Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases (original) (raw)
. Author manuscript; available in PMC: 2012 Jul 1.
Summary
Immunoglobulin E (IgE) antibodies have long been recognized as the antigen-specific triggers of allergic reactions. This review briefly introduces the established functions of IgE in immediate hypersensitivity and then focuses on emerging evidencefrom our own investigations as well as those of others that IgE plays important roles in protective immunity against parasites and exerts regulatory influences in the expression of its own receptors, FcεRI and CD23, as well as controlling mast cell homeostasis. We provide an overview of the multifaceted ways in which IgE antibodies contribute to the pathology of food allergy and speculate regarding potential mechanisms of action of IgE blockade.
Keywords: IgE, allergy, mast cell
Introduction
Immunoglobulin E (IgE), which originally evolved as an important component of adaptive immune responses to helminthic parasites, has in our times become infamous for itsrole as a troublesome instigator of allergic reactions. IgE-triggered immune responses have been termed ‘immediate hypersensitivity’ reactions, a term which conveys both the extreme sensitivity of the IgE system to antigens and the incredible speed of this immune response. Elevated production of IgE is present in subjects with the atopic conditions, asthma, allergic rhinitis, and atopic dermatitis. In this review, we cover the classical role of IgE in immediate hypersensitivity and discuss evidence that IgE mediates protective immunity during parasite infection. We describe recent advances in understanding of the roles of IgE in immune homeostasis, particularly with respect to IgE receptor regulation and mast cell biology, and explore new insights into the actions of IgE in food allergy.
IgE background
The history of IgE
IgE is the least abundant antibody class in circulation, and consequently, was not discovered until decades after IgG, IgM, IgD, and IgA. The serum concentration of IgE in normal individuals only reaches around 50 ng/ml, in contrast to IgG, which is present at concentrations on the order of 5–10 mg/ml. Production of IgE is influenced by both genetic and environmental factors. The paucity of IgE in circulation and its very short half-life (only a day or two in plasma, far shorter than the average three weeks for IgG) are the consequence both of the very small number of B cells committed to IgE synthesis and of the rapid absorption of IgE in tissues where it is tightly bound via FcεRI to mast cells. There, IgE may persist for several weeks (1, 2).
The basic structure of IgE has much in common with other immunoglobulin isotypes. Each IgE protein is a tetramer comprised of two identical pairs of heavy and light chains. Variable regions at the N-termini of the heavy and light chains create unique binding pockets that determine the antigen specificity of the antibody. The C-terminal regions of the heavy chains contain a constant region made up of four Cε repeats that confers the isotype-specific functions of IgE, including interaction with its cellular and soluble receptors. The incorporation of hydrophobic sequences encoded by M1 and M2 exons in transmembrane splice variants gives rise to membrane-bound IgE in B cells (2). The very low levels of IgE in serum prevented its discovery for many years. Characterization of other antibody isotypes had been facilitated by the isolation of transformed plasma cells from myeloma patients, which secreted large quantities of single isotypes. At the beginning of research into IgE, the component of plasma called reagin, that appeared to be uniquely capable of mediating anaphylactic responses, was not even confirmed to be an antibody, since it did not fix complement and failed to produce precipitin lines in agar diffusion reactions with antigen. Work by Prausnitz and Kustner demonstrated that antibodies within allergic sera were capable of transferring immediate hypersensitivity to the skin of non-allergic individuals, suggesting that reagin was indeed an antibody. Using this transfer of passive cutaneous anaphylaxis, it was established that reagin existed within the γ-globulin fraction of serum, was heat-labile, and did not cross the placenta. Eventually, a rare myeloma was found that produced antibodies capable of inhibiting the Prausnitz-Kustner test, signifying that this isotype was identical to the reagin molecule. Characterization of the reaginic isotype revealed a new immunoglobulin class and the name IgE was given in Lausanne in 1968, as has been recounted by Stanworth (3).
Regulation of IgE synthesis
Mature B cells leave the bone marrow producing IgD and IgM antibodies of a defined antigen specificity. They have the capacity to modify the isotype they produce to IgG, IgA, and IgE, each with distinct biological effector functions but retaining the originally committed antigenic specificity of the parent clone. This transition is referred to as ‘class switching’, a process in which both the B cell and helper-T cells encounter their cognate antigen.
Antigen binding to membrane-bound Ig triggers internalization and proteolytic degradation of the antigen into peptides that are bound to major histocompatibility complex (MHC) class II molecules and displayed at the cell surface for presentation to T-helper (Th) cells. T-cell help provides the critical signals that determine class switching in B cells both through direct cellular interactions and via secreted cytokines. In the case of IgE, CD154 (CD40L), transiently expressed on activated T cells, activates the nuclear factor κB (NF-κB)signaling pathway via constitutively expressed CD40 on the B cell. When the CD40 stimulus is accompanied by an IL-4 signal, the process of isotype switching is activated and directed towards IgE production. IL-4 binding to its receptor on B cells induces phosphorylation and nuclear translocation of signal transducer and activator of transcription6 (STAT6), leading to activation of transcription of the Cε exons. The resultant RNAs, known as ‘germline transcripts’, contain stop codons and hence do not encode functional proteins. However transcriptional activity at the Cε locus results in recruitment of a number of enzymes, including activation-induced cytidine deaminase (AID) to the locus initiating a targeted process of DNA excision and repair that results in splicing of the Cε exons to the VDJ cassette, located about 150 kb upstream and encoding the Ig domains (reviewed in 2). The product of this somatic genomic recombination event is a complete IgE gene, containing all the variable and constant (Fc) components of an IgE heavy chain. B cells that have completed this process are irreversibly committed to production of IgE antibodies and can further differentiate into plasma cells committed to IgE synthesis. Class switching is linked to cell division, and since the switch to IgE often proceeds via an intermediary IgG switch, an IgE response generally takes longer to develop than IgG production (2). The Cε gene is only expressed in B cells that have undergone somatic recombination of the heavy chain locus. Genetic deletion of Cε in mice generates animals devoid of IgE (IgE−/−), which we have used to probe the in vivo functions of IgE (4).
Cellular receptors for IgE
The high affinity receptor for IgE, FcεR1, is assembled from three subunits, an α subunit that binds the Fc region of IgE, a β subunit that provides important accessory signaling, and the FcR γ-chain, which is shared with FcγRIII and activates intracellular signaling pathways (2, 5). FcεR1 can be expressed as either an αβγ2 tetramer in mast cells and basophils, where it is responsible for immediate hypersensitivity reactions, or as an αγ2 trimer in a wide variety of other cell types. In mice FcεR1 expression is generally more restricted to the tetrameric isoform on mast cells and basophils (6). Recent reports, however, indicate that in some circumstances trimeric FcεRI can also be expressed in rodents on neurons and on dendritic cells (7, 8).
In humans, it is theorized that FcεR1 on antigen-presenting cells permits the transport of antigens captured by IgE in the tissues into peripheral lymph nodes in order to initiate immune responses (9). Unlike FcγRs, FcεR1 has a very avid Kd and is normally fully saturated at physiological concentrations of IgE. This remarkable occupancy is due to the extremely low Kd (~1 nm) for the affinity between FcεR1 and IgE, and allows IgE to persist when bound to cellular receptors for weeks or months despite its half-life of only a few days in serum (2).
CD23, also known as FcεRII, differs markedly in both form and function from FcεR1. CD23 is a calcium-dependent lectin consisting of a globular head structure that binds IgE atop a long stalk. It can exist in a membrane anchored form on the cell surface, or the head groups can be cleaved by proteases to be released as a free receptor (reviewed in 9). Although nominally the ‘low-affinity’ IgE receptor, monomeric CD23, has a Kd for binding IgE of approximately 0.1-1μM, a relatively strong interaction albeit not as tight as that of the FcεR1-IgE complex (Kd ~1 nm) (10). Moreover, because the leucine-zipper motifs in the stalk region allow three CD23 molecules to wind together in a trimer, the overall avidity of CD23 for IgE (10–100 nM) is not much less than that of FcεR1 (10).
CD23 is transcribed in two splice-isoforms—CD23a and CD23b—with CD23b being expressed on a broad array of cell types including T cells, dendritic cells, monocytes, neutrophils, and intestinal epithelial cells (10). Expression of CD23a is largely restricted to B cells, where it serves as a reservoir to absorb IgE and as such is the major regulator of serum IgE levels (11). Binding of IgE to membrane-bound CD23 inhibits further B-cell production of IgE, providing negative feedback (10). In humans but not mice, soluble CD23-IgE complexes can also interact with CD21, stimulating IgE synthesis when CD23 is trimeric or repressing it with monomeric CD23 (12–14). Interactions between CD23 and MHC class II may facilitate the processing of IgE-captured antigens into peptides, which can be loaded onto MHC class II for presentation to CD4+ helper T cells (10, 15, 16). Delivery of antigen as an immune complex with IgE to an antigen-presenting cell can generate 100-fold enhancement of both T and B-cell responses over immunization with the antigen alone (17, 18).
Beyond FcεR1 and CD23, several other receptors are capable of binding IgE, but much less is known about the importance of these interactions. In mice, IgE antibodies have been reported to interact with multiple IgG receptors: FcγRII, FcγRIII, and FcγRIV (19–21). Binding of IgE to FcγRII is a low affinity interaction occurring at high IgE levels that produces an inhibitory signal (2, 19). IgE-FcγRII binding on mouse B cells may substitute for the suppression of IgE synthesis by the IgE-CD23-CD21 complex in humans (2). FcγRIII binding of IgE was recently reported to reduce IL-12 production by dendritic cells, favoring Th2 polarization (22). IgE ligation of FcγRIV activates macrophages and promotes lung inflammation and so has been suggested to fulfill some of the functions of αγ2 FcεR1 expressed by human antigen-presenting cells (20, 21). The C-type lectin galectin-3 binds to both IgE and FcεR1 via carbohydrate residues and so can trigger the cross linking and degranulation of both mast cells and basophils (23, 24).
