Zinc transporter ZnT3 is involved in memory dependent on the hippocampus and perirhinal cortex (original) (raw)
. Author manuscript; available in PMC: 2012 Sep 30.
Published in final edited form as: Behav Brain Res. 2011 Apr 27;223(1):233–238. doi: 10.1016/j.bbr.2011.04.020
Abstract
Since zinc transporter ZnT3 is localized to the hippocampus and perirhinal cortex, we used ZnT3 knockout mice (KO) to analyze the role of ZnT3 in memory and behavior dependent on these brain regions. ZnT3KO mice were normal in initial learning in the standard water maze but had difficulty finding a second platform location. The mutants showed increased social interaction but were deficient in social and object recognition memory. These data suggest that ZnT3 is involved in certain types of spatial memory and behavior dependent on the hippocampus and perirhinal cortex.
Keywords: Spatial memory, Social recognition, Object recognition, Perirhinal cortex, Hippocampus
The human MTL is involved in the conscious memory for facts and events, often called declarative memory [1]. Analysis of memory deficits of patients has brought much insight into our understanding of memory [2, 3]. These studies of patients with MTL lesions have prompted developing models in monkeys and rodents designed to resemble memory deficits to allow for more precise functional dissection of MTL structures. Using this approach, much information was gathered on which MTL structures are responsible for various types of declarative memory. Still, very few genes are known to be enriched in the MTL, which is unfortunate because this would provide new insights into region-and circuitry-specific molecular mechanisms involved in declarative memory [4]. Zinc transporter ZnT3 is strongly enriched in MTL including the amygdala, hippocampus and perirhinal cortex [5]. ZnT3 is selectively located in the vesicles of zinc-secreting neurons and is responsible for most of the zinc released into the synapse [6]. The expression pattern of the ZnT3 protein is very similar to Zn2+ distribution [5, 7–9]. Zn2+ is involved in normal brain function [10–13] as well as in several pathological conditions, including Alzheimer’s disease [14]. In neurons, synaptic zinc may act as a neuromodulator [6, 15] and is involved in synaptic plasticity [9, 16–18].
ZnT3KO mice (a generous gift from Richard Palmiter, University of Washington) were maintained on C57BL/6J background (N>10) [8]. Homozygous KO mice and their wild-type (WT) littermates were generated by breeding ZnT3 heterozygous mice. All mice were maintained on a 12-h light/dark cycle. Behavioral experiments were conducted on 3–4 month old male littermates during the light phase of the cycle with experimenter blind to the genotype.
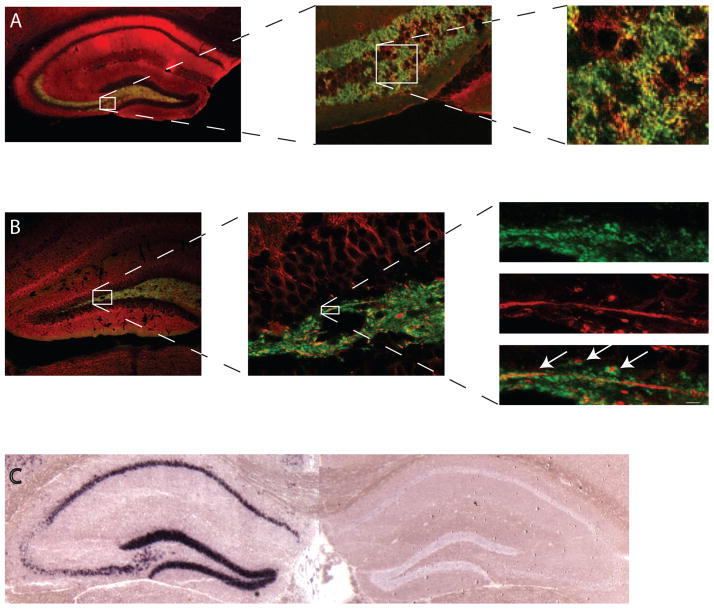
To analyze cell types expressing ZnT3 in MTL, we performed immunocytochemical co-localization experiments using antibodies against ZnT3 and synaptophysin (presynaptic marker) or MAP2a/b (dendritic marker). After incubation with the primary antibodies (rabbit anti-ZnT3 antibody (a gift from Richard Palmiter, 1:100), mouse anti-synaptophysin antibody (Sigma, 1:100), or mouse anti-MAP2a/b (Abcam, 1:200) in the presence of the M.O.M kit (Vector), sections were incubated with the biotinylated goat anti-rabbit antibody (Vector, 1:600) followed by fluorescein avidin (Vector, 1:800) and Cy3-conjugated donkey anti-mouse antibody (Jackson ImmunoResearch, 1:1600). Antibody labeling for synaptophysin showed co-localization with ZnT3 (Fig. 1A). Labeling for MAP2a/b showed proximity of the ZnT3-positive presynaptic structures to the MAP2-positive dendrites (Fig. 1B). These experiments confirmed ZnT3 presynaptic expression in the MTL principal neurons. Also, digoxigenin-RNA in situ hybridization performed on hippocampal brain slices showed ZnT3 RNA presence in WT but not ZnT3KO mice (Fig. 1C).
