The intracellular sensor Nod2 Promotes Intestinal Pathogen Eradication via the chemokine CCL2-Dependent Recruitment of Inflammatory Monocytes (original) (raw)
. Author manuscript; available in PMC: 2012 May 27.
Abstract
The intracellular sensor Nod2 is activated in response to bacteria, and the impairment of this response is linked to Crohn’s disease. However, the function of Nod2 in host defense remains poorly understood. We found that _Nod2_−/− mice exhibited impaired intestinal clearance of Citrobacter rodentium, an enteric bacterium that models human infection by pathogenic Escherichia coli. The increased bacterial burden was preceded by reduced CCL2 chemokine production, inflammatory monocyte recruitment, and Th1 cell responses in the intestine. Colonic stromal cells, but not epithelial cells or resident CD11b+ phagocytic cells, produced CCL2 in response to C. rodentium, which was impaired in _Nod2_−/− cells. Unlike resident phagocytic cells, inflammatory monocytes produced IL-12, a cytokine that induces adaptive immunity required for pathogen clearance. Adoptive transfer of Ly6Chi monocytes restored the clearance of the pathogen in infected _Ccr2_−/− mice. Thus, Nod2 mediates CCL2-CCR2-dependent recruitment of inflammatory monocytes, which is important in promoting bacterial eradication in the intestine.
Keywords: Intestinal bacteria, CCL2, inflammatory monocyte, Nod2, NLR
INTRODUCTION
Members of the nucleotide-binding oligomerization domain (NOD)-like receptor family function as intracellular pattern recognition receptors and regulators of host immunity by sensing microbial products and damage-associated signals (Shaw et al., 2008). The NOD-like receptor family member Nod2 recognizes peptidoglycan-derived molecules containing muramyl dipeptide (MDP) that are produced by both Gram-negative and Gram-positive bacteria (Girardin et al., 2003; Inohara et al., 2003) and may also sense viruses and oil adjuvants independently of MDP (Moreira et al., 2008; Sabbah et al., 2009). Upon MDP recognition, Nod2 induces the activation of the transcription factor NF-κB and the mitogen-activated protein kinases (MAPKs) (Inohara et al., 2003; Kobayashi et al., 2002; Park et al., 2007). The importance of Nod2 in inflammatory homeostasis is underscored by the observation that loss-of-function mutations in the NOD2 gene increase the susceptibility to Crohn’s disease (CD), a common inflammatory disorder of the bowel (Hugot et al., 2001; Ogura et al., 2001). In the small intestine, Nod2 has been suggested to control the normal microflora and bacterial pathogens by regulating the expression and/or release of anti-microbial peptides in Paneth cells (Biswas et al., 2010; Petnicki-Ocwieja et al., 2009). In the colon, Nod2-deficient mice are susceptible to colitis induced by trinitrobenzene sulfonate and adoptive transfer of OVA-specific CD4+ T cells followed by rectal administration of recombinant E. coli expressing OVA peptide (Penack et al., 2009; Watanabe et al., 2006). Thus, Nod2 may regulate the induction of pathogenic T helper 1 (Th1) cells in the intestine. However, the roles of Nod2 in the regulation of innate and adaptive immune responses triggered by bacterial pathogens remain poorly understood.
In the intestine, resident macrophages and dendritic cells (DCs) are hyporesponsive to microbial stimulation, which is thought to be important for preventing inappropriate activation of inflammatory responses to the normal microflora (Denning et al., 2007; Smythies et al., 2005). In response to inflammatory stimuli, circulating Gr1+CCR2+ monocytes that are proinflammatory migrate to tissues (Peters et al., 2004; Serbina et al., 2008). Gr1+ monocytes are recruited to the ileum after oral infection with the protozoan parasite Toxoplasma gondii, the cause of toxoplasmosis (Dunay et al., 2008). In this parasite model, the recruitment of Gr1+ monocytes to the small intestine is mediated by the expression of CCR2 on the monocyte population and local production of the ligand CCL2 (Dunay et al., 2008). Importantly, Gr1+ monocytes have been shown to control replication of T. gondii and tissue damage in the small intestine (Dunay et al., 2008). However, the innate immune sensors and cellular mechanisms that orchestrate the recruitment of Gr1+ monocytes to the sites of bacterial infection in the intestine remain poorly defined.
Citrobacter rodentium, a bacterial pathogen that naturally colonizes mice, is widely used to model human infections with enterohemorragic Escherichia coli (EHEC) and enteropathogenic E. coli (EPEC) (Borenshtein et al., 2008). Similar to the related EPEC and EHEC, C. rodentium induces marked infiltration of inflammatory cells 8–10 days after infection which correlates with the peak of bacterial colonization (Mundy et al., 2005). In wild-type mice, colonization of C. rodentium is resolved by day 21–23 after inoculation (Mundy et al., 2005). The mechanism by which C. rodentium is eradicated from the intestine remains poorly understood, but development of CD4+ T cell-dependent IgG responses against C. rodentium, but not CD8+ T cells or secretory IgA, are required for clearance (Bry and Brenner, 2004; Maaser et al., 2004; Simmons et al., 2003). However, the mechanisms that control the induction of protective adaptive immunity against C. rodentium remain poorly defined. In the current work, we showed that Nod2 controlled the clearance of C. rodentium by regulating the production of CCL2, the influx of Gr1+ inflammatory monocytes to the colon, and the induction of Th1 immune responses in the intestine.
RESULTS
Impaired clearance of C. rodentium in _Nod2_−/− mice
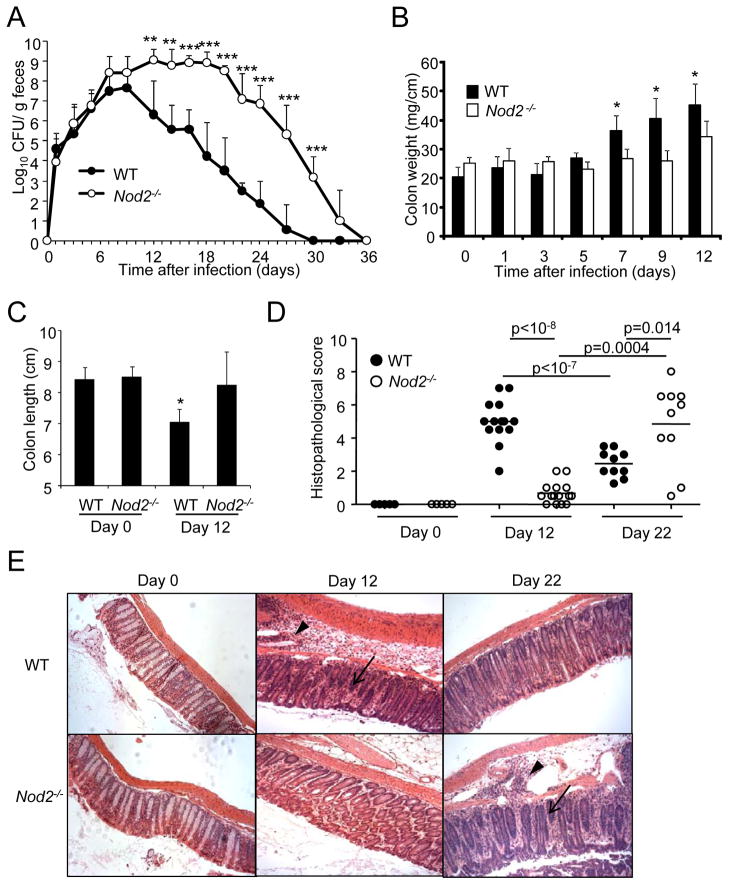
To assess the contribution of Nod2 to host defense against C. rodentium, we infected wild-type (WT) and _Nod2_−/− mice orally and the number of pathogenic bacteria in the feces was monitored for up to 36 days. Consistent with previous studies (Mundy et al., 2005), C. rodentium reached approximately 108 colony-forming units (CFU)/g on days 7–9 and the bacterial load declined over time to become undetectable by day 30 after infection in WT mice (Figure 1A). In contrast, the burden of C. rodentium in the feces of _Nod2_−/− mice remained 4–5 logs higher than in WT mice until day 20 and then declined reaching complete clearance by day 36 after infection (Figure 1A). As expected, WT mice developed an increase in the weight of the colons, reflecting inflammation, on days 7, 9, and 12; however, the colon weight of _Nod2_−/− mice remained largely unchanged despite comparable or even higher number of enteric pathogenic bacteria (Figure 1B). Another feature of inflammation, a decrease in colon length, was observed on day 12 after infection in WT, but not _Nod2_−/−, mice (Figure 1C). Consistently, there was inflammation in the colons of wild-type mice on day 12 after infection, but this was greatly attenuated in _Nod2_−/− mice (Figures 1D and 1E). However, there was overt colitis in Nod2−/− mice on day 22 post-infection when colonic inflammation had declined in WT mice (Figures 1D and 1E). Thus, the increased bacterial load in _Nod2_−/− mice was associated with reduced colonic inflammation during the early phase of the infection, but overt colitis at the late phase of infection. Overall, these results indicate that Nod2 deficiency results in impaired clearance of C. rodentium.
