A Gynecologic Oncology Group phase II trial of the protein kinase C-beta inhibitor, enzastaurin and evaluation of markers with potential predictive and prognostic value in persistent or recurrent epithelial ovarian and primary peritoneal malignancies (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 1.
Published in final edited form as: Gynecol Oncol. 2011 Mar 17;121(3):455–461. doi: 10.1016/j.ygyno.2011.02.013
Abstract
Purpose
Protein kinase C (PKC) activation contributes to proliferation and angiogenesis in epithelial ovarian or primary peritoneal carcinoma (EOC/PPC). A multi-institutional phase II trial was conducted to evaluate the efficacy and safety of PKCβ inhibitor enzastaurin in persistent or recurrent EOC/PPC and to explore potential prognostic and predictive biomarkers.
Methods
Eligible women with measurable platinum-sensitive and resistant EOC/PPC were treated with continuous administration of oral enzastaurin until disease progression or unacceptable toxicity. A two-stage sequential design was used to evaluate progression-free survival (PFS) ≥ 6-months, tumor response, and toxicity. Translational studies included sequencing of the TP53, PTEN, PIK3CA and PKCβII genes for somatic mutations, quantitative PCR assays for AKT2 and PTEN copy number alterations, and measurement of circulating VEGF-A plasma levels.
Results
Among 27 eligible and evaluable patients, 3 women with PFS ≥ 6-months (11%) and 2 women with partial responses (7%) were observed. One of them achieved a durable response and remains on the study. No grade 4 adverse events were observed. Most common grade 3 adverse events were constitutional (4) and gastrointestinal (3). Mutations in the TP53 gene and abnormal copy number in the PTEN gene were common (56% and 48% of cases, respectively).
Conclusions
Enzastaurin was tolerable but had insufficient activity to proceed with the second stage of accrual. However, 1 patient has been progression-free for 44 months. No association between a biomarker and response to enzastaurin has been found.
Exploratory analysis suggested an association between survival and PTEN copy number losses.
Keywords: Enzastaurin, ovarian cancer, VEGF PKCβ, TP53, PTEN, PIK3CA, AKT2
INTRODUCTION
Ovarian cancer remains the most lethal cancer of the female genital tract. The estimated incidence of ovarian cancer in the United States for 2009 is 21,550 with about 14,600 deaths [1]. Current first-line treatment for epithelial ovarian or primary peritoneal carcinoma (EOC/PPC) consists of platinum-based chemotherapy following surgical staging with cytoreduction; however, the majority of patients will develop a recurrence and their median overall survival (OS) is approximately two years [2].
Thus, new agents are needed to improve OS of women with EOC/PPC. Biologic agents with novel mechanisms of action are especially appealing in this disease because of their usually more favorable side effect profile and fewer overlapping toxicities with chemotherapy.
Enzastaurin hydrochloride (LY317615 – Eli Lilly) is an acyclic bisindolylmaleimide developed for the treatment of cancer based on its direct anti-proliferative and anti-angiogenic properties [3,4]. It is a potent selective oral inhibitor of protein kinase C (PKC), especially PKC beta (PKCβ), and an inhibitor of PI3 kinase (phospatidylinositol 3-kinase)/AKT signaling pathway [5]. PKC is a family of serine/threonine protein kinases which are high-affinity intracellular receptors to phorbol esters, natural products known to induce skin carcinogenesis in mice [6,7]. PKC beta (PKCβ) is one of the most active PKCs involved in regulating cell proliferation. Activated PKCβ has been found in multiple human tumors including EOC/PPC [8]. Enzastaurin is relatively inactive against other kinases, especially the tyrosine kinase family.
PKCβ as well as other PKCs activate AKT (also known as protein kinase B) which is frequently activated in a wide array of human cancers [9,10,11]. The activation of PI3 kinase (phospatidylinositol 3-kinase)/AKT cell signaling pathway promotes tumor cell survival and proliferation [12]. Enzastaurin blocks the key enzymes of this pathway and has demonstrated the ability to suppress the proliferation of cancer cell lines in vitro including EOC/PPC cell lines: OVCAR-3, OVCAR-4, and OVCAR-8 when tested against the NCI 60 cell line panel [13].
In addition, enzastaurin has anti-angiogenic activity and has been shown to suppress vascular endothelial growth factor (VEGF) production by human tumor xenografts [13,14]. EOC/PPC secrete large amounts of VEGF which stimulates angiogenesis and plays a crucial role in progression of EOC/PPC [15]. A monoclonal antibody to VEGF, bevacizumab, has demonstrated significant activity in ovarian cancer [16]. Thus, anti-angiogenic agents hold promise in the treatment of EOC/PPC.
Enzastaurin was well tolerated in a phase I trial with Grade I chromaturia (red discoloration of urine), fatigue, and GI side effects being the most common toxicities [12]. The observed dose limiting toxicity was changes in QTc defined as prolongation of QTc by more than 50 msecs over baseline [12].
The clinical development of enzastaurin, a potent inhibitor of the PKC and PTEN/PI3K/AKT signaling pathways, is of great interest in EOC/PPC. A translational research objective has been embedded in this phase II protocol to evaluate a panel of biomarkers relevant to enzastaurin and disease state.
As mentioned earlier, the evidence of deregulation of the PI3K/AKT/PTEN pathway in EOC/PPC has been reported previously and includes gain-of-function mutations and amplifications of PI-3-kinase [17]; amplification of AKT2 [18,19]; allelic imbalance and loss of heterozygosity in PTEN genes [20,21,22]. PTEN (_P_hosphatase and _TEN_sin homologue) is a key tumor suppressor and normally opposes the activation of the proto-oncogenic PI3K/AKT signaling pathway. Also, there are multiple nodes of crosstalk between PI3K/AKT/PTEN and p53 signaling pathways [23] which may result in cisplatin resistance in EOC/PPC [24]. Given these data, we assessed mutations and copy number changes in PIK3CA, AKT2, PKC, PTEN, and TP53 genes in this study. We sought to determine whether these genetic aberrations in the tumor might have prognostic value as opposed to the levels or activity of proteins encoded by them which may change in the course of the treatment. We also measured VEGF plasma levels before and after the first cycle of enzastaurin to find out whether the change in VEGF level could serve as a potential marker of early response to the drug.
