Synaptic Reliability Correlates with Reduced Susceptibility to Synaptic Potentiation by Brain-Derived Neurotrophic Factor (original) (raw)
Abstract
Recent studies have implicated brain-derived neurotrophic factor (BDNF) in use-dependent modification of hippocampal synapses. BDNF can rapidly potentiate synaptic transmission at glutamatergic synapses by enhancing transmitter release. Using simultaneous perforated patch recording from pairs and triplets of glutamatergic hippocampal neurons, we have examined how the initial state of the glutamatergic synapse determines its susceptibility to synaptic modification by BDNF. We found that the degree of synaptic potentiation by BDNF depends on the initial reliability and strength of the synapse: Relatively weak connections were strongly potentiated, whereas the effect was markedly reduced at stronger synapses. The degree of BDNF-induced potentiation strongly correlated with the initial coefficient of variation (CV) of the amplitude of excitatory postsynaptic currents (EPSCs) and inversely correlated with the initial paired–pulse facilitation, suggesting that synapses with lower release probability (Pr) are more susceptible to the action of BDNF. To determine whether saturation of Pr could have masked the potentiation effect of BDNF in the stronger synapses, we lowered the initial Pr either by reducing the extracellular Ca2+ concentration ([Ca2+]o) or by bath application of adenosine. Synapses that were initially strong remained unaffected by BDNF under these conditions of reduced Pr. Thus, the lack of BDNF effect on synaptic efficacy cannot simply be accounted for by saturation of Pr, but rather may be due to intrinsic changes associated with synaptic maturation that might covary with Pr. Finally, the dependence on initial synaptic strength was also found for divergent outputs of the same presynaptic neuron, suggesting that synaptic terminals with different degrees of responsiveness to BDNF can coexist within in the same neuron.
Studies of Xenopus neuromuscular synapses have shown that neurotrophins can modulate presynaptic transmitter release on a rapid time scale (Lohof et al. 1993; Stoop and Poo 1995). Neurotrophins have also been shown to modulate basal synaptic transmission at central synapses, such as the Schaffer collateral synapses in the CA1 region of the hippocampus, but results reported have not been consistent (Lessmann et al. 1994; Kang and Schuman 1995,1996; Figurov et al 1996; Kang et al. 1997; Frerking et al. 1998; Gottschalk et al. 1998; Lessmann and Heumann 1998). The possibility of synaptic modification by brain-derived neurotrophic factor (BDNF) is of particular interest to synaptic plasticity, because neurotrophins are synthesized and released in an activity-dependent manner (Gall and Isackson 1989; Zafra et al. 1990, 1991; Berzaghi et al. 1993; Castrén et al. 1993; Blöchl and Thoenen 1995, 1996; Goodman et al. 1996; Canossa et al. 1997; Wang and Poo 1997) and therefore may participate in processes underlying use-dependent synaptic modification such as long-term potentiation (LTP) (Thoenen 1995; Berninger and Poo 1996; McAllister et al. 1999). LTP in the CA1 region of the hippocampus is markedly impaired in mice deficient for one or both of the BDNF alleles (Korte et al. 1995; Patterson et al. 1996). Moreover, induction of LTP in hippocampal slices of BDNF-deficient mice could be rescued by acute re-expression of BDNF or treatment with recombinant BDNF protein (Korte et al. 1996; Patterson et al. 1996), arguing that impairment of LTP was not due to abnormal development of the hippocampus in the absence of BDNF. Rather, BDNF may be involved in the induction process in a more specific manner. Consistent with this interpretation, the induction of υ-burst LTP is blocked when BDNF function is abolished pharmacologically (Figurov et al. 1996; Kang et al. 1997).
Although there were several reports on synaptic modulation by BDNF at hippocampal synapses, there is disagreement on the precise nature of synaptic changes induced by BDNF. Kang and Schuman (1995, 1996) showed that BDNF can potentiate Schaffer collateral–CA1 synapses in slices of adult hippocampus, but other laboratories failed to observe such an effect (Figurov et al. 1996; Tanaka et al. 1997; Frerking et al 1998). In addition, BDNF may increase the reliability of synaptic transmission at high frequencies, suggesting a role for BDNF in regulating the pool of releasable vesicles (Figurov et al. 1996; Gottschalk et al. 1998; Pozzo-Miller et al. 1999). In dissociated cell cultures, a BDNF-induced potentiation of basal synaptic transmission similar to that observed in hippocampal slices has been found (Lessmann et al. 1994; Lessmann and Heumann 1998; Li et al. 1998a,b). However, potentiation occurred only at a subpopulation of glutamatergic synapses (Lessmann and Heumann 1998). Moreover, the locus of synaptic modification has been disputed (Levine et al. 1995, 1998; Lessmann and Heumann 1998; Li et al. 1998 a,b). Finally, BDNF was found to reduce inhibitory synaptic transmission in slices of adult hippocampus (Tanaka et al. 1997; Frerking et al. 1998).
To better understand the role of neurotrophins in synaptic plasticity, it is critical to evaluate precisely under which conditions modification may occur. For instance, in a recent study we found that interaction with the postsynaptic target cell is of crucial importance for endowing the synapse with responsiveness to BDNF: Potentiation of glutamatergic transmission by BDNF was only observed when the postsynaptic neuron was glutamatergic, but not when it was GABAergic (A.F. Schinder, B. Berninger, and M.-m. Poo, in prep.). Lessmann and Heumann (1998) observed that, for hippocampal synapses that underwent synaptic modification by neurotrophin-4 (NT-4), there was an inverse correlation between the magnitude of potentiation and the synaptic strength prior to exposure to NT-4. Here we have examined how the initial state of the glutamatergic synapses determines its susceptibility to BDNF using pairs and triplets of cultured hippocampal neurons, in which both pre- and postsynaptic neurons were glutamatergic. We found that BDNF-induced potentiation exhibits an inverse dependence on the initial synaptic strength. A detailed analysis of the dependence of BDNF-induced synaptic potentiation on initial presynaptic characteristics was then carried out and the consequences of manipulating release probability on the capability of the synapse to respond to BDNF were examined. Finally, we investigated whether divergent outputs of the same presynaptic neuron exhibit differential susceptibility to BDNF in accordance to their functional status.