Immediate hypersensitivity, the classical IgE-mediated response
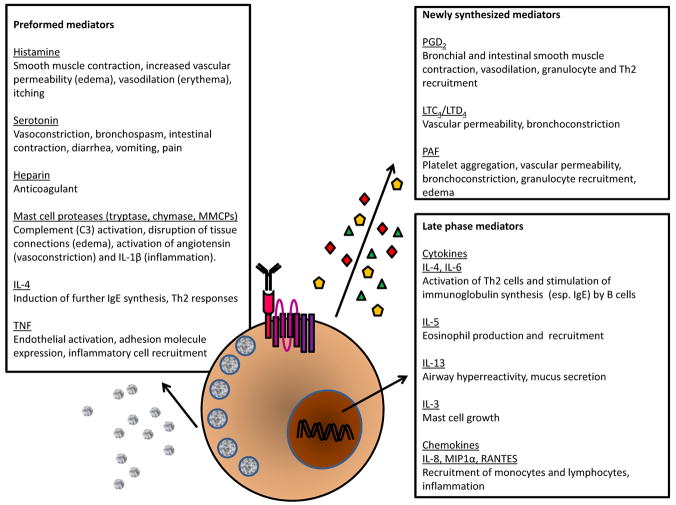
The best known functions of IgE are in instigating classic immediate hypersensitivity reactions, whereupon exposure to the offending allergen elicits symptoms of allergy only minutes later. Depending on the organ where sensitization and subsequent exposure have occurred, immediate hypersensitivity will manifest as urticaria in the skin, acute bronchospasm in the aeroallergen-challenged asthmatic, food-induced diarrhea and anaphylaxis in the gut, and systemic anaphylactic reactions to insect venoms and drugs. Crosslinking of IgE with antigen triggers the release of mast cell granules which contain preformed mediators and also induces de novo synthesis of lipid mediators, which produce the symptoms of immediate hypersensitivity within minutes (Fig. 1). Cytokine and chemokine secretion follow later hours and promote the accumulation and activation of inflammatory leukocytes in the tissue, which causes a second, late-phase response. This delayed reaction is also IgE and mast cell dependent (25) and reproduces many of the symptoms of earlyphase responses, including airway obstruction, cutaneous swelling, gastrointestinal distress, or anaphylaxis.
Fig. 1. The actions of mast cell mediators in immediate hypersensitivity.
Degranulation of mast cells (and basophils) triggers the secretion of a range of potent molecules that can be broadly divided into three categories: preformed mediators ready for immediate action (e.g. histamine), mediators synthesized within minutes (e.g. LTC4), and factors that require gene transcription and are involved in the late phase response hours later (e.g. cytokines). Fig. 1 summarizes some of the known actions of these mediators in evoking the symptoms of immediate hypersensitivity (138, 139). Abbreviations: PAF, platelet-activating factor; IL, interleukin; LT, leukotriene; PG, prostaglandin; RANTES, regulated upon activation, normal T cell expressed and secreted; MIP1α, macrophage inflammatory protein-1α.
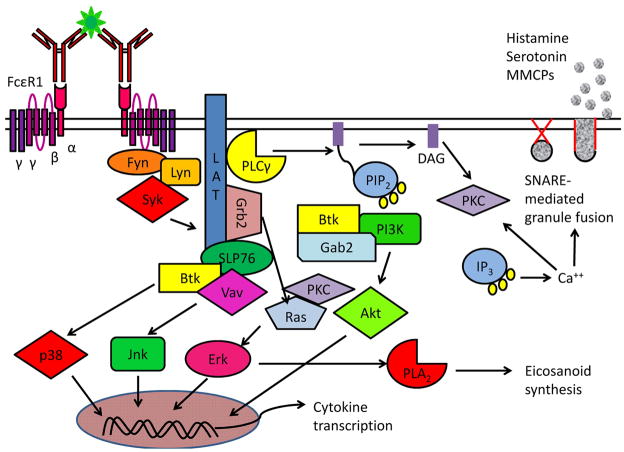
Mast cell degranulation begins when polyvalent antigen crosslinks multiple IgE molecules, causing FcεR1 aggregation and the activation of multiple signaling events (Fig. 2). Aggregation of intracellular immunoreceptor tyrosine-based activation motifs (ITAMs) on the FcεR1 β and γ chains stimulates phosphorylation by the protein tyrosine kinase (PTK) lyn and recruits other PTKs such as Syk and fyn as well as scaffolding proteins (LAT, SLP-76, Grb2) to assemble a signaling complex. Incorporation of phospholipase-Cγ (PLCγ) into the complex leads to hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), generating inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 diffuses to the endoplasmic reticulum, where it mediates the release of intracellular calcium stores. A resultant sudden increase in cytosolic calcium alters the conformation of the SNARE [SNAP (soluble NSF attachment protein) receptor] proteins that restrain the granules tethered to the inside of the cell, triggering granule fusion with the plasma membrane and dumping of preformed mediators (histamine, heparin, serotonin and mast cell proteases) into the extracellular space (9, 26–29).
Fig. 2. IgE triggering of immediate hypersensitivity.
Complex allergens contain multiple epitopes for IgE binding, prompting the aggregation of IgE:FcεR1and fyn and or lyn-mediated phosphorylation of ITAMs on the clustered β and γ chains. Syk is recruited to the phosphorylated FcεR1γ chain ITAMs and triggers the formation of a signaling complex by phosphorylating LAT, thus creating docking sites for Grb2, PLCγ and Gads/SLP-76. PLCγ is recruited to this membrane associated complex and then cleaves PIP2, creating the messenger molecules DAG and IP3, which drive PKC activation and intracellular calcium release respectively. The combined actions of PKC and calcium-mediated changes in SNARE protein conformations trigger the exocytosis of granules, releasing several mediators of immediate hypersensitivity. Simultaneous activation of the three major MAPK cascades, Erk, Jnk, and p38 MAPK, results in activation of transcription of cytokines and other late phase mediators as well as stimulation of arachadonic acid metabolism and production of prostaglandins and leukotrienes. Significant crosstalk between activation pathways is possible, including DAG-mediated activation of PKC, which can enhance Ras-Erk signaling. Fyn also phosphorylates Gab2, which in turn increases PI3K activity, prompting the conversion of PIP2 to PIP3 and favoring the localization of Btk and Akt to the plasma membrane. Akt contributes to cytokine production as well as inhibiting apoptosis pathways.
Parallel signaling pathways activated by FcεR1 aggregation converge with DAG-induced protein kinase C (PKC) activity and calcium influx to activate the transcription of cytokines and eicosanoid metabolism. Guanine nucleotide exchange factor activation by the LAT/SLP-76signaling complex initiates multiple MAPK pathways, including Ras-Erk, Jnk, p38, and Akt, promoting the transcription of cytokine genes. Downstream of Ras and Erk, phospholipase A2 (PLA2) prompts the release of mast cell arachadonic acid stores to promote the synthesis of eicosanoid mediators, including prostaglandins and leukotrienes, which are important instigators of chemotaxis, vasodilation, and bronchoconstriction (27, 28, 30).
The majority of the symptoms of immediate hypersensitivity responses in the airway or vasculature can be traced to the actions of factors deriving from the mast cell granules or eicosanoid synthesis. For instance, histamine stimulates nerve endings to produce itching, as well as the mucus secretion and smooth muscle contraction that block the airway (27, 31). Serotonin acts on the nervous system, giving rise to tachycardia, bronchoconstriction, diarrhea, and pain (30, 31). Vasoactive amines (histamine, serotonin) and lipid mediators (platelet-activating factor, prostaglandins, leukotrienes) increase cutaneous blood flow and enhance local vascular permeability such that plasma proteins leak out to cause swelling and erythrocytes trapped behind in the widened arterioles redden the area (erythema) (2, 32). In systemic anaphylaxis, this enhanced vascular permeability also drives a sudden loss in blood pressure (hypotension), since fluid rushes to the extremities, with a corresponding loss in core body temperature (32).
As the acute symptoms of immediate hypersensitivity abate, IgE-stimulated mast cells produce a range of chemokines (RANTES, eotaxin, MIP1α) and cytokines (IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, IL-25, IL-33, TNFα, GM-CSF) that orchestrate the influx and activation of cell types associated with allergy (33–35). Eotaxin and RANTES recruit T cells, which are potent producers of mast cell growth factors, and eosinophils, which proliferate in the presence of IL-5 (36). IL-4 upregulates very late antigen-4 (VLA-4)on the vascular endothelium, leading to the recruitment of vascular cell adhesion molecule-1 (VCAM-1) expressing T cells, basophils, eosinophils, and monocytes. IL-4also encourages B cells to produce more IgE, replenishing the IgE consumed in the degranulation reaction (37). Mast cells and basophils multiply in response to IL-3 and decrease their threshold for degranulation due to IL-4 (38–42). Together, these processes set the stage for the induction of a T cell and eosinophil-dominant allergic tissue inflammation, which underlies chronic allergic diseases such as asthma, atopic dermatitis, and allergic rhinitis while also providing amplifying the pathways for future immediate hypersensitivity arising in the event of repeated allergen encounters.
New evidence for IgE-independent immediate hypersensitivity
Over the past couple of decades, work by several groups, including our own, has challenged the view that all immediate hypersensitivity reactions are triggered by IgE. Some of the first evidence that IgE was not unique in being able to mediate anaphylaxis came, interestingly enough, through demonstrations that the IgG fraction of serum could passively transfer cutaneous sensitivity, similarly to what was originally described for the reagin molecule (43). In contrast to reagin-mediated reactions, this IgG response was short in latency (IgG injected into the skin is not tightly bound to FcγRs, and hence quickly diffused away with resultant loss of sensitivity, while injected IgE was tightly bound to dermal mast cells via FcεRI and persisted for weeks) and the activity was heat stable (in contrast to IgE which was heat-labile).
