Structural Basis of Defects in the Sacsin HEPN Domain Responsible for Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS) (original) (raw)
Abstract
Sacsin is a 520-kDa protein mutated in the early-onset neurodevelopmental and neurodegenerative disease autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS). The C terminus of the protein contains an HEPN (higher eukaryotes and prokaryotes nucleotide-binding) domain of unknown function. Here, we determined the high-resolution 1.9-Å crystal structure of the HEPN domain from human sacsin. The structure is composed of five parallel α-helices with a large loop of several short helical segments. Two HEPN protomers assemble as a dimer to form a large positively charged cavity at the dimer interface that binds GTP and other nucleotides. The crystal structure reveals that the ARSACS N4549D mutation disrupts dimerization and protein folding. This study provides novel insights into the oligomerization state of sacsin and functions that are lost in mutations that cause ARSACS.
Keywords: Biophysics, Crystal Structure, Neurodegeneration, Neurological Diseases, X-ray Crystallography
Introduction
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS)3 is an early-onset neurodevelopmental and degenerative disease associated with atrophy of the upper cerebellar vermis and absence of Purkinje cells (1). The SACS gene was originally identified as causing ARSACS in the Charlevoix-Saguenay and Lac-Saint-Jean regions of Quebec (2), but its occurrence was later documented in many populations worldwide, suggesting that it may be one of the most common recessive ataxias (for review, see Ref. 3). In ARSACS patients, the SACS gene carries missense, nonsense, and frameshift mutations (2). The gene codes for a 520-kDa protein called sacsin, which is highly expressed in neurons. Sacsin has been proposed to act as a co-chaperone of the Hsp70 chaperone system (4), but the underlying basis of the neurological defects in ARSACS is unknown. Sacsin knockdown in tissue culture results in increased toxicity in cells expressing polyglutamine-expanded ataxin-1, suggesting a link between sacsin and other forms of ataxia (4). Sacsin has also been identified as a potential substrate of the ubiquitin ligase Ube3A responsible for Angelman syndrome, a neurodevelopmental disorder with a motor component that is similar to that seen in ARSACS (5).
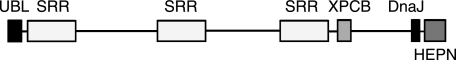
Analysis of the structural domains in sacsin provides insight into its physiological function and involvement in neurodegeneration (Fig. 1). At the N terminus, sacsin contains a ubiquitin-like domain that can interact with the proteasome (4). This is followed by three supradomains (termed sacsin repeat regions) that possess ATPase activity (6). Toward the C terminus, sacsin contains a 75-residue domain with sequence similarity to the helical XPC-binding domain from human HR23A, which has been implicated in interactions with the ubiquitin ligase Ube3A (5). The C-terminal region of the protein also contains a protein-protein interaction J-domain (2), which is typically associated with DnaJ-like co-chaperones involved in regulation of the Hsp70 heat shock system (7). The J-domain of sacsin genetically complements DnaJ of Agrobacterium tumefaciens (4) and is able to increase the ATPase activity of Hsp70 in in vitro assays (6). Finally, bioinformatics studies have predicted an HEPN (higher eukaryotes and prokaryotes nucleotide-binding) domain at the extreme C terminus of sacsin (8). Although a number of crystal structures of bacterial HEPN domains have been determined (Protein Data Bank codes 1UFB, 1WOL, and 2HSB) (9, 10), their function remains obscure. Many occur associated with nucleotidyltransferase domains either as separate proteins or together in a single polypeptide. The HEPN domain-containing protein kanamycin nucleotidyltransferase is an enzyme that deactivates aminoglycoside antibiotics. The structure of kanamycin nucleotidyltransferase shows that the protein is a dimer with two ATP- and kanamycin-binding sites (9).
FIGURE 1.
Domain organization of sacsin. UBL, ubiquitin-like domain; SRR, sacsin repeat region; XPCB, XPC-binding domain.