Figure 1. _Nod2_−/− exhibit impaired clearance of C. rodentium.
(A) Bacterial numbers in stool from WT (n= 10) or _Nod2_−/− mice (n= 10) infected orally with C. rodentium were determined by CFU assay. (B) Colon weight of uninfected wild-type (WT) or _Nod2_−/− mice, and infected WT (n=10) or _Nod2_−/− mice (n=10) at days 1, 3, 5, 7, 9, or 12 post-infection (p.i.). (C) Colon length of uninfected WT (n=10) or Nod2−/− mice (n=10), and infected WT (n=10) or Nod2−/− mice (n=10) at day 12 p.i. (D) Histopathological scores of inflammation at day 0 and at day 12 and 22 p. i. of mice inoculated with C. rodentium. Each dot represents an individual mouse. (E) H&E staining of colon slides from representative uninfected and infected WT and Nod2−/− mice. Arrow heads and arrows denote marked submucosal inflammatory cellular infiltrates/edema and epithelial damage/inflammation in the mucosa, respectively. Magnification ×200. Data are means ± SD. *,** and *** denote significant differences between WT and Nod2−/− mice at p<0.05, p<0.01, and P<0.001, respectively. Results are representative of at least 3 independent experiments.
CCL2 production is reduced in _Nod2_−/− mice infected with C. rodentium
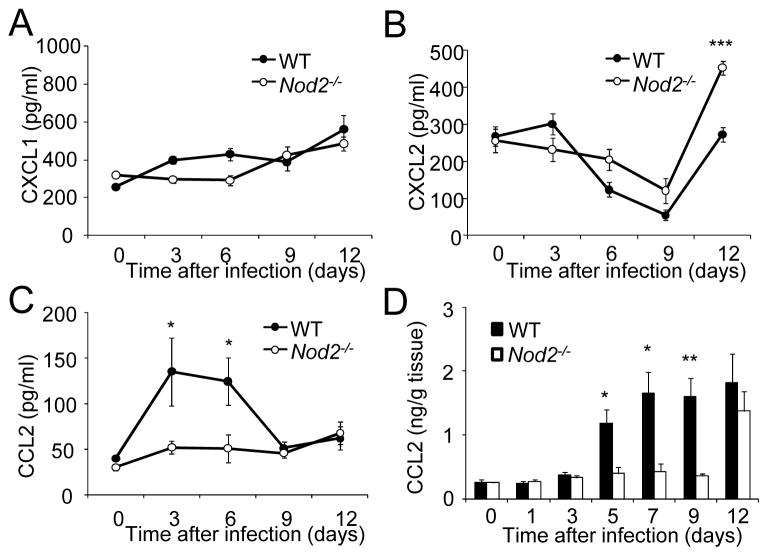
To begin to understand the impairment of C. rodentium clearance in _Nod2_−/− mice, we examined the production of chemokines as these molecules drive the recruitment of inflammatory cells to the intestine in response to bacterial infection (Johnston and Butcher, 2002). Production of CXCL1, a chemokine that mediates the influx of neutrophils, was comparable in the sera of uninfected and infected WT and _Nod2_−/− mice (Figure 2A). Similarly, production of CXCL2, another chemokine involved in neutrophil recruitment, was comparable on day 3, 6, and 9 in the sera of WT and _Nod2_−/− mice, although it was significantly elevated in _Nod2_−/− mice on day 12 post-infection (Figure 2B). Notably, the amounts of CCL2, a monocyte chemoatractant, were reduced in the serum of _Nod2_−/− mice on day 3 and 6 after infection when the bacterial burden was comparable in WT and mutant mice (Figure 2C). CCL2 production was also impaired in the colon of _Nod2_−/− mice on day 5, 7 and 9 after infection when compared to WT mice (Figure 2D). These results indicate that Nod2 positively regulates the production of CCL2 during the early phase of C. rodentium infection.
Figure 2. Nod2−/− mice are impaired in their ability to produce CCL2 in response to C. rodentium.
Serum CXCL1 (A), CXCL2 (B), and CCL2 (C) levels at day 0, 3, 6, 9, and 12 after infection of WT (n=10) or Nod2−/− mice (n=10) with C. rodentium. (D) CCL2 levels in colon homogenates derived from WT and Nod2−/− mice on day 0, 1, 3, 5, 7, 9, and 12 p. i. with C. rodentium. Data are means ± SE. * and ** denote significant differences between WT and Nod2−/− mice at p<0.05 and p<0.01, respectively. Results are representative of at 2 independent experiments.
