues not already _S_-nitrosylated. Proteins were then precipitated in acetone and resuspended in (HENS buffer 100 mm Hepes, 1 mm EDTA, 0.1 mm neocuproine, 1% SDS (w/v), pH 8.0). Next, _S_-nitrosylated proteins were reduced with ascorbic acid and biotinylated using biotin-HPDP (biotin-conjugated to sulfhydryl-reactive _N_-(6-(biotinamido)hexyl)-3′-(2′-pyridyldithio)-propionamide; Pierce). Following a second acetone precipitation and resuspension, proteins were incubated overnight at 4 °C in the presence of streptavidin-agarose beads and biotinylated proteins resolved on 12% SDS-PAGE for subsequent transfer to PVDF for immunoblot analysis.
Recombinant Proteins and Activation Assays
The GST-VAMP2 protein (soluble, TM domain deleted) was generated in E. coli and purified by glutathione-agarose affinity chromatography as described previously (21) for use in the Syntaxin 4 accessibility assay. GST-VAMP2 linked to Sepharose beads was combined with 2.5 mg of detergent cell lysate for 2 h at 4 °C in Nonidet P-40 lysis buffer, followed by three stringent washes with lysis buffer, and associated Syntaxin 4 protein was resolved on 12% SDS-PAGE and detected by immunoblotting.
Co-immunoprecipitation and Immunoblotting
MIN6 β-cells were preincubated in MKRBB for 2 h followed by stimulation with glucose (20 mm). Cells were subsequently lysed in 1% Nonidet P-40 lysis buffer. Cleared detergent cell lysates (2 mg) were combined with primary antibody for 2 h at 4 °C followed by a second incubation with protein G plus-agarose for 2 h. The resultant immunoprecipitates were subjected to 12% SDS-PAGE followed by transfer to PVDF membrane for immunoblotting. Syntaxin 4, GST, Munc18c, and VAMP2 antibodies were used at 1:5000; FLAG antibody was used at 1:2000; SNAP25 and Munc18-1 antibodies were used at 1:1000; and Cdc42 antibody was used at 1:250. Secondary antibodies conjugated to horseradish peroxidase were diluted at 1:5000 for visualization by immunoblot as described above.
Immunofluorescence and Confocal Microscopy
MIN6 cells were grown to 40% confluence on glass coverslips and transfected with FLAG-WT- or C141S-Syntaxin 4 plasmid DNA. After 48 h of incubation, cells were placed in MKRBB for 2 h followed by fixation and permeabilization in 4% paraformaldehyde and 0.1% Triton X-100 for 10 min at 4 °C. Fixed cells were blocked in 1% BSA and 5% donkey serum for 1 h at room temperature, followed by incubation with anti-FLAG (M2) antibody (1:100) for 1 h and then washed with PBS and incubated with donkey anti-mouse IgG-conjugated FITC (1:100) antibody (Jackson ImmunoResearch Laboratories) for 1 h. Cells were washed again in PBS, overlaid with Vectashield mounting medium, and mounted for confocal fluorescence microscopy using a Zeiss 510 confocal microscope. Images were captured at the mid-plane of the cell cluster using a 100× objective with a 3-fold zoom.
Computer Modeling and Statistical Analysis
The three-dimensional structure of the Syntaxin 4 in association with Munc18c was shown previously (4), guided by the crystallized structure of the Syntaxin 1A-Munc18 complex (Protein Data Bank code 3C98) and the crystal structure of Munc18c (Protein Data Bank code 2PJX). Coordinates and putative crystal structure of Syntaxin 4 are available with the RCSB under code 2PJX. All representations of three-dimensional structures in the manuscript were generated with PyMOL. All data were evaluated for statistical significance using Student's t test, where appropriate.
RESULTS
Syntaxin 4 Is S-Nitrosylated in Response to Glucose Stimulation
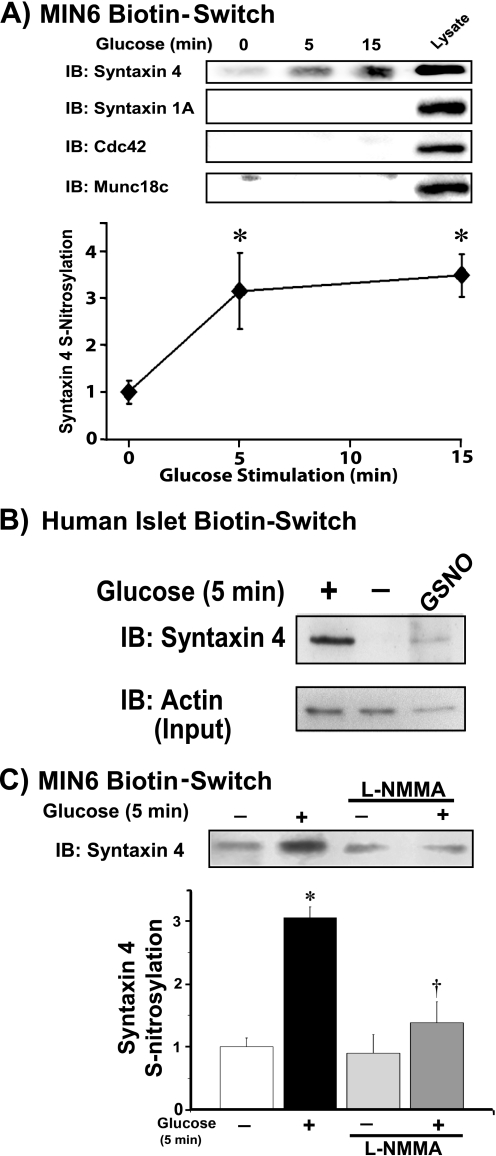
Given previous findings in neuronal model systems of exocytosis (13, 14, 25) and that NOS and NO are involved in glucose-stimulated insulin secretion (26–31), we employed the biotin-switch/immunoblot assay to determine if and which key exocytotic proteins undergo _S_-nitrosylation in response to glucose stimulation in clonal MIN6 mouse pancreatic β-cells. Following biotin-switch purification of _S_-nitrosylated proteins, we assayed for the presence of known operative SNARE proteins in insulin granule exocytosis. Additionally, we included Cdc42, which contains a cysteine residue known to be critical for post-translational protein geranylgeranylation, and therefore used as a control for nonspecific signal in our assay. In response to acute glucose stimulation spanning the initial insulin secretory response period, a 3-fold increase in _S_-nitrosylation exclusively of Syntaxin 4 was detected reproducibly as early as 5 min (Fig. 1A). Failure to detect _S_-nitrosylation of Cdc42 supported the notion that glucose specifically leads to _S_-nitrosylation of Syntaxin 4.