Here, we determined the first structure of a eukaryotic HEPN domain. The high-resolution crystal structure reveals the molecular basis of the defect in the sacsin missense mutation N4549D, which causes ARSACS (11). The structure reveals a dimeric assembly with a large positively charged cavity that is unique to the HEPN domain of sacsin. Isothermal titration calorimetry and NMR titrations showed that the HEPN domain binds GTP with low-micromolar affinity. Loss of these functions by either missense or truncation mutations leads to the neurological disease ARSACS (2, 11, 12).
EXPERIMENTAL PROCEDURES
Protein Expression, Preparation, and Purification
The HEPN domain (residues 4439–4579 and 4441–4579) of human sacsin was cloned into pGEX-4T-1 (Amersham Biosciences) and expressed in Escherichia coli BL21(DE3) cell in rich LB medium as an N-terminal GST-tagged fusion. For labeling for NMR experiments, cells were grown in M9 minimal medium with [15N]NH4Cl and [U-13C]glucose. For production of a selenomethionine-labeled protein, the expression plasmid was transformed into the E. coli methionine auxotroph strain DL41(DE3), and the protein was produced using LeMaster medium (13). Cells were harvested and broken in PBS (pH 7.4). The fusion protein was purified by affinity chromatography on glutathione-Sepharose resin, and the tag was removed by cleavage with thrombin, leaving a two-residue (Gly-Ser) N-terminal extension. The HEPN domain was additionally purified by size-exclusion chromatography.
Crystallization
Initial crystallization conditions were identified by hanging drop vapor diffusion using a PACT screen (Qiagen). The best crystals were obtained by equilibrating a 1.0-μl drop of a protein (8 mg/ml) in 20 mm sodium acetate (pH 5.5), 0.3 m NaCl, and 5 mm DTT; mixed with 1.0 μl of reservoir solution containing 7% (w/v) PEG 3350, 0.2 m sodium malonate, and 0.1 m BisTris (pH 6.0); and suspended over 1 ml of reservoir solution. Crystals grew in 1–3 days at 22 °C. For data collection, 25% ethylene glycol was added for cryoprotection, and the crystals were flash-cooled in an N2 cold stream.
Structure Solution and Refinement
Diffraction data from a selenomethionine-labeled crystal were collected using a single-wavelength (0.9780 Å) anomalous dispersion regime on an ADSC Quantum 210 CCD detector (Area Detector Systems Corp.) at beamline A1 at the Cornell High Energy Synchrotron Source (Table 1). Data processing and scaling were performed with HKL2000 (14). The positions of the selenium atoms and structure factor phases were determined using the program SHELX (15). The crystals contained four molecules in the asymmetric unit corresponding to Vm = 2.39 Å3 Da−1 and a solvent content of 48.6%. Density modification with the program ARP/wARP (16) allowed for automated model building of ∼70% of the residues. The partial model obtained from ARP/wARP was extended manually with the program Coot (17) and improved by refinement using REFMAC (18) and model refitting, followed by TLS (translation-libration-screw) refinement (19). The final model was 99% complete for all four chains and included three malonate ions. The atomic coordinates and structure factors (code 3o10) have been deposited in the Protein Data Bank.
TABLE 1.
Data collection and refinement statistics
The highest resolution shell is shown in parentheses. r.m.s.d., root mean square deviation.
