A phase II trial of dasatinib in advanced melanoma (original) (raw)
. Author manuscript; available in PMC: 2012 May 15.
Published in final edited form as: Cancer. 2010 Nov 29;117(10):2202–2208. doi: 10.1002/cncr.25766
Abstract
Rationale
Inhibiting Src kinases (non-receptor tyrosine kinase signaling intermediates) reduces melanoma cell proliferation and invasion. Dasatinib inhibits c-kit, PDGFβR and EPHA2 and src kinases c-Src, c-Yes, Lck and Fyn. We conducted a phase 2 trial of dasatinib in melanoma to assess response rate (RR), progression-free survival (PFS) and toxicity.
Methods
Adults with stage III/IV chemotherapy-naive unresectable melanoma, were eligible. Dasatinib was initially administered at 100mg PO BID continuously to 17 patients. Due to toxicity, the starting dose was decreased to 70mg BID. Tumor assessments occurred every 8 weeks.
Results
Thirty nine patients were enrolled, 36 evaluable for activity and toxicity. Five, four and three had acral-lentiginous, ocular or mucosal primaries. Two had confirmed partial responses lasting 64 and 24 weeks; RR-5%. Three had minor responses lasting 136, 64 and 28 weeks, and one who was responding discontinued due to non-compliance. Median PFS was eight weeks; 6-month PFS rate −13 %. One patient with an exon-13 c-kit mutation had a partial response, while disease in another with an exon-11 c-kit mutation progressed. Common toxicities were fatigue, dyspnea and pleural effusions.
Conclusions
Daily dasatinib has minimal activity in unselected melanoma patients, excluding those with c-kit mutations. The study did not meet the pre-specified endpoints of 30% response rate or six-month PFS. Dasatinib was overall poorly tolerated, often requiring dose reduction or interruption. Because activity was observed in a small subset without c-kit mutations, identifying predictive biomarkers is important for future development of dasatinib in melanoma alone or in combination trials.
Keywords: src kinases, dasatinib, melanoma
Introduction
Systemic therapy options for metastatic melanoma are limited. Immune modulation, such as high dose interleukin-2, anti CTLA-4 and adoptive transfer of tumor-specific T lymphocytes can induce durable responses in 5–20% of patients, but not all are appropriate candidates for these therapies 1, 2. Cytotoxic chemotherapy induces responses in approximately 10–15%, but responses are rarely durable, and there is no proven impact on overall survival. Recently, an inhibitor of mutated B-Raf, was shown to cause shrinkage of tumors harbor BRAFV600E mutations 3, 4. Only 40–50% of melanomas have BRAF mutantions, and although responses to the inhibitor were impressive, most patients develop resistance over time. Similar results have been published for c-kit inhibitors in melanoma patients whose tumors harbor activating c-kit mutations 5; however, these mutations are relatively rare 6. Therefore, for the vast majority of melanoma patients, additional, well tolerated, molecular targeted therapies are needed.
Dasatinib is an orally administered small molecule inhibitor of tyrosine kinases including src-family kinases (c-src, yes, lck and fyn), bcr-abl, c-kit, PDGFβ receptor, and EPHA2 7. The IC50 for enzyme inhibition for these kinases is in the low nanomolar range (<1nM for c-src, yes and fyn and >20nM for PDGFβR) 8. Dasatinib was approved by the Federal Drug Administration for chronic myelogenous leukemia and gastrointestinal stromal tumors 9, 10. It was generally well tolerated in earlier studies. Based on toxicities in the phase I trial for solid tumors, a starting dose of 70mg to 120mg twice daily was recommended for phase II studies 11, 12. Common toxicities in previous studies include anemia, diarrhea, nausea, and vomiting, hemorrhage, fatigue, dyspnea, anorexia, dehydration, fluid retention, pleural and pericardial effusion, a moderate increase in QTcF, elevated creatinine and depression 12.