Figure 1.
Localization of ZnT3 protein in the hippocampus. A, There is a strong overlap (yellow) between antibodies against ZnT3 (green) and synaptophysin (red), a presynaptic marker. B, Double-labeling using anti-ZnT3 (green) and anti-MAP2a/b (red), a dendritic marker, shows no co-localization but proximity between the ZnT3- and MAP2-labeled neurons (arrows). Scale bar is 1 μm. C, ZnT3 mRNA is present in the hippocampus in WT mice (left panel) but not in KO mice (right panel).
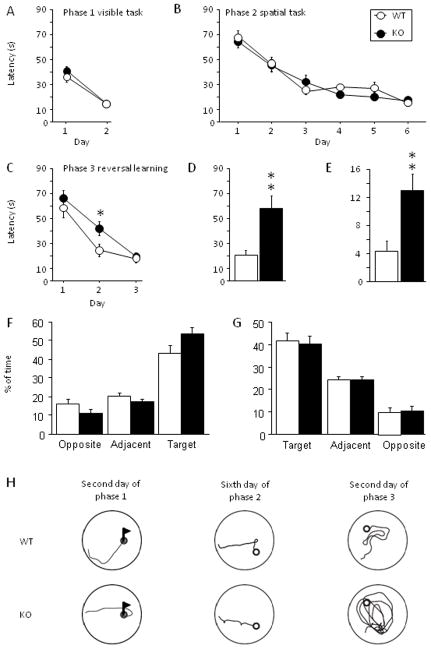
To test hippocampus-dependent memory in ZnT3KO mice, we first analyzed their performance in the standard water maze protocol [19]. The training apparatus was a circular pool (1.8 m diameter) filled with water, which was made opaque by the addition of white nontoxic latex paint. The pool was surrounded by numerous visual cues, which were kept in constant locations for the entire training period. A circular escape platform (13 cm diameter) was submerged ~0.8 cm below the surface of the water. The task was composed of three training phases as previously described [20]. The first phase consisted of two days of training the mice to reach a visible platform (procedural memory) followed by the second phase consisting of six days of training to find a hidden platform (spatial memory). The third phase was a transfer task consisting of three days with a hidden platform located in the quadrant opposite to the location of the platform during the spatial phase. For each phase, four trials, 90-s maximum with a 15-min inter-trial interval (ITI), were given daily. Statistical analysis was run using Statview (SAS institute). In the first phase, both KO and WT mice learned to locate the visible platform (F(1,18) = 79.53; P < 0.001) regardless of their genotypes (_P_ > 0.306; Fig. 2A). In addition, during phase 1 no difference was detected between genotypes in swim speed (data not shown; Ps > 0.479) showing that ZnT3KO mice did not have visual, sensory, motor or motivational deficits consistent with previously published work [7, 21]. During the second phase, both genotypes were able to find the platform (F(5,95) = 41.54; P < 0.001) and displayed a similar level of performance (_P_ > 0.398; Fig. 2B). During phase 2, no difference between WT and KO mice was detected in swim speed (data no shown; Ps > 0.125) as well as in time spent near the wall (data not shown; Ps > 0.190). When analyzed as a probe test, the first trial of the transfer test (phase 3; Fig. 2F) revealed that both genotypes spent significantly more time in the target quadrant (old platform location in phase 2) than in the other quadrants (F(3,57) = 60.717; P < 0.001) but without difference between WT and KO mice (_P_s > 0.078). This analysis confirmed that both genotypes used a spatial strategy during phase 2. In the transfer test, the mutants showed a deficit in learning the new location of the platform (Fig. 2C–E). More specifically, on the second day of the transfer test ZnT3KO mice spent more time searching for the platform than did control mice (Scheffe’s F, P < 0.035). Further analysis of the first trial of the second day showed that the mutants needed more time to reach the platform (unpaired student t-test, t = 3.32; _P_ < 0.004; Fig. 2D) because they spent more time swimming in the area of the former location of the platform (unpaired student t-test, t = 3.21; _P_ < 0.005, Fig. 2E). Analysis of swim speed did not reveal any differences between the genotypes (data not shown; _Ps_ > 0.063). The probe test performed 24 h after the third day of transfer (Fig. 2G) showed that both genotypes used a spatial strategy during spending 40% of the time in the target quadrant (F(3,57) = 23.181; P < 0.001) without any difference between genotype (Ps = 0.397).