Recruitment of CD11b+Gr1+F4/80+ phagocytic cells to the colon is impaired in _Nod2_−/− mice
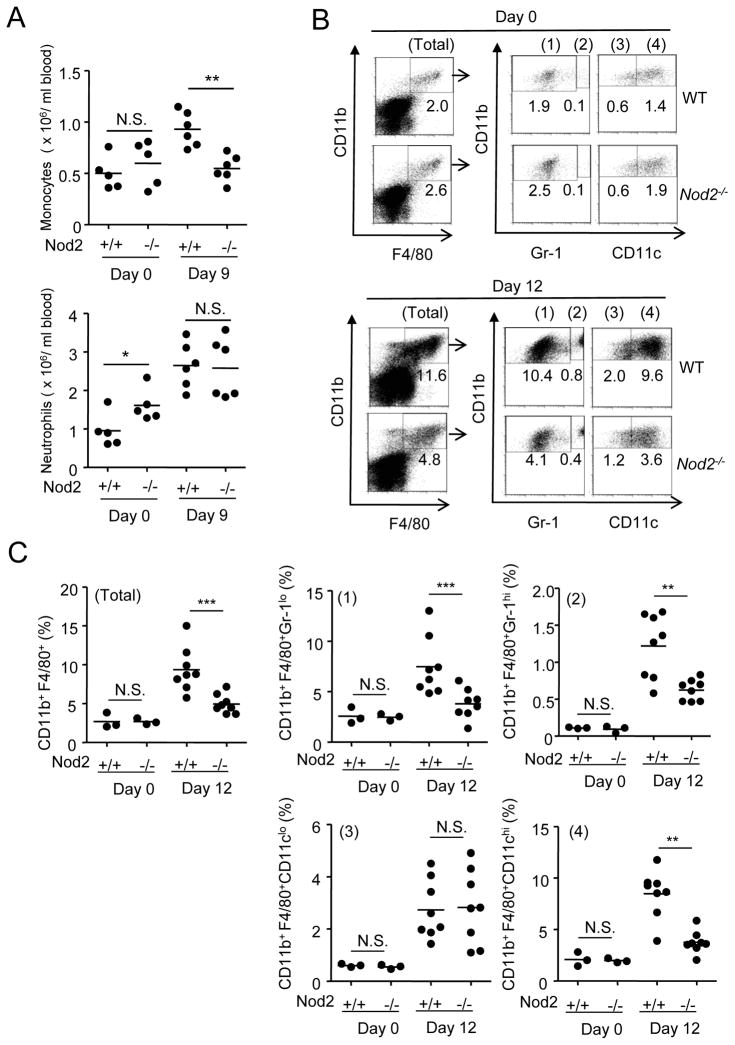
We assessed the influx of monocytes and neutrophils in peripheral blood of WT and _Nod2_−/− mice after oral infection with C. rodentium. On day 9 after infection, there was an increased in monocytes in WT, but not _Nod2_−/− mice (Figure 3A). Although _Nod2_−/− mice had a mild elevation in the number of peripheral blood neutrophils before infection, the number of neutrophils after infection was comparable in WT and _Nod2_−/− mice (Figure 3A). In the absence of infection, the number of resident cells that co-express CD11b and F4/80, that are markers of monocytes and macrophages, was comparable in the colons of WT and _Nod2_−/− mice (Figure 3B). Similarly, the percentage of neutrophils (CD11b+Gr1hiF4/80−) was similar in the colons of uninfected WT and _Nod2_−/− mice (Figure S1). In infected animals, there was an increase in the influx of neutrophils (CD11b+Gr1hiF4/80− ) to the colon; however, this was minimally affected by the absence of Nod2 (Figure S1). When the intestinal cells were analyzed 12 days after infection, there was a robust increase in the percentage of CD11b+F4/80+ phagocytic cells in the colons of WT mice that included Gr1hi and Gr1lo as well as CD11chi and CD11clo cell populations (Figures 3B and 3C). Notably, the influx of CD11b+F4/80+ Gr1hi cells (inflammatory monocytes) and CD11b+F4/80+Gr1lo cells (macrophages or DCs) was reduced in the colons of _Nod2_−/− mice (Figures 3B and 3C). Furthermore, CD11b+F4/80+CD11chi DCs increased after infection, and this population was also reduced in the infected colons of _Nod2_−/− mice (Figures 3B and 3C). In contrast, the influx of CD11b+F4/80+CD11clo (macrophages) was not affected by the absence of Nod2 (Figures 3B and 3C). These studies indicate that Nod2 regulates the influx of circulating monocytes to the blood and colon in response to C. rodentium infection.
Figure 3. Impaired influx of CD11b+Gr1+F4/80+ cells to the colon in Nod2−/− mice infected with C. rodentium.
(A) number of monocytes (CD11b+F4/80+Gr-1hi) and neutrophils (CD11b+F4/80−Gr-1hi) in the blood of uninfected and infected WT or Nod2−/− mice (n= 5–6 mice per group) at day 9 p.i. (B) Cells were isolated from the colon of WT or Nod2−/− mice on day 0, and 12 after oral infection with C. rodentium, and stained for CD11b, F4/80, and Gr-1 or CD11c mAb. CD11b+F4/80+ double-positive cells (Total fraction) were gated and percentage of CD11b+F4/80+Gr-1lo (1), CD11b+F4/80+Gr-1hi (2), CD11b+F4/80+CD11clo (3), and CD11b+F4/80+CD11chi (4) cells in the gated population was determined. (C) Quantitated data pooled from several experiments; each dot represents one mouse. Horizontal bars indicate mean values. ** and *** denote significant differences between WT and _Nod2−/−_mice at p<0.01, and P<0.001, respectively. Results are representative of at least 3 independent experiments.
CCL2 and CCR2 regulate the colonic recruitment of CD11b+F4/80+ phagocytic cells and clearance of C. rodentium
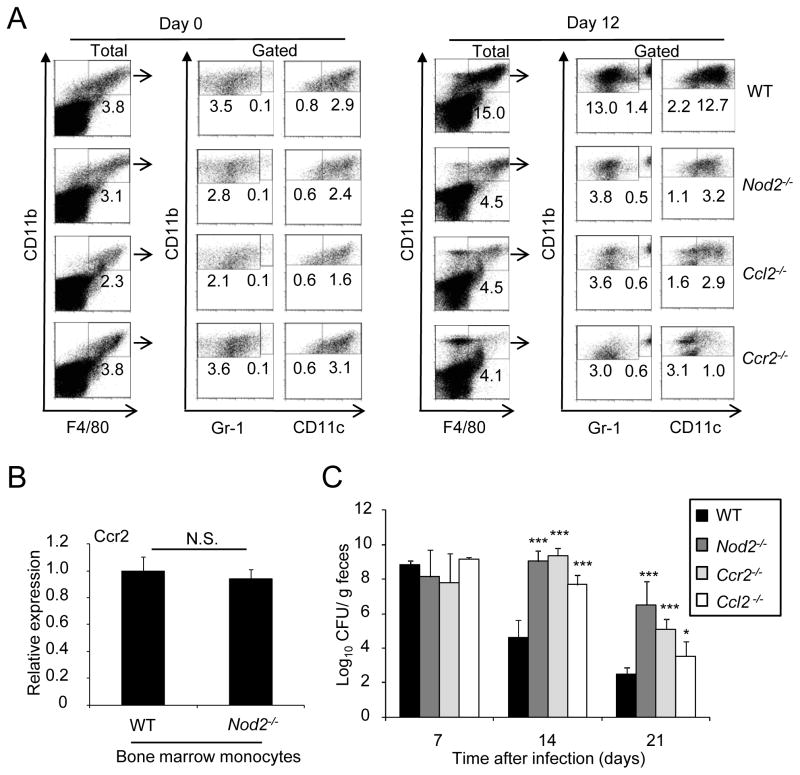
To determine whether CCL2 and its receptor CCR2 regulate the intestinal influx of monocytes in response to C. rodentium infection, we evaluated mononuclear phagocytic cell populations in the colons of WT, _Ccl2_−/−, and _Ccr2_−/− mice after oral inoculation with the pathogen and compared that with _Nod2_−/− mice, which were used as a reference control. The recruitment of CD11b+F4/80+ Gr1hi (inflammatory monocytes), CD11b+F4/80+Gr1lo (macrophage or DCs) and CD11b+F4/80+CD11chi (DCs) to the colon was reduced in both _Ccl2_−/− and _Ccr2_−/− mice (Figure 4A). In contrast, the influx of CD11b+F4/80+CD11clo (macrophage) cell population was not altered in _Ccl2_−/−, _Ccr2_−/−, and _Nod2_−/− mice (Figure 4A). Analysis of CCR2 expression in bone marrow monocytes revealed comparable amounts in WT and _Nod2_−/− cells (Figure 4B). To determine whether CCL2 and CCR2 regulate the clearance of C. rodentium, we infected WT and mutant mice with the pathogen and monitored the bacterial burden in the feces. The number of C. rodentium on day 7 after infection was comparable in WT, _Ccl2_−/− and _Ccr2_−/− mice (Figure 4C). Importantly, the bacterial burden was increased ~ 3–4 log on day 14 and ~2–4 log on day 21 in the feces of _Ccr2_−/− and _Ccl2_−/− mice when compared to wild-type mice (Figure 4C). The clearance of C. rodentium in _Ccr2_−/− mice was comparable to that observed in _Nod2_−/− mice, although we observed a slightly greater impairment in _Ccl2_−/− mice than in _Nod2_−/− and _Ccr2_−/− mice on day 21 after infection (Figure 4C). Thus, the CCL2-CCR2 axis regulates the influx of inflammatory monocytes to the colon in response to C. rodentium infection, and this correlates with impaired clearance of the pathogen.
Figure 4. CCL2 and CCR2 regulate the colonic recruitment of CD11b+F4/80+ cells and clearance of C. rodentium.