A definitive approach to the question of a requisite role of IgE in anaphylaxis was really made possible by the generation of IgE deficient (IgE−/− ) mice by gene targeting in 1994, a time when the use of this technology was just coming into the mainstream of immunological investigation. Our initial studies of the IgE−/− mouse proved definitively that active systemic anaphylaxis in rodents can also arise by IgE-independent mechanisms. Further studies by other investigators demonstrated that IgG1 could mediate anaphylaxis in mice (44) and that anaphylaxis could occur in the absence of FcεR1 or mast cells (45). Eventually it became clear that this pathway functioned independently of mast cells altogether through IgG binding to FcγRIII on macrophages, with platelet-activating factor (PAF) substituting for histamine to increase vascular permeability and bronchoconstriction (6, 44). Although mast cells and basophils express FcγRIII and are capable of degranulating in response to IgG, they are evidently not required for IgG-mediated anaphylaxis in rodent models. The interaction between IgG and FcγRIII is so much weaker than IgE and FcεR1 that the concentrations of IgG and antigen required for IgE-independent anaphylaxis are not normally observed outside the laboratory, and so the presence of IgG-dependent anaphylaxis has never been definitively established in humans (45). Nevertheless, all the requisite factors exist in humans for IgG-mediated anaphylaxis and the presence of immediate allergic reactions in some subjects in whom allergen-specific IgE cannot be detected by sensitive methods suggests that IgE-independent pathways are operative. This possibility may have implications for our understanding of acute reactions to protein-based therapies such as monoclonal antibody treatments (45).
IgE in immunity to parasites
The IgE−/− mouse provided an invaluable experimental tool to study the functions of IgE in other immune functions, including the host response to helminthic parasites. In Westernized societies the rates of atopic disorders are increasing (46, 47), and it is clear that the health costs arising from allergies driven by dysregulated IgE production are enormous. Why then does IgE exist? Evolution would be expected to select against individuals with a predisposition for anaphylaxis unless there were some stronger selective advantage conferred by the presence of IgE antibodies along with their family of receptors and dedicated effector cell lineages driving their persistence. The nature of this evolutionary pressure is suggested by epidemiological observations of high IgE titers in helminth-infected populations indicating that IgE may defend against metazoan parasites. Since humans have been infected by intestinal helminths for many thousands if not millions of years, there has been time for selection pressures to act (48).
IgE functions in worm elimination and granuloma formation in Schistosoma mansoni infestation
Support for a role of IgE in parasite immunity was found when it was discovered that human eosinophils and even platelets could use IgE in vitro to kill the blood fluke Schistosoma mansoni (49–51). Importantly, IgE titers have been shown to positively predict resistance to reinfection in _S. mansoni-_infected populations (52, 53). Attempts to study the role of IgE in rodent models of schistosomiasis have produced conflicting results. In mice it has been difficult to model the low-level infection, reinfection, and superinfection that occurs in populations in the parts of the world where S. mansoni is endemic. Furthermore, the broader expression of FcεR1 in humans permits IgE-mediated killing of schistosomes by eosinophils and platelets, defense mechanisms which might not be operative in mice (54).
We again took advantage of IgE−/− mice to address this question and found that IgE enhances granuloma formation in the liver while assisting in the clearance of adult worms during primary infection (55). By contrast, Jankovic and colleagues (56) found that deletion of FcεR1 did not alter worm burden and in fact increased liver pathology and granuloma volume. Other studies using less specific means of enhancing or reducing IgE levels have found little impact of IgE on the progression of murine schistosomiasis (57–59). Importantly, we showed that IgE−/− mice had impaired production of parasite-specific IgG1 antibodies, which have been implicated in anti-schistosomal immunity (55, 60). Thus, it is possible that the protective effect of IgE we observed was not due to IgE interacting with FcεR1 on mast cells or basophils, but rather to processing of IgE and parasite antigen via CD23 for presentation on MHC class II, facilitating the production of protective antibodies by B cells. Both our study and that of Jankovic et al. suggest that IL-4 production by FcεR1-bearing basophils and mast cells is not required for the induction of the parasite-specific Th2 response in schistosomiasis (55, 56).
IgE-mediated expulsion of adult worms and responses to larval cysts in mice infected with Trichinella spiralis
Infection with the parasite Trichinella spiralis is accompanied by intestinal mastocytosis and heightened IgE responses (61). Elimination of T.spiralis requires expulsion of the adult worms from the gut and destruction of the larval cysts deposited in the muscles. We found that IgE accelerated the removal of worms from the intestine and reduced the viability of larval parasites in muscle (62). IgE-sufficient animals showed intense deposition of IgE around the necrotic cysts, findings that are consistent with the release of toxic granules from mast cells onto the parasites in response to IgE. T. spiralis infection drives a marked splenic mastocytosis and elevated serum levels of mouse mast cell protease-1 (MMCP-1), consistent with a systemic expansion of mast cells driven by the parasite. This mast cell increase was dramatically attenuated in IgE−/− mice, implicating IgE antibodies in mast cell homeostasis, a finding which would be borne out by our own group and by others both in subsequent studies on cultured mast cells and in investigations of mast cell responses in allergen challenged mice (see below). Protective roles for mast cells during T. spiralis infection have also been observed using mast cell deficient W/Wv mice and by antibody inhibition of c-Kit (61).
IgE effects on the expression of its receptors
Both the availability of IgE−/− mice and the introduction of the anti-IgE monoclonal antibody, omalizumab, into clinical use have shed light on previously unappreciated functions of IgE in positively regulating the expression of its own receptors, FcεRI and CD23. Most recently it has become evident that various facets of mast cell biology, including survival, activation, migration, adhesion, and production of mediators are also directly affected by IgE antibodies, even in the absence of antigen and hence referred to as antigen-independent effects of IgE (reviewed in 63,64).
IgE antibodies enhance expression of CD23
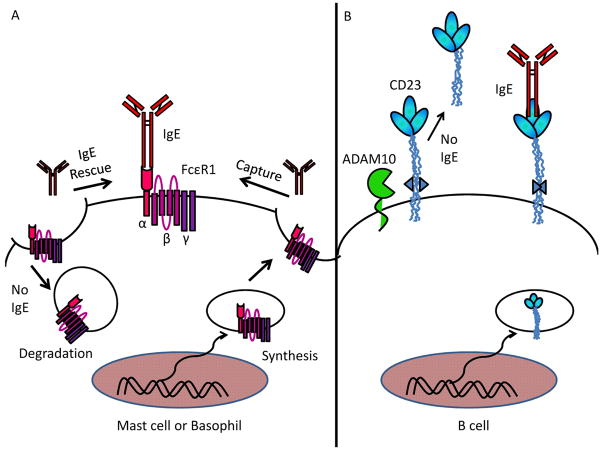
Studies on IgE-deficient mice and later investigations of the effects of IgE blockade with omalizumab in human subjects have revealed that IgE enhances the expression of both its high and low affinity receptors (Fig. 3). We first made this observation when flow cytometric analysis of B cells from IgE−/− mice indicated reduced surface expression of CD23 along with diminished IgE-binding capacity (65). CD23 protein expression was readily restored to wildtype levels by intravenous infusion of IgE (no antigen required). IgE−/− splenocytes responded normally to other stimuli (IL-4, CD40L) that upregulate CD23, however, and mRNA transcript levels for CD23 were equivalent in IgE−/− and wildtype mice, consistent with reports by Conrad and colleagues (66)that the presence of IgE does not alter CD23 transcription. Instead, the IgE bound form of CD23 was found to be protected from protease cleavage by ADAM-10 (a disintegrin and metalloproteinase-10), allowing it to remain attached to the cell surface (9, 66). This stabilization of CD23 by IgE likely has ramifications for facilitated antigen presentation, since preferential maintenance of IgE-CD23 complexes on the B-cell surface would increase the odds of IgE-mediated antigen uptake and could thus amplify allergic responses. On the other hand however, IgE inhibits on its own synthesis by interacting with surface-bound CD23, so IgE-mediated upregulation of CD23, while enhancing antigen uptake and presentation, might simultaneously result in greater negative feedback on IgE production.
Fig. 3. IgEregulation of its own receptors.
(A) Upregulation of FcεR1by IgE occurs through the prevention of receptor internalization and degradation, capture of internal pools of FcεR1 at the cell surface and continued basal transcription. (B) IgE binding to CD23 prevents ADAM10-mediated cleavage of CD23. Transcription of CD23 and FcεR1 is unaffected by IgE binding.
IgE regulates levels of FcεRI
We have similarly observed that IgE is a potent influence in maintaining the high density of FcεR1 normally present on mast cells and basophils. It has been appreciated for some time that IgE serum levels and FcεR1 expression are regulated in tandem inatopic patients, but it has not been possible to distinguish between correlations and cause (67–70). The first suggestion that IgE was directly responsible for enhancing FcεR1 expression came from two studies wherein a mast cell-like leukemia line upregulated FcεR1 when incubated with IgE in vitro (70, 71). We found that this mechanism is indeed operative in vivo at physiologic IgE levels. IgE−/− mice showed greatly reducedFcεR1 expression on both circulating basophils and tissue mast cells, and both cell types rapidly upregulated FcεR1 in response to IgE infusion (72, 73). The phenomenon could be reproduced ex vivo; freshly harvested mast cells or bone marrow-derived mast cells (BMMCs) exhibited markedly increased surface levels of FcεRI when cultured in the presence of IgE. Eventually these findings were extended to human mast cells, basophils, and dendritic cells when it was shown that the anti-IgE therapeutic omalizumab can reduce FcεR1 levels on each of these cell types (74–78). As was the case with CD23, this effect is antigen-independent. Binding of IgE to FcεR1 stabilizes the receptor, preventing internalization and protease degradation (79). As FcεR1 continues to be synthesized within a cell, the presence of IgE favors the capture and accumulation of FcεR1 at the cell surface (79).