| HEPN | |
|---|---|
| Data collection | |
| Space group | P3221 |
| Cell dimensions | |
| a, b, c (Å) | 72.58, 72.58, 201.18 |
| α, β, γ | 90.00°, 90.00°, 120.00° |
| Resolution (Å) | 50-1.90 (1.93-1.90) |
| _R_sym | 0.072 (0.189) |
| I/σ_I_ | 24.4 (6.9) |
| Completeness (%) | 98.1 (86.2) |
| Redundancy | 9.6 (5.2) |
| Refinement | |
| Resolution (Å) | 67.1-1.90 |
| No. reflections | 45,815 |
| _R_work/_R_free | 0.184/0.225 |
| No. of atoms | |
| HEPN | 4401 |
| Malonate | 21 |
| Water | 407 |
| _B_-factors | |
| HEPN | 20.0 |
| Malonate ions | 28.2 |
| Water | 35.1 |
| r.m.s.d. | |
| Bond lengths (Å) | 0.016 |
| Bond angles | 1.42° |
| Ramachandran statistics (%) | |
| Most favored regions | 93.2 |
| Additional allowed regions | 6.8 |
NMR Spectroscopy
NMR resonance assignments of the HEPN domain from human sacsin (residues 4439–4579) were carried out using 15N- and 13C,15N-labeled HEPN samples. Protein signal assignments were performed using standard HNCA, HNCACB, CBCA(CO)NH, and 15N NOESY heteronuclear multiple quantum coherence experiments. NMR samples contained 0.5 mm protein in 20 mm BisTris and 70 mm NaCl (pH 6.5). Pulsed field gradient measurements of the translational diffusion coefficient were done with a gradient duration of 3.5 ms and a diffusion time of 150 ms at 303 K (20). The gradient strength was calibrated using lysozyme (D = 1.34 × 10−6). For NMR titrations, ligands were added to 0.2 mm 15N-labeled HEPN domain, and a weighted average ((ΔδH2 + (0.2 × ΔδN)2)1/2) of the changes in the 1H and 15N chemical shifts was calculated. Binding affinities were determined from a plot of chemical shift changes as a function of the free ligand. All NMR experiments were performed at 303 K using Varian 800-MHz and Bruker 600-MHz spectrometers.
Isothermal Titration Calorimetry Measurements
Experiments were carried out on a MicroCal iTC200 titration calorimeter (GE Healthcare) in 20 mm BisTris and 70 mm NaCl (pH 6.5) at 298 K. Titration experiments were performed with 50 mm HEPN domain in the cell and 0.3–1 mm nucleotide in the syringe to ensure a final nucleotide/HEPN dimer molar ratio of at least 2:1. Calorimetric data were processed using software provided by the manufacturer.
Multi-angle Light Scattering Measurements
Size-exclusion chromatography and multi-angle light scattering experiments were performed using an analytical BioSuite 250 4-μm UHR SEC column (Waters) equilibrated in 20 mm sodium acetate and 300 mm NaCl (pH 5.5) with in-line static light scattering by absorbance and differential refractive index detectors (miniDAWN TREOS and Optilab-rEX, Wyatt Technology Corp.). The sacsin HEPN domain was loaded at 2 mg/ml with a sample size of 50 μl at 22 °C. Data were analyzed with the ASTRA software package (Wyatt Technology Corp.).
RESULTS AND DISCUSSION
Crystal Structure of the HEPN Domain
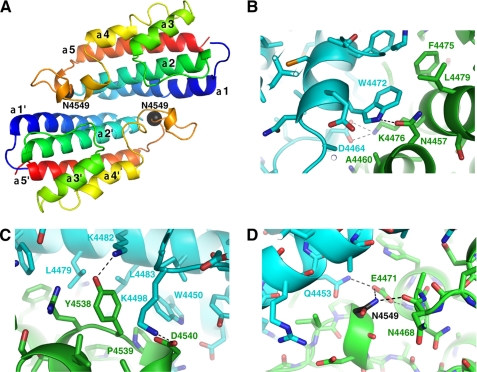
To gain insight into the functional role of the HEPN domain in sacsin, we crystallized the domain (residues 4441–4579) and determined its structure at 1.9-Å resolution using selenomethionine-labeled protein. The asymmetric unit contains four HEPN molecules (protomers) arranged as two dimers (supplemental Fig. 1). Each protomer is composed of a five-helix bundle with three long antiparallel helices (α1, α2, and α5) that provide the majority of the fold and two shorter helices (α3 and α4) that stack at a slight angle to the long helices. A long ordered loop between helices α4 and α5 contains three single-turn helical segments.
The HEPN protomers are arranged pairwise as symmetric dimers with a large buried surface of 3244 Å2 (Fig. 2A). The intermolecular contacts run along the length of the α1-α2 surface. At the heart of the dimer, the side chain of Trp-4472 of one chain contacts Phe-4475, Lys-4476, Lys-4479, and Asn-4457 of the opposite chain (Fig. 2B). The α4-α5 loop also contributes to the dimer interface with key interactions from Tyr-4538, Pro-4539, and Asp-4540 (Fig. 2C).