METHODS
Patients
This was a multi-center phase II trial of enzastaurin as a single-agent in women with advanced EOC/PPC. To be eligible for the trial, patients must have had histologic documentation of diagnosis with measurable disease according to RECIST (1.0) criteria [25].
Patients were required to have 1 platinum-based chemotherapy regimen and allowed to have 1 additional cytotoxic regimen. Patients needed a GOG Performance Status of 0, 1, or 2, or just 0 or 1 depending on prior chemotherapy. Patients were required to have a platinum-free interval of less than 12 months or persistent disease. Patients with platinum-resistant and platinum-sensitive disease (i.e. had Platinum Free Interval between 6 and 12 months) were included. Only patients with adequate bone marrow, renal, and liver functions were eligible.
All women provided written informed consent and participating institutions obtained annual Institutional Review Board (IRB) approval for this trial including the correlative studies.
Drug Administration
Enzastaurin was administered orally in a fed state at a loading dose of 375 mg TID on day 1 followed by continuous treatment with 500 mg daily until disease progression or adverse effects prohibited further therapy. The length of one cycle was 28 days.
Clinical Management, Assessments and Testing
Pretreatment evaluation consisted of history and physical exam, CXR, ECG, CBC, serum chemistries, urinalysis, CA125, PT/PTT, and radiographic documentation of measurable disease by CT or MR scan.
During the study, interval visits, CBC, serum chemistries and CA125 were obtained at the start of each cycle (every 28 days). CT or MR scan to evaluate for response was performed every other cycle for the first 6 months. Evaluation of response was per formed by RECIST (1.0) criteria [25]. Dose reduction to 250 mg of Enzastaurin daily was required for severe hematologic and non-hematologic toxicities.
Clinical End Points
The primary efficacy clinical end-points of the study were the frequency of patients who were alive and progression-free at 6 months (PFS at 6 months) or who had a tumor response. Another primary endpoint was the toxicity of enzastaurin. Secondary clinical endpoints included the duration of PFS and OS as well as the assessment of the impact of platinum sensitivity, initial performance status, and age on PFS and OS.
Translational Research Objectives
The translational research objectives were to assess markers that could be relevant to the effectiveness of enzastaurin (Supplemental Figure 1) including somatic mutations in genes such as TP53, PTEN, PIK3CA and PKCβII in DNA extracted from tumor before initiation of treatment, number of copies of PTEN and AKT2, and VEGF-A concentration in plasma pre-cycle 1 and pre-cycle2.
Specimens
Archival formalin-fixed and paraffin-embedded (FFPE) primary, metastatic and/or recurrent tumor collected prior to starting enzastaurin treatment was a requirement for all patients who consented to the study participation. Twenty-three patients had their biomarkers tested from their primary tumors, and four had it tested from metastatic disease at the primary diagnosis. Only one patient had testing done on recurrent tumor. Therefore the results of this study were obtained primarily from the analysis of tissue procured before initial chemotherapy.
A block or 20 unstained slides for each patient was submitted for analysis. Plasma was prepared from blood drawn before the first (pre-cycle 1) and the second (pre-cycle 2) cycles of enzastaurin treatment respectively, frozen at ≤−70°C and shipped to the GOG Tissue Bank.
DNA Extraction
Genomic DNA was extracted from FFPE tumor tissue using a Trimgen DNA purification kit (Trimgen Corp, Sparks, MD) according to the kit instructions.
Mutation analysis for TP53, PTEN, PIK3CA and PKCβII
Polymerase chain reaction (PCR) primers flanking the desired exons were used to amplify TP53 (exons 5–9), PTEN (exons 2, 3, 5, 6 and 7), PIK3CA (exons 1, 9 and 20) and PKCβII (exons 2–17). Primer sequences are available in supplemental Table 1. PCR amplicons were subjected to direct sequencing using same primers for PCR amplification and ABI BigDye Terminator kit v1.1 (Applied Biosystems, Foster City, CA) according to manufacturer’s instructions. Sequence variations were determined by using Sequencher software 4.7 (Gene Codes Corporation, Ann Arbor, MI) compared with GenBank genomic sequences for each gene. All of the sequence variations were confirmed by multiple, independent PCR amplifications and repeated sequencing reactions.
Copy number assessments for PTEN and AKT2
Copy number analysis of AKT2, PTEN (using two independent probes, intron 1 and intron 5) was performed using quantitative PCR (qPCR). Custom TaqMan assays were obtained from ABI (Supplemental Table 2): All probes were labeled with FAM as the reporter dye and TAMRA as the quencher. Each sample was run in triplicate on an ABI Prism 7900HT analyzer using Caveolin-1 as a reference gene. A standard curve was generated using normal human genomic DNA and data was analyzed using the standard curve method.
Enzyme-linked immunosorbent assay for VEGF
VEGF-A concentrations were quantified using the Quantikine Human VEGF ELISA kit, catalog # DVE00 (R&D Systems, Inc; Minneapolis, MN) and the VEGF165 standard in pre-cycle 1 and pre-cycle 2 plasma specimens according to the kit instructions.
Statistical Design
The study was designed to detect cytostatic activity by examining delays in tumor progression through the frequency of patients PFS at 6 months and cytotoxic activity through the frequency of tumor response. Activity by either measure was considered sufficient to declare the drug worthy of further investigation in a phase III trial. A bivariate 2-stage design was used to limit needless exposure when the drug was inactive [26].
With 27 eligible and evaluable patients, the study required more than 4 patients PFS at 6 months or more than 3 patients with responses before the study could proceed to the second stage accrual. The cumulative targeted sample size for the second stage was 52 patients. The study would require more than 12 patients PFS at 6 months or more than 8 patients with responses before the agent would be declared interesting.