Several receptor tyrosine kinases (RTKs) and downstream intermediates inhibited by dasatinib have been shown to play a role in melanoma cell growth and metastasis. Aberrant expression of basic fibroblast growth factor (FGF) is one of the common early events in progression of melanocytes to melanoma, and is associated with melanoma growth and survival 13. In melanoma cells, suppression of FGFR1 is associated with decreased Src-kinase activity, resulting in decreased melanoma proliferation and tumorigenesis 14. C-Src activation results in proliferation, invasion, angiogenesis and motility of cancer cells, and in many malignancies is active in advanced stage disease 15. Src-activated Stat3 signaling is necessary for growth and survival of melanoma cells 16. Stat5 activation in melanoma cells is also mediated through Src kinases, and is important for cell survival 17. Several investigators reported increased src-kinase activity in melanoma cells 18. In addition to Src, other dasatinib targets have been associated with melanoma proliferation or survival. Activation of platelet derived growth factor receptors (PDGFβR) in B16 melanoma cells promotes angiogenesis, tumor growth and inhibition of apoptosis 19. The Eph receptor family is upregulated in melanoma, and contributes to the malignant process 20.
The need for improved systemic therapies for metastatic melanoma coupled with pre-clinical evidence that Src kinases are important intracellular signaling intermediates in melanoma cells provided the rationale for initiating a phase II single agent trial of dasatinib in unresectable stage III and IV melanoma.
Patients and Methods
Prior to activation, the study was approved by the Cancer Therapy Evaluation Program of the National Cancer Institute and Institutional Review Boards at Yale University and the University of Minnesota.
Inclusion and exclusion criteria
Eligible patients had histologically confirmed, unresectable stage III/IV melanoma of cutaneous, mucosal or ocular origin. Measurable disease by RECIST criteria 21 and disease progression in the 6 months prior to enrollment was required. No prior chemotherapy was allowed; up to 2 prior immunotherapies or biological therapies were acceptable. Prior radiation to non-measurable sites was permissible. Patients had to be >18 years old, able to understand and willing to sign informed consent, have an ECOG performance status of 0 or 1 and have a life expectancy of ≥3 months. Adequate organ and bone marrow function was required; WBC>3,000/μL, platelets >100,000/μL, hemoglobin ≥9.0gm/dl, total bilirubin ≤1.5mg/ml, AST(SGOT) and ALT(SGPT)≤2.5 institutional upper limit of normal, and creatinine ≤1.5X institutional upper limit of normal.
Patients with active brain metastases were excluded. Treated brain metastases stable for ≥8 weeks were allowed. Patients requiring immune suppressant agents (including steroids), pro-arrhythmic drugs, potent inhibitors or inducers of CYP3A4 and patients with a QTc>480 msec were ineligible. Other exclusion criteria included pregnancy, concurrent active malignancies and HIV infection.
Study design and treatment
This single arm phase II study was open at Yale University and the University of Minnesota. Dasatinib was initially administered at 100mg orally BID. After enrolling 17 patients, the starting dose was reduced to 70mg BID. Each cycle was 28 days. There were no planned breaks between cycles. Patients continued treatment until progressive disease or intolerable toxicity. Dose modification criteria were standard; Grade 2 toxicities required interruption of treatment until toxicity resolved to ≤Grade 1. Grade 3 hematologic toxicities required dose reduction while Grade 4 toxicities required discontinuation of study drug. Grade 3 or 4 non-hematologic toxicities similarly required cessation of Dasatinib. For the first 17 patients, dose level -1 was 100mg in the am, 50mg in the pm, dose level -2: 50mg PO BID. Subsequently, doses were modified as follows; dose level 0: 70mg PO BID, dose level -1: 50mg PO BID, dose level -2: 100mg PO daily and dose level -3: 70mg PO daily.
Assessment of toxicity and response
The National Cancer Institute Common Toxicity Criteria version 3.0 was used to grade adverse events. Brain imaging was performed at baseline and every 16 weeks. Imaging of chest, abdomen and pelvis, and limbs when necessary, was performed by CT, MRI and/or PET at baseline and every 8 weeks (2 cycles). Response was assessed by RECIST criteria21.