FIGURE 1.
Syntaxin 4 is specifically _S_-nitrosylated in response to acute glucose stimulation in a NOS-dependent manner. A, MIN6 β-cells were preincubated for 2 h in glucose-free MKRBB followed by stimulation for 5 or 15 min with 20 mm glucose. Clarified detergent cell lysates were subjected to a biotin-switch assay, proteins were resolved on 12% SDS-PAGE and immunoblotted (IB) for known insulin exocytosis proteins, as described under “Experimental Procedures.” Data are representative of three independent studies, quantified by optical density scanning and each normalized to unstimulated = 1.0. *, p < 0.05 versus unstimulated. B, human islets were preincubated for 2 h in glucose-free Kreb's ringer buffer followed by 5 min glucose stimulation; GSNO served as a positive control for the biotin-switch reaction. Biotin switch assays were conducted as described above. Mouse islets responded similarly (not shown). Actin abundance in starting islet lysates was determined by immunoblot (Input). C, biotin-switch immunoblot for _S_-nitrosylation of Syntaxin 4 protein following 1 h of preincubation with 500 μm general NOS inhibitor, l-NMMA, in the absence or presence of a 5-min glucose stimulation. Data represent three independent experiments, each normalized to unstimulated = 1 for bar graph quantitation. *, p < 0.05 versus nonstimulated vehicle control; †, p < 0.05 versus glucose-stimulated vehicle control.
Importantly, the rapid glucose-induced _S_-nitrosylation of Syntaxin 4 was replicated fully in human islets (Fig. 1B); the NO donor GSNO was used as a positive control for detection of human islet Syntaxin 4 _S_-nitrosylation in the biotin-switch assay. However, given the limited availability of high quality human islets, and having established the suitability of the MIN6 β-cells in replicating the _S_-nitrosylation effect, we performed further mechanistic studies of Syntaxin 4 _S_-nitrosylation using the MIN6 cell model system. The MIN6 cell line is considered to have insulin content and GSIS similar to that of normal islets, where normal responses occur at 16–20 mm glucose (32). MIN6 cells were incubated with the NOS inhibitor l-NMMA to confirm that this modification was dependent upon NOS function. Indeed, glucose-mediated _S_-nitrosylation of Syntaxin 4 was attenuated in response to l-NMMA (Fig. 1C). Although previously identified as a target of S-nitrosylation in PC12 cells, Syntaxin 1A was not found targeted in these assays of MIN6 β-cells. Moreover, Munc18c, the SM partner of Syntaxin 4, was not a target of _S_-nitrosylation. Other exocytotic factors of insulin granule exocytosis also failed to exhibit _S_-nitrosylation (supplemental Fig. 1). Taken together, these findings suggested Syntaxin 4 _S_-nitrosylation to be selective, and because it occurred in human islets, to be physiologically relevant to insulin granule exocytosis.
Syntaxin 4 Is Specifically S-Nitrosylated at Cys141
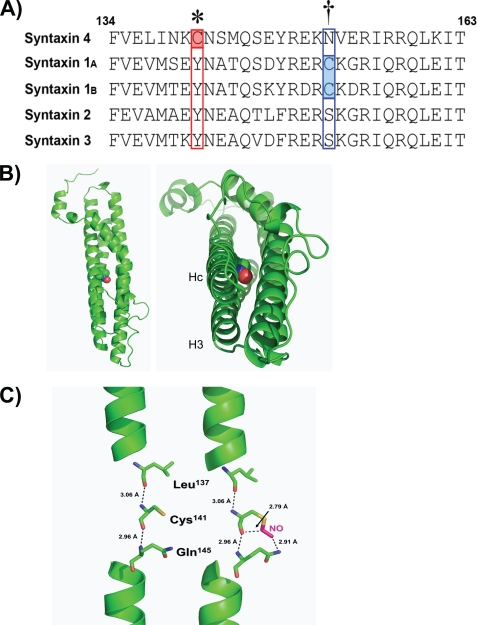
Next, sequence examination was used to determine the nature and location of the _S_-nitrosylation of Syntaxin 4. Syntaxin 4 contained only two cysteine residues, existing in the third of the three N-terminal coiled-coil domains, designated Hc, and the other in the transmembrane spanning domain at the far C terminus (Fig. 2A). Situated within the Hc domain of Syntaxin 4, Cys141 had characteristic features of an _S_-nitrosylation site, with a lysine residue at the −1 site and both acidic and basic residues in close proximity (33, 34). Alignment against other known plasma membrane-localized Syntaxin isoforms expressed in the β-cell revealed only Syntaxin 4 to contain a cysteine at this site, although Syntaxin 1A/1B did contain a cysteine residue within the Hc domain; the flanking residues differ slightly yet retain the identified characteristics of a potential _S_-nitrosylation site (35). Comparative analysis of the putative structure of Syntaxin 4 revealed that an _S_-nitrosylation modification of the cysteine sulfhydryl chain could potentially affect the closed conformation, as the modification resides squarely in the middle of this 4-helical bundle (Fig. 2B, red ball). Furthermore, rather than altering direct interactions with the other α-helices, which are positionally juxtaposed to Hc, the modification was predicted to change the interaction of Cys141 with other residues within the Hc α-helix, namely Gln145 (Fig. 2C). This led us to speculate that this modification may alter the ability of Hc to closely associate with the H3 (“SNARE”) domain by virtue of a “frameshift” within the Hc helix.
FIGURE 2.
Mapping and structural analysis of _S_-nitrosylation of Syntaxin 4. A, the amino acid sequence alignment of the known plasma membrane-localized Syntaxin isoforms expressed in pancreatic β-cells specifically within the Hc region of each protein. Syntaxin 4 contains two cysteine residues (Cys141 and Cys279, the latter of which is positioned within the putative transmembrane domain); red and blue boxed regions denote locations of Hc domain cysteines present in Syntaxin 4 and Syntaxin 1A/1B, respectively. B, predicted three-dimensional structure of the 4-helical bundle of Syntaxin 4 (4) showing the location of _S_-nitrosylated Cys141 (red ball) in the vertical (left) and horizontal (right) planes. C, predicted alteration of intramolecular interactions following _S_-nitrosylation of Cys141 within Syntaxin 4. _S_-Nitrosylation at Cys141 results in an additional potential interaction with Gln145.