FIGURE 2.
Crystal structure of the HEPN domain from sacsin. A, the schematic shows that the ARSACS mutation N4549D occurs at the dimer interface between two protomers (colored blue to red from the N to C terminus). Each protomer is composed of five α-helices and a large loop that contains Asn-4549 (black sphere). B, the enlarged view of the α1-α2 dimer interface centered around Trp-4472. The two protomers are colored in green and cyan, and key residues are labeled. Hydrogen bonds are shown as dashed lines. C, the close-up view shows the interface between the α4-α5 loop of one protomer and the α1-α2 surface of another protomer. D, mutation of Asn-4549 disrupts a hydrogen bond with the backbone carbonyl of Asn-4468 and the salt bridge with the side chain of Glu-4471.
Mutation of Asn-4549 Disrupts the Dimer Interface
The structure allows evaluation of the effect of the N4549D mutation on the folding and stability of the sacsin HEPN domain. Asn-4549 is in the α4-α5 loop at the edge of the dimer interface (Fig. 2D). Replacement of this asparagine with aspartic acid will destabilize HEPN folding through the introduction of a charge at the dimer interface and loss of two polar contacts. In support of this, bacterial expression of HEPN mutant N4549D yielded insoluble protein that was unable to fold correctly and dimerize (supplemental Fig. 2).
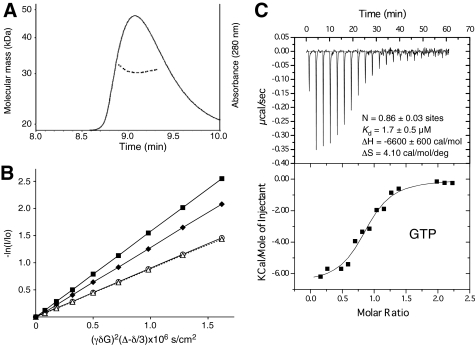
Sacsin Is a Dimer
We used two experimental approaches to verify that the dimer observed in the crystal is not an artifact of crystal packing. Size-exclusion chromatography showed that the protein elutes as a single peak with a molecular mass of 31 kDa as determined by multi-angle light scattering (Fig. 3A and supplemental Fig. 3). This is very close to the theoretical molecular mass of 32 kDa for the HEPN dimer. We also used NMR translational self-diffusion measurements to estimate the molecular mass in solution. The ratio of the diffusion constants for the HEPN domain (residues 4439–4579) and lysozyme (14.5 kDa) was 0.7, which is consistent with a dimeric mass (Fig. 3B). Taken together, these results confirm that the sacsin HEPN domain is a dimer in solution and that the full-length protein is very likely a dimer in the cell.
FIGURE 3.
The sacsin HEPN domain forms a dimer in solution. A, size-exclusion chromatography of sacsin HEPN (residues 4441–4579) with in-line molecular mass determination by multi-angle light scattering. B, NMR self-diffusion experiments for the HEPN domain (residues 4439–4579) alone (○) and in the presence of ATP at a 1:5 ratio (△; dashed line), chicken lysozyme (♦), and bovine ubiquitin (■). The slopes are equal to the translational diffusion coefficients. C, isothermal calorimetric titrations of the HEPN domain (residues 4439–4579) from sacsin with GTP. Upper panel, thermogram; lower panel, data analysis after integration (points) and the fit (continuous line) to determine the stoichiometry (N), affinity, enthalpy, and entropy.
The HEPN Domain Binds GTP
On the basis of the association of bacterial HEPN domains with nucleotidyltransferases, we tested the sacsin domain for the binding of nucleotides by NMR spectroscopy and isothermal titration calorimetry. GTP bound best with an affinity of 1.7 μm and a molar ratio of one GTP/HEPN dimer (Fig. 3C). GDP and ATP also bound but with 3- and 10-fold lower affinity (Table 2). Given cellular concentrations, this suggests that ∼10–30% of sacsin molecules may have ATP-bound. We detected no GTPase activity with the purified HEPN domain (supplemental Fig. 4).
TABLE 2.