C-kit mutational analyses
Pre-treatment tumor biopsies were reviewed by a pathologist and areas of invasive tumor were identified. DNA was extracted and sequencing of exons 11, 13, 17 and 18 of the c-kit gene was conducted using standard methods 22.
Statistical methods
Other agents used for metastatic melanoma have response rates of <20%. In a recent randomized trial of 777 patients with untreated metastatic disease, approximately 30% had PFS >120 days and approximately 20% had PFS >180 days. The median PFS ranged from 1.6 to 2.4 months 23. For agents such as dasatinib, prolonged stable disease may be an indication of activity. For this study, a RR or 6 month PFS rate consistent with ≥30% was considered worthy of further study. For this phase II trial, a Simon’s optimum two-stage design was used 24. The pre-determined criterion for moving to the second stage was three patients of the first 18 achieving either 6 months PFS or partial or complete response. The purpose was to differentiate RR ≤10% from RR ≥30% and to differentiate 6-month PFS ≤10% from PFS ≥30% (corresponding to median PFS of 3.5 versus 1.8 months). If at least 7 responses (≥20%), or at least 7 instances of 6-month PFS, were observed among the evaluable patients, this agent would be considered worthy of further testing in melanoma. This design would yield ≥90% power to detect a true objective RR or true 6-month PFS rate of at least 30% and would give ≥.95 probability of a negative result if the true RR was ≤10% and the true 6-month PFS rate was ≤10% (median PFS of 1.8 months).
Results
Patient characteristics
Enrollment demographics are shown in Table 1. Thirty nine patients were enrolled at Yale University and University of Minnesota between February 2007 and June 2009, of whom 36 were evaluable for response and toxicity. Three were excluded from toxicity assessments for early withdrawal (<1 cycle) for non-compliance (withdrawal from study due to voluntary discontinuation of therapy without protocol-specified toxicities or disease progression). These patients withdrew for personal and social reasons. All drug activity analyses are based on the 39 enrolled patients. The mean age was 64 years, 67% male, 44% and 56% had an ECOG performance status of 0 and 1, and 28%, 39%, and 33% had M1a, M1b, and M1c disease, respectively. Five, four and three patients had acral-lentiginous, ocular or mucosal primaries.
Table 1.
Enrollment Demographics
| Patients evaluable for safety and activity | N=39 |
|---|---|
| Age | |
| Mean | 64 years |
| Range | 37–84 years |
| Gender | |
| Male | 26 (67%) |
| Female | 13 (33%) |
| ECOG performance status | |
| 0 | 17 (44%) |
| 1 | 22 (56%) |
| Prior treatments | |
| None | 31 (79%) |
| 1 | 5 (13%) |
| 2 | 3 (8%) |
| Site of primary melanoma | |
| Cutaneous | 22 (56%) |
| Acral-lentiginous | 5 (13%) |
| Unknown primary | 3 (8%) |
| Mucosal melanoma | 5 (13%) |
| Ocular melanoma | 4 (10%) |
| Stage | |
| M1a | 11 (28%) |
| M1b | 15 (39%) |
| M1c | 13 (33%) |
Dose delivery
Toxicity assessments and AEs are based on the 36 evaluable patients. The first 17 patients began at 100mg PO BID. Twelve (71%) had drug held for toxicity. The most common adverse events (AEs) requiring cessation of treatment were dyspnea (7 patients, 41%), pleural effusions (6 patients, 35%), fatigue (11 patients, 65%), and diarrhea (2 patients, 12%). The median time on 100mg PO BID was 4 weeks.
Nineteen patients were treated at a starting dose of 70mg PO BID. Dasatinib was held and/or dose-reduced due to toxicity in 9 (47%) of patients. AEs resulting in holding treatment were similar to the 100mg PO BID dose level and included dyspnea (5 patients, 26%), pleural effusion (3 patients, 16%), fatigue (6 patients, 32%) and anorexia (2 patients, 11%). The median time on 70 mg BID dose was 7.5 weeks.