(A) Cells were isolated from the colon of WT, Nod2−/−, Ccl2−/−, or Ccr2−/− mice on day 0, and 12 after oral infection with C. rodentium, and stained for CD11b, F4/80, and Gr-1 or CD11c mAb. CD11b+F4/80+ double-positive cells (% of total cells, left panel) were gated and percentage of CD11b+F4/80+Gr-1lo, CD11b+F4/80+Gr-1hi, CD11b+F4/80+CD11clo, and CD11b+F4/80+CD11chi cells in the gated population was determined. (B) Gene expression of Ccr2 was compared between bone marrow monocytes from WT or Nod2−/− mice by real-time RT-PCR. mRNA expression of Ccr2 was normalized to that of -actin. (C) C. rodentium numbers in stool from infected WT, Nod2−/−, Ccl2−/−, or Ccr2−/− mice (n= 10/group) at indicated time p. i. were determined by CFU assay. Data are means ± SD. * and *** denote significant differences between WT and Nod2−/−, Ccl2−/−, or Ccr2−/− mice at p<0.05 and P<0.001, respectively. Results are representative of at least 3 independent experiments.
The regulation of CCL2 by Nod2 is independent of T cells and mediated by hematopoietic and non-hematopoietic cells
Because T cell-dependent responses are critical for C. rodentium clearance, we asked whether the regulation of CCL2 and monocyte influx by Nod2 in the colon requires T cells. To address this question, we generated _Nod2_−/− mice in the _Tcrb_−/− background that lack peripheral T cells and compared the response of these animals to Nod2+/+_Tcrb_−/− mice. As it was observed in mice with intact numbers of T cells, Nod2 was important for the colonic production of CCL2 in response to oral infection with C. rodentium despite the absence of T cells (Figure S2A). Furthermore, Nod2 regulated the influx of mononuclear phagocytic cells to the colon in infected animals in the absence of T cells (Figure S2B). To determine whether Nod2 acts in bone marrow-derived cells or in stromal or epithelial cells to regulate the production of CCL2, we generated chimeric mice by reciprocal bone marrow transfer into lethality-irradiated recipients to generate four groups of chimeric mice. Because Nod2 exhibits an intrinsic function in T cells to regulate T cell survival and/or proliferation during homeostatic proliferation (Shaw et al., 2009), we used _Nod2_−/−_Tcrb_−/− and Nod2+/+_Tcrb_−/− to construct the chimeric animals to avoid secondary effects related to the intrinsic function of Nod2 in T cells. These experiments revealed that both bone marrow-derived and radio-resistant cell populations contribute to Nod2-dependent CCL2 production in response to intestinal infection with C. rodentium, although non-hematopoietic cells appear to play a more important role (Figure S2C).
_Nod2_−/− intestinal stromal cells are impaired in CCL2 production
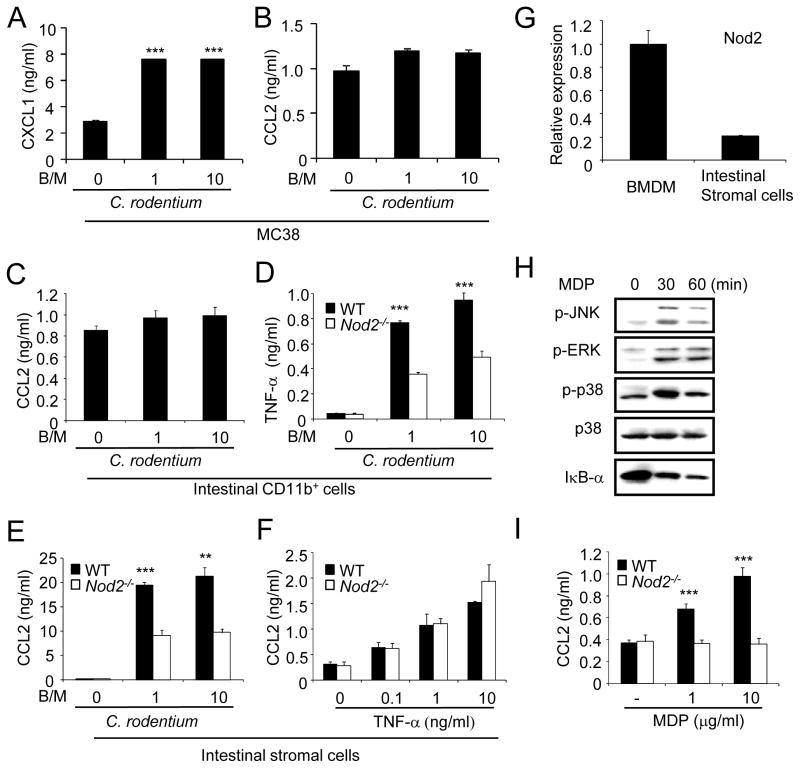
To determine which cells produce CCL2 in response to C. rodentium, we infected colonic epithelial cells, intestinal stromal cells and resident CD11b+ mononuclear cell populations with C. rodentium and measured CCL2 in the culture supernatants. Because primary epithelial cells are not suitable for analysis due to loss of viability during cell isolation, we infected MC38, an epithelial cell line derived from the mouse colon. Infection of MC38 cells with C. rodentium elicited CXCL1 production, but not CCL2 (Figures 5A and 5B). Similarly, CD11b+ intestinal cells that are comprised largely of resident macrophages and DCs did not produce CCL2 in response to C. rodentium infection (Figure 5C), although they secreted TNF-α which was reduced in _Nod2_−/− cells (Figure 5D). Notably, infection of primary stromal cells isolated from the mouse colon with C. rodentium or EPEC elicited robust amounts of CCL2 (~ 20–40 ng/ml), which was significantly reduced in stromal cells deficient in Nod2 (Figure 5E and Figure S3). Furthermore, stimulation of stromal cells with TNF-α induced the production of CCL2, although this was independent of Nod2 (Figure 5F). Nod2 mRNA was expressed in intestinal stromal cells, although at lower levels than in bone-marrow macrophages (Figure 5G). Importantly, stimulation of stromal cells with MDP induced MAPK activation and degradation of IκB-α (Figure 5H). Furthermore, MDP induced secretion of CCL2 which was abrogated in stromal cells deficient in Nod2 (Figure 5I). These results indicate that colonic stromal cells are important producers of CCL2 in response to C. rodentium infection and this process is regulated by Nod2.
Figure 5. Nod2−/− intestinal stromal cells are impaired in CCL2 production. robust amounts of CCL2 in response to C. rodentium which is impaired in Nod2−/− mice.
(A, B) MC38 epithelial cells were infected with live C. rodentium at bacterial/macrophage ratio (B/M) of 0 (uninfected), 1, or 10. Cell-free supernatants were analyzed by ELISA for production of CXCL1 (A) and CCL2 (B). *** denotes significant differences between uninfected and _C. rodentium_-infected cultures at p<0.001. (C) Purified intestinal CD11b+ cells from the colon were infected with C. rodentium at B/M of 0, 1, or 10. Cell-free supernatants were analyzed by ELISA for production of CCL2. (D) Intestinal CD11b+ cells from the colons of WT or Nod2−/− mice were infected with C. rodentium at B/M of 0, 1, or 10. Cell-free supernatants were analyzed by ELISA for production of TNF-α. (E) Colonic stromal cells (Intestinal stromal cells) from WT or Nod2−/− mice were treated with TNF- α at concentration of 0, 0.1, 1, or 10 ng/ml. (F) Intestinal stromal cells from WT or Nod2−/− mice were infected with C. rodentium at the indicated B/M. (G) Nod2 gene expression in bone marrow-derived macrophages and intestinal stromal cells by real-time RT-PCR. mRNA expression of Nod2 was normalized to that of -actin. (H) Intestinal stromal cells were left untreated or stimulated with MDP (10 g/ml) for the indicated times. Cell extracts were immunoblotted with Abs that detect total and phosphorylated (activated) forms of the indicated proteins. (I) Intestinal stromal cells were stimulated with MDP or left untreated for 24 h. Data are means ± SD. **, and *** denote significant differences between cell cultures from WT and Nod2−/− mice at p<0.01, or p<0.001, respectively. Results are representative of at least 3 independent experiments.