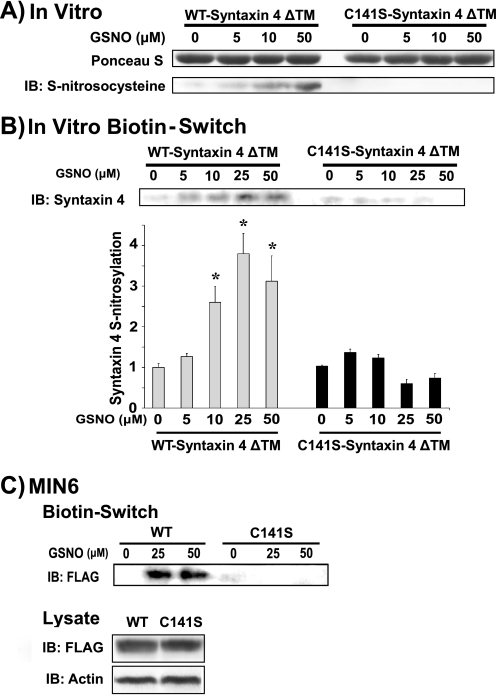
To investigate whether Cys141 sufficed as the specific site of _S_-nitrosylation within Syntaxin 4, we generated WT- and C141S-Syntaxin 4 bacterial expression constructs (lacking the transmembrane domain, ΔTM, to permit solubility in vitro). Under _in vitro S_-nitrosylation conditions, the _S_-nitrosylation of WT- but not C141S-Syntaxin 4-C141S-ΔTM was detected, via immunoblotting with an antibody specific to _S_-nitrosocysteine (Fig. 3A) or by the biotin-switch assay (Fig. 3B). In both cases, we detected _S_-nitrosylation at physiologically relevant levels of NO (36) and in a concentration-dependent manner. Subsequently, we tested full-length constructs of FLAG-tagged Syntaxin 4 transfected into MIN6 cells (Fig. 3C) to investigate the potential for the cysteine of the transmembrane domain to contribute to the nitrosylation signal. In similar fashion as truncated Syntaxin 4 constructs, WT-Syntaxin 4 was _S_-nitrosylated, whereas C141S-Syntaxin 4 was resistant to GSNO-induced nitrosylation, despite equivalent expression in the cell lysates. These data supported the concept that Cys141 served as the specific and sole site of _S_-nitrosylation in Syntaxin 4.
FIGURE 3.
Cys141 is the site of _S_-nitrosylation within Syntaxin 4. Recombinant purified soluble (ΔTM) WT-Syntaxin 4 or C141S-Syntaxin 4 proteins were subjected in vitro to treatment with 0–50 μm GSNO (1 h at 37 °C) for detection of _S_-nitrosylation, by immunoblotting (IB) for protein resolved on 12% SDS-PAGE with anti-S-nitrosocysteine antibody (A) or biotin-switch immunoblot assay (B), as described in the legend to Fig. 1 bar graph shows quantification of three independent studies by optical density scanning, normalized to unstimulated = 1, *, p < 0.05 versus unstimulated. C, biotin-switch assay using lysates prepared from MIN6 cells transiently transfected to express FLAG-tagged full-length WT- or C141S-Syntaxin 4. Lysate immunoblots show equivalent expression of WT- and C141S-Syntaxin 4 proteins in cells, relative to actin content.
S-Nitrosylation of Syntaxin 4 Positively Correlates with Binding to VAMP2
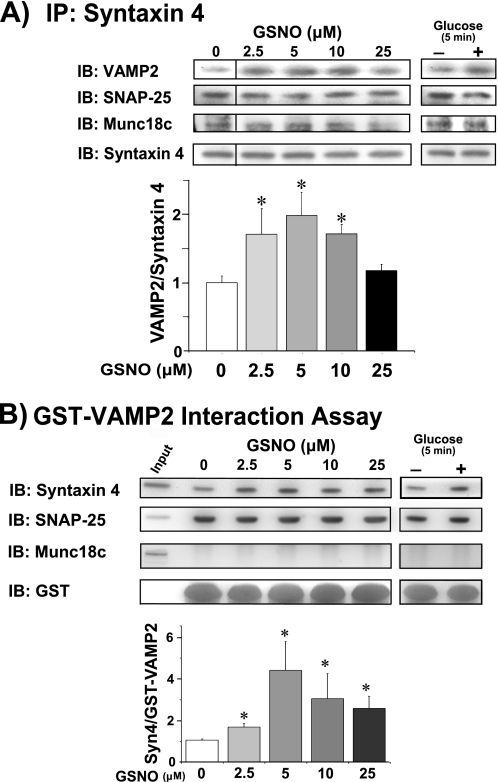
Syntaxin 4-VAMP2 complexes form within 5 min in response to the glucose stimulus in MIN6 β-cells (5). To determine whether _S_-nitrosylation correlated with increased or decreased Syntaxin 4 accessibility toward binding to VAMP2, GSNO exposure was used. Following exposure of lysates to GSNO (0–25 μm), we observed a significant increase in endogenous Syntaxin 4-VAMP2 binding, as assessed by anti-Syntaxin 4 coimmunoprecipitation (Fig. 4A). SNAP-25 binding to Syntaxin 4 was static, unchanged in response to GSNO treatment or glucose stimulation, consistent with its participation in transient binary Syntaxin 4-SNAP-25 complexes (21, 37). Munc18c was also co-immunoprecipitated by Syntaxin 4, however, because Munc18c-Syntaxin 4 binary complexes are of high affinity (KD = 32 nm) and are prevalent in MIN6 cells (4), this type of assay is not conclusive for determination of Munc18c protein binding to the SNARE core complex. Notably, Munc18c binding to the SNARE core complex has yet to be detected in β-cells.
FIGURE 4.
NO-mediated _S_-nitrosylation of Syntaxin 4 induces its activation and association with VAMP2. MIN6 cells were preincubated for 2 h in glucose-free MKRBB followed by stimulation for 5 min with 20 mm glucose or left unstimulated for incubation with the NO donor GSNO (0–25 μm for 1 h at 37 °C). Lysates were subsequently used for anti-Syntaxin 4 immunoprecipitation and immunoblot (IB) detection of VAMP2, SNAP-25, and Munc18c (A); the bar graph shows quantification of the ratio of VAMP2/Syntaxin 4 and normalized to untreated = 1 in each of three independent experiments. Syntaxin 4 activation assays were performed using recombinant GST-VAMP2 as bait to precipitate accessible Syntaxin 4 (Syn4) and SNAP-25 (B); the bar graph shows quantification of the ratio of Syn4/GST-VAMP2 fusion protein and normalized to untreated = 1 in each of three independent experiments. *, p < 0.05 versus untreated and unstimulated. Munc18c failed to precipitate with GST-VAMP2, despite its presence in corresponding input lysate.