Drug related AEs
AEs are summarized in Table 2. The most common AEs were fatigue (35 patients, 97%), dyspnea (31 patients, 86%), pleural effusion (17 patients, 47%), nausea (29 patients, 81%) and anorexia (26 patients, 72%). Most AEs were grade 1 or 2. The most common grade 3 or 4 events were fatigue, dyspnea and pleural effusion. Pleural effusions were commonly successfully managed with a short course of prednisone and/or furosemide.
Table 2.
Drug related clinical adverse events
| Adverse Event | All Grades | Grade 3 | Grade 4 |
|---|---|---|---|
| Fatigue | 30 (83%) | 5 (14%) | |
| Dyspnea | 27 (75%) | 2 (6%) | 2 (6%) |
| Pleural Effusion | 14 (39%) | 1 (3%) | 2 (6%) |
| Nausea | 26 (74%) | 3 (8%) | |
| Emesis | 9 (26%) | ||
| Diarrhea | 14 (40%) | 2 (6%) | |
| Weight Loss | 8 (23%) | ||
| Rash | 17 (49%) | ||
| Pruritis | 7 (20%) | ||
| Anorexia | 23 (66%) | 3 (8%) | |
| Xerostomia | 13 (37%) | ||
| Heartburn | 6 (17%) | ||
| Taste alteration | 8 (23%) | ||
| Edema | 5 (14%) | ||
| Cough | 13 (37%) |
There were no grade 3 or 4 laboratory AEs. Grade 1–2 events included hypocalcemia (12 patients, 45%), elevations in transaminases (9 patients, 27%), creatinine (8 patients, 23%) and alkaline phosphatase (3 patients, 8%). Hematologic toxicities included neutropenia (3 patients, 8%), thrombocytopenia (4 patients, 11%) and anemia (12 patients, 34%). With the exception of anemia-related fatigue, laboratory abnormalities were not associated with symptoms and resolved with cessation of therapy.
Activity
Table 3 lists all patients demonstrating tumor regression for >16 weeks, their primary site of disease, sites of metastatic disease, and PFS. Of the 36 patients evaluable for activity, two had a confirmed partial response (PR), lasting 64 and 24 weeks, for a RR of 5% (95% CI 1.537–18.145). Tumor regression that did not meet PR criteria was seen in 3 patients (8%), lasting 136, 64 and 28 weeks. The patient with a minor response lasting 64 weeks had 28% reduction in tumor burden. One patient with 27% reduction in tumor burden withdrew from the study after completing the fourth cycle. Regression of liver lesions was observed after 2 cycles in one other patient (not listed in Table 3), but the disease progressed after the fourth cycle. Overall, sites of response included subcutaneous tissues, lymph nodes, lung, liver and skin.
Table 3.
Summary of patients with tumor regression
| Patient# | Best response0 | % reduction | C-kit Mutations | Primary site | Metastatic sites | Duration* | Reason off study |
|---|---|---|---|---|---|---|---|
| 13 | PR | 56% | Mutation, Exon 13 | Subsungal | Lungs, nodes, soft tissue | 24 weeks | POD |
| 28 | PR | 60% | WT | Chest wall | Lung | 64 weeks | POD |
| 1 | SD | 28% | WT | Ear | Lungs, nodes | 64 weeks | POD |
| 31 | SD | 27% | WT | Lower extremity | Lymph nodes | 16 weeks | Non- compliance |
| 2 | SD | 9% | WT | Lower extremity | Soft tissue | 136 weeks | POD |
| 32 | SD | 21% | WT | Cheek | muscle | 28 weeks | POD |
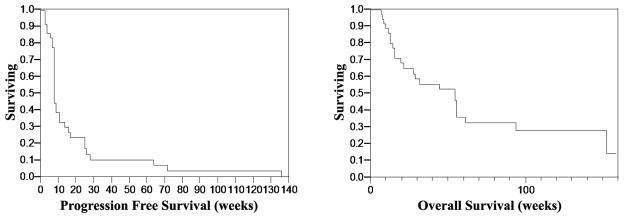
Progression-free and overall survival curves are shown in Figure 1. Median PFS was 8 weeks; range 3–136 weeks. Median overall survival was 55 weeks; range 7–159 weeks. As of May 2010, six patients (16%) remain alive, and three (8%) are lost to follow-up.