Impaired colonic Th1 cell responses and antibody production against C. rodentium in _Nod2_−/− mice
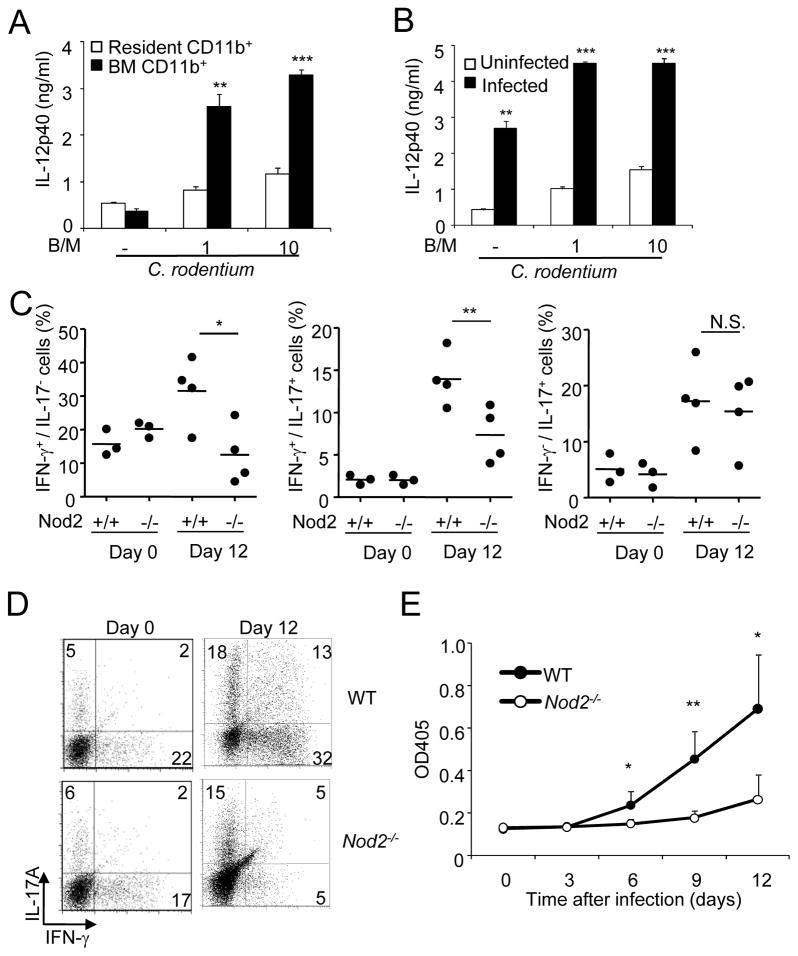
Development of T-cell mediated IgG production against C. rodentium is critical for pathogen eradication (Bry and Brenner, 2004; Maaser et al., 2004; Simmons et al., 2003). Infection of bone marrow CD11b phagocytes with C. rodentium elicited significantly more IL-12, a cytokine that is important for Th1 cell responses, than in resident CD11b cells purified from the colon of uninfected mice (Figure 6A). Notably, CD11b+ cells isolated from infected colons produced IL-12 at amounts comparable to those elicited in bone marrow-derived CD11b+ cells, and at much higher levels than colonic CD11b+ cells from uninfected mice (Figure 6B). Further analysis revealed that CD11b+ cells purified from infected colons expressed markers of classically activated macrophages and increased expression of Arg1 and Ym1 that are induced by microbial stimulation (Qualls et al., 2010), when compared to those found in cells isolated from the colons of uninfected animals (Figure S4A). To determine whether Nod2 regulates Th1 cell responses in vivo, we purified CD4+ T cells from the colons of WT and _Nod2_−/− mice after infection. On day 12 after infection, the induction of IFN-γ-producing CD4+ T cells and doubly producing IFN-γ- and IL-17 CD4+ T cells was impaired in the colon of infected _Nod2_−/− mice when compared to WT mice (Figures 6C and 6D). However, similar numbers of IFN-γ-and IL-17-producing CD4+ T cells were noted in WT and _Nod2_−/− mice on day 22 after infection when reduced loads of the pathogen were observed in both WT and mutant mice (Figure S4B) [Au: lease see comment above.]. The reduction in Th1 responses was specific in that the induction of IL-17-producing CD4+ T cells was comparable in _Nod2_−/− and WT mice (Figures 6C and 6D). Importantly, serum IgG concentrations against C. rodentium were reduced significantly in _Nod2_−/− mice, when compared to WT mice (Figure 6E). Collectively, these results indicate that Nod2 regulates intestinal Th1 cell responses and IgG production against C. rodentium in vivo.
Figure 6. Impaired colonic Th1 responses and antibody production against C. rodentium in Nod2−/− mice.
(A) Colonic (resident) and bone marrow CD11b+ cells from WT mice were infected with C. rodentium at a bacterial/macrophage ratio (B/M) of 0, 1, or 10. Cell-free supernatants were analyzed by ELISA for production of IL-12p40. ** and ***, denote significant differences between cell cultures from WT and Nod2−/− mice infected with C. rodentium at p<0.01, or p<0.001, respectively. (B) CD11b+ cells purified from the colon of uninfected mice or mice inoculated with C. rodentium for 12 days were infected with C. rodentium at indicated B/M. Cell-free supernatants were analyzed by ELISA for production of IL-12p40. ** and ***, denote significant differences between cell cultures from uninfected and infected with C. rodentium at p<0.01, or p<0.001, respectively. (C) Intracellular production of IFN-γ, and IL-17A was assessed in isolated colonic CD4+ T cells from WT or Nod2−/− mice on day 0 and 12 after oral infection with C. rodentium. Each dot represents one mouse and horizontal bars indicate the mean values. (D) Raw data from representative experiment used to generate results in C. Numbers indicate the percentage of cells in each quadrant. (E) Serum levels of _C. rodentium_-specific IgG response (mean OD plus SD) in samples from WT or Nod2−/− mice infected with C. rodentium on day 0, 3, 6, 9, and 12. Data are means ± SD. Results are representative of at least 3 independent experiments.