Antigen-independent IgE effects on mast cells
Effects of monoclonal IgE antibodies on cultured mast cells
A major shift in understanding of the interplay between IgE and mast cells has occurred over the past 10years, as several groups, including ours, have found that IgE antibodies significantly affect mast cell proliferation and survival both in culture and in in vivo settings. In 2001, back-to-back papers by Asai et al. and Kalesnikoff et al. in Immunity (80, 81) reported the ability of IgE to enhance the survival of mast cells. This was a paradigm-shifting finding, suggesting a previously unappreciated function for IgE antibodies in regulating mast cell homeostasis and, importantly, showing that this function of IgE was completely independent of the presence of antigen. Both groups studied the growth of murine BMMCs, which can be generated by culture of bone marrow in the presence of IL-3 (and/or SCF). BMMCs undergo apoptosis if these cytokine growth factors are removed from their cultures and, importantly, are normally maintained in medium devoid of IgE. In these studies, the addition of IgE was shown to protect BMMCs from cell death induced by withdrawal of IL-3 (80, 81). Interestingly, this effect could not be replicated by IgG and was fully expressed in the absence of antigen. In fact, IgE effects were inhibited if specific antigen was introduced (80, 81). IgE alone was later shown to similarly increase the survival of human lung mast cells cultured ex vivo (and here the effect was attributed to induction of IL-6) (82).
The explanations offered by the two groups regarding mechanisms whereby IgE might promote mast cell survival, however, diverged greatly and remain elusive to this day. Asai and colleagues (80) found that oneIgE anti-DNP monoclonal antibody (clone DNP-ε-206) stimulated neither the usual mast cell signaling pathways that are activated by antigen:IgE:FcεRI nor production of any cytokines that might enhance survival. In contrast, Kalesnikoff et al. (81) provided evidence that a distinct anti-DNP IgE (SPE-7) stimulated cytokine secretion and that this was due to activation of the same MAPK cascades known to be induced following FcεR1 aggregation. In further contrast to the DNP-ε-206 clone, the SPE-7 antibody elevated expression of Bcl-XL, a mitochondrial protein that inhibits apoptosis, and prevented the DNA fragmentation associated with apoptosis (81). Follow up work showed a spectrum of activities for different IgE antibodies, with a general correlation between the ability to induce FcεR1 aggregation and replication of signaling events typically induced by classicalIgE and antigen crosslinking (63, 83). It is unclear why some IgE antibodies are more potent than others and how IgE aggregates FcεR1 in the absence of antigen. The exceptional density of FcεR1 on the surface of mast cells may allow low affinity interactions with alternative ligands or between glycosylated IgE molecules to exert influences in the setting of the high IgE concentrations used for these experiments. Interestingly, structure studies of the potent IgE SPE-7 have established the presence of several distinct physical conformations of its antigen binding site existing in equilibrium. Peptide display screening revealed that SPE-7 not only recognizes the DNP hapten but also binds to the self-antigen thioredoxin with low affinity, an observation that has raised the possibility that ‘antigen-independent’ effects of IgE antibodies may result, in fact, in some cases from auto reactivity conferred by alternative conformations in a highly amplified signaling system(84).
Differences in cytokine stimulation between various IgE clones have allowed researchers to determine that at least two anti-apoptotic pathways are operative in IgE-treated mast cells. ‘Cytokinergic’ IgE molecules(such as SPE-7)that stimulate production of significant quantities of cytokines (predominantly IL-6, TNFα, and IL-3) can promote mast cell survival through paracrine effects (64, 85). This pathway is IL-3 dependent (64, 85). Poorly cytokinergic IgEs only enhance the survival of mast cells that interact directly with the IgE molecule (and not co-cultured FcεRI−/− mast cells). The basis for this growth-enhancing effect is not understood but it is dependent on FcεR1 and signaling through Syk (64).
In addition to keeping mast cells alive, IgE acting in the absence of antigen has been shown to enhance multiple aspects of mast cell activation, promoting the acquisition of an inflammatory phenotype. In keeping with its anti-apoptotic effects, IgE enhances the survival of mast cell progenitors cultured in vitro and increases the rate at which they mature into mast cells (86). In mature mouse mast cells, IgE increases mast cell granularity and the synthesis of preformed mediators, including histamine, TNFα and MMCPs (86). Pretreatment with IgE primes mast cells to secrete IL-4 and enhances the release of IL-6 and serotonin when the cells are challenged with antigen (72). Importantly, prior exposure to IgE decreases the concentration of antigen required to trigger mast cell degranulation and markedly increases the release of mediators upon antigen challenge (72). The greater production of mediators may be explained in part by stabilization of surface FcεR1 by IgE, resulting in a larger pool of FcεR1 which in turn provides more intense signals for degranulation (72). Stimulation of eicosanoid and chemokine production by IgE also facilitates the upregulation of integrins and adhesion to fibronectin, which leads mast cells to migrate towards higher IgE concentrations and accumulate at sites of allergic inflammation (64, 87–89).
All together, these studies indicate that IgE acting in the absence of antigen can be an important growth and maturation factor for mast cells. By recruiting mast cells and decreasing their threshold for degranulation, IgE could theoretically amplify developing allergic responses in vivo (26, 86, 90, 91). The observations from cell culture systems raise the very important possibility that IgE blockade in human allergic diseases, which is now routinely practiced, might have effects extending far beyond inhibition of allergen driven mast cell activation via FcεRI:IgE. By putting a damper on diverse aspects of IgE-driven mast cell and mast cell progenitor functions, it is possible that anti-IgE therapy might have the potential to ‘reset’ the immune system in allergic patients as well as preventing acute episodes of immediate hypersensitivity (92).
Effects of polyclonal IgE, produced during active allergic responses, on mast cell homeostasis
While analyses of IgE effects on cultured mast cells have generated interesting insights regarding potential IgE functions, conclusions from such studies must be interpreted with caution. Experiments performed on highly enriched cultured cells, using single clones of monoclonal IgE antibodies, may not fully recapitulate the effects of polyclonal IgE on tissue mast cells duringphysiologic immune responses. Therefore we have recently begun to investigate the immunological effects of polyclonal IgE and the effects of IgE on mast cell responses during allergen challenge in vivo. To extend the concepts regarding antigen-independent effects of monoclonal IgE to polyclonal IgE generated during an active allergic response in vivo, we compared mast cell effects of serum from normal mice to serum from animals subjected to epicutaneous allergen exposure in a protocol that elicits allergic skin inflammation and high IgE responses. Atopic sera enhanced mast cell survival and IL-6 production by BMMCs, whereas the same sera depleted of IgE or control sera derived from IgE−/− mice had a reduced ability to do so. In the same study, IgE present in sera from atopic dermatitis patients was found to induce production of the chemokine IL-8 by human mast cells. Consistent with the data previously reported for the cytokinergic monoclonal IgE, SPE-7, polyclonal mouse IgE activated the phosphorylation of LAT, PLCγ, and p38 MAPK and was dependent on FcεR1 for it activity. Although IgE was clearly not the only factor in atopic serum capable of influencing mast cell biology, these findings provided a crucial demonstration that polyclonal IgE at physiologic concentrations has cytokine-inducing and anti-apoptotic effects on mast cells (93).
IgE effects on mast cell homeostasis during an active allergic response to inhaled mold allergen
In atopic disease and helminth infections, mast cell numbers and IgE levels arise in parallel creating an enhanced pool of sensitized effector cells of hypersensitivity (61, 62, 94–96). Our studies with T. spiralis infection provided evidence that IgE antibodies regulate mast cell homeostasis in vivo (62). This observation along with the knowledge that mast cell accumulation can occur in the airways and our observations of IgE effects on cultured mast cells prompted us to investigate the role of IgE in mast cell homeostasis in vivo following allergen inhalation.
We have shown that sensitization of mice with an extract of the fungus Aspergillus fumigatus (Af) induces vigorous IgE responses along with allergic airway inflammation and bronchial hyperresponsiveness (97–99). We recently reported that this protocol drives a strong mast cell expansion in the bronchus, trachea, and spleen, and that this effect is IgE dependent (95). Mast cells arise in situ in mucosal and connective tissues where they develop from blood borne progenitors. These progenitor cells can be identified by functional and flow cytometric assays. As expected we observed an accumulation of mast cell progenitors in the lungs following Af inhalation but the numbers of progenitors were similar in the presence or absence of IgE, suggesting that IgE exerts its influence on tissue mast cell homeostasis at the level of the mature mast cell. Consistent with this interpretation of the findings, wefound that survival of mature BMMCs injected into _Af_-treated IgE-sufficient mice was markedly enhanced compared to that of BMMCs transferred into IgE−/− mice. Furthermore, greater numbers of the BMMCs recovered from IgE−/− mice bound Annexin Vindicating ongoing apoptosis and suggesting that the IgE provides an important mast cell survival signal (95).
IgE antibody influence on dermal mast cell function and immune sensitization
Our investigations into the antigen-independent effects of IgE on immune responses took a twist when we made the completely unexpected observation that IgE−/− mice fail to mount contact sensitivity responses. Contact sensitivity is elicited when a sensitizing cutaneous application of a chemical hapten is followed several days later by reexposure to the same hapten at a distant skin type (usually the ear in mice). Challenged mice exhibit the gradual onset of skin swelling, peaking at 48–72 h. Contact sensitivity is a form of delayed type hypersensitivity, the type of immune response thought to be entirely T-cell driven and independent of effector mechanisms of immediate hypersensitivity. The rash of poison ivy, which arises after a sensitized individual is exposed to the plant, is an example of a contact sensitivity reaction in humans. Haptens, such as urushiol, the oily culprit in poison ivy and its relatives, are non-protein molecules that covalently bind to self-proteins, altering their epitopes so that when the haptenated proteins are presented on MHC molecules they activate T-cell responses (100).
Contact sensitivity responses can be divided into two arms: the sensitization phase in which dendritic cells bearing haptenated proteins migrate from the exposed skin to the draining lymph nodes to prime T cells, and the elicitation phase wherein a second application of the chemical hapten to the ear provokes a T-cell-driven inflammatory response, complete with local accumulation of granulocytes and lymphocytes, production of inflammatory cytokines and edema (100). The severity of contact sensitivity had been reported to correlate with hapten-specific and overall IgE levels (101), and some (but not all) reports suggested that mast cells could augment certain solvent and hapten protocols (102–105). We were therefore most interested to evaluate the contact sensitivity response of IgE−/− mice. Our findings provided the first strong in vivo evidence for antigen-independent effects of IgE in immune sensitization.