Figure 1.
Progression-free (Panel A) and overall (Panel B) survival in patients treated with dasatinib.
C-kit expression and mutations
Dasatinib is a known potent inhibitor of c-kit, and by the time this study was activated, reports were published documenting excellent clinical responses in melanoma patients whose tumors had activating c-kit mutations treated with c-kit inhibitors 5, 25. Therefore, in patients demonstrating a tumor response of any kind to dasatinib, and in selected other patients in whom c-kit overexpression and/or mutation was expected based on prior literature reports, we determined c-kit mutation and/or expression by immunohistochemistry (IHC) in pre-treatment tumor samples. Only one responder, a patient with a primary subungual melanoma, had a K642E substitution mutation in exon 13 of c-kit. The other patients demonstrating some tumor reduction all had wild-type c-kit (Table 3).
Tissue was available on 5 of 8 other patients with primary acral-lentiginous or mucosal melanomas. An exon 11 deletion mutation was found in one other patient who did not respond to dasatinib. One patient had strong c-kit expression by IHC (3+ on a scale of 0–3), but no mutation, and did not respond. No other responses were observed among this group. Tissue was available for IHC (but insufficient for mutational analyses) on three patients with primary ocular melanoma. All had expression by IHC, none responded.
Discussion
Dasatinib is an inhibitor of several tyrosine kinases that play a role in melanoma pathogenesis, including src family members, and the EPHA2, PDGFβ and c-kit receptors. We conducted a phase II clinical trial of dasatinib in 39 unselected patients with advanced melanoma.
One of the primary endpoints was to assess toxicity. The starting dose was based on the maximum tolerated dose in the phase I solid tumor trial. Toxicity was intolerable, and a surprisingly high rate of toxicity was seen at lower doses as well. While the majority of adverse AEs were grade 1/2, and all resolved with holding therapy, the chronicity of the toxicities resulted in impaired quality of life and frequent cessation of therapy. Dyspnea and fatigue secondary to pleural effusions were fairly easily managed with temporary treatment cessation and a short course of prednisone, with/without furosemide. The increased frequency and severity of AEs in our study compared with the phase I study likely reflects the improved life expectancy of the patients in this phase II trial and the longer resultant duration of treatment.
The objective RR from dasatinib was 5%. Evidence of tumor regression, confirmed after 4 cycles of therapy, was observed in 5 other patients, including two with PFS exceeding one year. Median PFS was only eight weeks. Pre-clinical studies published after initiation of this trial showed variable inhibition of melanoma cell growth by dasatinib in vitro. Eustace et al reported an IC50 in the nanomolar range for only 1/5 cell lines 26, Homsi et al showed variable sensitivity in three cell lines 27, Buettner et all showed little to no effect on viability 28, and Woodman et al demonstrated activity in c-KIT mutant cell lines 25. In our own studies, 2/8 cell lines were growth-inhibited by concentrations <300nM, while the other six were significantly more resistant (Jilaveanu et al, submitted). Peak serum concentrations reached just over 200ηM in patients on the highest dose level in the phase I solid tumor trial (120mg), and the elimination half-life was approximately 4 hours 8, 12. Thus, although serum drug concentrations might not accurately reflect intracellular levels, the pharmacokinetic data raise concerns that levels of drug necessary for cell growth inhibition may not be achievable in most patients. Of note, in the phase I studies, clinically tolerable doses were sufficient to inhibit c-Src in post-treatment tumor biopsy samples. However, inhibition of Src-kinases may not be sufficient to mediate a measurable anti-tumor effect in most patients. The phase II clinical study was not designed to detect other potentially meaningful biological effects of src inhibition, such as reduction in motility or invasion 28.