Ly6Chi monocytes traffic to the colon and restore clearance of C. rodentium in Ccr2−/− mice
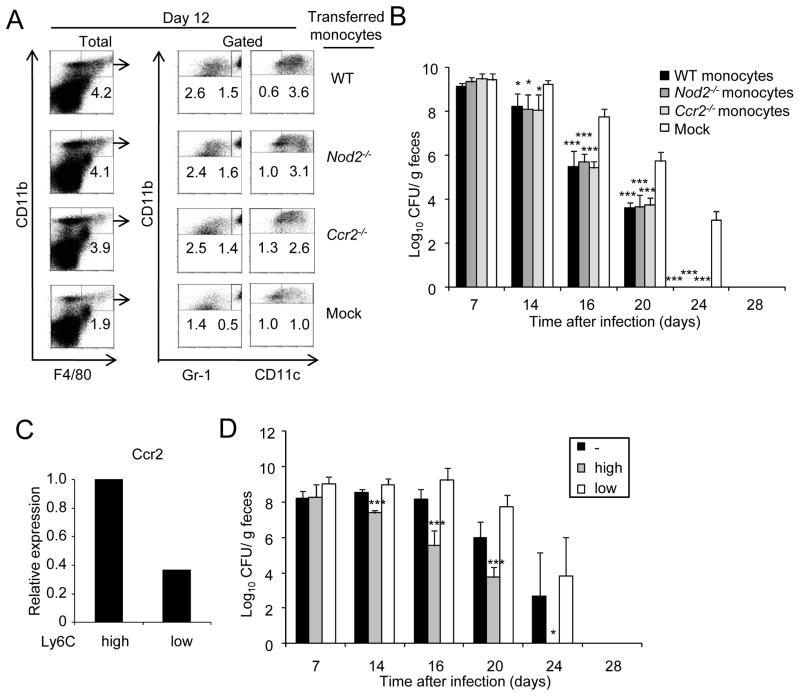
We next assessed the ability of monocytes to promote the clearance of C. rodentium in infected mice. To address this question, we took advantage of our observation that the influx of monocytes to the colon in response to C. rodentium infection is impaired in _Ccr2_−/− mice (Figure 4A). Using an adoptive transfer protocol, we found that intravenous injection of bone marrow monocytes into _Ccr2_−/− mice induced the influx of CD11b+F4/80+Gr1hi cells into the colon of previously infected _Ccr2_−/− mice whereas mock transfer did not (Figure 7A). In addition, transfer of monocytes from _Ccr2_−/− mice also induced the influx of CD11b+F4/80+Gr1hi cells (Figure 7A). The latter is consistent with previous observations that CCR2 is critical for monocyte emigration from the bone marrow, but not for circulation into tissues in response to Listeria or T. gondii infection (Dunay et al., 2008; Serbina and Pamer, 2006). Importantly, adoptive transfer of monocytes from _Nod2_−/− mice was as effective as that of wild-type monocytes in eliciting the influx of CD11b+F4/80+Gr1hi to the colon of infected _Ccr2_−/− recipient mice (Figure 7A). These results indicate that Nod2 controls the colonic recruitment of monocytes through the production of CCL2, but Nod2 is not required within monocytes to traffic to the intestine in response to pathogen infection. _Ccr2_−/− mice transferred with monocytes from wild-type, _Ccr2_−/− and _Nod2_−/− mice effectively cleared C. rodentium with a kinetics comparable to that observed in intact WT mice (Figure 7B). In contrast, the bacterial burden in the feces of _Ccr2_−/− mice that did not receive monocytes was 2–3 logs higher than in mice injected with either wild-type, _Ccr2_−/− or _Nod2_−/− monocytes at day 16, 20, and 24 following infection (Figure 7B). To determine the ability of monocyte populations to promote pathogen clearance, we sorted bone marrow monocytes into Ly6ChiCD11b+ and Ly6CloCD11b+ subsets and adoptively transferred the purified cells to _Ccr2_−/− mice previously infected with C. rodentium. Ly6ChiCD11b+ monocytes expressed higher amounts of CCR2 than Ly6CloCD11b+ monocytes (Figure 7C). Importantly, Ly6ChiCD11b+, but not Ly6CloCD11b+ monocytes, promoted the clearance of the pathogen (Figure 7D). These results indicate that the influx of Ly6Chi monocytes to the colon is important to clear C. rodentium in the intestine.
Figure 7. Ly6Chi but not Ly6Clo monocytes promote clearance of C. rodentium.
(A) Bone marrow monocytes from WT, Nod2−/−, and Ccr2−/− mice were adoptively transferred into Ccr2−/− mice previously challenged orally with C. rodentium. Four days after oral infection with C. rodentium, mice received the cells or received PBS (mock) by i.v. injection. Cells were isolated from the colon of WT monocytes → Ccr2−/− mice, Nod2−/− monocytes → Ccr2−/− mice, Ccr2−/− monocytes → Ccr2−/− mice or received no monocytes (mock) on day 0, and 12 after oral infection with C. rodentium. Intestinal cells were stained for CD11b, F4/80, and Gr-1 or CD11c. CD11b+F4/80+ double-positive cells (% of total cells, left panel) were gated and percentage of CD11b+F4/80+Gr-1lo, CD11b+F4/80+Gr-1hi, CD11b+F4/80+CD11clo, and CD11b+F4/80+CD11chi cells in the gated population was determined. Results are representative of at least two experiments. (B) C. rodentium numbers in stool from infected WT monocytes → Ccr2−/− mice, Nod2−/− monocytes → Ccr2−/− mice, Ccr2−/− monocytes → Ccr2−/− mice or mock → Ccr2−/− mice were determined by CFU assay. (C) Expression of Ccr2 in bone marrow Ly6Chi or Ly6Clo monocytes by real-time RT-PCR. mRNA expression of Ccr2 was normalized to that of β-actin. (D) C. rodentium numbers in stool from infected Ly6Chi monocytes → Ccr2−/− mice, Ly6Clo monocytes → Ccr2−/− mice, or mock → Ccr2−/− mice were determined by CFU assay (n= 5 mice per group). Data are means ± SD. Results are representative of 2 independent experiments.
DISCUSSION
We demonstrate that Nod2 regulates the clearance of C. rodentium by controlling the production of CCL2 and the influx of inflammatory monocytes to the site of infection. Unlike resident macrophages and DCs isolated from uninfected colons, monocytes and CD11b+ phagocytic cells purified from colons infected with C. rodentium elicited the production of robust amounts of IL-12, a cytokine that is critical for the induction of Th1 cell immune responses and eradication of this pathogen (Bry and Brenner, 2004; Maaser et al., 2004; Simmons et al., 2003). Consistently, we found that Nod2 regulates the development of Th1 cells in response to C. rodentium in the intestine and the production of IgG against the bacterium. Adoptive transfer experiments demonstrated the critical role of Ly6Chi inflammatory monocytes in the clearance of C. rodentium in vivo. These findings reveal a previously unrecognized role for Nod2 in the regulation of protective Th1 cell responses against an enteric pathogen in the intestine via CCL2-dependent recruitment of inflammatory monocytes.
Recent studies have revealed the presence of two major subsets of circulating monocytes with different surface markers and functions (Geissmann et al., 2003; Geissmann et al., 2010). The inflammatory Gr1+Ly6Chi inflammatory monocyte subset gives rise to macrophages and DCs in systemic and lung infection models (Peters et al., 2004; Serbina and Pamer, 2006) and is required for resistance against T. gondii in the small intestine of mice (Dunay et al., 2008). We showed here that Nod2 regulates the influx of Gr1+ inflammatory monocytes to the colon in the setting of infection with C. rodentium. In addition to Gr1+ monocytes, the presence of CD11b+F4/80+Gr1lo and CD11b+F4/80+CD11chi cells was reduced in the colon of _Nod2_−/− infected animals. Because Gr1+ inflammatory monocytes differentiate into resident CD11b+F4/80+Gr1lo macrophages or DCs and CD11b+F4/80+CD11chi DCss under basal conditions (Bogunovic et al., 2009; Varol et al., 2009), Nod2 likely controls the presence of CD11b+F4/80+Gr1lo and CD11b+F4/80+CD11chi cells in the colon by inducing the influx of Gr1+ monocyte progenitors into the peripheral blood and into the colon. This possibility is also supported by the observation that the influx of monocytes in peripheral blood triggered by infection is impaired in _Nod2_−/− mice. Furthermore, the increased number of colonic CD11b+F4/80+Gr1lo and CD11b+F4/80+CD11chi elicited by C. rodentium infection is reduced in _Ccl2_−/− and _Ccr2_−/− mice, consistent with the known role of CCL2 in controlling the influx of Gr1+ monocytes to sites of infection (Dunay et al., 2008; Serbina and Pamer, 2006). In contrast, neither Nod2 nor CCL2-CCR2 affected the increase of colonic CD11b+F4/80+CD11clo cells induced by C. rodentium infection. The latter population is heterogenous, but is likely to include resident macrophages that are derived from circulating Gr1−CCR2− monocytes that patrol the luminal surface of vascular endothelium and traffic to surrounding tissues for damage or infection (Auffray et al., 2007).