Figure 2.
ZnT3KO mice have normal learning in the water maze, but showed deficits in reversal learning. A, No difference was found between the genotypes (10 WT and 11 KO) in the visible platform task in the water maze (phase 1). B, Both genotypes learned similarly in the spatial memory task (phase 2). C, In the transfer task (reversal learning), both genotypes learned the new location of the platform, but ZnT3KO mice needed significantly more time to find the platform during the second day (phase 3). D and E, First trial of the second day of the transfer task. D, Mutants needed significantly more time to find the platform. E, Time spend on the former platform location during the first trial of the second day of transfer, showing that ZnT3KO mice spent significantly more time in the previous platform location. F, First trial of phase 3 analyzed as a probe trial showed that both genotypes used a spatial strategy during phase 2. G, Probe trial performed 24 h after the end of phase 3 showed that WT and KO mice used spatial strategy during phase 3. H, Representative swim paths used by KO and WT mice during each phase. Pictures on the left represent swim paths used on day 2 of phase 1 (visible platform). Pictures in the middle represent swim paths that were used on day 6 of phase 2 (spatial phase). Pictures on the right represent swim paths used during day 2 of phase 3 (reversal – transfer) when KO mice spent significantly more time finding the platform. Results are presented as mean ± SEM. *, P < 0.05; **, P < 0.01.
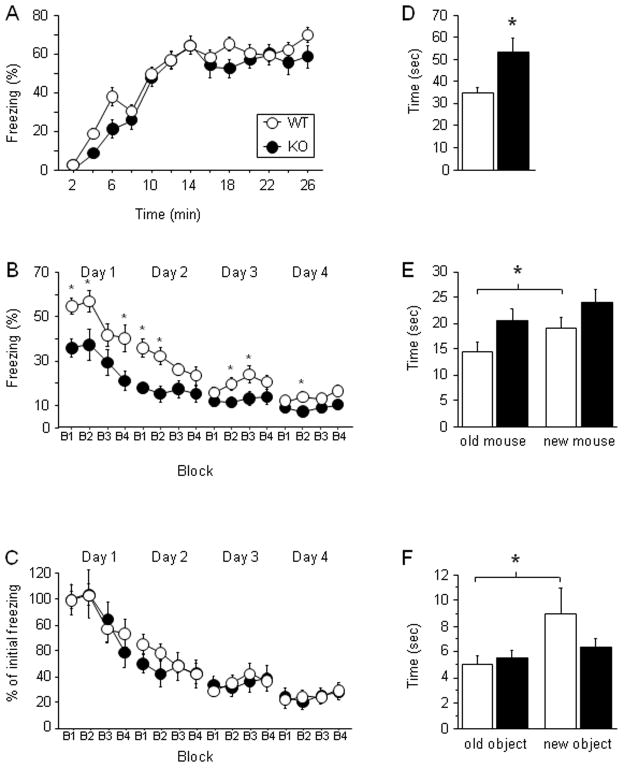
To examine ZnT3KO mice in another memory paradigm dependent on the hippocampus, we turned to contextual fear conditioning and extinction. During training, mice received 10 shocks (2 s, 0.7 mA) with an average of a 75-s ITI explicitly unpaired with 10 tones (30 s, 2.8 kHz, 85 db). The percentage of time spent freezing was measured by FreezeView software (Coulbourn Instruments). During the acquisition phase, all mice learned the task (F(12, 360) = 99.14; P < 0.001) with a similar rate of progression between the genotypes (_P_ > 0.070; Fig. 3A). Both groups learned equally well as shown by the average of the last 3 minutes of the acquisition (P > 0.129, data not shown). The extinction of fear memory was performed in the acquisition context 5 h following training. The mice were exposed to the acquisition context for 10 min a day for 4 days total. At the beginning of the first extinction session, ZnT3KO mice froze less than WT mice (unpaired student t-test, t = 3.34; P < 0.002), showing a deficit in contextual fear memory. During extinction, although both groups extinguished through all 4 days of extinction (_F_(15, 450) = 35.99; _P_ < 0.001; Fig. 3B), the ANOVA showed genotype effect between ZnT3KO and WT mice (_F_(1, 30) = 8.88; _P_ < 0.006) with ZnT3KO mice freezing less than WT littermates. Moreover, a significant interaction between genotype and extinction sessions was found (_F_(15, 450) = 2.30; _P_ < 0.004). Interestingly, analysis performed on curves normalized to the initial learning (Fig. 3C) showed that both genotypes extinguished with the same rate (_Ps_ > 0.735).
Figure 3.