After this trial was written, reports of c-kit mutations in up to 30% of acral-lentiginous melanomas and 39% of mucosal melanomas were published 29. Over the past 4 years, several investigators have reported dramatic tumor regression in melanoma patients whose tumors had c-kit mutations, as seen in GIST patients. In contrast, c-kit mutations were generally not found in cutaneous melanomas arising in intermittently sun-exposed skin or in ocular melanomas, although the latter often demonstrated c-kit overexpression by IHC 30, 31. Two of our 9 patients with primary acral-lentiginous or mucosal primaries had documented c-kit mutations. Both had subungual primaries, one achieved a partial response to dasatinib. No confirmed responses were observed in c-kit overexpressing tumors lacking a c-kit mutation, although we did not assess gene amplification status. Our data are consistent with other reports 5, 25. Upon disease progression, the responding patient with a tumor c-kit mutation developed an excellent response to sorafenib, another c-kit inhibitor, in combination with temozolomide. The systemic response was durable, but she ultimately developed uncontrollable brain metastases. This limited single patient experience suggests that different c-kit mutations may respond variably to c-kit inhibitors, that progression on one c-kit inhibitor does not preclude response to another, and lack of drug CNS penetration and consequent CNS disease progression may be a problem.
Other than the patient with a subungal melanoma and a c-kit mutation, other patients who had tumor reduction had tumors that were wild type for c-kit, indicating that other pathways and molecules targeted by dasatinib might be driving proliferation in these tumors. In our in vitro studies, we attempted to identify predictors of sensitivity to dasatinib, and found that high caveolin-1 levels are associated with in vitro growth inhibition (Jilaveanu et al, submitted). Although the number of patients in whom activity was seen in this trial is small, there might be an association between pre-treatment caveolin-1 tumor levels and tumor reduction. This is consistent with findings in other diseases 32, 33, and requires validation in additional trials of dasatinib for melanoma. The biological relationship between dasatinib target inhibition, caveolin-1 expression, and the mechanism of growth inhibition remains unclear.
Based on the study design, an overall response rate ≥20% or 6-month PFS ≥20%, single agent dasatinib would have been considered worthy of further study in this disease. This is a relatively stringent requirement for a multi-target kinase inhibitor without preselection of patients based on tumor biology. Our results do suggest that dasatinib has some activity in melanoma, and further evaluation of patient subsets such as caveolin-1 over-expressers and c-kit mutated tumors are ongoing in the preclinical and clinical setting, respectively. Further evaluation of dasatinib in carefully selected melanoma patients might be warranted.
As with other anti-neoplastic agents, increased activity might be seen with addition of drugs that work by alternative mechanisms. Combinations with other small molecule inhibitors might result in severe toxicities, as has been the experience when combining small molecule inhibitors in other diseases 34. Addition of cytotoxic chemotherapy with limited overlapping toxicities might be better tolerated. Our recent in vitro studies, and those of others, suggest that addition of cisplatin or dacarbazine might enhance dasatinib activity 27. A trial of a chemotherapy-dasatinib combination has been initiated at another center. It may be important to identify those patients that have the best chance for responding to the combination, for example, by pre-selecting for high caveolin-1 expression in tumor, and for factors permissive of response to the chemotherapy, such as low MGMT expression and an intact DNA mismatch repair pathway.
In summary, single agent dasatinib has minimal activity in unselected melanoma patients, and at doses used in this trial, produces poorly tolerated toxicities in most patients. Tumor regression was noted in approximately 14% of patients without tumor c-kit mutations and one of two with c-kit mutations. This level of activity suggests that biomarker-based patient pre-selection may identify a subset of patients who could potentially derive benefit from dasatinib as a single agent or in combination with other agents, and predictors of sensitivity/response need to be studied further. Because of the AEs, future combination studies should be based on biological activity achievable at lower serum concentrations (perhaps sufficient to inhibit src) and molecularly defined patient subsets.
Acknowledgments
This work was supported by the Yale SPORE in Skin Cancer, 1 P50 CA121974 (to R. Halaban), NIH R01 grant CA115756 (to H. Kluger) and the Milstein-Meyer Funds for Melanoma Research at Yale. The trial was sponsored by the Cancer Therapy Evaluation Program at the National Cancer Institute.