Under basal conditions, lamina propria CD11b+F4/80+CD11c− macrophages express several anti-inflammatory molecules including IL-10, but little or no proinflammatory cytokines even after microbial stimulation (Denning et al., 2007). In contrast, lamina propria CD11b+CD11c+ cells comprised several DC subpopulations that promote Th17 cell differentiation, T cell regulatory activity and activation of T cells in mesenteric lymph nodes (Denning et al., 2007; Murai et al., 2009; Schulz et al., 2009). In line with previous observations that lamina propria macrophages and DCs are poor drivers of Th1 cell responses (Monteleone et al., 2008), infection of the resident CD11b+ colonic population with C. rodentium elicited significantly less IL-12 production than bone marrow CD11b+ phagocytes. Furthermore, C. rodentium induced robust production of IL-12 from CD11b+ cells isolated from infected colons, but not from CD11b+ cells residing in the uninfected intestine. Although the CD11b+ population isolated from infected colon contain multiple subsets, our results suggest that the inflammatory monocyte population recruited to the site of bacterial infection can induce Th1 cell responses. Consistent with a role of inflammatory monocytes in eliciting Th1 cell responses against the pathogen, IFN-γ-producing T cells and IL-17-producing T cells were induced in the colon after C. rodentium. However, only T cells producing IFN-γ were significantly reduced early in the colon of infected _Nod2_−/− mice which correlated with impaired influx of inflammatory monocytes. Although Nod2 is likely to influence host defense against enteric pathogens by several mechanisms (Biswas et al., 2010; Cruickshank et al., 2008; Watanabe et al., 2006), these results suggest that Nod2 controls through CCL2 the recruitment of Gr1+ monocytes to drive Th1 cell responses locally in the intestine. Thus, Gr1+ inflammatory monocytes may control infection not only by promoting intracellular killing of microbes (Dunay et al., 2008), but also by inducing specific adaptive immunity against the invasive pathogen. In the infection model of C. rodentium, CD4+-dependent IgG production against the pathogen is critical for pathogen clearance (Bry and Brenner, 2004; Bry et al., 2006; Simmons et al., 2003). Importantly, the IgG response against C. rodentium was impaired in _Nod2_−/− mice. Collectively, these results suggest that by regulating the influx of inflammatory monocytes is important for the development of Th1 responses that mediate the enteric eradication of C. rodentium.
Intestinal stromal cells are comprised of several populations including myofibroblasts located directly subjacent to the epithelial basement membrane and interstitial fibroblasts (Powell et al., 1999; Smith et al., 1997). Intestinal stromal cells can produce several pro-inflammatory mediators including cytokines, chemokines, and metabolites of arachidonic acid in response to various TLR ligands (Otte et al., 2003). We found that infection of colonic stromal cells with C. rodentium elicited copious amounts of CCL2 while resident CD11b+ cells did not. Given the close contact of stromal cells with the epithelium and their abundance in the lamina propria, these results suggest that these cells are critically important for the initial sensing of invasive pathogens and eliciting the influx of Gr1+ inflammatory monocytes. A role of stromal cells in CCL2 production in response to C. rodentiun is further supported by several findings. First, CCL2 levels were greatly attenuated in intestinal stromal cells from Nod2−/− mice. Second, the mouse chimeras experiments revealed that Nod2 acts in both radiation-resistant non-hematopoietic cells and bone-marrow derived cells to induce CCL2 in response to C. rodentium infection, although the role of non-hematopoietic cells was more important. The mechanism by which Nod2 impacts on CCL2 production via hematopoietic cells remain to be further investigated. Because infection of resident CD11b+ cells with C. rodentium elicited TNF- α production and this response was attenuated in Nod2 cells, it is possible that the mechanism involves impaired secretion of CCL2 by stromal cells in response to TNF- α stimulation. Although our results are consistent with stromal cells being important drivers of CCL2 production in the intestine, our results may not apply to all tissues given that CCL2 is produced by several cell types and that the location and abundance of stromal cells vary among different organs (Colotta et al., 1992; Struyf et al., 1998). Further studies using different models of microbial infection will be necessary to understand the role of different cell types in the production of CCL2.
Our findings regarding the role of Nod2 in this model of intestinal infection with C. rodentium may have implications for understanding the pathogenesis of CD. A remarkable finding was that Nod2−/− mice exhibited reduced inflammation during the early phase of the C. rodentium infection, but at later stages the colon of mutant mice developed severe colitis. Because the induction of colitis in Nod2−/− was preceded by impaired clearance of the enteric bacterium, a possible interpretation is that the failure to clear the bacterium elicited the over activation of Nod2-independent pathways to clear the pathogen. This scenario is consistent with the observation that Nod2−/− mice exhibited impaired clearance of C. rodentium, but ultimately the mutant mice eliminated the bacterium. Because C. rodentium can be recognized by several pattern recognition receptors including TLR2 and TLR4 (Gibson et al., 2008; Khan et al., 2006), we suggest that in the absence of Nod2, the mutant mice mount a compensatory inflammatory responses that leads to pathogen eradication. Consistent with this model, colitis triggered by adoptive transfer of OVA-specific T cells into Nod2−/− recipient mice followed by challenge with recombinant E. coli expressing OVA peptide is attenuated in the absence of TLR2 (Watanabe et al., 2006). Although several interpretations of the latter experiments are possible including a regulatory role for Nod2 in TLR-mediated responses (Watanabe et al., 2006), one possibility is that TLR2 is needed to drive pro-inflammatory T cells in the absence of Nod2. We have shown that Nod2 is important to induce Th1 responses in the colon of mice infected with C. rodentium. However, the impairment of both Th1 responses and pathogen clearance was partial, indicating that other innate signaling pathways activated by the pathogen can contribute to drive Th1 responses leading to pathogen clearance. Because human CCR2+ inflammatory monocytes, but not resident macrophages/DCs, can respond robustly to microbial stimulation and accumulate in intestinal tissue of CD (Kamada et al., 2008), one interesting possibility is that CCR2+ inflammatory monocytes maintain Th1 responses that are characteristic of IBD.
EXPERIMENTAL PROCEDURES
Infection of Mice
C57BL/6 mice were from Jackson Laboratories. _Nod2_−/− mice in C57BL/6 background have been described (Kim et al., 2008). _Ccl2_−/− and _Ccr2_−/− mice in C57BL/6 background were a gift of Dr. Kenneth James Pienta, the University of Michigan. Mice were orally inoculated with kanamycin (Kn)-resistant wild-type C. rodentium strain DBS120, a gift of Dr. David Schauer, Massachusetts Institute of Technology. Mice were infected by oral gavage with 0.2 ml of PBS containing approximately 1 × 109 CFU of C. rodentium. Histologic assessment was performed in a blinded fashion by using a scoring system (Chen et al., 2008). EPEC strain MOR6 was a gift of Dr. M. O’Riordan, the University of Michigan. The animal studies were conducted under approved protocols by the University of Michigan Committee on Use and Care of Animals.
Cytokine Levels
Mouse chemokines and cytokines were measured with enzyme-linked immunoabsorbent assay (ELISA) kits (R & D Systems, Minneapolis, MN).
Bone marrow and Intestinal Cell Isolation
Colonic cells were isolated using a modified protocol described previously (Takada et al., 2010). Colonic macrophage/DCs cells or bone marrow monocytes were further purified by positive selection using a magnetic cell separation system (MACS; Miltenyi Biotec, Auburn, CA) with anti-mouse CD11b microbeads (Takada et al., 2010). Analyses of purified populations showed that the purified BM cells were > 80% CD11b+F4/80+ while the intestinal cells were > 80% CD11b+ and contained both F4/80+ and CD11c+ cells. Stromal cells were isolated from the intestinal mononuclear cell fraction by plating and incubating intestinal mononuclear cells overnight. The attached fibroblast-like cells were further culture for 7 days before use (Lawrance et al., 2003).
Flow Cytometric Analysis
Cells were stained with labeled mAbs for mouse F4/80, CD11b, CD11c, and Gr-1 (purchased from BD Pharmingen or eBiosciences [San Diego, CA]) for 20 min at 4°C. After staining, cells were analyzed using a FACS Calibur system. CellQuest (BD Biosciences, San Jose, CA) or Flowjo (TreeStar, Ashland, OR) software was used for data analysis.
In vitro bacterial Infection
MC38 cells (a gift of Dr. Yang Liu from the University of Michigan), monocytes, macrophages/DCs, or stromal cells were infected for 60 min at 37° C, and 50 g/ml of gentamycin was added to limit the growth of extracellular bacteria.
cDNA synthesis and real-time RT-PCR
Total RNA was extracted using Total RNA Kit I (OMEGA Bio-Tek. Inc.) according to the manufacturer’s instructions and cDNA was synthesized using High Capacity RNA-to-cDNA Kit (Applied Biosystems). Real-time PCR was performed using SYBR Green master mix (Applied Biosystems). Expression of each target gene was normalized to that of β-actin.