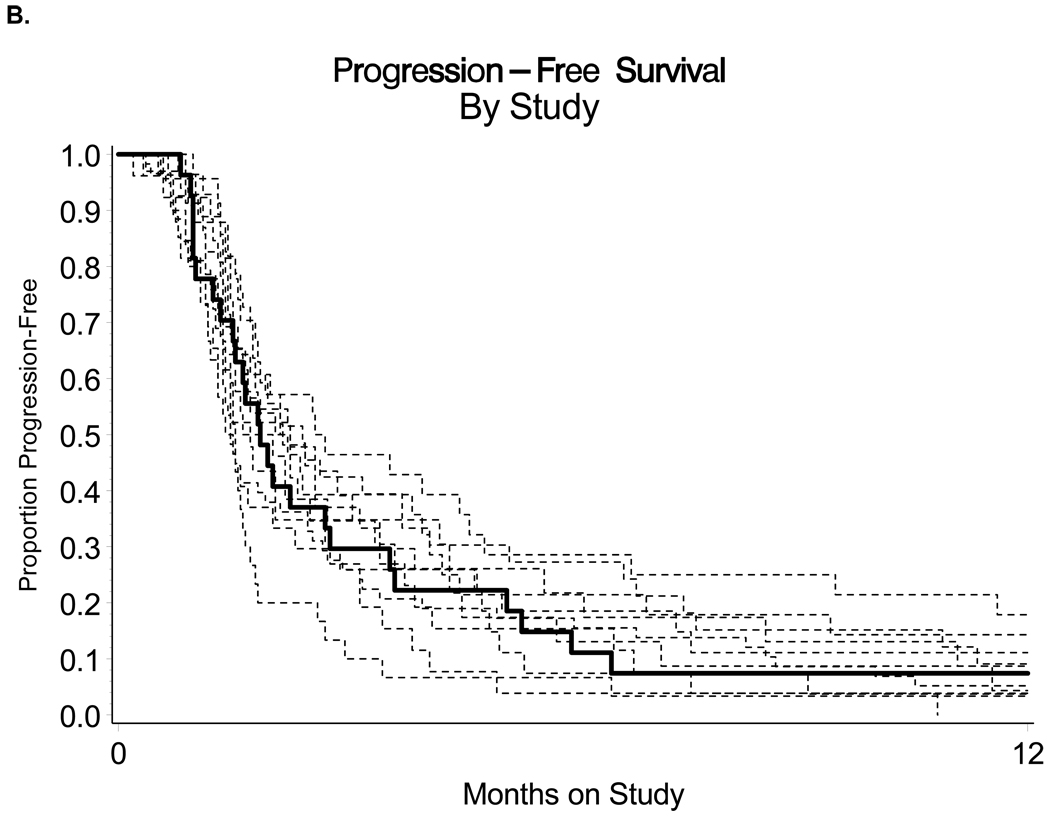
The sample sizes were targeted so that the probability of declaring the regimen active when in fact it is inactive (pr≤10% and ps≤15%) was limited to 10% with approximately 90% power (pr>25% or ps>35%) where pr and ps are the probabilities of response and PFS at 6 months respectively. The null probabilities were obtained from an analysis of historical controls where the agents under investigation were deemed to have minimal activity (Figure 1B) [27–37]. The frequency and severity of adverse events were evaluated with National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE v3) and tabulated according to the organ system affected. Cox proportional hazards models were used where feasible to assess any possible association between a biomarker of interest and PFS or OS [38]. Associations within biomarkers and clinical characteristics were examined with Kendall’s Tau-b, Spearman’s correlation coefficient, and Fisher’s Exact Test as feasible [39–41]. Clinical characteristics examined with biomarker data included response, PFS ≥6 months, performance status, age group, number of prior regimens, and grade of tumor tissue. Since all except one of the patients had disease progression, exact Wilcoxon or Kruskal-Wallis tests were conducted to assess associations between mutation data and PFS [42]. Any analysis with a p-value less than 5% was designated as being “suggestive” of a possible association. Power to detect true associations was limited. Suggestive associations should be interpreted as hypothesis-generating.
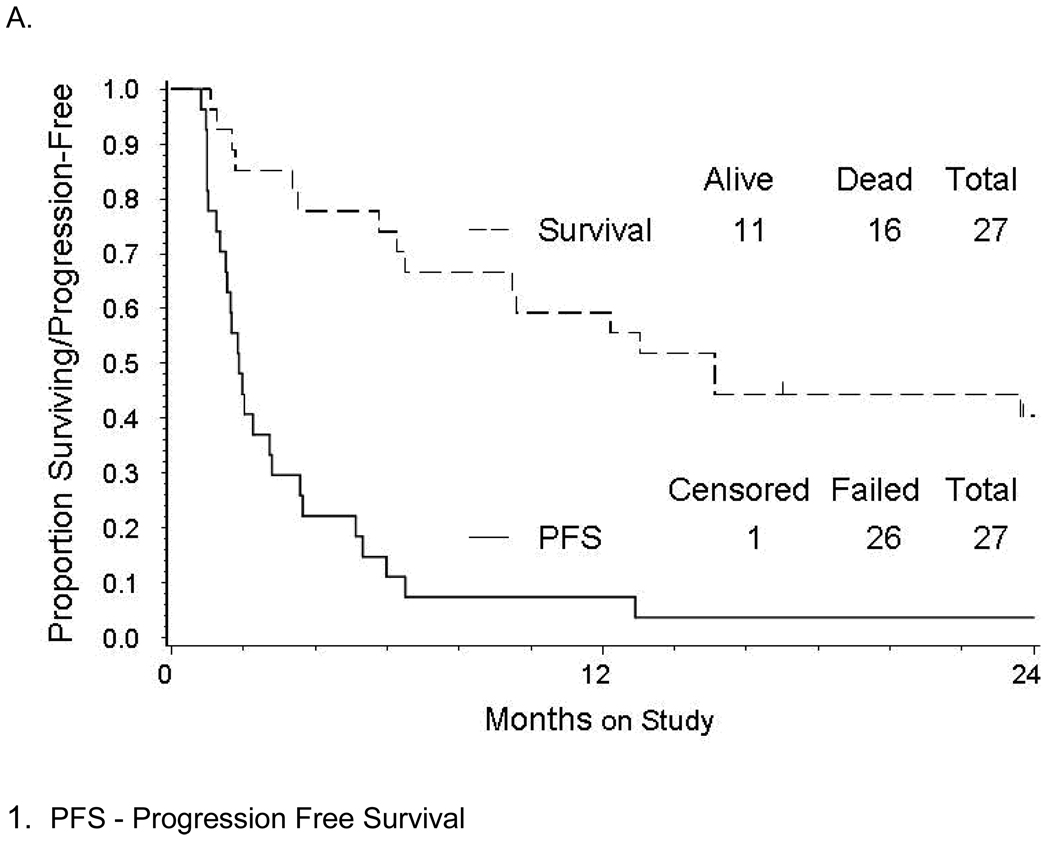
Figure 1.