Materials and Methods
CELL CULTURE
Low-density cultures of dissociated embryonic rat hippocampus were prepared as described previously (Fitzsimonds et al. 1997). Hippocampi were removed from embryonic day-18 rats, treated with trypsin (15 min, 37°C), followed by washing and gentle trituration. Dissociated cells were plated at 20,000–60,000 cells/ml on poly-l-lysine coated coverslips in 35-mm dishes. The plating medium was DMEM (BioWhittaker, Walkersville, MD) supplemented with 10% heat-inactivated FBS (Hyclone, Logan, UT) and 10% Ham’s F12 with glutamine. The culture medium was changed to the above medium containing 20 mm KCl 24 hr after plating. Both glial and neuronal cells were present under these conditions. Neurons were used for electrophysiological recordings after 7–15 days in culture.
ELECTROPHYSIOLOGY
Whole-cell perforated patch recording (Hamill et al. 1981; Horn and Marty 1988; Rae et al. 1991) from two or three neurons was performed simultaneously, with amphotericin B (ICN) for perforation. The micropipettes were made from borosilicate glass capillaries (Kimax) with a resistance of 2–2.5 MΩ. Pipettes were tip filled with internal solution and then back filled with internal solution containing 200 μg/ml amphotericin B. The internal solution contained the following: 136.5 mm K-gluconate, 17.5 mm KCl, 9 mm NaCl, 1mm MgCl2, 10 mm HEPES, 0.2 mm EGTA (pH 7.4), osmolarity 300 mOsm. The external solution contained the following: 150 mm NaCl, 3 mm KCl, 3 mm CaCl2, 2 mm MgCl2, 10 mm HEPES, 5 mm glucose (pH 7.4), osmolarity 310 mOsm. In some experiments, CaCl2 was reduced to 0.5 mm and MgCl2 increased to 3 mm to decrease release probability. All experiments were performed at room temperature. The bath was constantly perfused with fresh external solution at a rate of 0.5 ml/min. Neurons were visualized by phase-contrast microscopy through a Nikon inverted microscope. Recordings were carried out by patch clamp amplifiers (Axopatch 1D or 200B, Axon Instruments, Foster City, CA). Signals filtered at 5 kHz were sampled at 10 kHz and analyzed with PClamp 6.0 software (Axon Instruments). Series resistance was assessed at 5–10 min intervals and compensated at 80% (lag 35–100 μsec). Data were rejected from analysis if the leak current exceeded 100 pA or if a change in excitatory postsynaptic current (EPSC) amplitude correlated with a change in series resistance. For assessing synaptic connectivity, each neuron was stimulated at low frequency (0.05–0.1 Hz) by 1 msec step-depolarization from 70 to + 50 mV in voltage-clamp mode, and responses from all neurons were recorded. Under these conditions, both inhibitory postsynaptic currents (IPSCs) and EPSCs were inward at the holding potential (−70 mV). EPSCs were completely blocked by 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and IPSCs by 20 μm bicuculline, indicating that they were mediated by AMPA and GABAA receptors, respectively. Inhibitory postsynaptic currents had distinctly longer decay times and more negative reversal potentials (approximately −50 mV) than EPSCs (∼0 mV). These properties were used to distinguish the identities of postsynaptic neurons (excitatory versus inhibitory) when reciprocally connected. If present, autaptic responses were only used for identification of the neuronal type and not for assaying synaptic effects of BDNF. In this study, only synaptic connections among glutamatergic neurons were analyzed, as glutamatergic synapses onto GABAergic neurons have been found unresponsive to BDNF (A.F. Schinder, B. Berninger, and M.-m. Poo, in prep.). Experiments involving divergent outputs in a triplet (Fig. 5, below) were performed only when connections were unambiguously monosynaptic (delay from end of stimulus to onset of response <4 msec).
Figure 5.
Differential synaptic potentiation by BDNF at divergent outputs of a single presynaptic neuron. (A, B) Two examples of triplet recordings in which a single presynaptic glutamatergic neuron projected onto two glutamatergic neurons (see diagram at top), with differing synaptic initial synaptic strength. Each data point represents the peak amplitude of the EPSC, normalized to the mean amplitude during the control period (prior to BDNF treatment), as indicated by the dotted line. Filled and open symbols depict EPSCs for initially relatively weak and strong connections, respectively. Shown at top are sample traces (averages of 10 events) at the time indicated. Scales, 200 pA, 5 msec (A) and 50 pA, 5 msec (B). Initial amplitudes of the EPSCs were 460 and 1010 pA in A and 42 and 336 pA in B.
BDNF TREATMENT
Human recombinant BDNF (20–100 ng/ml; Promega) was dissolved in 5 ml of external solution containing 0.05% BSA (Sigma). BSA was added during the control period and did not affect basal synaptic transmission. Once BDNF perfusion started, the same external solution was recycled through the peristaltic pump for the remainder of the experiment. Recycling external solution without BDNF did not affect synaptic transmission (see Fig. 2, below). No recycling was used in experiments in which adenosine (Sigma) was applied, because the latter was found to bind to the Tygon tubing of the peristaltic system (Fisher Scientific Company, Pittsburgh, PA). Instead, the bath was constantly perfused with fresh solution.
Figure 2.