References
- 1.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–9. doi: 10.1200/JCO.2008.16.5449. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18809613. [DOI] [PMC free article] [PubMed]
- 2.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591–8. doi: 10.1158/1078-0432.CCR-09-1024. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19671877. [DOI] [PubMed]
- 3.Jilaveanu LB, Aziz SA, Kluger HM. Chemotherapy and biologic therapies for melanoma: do they work? Clin Dermatol. 2009;27(6):614–25. doi: 10.1016/j.clindermatol.2008.09.020. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19880049. [DOI] [PubMed]
- 4.Flaherty KT, Hodi FS, Bastian BC. Mutation-driven drug development in melanoma. Curr Opin Oncol. 22(3):178–83. doi: 10.1097/cco.0b013e32833888ee. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20401974. [DOI] [PMC free article] [PubMed]
- 5.Hodi FS, Friedlander P, Corless CL, Heinrich MC, Mac Rae S, Kruse A, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26(12):2046–51. doi: 10.1200/JCO.2007.14.0707. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18421059. [DOI] [PubMed]
- 6.Garrido MC, Bastian BC. KIT as a therapeutic target in melanoma. J Invest Dermatol. 130(1):20–7. doi: 10.1038/jid.2009.334. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19847190. [DOI] [PMC free article] [PubMed]
- 7.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47(27):6658–61. doi: 10.1021/jm049486a. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15615512. [DOI] [PubMed]
- 8.Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, et al. 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem. 2006;49(23):6819–32. doi: 10.1021/jm060727j. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17154512. [DOI] [PubMed]
- 9.Pavlu J, Marin D. Dasatinib and chronic myeloid leukemia: two-year follow-up in eight clinical trials. Clin Lymphoma Myeloma. 2009;9(6):417–24. doi: 10.3816/CLM.2009.n.083. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19951880. [DOI] [PubMed]
- 10.von Mehren M. Beyond imatinib: second generation c-KIT inhibitors for the management of gastrointestinal stromal tumors. Clin Colorectal Cancer. 2006;6(Suppl 1):S30–4. doi: 10.3816/ccc.2006.s.005. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17419150. [DOI] [PubMed]
- 11.Araujo J, Logothetis C. Dasatinib: A potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. doi: 10.1016/j.ctrv.2010.02.015. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20226597. [DOI] [PMC free article] [PubMed]
- 12.Demetri GD, Lo Russo P, MacPherson IR, Wang D, Morgan JA, Brunton VG, et al. Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res. 2009;15(19):6232–40. doi: 10.1158/1078-0432.CCR-09-0224. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19789325. [DOI] [PubMed]
- 13.Halaban R, Kwon BS, Ghosh S, Delli Bovi P, Baird A. bFGF as an autocrine growth factor for human melanomas. Oncogene Res. 1988;3(2):177–86. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3226725. [PubMed]
- 14.Yayon A, Ma YS, Safran M, Klagsbrun M, Halaban R. Suppression of autocrine cell proliferation and tumorigenesis of human melanoma cells and fibroblast growth factor transformed fibroblasts by a kinase-deficient FGF receptor 1: evidence for the involvement of Src-family kinases. Oncogene. 1997;14(25):2999–3009. doi: 10.1038/sj.onc.1201159. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9223663. [DOI] [PubMed]
- 15.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470–80. doi: 10.1038/nrc1366. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15170449. [DOI] [PubMed]
- 16.Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21(46):7001–10. doi: 10.1038/sj.onc.1205859. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12370822. [DOI] [PubMed]
- 17.Mirmohammadsadegh A, Hassan M, Bardenheuer W, Marini A, Gustrau A, Nambiar S, et al. STAT5 phosphorylation in malignant melanoma is important for survival and is mediated through SRC and JAK1 kinases. J Invest Dermatol. 2006;126(10):2272–80. doi: 10.1038/sj.jid.5700385. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16741510. [DOI] [PubMed]