Binding affinities of ligands for the sacsin HEPN domain
| Ligand | Kd |
|---|---|
| μ_m_ | |
| GTP | 1.7 |
| GDP | 5.1 |
| ATP | 22 |
| ADP | 54a |
| Pyrophosphate | 101a |
| Orthophosphate | 1100a |
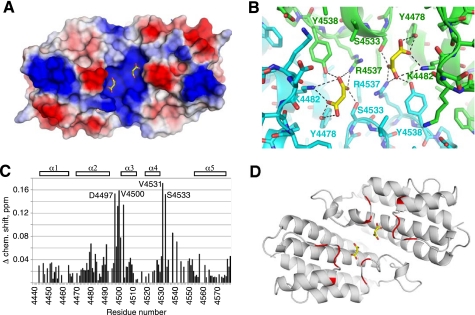
Inspection of the electron density map revealed the presence in the crystal of two bound malonate ions that were present in the crystallization buffer at 0.2 m. The malonate ions are symmetrically positioned close to each other and occupy a large positively charged cavity formed at the HEPN dimer interface (Fig. 4A). Both protomers are involved in binding each malonate ion (Fig. 4B), so loss of dimerization would disrupt binding of the negatively charged ions.
FIGURE 4.
The HEPN structure contains a binding surface for negatively charged ligands. A, electrostatic potential surface representation of the HEPN dimer identifying a large positively charged pocket with two bound malonate ions (yellow). Positive charge is shown in blue, and negative charge is shown in red. B, enlarged view showing the binding pocket with malonate and hydrogen bonds to both HEPN protomers. C, identification of the GTP-binding site in solution. The plot of NMR chemical shift changes in the 15N-labeled HEPN domain upon addition of GTP shows regions involved in binding. D, mapping of the chemical shifts onto the HEPN structure. The residues that shift by >0.1 ppm upon GTP binding are colored red. The largest chemical shift changes occur in the loops that contribute to the basic pocket.
We turned to NMR spectroscopy for confirmation that the positively charged cavity is the GTP-binding site. The 1H-15N correlation spectrum of the HEPN domain (residues 4439–4579) displays good dispersion of signals characteristic of a well folded protein (supplemental Fig. 5). The addition of GTP or other ligands resulted in specific chemical shift changes (supplemental Fig. 6) that could be used to estimate the binding affinities (Table 2). Furthermore, assignment of the NMR signals allowed us to identify the α2-α3 and α4-α5 loops of the HEPN domain as the regions that were most affected by GTP binding (Fig. 4C). Mapping of the changes onto the crystal structure confirmed that GTP binds to the positively charged cavity occupied by malonate ions in the crystal structure (Fig. 4D).
Comparison with Other HEPN Domains
HEPN domains are widely distributed in eubacteria and archaea but are restricted to animals in eukaryotes (8, 21). Depending on their associations, they can be broken into 1) single HEPN domains found in small proteins without other protein domains, 2) HEPN domains found associated with nucleotidyltransferase domains, and 3) sacsin-like HEPN domains found in metazoans. The bacterial HEPN domains have been suggested to be part of prokaryotic toxin-antitoxin systems as mobile selfish genetic elements (21). In humans, the HEPN domain occurs only in the protein sacsin. A second more distantly related sacsin-like protein occurs in fish, birds, and invertebrates (Fig. 5A) (6).
FIGURE 5.
Comparison of HEPN structures and their dimeric arrangement. A, an unrooted phylogenetic tree of HEPN domains in animals shows the existence of two distinct groupings, a diverse form than includes invertebrates and a more conserved form found in vertebrates, including humans. B, an overlay of the HEPN domains from human sacsin (white) and TT1696 from Thermus thermophilus (Protein Data Bank code 1UFB; black) shows strong similarity in both the monomer structure and dimeric arrangement. C, an overlay of HEPN protomers from sacsin (white) and TM0613 from Thermotoga maritima (Protein Data Bank code 1O3U; black) shows a conserved monomer structure but a strikingly different dimeric arrangement.