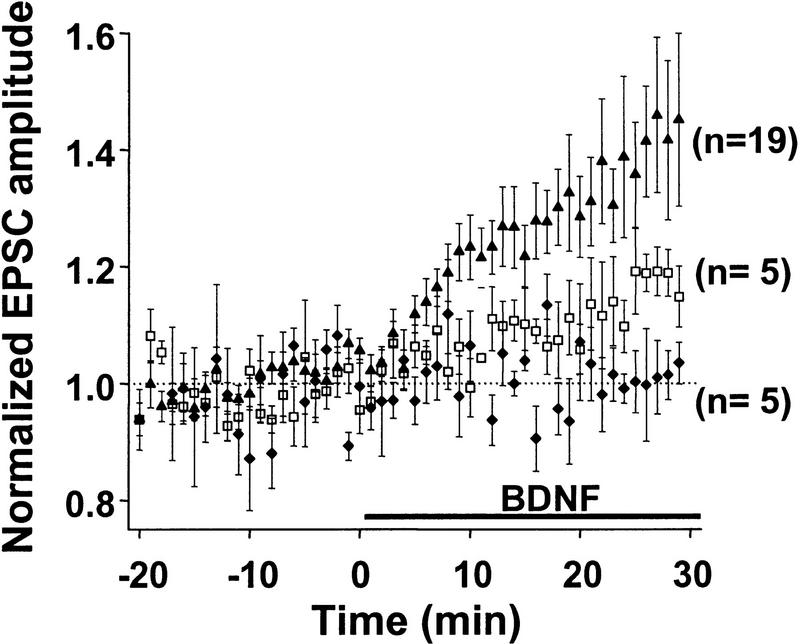
Dose dependence of the potentiation effect of BDNF. Average time course of synaptic potentiation following exposure to 0 (♦), 20 (□), and 100 (▴) ng/ml of BDNF. Prior to averaging, EPSC amplitudes from each experiment were normalized to the mean amplitude recorded during control period (before treatment). Data points represent mean ± s.e.m. n refers to the number of experiments.
Results
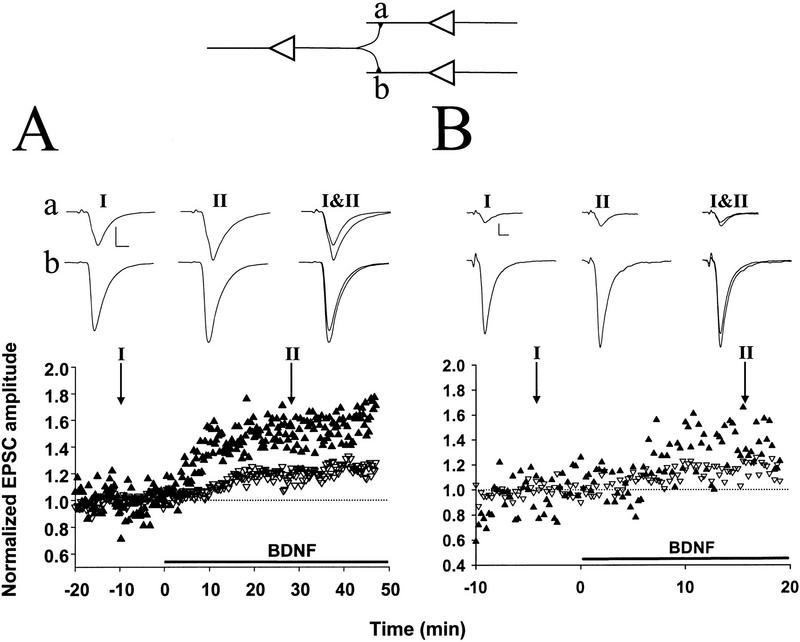
Because of the target specificity of the BDNF effect (A.F. Schinder, B. Berninger, and M.-m. Poo, in prep), we have chosen to study pairs or triplets of hippocampal neurons consisting of glutamatergic neurons only. Excitatory postsynaptic currents were evoked by a brief step depolarization of a designated presynaptic neuron, with perforated patch recording (Hamill et al. 1981; Horn and Marty 1988; Rae et al. 1991). Application of exogenous of BDNF (100 ng/ml) rapidly and markedly increased the amplitude of EPSCs in a subpopulation of these synaptic connections. In the case shown in Figure 1A, the synapse exhibited an average EPSC of ∼80 pA during the control period (prior to BDNF treatment). Clear strengthening of synaptic efficacy was detected after 10 min in the presence of BDNF, and the EPSC amplitude reached a plateau value of ∼140 pA after 20 min. In contrast, for the case shown in Figure 1B, in which the synapse showed initial EPSCs of ∼1 nA, no effect of BDNF on synaptic transmission was observed, suggesting that the potentiation effect may depend on the initial synaptic strength. Results from all recordings (including those showing no BDNF effect) are summarized in Figure 2. The average EPSC amplitude at 20–30 min after the onset of BDNF application (100 ng/ml) was 134 ± 8% (s.e.m.; n = 19) of the control values (prior to the exposure to BDNF). The effect of BDNF on synaptic transmission was dose dependent (Fig. 2): The average EPSC amplitude after exposure to 0 and 20 ng/ml of BDNF was 97 ± 6% (n = 5) and 110 ± 6% (n = 5) of the control values, respectively.
Figure 1.
Potentiation of glutamatergic transmission by BDNF depends on the initial synaptic strength. (A) Example of a recording of glutamatergic synaptic transmission with a low initial EPSC amplitude (83 pA) prior to exposure to BDNF. Both the designated pre- and postsynaptic neurons were glutamatergic, as judged from the reversal potentials of the synaptic currents recorded in response to stimulation of each neuron in the pair. The presynaptic neuron was stimulated with paired–pulses (50 msec apart) once every 15 sec. Each data point depicts the peak amplitude of the first EPSC normalized to the mean amplitude recorded during the control period (prior to BDNF treatment, dotted line). Duration of exposure to BDNF (100 ng/ml) is depicted by the thick line below. Shown above are sample traces (averages of 10 events) recorded at the time marked by I and II. Scales, 40 pA, 10 msec. (B) Example of a recording of glutamatergic synaptic transmission onto another glutamatergic neuron with a large initial amplitude (1034 pA) prior to exposure to BDNF. Scales, 200 pA, 10 msec.