Because endogenous SNARE complexes are composed of both trans- and cis-SNARE configurations, which cannot be distinguished by coimmunoprecipitation, we used an additional assay of Syntaxin 4 “activation.” Activation was gauged by the ability of endogenous Syntaxin 4 to bind to an exogenous GST-VAMP2 probe in response to appropriate stimuli, such as glucose (Fig. 4B). Mimicking this, the level of Syntaxin 4 activation peaked at 5 μm GSNO and declined as the dose approached levels more commonly associated with pathological stimulatory conditions (36). SNAP-25 also precipitated with GST-VAMP2, again in a static, GSNO- and glucose-independent manner. Munc18c failed to precipitate with GST-VAMP2 under any conditions tested, despite its presence in the input lysate, yet consistent with its known role in binary Syntaxin 4 complexes. Overall, the decline in Syntaxin 4 activation with pathological GSNO dosages alluded to a potential vulnerability of this signaling pathway to dysregulation in inflammatory situations.
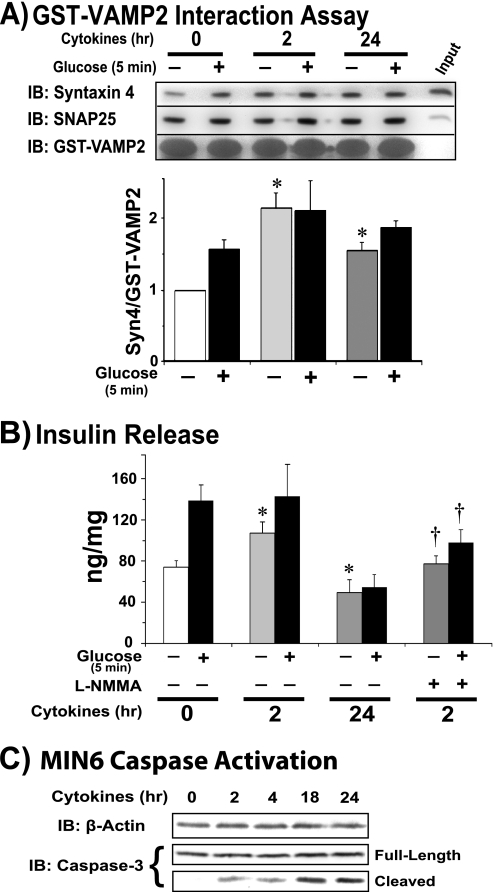
Acute Inflammatory Cytokine Signaling Induces S-Nitrosylation of Syntaxin 4
Given that cytokines are known to evoke production of NO in β-cells (31), we tested the possibility that acute cytokine stimulation might elicit Syntaxin 4 nitrosylation and/or activation, in sync with cytokine-induced dysregulation of GSIS. Inflammatory cytokine treatment leads to induction of cellular responses including changes in gene expression such as up-regulation of inducible NOS (NOS2), whereby inducible NOS protein levels rise, along with its contribution to the oxidative state of the cell. Previous work using l-NMMA indicated that the inhibitory actions of cytokines (IL-1β and/or IFNγ) upon non-obese diabetic mouse islet or rat islet GSIS were mediated by NO (31, 38, 39). As such, we examined Syntaxin 4 activation and acute GSIS (5 min stimulation) in response to variable cytokine exposure periods to include time points early within a time period where inducible NOS contribution would be either below pathological levels (2 h) or well into a pathological state (24 h). Because MIN6 β-cells are mouse-derived, a cytokine mixture including TNFα, IL-1β, and IFN-γ was required to elicit the full response (39). Syntaxin 4 activation in cells stimulated with inflammatory cytokines resulted in elevated activation under basal (unstimulated) conditions in cells pretreated for either 2 or 24 h (Fig. 5A). SNAP-25 also precipitated with GST-VAMP2, in a static, glucose-independent manner. Munc18c failed to precipitate with GST-VAMP2 under any conditions tested (data not shown).
FIGURE 5.
Acute cytokine-mediated signaling induces inappropriate Syntaxin 4 activation. MIN6 cells were preincubated with cytokine mixture for 2 h in glucose-free MKRBB prior to the 5-min glucose stimulation; for the 24-h time point, cytokines were added to DMEM for 22 h prior to and during the MKRBB incubation. A, lysates were subsequently used for Syntaxin 4 activation assays, using recombinant GST-VAMP2 as bait to precipitate accessible Syntaxin 4 (Syn4) and SNAP-25; the bar graph shows quantification of the ratio of Syn4/GST-VAMP2 fusion protein and normalized to untreated = 1 in each of three independent experiments. *, p < 0.05 versus untreated and unstimulated. B, insulin secretion into the buffer was quantitated. NOS inhibitor l-NMMA (500 μm) was added to a duplicate set of cells subjected to the 2-h cytokine treatment for the last 15 min of the preincubation period, immediately prior to glucose stimulation (20 mm). The bar graph represents the mean ± S.E. of insulin secretion quantified by radioimmunoassay and is adjusted for protein content of corresponding cells in each of six experiments. *, p < 0.05 versus untreated unstimulated. C, time-dependent induction of apoptotic signaling in MIN6 cells following the onset of cytokine exposure, as detected by immunoblotting (IB) lysates for presence of full-length and cleaved, activated caspase-3 (56).
Acute GSIS in MIN6 cells represents ∼1.5–2-fold increase within this acute 5-min glucose-stimulatory regime, as reported (32, 40–42) and shown in Fig. 5B (bars 1 and 2). Coordinately, 2 h of cytokine pretreatment caused a >40% increase in basal insulin secretion, consistent with prior reports (43–45), without potentiating acute GSIS (Fig. 5B, bars 3 and 4). Moreover, l-NMMA treatment of cells subjected to cytokines for 2 h abrogated the cytokine-induced increase in basal insulin release, as well as the acute glucose-induced increase. Cytokine treatment for 24 h had the expected toxic effects, based upon prior reports (44–46), with diminished levels of both basal and glucose-stimulated insulin release (Fig. 5B, bars 5 and 6); importantly, this was concurrent with persistent elevation of Syntaxin 4 basal activation compared with that of untreated cells. This corresponds with the time line of caspase-3 cleavage-mediated activation (Fig. 5C), where 2 h treatment had only minimal effect versus 24 h treatment.