Crystal structures of several bacterial HEPN domains have been determined, but their sequence similarity to human sacsin is <30%. Although all of the proteins are symmetric dimers, the bacterial structures show considerable plasticity in the relative arrangement of the two protomers. The HEPN dimer of TT1696 is quite similar to sacsin, whereas the dimer from TM0613 displays a different arrangement of protomers (Fig. 5, B and C). The majority of proteins composed of a single HEPN domain show an arrangement similar to TT1696. In both cases, HEPN helices α1 and α2 contribute much of the dimerization surface. In the kanamycin nucleotidyltransferase structures, the HEPN domain plays a dual role in nucleotide binding and dimerization and bears little similarity to sacsin (9).
In sacsin, the HEPN domain is immediately preceded by a J-domain, which is similar to the domain from DNAJB6 (also known as MRJ). DNAJB6 is an Hsp40 homolog that has been associated with several neurodegenerative disorders: Parkinson disease (22, 23) and polyglutamine expansion diseases (24) such as Huntington disease (25). The close physical association of the sacsin J-domain and HEPN domains suggests that the nucleotide-binding activity of the HEPN domain may be related to the hypothesized function of sacsin as a co-chaperone (4, 6). The HEPN domain could increase the local concentration of GTP or ATP to promote nucleotide exchange onto Hsp70. Sacsin has been found associated with mitochondria and may play a role in mitochondrial quality control, as observed for other proteins associated with neurodegenerative diseases (4, 26).
Conclusions
The gigantic size of the SACS gene and translated protein has hindered biochemical studies of ARSACS. Presently, much more is known about the genetics of ARSACS, including its possible link with other neurological diseases, than about the function of sacsin in cells. As a first step, we have characterized the structure and function of the C-terminal HEPN domain and shown that it dimerizes and contains a high-affinity binding site for GTP. Loss of these functions either by a missense mutation (11) or by premature termination leads to ARSACS disease (2). Much still needs to be learned about sacsin and its involvement in neurodevelopment and neurodegeneration. Future studies should help determine the function of other domains in sacsin, its interactions within the cell, and the role of sacsin in maintaining neuronal health.
Supplementary Material
Supplemental Data
Acknowledgments
We thank François Blondeau for help in the initial stages of the project. Data acquisition at the Macromolecular Diffraction (MacCHESS) facility at the Cornell High Energy Synchrotron Source was supported by National Science Foundation Award DMR 0225180 and National Institutes of Health Grant RR01646.
*
This work was supported by the Fondation de l'Ataxie Charlevoix-Saguenay, the Neuromuscular Research Partnership Program of the Canadian Institutes of Health Research, Muscular Dystrophy Canada, and the ALS Society of Canada.
The atomic coordinates and structure factors (code 3o10) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
3
The abbreviations used are:
ARSACS
autosomal recessive spastic ataxia of Charlevoix-Saguenay
BisTris
2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1.Bouchard J. P., Richter A., Mathieu J., Brunet D., Hudson T. J., Morgan K., Melançon S. B. (1998) Neuromusc. Disord. 8, 474–479 [DOI] [PubMed] [Google Scholar]
- 2.Engert J. C., Bérubé P., Mercier J., Doré C., Lepage P., Ge B., Bouchard J. P., Mathieu J., Melançon S. B., Schalling M., Lander E. S., Morgan K., Hudson T. J., Richter A. (2000) Nat. Genet. 24, 120–125 [DOI] [PubMed] [Google Scholar]