Contact sensitivity reactions were markedly impaired in IgE−/− mice but could be restored by treatment with IgE. Surprisingly, the specificity of the restoring IgE was irrelevant, and exogenous IgE only restored contact sensitivity if given before immune sensitization. These observations strongly suggested a role for IgE antibodies in facilitating immune sensitization rather than in the specific effector immune response giving rise to skin edema and cellular infiltration. Four different monoclonal IgEs were tested with equal success, but a control IgG was ineffective. It is quite likely that these findings are physiologically relevant because IgE concentrations reached normal levels for clean laboratory BALB/c mice within a few hours of intravenous injection (102). IgE titers in atopic humans can be far higher.
Splenocytes from IgE−/− mice treated with the sensitizer oxazolone were unable to transfer substantial reactivity into naive mice, whereas splenocytes from wildtype animals transferred inflammation equally well into naive IgE−/− and wildtype mice. These findings further strengthened our conclusions regardingrole for IgE in the sensitization phase. Both mast cells and FcεRI were critical for complete immune sensitization and elicitation of ear swelling, as evidenced by transfers using mast cell-deficient W/Wv and IgE receptor-deficient mice.
Hapten treatment upregulated the transcripts for multiple genes in the skin, including TNFα, IL-6, IL-1β, MCP-1 and MMCP-6, all of which were present at lower levels in IgE−/− mice but could be enhanced by IgE treatment. These cytokines and the protease MMCP-6 are known to be secreted by mast cells stimulated with IgE alone (64, 81, 86)and act on dendritic cells to enhance antigen presentation, adhesion, and migration into lymph nodes (104, 106–110). Local administration of IL-1β, IL-6, or MCP-1 prior to sensitization was sufficient to restore ear swelling in IgE−/− mice.
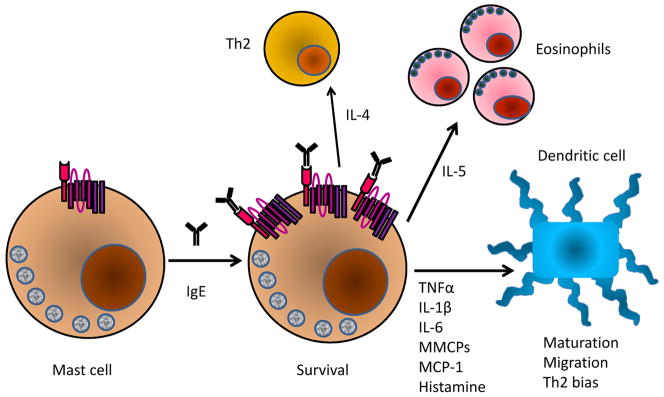
Dendritic cell emigration from skin to the draining lymph nodes is a crucial first step in the generation of immune responses to contact sensitizers. This dendritic cell emigration, which was readily observed in wildtype mice, was markedly impaired in the skin of hapten-exposed IgE−/− mice. All together, these findings suggested that basal levels of IgE antibodies modulate mast cell phenotype and cytokine secretion through antigen-independent effects in vivo, and that the physiologic steady-state release of mast cell factors provides important tonic signaling to other immune cells, particularly dendritic cells, and alters the initiation of immune responses (Fig. 4).
Fig. 4. IgE effects on mast cell function and immune responses.
Stimulation of mast cells with IgE enhances FcεR1 levels and the sensitivity to antigen. IgE triggers the secretion of IL-4, which enhances Th2 responses, IL-5, which drives development and recruitment eosinophils, as well as a variety of mediators that alter the maturation, migration, and phenotype of dendritic cells.
IgE modulation of immune responses to pulmonary chemical sensitizers Occupational asthma arises in individuals subjected tochronic inhalation of chemicals present in the workplace. The mechanisms of this disease are believed to mirror those of contact sensitivity so we were interested to assess whether the defect in cutaneous immune responses observed in IgE−/− mice would extend to pulmonary reactions. We adapted a mouse model in which repeated inhalation of a chemical hapten recreated multiple features typical of occupational asthma, including granulocytic infiltration, mucus production and bronchial hyperreactivity (111). All of these phenotypes were reduced in IgE−/− mice and could be corrected by restoration of IgE to physiologic levels prior to sensitization, an effect which was again shown to be independent of the specificity of the infused IgE antibodies. The administration of irrelevant IgE in this system did not completely reconstitute the degree of leukocyte lung infiltrate seen in wildtype mice, but did enhance the infiltration of lymphocytes, particularly NK cells, and eosinophils (111). Interestingly, serum levels of MMCP-1 were elevated in sensitized and challenged wildtype mice, presumably reflecting degranulation of mast cells during the elicitation of the chemical response. MMCP-1 levels remained low in IgE−/− mice except in those receiving IgE treatment, consistent with in vitro data showing protease upregulation by mast cells stimulated with IgE in the absence of antigen (89).
IgE and food allergy
The role of IgE antibodies in gut immune homeostasis and allergic sensitization Although less common than allergic rhinitis, atopic dermatitis, and asthma, the prevalence of food allergy is rising, and IgE-mediated food reaction scan be severe (112). The clinical observation that the presence of allergen-specific IgE can almost always be demonstrated in patients undergoing food anaphylaxis has implicated IgE antibodies as the key effectors. However, it is also clear that the presence of IgE antibodies is not sufficient to confer anaphylactic sensitivity. Some children with measurable levels of food-specific IgE can ingest those foods without incident. This observation suggests that additional effector mechanisms must be in place for the full expression of IgE-mediated food allergy. Recently, we have been able to develop a novel animal model in which to investigate this issue. Exposure of the gastrointestinal immune system to food allergens is regulated. In the healthy gut, tight junctions between epithelial cells lining the intestine prevent the transit of intact protein antigens. Proteins and other antigens can still be sampled by the extended dendrites of dendritic cells, M cells, or through uptake of antigen complexed with secreted IgA. These pathways lead to non-inflammatory presentation of antigen (113). Specialized intestinal dendritic cells secrete TGFβ and retinoic acid in order to polarize T cells toward a regulatory phenotype (114). Under some circumstances, the induction of allergic responses is favored in the intestinal mucosa. IgE synthesis by resident plasma cells can maintain a relatively high level of local IgEwhich, in turn, sustains elevated FcεR1 expression on mucosal mast cells (9, 115, 116). Moreover, in the allergic or inflamed gut, intestinal epithelial cells, and dendritic cells upregulate both high and low affinity IgE receptors (10, 117, 118). Mast cell precursors are enriched in the gut, but it is unclear whether local IgE production influences the recruitment, survival, and activation of intestinal mast cells, as we have found in other tissues (62, 91, 95).
When IgE is produced against food antigens, CD23-mediated transport of IgE-antigen complexes back through the cell mediates transfer of intact allergens into the serosa (119). Both isoforms of CD23 are present on intestinal epithelial cells, with CD23a expressed mainly on basolateral surfaces, while CD23b is restricted to the apical membrane (10). The different CD23 isoforms likely regulate the kinetics of free IgE transport to the lumen for antigen sampling and recapture for paracellular uptake of allergens to activate mast cells (10, 118, 120, 121).
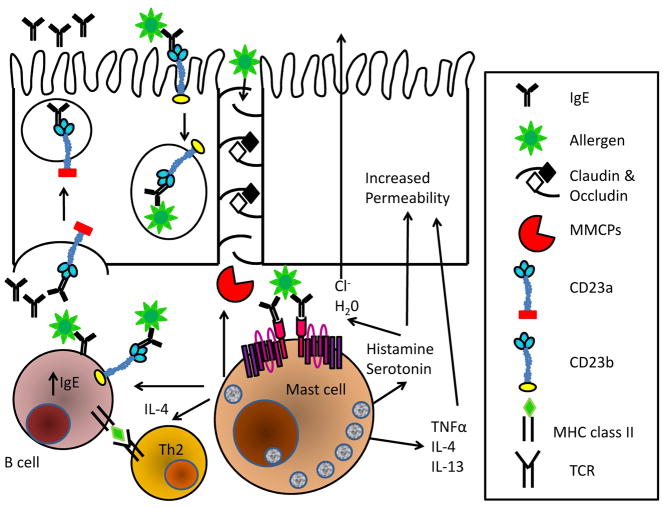
Activation of mucosal mast cells by IgE:antigen results in release of MMCPs that break down the claudin and occludin proteins forming the tight junctions, as well as inflammatory cytokines that alter ion transport and paracellular permeability in the intestinal epithelium (113, 122–125). Disruption of the intestinal epithelium contributes to the ‘leaky gut’ phenotype that has been suggested to contribute to the etiology of several disorders, including food allergy (113, 124). In the setting of impaired intestinal integrity, intact allergens can squeeze past degraded tight junctions raising the odds that allergic sensitization will occur (Fig. 5). The interaction between CD23 and IgE that so greatly enhances antibody production to the IgE-bound antigen also has the potential to drive strong responses to unrelated antigen encountered in the same context, sensitizing the immune system to other allergens via epitope spreading (9, 10).
Fig. 5. Actions of IgE in food allergic reactions.
IgE secreted by resident B cells is exported to the intestinal lumen via CD23 expressed on epithelial cells. Transcytosis of IgE:Ag viaCD23 facilitates capture of intact antigen to the mucosa where it can trigger the degranulation of mast cells. Released MMCPs digest the tight junction proteins, occuldin and claudin, thus permitting direct passage of intact allergens to the mucosa. Serotonin and histamine promote the secretion of chloride ions, drawing water into the lumen and giving rise to diarrhea. Mast cell cytokine secretion enhances intestinal permeability, drives T cells towards a Th2 phenotype and increases IgE synthesis. Antigen focusing by CD23:IgE dramatically enhances B and T-cell responses to allergens, potentiating responses on future allergen encounter and facilitating allergic epitope spreading.