(A) Kaplan-Meier estimate of progression-free survival (solid line) and overall survival (dashed line) for women treated with enzastaurin, and (B) the PFS1for those treated with enzastaurin (solid line) compared with non-randomized GOG historical controls (dashed lines).
RESULTS
Patient Eligibility and Characteristics
Twenty-eight patients were enrolled onto the study from November, 2006 to May, 2007. One was ineligible due to improper treatment prior to study entry, leaving 27 for analysis. Patient characteristics are listed in Table 1. Twenty-three patients discontinued treatment for disease progression. One patient achieved a durable partial response and remains on the study at the time of writing this manuscript. Two refused further treatment, and 1 was discontinued for toxicity (grade 2 vomiting) as permitted by the protocol.
Table 1.
Characteristics and Outcomes for the 27 Eligible Patients
| Characteristics | Cases | % |
|---|---|---|
| Age | ||
| 40–49 | 4 | 14.8 |
| 50–59 | 10 | 37.0 |
| 60–69 | 6 | 22.2 |
| 70–79 | 7 | 25.9 |
| Race | ||
| African American | 1 | 3.7 |
| White | 26 | 96.3 |
| Performance Status | ||
| 0 | 16 | 59.3 |
| 1 | 11 | 40.7 |
| Site of Disease | ||
| Ovary | 24 | 88.9 |
| Peritoneum | 3 | 11.1 |
| Cell Type | ||
| Adenocarcinoma, Unspecified. | 2 | 7.4 |
| Endometrioid Adenocarcinoma | 1 | 3.7 |
| Mixed Epithelial Carcinoma | 2 | 7.4 |
| Serous Adenocarcinoma | 22 | 81.5 |
| Grade | ||
| 1: Well differentiated | 1 | 3.7 |
| 2: Moderately differentiated | 6 | 22.2 |
| 3: Poorly differentiated | 20 | 74.1 |
| Prior Regimens | ||
| 1 | 14 | 51.9 |
| 2 | 13 | 48.1 |
| Platinum Sensitivity (Platinum-Free Interval) | ||
| Refractory / Resistant (<6 month) | 16 | 59.3 |
| Sensitive (≥6 month) | 11 | 40.7 |
| Tumor Response | ||
| Objective Response | 2†* | 7.4 |
| Stable Disease | 6 | 22.2 |
| Increasing Disease | 17 | 63.0 |
| Non-evaluable | 2 | 7.4 |
| PFS ≥6 months | ||
| No | 24 | 88.9 |
| Yes | 3‡ | 11.1 |
| Cycles | ||
| One | 6 | 22.2 |
| Two | 12 | 44.4 |
| Three | 3 | 11.1 |
| ≥ Four | 6 | 22.2 |
| Disease and Vital Status | ||
| Alive without progression | 1 | 3.7 |
| Alive with progression | 10 | 37.0 |
| Dead | 16* | 59.3 |
Adverse events
A summary of adverse events is provided in Table 2. Only events deemed at least possibly related to enzastaurin were included. Overall, enzastaurin was well tolerated with no grade 4 toxicities. The most common severe side effects observed in the study were constitutional (4), primarily fatigue, gastrointestinal (3), and anemia (1). One patient had grade 3 dyspnea. The grade 3 neurological and metabolic reactions were dizziness and hyperkalemia, respectively. We had not observed QTc changes in our study group and only 2 patients developed grade 1 thrombocytopenia. No patient required a dose reduction of enzastaurin.
Table 2.