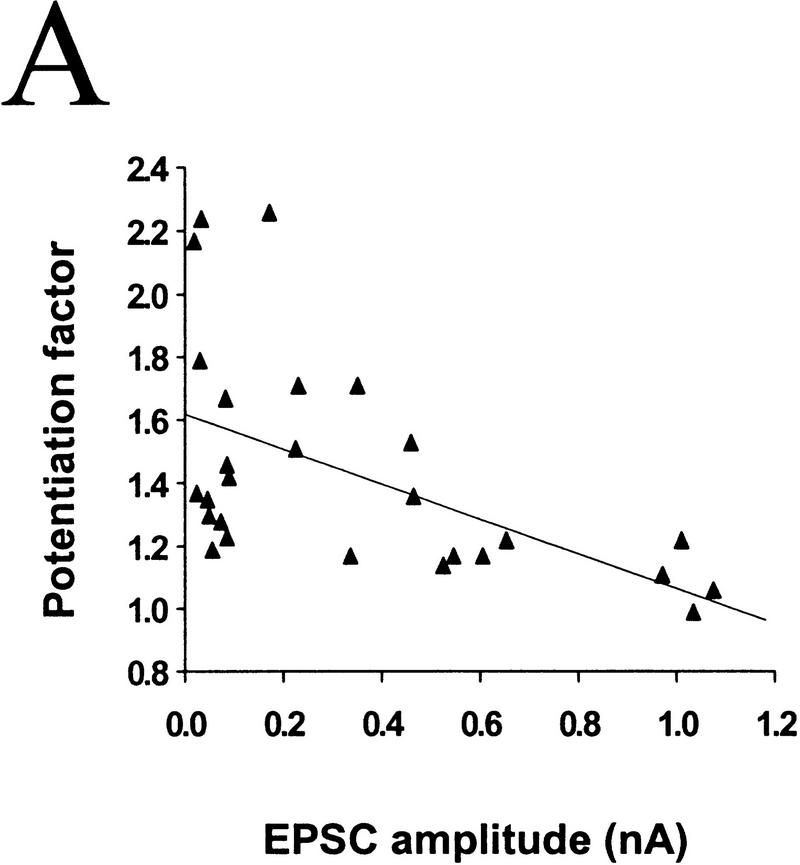
To assess whether differences in the initial synaptic strength account for the variability in the response of these synapses to BDNF, we compared the degree of potentiation by BDNF for synaptic connections of different initial EPSC amplitudes. Marked synaptic potentiation was observed in most cases of initially weak synaptic connections, whereas synapses with high EPSC amplitudes were much less affected. For synapses with EPSC amplitudes of <400 pA, exposure to 100 ng/ml BDNF resulted in a potentiation of 158 ± 15% (_n_ = 7), whereas for those with amplitudes between 400 and 800 pA and >800 pA synaptic potentiation was 124 ± 7% (n = 6) and 105 ± 7% (n = 4), respectively. The correlation between the degree of potentiation and the initial EPSC amplitude is shown in Figure 3A (r = −0.552, P = 0.0028, ANOVA).
Figure 3.
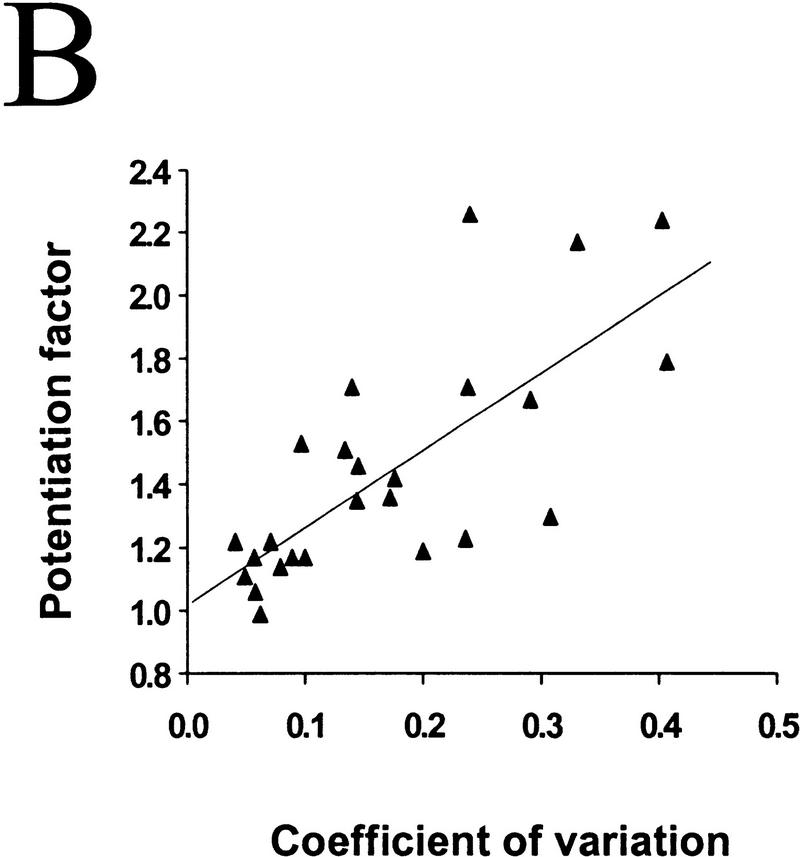
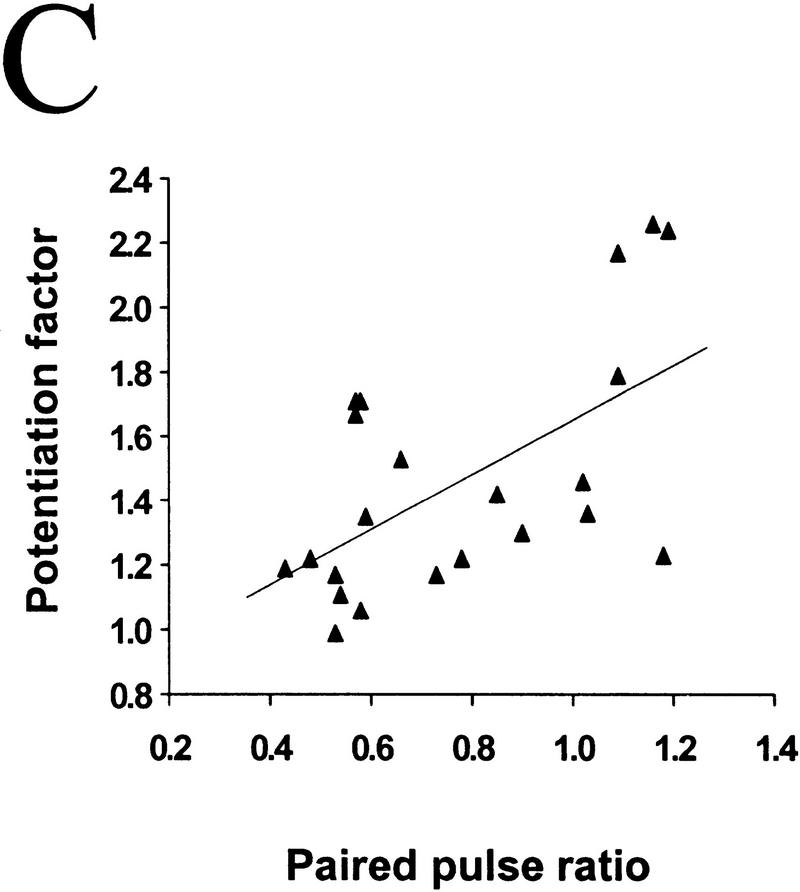
The degree of synaptic potentiation by BDNF correlates with initial synaptic strength, CV, and paired–pulse facilitation. (A) Relationship between synaptic potentiation and initial EPSC amplitude. Initial EPSC amplitude was calculated for 40 events during 10 min of control period. The potentiation factor is defined as the ratio of mean EPSC amplitude during the last 10 min of the experiment following BDNF treatment to that during the control period. Each point refers to the result of one experiment. The line represents the best linear fit of the data (r = −0.552, P = 0.0028, ANOVA). (B) Relationship between synaptic potentiation and the initial CV. Initial CV was determined as ς/mean, in which ς and mean are the standard deviation and mean value of the EPSC amplitude during the control period. The line represents the best linear fit of the data (r = 0.741, P < 0.0001, ANOVA). (C) Relationship between synaptic potentiation and initial paired–pulse facilitation. The paired–pulse ratio is the ratio of the amplitude of the second EPSC to that of the first. The initial paired–pulse ratio was computed as the average value observed during the control period. The line represents the best linear fit of the data (r = 0.586, P = 0.0042, ANOVA).