Application of murine models to study mechanisms of food allergy The default immune response to ingested antigens in mice, like in humans, is tolerance induction. As a result, it has been difficult to recapitulate the syndrome of food anaphylaxis in mouse models based on enteral sensitization and challenge. Therefore, most murine investigations of food allergy rely parenteral sensitization (with adjuvant) prior to enteral challenge (126). Oral challenge in these models stimulates allergic diarrhea but fails to fully reproduce the features of systemic anaphylaxis, such as hypothermia, seen in severe human food allergy (112, 127, 128). Despite these limitations, however, such studies have provided some important insights. In parenterally sensitized mice, subsequent recurrent ingested exposure to food allergens drives an intestinal mastocytosis. This mast cell response is required for the expression of diarrhea following challenge. The cytokines IL-4 and, to a lesser extent, IL-13 drive intestinal mast cell expansion and activation and IgE production (128, 129), and transgenic intestinal overexpression of IL-9 is sufficient to promote allergic diarrhea, mastocytosis, and to disrupt intestinal integrity (130). Genetic deletion of mast cell chymases (MMCP-1, MMCP-4) or stabilization of mast cells with cromolyn sodium restores intestinal integrity and can prevent diarrhea occurrence (122, 123, 130).
Allergic predisposition in humans has been linked to genetic polymorphisms in the IL-4Rαchain (131, 132). We have recently characterized a line of knock-in mice, F709, that harbor an activating allele of IL-4Rα. These animals have heightened allergic responses as evidenced by IgE production, Th2 differentiation of antigen-stimulated T cells, and increased allergic airway inflammation and bronchial hyperresponsiveness in murine asthma models (133). Recently, we have established that these animals can be enterally sensitized to the model food allergen, ovalbumin, and that they display robust systemic anaphylaxis upon enteral challenge (96). The allergic response is characterized by enhanced serum levels of IgE, massive expansion of intestinal mast cells, diarrhea, mast cell degranulation, increased gut permeability, and systemic vasodilation leading to hypothermia. This is a unique murine model in that it represents the first system in which mice sensitized exclusively via enteral exposure to allergen display robust systemic anaphylaxis upon challenge. Food allergy reactions exhibited by F709 mice are absolutely IgE and FcεR1dependent. Bone marrow chimera experiments demonstrated a major role for the F709 genotype on hematopoietic cells but also revealed contributions of IL-4 signaling on non-hematopoietic cells. We anticipate that further studies using the F709 mice will improve our understanding of the etiology of food allergic disease. We intend to use this mouse model to further extend understanding of the role of IgE antibodies in allergic pathogenesis, particularly with respect to mast cell homeostasis, gut permeability, and active transepithelial food allergen transport.
Conclusion
Our evolving understandings of the diverse effects ofIgE antibodies on both the expression of its receptors and on the phenotype and survival of, mast cells, the critical effector cells of hypersensitivity, suggest that IgE is an optimal target in allergy treatment. In fact, the full gamut of IgE effects remains to be understood. Roles for activated mast cells, driven by IgE responses in chronic allergic diseases likely extend far beyond the instigation of occasional disease flares via immediate hypersensitivity mechanisms. Airway mast cells likely have critical functions in sustaining chronic inflammation via the synthesis of cytokines, such as IL-4, IL-5, and IL-13, which promote Th2 cell development, further IgE synthesis and the differentiation and recruitment of eosinophils (37, 134–137). Additional cell types might further amplify this positive feedback, particularly inatopic human subjects given the much broader expression of FcεR1. Facilitated antigen presentation occurring via CD23 could significantly impact B-cell production of IgE, and might promote the spreading of reactivity to other epitopes within given allergens or to unrelated antigens delivered in the same allergic context (9, 10). Anti-IgE therapy, beyond inhibiting IgE-mediated acute reactions following allergen exposure, might actually break some of these positive feedback loops and reset the immune system to a less atopicstate, perhaps even providing an environment permissive for allergen tolerance.
Acknowledgments
This work was supported by the National Institutes of Health grants NIAID R01AI054471 and R21AI087666. The authors declare no conflicts of interest.
References
- 1.Kubo S, Nakayama T, Matsuoka K, Yonekawa H, Karasuyama H. Long term maintenance of IgE-mediated memory in mast cells in the absence of detectable serum IgE. J Immunol. 2003;170:775–780. doi: 10.4049/jimmunol.170.2.775. [DOI] [PubMed] [Google Scholar]
- 2.Gould HJ, et al. The biology of IGE and the basis of allergic disease. Annual review of immunology. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 3.Stanworth DR. The discovery of IgE. Allergy. 1993;48:67–71. doi: 10.1111/j.1398-9995.1993.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 4.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 5.Lin S, Cicala C, Scharenberg AM, Kinet JP. The Fc(epsilon)RIbeta subunit functions as an amplifier of Fc(epsilon)RIgamma-mediated cell activation signals. Cell. 1996;85:985–995. doi: 10.1016/s0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- 6.Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet JP. Absence of Fc epsilonRI alpha chain results in upregulation of Fc gammaRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between Fc epsilonRI and Fc gammaRIII for limiting amounts of FcR beta and gamma chains. The Journal of clinical investigation. 1997;99:915–925. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grayson MH, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. The Journal of experimental medicine. 2007;204:2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Kleij H, et al. Evidence for neuronal expression of functional Fc (epsilon and gamma) receptors. The Journal of allergy and clinical immunology. 125:757–760. doi: 10.1016/j.jaci.2009.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nature reviews. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 10.Acharya M, et al. CD23/FcepsilonRII: molecular multi-tasking. Clinical and experimental immunology. 162:12–23. doi: 10.1111/j.1365-2249.2010.04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng LE, Wang ZE, Locksley RM. Murine B cells regulate serum IgE levels in a CD23-dependent manner. J Immunol. 185:5040–5047. doi: 10.4049/jimmunol.1001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aubry JP, Pochon S, Graber P, Jansen KU, Bonnefoy JY. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992;358:505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- 13.Kilmon MA, Ghirlando R, Strub MP, Beavil RL, Gould HJ, Conrad DH. Regulation of IgE production requires oligomerization of CD23. J Immunol. 2001;167:3139–3145. doi: 10.4049/jimmunol.167.6.3139. [DOI] [PubMed] [Google Scholar]
- 14.McCloskey N, et al. Soluble CD23 monomers inhibit and oligomers stimulate IGE synthesis in human B cells. The Journal of biological chemistry. 2007;282:24083–24091. doi: 10.1074/jbc.M703195200. [DOI] [PubMed] [Google Scholar]
- 15.Karagiannis SN, et al. Endocytosis and recycling of the complex between CD23 and HLA-DR in human B cells. Immunology. 2001;103:319–331. doi: 10.1046/j.1365-2567.2001.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores-Romo L, Johnson GD, Ghaderi AA, Stanworth DR, Veronesi A, Gordon J. Functional implication for the topographical relationship between MHC class II and the low-affinity IgE receptor: occupancy of CD23 prevents B lymphocytes from stimulating allogeneic mixed lymphocyte responses. European journal of immunology. 1990;20:2465–2469. doi: 10.1002/eji.1830201116. [DOI] [PubMed] [Google Scholar]
- 17.Hjelm F, Karlsson MC, Heyman B. A novel B cell-mediated transport of IgE-immune complexes to the follicle of the spleen. J Immunol. 2008;180:6604–6610. doi: 10.4049/jimmunol.180.10.6604. [DOI] [PubMed] [Google Scholar]
- 18.Getahun A, Hjelm F, Heyman B. IgE enhances antibody and T cell responses in vivo via CD23+ B cells. J Immunol. 2005;175:1473–1482. doi: 10.4049/jimmunol.175.3.1473. [DOI] [PubMed] [Google Scholar]
- 19.Takizawa F, Adamczewski M, Kinet JP. Identification of the low affinity receptor for immunoglobulin E on mouse mast cells and macrophages as Fc gamma RII and Fc gamma RIII. The Journal of experimental medicine. 1992;176:469–475. doi: 10.1084/jem.176.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano M, et al. IgEb immune complexes activate macrophages through FcgammaRIV binding. Nature immunology. 2007;8:762–771. doi: 10.1038/ni1477. [DOI] [PubMed] [Google Scholar]
- 21.Mancardi DA, Iannascoli B, Hoos S, England P, Daeron M, Bruhns P. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. The Journal of clinical investigation. 2008;118:3738–3750. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blink SE, Fu YX. IgE regulates T helper cell differentiation through FcgammaRIII mediated dendritic cell cytokine modulation. Cellular immunology. 264:54–60. doi: 10.1016/j.cellimm.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frigeri LG, Liu FT. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J Immunol. 1992;148:861–867. [PubMed] [Google Scholar]
- 24.Frigeri LG, Zuberi RI, Liu FT. Epsilon BP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (Fc epsilon RI) and activates mast cells. Biochemistry. 1993;32:7644–7649. doi: 10.1021/bi00081a007. [DOI] [PubMed] [Google Scholar]
- 25.Shampain MP, Behrens BL, Larsen GL, Henson PM. An animal model of late pulmonary responses to Alternaria challenge. The American review of respiratory disease. 1982;126:493–498. doi: 10.1164/arrd.1982.126.3.493. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nature reviews. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 27.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. The Journal of allergy and clinical immunology. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. quiz 647–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunological reviews. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castle JD, Guo Z, Liu L. Function of the t-SNARE SNAP-23 and secretory carrier membrane proteins (SCAMPs) in exocytosis in mast cells. Molecular immunology. 2002;38:1337–1340. doi: 10.1016/s0161-5890(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 30.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. The Journal of allergy and clinical immunology. 125:S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. The Journal of allergy and clinical immunology. 2002;110:341–348. doi: 10.1067/mai.2002.126811. [DOI] [PubMed] [Google Scholar]