Adverse Events Sorted by Grade and Number of Patients Experiencing Events
| CTCAE1 v3.0 Grade | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | Total | |
| Leukopenia | 25 | 2 | 0 | 0 | 0 | 0 | 27 |
| Thrombocytopenia | 25 | 2 | 0 | 0 | 0 | 0 | 27 |
| Neutropenia | 26 | 1 | 0 | 0 | 0 | 0 | 27 |
| Anemia | 9 | 10 | 7 | 1 | 0 | 0 | 27 |
| Other Hematologic | 26 | 1 | 0 | 0 | 0 | 0 | 27 |
| Cardiac | 25 | 1 | 1 | 0 | 0 | 0 | 27 |
| Constitutional | 9 | 9 | 5 | 4 | 0 | 0 | 27 |
| Dermatologic | 22 | 3 | 2 | 0 | 0 | 0 | 27 |
| Nausea | 18 | 6 | 2 | 1 | 0 | 0 | 27 |
| Vomiting | 22 | 3 | 2 | 0 | 0 | 0 | 27 |
| Gastrointestinal | 11 | 8 | 6 | 2 | 0 | 0 | 27 |
| Genitourinary/Renal | 24 | 3 | 0 | 0 | 0 | 0 | 27 |
| Hemorrhage | 26 | 0 | 1 | 0 | 0 | 0 | 27 |
| Infection | 26 | 0 | 1 | 0 | 0 | 0 | 27 |
| Lymphatics | 20 | 4 | 3 | 0 | 0 | 0 | 27 |
| Metabolic | 20 | 5 | 1 | 1 | 0 | 0 | 27 |
| Neurosensory | 26 | 1 | 0 | 0 | 0 | 0 | 27 |
| Other Neurological | 25 | 1 | 0 | 1 | 0 | 0 | 27 |
| Pain | 19 | 6 | 2 | 0 | 0 | 0 | 27 |
| Pulmonary | 23 | 2 | 1 | 1 | 0 | 0 | 27 |
Clinical Activity
Patients received a median of 2 cycles of therapy (range 1 – 39). Three patients had 5 or more cycles. The median follow-up time among the 11 patients alive at the time of data freeze was 26.5 months (range 17 – 30). The observed number of patients who were progression free at 6 months was 3 (11.1%; 90% CI 3.1% – 26.3%), and the observed number of patients with responses was 2 (7.4%; 90% CI 1.3% – 21.5%). One patient had a response and was progression free at 6 months. Seventeen patients had increasing disease (63%). As a single agent, enzastaurin did not demonstrate sufficient activity to proceed to a second stage of accrual and the study was terminated. The median PFS was observed to be 1.87 months (90% CI 1.54 – 2.73). The median OS was 15.1 months (Figure 1A). Survival analyses involving PFS and OS did not show any suggestive relationships with platinum sensitivity, age, or performance status. Interestingly, as of January 2011, 1 patient remained on the study. She completed 49 cycles of treatment with enzastaurin and achieved a durable partial response lasting for 44 months. This patient was a 52-year-old Caucasian woman with a performance status of 0 at the time of enrollment with adenocarcinoma of unspecified cellular type. She initially completed 8 cycles of Carboplatin and Taxol with a platinum-free interval of about 3.6 months and then, was treated with 3 cycles of Doxil.
Translational Research
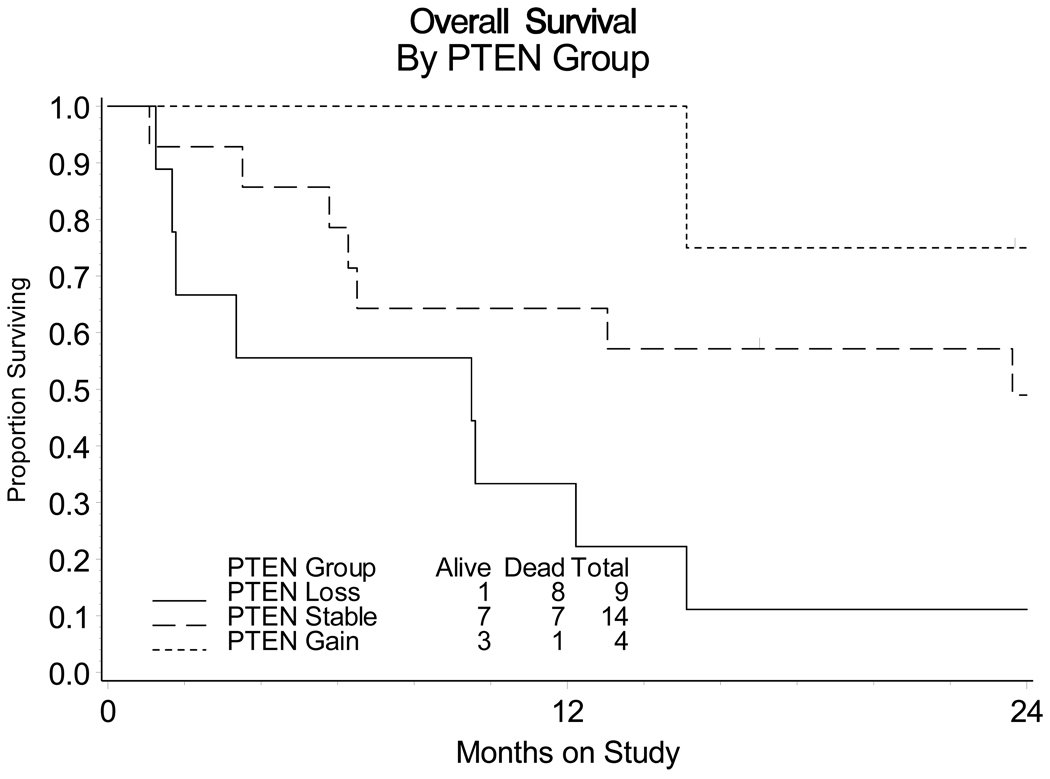
Possible associations were detected among some of the biomarkers and the clinical parameters. However, no associations between the biomarkers and tumor response or resistance to enzastaurin were observed. Mutations were common in TP53 (56%; 15/27), although likely underestimated since only exons 5–9 were analyzed, but were less frequent in PKCβII (15%; 4/27), PIK3CA (0%; 0/27), and PTEN (0%; 0/27) (Table 3). A previously reported mutation in PIK3CA (c.1634A>C; p.E545A) [43] was found in 8 of the 27 samples; however, based on its frequency in this sample set, we suspect that this alteration is likely a benign polymorphism. Gene copy number alterations were found for AKT2 (15%; 4/27) and PTEN (48% with a gain or loss; 15% [4/27] with a gain and 33% [9/27] with a loss) (Table 3), which is consistent with our recent studies showing frequent genetic abnormalities of the PI3K/AKT pathway in primary ovarian cancer [unpublished data]. We observed that 1 patient of 9 with PTEN loss was alive, while 10 patients were alive among 18 patients with the stable PTEN and gain in PTEN within 24 months since the enrollment in the study, Of potential significance, an exploratory analysis suggested an association between copy number loss of the PTEN gene and poor overall survival (OS) (Figure 2). There were 4 cases that showed gains in PTEN copy number using two independent qPCR probes; however, this may reflect the loss of the reference gene, Caveolin-1, in a subset of the tumors. Interestingly, these individuals had the best prognosis (Figure 2). Circulating VEGF levels were also measured. The Q1, M, and Q3 (first, median, and third quartiles) for VEGF at baseline were 33, 53, and 102 pg/ml, respectively. The Q1, M, and Q3 for VEGF pre-cycle 2 were 53, 87, and 127 pg/ml, respectively. These values are in the low range for cancer patients and there were no significant differences noted before and after the first cycle of treatment (pre-cycle 1 versus pre-cycle 2) when the sign test was applied.
