Emi1 regulates the anaphase-promoting complex by a different mechanism than Mad2 proteins (original) (raw)
Abstract
The anaphase-promoting complex/cyclosome (APC) ubiquitin ligase is activated by Cdc20 and Cdh1 and inhibited by Mad2 and the spindle assembly checkpoint complex, Mad2B, and the early mitotic inhibitor Emi1. Mad2 inhibits APCCdc20, whereas Mad2B preferentially inhibits APCCdh1. We have examined the mechanism of APC inhibition by Emi1 and find that unlike Mad2 proteins, Emi1 binds and inhibits both APCCdh1 and APCCdc20. Also unlike Mad2, Emi1 stabilizes cyclin A in the embryo and requires zinc for its APC inhibitory activity. We find that Emi1 binds the substrate-binding region of Cdc20 and prevents substrate binding to the APC, illustrating a novel mechanism of APC inhibition.
Keywords: Emi1, Cdc20, Cdh1, APC, Mad2, mitosis
The anaphase-promoting complex/cyclosome (APC) is a ubiquitin ligase that controls mitotic progression by ubiquitylating key mitotic regulators, including the anaphase inhibitor securin and the mitotic cyclins A and B, targeting them for destruction by the 26S proteasome (for review, see Page and Hieter 1999; Zachariae and Nasmyth 1999). The APC is present throughout the cell cycle, but selective binding of the activator proteins Cdc20 or Cdh1 results in a peak of APCCdc20 activity in mitosis and APCCdh1 activity in late mitosis and G1 (Sigrist and Lehner 1997; Visintin et al. 1997; Fang et al. 1998b; Kramer et al. 1998, 2000; Lorca et al. 1998; Prinz et al. 1998; Zachariae et al. 1998).
Cdc20 and Cdh1 target for ubiquitylation proteins containing a destruction box motif (D-box; Glotzer et al. 1991). Cdh1 also recognizes proteins with a KEN-box motif (Pfleger and Kirschner 2000). APC substrates were recently found to bind and be recruited directly to the APC by Cdc20/Cdh1 in a D-box- and KEN-box-dependent manner (Burton and Solomon 2001; Hilioti et al. 2001; Pfleger et al. 2001a).
APC substrate destruction is temporally regulated: cyclin A in prometaphase, securin at metaphase-anaphase, and the mitotic polo-like kinase upon mitotic exit (Cohen-Fix et al. 1996; Shirayama et al. 1998; den Elzen and Pines 2001; Geley et al. 2001). Tight regulation of APC activity ensures the sequential destruction of APC substrates and the correct timing of mitotic events. We recently identified the APC inhibitor Emi1, which binds Cdc20 to inhibit premature APC activation in mitosis (Reimann et al. 2001). In Xenopus embryos, Emi1 is required for cyclin B accumulation and mitotic entry and Emi1 destruction is required for mitotic exit.
APCCdc20 activity is also regulated by the spindle assembly checkpoint (SC), a pathway that delays sister chromatid separation until chromosome alignment at metaphase (for review, see Shah and Cleveland 2000). The SC protein Mad2 acts at unattached kinetochores in prometaphase to inhibit the APC until chromosome alignment, and is activated following spindle damage. Mad2 binds and inhibits Cdc20 in vitro (Fang et al. 1998a; Hwang et al. 1998; Kallio et al. 1998; Kim et al. 1998). BubR1, another SC component, also forms a complex with Cdc20 and inhibits APC activation by Cdc20 in vitro (Sudakin et al. 2001; Tang et al. 2001). The Mad2-like protein Mad2B was recently identified as an APCCdh1 inhibitor in vitro and in vivo (Chen and Fang 2001; Pfleger et al. 2001b). Mad2 and Mad2B have been proposed to inhibit APC activity by inhibiting substrate release from APCCdc20 and APCCdh1, respectively (Pfleger et al. 2001b).
To understand how Emi1 regulates APC activity, we investigated its APC inhibitory activity in several different assays. We find that Emi1 inhibits Cdh1–APC as well as Cdc20–APC activation, acting more broadly than either Mad2 or Mad2B. Unlike Mad2 or Mad2B, Emi1 can inhibit APC already activated by Cdc20 or Cdh1. Emi1 binds the Cdc20 N terminus in the substrate-binding region, and directly inhibits substrate binding to Cdc20, potentially explaining its mechanism of APC inhibition.