It was shown previously that BDNF-induced synaptic potentiation of glutamatergic synapses in hippocampal cultures (Lessmann and Heumann 1998; A.F. Schinder, B. Berninger, M.-m. Poo, in prep.) and neuromuscular synapses in Xenopus cultures (Boulanger and Poo 1999) is accompanied by a decrease in the coefficient of variation (CV) and a reduction in the paired-pulse facilitation. The CV is a measure of the fluctuation of the postsynaptic response which, according to classical quantal theory, is solely determined by presynaptic properties, that is, the release probability (Pr) and the number of release sites (N). Paired–pulse facilitation (or depression) of the postsynaptic response is commonly observed for the second of two stimulation pulses applied in close succession, and has been shown to be a function of Pr. A decrease in CV and paired–pulse facilitation therefore suggests that the effect of BDNF can be largely accounted for by an enhanced transmitter release, presumably due to either an increase in Pr and/or in N. This is also consistent with the finding of increased frequency of miniature EPSCs after BDNF treatment of hippocampal and neuromuscular synapses (Lohof et al. 1993, Lessmann et al. 1994; Li et al. 1998 a,b). Because BDNF exerts its effect mainly by changing presynaptic release properties, it is reasonable to expect synapses with lower release capability to be more susceptible to potentiation by BDNF. We found that BDNF-induced synaptic potentiation significantly correlated with the initial CV (Fig. 3B; r = 0.74, P < 0.001, ANOVA) and the extent of paired–pulse facilitation (Fig. 3C; r = 0.586, P = 0.0042, ANOVA). Thus, synapses with low initial Pr and/or N are more responsive to BDNF than those synapses with a high initial Pr and/or N.