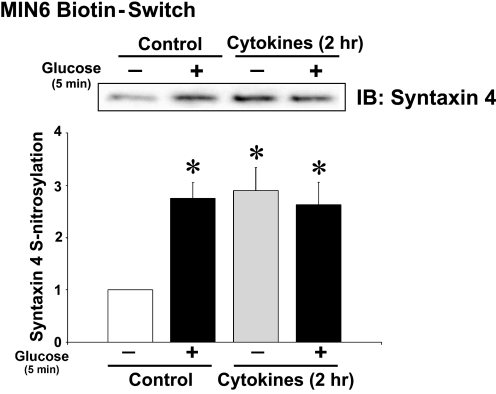
To determine whether the increased Syntaxin 4 activation correlated with an induction of Syntaxin 4 _S_-nitrosylation following a 2-h cytokine treatment, biotin-switch assays were performed. Compared with control unstimulated cells, 2 h of cytokine treatment indeed increased the basal level of Syntaxin 4 _S_-nitrosylation by nearly 3-fold, similar to that otherwise induced by acute glucose stimulation (Fig. 6).
FIGURE 6.
Short term cytokine treatment induces _S_-nitrosylation of Syntaxin 4. Biotin-switch immunoassay of lysates prepared from MIN6 cells stimulated with cytokines for the short term 2-h period followed by acute glucose stimulation for 5 min. Proteins captured in the biotin-switch were resolved on 12% SDS-PAGE for immunoblotting (IB) with anti-Syntaxin 4 antibody. The bar graph represents the mean ± S.E. for three experiments. *, p < 0.05 versus untreated and unstimulated.
Defective Binding and Function of C141S-Syntaxin 4 Mutant
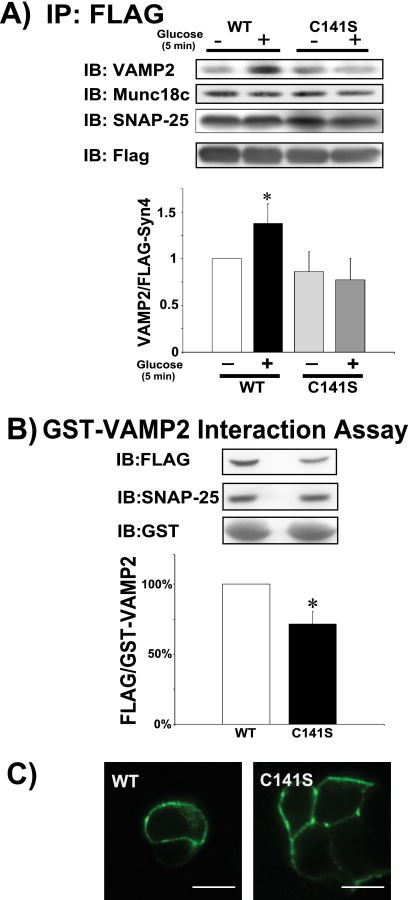
To delineate the requirement for _S_-nitrosylation of Syntaxin 4 at Cys141 for VAMP2 binding, Syntaxin 4 activation, and/or GSIS, we transfected MIN6 pancreatic β-cells with expression constructs of either FLAG-tagged full-length WT- or mutant C141S-Syntaxin 4. Immunoprecipitation of the FLAG-tagged WT-Syntaxin 4 yielded an increased association of VAMP2 from lysates prepared from glucose-stimulated cells (Fig. 7A); the C141S mutant failed to respond to the glucose stimulus. SNAP-25 binding to WT- and C141S-Syntaxin 4 proteins was static, unchanged in response to glucose stimulation. Similarly, Munc18c complexed with WT- and C141S-Syntaxin 4 proteins equitably. GST-VAMP2 interaction assays similarly showed a significant decrease in glucose-induced C141S-Syntaxin 4 activation, relative to WT-Syntaxin 4 (Fig. 7B). SNAP-25 also precipitated with GST-VAMP2 from lysates expressing WT- or C141S-Syntaxin 4 proteins. Munc18c failed to precipitate with GST-VAMP2 under any conditions tested (data not shown). The paucity of C141S-Syntaxin 4 binding and function was not due to cellular mislocalization of the C141S-Syntaxin 4 protein, as anti-FLAG immunolocalization placed both wild-type and mutant proteins at the plasma membrane (Fig. 7C).
FIGURE 7.
C141S-Syntaxin 4 shows attenuated glucose-induced activation and binding to VAMP2. Cleared detergent lysates were prepared from MIN6 cells transiently transfected to express FLAG-tagged full-length WT- or C141S-Syntaxin 4 and acutely stimulated with glucose for 5 min for use in anti-FLAG immunoprecipitation reactions for subsequent immunoblot (IB) detection of VAMP2, SNAP-25, and Munc18c (A). The bar graph shows quantification of the ratio of VAMP2/FLAG-Syn4 normalized to unstimulated WT = 1. *, p < 0.05 versus WT unstimulated. B, syntaxin 4 activation assays were performed using recombinant GST-VAMP2 as bait to precipitate accessible Syntaxin 4 (Syn4) and SNAP-25; the bar graph shows quantification of the ratio of FLAG-Syn4/GST-VAMP2 fusion protein. Glucose-stimulated WT-Syn4 binding was set equal to 100% and C141S-Syn4 binding compared thereto in each of three independent experiments, *, p < 0.05. C, confocal immunofluorescent microscopy was used to localize the WT- and C141S-Syn4 proteins expressed in MIN6 cells. Scale bar, 10 μm. Images are representative of at least 20 cells for each construct.
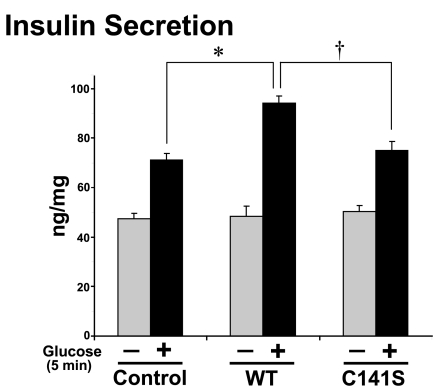
Consistent with these binding deficiencies, C141S-Syntaxin 4 failed to enhance GSIS akin to that of WT-Syntaxin 4 (Fig. 8). Previous studies have shown the potentiating effect of overexpressing WT-Syntaxin 4 upon GSIS, both in MIN6 cells and in islets from a transgenic WT-Syntaxin 4-overexpressing mouse model (12, 47). In both cases, basal secretion remains unaffected, consistent with the concept that an activation step is required and that the mere increase in Syntaxin 4 abundance is insufficient for initiation of insulin secretion. Coordinately, C141S-expressing cells did not elicit a dominant-negative response, but rather responded in a manner similar to that of cells transfected with empty vector (control), with the expected ∼1.5-fold increase in response to the acute 5-min glucose stimulation (albeit a slightly smaller index when compared with nontransfected cells). This contrasts with the enhanced acute GSIS of cells expressing WT-Syntaxin 4, consistent with the observed potentiation of first-phase GSIS in Syntaxin 4-overexpressing islets (12). These data suggested that Cys141 might be required to play an important positive role in GSIS.