Contextual fear, social interaction, and object recognition are deficient in ZnT3KO mice. A, B and C, Contextual fear conditioning. A, Both genotypes (16 WT and 16 KO) had a similar rate of freezing during the acquisition phase. B, During the extinction phase, ZnT3KO mice froze less than WT littermates. Percent of freezing to the context is shown by 4 blocks of 150 s during 4 days of extinction. C, Extinction normalized to the initial freezing showed no difference in the rate of extinction between WT and KO mice. D, ZnT3KO mice (n = 9) have a significant increase in social interactions compared to WT littermates (n = 10). E, During social memory test, ZnT3KO mice failed to recognize an unfamiliar mouse from the familiar mouse compared to the control mice. F, Object recognition. During testing ZnT3KO mice (n = 11) failed to discriminate between the old object and the new object compared to control mice (n = 11). Results are presented as mean ± SEM. Results are presented as mean ± SEM. *, P < 0.05.
ZnT3KO mice were also examined in hippocampus-dependent social memory. Before the beginning of the experiment, mice were housed individually for a week and then were exposed to a stranger mouse for 15 min each day for 2 days for habituation. The social interaction test consisted of placing an intruder into the home cage of the experimental mouse for 5 min. The investigating behavior of the host towards the intruder was video recorded. For social discrimination, after a 24-hr interval, the same intruder was placed into the cage of the host together with a novel mouse. The investigating behavior of the host towards the old mouse vs. new mouse was video recorded for 5 min. Video recordings were analyzed off-line by two independent observers. During social interactions, ZnT3KO mice spent significantly more time with a stranger mouse compared to the control mice (unpaired student t-test, t = 2.84; P < 0.012; Fig. 3D). However, this did not lead to an increase in aggressive behavior (data not shown). During the social discrimination phase, WT mice spent significantly more time with an unfamiliar mouse than with a familiar mouse (paired student t-test, t = 2.68; _P_ < 0.025), whereas ZnT3KO mice failed to discriminate between the two mice (_P_ > 0.192; Fig. 3E).
Because ZnT3 is expressed in the perirhinal cortex, we analyzed ZnT3KO mice in object recognition using protocol sensitive to perirhinal lesions [22]. Mice were placed individually in the corner of an arena (43.2-cm × 43.2-cm) and were allowed to explore two objects (out of total three objects used in the experiment) for 8 min which were placed equidistantly from the wall. During the presentation session no preference was detected for one object over the other for both genotypes (P > 0.578; data not shown). After a 24-hr delay, each mouse was returned to the arena, which now contained a novel object and one of the old objects. Testing lasted for 5 min and was video recorded; the time spent exploring a novel object vs. an old object was analyzed off-line. WT mice spent more time investigating the novel object during the retention session (paired student t-test, t = 2.63; P < 0.025), while ZnT3KO mice spent an equal amount of time with both the new and the old object (_P_ > 0.200; Fig. 3F).