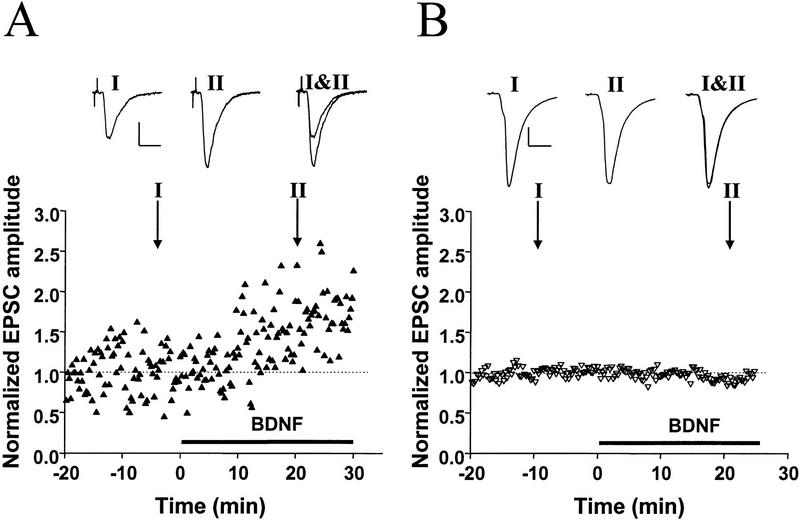
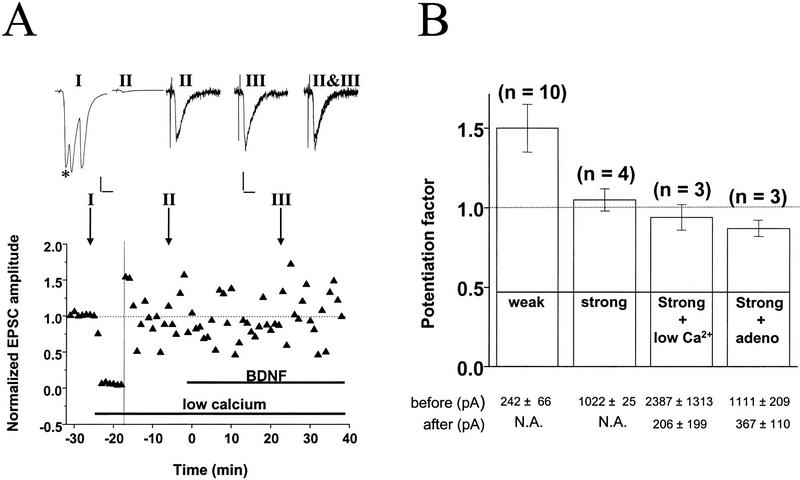
To determine whether BDNF-induced synaptic potentiation may be masked at synapses with high initial Pr, we performed two manipulations to lower Pr. For these experiments we selected synapses with high initial amplitude, which typically show no potentiation induced by BDNF under control conditions ([Ca2+]o = 3 mm, no adenosine). In the first set of experiments, after recording a baseline to determine the initial EPSC amplitude, we lowered [Ca2+]o from 3 to 0.5 mm. As shown for a synapse with an initial EPSC amplitude of ∼1050 pA (Fig. 4A), lowering [Ca2+]o reduced the EPSC amplitude by 97%, indicating a drastic reduction in Pr. Although these conditions resulted in an initial EPSC amplitude of approximately −30 pA for the control period, subsequent BDNF treatment did not significantly enhance synaptic transmission. All experiments involving lowering of [Ca2+]o are summarized in Figure 4B. On the average, EPSC amplitudes decreased from −2387 ± 1313 pA to −206 ± 199 pA, whereas CV increased from 0.033 ± 0.01 to 0.44 ± 0.10 and paired–pulse facilitation increased from 0.43 ± 0.01 to 1.73 ± 0.35. Despite the strong reduction in Pr, however, no potentiation by BDNF was revealed (94 ± 8 % of control period, n = 3).
Figure 4.
Lowering Pr at stronger synapses does not unmask response to BDNF. (A) Example for absence of BDNF effect after lowering Pr by reducing [Ca2+]o. Initial EPSC was ∼−1050 pA and was reduced to −30 pA after reducing [Ca2+]o from 3 to 0.5 mm and elevating [Mg2+]o from 2 to 3 mm. The vertical line depicts the time point following lowering [Ca2+]o, after which EPSCs amplitudes were normalized to the new mean value recorded during the last 10 min prior to exposure to BDNF (100 ng/ml). Shown at top are sample traces of EPSCs (average of 10 events). Scales, 200 pA, 10 msec (left two traces), and 10 pA, 10 msec (right three traces). Duration of low [Ca2+]o and BDNF treatments are indicated by the thick lines below. (*) Monosynaptic EPSCs monitored. Note that polysynaptic currents disappeared after the low [Ca2+]o treatment. (B) Summary of results from experiments in which Pr was reduced by either treatment with low [Ca2+]o or with adenosine. Potentiation factor observed after the BDNF treatment was defined as in Fig. 3. Bars represent average values obtained from all experiments on weak synapses (EPSC amplitude <600 pA) and strong synapses (>800 pA) in the normal recording medium as well as experiments on strong synapses (>800 pA) in the presence of low [Ca2+]o and adenosine. The average EPSC amplitude before and after the low [Ca2+]o or adenosine treatment is shown at bottom. (N.A.), Not applicable. Error bars, s.e.m.
As an alternative way of reducing Pr, we applied adenosine (1–2 μm), which is known to inhibit presynaptic Ca2+ influx (Wu and Saggau 1994). This treatment resulted in a decrease in EPSC amplitude (from −1111 ± 209 to −367 ± 110 pA), with CV increasing from 0.06 ± 0.01 to 0.18 ± 0.02 and paired–pulse facilitation increasing from 0.46 ± 0.04 to 0.96 ± 0.43, reflecting a strong reduction in Pr. As summarized in Figure 4B, no potentiation was observed in response to BDNF after treatment with adenosine (87 ± 5% of control period, n = 3). Taken together with the low [Ca2+]o experiments, these results demonstrate that lowering Pr at initially strong synapses is not sufficient to allow synaptic potentiation by BDNF.
Is the susceptibility to synaptic modification by BDNF a global property of the presynaptic neuron or a property of its individual presynaptic terminals? To address this question we recorded from triplets, in which a single presynaptic neuron projected divergent outputs to the two other neurons with substantially different synaptic efficacy. Results from two experiments are shown in Figure 5. In both cases, BDNF induced a higher degree of potentiation in the synaptic connection with initially weaker synaptic response. Thus, the size dependence of synaptic modification by BDNF is also found for divergent outputs from the same presynaptic neuron, suggesting that individual synaptic terminals may undergo independent synaptic modification.