Table 3.
Results of translational research for the study cohort
| HighVEGF 1 | Mutations | Copy Number Alterations | ||||||
|---|---|---|---|---|---|---|---|---|
| TP532 | PKCβII | PIK3CA4 | PTEN5 | AKT2 Gain6 | PTEN | |||
| Gain6,7 | Loss8 | Either | ||||||
| 10/20 | 15/27 | 4/27 | 0/27 | 0/27 | 4/27 | 4/27 | 9/27 | 13/27 |
Figure 2.
Overall survival of patients in the study cohort based on PTEN copy number in tumors
Kaplan-Meier survival distributions for PTEN loss (solid line), PTEN stable (long dashed line) and PTEN gain (short dashed line) and log-rank (permutation test) suggest potential association between copy number alterations (CNAs) in PTEN and survival.
The previously mentioned patient who had achieved durable response had relatively normal levels of biomarkers relative to the cohort under examination (Table 4). Her tumor biomarkers were unremarkable, i.e., wild type for TP53, PKCβII, PIK3CA, and PTEN, no evidence of copy number alterations in AKT and PTEN, and no elevation of plasma VEGF level at baseline. The pre-treatment VEGF fell within the first (Q1) and third quartiles (Q3) of the sample. However, the sample first quartile, median, and third quartile estimates for the second VEGF measurements after 1st cycle of enzastaurin among the 16 available specimens were 52.5, 86.9, and 127.4 pg/ml respectively while the selected patient’s level was 26.8 pg/ml (standardized score from a mean of 93.6 pg/ml and standard deviation of 53.2 pg/ml was −1.26). Although not a statistical outlier, this patient’s VEGF measurement after 1st cycle of enzastaurin was low.
Table 4.
Descriptive marker data for 2 patients who experienced partial responses (PRs) on Enzastaurin
| Two PRs | VEGF1 Precycle 2 | Genomic Alterations 2 | Copy NumberAlterations 3 | ||||
|---|---|---|---|---|---|---|---|
| PIK3CA | PTEN | TP53 | PKCβII | AKT2 | PTEN | ||
| PFS≥39 months; Still on study | Low 26.82 Z=−1.25 | c.1658G>C p.S553T; c.1634A>C p.E545A; c.1659del T p.H554TfsX5; c.352+40 A>G; c.352+136 ins 3 | c.210-34 del T | Wt4 | c.380 + 31 G>A | None | None |
| PFS <6 month PFS = 5.32 | Normal 57.24 Z=−0.68 | c.1658G>C p.S553T; c.1634A>C p.E545A; c.1659del T p.H554TfsX5 | c.210-34 del T | c.818G>A p.R273H | c.380 + 31 G>A | None | None |
DISCUSSION
Our result showed that enzastaurin as a single agent was well tolerated by women with ovarian cancer. Although there were 2 cases of partial response and 3 cases of stable disease for longer than 6 months, the study did not meet the criteria for the second stage of accrual and was terminated. These results are consistent with the results of other singe-agent phase II trials with enzastaurin in other solid tumors which have been negative to date [44–46]. They are also consistent with phase II trials of other PKC inhibitors, bryostatin and aprinocarsen (PKCα inhibitor), in ovarian cancer [47–49]. Although our results are disappointing as far as targeting PKC in ovarian cancer, it is very likely that insufficient activity of enzastaurin is related to multiplicity of signal transduction pathways in cancer cells. This gives a rationale to using combinations of PKC inhibitors with non-cross resistant inhibitors of other important pathways in ovarian cancer, particularly VEGF inhibitors. A phase I trial using enzastaurin and bevacizumab in heavily-pretreated patients with gynecologic cancers showed very encouraging results with 32% of patients achieving a response and 48% of patients being progression-free at 6 months [50].
Enzastaurin continues to be studied in other cancers. A cytostatic mechanism of action along with favorable side effect profile makes enzastaurin a candidate for clinical trials evaluating it in the maintenance setting for malignant diseases [46, 51]. Enzastaurin in combination with various chemotherapeutic agents is also being evaluated in phase II trials.
Our translational research data have not definitively established associations between the studied biomarkers and clinical outcomes, although interesting observations have been made. The lack of correlation between biomarkers relevant to enzastaurin and clinical outcomes has been noted in another study [52]. It must be stressed that our genomic studies have focused on mutations and changes in gene copy numbers. We have not performed a full assessment of the genes and proteins in the PI3K/AKT/PTEN signaling pathway. If enzastaurin development continues new clinical trials should include downstream evaluation of these genes and their products including mRNA quantification and direct measurement of protein expression by immunoblotting or immunohistochemistry.
CONCLUSIONS
Single agent Enzastaurin was well tolerated but showed limited activity in unselected persistent or recurrent EOC/PPC. One patient has achieved a PR and remains progression-free on enzastaurin for more than 3 years. None of the evaluated biomarkers showed correlations with tumor response. Potential association was observed between survival and PTEN gene copy number alterations in the tumor which requires validation in a larger sample. Trials are needed to evaluate combinations of enzastaurin with cytotoxic chemotherapy and/or targeted agents, especially anti-angiogenic drugs.
Supplementary Material
01
ACKNOWLEDGEMENT
The authors thank Ms. Kim Blaser (Gynecologic Oncology Group) for her assistance in formatting and editing this manuscript, Ms. Sandy Dascomb (Gynecologic Oncology Group) for coordinating the clinical data of this study, Ms. Meg Colahan (Gynecologic Oncology Group) for managing the protocol, Dr. Betsy Bove and Mr. Frank Zambito (Fox Cancer Center) for excellent technical support. We also thank the GOG Publications Subcommittee, Dr. Janet Plate (Rush University), and Dr. Katherine Look (Eli Lilly Pharmaceutical Company) for their critical review and thoughtful suggestions for the manuscript. We also gratefully acknowledge the support of Eli Lilly Pharmaceutical Company.