- 32.Makabe-Kobayashi Y, et al. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. The Journal of allergy and clinical immunology. 2002;110:298–303. doi: 10.1067/mai.2002.125977. [DOI] [PubMed] [Google Scholar]
- 33.Burd PR, Thompson WC, Max EE, Mills FC. Activated mast cells produce interleukin 13. The Journal of experimental medicine. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PloS one. 5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda K, et al. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–3596. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 36.Rothenberg ME, Hogan SP. The eosinophil. Annual review of immunology. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 37.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. The Journal of clinical investigation. 1997;99:1492–1499. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shelburne CP, Ryan JJ. The role of Th2 cytokines in mast cell homeostasis. Immunological reviews. 2001;179:82–93. doi: 10.1034/j.1600-065x.2001.790109.x. [DOI] [PubMed] [Google Scholar]
- 39.Feuser K, Feilhauer K, Staib L, Bischoff SC, Lorentz A. Akt cross-links IL-4 priming, stem cell factor signaling, and IgE-dependent activation in mature human mast cells. Molecular immunology. 48:546–552. doi: 10.1016/j.molimm.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Lorentz A, Schwengberg S, Sellge G, Manns MP, Bischoff SC. Human intestinal mast cells are capable of producing different cytokine profiles: role of IgE receptor cross-linking and IL-4. J Immunol. 2000;164:43–48. doi: 10.4049/jimmunol.164.1.43. [DOI] [PubMed] [Google Scholar]
- 41.Lorentz A, et al. IL-4-induced priming of human intestinal mast cells for enhanced survival and Th2 cytokine generation is reversible and associated with increased activity of ERK1/2 and c-Fos. J Immunol. 2005;174:6751–6756. doi: 10.4049/jimmunol.174.11.6751. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi M, et al. IgE enhances Fc epsilon receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fc epsilon receptor I expression and mediator release. J Immunol. 1999;162:5455–5465. [PubMed] [Google Scholar]
- 43.Nussenzweig RS, Merryman C, Benacerraf B. Electrophoretic Separation and Properties of Mouse Antihapten Antibodies Involved in Passive Cutaneous Anaphylaxis and Passive Hemolysis. The Journal of experimental medicine. 1964;120:315–328. doi: 10.1084/jem.120.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE-or IgG1-dependent passive anaphylaxis. The Journal of clinical investigation. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkelman FD. Anaphylaxis: lessons from mouse models. The Journal of allergy and clinical immunology. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516–507. [DOI] [PubMed] [Google Scholar]
- 46.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. The Journal of allergy and clinical immunology. 2003;112:1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 47.Schafer T, et al. Epidemiology of food allergy/food intolerance in adults: associations with other manifestations of atopy. Allergy. 2001;56:1172–1179. doi: 10.1034/j.1398-9995.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 48.Aspock H, Auer H, Picher O. Trichuris trichiura eggs in the Neolithic glacier mummy from the Alps. Parasitology Today. 1996;12:255–256. [Google Scholar]
- 49.Gounni AS, et al. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 50.Joseph M, Auriault C, Capron A, Vorng H, Viens P. A new function for platelets: IgE-dependent killing of schistosomes. Nature. 1983;303:810–812. doi: 10.1038/303810a0. [DOI] [PubMed] [Google Scholar]
- 51.Capron M, Capron A. Immunoglobulin E and effector cells in schistosomiasis. Science (New York, NY) 1994;264:1876–1877. doi: 10.1126/science.8009216. [DOI] [PubMed] [Google Scholar]
- 52.Pinot de Moira A, Fulford AJ, Kabatereine NB, Ouma JH, Booth M, Dunne DW. Analysis of complex patterns of human exposure and immunity to Schistosomiasis mansoni: the influence of age, sex, ethnicity and IgE. PLoS neglected tropical diseases. :4. doi: 10.1371/journal.pntd.0000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunne DW, Butterworth AE, Fulford AJ, Ouma JH, Sturrock RF. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Memorias do Instituto Oswaldo Cruz. 1992;87 (Suppl 4):99–103. doi: 10.1590/s0074-02761992000800014. [DOI] [PubMed] [Google Scholar]
- 54.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annual review of immunology. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 55.King CL, Xianli J, Malhotra I, Liu S, Mahmoud AA, Oettgen HC. Mice with a targeted deletion of the IgE gene have increased worm burdens and reduced granulomatous inflammation following primary infection with Schistosoma mansoni. J Immunol. 1997;158:294–300. [PubMed] [Google Scholar]
- 56.Jankovic D, et al. Fc epsilonRI-deficient mice infected with Schistosoma mansoni mount normal Th2-type responses while displaying enhanced liver pathology. J Immunol. 1997;159:1868–1875. [PubMed] [Google Scholar]
- 57.Amiri P, Haak-Frendscho M, Robbins K, McKerrow JH, Stewart T, Jardieu P. Anti-immunoglobulin E treatment decreases worm burden and egg production in Schistosoma mansoni-infected normal and interferon gamma knockout mice. The Journal of experimental medicine. 1994;180:43–51. doi: 10.1084/jem.180.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El Ridi R, Ozaki T, Kamiya H. Schistosoma mansoni infection in IgE-producing and IgE-deficient mice. The Journal of parasitology. 1998;84:171–174. [PubMed] [Google Scholar]
- 59.El Ridi R, Ragab S, Lewis S, Afifi A. Role of IgE in primary murine Schistosomiasis mansoni. Scandinavian journal of immunology. 2001;53:24–31. doi: 10.1046/j.1365-3083.2001.00835.x. [DOI] [PubMed] [Google Scholar]
- 60.Delgado V, McLaren DJ. Evidence for enhancement of IgG1 subclass expression in mice polyvaccinated with radiation-attenuated cercariae of Schistosoma mansoni and the role of this isotype in serum-transferred immunity. Parasite immunology. 1990;12:15–32. doi: 10.1111/j.1365-3024.1990.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe N, Bruschi F, Korenaga M. IgE: a question of protective immunity in Trichinella spiralis infection. Trends in parasitology. 2005;21:175–178. doi: 10.1016/j.pt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 62.Gurish MF, et al. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139–1145. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 63.Bryce PJ, Oettgen HC. Antigen-independent effects of immunoglobulin E. Current allergy and asthma reports. 2005;5:186–190. doi: 10.1007/s11882-005-0036-6. [DOI] [PubMed] [Google Scholar]
- 64.Kawakami T, Kitaura J. Mast cell survival and activation by IgE in the absence of antigen: a consideration of the biologic mechanisms and relevance. J Immunol. 2005;175:4167–4173. doi: 10.4049/jimmunol.175.7.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kisselgof AB, Oettgen HC. The expression of murine B cell CD23, in vivo, is regulated by its ligand, IgE. International immunology. 1998;10:1377–1384. doi: 10.1093/intimm/10.9.1377. [DOI] [PubMed] [Google Scholar]
- 66.Lee WT, Rao M, Conrad DH. The murine lymphocyte receptor for IgE. IV. The mechanism of ligand-specific receptor upregulation on B cells. J Immunol. 1987;139:1191–1198. [PubMed] [Google Scholar]
- 67.Saini SS, Klion AD, Holland SM, Hamilton RG, Bochner BS, Macglashan DW., Jr The relationship between serum IgE and surface levels of FcepsilonR on human leukocytes in various diseases: correlation of expression with FcepsilonRI on basophils but not on monocytes or eosinophils. The Journal of allergy and clinical immunology. 2000;106:514–520. doi: 10.1067/mai.2000.108431. [DOI] [PubMed] [Google Scholar]
- 68.Malveaux FJ, Conroy MC, Adkinson NF, Jr, Lichtenstein LM. IgE receptors on human basophils. Relationship to serum IgE concentration. The Journal of clinical investigation. 1978;62:176–181. doi: 10.1172/JCI109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sihra BS, Kon OM, Grant JA, Kay AB. Expression of high-affinity IgE receptors (Fc epsilon RI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: relationship to total serum IgE concentrations. The Journal of allergy and clinical immunology. 1997;99:699–706. doi: 10.1016/s0091-6749(97)70033-2. [DOI] [PubMed] [Google Scholar]
- 70.Furuichi K, Rivera J, Triche T, Isersky C. The fate of IgE bound to rat basophilic leukemia cells. IV. Functional association between the receptors for IgE. J Immunol. 1985;134:1766–1773. [PubMed] [Google Scholar]
- 71.Quarto R, Kinet JP, Metzger H. Coordinate synthesis and degradation of the alpha-, beta-and gamma-subunits of the receptor for immunoglobulin E. Molecular immunology. 1985;22:1045–1051. doi: 10.1016/0161-5890(85)90107-5. [DOI] [PubMed] [Google Scholar]
- 72.Yamaguchi M, et al. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. The Journal of experimental medicine. 1997;185:663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lantz CS, et al. IgE regulates mouse basophil Fc epsilon RI expression in vivo. J Immunol. 1997;158:2517–2521. [PubMed] [Google Scholar]
- 74.MacGlashan DW, Jr, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 75.MacGlashan D, Jr, et al. In vitro regulation of FcepsilonRIalpha expression on human basophils by IgE antibody. Blood. 1998;91:1633–1643. [PubMed] [Google Scholar]
- 76.Saini SS, et al. Down-regulation of human basophil IgE and FC epsilon RI alpha surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]
- 77.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. The Journal of allergy and clinical immunology. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 78.Prussin C, Metcalfe DD. 4. IgE, mast cells, basophils, and eosinophils. The Journal of allergy and clinical immunology. 2003;111:S486–494. doi: 10.1067/mai.2003.120. [DOI] [PubMed] [Google Scholar]
- 79.Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol. 2001;167:1290–1296. doi: 10.4049/jimmunol.167.3.1290. [DOI] [PubMed] [Google Scholar]
- 80.Asai K, et al. Regulation of mast cell survival by IgE. Immunity. 2001;14:791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 81.Kalesnikoff J, et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 82.Cruse G, Cockerill S, Bradding P. IgE alone promotes human lung mast cell survival through the autocrine production of IL-6. BMC immunology. 2008;9:2. doi: 10.1186/1471-2172-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kitaura J, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science (New York, NY) 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 85.Kohno M, Yamasaki S, Tybulewicz VL, Saito T. Rapid and large amount of autocrine IL-3 production is responsible for mast cell survival by IgE in the absence of antigen. Blood. 2005;105:2059–2065. doi: 10.1182/blood-2004-07-2639. [DOI] [PubMed] [Google Scholar]