Discussion
In this study we have shown that the functional status of glutamatergic synapses determines the extent of synaptic modification by BDNF: Higher degree of synaptic potentiation was observed at synapses displaying a smaller initial EPSC amplitude and higher CV and paired–pulse facilitation. The absence of synaptic modification at strong synapses was not due to mere occlusion of a BDNF effect by a high initial Pr, because lowering Pr either by reducing [Ca2+]o or exposure to adenosine was not sufficient to reveal significant potentiation by BDNF. Finally, triple recordings showed that divergent outputs of the same presynaptic neuron can exhibit a differential response to BDNF in accordance with their synaptic strength.
The magnitude of synaptic potentiation by BDNF was found to be inversely correlated with the initial size of the EPSC. In the present culture, the average EPSC amplitudes increased over time in culture. (162 ± 24 pA for 7–10 days in vitro (DIV) (n = 37), and 408 ± 52 for 11–15 DIV (n = 46). Synaptic connections with EPSC amplitude >800 pA appeared typically only after 11 DIV. Thus, the loss of responsiveness to BDNF may be a consequence of synaptic maturation, with immature synapses being more susceptible than mature synapses. Strong synaptic connections typically exhibit a low CV, indicating that growth in EPSC amplitude at these hippocampal synapses may be due to an increase in Pr and/or N. Long-term treatment of BDNF has been shown to promote formation of synaptic connections in cultures prepared from E16 but not E18 rat embryos (Vicario-Abejon et al. 1998), suggesting that synaptic maturation may require exposure to BDNF. During hippocampal development, Schaffer collateral—CA1 synapses were shown to grow in strength predominantly by an increase in the number of release sites (Hsia et al. 1998). Acute effects of BDNF observed here might also involve rapid formation of new synaptic contacts, leading to an increased N and reduced CV. As connections among neurons mature, there may be a maximal density of synaptic contacts that can be accommodated on each neuron. The reduced effect of BDNF at stronger connections may reflect a diminished ability of the neuron to add new synapses. Our findings on the dependence of synaptic modification on the initial strength reveal an intriguing parallel to a previous study of LTP in the same culture system (Bi and Poo 1998). In the latter study, the magnitude of synaptic potentiation induced by correlated pre- and postsynaptic spiking was also found to depend on the initial synaptic strength. This apparent similarity of BDNF and activity-dependent synaptic modification with respect to initial conditions suggests that these processes may share common mechanisms.
Potentiation of hippocampal and neuromuscular synapses by BDNF was shown previously to be accompanied by a decrease in paired–pulse facilitation (Kang and Schuman 1995; Lessmann and Heumann 1998; Boulanger and Poo 1999; A.F. Schinder, B. Berninger, M.-m. Poo, in prep.). Moreover, exposure to BDNF results in an increase in the frequency of miniature EPSCs at these synapses (Lohof et al. 1993; Lessmann et al. 1994; Li et al. 1998b). These findings argue that the effect of BDNF may involve an increased Pr. The fact that synapses with low paired–pulse facilitation showed a reduced response to BDNF suggested that the lack of BDNF effect is due to a saturation of Pr. To test this possibility, we manipulated Pr at synapses with a high initial EPSC amplitude by either reducing [Ca2+]o or applying adenosine. From the decrease in the EPSC amplitude following these treatments we estimate that Pr was reduced by 50%–97%. Despite this drastic reduction in Pr, we did not observe an effect of BDNF on these synapses. We therefore conclude that a low _Pr_—although it may still be a prerequisite—is not sufficient to render hippocampal synapses susceptible to BDNF, and other synaptic properties are critical for setting the degree of susceptibility to the action of BDNF.
The reduction in the responsiveness to BDNF associated with the maturation of synaptic connections could be a consequence of changes in neurotrophin signaling or in transmitter release machinery. For instance, the expression of truncated TrkB receptors lacking a functional tyrosine kinase domain increases during brain development (Allendoerfer et al. 1994; Fryer et al. 1996). Because truncated TrkB receptors may act in a dominant-negative manner (Eide et al. 1996), increased levels of these receptors may account for the reduced action of BDNF at mature synapses. Alternatively, changes in the release machinery, for example, (dis)appearance of synaptic proteins that are targeted by BDNF and influence Pr, could account for the differential response to BDNF. Clear differences in response to neurotrophin-3 (NT-3) have been observed for frog neuromuscular synapses formed by the axon tip or by the cell body, indicating that certain regulatory components of the release machinery in these two compartments are functionally distinct (Chang and Popov 1999). That differential BDNF effects are observed at divergent outputs of the same presynaptic neuron further suggests that functional differences may appear at different terminals of the hippocampal neuron as a consequence of the heterogeneous pace of maturation or target-cell-specific retrograde signaling. Differential presynaptic properties and synaptic plasticity at different nerve terminals of the same neuron have been found in a number of systems (Laurent and Sivaramakrishnan 1992; McMahon and Kauer 1997; Bi and Poo 1998; Maccaferri et al. 1998; Markram et al. 1998; Reyes et al. 1998; A.F. Schinder, B. Berninger, M.-m. Poo, in prep.).
We have recently observed that the responsiveness of glutamatergic synapses to BDNF is dependent on whether the postsynaptic target neuron is glutamatergic or GABAergic in nature (A.F. Schinder, B. Berninger, and M.-m. Poo, in prep.). Synaptic potentiation could be induced by BDNF only when the postsynaptic neuron was glutamatergic. This differential responsiveness to BDNF of divergent outputs from the same presynaptic neuron supports the notion of a localized synaptic action of BDNF. At developing Xenopus neuromuscular synapses, potentiation of transmitter release was restricted to synapses on myocytes overexpressing NT-4, without affecting nearby synapses formed by the same neuron onto control myocytes (Wang et al. 1998), suggesting again localized synaptic action of neurotrophin.