In summary, our work shows that ZnT3 is involved in several types of memory dependent on MTL: spatial memory, contextual fear conditioning/extinction and social memory are dependent on the hippocampus, and object recognition is dependent on the perirhinal cortex and hippocampus. We previously showed that ZnT3, in addition to be strongly expressed in the amygdala, is highly enriched in the hippocampus and perirhinal cortex [7]; the deficits in the behaviors controlled by these two brain areas in ZnT3KO mice show the role of ZnT3 in the hippocampo-perirhinal neural circuitry. Because ZnT3 is the major transporter of synaptically released zinc in the brain, our experiments also suggest Zn2+ involvement in these behaviors.
In the standard water maze, ZnT3KO mice learned normally the location of the platform, as reported previously by Cole et al. [21] and by Adlard et al. [23] for young adult KO mice (but not older KO mice). However, during reversal learning they spent more time finding new platform location compared to the WT mice, which is also consistent with the study by Cole et al. [21]. Since in the transfer task we found differences on day 2 but not on day 1, we analyzed the percentage of time spent by the WT and the KO mice in each quadrant on day 1 of the transfer task. Both genotypes spent a lot of time looking for the platform in the original platform location. This explains why there was no difference on day 1 of the transfer task. The WT mice on day 1 of the transfer task did not learn yet the new location, but on day 2 they learned it better than the KO mice. Interestingly, normal initial learning but difficulty in finding a second platform location in ZnT3KO mice is reminiscent of the spatial memory deficit found in patient H.M., who was able to locate an invisible sensor during initial learning but not the second sensor location in a spatial memory task designed to be a human analogue of the Morris water maze [24]. In humans, this spatial task is thought to be dependent on the parahippocampal cortex [25], which is likely to be normal in ZnT3KO mice since this brain area does not express ZnT3 [7]. By contrast, finding new routes requires the intact hippocampus, which is deficient in ZnT3KO mice [26, 27].
In contextual fear conditioning and extinction, ZnT3KO mice showed deficiency as well. Mice were trained with the CS and US presented in an explicitly unpaired fashion; this protocol favors hippocampal vs. amygdala involvement and supports conditioning to the context by minimizing CS-US contingency [28, 29]. Although ZnT3KO mice learned normally during fear conditioning training, 5 h later when tested in the same context (at the beginning of the extinction session) they froze much less than the WT mice. When we normalized the extinction curves to the initial freezing we found that the rate of extinction was similar between the WT and KO mice. These data suggest that the mutants are able to learn and extinguish normally the CS-US association; however, they are deficient in retaining/recalling fear memory for the context after initial learning. Studies of the hippocampal involvement in contextual fear conditioning have provided controversial results [28, 30–34]. Phillips and LeDoux [28] showed that pretraining hippocampal lesions do not affect foreground contextual conditioning (unpaired); however, they pointed out that they cannot rule out a role for the hippocampus in the foreground conditioning. Trifilieff et al. [35] showed an enhancement of synaptic efficacy in the CA1 of the dorsal hippocampus after unpaired fear conditioning. They also showed that injections of the MEK inhibitor U0126 in the dorsal hippocampus 30 min before or 7.5 h after conditioning impaired memory for the context in the unpaired fear conditioning protocol. Thus, there is some evidence for the role of the dorsal hippocampus in foreground conditioning. An earlier work by Cole et al. [21] did not find abnormalities in contextual fear conditioning in ZnT3KO mice. The differences in our results and theirs may be in the strain background. Their mice were on a mixed C57 × 129 background while our mice are backcrossed to the C57 strain. There are also some differences in the protocols: during training we used 10 explicitly unpaired CS-US presentations while Cole et al. [21] used 3 paired CS-US presentations. A recent paper by Sindreu et al. [26] also examined ZnT3KO mice in contextual fear learning. They showed that ZnT3KO mice were normal in gradual acquisition of contextual fear conditioning over 5 days (with a mild shock, 0.4 mA); the mutants however were deficient in contextual discrimination. Overall, our results and others show that ZnT3 is involved in some straight-forward contextual learning but not in all behavioral tasks where contextual memory is tested. Hippocampal ZnT3 is likely involved in contextual fear learning, although ZnT3 expression in the amygdala and perirhinal cortex may also contribute to this phenotype.