- 3.Takiyama Y. (2007) Cerebellum, 1–7 [DOI] [PubMed] [Google Scholar]
- 4.Parfitt D. A., Michael G. J., Vermeulen E. G., Prodromou N. V., Webb T. R., Gallo J. M., Cheetham M. E., Nicoll W. S., Blatch G. L., Chapple J. P. (2009) Hum. Mol. Genet. 18, 1556–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greer P. L., Hanayama R., Bloodgood B. L., Mardinly A. R., Lipton D. M., Flavell S. W., Kim T. K., Griffith E. C., Waldon Z., Maehr R., Ploegh H. L., Chowdhury S., Worley P. F., Steen J., Greenberg M. E. (2010) Cell 140, 704–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson J. F., Siller E., Barral J. M. (2010) J. Mol. Biol. 400, 665–674 [DOI] [PubMed] [Google Scholar]
- 7.Minami Y., Höhfeld J., Ohtsuka K., Hartl F. U. (1996) J. Biol. Chem. 271, 19617–19624 [DOI] [PubMed] [Google Scholar]
- 8.Grynberg M., Erlandsen H., Godzik A. (2003) Trends Biochem. Sci. 28, 224–226 [DOI] [PubMed] [Google Scholar]
- 9.Pedersen L. C., Benning M. M., Holden H. M. (1995) Biochemistry 34, 13305–13311 [DOI] [PubMed] [Google Scholar]
- 10.Erlandsen H., Canaves J. M., Elsliger M. A., von Delft F., Brinen L. S., Dai X., Deacon A. M., Floyd R., Godzik A., Grittini C., Grzechnik S. K., Jaroszewski L., Klock H. E., Koesema E., Kovarik J. S., Kreusch A., Kuhn P., Lesley S. A., McMullan D., McPhillips T. M., Miller M. D., Morse A., Moy K., Ouyang J., Page R., Robb A., Quijano K., Schwarzenbacher R., Spraggon G., Stevens R. C., van den Bedem H., Velasquez J., Vincent J., Wang X., West B., Wolf G., Hodgson K. O., Wooley J., Wilson I. A. (2004) Proteins 54, 806–809 [DOI] [PubMed] [Google Scholar]
- 11.Richter A. M., Ozgul R. K., Poisson V. C., Topaloglu H. (2004) Neurogenetics 5, 165–170 [DOI] [PubMed] [Google Scholar]
- 12.Baets J., Deconinck T., Smets K., Goossens D., Van den Bergh P., Dahan K., Schmedding E., Santens P., Rasic V. M., Van Damme P., Robberecht W., De Meirleir L., Michielsens B., Del-Favero J., Jordanova A., De Jonghe P. (2010) Neurology 75, 1181–1188 [DOI] [PubMed] [Google Scholar]
- 13.Hendrickson W. A., Horton J. R., LeMaster D. M. (1990) EMBO J. 9, 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 15.Sheldrick G. M., Schneider T. R. (1997) Methods Enzymol. 277, 319–343 [PubMed] [Google Scholar]
- 16.Perrakis A., Sixma T. K., Wilson K. S., Lamzin V. S. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 448–455 [DOI] [PubMed] [Google Scholar]
- 17.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 18.Murshudov G. N., Vagin A. A., Lebedev A., Wilson K. S., Dodson E. J. (1999) Acta Crystallogr. D Biol. Crystallogr. 55, 247–255 [DOI] [PubMed] [Google Scholar]
- 19.Winn M. D., Murshudov G. N., Papiz M. Z. (2003) Methods Enzymol. 374, 300–321 [DOI] [PubMed] [Google Scholar]
- 20.Altieri A. S., Hinton D. P., Byrd R. A. (1995) J. Am. Chem. Soc. 117, 7566–7567 [Google Scholar]
- 21.Makarova K. S., Wolf Y. I., Koonin E. V. (2009) Biol. Direct 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose J. M., Novoselov S. S., Robinson P. A., Cheetham M. E. (2011) Hum. Mol. Genet. 20, 16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durrenberger P. F., Filiou M. D., Moran L. B., Michael G. J., Novoselov S., Cheetham M. E., Clark P., Pearce R. K., Graeber M. B. (2009) J. Neurosci. Res. 87, 238–245 [DOI] [PubMed] [Google Scholar]
- 24.Hageman J., Rujano M. A., van Waarde M. A., Kakkar V., Dirks R. P., Govorukhina N., Oosterveld-Hut H. M., Lubsen N. H., Kampinga H. H. (2010) Mol. Cell 37, 355–369 [DOI] [PubMed] [Google Scholar]
- 25.Chuang J. Z., Zhou H., Zhu M., Li S. H., Li X. J., Sung C. H. (2002) J. Biol. Chem. 277, 19831–19838 [DOI] [PubMed] [Google Scholar]
- 26.Youle R. J., Narendra D. P. (2011) Nat. Rev. Mol. Cell Biol. 12, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data