What might be the functional significance of a developmental regulation in the synaptic response to BDNF? Our findings suggest that synaptic modification by BDNF may play an important role during specific phases of neural development, when synaptic plasticity is most prominent. This is consistent with previous observations on the effects of neurotrophins on the synaptic organization of the visual cortex during the critical period of development (Domenici et al. 1991, 1994; Cabelli et al. 1995, 1996; Galuske et al. 1996). The fact that synapses originating from the same presynaptic neuron can express different degrees of responsiveness to BDNF suggests that, if new synapse formation were to occur during adulthood, such synapses may be more modifiable by BDNF than their more mature sister synapses.
Acknowledgments
We thank X.-Y. Wang for culture preparation and Professor S. Varón for support. B.B. was a recipient of a long-term fellowship of the Human Frontier Science Program Organization. This work was supported by a grant from the National Institutes of Health (NS37831).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Allendoerfer KL, Cabelli RJ, Escandén E, Kaplan DR, Nikolics K, Shatz CJ. Regulation of neurotrophin receptors during the maturation of the mammalian visual system. J Neurosci. 1994;14:1795–1811. doi: 10.1523/JNEUROSCI.14-03-01795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B, Poo M-m. Fast actions of neurotrophic factors. Curr Opin Neurobiol. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- Berzaghi MP, Cooper J, Castrén E, Zafra F, Sofroniew G, Thoenen H, Lindholm D. Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J Neurosci. 1993;13:3818–3826. doi: 10.1523/JNEUROSCI.13-09-03818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G-q, Poo M-m. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blöchl A, Thoenen H. Characterization of nerve growth factor (NGF) release from hippocampal neurons: Evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- ————— Localization of cellular storage compartments and sites of constitutive and activity-dependent release of nerve growth factor (NGF) in primary cultures of hippocampal neurons. Mol Cell Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- Boulanger L, Poo M-m. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nature Neurosci. 1999;2:346–351. doi: 10.1038/7258. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- Canossa M, Griesbeck O, Berninger B, Campana G, Kolbeck R, Thoenen H. Neurotrophin release by neurotrophins: Implications for activity-dependent neuronal plasticity. Proc Natl Acad Sci. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Chang S, Popov SY. Long-range signaling within growing neurites mediated by neurotrophin-3. Proc Natl Acad Sci. 1999;96:4095–4100. doi: 10.1073/pnas.96.7.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici L, Berardi N, Carmignoto G, Vantini G, Maffei L. Nerve growth factor prevents the amblyopic effects of monocular deprivation. Proc Natl Acad Sci. 1991;88:8811–8815. doi: 10.1073/pnas.88.19.8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici L, Cellerino A, Berardi N, Cattaneo A, Maffei L. Antibodies to nerve growth-factor (NGF) prolong the sensitive period for monocular deprivation in the rat. Neuroreport. 1994;5:2041–2044. doi: 10.1097/00001756-199410270-00013. [DOI] [PubMed] [Google Scholar]
- Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Fitzsimonds RM, Song HJ, Poo M-m. Propagation of activity-dependent synaptic depression in simple neural networks. Nature. 1997;388:439–448. doi: 10.1038/41267. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF. Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol. 1996;374:21–40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Gall CM, Isackson PJ. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- Galuske RA, Kim DS, Castrén E, Thoenen H, Singer W. Brain-derived neurotrophic factor reversed experience-dependent synaptic modifications in kitten visual cortex. Eur J Neurosci. 1996;8:1554–1559. doi: 10.1111/j.1460-9568.1996.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophys. 1998;79:2013–2024. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- ————— A requirement for local protein synthesis in neurotrophin-induced hippocampal plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: Different roles for TrkB signaling in hippocamapl long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, Sivaramakrishnan A. Single local interneurons in the locust make central synapses with different properties of transmitter release on distinct postsynaptic neurons. J Neurosci. 1992;12:2370–2380. doi: 10.1523/JNEUROSCI.12-06-02370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V, Heumann R. Modulation of unitary glutamatergic synapses by neurotrophin-4/5 or brain-derived neurotrophic factor in hippocampal microcultures: Presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience. 1998;86:399–413. doi: 10.1016/s0306-4522(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurons. Neuroreport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci. 1998;18:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Xu Y, Ju D, Lester HA, Davidson N, Schuman EM. Expression of a dominant negative TrkB receptor, T1, reveals a requirement for presynaptic signaling in BDNF-induced synaptic potentiation in cultured hippocampal neurons. Proc Natl Acad Sci. 1998a;95:10884–10889. doi: 10.1073/pnas.95.18.10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in hippocampal neurons. J Neurosci. 1998b;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo M-m. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Tóth K, McBain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci. 1998;95:5323–5328. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron. 1997;18:295–305. doi: 10.1016/s0896-6273(00)80269-x. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TAS, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng Z-H, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Meth. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Stoop R, Poo M-m. Potentiation of transmitter release by ciliary neurotrophic factor requires somatic signaling. Science. 1995;267:695–699. doi: 10.1126/science.7839148. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in the rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejón C, Collin C, McKay RDG, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-h, Poo M-m. Potentiation of developing synapses by postsynaptic release of neurotrophin-4. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- Wang X-h, Berninger B, Poo M-m. Localized synaptic actions of neurotrophin-4. J Neurosci. 1998;18:4985–4992. doi: 10.1523/JNEUROSCI.18-13-04985.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity-dependent regulation of BDNF and NGF mRNA in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Castrén E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]