The study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the GOG Tissue Bank (CA 27469, CA 11479) and the GOG Statistical and Data Center (CA 37517), CA 140323 and Ovarian Cancer Research Fund (to Dr. Andrew Godwin) and Eli Lilly Pharmaceutical Company. The following GOG member institutions participated in this protocol: Abington Memorial Hospital, University of Washington, University of Iowa Hospitals and Clinics, Rush-Presbyterian-St. Luke's Medical Center, University of Massachusetts Medical School, Fox Chase Cancer Center, University of Oklahoma and Community Clinical Oncology Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer.J.Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF. Treatment goals in ovarian cancer. Int.J.Gynecol.Cancer. 2005;15 Suppl 1:3–11. doi: 10.1111/j.1525-1438.2005.15351.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen YB, LaCasce AS. Enzastaurin. Expert Opin Investig Drugs. 2008;17:939–944. doi: 10.1517/13543784.17.6.939. [DOI] [PubMed] [Google Scholar]
- 4.Ma S, Rosen ST. Enzastaurin. Curr Opin Oncol. 2007;19:590–595. doi: 10.1097/CCO.0b013e3282f10a00. [DOI] [PubMed] [Google Scholar]
- 5.Green LJ, Marder P, Ray C, Cook CA, Jaken S, Musib LC, et al. Development and validation of a drug activity biomarker that shows target inhibition in cancer patients receiving enzastaurin, a novel protein kinase C-beta inhibitor. Clin Cancer Res. 2006;12:3408–3415. doi: 10.1158/1078-0432.CCR-05-2231. [DOI] [PubMed] [Google Scholar]
- 6.Fields AP, Murray NR. Protein kinase C isozymes as therapeutic targets for treatment of human cancers. Adv.Enzyme Regul. 2008;48:166–178. doi: 10.1016/j.advenzreg.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonelli A, Mischiati C, Guerrini R, Voltan R, Salvadori S, Zauli G. Perspectives of protein kinase C (PKC) inhibitors as anti-cancer agents. Mini Rev Med Chem. 2009;9:498–509. doi: 10.2174/138955709787847967. [DOI] [PubMed] [Google Scholar]
- 8.Gokmen-Polar Y, Murray NR, Velasco MA, Gatalica Z, Fields AP. Elevated protein kinase C betaII is an early promotive event in colon carcinogenesis. Cancer Res. 2001;61:1375–1381. [PubMed] [Google Scholar]
- 9.Aeder SE, Martin PM, Soh JW, Hussaini IM. PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene. 2004;23:9062–9069. doi: 10.1038/sj.onc.1208093. [DOI] [PubMed] [Google Scholar]
- 10.Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, et al. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- 11.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 12.Carducci MA, Musib L, Kies MS, Pili R, Truong M, Brahmer JR, et al. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol. 2006;24:4092–4099. doi: 10.1200/JCO.2005.05.3447. [DOI] [PubMed] [Google Scholar]
- 13.Graff JR, McNulty AM, Hanna KR, Konicek BW, Lynch RL, Bailey SN, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 14.Keyes KA, Mann L, Sherman M, Galbreath E, Schirtzinger L, Ballard D, et al. LY317615 decreases plasma VEGF levels in human tumor xenograft-bearing mice. Cancer Chemother Pharmacol. 2004;53:133–140. doi: 10.1007/s00280-003-0713-x. [DOI] [PubMed] [Google Scholar]
- 15.Santin AD, Hermonat PL, Ravaggi A, Cannon MJ, Pecorelli S, Parham GP. Secretion of vascular endothelial growth factor in ovarian cancer. Eur J Gynaecol Oncol. 1999;20:177–181. [PubMed] [Google Scholar]
- 16.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 17.Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11:2875–2878. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- 18.Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U.S.A. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 20.Saito M, Okamoto A, Kohno T, Takakura S, Shinozaki H, Isonishi S, et al. Allelic imbalance and mutations of the PTEN gene in ovarian cancer. Int J Cancer. 2000;85:160–165. [PubMed] [Google Scholar]
- 21.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 22.Lu QJ, Zhao XD, Song JY, Li XH, Ma Y, Meng H, et al. Research on the mechanisms of PTEN gene inactivation in ovarian cancer. Zhonghua Bing Li Xue Za Zhi. 2005;34:266–269. [PubMed] [Google Scholar]
- 23.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Sill MW, Yothers G. A method for utilizing bivariate efficacy outcome measures to screen agents for activity in 2-stage phase II clinical trials, Technical Report 06–08. Department of Biostatistics, University at Buffalo; 2006. [Google Scholar]
- 27.Markman M, Blessing JA, DeGeest K, Morgan M, Look KY, Herzog TJ, et al. Lack of efficacy of 24-h infusional topotecan in platinum-refractory ovarian cancer: A Gynecologic Oncology Group trial. Gynecol Oncol. 1999;75:444–446. doi: 10.1006/gyno.1999.5640. [DOI] [PubMed] [Google Scholar]
- 28.Markman M, Blessing JA, Moore D, Ball H, Lentz SS. Altretamine (hexamethylmelamine) in platinum-resistant and platinum-refractory ovarian cancer: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 1998;69:226–229. doi: 10.1006/gyno.1998.5016. [DOI] [PubMed] [Google Scholar]
- 29.Muggia FM, Blessing JA, Homesley HD, Sorosky J. Tomudex (ZD1694, NSC 639186) in platinum-pretreated recurrent epithelial ovarian cancer: a phase II study by the Gynecologic Oncology Group. Cancer Chemother Pharmacol. 1998;42:68–70. doi: 10.1007/s002800050786. [DOI] [PubMed] [Google Scholar]
- 30.Manetta A, Blessing JA, Hurteau JA. Evaluation of cisplatin and cyclosporin A in recurrent platinum-resistant ovarian cancer: a phase II study of the gynecologic oncology group. Gynecol Oncol. 1998;68:45–46. doi: 10.1006/gyno.1997.4887. [DOI] [PubMed] [Google Scholar]