FIGURE 8.
C141S-Syntaxin 4 fails to acutely potentiate glucose-stimulated insulin secretion. MIN6 cells transiently transfected to express FLAG-tagged full-length WT- or C141S-Syntaxin 4 were preincubated for 2 h in glucose-free MKRBB followed by stimulation for 5 min with 20 mm glucose or left unstimulated. Insulin secretion was quantified by radioimmunoassay adjusted for protein content of corresponding cells in five independent experiments. *, p < 0.05 versus control; †, p < 0.05 versus WT-Syn4.
DISCUSSION
In this study, we present for the first time evidence demonstrating specific _S_-nitrosylation of Cys141 in Syntaxin 4 and the role of this modification in insulin granule exocytosis, under both normal and pathophysiological conditions. During normal metabolic conditions, this _S_-nitrosylation modification may serve as a proexocytotic signal in pancreatic β-cells, enhancing activation of Syntaxin 4 to a state conducive to association with incoming VAMP2-bound insulin granules to form SNARE core complexes (Fig. 9, SNAP-25 not shown). Furthermore, this modification is dependent on NOS function, as we observed a significant loss of signal and exocytotic function through pharmacological inhibition of NOS with l-NMMA. We mapped the modification to a single cysteine residue, Cys141, and showed that its mutation to C141S attenuated its ability to bind and function in a glucose-responsive manner. Strikingly, acute cytokine treatment mimicked the effects of glucose upon Syntaxin 4 activation, coordinate with elevated basal insulin release, effectively negating the stimulus-regulated manner of exocytosis. This occurred prior to evidence of abundant cytokine-induced caspase activation, suggestive of an early and previously unrecognized effect of cytokines upon β-cell insulin granule exocytosis. Given that insulin exocytosis defects can often precede β-cell failure and death, the cytokine-induced _S_-nitrosylation of Syntaxin 4 may provide a mechanistic basis for this early exocytotic dysfunction.
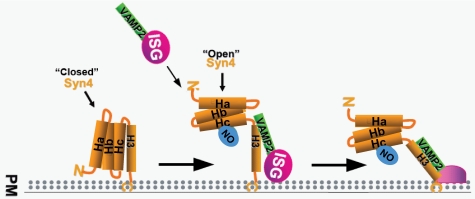
FIGURE 9.
Proposed mechanistic effect of Syntaxin 4 _S_-nitrosylation in β-cells. Under basal unstimulated conditions, Syntaxin 4 maintains a “closed” conformation. Upon acute glucose stimulation, Syntaxin 4 Cys141 undergoes _S_-nitrosylation, coordinate with the timing of its activation, presumed to represent a transition to an “open” conformation that is more accessible to incoming VAMP2-bound insulin secretory granules (ISG). This is expected to subsequently facilitate insulin granule docking and fusion with the plasma membrane (PM) to permit insulin release from the β-cell.
Unlike neuronal model systems where _S_-nitrosylation of Syntaxin 1A was observed (14), glucose-stimulation of pancreatic β-cells over a 5–15-min time course failed to yield detectable _S_-nitrosylated Syntaxin 1A. Remarkably, the cysteine residues in each Syntaxin isoform exist in their Hc domains, which share 65% sequence similarity with a conserved α-helical structure (48). Although Syntaxin 4 has yet to be crystallized, a version threaded from the Syntaxin 1A crystal structure shows tremendous overlap, suggesting that post-translational modification of each by S-nitrosylation might exert a similar “opening” effect to increase the accessibility to VAMP2 for SNARE core complex formation. Alternatively, differential _S_-nitrosylation of Syntaxin 4 versus Syntaxin 1A may be related to their differential pairing with SM proteins Munc18c and Munc18-1, respectively, or other isoform specific interactions, as syntaxins are well recognized as having numerous binding partners (49). In addition, t- and v-SNARE activation triggers have previously been proposed, such that activated SNAREs might bind and catalyze vesicle fusion more efficiently, although only a few candidate activators have been identified, such as Cdc42 and myosin Va (9, 50), both of which would require an upstream signal to carry out an activator function. In this regard, NO synthesis occurs well within the timeframe of glucose entry and metabolism in the β-cell, and as such, may be a plausible activation signal.
Because glucose does not stimulate inducible NOS induction to any significant degree within the acute 5-min period in which _S_-nitrosylation of Syntaxin 4 occurs, this might suggest that glucose can trigger the rapid generation of NO as a rapid and transient post-translational modification mechanism. This mechanism may be exploited under conditions of acute inflammatory signaling in pancreatic β-cells, prior to the later transcriptional alterations associated with cytokine-mediated inflammatory stimulation, including up-regulation and activation of inducible NOS (NOS2). Indeed, induction of inducible NOS has been implicated as a critical factor in cytokine-mediated β-cell dysfunction and subsequent destruction (51), though the specific role of NO is not entirely understood. This is supported by previous reports where 6 h of cytokine exposure was required to observe significant secretion suppression and onset of apoptosis (46). Along these same lines, the vast majority of studies which conclude in favor of secretion suppression and cell death rely on extended cytokine exposure (12–48 h) (52). Conversely, reports have also found that low levels of NO, arising from constitutive, calcium-dependent NOS isoforms, and neuronal NOS (NOS1) in particular, provide protection against cytokine-induced insult. Furthermore, cytokine administration has even been shown to confer anti-diabetogenic effects on β-cells (43). However, one could speculate that when NO levels rise to a critical threshold or remain constitutively present at elevated concentrations and/or in the presence of additional intracellular oxidants, the dual nature role of NO then would serve as a negative regulator and damaging agent, resulting initially in promotion of inappropriate secretion. To a certain extent, this hypothetical model is supported by human clinical data, whereby systemic insulin levels are elevated for extended periods in the presence of chronic inflammatory signaling prior to onset of clinically defined diabetes (53). Thus, _S_-nitrosylation of Syntaxin 4, and potentially other as-of-yet unidentified targets, could represent a potential site of initial mechanistic disruption leading to eventual pathology.