We found that ZnT3KO mice have deficits in social recognition. Long-term social memory in mice has been shown to be dependent on the hippocampus [36]. Interestingly, Kogan et al. found that housing of mice in groups allows long-term social memory to be formed, but when the mice were housed individually, they could retain memory for 30 min only. We keep our mice in groups and house them individually for one week before the experiments. The WT mice were capable of learning social recognition, although the mutants showed deficit. This discrepancy between our results and those by Kogan et al. may be attributed to differences in the protocols, such as analyzing mouse interactions for 5 min in our protocol versus 2 min by Kogan et al. An increase in social interactions in KO mice may be attributed to the deficits in memory for social recognition in the mutants. An initial interest during an encounter between mice of the same sex is driven by novelty and later should cease in WT mice due to lack of novelty. Since the mutants have a memory deficiency for social recognition (as we showed) they continue interacting. Our previous work as well as others demonstrated that many of the mice that are knockouts for amygdala-enriched genes had an increase in social interactions [37, 38]. This may be due to the fact that the amygdala projections to the areas of the hippocampus and cortex that control social interactions are deficient thus leading to abnormally long social interactions. Decreased amygdala function leads to decreased danger assessment and thus the KO animals spend more time interacting.
ZnT3KO mice were deficient in object recognition, which is controlled by the hippocampus and perirhinal cortex. According to one view, the perirhinal cortex is critically important for recognition of the object’s novelty, while the hippocampus contributes to the performance of certain object recognition tasks when contextual information becomes important [39]. The protocol we were using was adapted from the study where perirhinal lesions affected performance in rats [22], thus our results may point to ZnT3 expression in the perirhinal cortex as important in object recognition. Another possibility is that recollection and familiarity are controlled by both the hippocampus and perirhinal cortex and that methods commonly used to differentiate between recollection and familiarity actually distinguish between strong and weak memories [40, 41]. It is interesting in this regard that our recently published work suggested that ZnT3 is involved in weak or complex fear memories but may be dispensable for learned fear acquired in standard cued conditioning with several CS-US presentations [7].
In conclusion, our present results show that ZnT3 is involved in several forms of spatial memory and behavior dependent on the hippocampus and perirhinal cortex. Our current data together with our earlier work on the role of ZnT3 in the amygdala-dependent fear learning [7] and work by Sindreu et al. on the role of hippocampal ZnT3 in contextual discrimination [26] suggest that ZnT3 is important in cognitive function where re-learning or attention to visual/auditory/contextual details is required.
Acknowledgments
We are grateful to the NARSAD, NIH, NSF, and Whitehall Foundation for financial support and A. Manzar, A. Wong, Trevor Baybutt and Olivia Friebely for technical assistance. Research funded in part by the New Jersey Governor’s Council for Medical Research and Treatment of Autism, Special Child Health and Early Intervention Services, New Jersey Department of Health and Senior Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Squire LR, Zola-Morgan S. Memory: brain systems and behavior. Trends Neurosci. 1988;11:170–5. doi: 10.1016/0166-2236(88)90144-0. [DOI] [PubMed] [Google Scholar]
- 2.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squire LR. The legacy of patient H.M. for neuroscience. Neuron. 2009;61:6–9. doi: 10.1016/j.neuron.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda M, Mayford MR. CaMKII activation in the entorhinal cortex disrupts previously encoded spatial memory. Neuron. 2006;50:309–18. doi: 10.1016/j.neuron.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:14934–9. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol. 2005;566:747–58. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martel G, Hevi C, Friebely O, Baybutt T, Shumyatsky GP. Zinc transporter 3 is involved in learned fear and extinction, but not in innate fear. Learn Mem. 2010;17:582–90. doi: 10.1101/lm.1962010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci U S A. 1999;96:1716–21. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kodirov SA, Takizawa S, Joseph J, Kandel ER, Shumyatsky GP, Bolshakov VY. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc Natl Acad Sci U S A. 2006;103:15218–23. doi: 10.1073/pnas.0607131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–96. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM. Endogenous zinc inhibits GABA(A) receptors in a hippocampal pathway. J Neurophysiol. 2004;91:1091–6. doi: 10.1152/jn.00755.2003. [DOI] [PubMed] [Google Scholar]