- 86.Kashiwakura J, et al. Pivotal advance: IgE accelerates in vitro development of mast cells and modifies their phenotype. Journal of leukocyte biology. 2008;84:357–367. doi: 10.1189/jlb.1207841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kitaura J, et al. Regulation of highly cytokinergic IgE-inducedmast cell adhesion by Src, Syk, Tec, and protein kinase C family kinases. J Immunol. 2005;174:4495–4504. doi: 10.4049/jimmunol.174.8.4495. [DOI] [PubMed] [Google Scholar]
- 88.Kitaura J, et al. IgE-and IgE+Ag-mediated mast cell migration in an autocrine/paracrine fashion. Blood. 2005;105:3222–3229. doi: 10.1182/blood-2004-11-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lam V, et al. IgE alone stimulates mast cell adhesion to fibronectin via pathways similar to those used by IgE + antigen but distinct from those used by Steel factor. Blood. 2003;102:1405–1413. doi: 10.1182/blood-2002-10-3176. [DOI] [PubMed] [Google Scholar]
- 90.Jones TG, et al. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. J Immunol. 2009;183:5251–5260. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gurish MF, Boyce JA. Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell. The Journal of allergy and clinical immunology. 2006;117:1285–1291. doi: 10.1016/j.jaci.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 92.Fried AJ, Oettgen HC. Anti-IgE in the treatment of allergic disorders in pediatrics. Current opinion in pediatrics. doi: 10.1097/MOP.0b013e3283404201. [DOI] [PubMed] [Google Scholar]
- 93.Kashiwakura J, et al. Polyclonal IgE induces mast cell survival and cytokine production. Allergol Int. 2009;58:411–419. doi: 10.2332/allergolint.08-OA-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harari Y, Russell DA, Castro GA. Anaphylaxis-mediated epithelial Cl-secretion and parasite rejection in rat intestine. J Immunol. 1987;138:1250–1255. [PubMed] [Google Scholar]
- 95.Mathias CB, et al. IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation. J Immunol. 2009;182:2416–2424. doi: 10.4049/jimmunol.0801569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathias CB, et al. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. The Journal of allergy and clinical immunology. doi: 10.1016/j.jaci.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van de Rijn M, Mehlhop PD, Judkins A, Rothenberg ME, Luster AD, Oettgen HC. A murine model of allergic rhinitis: studies on the role of IgE in pathogenesis and analysis of the eosinophil influx elicited by allergen and eotaxin. The Journal of allergy and clinical immunology. 1998;102:65–74. doi: 10.1016/s0091-6749(98)70056-9. [DOI] [PubMed] [Google Scholar]
- 98.Mehlhop PD, et al. CD40L, but not CD40, is required for allergen-induced bronchial hyperresponsiveness in mice. American journal of respiratory cell and molecular biology. 2000;23:646–651. doi: 10.1165/ajrcmb.23.5.3954. [DOI] [PubMed] [Google Scholar]
- 99.Mehlhop PD, et al. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. The Journal of experimental medicine. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagai H, Inagaki N, Tanaka H. Role of IgE for the onset of allergic cutaneous response caused by simple chemical hapten in mice. International archives of allergy and immunology. 1999;118:285–286. doi: 10.1159/000024101. [DOI] [PubMed] [Google Scholar]
- 102.Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE on mast cells. Novartis Foundation symposium. 2005;271:15–24. discussion 24–38, 95-19. [PubMed] [Google Scholar]
- 103.Askenase PW, et al. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J Immunol. 1983;131:2687–2694. [PubMed] [Google Scholar]
- 104.Biedermann T, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. The Journal of experimental medicine. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mekori YA, Galli SJ. Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. J Immunol. 1985;135:879–885. [PubMed] [Google Scholar]
- 106.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cell migration in mice requires intact type I interleukin 1 receptor (IL-1RI) function. Archives of dermatological research. 1999;291:357–361. doi: 10.1007/s004030050422. [DOI] [PubMed] [Google Scholar]
- 107.Cumberbatch M, Griffiths CE, Tucker SC, Dearman RJ, Kimber I. Tumour necrosis factor-alpha induces Langerhans cell migration in humans. The British journal of dermatology. 1999;141:192–200. doi: 10.1046/j.1365-2133.1999.02964.x. [DOI] [PubMed] [Google Scholar]
- 108.Gordon JR. Monocyte chemoattractant peptide-1 expression during cutaneous allergic reactions in mice is mast cell dependent and largely mediates the monocyte recruitment response. The Journal of allergy and clinical immunology. 2000;106:110–116. doi: 10.1067/mai.2000.107036. [DOI] [PubMed] [Google Scholar]
- 109.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 110.Wang B, et al. Depressed Langerhans cell migration and reduced contact hypersensitivity response in mice lacking TNF receptor p75. J Immunol. 1997;159:6148–6155. [PubMed] [Google Scholar]
- 111.Mathias CB, Freyschmidt EJ, Oettgen HC. Immunoglobulin E antibodies enhance pulmonary inflammation induced by inhalation of a chemical hapten. Clin Exp Allergy. 2009;39:417–425. doi: 10.1111/j.1365-2222.2008.03140.x. [DOI] [PubMed] [Google Scholar]
- 112.Allen KJ, Martin PE. Clinical aspects of pediatric food allergy and failed oral immune tolerance. Journal of clinical gastroenterology. 44:391–401. doi: 10.1097/MCG.0b013e3181d7760b. [DOI] [PubMed] [Google Scholar]
- 113.Perrier C, Corthesy B. Gut permeability and food allergies. Clin Exp Allergy. 41:20–28. doi: 10.1111/j.1365-2222.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 114.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smurthwaite L, et al. Persistent IgE synthesis in the nasal mucosa of hay fever patients. European journal of immunology. 2001;31:3422–3431. doi: 10.1002/1521-4141(200112)31:12<3422::aid-immu3422>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 116.Negrao-Correa D, Adams LS, Bell RG. Intestinal transport and catabolism of IgE: a major blood-independent pathway of IgE dissemination during a Trichinella spiralis infection of rats. J Immunol. 1996;157:4037–4044. [PubMed] [Google Scholar]
- 117.Untersmayr E, et al. The high affinity IgE receptor Fc epsilonRI is expressed by human intestinal epithelial cells. PloS one. 5:e9023. doi: 10.1371/journal.pone.0009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu LC, Montagnac G, Yang PC, Conrad DH, Benmerah A, Perdue MH. Intestinal epithelial CD23 mediates enhanced antigen transport in allergy: evidence for novel splice forms. American journal of physiology. 2003;285:G223–234. doi: 10.1152/ajpgi.00445.2002. [DOI] [PubMed] [Google Scholar]
- 119.Yang PC, Berin MC, Yu LC, Conrad DH, Perdue MH. Enhanced intestinal transepithelial antigen transport in allergic rats is mediated by IgE and CD23 (FcepsilonRII) The Journal of clinical investigation. 2000;106:879–886. doi: 10.1172/JCI9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tu Y, et al. CD23-mediated IgE transport across human intestinal epithelium: inhibition by blocking sites of translation or binding. Gastroenterology. 2005;129:928–940. doi: 10.1053/j.gastro.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 121.Berin MC, Li H, Sperber K. Antibody-mediated antigen sampling across intestinal epithelial barriers. Annals of the New York Academy of Sciences. 2006;1072:253–261. doi: 10.1196/annals.1326.002. [DOI] [PubMed] [Google Scholar]
- 122.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Groschwitz KR, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:22381–22386. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. The Journal of allergy and clinical immunology. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ceponis PJ, Botelho F, Richards CD, McKay DM. Interleukins 4 and 13 increase intestinal epithelial permeability by a phosphatidylinositol 3-kinase pathway. Lack of evidence for STAT 6 involvement. The Journal of biological chemistry. 2000;275:29132–29137. doi: 10.1074/jbc.M003516200. [DOI] [PubMed] [Google Scholar]
- 126.Berin MC, Mayer L. Immunophysiology of experimental food allergy. Mucosal immunology. 2009;2:24–32. doi: 10.1038/mi.2008.72. [DOI] [PubMed] [Google Scholar]
- 127.Brandt EB, et al. Mast cells are required for experimental oral allergen-induced diarrhea. The Journal of clinical investigation. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang M, et al. Peanut-induced intestinal allergy is mediated through a mast cell-IgE-FcepsilonRI-IL-13 pathway. The Journal of allergy and clinical immunology. 26:306–316. 316, e301–312. doi: 10.1016/j.jaci.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brandt EB, et al. Targeting IL-4/IL-13 signaling to alleviate oral allergen-induced diarrhea. The Journal of allergy and clinical immunology. 2009;123:53–58. doi: 10.1016/j.jaci.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Forbes EE, et al. IL-9-and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. The Journal of experimental medicine. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ober C, et al. Variation in the interleukin 4-receptor alpha gene confers susceptibility to asthma and atopy in ethnically diverse populations. American journal of human genetics. 2000;66:517–526. doi: 10.1086/302781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. The New England journal of medicine. 1997;337:1720–1725. doi: 10.1056/NEJM199712113372403. [DOI] [PubMed] [Google Scholar]
- 133.Tachdjian R, et al. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. The Journal of allergy and clinical immunology. 125:1128–1136. doi: 10.1016/j.jaci.2010.01.054. e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kung TT, et al. Mast cells modulate allergic pulmonary eosinophilia in mice. American journal of respiratory cell and molecular biology. 1995;12:404–409. doi: 10.1165/ajrcmb.12.4.7695919. [DOI] [PubMed] [Google Scholar]
- 135.Kobayashi T, et al. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. J Immunol. 2000;164:3855–3861. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- 136.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. The Journal of experimental medicine. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kitawaki T, et al. IgE-activated mast cells in combination with pro-inflammatory factors induce Th2-promoting dendritic cells. International immunology. 2006;18:1789–1799. doi: 10.1093/intimm/dxl113. [DOI] [PubMed] [Google Scholar]