Given the potential for pleiotropic effects and the fact that reactive species, including NO, are able to affect a large number of potential targets, elucidating the true effect of NO signaling continues to pose both a technical and pharmacological challenge (e.g. reliance upon nonspecific inhibitors such as l-NMMA, lack of Syntaxin 4-specific _S_-nitrosylation inhibitors). A survey of the current literature shows a number of contradictions as to what role NO is actually serving in exocytosis. Therefore, it is entirely possible that the overall response to the signal is a summation of positive and negative NO-sensitive regulators, and as such is highly dependent upon the given cellular and subcellular circumstances, in addition to concentration effects. This may explain, in part, why general attempts at therapeutic intervention with antioxidant compounds have achieved little in terms of measurable clinical benefit (54). Here, we have endeavored to isolate the function of NO on one specific protein involved in pancreatic β-cell exocytosis during normal physiologic signaling events and in vitro using physiologically relevant concentrations of NO. To that end, our data suggest the influence of NO upon Syntaxin 4 to be a prosecretory effect under very low concentrations and within a very short time frame within this model, but we cannot rule out that at elevated concentrations such as inflammatory conditions, the concentrations of NO as well as the overall bioavailability of NO could contribute to an altogether different cellular response. This is exemplified by our finding that high levels of exogenous NO caused a reduction in Syntaxin 4 activation and VAMP2 binding (Fig. 4), relative to that induced at lower doses. An alternative explanation could arise from the fact that NO in the presence of superoxide anion (O2−) can drive the formation of peroxynitrite (ONOO−), which can subsequently react with protein tyrosine residues to form 3-nitrotyrosine as one example, and has been shown to have deleterious effects in pancreatic islets (55). Further study will be necessary to identify and characterize the effect of these protein modifications on insulin exocytosis and general islet health with an appreciation for highly nuanced physiological and pathophysiological differences.
Supplementary Material
Supplemental Data
Acknowledgments
We thank Dr. Tom Hurley for assistance with the PyMOL structures. We are grateful to Drs. Raghu Mirmira, Patrick Fueger, and Bernard Maier for critical review of the manuscript. We also thank Drs. Eunjin Oh, Zhanxiang Wang, and Jenna Jewell for technical assistance. Isolated human pancreatic islets from independent cadaver donors were obtained from the Islet Cell Resource Centers of the Integrated Islet Distribution Program (IIDP).
*
This work was supported, in whole or in part, by National Institutes of Health Grants K01 DK087811 and F32 HL090198 (to D. A. W.) and R01 DK067912 and DK76614 (to D. C. T.) and National Institutes of Health and American Heart Association Predoctoral Fellowship T32 DK64466 (to M. A. K.).
2
The abbreviations used are:
SNARE
SNAP receptor
MKRBB
modified Krebs-Ringer bicarbonate buffer
SNAP
soluble NSF attachment protein
SM
Sec1/Munc18 protein
GSIS
glucose-stimulated insulin secretion
GSNO
_S_-nitrosoglutathione
l-NMMA
l-NG-monomethyl arginine citrate
TM
transmembrane domain
t-SNARE
SNARE proteins on the target membrane
v-SNARE
SNARE proteins on the vesicle membrane.
REFERENCES
- 1.Südhof T. C., Rothman J. E. (2009) Science 323, 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nili U., de Wit H., Gulyas-Kovacs A., Toonen R. F., Sørensen J. B., Verhage M., Ashery U. (2006) Neuroscience 143, 487–500 [DOI] [PubMed] [Google Scholar]
- 3.Fujita Y., Sasaki T., Fukui K., Kotani H., Kimura T., Hata Y., Südhof T. C., Scheller R. H., Takai Y. (1996) J. Biol. Chem. 271, 7265–7268 [DOI] [PubMed] [Google Scholar]
- 4.Jewell J. L., Oh E., Bennett S. M., Meroueh S. O., Thurmond D. C. (2008) J. Biol. Chem. 283, 21734–21746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh E., Thurmond D. C. (2006) J. Biol. Chem. 281, 17624–17634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmelzle K., Kane S., Gridley S., Lienhard G. E., White F. M. (2006) Diabetes 55, 2171–2179 [DOI] [PubMed] [Google Scholar]
- 7.Umahara M., Okada S., Yamada E., Saito T., Ohshima K., Hashimoto K., Yamada M., Shimizu H., Pessin J. E., Mori M. (2008) Endocrinology 149, 40–49 [DOI] [PubMed] [Google Scholar]
- 8.Chen G., Liu P., Thurmond D. C., Elmendorf J. S. (2003) FEBS Lett. 534, 54–60 [DOI] [PubMed] [Google Scholar]
- 9.Jahn R., Scheller R. H. (2006) Nat. Rev. Mol. Cell Biol. 7, 631–643 [DOI] [PubMed] [Google Scholar]
- 10.Wheeler M. B., Sheu L., Ghai M., Bouquillon A., Grondin G., Weller U., Beaudoin A. R., Bennett M. K., Trimble W. S., Gaisano H. Y. (1996) Endocrinology 137, 1340–1348 [DOI] [PubMed] [Google Scholar]
- 11.Sadoul K., Berger A., Niemann H., Weller U., Roche P. A., Klip A., Trimble W. S., Regazzi R., Catsicas S., Halban P. A. (1997) J. Biol. Chem. 272, 33023–33027 [DOI] [PubMed] [Google Scholar]
- 12.Spurlin B. A., Thurmond D. C. (2006) Mol. Endocrinol. 20, 183–193 [DOI] [PubMed] [Google Scholar]
- 13.Meffert M. K., Calakos N. C., Scheller R. H., Schulman H. (1996) Neuron 16, 1229–1236 [DOI] [PubMed] [Google Scholar]
- 14.Palmer Z. J., Duncan R. R., Johnson J. R., Lian L. Y., Mello L. V., Booth D., Barclay J. W., Graham M. E., Burgoyne R. D., Prior I. A., Morgan A. (2008) Biochem. J. 413, 479–491 [DOI] [PubMed] [Google Scholar]
- 15.Gray J. P., Heart E. (2010) Toxicol. Mech. Methods 20, 167–174 [DOI] [PubMed] [Google Scholar]