- 12.Mott DD, Benveniste M, Dingledine RJ. pH-dependent inhibition of kainate receptors by zinc. J Neurosci. 2008;28:1659–71. doi: 10.1523/JNEUROSCI.3567-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirzel K, Muller U, Latal AT, Hulsmann S, Grudzinska J, Seeliger MW, et al. Hyperekplexia phenotype of glycine receptor alpha1 subunit mutant mice identifies Zn(2+) as an essential endogenous modulator of glycinergic neurotransmission. Neuron. 2006;52:679–90. doi: 10.1016/j.neuron.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande A, Kawai H, Metherate R, Glabe CG, Busciglio J. A role for synaptic zinc in activity-dependent Abeta oligomer formation and accumulation at excitatory synapses. J Neurosci. 2009;29:4004–15. doi: 10.1523/JNEUROSCI.5980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frederickson CJ, Hernandez MD, McGinty JF. Translocation of zinc may contribute to seizure-induced death of neurons. Brain Res. 1989;480:317–21. doi: 10.1016/0006-8993(89)90199-6. [DOI] [PubMed] [Google Scholar]
- 16.Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57:546–58. doi: 10.1016/j.neuron.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci. 2006;26:7181–8. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci. 2001;21:5520–7. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 20.Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, et al. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–18. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 21.Cole TB, Martyanova A, Palmiter RD. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Res. 2001;891:253–65. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- 22.Albasser MM, Davies M, Futter JE, Aggleton JP. Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav Neurosci. 2009;123:115–24. doi: 10.1037/a0013829. [DOI] [PubMed] [Google Scholar]
- 23.Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J Neurosci. 2010;30:1631–6. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohbot VD, Corkin S. Posterior parahippocampal place learning in H.M. Hippocampus. 2007;17:863–72. doi: 10.1002/hipo.20313. [DOI] [PubMed] [Google Scholar]
- 25.Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36:1217–38. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 26.Sindreu C, Palmiter RD, Storm DR. Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1019166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh SW, Won SJ, Hamby AM, Yoo BH, Fan Y, Sheline CT, et al. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J Cereb Blood Flow Metab. 2009;29:1579–88. doi: 10.1038/jcbfm.2009.80. [DOI] [PubMed] [Google Scholar]
- 28.Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- 29.Desmedt A, Garcia R, Jaffard R. Differential modulation of changes in hippocampal-septal synaptic excitability by the amygdala as a function of either elemental or contextual fear conditioning in mice. J Neurosci. 1998;18:480–7. doi: 10.1523/JNEUROSCI.18-01-00480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–91. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–74. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 33.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–74. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 34.Bast T, Zhang WN, Feldon J. Hippocampus and classical fear conditioning. Hippocampus. 2001;11:828–31. doi: 10.1002/hipo.1098. [DOI] [PubMed] [Google Scholar]
- 35.Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, et al. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem. 2006;13:349–58. doi: 10.1101/lm.80206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Martel G, Nishi A, Shumyatsky GP. Stathmin reveals dissociable roles of the basolateral amygdala in parental and social behaviors. Proc Natl Acad Sci U S A. 2008;105:14620–5. doi: 10.1073/pnas.0807507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada E, Watase K, Yamada K, Ogura H, Yamano M, Inomata Y, et al. Generation and characterization of mice lacking gastrin-releasing peptide receptor. Biochem Biophys Res Commun. 1997;239:28–33. doi: 10.1006/bbrc.1997.7418. [DOI] [PubMed] [Google Scholar]
- 39.Winters BD, Saksida LM, Bussey TJ. Implications of animal object memory research for human amnesia. Neuropsychologia. 2010;48:2251–61. doi: 10.1016/j.neuropsychologia.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]