- 31.Markman M, Blessing JA, Alvarez RD, Hanjani P, Waggoner S, Hall K. Phase II evaluation of 24-h continuous infusion topotecan in recurrent, potentially platinum-sensitive ovarian cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2000;77:112–115. doi: 10.1006/gyno.2000.5755. [DOI] [PubMed] [Google Scholar]
- 32.Plaxe SC, Blessing JA, Morgan MA, Carlson J Gynecologic Oncology Group. Phase II trial of pyrazoloacridine in recurrent platinum-resistant ovarian cancer: a Gynecologic Oncology Group study. Am J Clin Oncol. 2002;25:45–47. doi: 10.1097/00000421-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Fracasso PM, Blessing JA, Morgan MA, Sood AK, Hoffman JS. Phase II study of oxaliplatin in platinum-resistant and refractory ovarian cancer: a gynecologic group study. J Clin Oncol. 2003;21:2856–2859. doi: 10.1200/JCO.2003.03.077. [DOI] [PubMed] [Google Scholar]
- 34.Fracasso PM, Brady MF, Moore DH, Walker JL, Rose PG, Letvak L, et al. Phase II study of paclitaxel and valspodar (PSC 833) in refractory ovarian carcinoma: a gynecologic oncology group study. J Clin Oncol. 2001;19:2975–2982. doi: 10.1200/JCO.2001.19.12.2975. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman MA, Blessing JA, Lentz SS Gynecologic Oncology Group Study. A phase II trial of dolastatin-10 in recurrent platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;89:95–98. doi: 10.1016/s0090-8258(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman MA, Blessing JA, Morgan M. Phase II trial of CI-958 in recurrent platinum-refractory ovarian carcinoma. A Gynecologic Oncology Group Study. Gynecol Oncol. 2000;79:463–465. doi: 10.1006/gyno.2000.5984. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman MA, Blessing JA, Nuñez ER. A phase II trial of CI-958 in recurrent platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol.Oncol. 2001;81:433–435. doi: 10.1006/gyno.2001.6182. [DOI] [PubMed] [Google Scholar]
- 38.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society Series B. 1972;34:187–220. [Google Scholar]
- 39.Kendall MG. The treatment of ties in rank problems. Biometrika. 1945;33:239–251. doi: 10.1093/biomet/33.3.239. [DOI] [PubMed] [Google Scholar]
- 40.Spearman C. The proof and measurement of association between two things. Amer. J. Psychol. 1904;15:72–101. [PubMed] [Google Scholar]
- 41.Fisher RA. Statistical Methods for Research Workers. Edinburgh: Oliver and Boyd; 1934. 1970. [Google Scholar]
- 42.Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York: John Wiley & Sons; 1973. [Google Scholar]
- 43.Baohua Y, Xiaoyan Z, Tiecheng Z, Tao Q, Daren S. Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn Mol Pathol. 2008;17:159–165. doi: 10.1097/PDM.0b013e31815d0588. [DOI] [PubMed] [Google Scholar]
- 44.Kreisl TN, Kotliarova S, Butman JA, Albert PS, Kim L, Musib L, et al. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12:181–189. doi: 10.1093/neuonc/nop042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh Y, Herbst RS, Burris H, Cleverly A, Musib L, Lahn M, et al. Enzastaurin, an oral serine/threonine kinase inhibitor, as second- or third-line therapy of non-small-cell lung cancer. J Clin Oncol. 2008;26:1135–1141. doi: 10.1200/JCO.2007.14.3685. [DOI] [PubMed] [Google Scholar]
- 46.Morschhauser F, Seymour JF, Kluin-Nelemans HC, Grigg A, Wolf M, Pfreundschuh M, et al. A phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Ann Oncol. 2008;19:247–253. doi: 10.1093/annonc/mdm463. [DOI] [PubMed] [Google Scholar]
- 47.Advani R, Peethambaram P, Lum BL, Fisher GA, Hartmann L, Long HJ, et al. A Phase II trial of aprinocarsen, an antisense oligonucleotide inhibitor of protein kinase C alpha, administered as a 21-day infusion to patients with advanced ovarian carcinoma. Cancer. 2004;100:321–326. doi: 10.1002/cncr.11909. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong DK, Blessing JA, Look KY, Schilder R, Nunez ER. A randomized phase II evaluation of bryostatin-1 (NSC #339555) in recurrent or persistent platinum-sensitive ovarian cancer: a Gynecologic Oncology Group Study. Invest New Drugs. 2003;21:373–377. doi: 10.1023/a:1025490818450. [DOI] [PubMed] [Google Scholar]
- 49.Clamp AR, Blackhall FH, Vasey P, Soukop M, Coleman R, Halbert G, et al. A phase II trial of bryostatin-1 administered by weekly 24-hour infusion in recurrent epithelial ovarian carcinoma. Br J Cancer. 2003;89:1152–1154. doi: 10.1038/sj.bjc.6601285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armstrong DK, Ermisch S, Collins C, Nicol S, Wang T, Zhang Z, et al. A phase I study of enzastaurin and bevacizumab in patients with advanced cancer: Response and clinical benefit in ovarian and other gynecologic cancers. Gynecol Oncol. 2010;116:S16. [Google Scholar]
- 51.Glimelius B, Lahn M, Gawande S, Cleverly A, Darstein C, Musib L, et al. A window of opportunity phase II study of enzastaurin in chemonaive patients with asymptomatic metastatic colorectal cancer. Ann Oncol. 2010;21:1020–1026. doi: 10.1093/annonc/mdp521. [DOI] [PubMed] [Google Scholar]
- 52.Richards DA, Kuefler PR, Becerra C, Wilfong LS, Gersh RH, Boehm KA, et al. Gemcitabine plus enzastaurin or single-agent gemcitabine in locally advanced or metastatic pancreatic cancer: Results of a Phase II, randomized, noncomparative study. Invest.New Drugs. 2009 Aug 28; doi: 10.1007/s10637-009-9307-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01