- 18.O’Connor TJ, Neufeld E, Bechberger J, Fujita DJ. pp60c-src in human melanocytes and melanoma cells exhibits elevated specific activity and reduced tyrosine 530 phosphorylation compared to human fibroblast pp60c-src. Cell Growth Differ. 1992;3(7):435–42. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1384653. [PubMed]
- 19.Furuhashi M, Sjoblom T, Abramsson A, Ellingsen J, Micke P, Li H, et al. Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res. 2004;64(8):2725–33. doi: 10.1158/0008-5472.can-03-1489. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15087386. [DOI] [PubMed]
- 20.Fang WB, Brantley-Sieders DM, Parker MA, Reith AD, Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24(53):7859–68. doi: 10.1038/sj.onc.1208937. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16103880. [DOI] [PubMed]
- 21.Sohaib SA, Turner B, Hanson JA, Farquharson M, Oliver RT, Reznek RH. CT assessment of tumour response to treatment: comparison of linear, cross-sectional and volumetric measures of tumour size. Br J Radiol. 2000;73(875):1178–84. doi: 10.1259/bjr.73.875.11144795. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11144795. [DOI] [PubMed]
- 22.Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24(29):4764–74. doi: 10.1200/JCO.2006.06.2265. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16954519. [DOI] [PubMed]
- 23.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–45. doi: 10.1200/JCO.2006.06.0483. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16966688. [DOI] [PubMed]
- 24.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2702835. [DOI] [PubMed]
- 25.Woodman SE, Trent JC, Stemke-Hale K, Lazar AJ, Pricl S, Pavan GM, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009;8(8):2079–85. doi: 10.1158/1535-7163.MCT-09-0459. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19671763. [DOI] [PMC free article] [PubMed]
- 26.Eustace AJ, Crown J, Clynes M, O’Donovan N. Preclinical evaluation of dasatinib, a potent Src kinase inhibitor, in melanoma cell lines. J Transl Med. 2008;6:53. doi: 10.1186/1479-5876-6-53. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18823558. [DOI] [PMC free article] [PubMed]
- 27.Homsi J, Cubitt CL, Zhang S, Munster PN, Yu H, Sullivan DM, et al. Src activation in melanoma and Src inhibitors as therapeutic agents in melanoma. Melanoma Res. 2009;19(3):167–75. doi: 10.1097/CMR.0b013e328304974c. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19434004. [DOI] [PubMed]
- 28.Buettner R, Mesa T, Vultur A, Lee F, Jove R. Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol Cancer Res. 2008;6(11):1766–74. doi: 10.1158/1541-7786.MCR-08-0169. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19010823. [DOI] [PMC free article] [PubMed]
- 29.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–6. doi: 10.1200/JCO.2006.06.2984. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16908931. [DOI] [PubMed]
- 30.Pereira PR, Odashiro AN, Marshall JC, Correa ZM, Belfort R, Jr, Burnier MN., Jr The role of c-kit and imatinib mesylate in uveal melanoma. J Carcinog. 2005;4:19. doi: 10.1186/1477-3163-4-19. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16236162. [DOI] [PMC free article] [PubMed]
- 31.Smalley KS, Sondak VK, Weber JS. c-KIT signaling as the driving oncogenic event in sub-groups of melanomas. Histol Histopathol. 2009;24(5):643–50. doi: 10.14670/HH-24.643. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19283671. [DOI] [PubMed]
- 32.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67(5):2226–38. doi: 10.1158/0008-5472.CAN-06-3633. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17332353. [DOI] [PubMed]
- 33.Konecny GE, Glas R, Dering J, Manivong K, Qi J, Finn RS, et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br J Cancer. 2009;101(10):1699–708. doi: 10.1038/sj.bjc.6605381. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19861960. [DOI] [PMC free article] [PubMed]
- 34.Sosman J, Puzanov I. Combination targeted therapy in advanced renal cell carcinoma. Cancer. 2009;115(10 Suppl):2368–75. doi: 10.1002/cncr.24234. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19402058. [DOI] [PubMed]