- 16.Leloup C., Tourrel-Cuzin C., Magnan C., Karaca M., Castel J., Carneiro L., Colombani A. L., Ktorza A., Casteilla L., Pénicaud L. (2009) Diabetes 58, 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzo M. A., Piston D. W. (2003) J. Cell Biol. 161, 243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malmgren S., Nicholls D. G., Taneera J., Bacos K., Koeck T., Tamaddon A., Wibom R., Groop L., Ling C., Mulder H., Sharoyko V. V. (2009) J. Biol. Chem. 284, 32395–32404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenzen S. (2008) Biochem. Soc. Trans. 36, 343–347 [DOI] [PubMed] [Google Scholar]
- 20.Thurmond D. C., Ceresa B. P., Okada S., Elmendorf J. S., Coker K., Pessin J. E. (1998) J. Biol. Chem. 273, 33876–33883 [DOI] [PubMed] [Google Scholar]
- 21.Jewell J. L., Luo W., Oh E., Wang Z., Thurmond D. C. (2008) J. Biol. Chem. 283, 10716–10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min J., Okada S., Kanzaki M., Elmendorf J. S., Coker K. J., Ceresa B. P., Syu L. J., Noda Y., Saltiel A. R., Pessin J. E. (1999) Mol. Cell 3, 751–760 [DOI] [PubMed] [Google Scholar]
- 23.Ke B., Oh E., Thurmond D. C. (2007) J. Biol. Chem. 282, 21786–21797 [DOI] [PubMed] [Google Scholar]
- 24.Forrester M. T., Foster M. W., Benhar M., Stamler J. S. (2009) Free Radic. Biol. Med. 46, 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prior I. A., Clague M. J. (2000) Biochim. Biophys. Acta 1475, 281–286 [DOI] [PubMed] [Google Scholar]
- 26.Laffranchi R., Gogvadze V., Richter C., Spinas G. A. (1995) Biochem. Biophys. Res. Commun. 217, 584–591 [DOI] [PubMed] [Google Scholar]
- 27.Nakata M., Yada T. (2003) Pancreas 27, 209–213 [DOI] [PubMed] [Google Scholar]
- 28.Smukler S. R., Tang L., Wheeler M. B., Salapatek A. M. (2002) Diabetes 51, 3450–3460 [DOI] [PubMed] [Google Scholar]
- 29.Spinas G. A., Laffranchi R., Francoys I., David I., Richter C., Reinecke M. (1998) Diabetologia 41, 292–299 [DOI] [PubMed] [Google Scholar]
- 30.Vincent S. R. (1992) Science 258, 1376–1378 [DOI] [PubMed] [Google Scholar]
- 31.Corbett J. A., Mikhael A., Shimizu J., Frederick K., Misko T. P., McDaniel M. L., Kanagawa O., Unanue E. R. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 8992–8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 33.Greco T. M., Hodara R., Parastatidis I., Heijnen H. F., Dennehy M. K., Liebler D. C., Ischiropoulos H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7420–7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hess D. T., Matsumoto A., Kim S. O., Marshall H. E., Stamler J. S. (2005) Nat. Rev. Mol. Cell Biol. 6, 150–166 [DOI] [PubMed] [Google Scholar]
- 35.Hao G., Derakhshan B., Shi L., Campagne F., Gross S. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1012–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakada S., Ishikawa T., Yamamoto Y., Kaneko Y., Nakayama K. (2003) Pflugers Arch 447, 305–311 [DOI] [PubMed] [Google Scholar]
- 37.Weninger K., Bowen M. E., Choi U. B., Chu S., Brunger A. T. (2008) Structure 16, 308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas H. E., Darwiche R., Corbett J. A., Kay T. W. (2002) Diabetes 51, 311–316 [DOI] [PubMed] [Google Scholar]
- 39.Cetkovic-Cvrlje M., Eizirik D. L. (1994) Cytokine 6, 399–406 [DOI] [PubMed] [Google Scholar]
- 40.Nevins A. K., Thurmond D. C. (2003) Am. J. Physiol. Cell Physiol 285, C698–710 [DOI] [PubMed] [Google Scholar]
- 41.Thurmond D. C., Gonelle-Gispert C., Furukawa M., Halban P. A., Pessin J. E. (2003) Mol. Endocrinol. 17, 732–742 [DOI] [PubMed] [Google Scholar]
- 42.Ishihara H., Asano T., Tsukuda K., Katagiri H., Inukai K., Anai M., Kikuchi M., Yazaki Y., Miyazaki J. I., Oka Y. (1993) Diabetologia 36, 1139–1145 [DOI] [PubMed] [Google Scholar]
- 43.Corbett J. A., McDaniel M. L. (1992) Diabetes 41, 897–903 [DOI] [PubMed] [Google Scholar]
- 44.Donath M. Y., Størling J., Berchtold L. A., Billestrup N., Mandrup-Poulsen T. (2008) Endocr. Rev. 29, 334–350 [DOI] [PubMed] [Google Scholar]
- 45.Spinas G. A., Palmer J. P., Mandrup-Poulsen T., Andersen H., Nielsen J. H., Nerup J. (1988) Acta Endocrinol. 119, 307–311 [DOI] [PubMed] [Google Scholar]
- 46.Wu J. J., Chen X., Cao X. C., Baker M. S., Kaufman D. B. (2001) J. Surg. Res. 101, 190–195 [DOI] [PubMed] [Google Scholar]
- 47.Spurlin B. A., Park S. Y., Nevins A. K., Kim J. K., Thurmond D. C. (2004) Diabetes 53, 2223–2231 [DOI] [PubMed] [Google Scholar]
- 48.Bennett M. K., García-Arrarás J. E., Elferink L. A., Peterson K., Fleming A. M., Hazuka C. D., Scheller R. H. (1993) Cell 74, 863–873 [DOI] [PubMed] [Google Scholar]
- 49.Goh C. S., Cohen F. E. (2002) J. Mol. Biol. 324, 177–192 [DOI] [PubMed] [Google Scholar]
- 50.Watanabe M., Nomura K., Ohyama A., Ishikawa R., Komiya Y., Hosaka K., Yamauchi E., Taniguchi H., Sasakawa N., Kumakura K., Ushiki T., Sato O., Ikebe M., Igarashi M. (2005) Mol. Biol. Cell 16, 4519–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnush M., Scarim A. L., Heitmeier M. R., Kelly C. B., Corbett J. A. (1998) J. Immunol. 160, 2684–2691 [PubMed] [Google Scholar]
- 52.Mathis D., Vence L., Benoist C. (2001) Nature 414, 792–798 [DOI] [PubMed] [Google Scholar]
- 53.Leahy J. L. (1990) Diabetes Care 13, 992–1010 [DOI] [PubMed] [Google Scholar]
- 54.Scott J. A., King G. L. (2004) Ann. N.Y. Acad. Sci. 1031, 204–213 [DOI] [PubMed] [Google Scholar]
- 55.Koeck T., Corbett J. A., Crabb J. W., Stuehr D. J., Aulak K. S. (2009) Arch Biochem. Biophys 484, 221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boatright K. M., Renatus M., Scott F. L., Sperandio S., Shin H., Pedersen I. M., Ricci J. E., Edris W. A., Sutherlin D. P., Green D. R., Salvesen G. S. (2003) Mol. Cell 11, 529–541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data