Immunoblotting
Cell lysates were probed with antibodies specific for mouse IκB-α, p38, phospho-p38, phospho-ERK, and phospho-JNK (Cell Signaling Technology, Beverly, MA).
ELISA for anti-Citrobacter IgG
_C. rodentium_-specific IgG was quantified by coating 96-well plates with heat-killed C. rodentium as described (Bry and Brenner, 2004).
Bone Marrow Chimeric Mice
To generate BM chimeric mice, Nod2+/+TCR −/− or Nod2−/−TCR −/− recipients received a single lethal dose of 9 Gy total body irradiation from a 137Cesium source. On the same day, mice were injected i.v. with 5 × 106 BM cells from Nod2+/+TCR −/− or Nod2−/−TCR −/− mice and then rested for 8 weeks before use.
Adoptive Transfer
Bone-marrow cells from wild-type, Nod2−/−, Ccr2−/− mice were harvested, washed in FACS buffer and monocytes were isolated by The EasySep® mouse monocyte enrichment Kit (STEMCELL Technologies Inc, Vancouver, Canada). To separate Ly6Chigh and Ly6Clow monocytes, bone marrow monocytes were incubated with anti-CD11b and anti-Ly6C mAb and double-positive (CD11b+Ly6C+) and single-positive (CD11b+Ly6C−) cells sorted by flow cytometry. Monocytes were counted and transferred by i.v. inoculation of 1 × 106 cells in the tail vein of Ccr2−/− mice 4 days after oral infection with C. rodentium. Bacteria clearance was measured every week until 4 weeks after oral infection. Alternatively, colons were collected 12 days after oral infection, and colonic cells were isolated for flow cytometric analysis.
Statistical Analysis
Statistical significance between groups was determined by two tailed _t_-test with unequal variance (Aspin-Welch’s _t_-test). In vivo chemokine production and bacterial counts of infected mice were analyzed by Mann-Whitney test. Differences were considered significant when p values were <0.05.
Supplementary Material
01
Acknowledgments
We thank Richard Flavell, Tak Mak and Kenneth Pienta for generous supply of mutant mice and David Schauer for bacterial strains. Sharon Koonse for excellent animal husbandry, and Joel Whitfield for ELISA assays. This work was supported by NIH grant R01 DK61707 and funds to the Michigan Comprehensive Cancer Center Immunology Monitoring Core from the University of Michigan’s Cancer Center Support Grant (5 P30 CA46592). Y.-G. Kim was supported by training funds from the University of Michigan Comprehensive Cancer Center, N. K. by a Fellowship from the Uehara Memorial Foundation, L. F. by a Career Development Award from the Crohn’s and Colitis Foundation of America, and M. H. S. by training grant 2T32 HL007517 from the NIH.
Abbreviations
DC
dendritic cell
MAPK
mitogen-activated protein kinase
MDP
muramyl dipeptide
NLR
nucleotide-binding oligomerization domain-like receptors
PGN
peptidoglycan
TLR
Toll-like receptor
WT
wild-type
Kn
Kanamycin
p.i
post- infection
Footnotes
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Biswas A, Liu YJ, Hao L, Mizoguchi A, Salzman NH, Bevins CL, Kobayashi KS. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc Natl Acad Sci U S A. 2010;107:14739–14744. doi: 10.1073/pnas.1003363107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenshtein D, McBee ME, Schauer DB. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr Opin Gastroenterol. 2008;24:32–37. doi: 10.1097/MOG.0b013e3282f2b0fb. [DOI] [PubMed] [Google Scholar]
- Bry L, Brenner MB. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J Immunol. 2004;172:433–441. doi: 10.4049/jimmunol.172.1.433. [DOI] [PubMed] [Google Scholar]
- Bry L, Brigl M, Brenner MB. CD4+-T-cell effector functions and costimulatory requirements essential for surviving mucosal infection with Citrobacter rodentium. Infect Immun. 2006;74:673–681. doi: 10.1128/IAI.74.1.673-681.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Shaw MH, Redondo G, Nunez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Borre A, Wang JM, Tattanelli M, Maddalena F, Polentarutti N, Peri G, Mantovani A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J Immunol. 1992;148:760–765. [PubMed] [Google Scholar]
- Cruickshank SM, Wakenshaw L, Cardone J, Howdle PD, Murray PJ, Carding SR. Evidence for the involvement of NOD2 in regulating colonic epithelial cell growth and survival. World J Gastroenterol. 2008;14:5834–5841. doi: 10.3748/wjg.14.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DL, Ma C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, Khan MA, Vallance BA. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:388–403. doi: 10.1111/j.1462-5822.2007.01052.x. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- Johnston B, Butcher EC. Chemokines in rapid leukocyte adhesion triggering and migration. Semin Immunol. 2002;14:83–92. doi: 10.1006/smim.2001.0345. [DOI] [PubMed] [Google Scholar]
- Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Ma C, Knodler LA, Valdez Y, Rosenberger CM, Deng W, Finlay BB, Vallance BA. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect Immun. 2006;74:2522–2536. doi: 10.1128/IAI.74.5.2522-2536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- Lawrance IC, Wu F, Leite AZ, Willis J, West GA, Fiocchi C, Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, Eckmann L. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect Immun. 2004;72:3315–3324. doi: 10.1128/IAI.72.6.3315-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone I, Platt AM, Jaensson E, Agace WW, Mowat AM. IL-10-dependent partial refractoriness to Toll-like receptor stimulation modulates gut mucosal dendritic cell function. Eur J Immunol. 2008;38:1533–1547. doi: 10.1002/eji.200737909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LO, Smith AM, DeFreitas AA, Qualls JE, El Kasmi KC, Murray PJ. Modulation of adaptive immunity by different adjuvant-antigen combinations in mice lacking Nod2. Vaccine. 2008;26:5808–5813. doi: 10.1016/j.vaccine.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–1878. doi: 10.1016/s0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Nunez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- Penack O, Smith OM, Cunningham-Bussel A, Liu X, Rao U, Yim N, Na IK, Holland AM, Ghosh A, Lu SX, et al. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J Exp Med. 2009;206:2101–2110. doi: 10.1084/jem.20090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W, Cyster JG, Mack M, Schlondorff D, Wolf AJ, Ernst JD, Charo IF. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J Immunol. 2004;172:7647–7653. doi: 10.4049/jimmunol.172.12.7647. [DOI] [PubMed] [Google Scholar]
- Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183–201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, Kaplan G, Watowich SS, Murray PJ. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci Signal. 2010;3:ra62. doi: 10.1126/scisignal.2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Shaw MH, Reimer T, Kim YG, Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20:377–382. doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MH, Reimer T, Sanchez-Valdepenas C, Warner N, Kim YG, Fresno M, Nunez G. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol. 2009;10:1267–1274. doi: 10.1038/ni.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, Goldin R, Lewis DJ, MacDonald TT, Strugnell RA, et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2003;71:5077–5086. doi: 10.1128/IAI.71.9.5077-5086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf S, Van Collie E, Paemen L, Put W, Lenaerts JP, Proost P, Opdenakker G, Van Damme J. Synergistic induction of MCP-1 and -2 by IL-1beta and interferons in fibroblasts and epithelial cells. J Leukoc Biol. 1998;63:364–372. doi: 10.1002/jlb.63.3.364. [DOI] [PubMed] [Google Scholar]
- Takada Y, Hisamatsu T, Kamada N, Kitazume MT, Honda H, Oshima Y, Saito R, Takayama T, Kobayashi T, Chinen H, et al. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol. 2010;184:2671–2676. doi: 10.4049/jimmunol.0804012. [DOI] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473–485. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01