Results
Emi1 binds Cdh1 and inhibits APCCdh1 activity
Studies of the likely Drosophila homolog of Emi1, Regulator of cyclin A (Rca1), show that Rca1 overexpression in G1 cells stabilizes cyclin A (Dong et al. 1997). Cdh1 activates the APC to ubiquitylate cyclin A and other G1 substrates (for review, see Zachariae and Nasmyth 1999). Because Emi1 binds and inhibits Cdc20, we considered whether Emi1 also inhibits the related protein Cdh1. Baculovirus-expressed Emi1 and Cdh1 coimmunoprecipitated from insect cell lysate and 35S-labeled Cdh1 precipitated with GST–Emi1 protein (Fig. 1A). Human Emi1 and Cdh1 also form a complex in vivo (J. Hsu, J. Reimann, C. Sorensen, J. Lukas, and P. Jackson, in prep.).
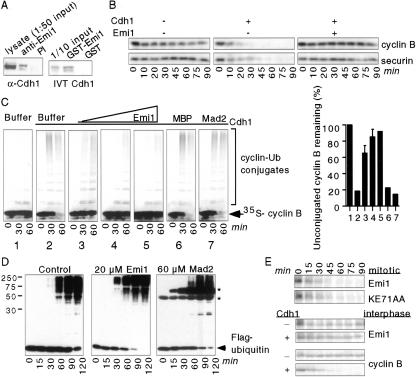
Figure 1.
Emi1 binds Cdh1 and inhibits APCCdh1 activity. (A) Emi1 interacts with Cdh1. SF9 cells were coinfected with baculoviruses expressing Emi1 and Cdh1, lysed, and lysates precipitated with preimmune (PI) or anti-Emi1 antisera and analyzed for Cdh1 by immunoblotting (left). GST–Emi1 or GST was incubated with 35S-labeled in vitro translated (IVT) Cdh1, bound to glutathione agarose, and analyzed by SDS-PAGE and autoradiography (right). (We typically observe ∼20–30% of input 35S-labeled Cdh1 precipitating with GST–Emi1.) (B) Emi1 inhibits APCCdh1 activity in Xenopus egg extracts. 35S-labeled IVT N terminus _X_cyclin B or _X_securin was incubated in Xenopus interphase extracts treated with buffer, buffer + IVT Cdh1, or IVT Cdh1 plus MBP–Emi1 (1 μM). Aliquots were removed at the indicated times and analyzed by SDS-PAGE and autoradiography. (C) Emi1, but not Mad2, inhibits APCCdh1-dependent activation in vitro. IVT Cdh1 (panels 2_–_7) or rabbit reticulocyte lysate (1) was incubated with buffer (1 and 2), MBP–Emi1 (3, 1 μM; 4, 3 μM; 5, 10 μM), 10 μM MBP (6), or 80 μM GST–Mad2 (7). APC immunopurified from interphase egg extracts was then incubated with the Cdh1/protein mixtures. The ability of treated APC to ubiquitylate 35S-labeled N-terminal cyclin B fragment was assayed. The percentage of cyclin B remaining unconjugated to ubiquitin after 60 min was quantitated on a PhosphorImager (graph). (D) Neither Emi1 nor Mad2 inhibits the substrate-independent reaction of the APC. Baculovirus-expressed and purified APC2/APC11 was incubated with 20 μM MBP (control), 20 μM MBP–Emi1, or 60 μM GST–Mad2 in the presence of E1, E2, ATP, and Flag-tagged ubiquitin. Aliquots were taken at the indicated times and analyzed for the formation of polyubiquitin chains by immunoblotting with αFlag antibodies. (*, GST–Mad2; αFlag antibody cross-reacts with GST, which is ubiquitylated in this assay.) (E) Emi1 destruction is not mediated by APCCdh1. 35S-labeled IVT wild-type Emi1, KE71AA mutant (substitution of K 71 and E 72 with alanines), or N terminus cyclin B fragment was added to mitotic extracts, interphase extracts, or Cdh1-supplemented interphase extracts. Aliquots were removed at the indicated times and analyzed for substrate destruction by SDS-PAGE and autoradiography.
Next, we tested whether Emi1 inhibits APCCdh1 activity in Xenopus egg extracts. Radiolabeled in vitro translated (IVT) cyclin B and securin are stable in interphase extracts, where the APC is inactive (Fig. 1B). Addition of IVT Cdh1 to these extracts activated the APC for cyclin B and securin destruction. Emi1 addition to these Cdh1-supplemented extracts stabilized cyclin B and securin (Fig. 1B). Emi1 also inhibited Cdh1 activation of APC immunopurified from interphase extracts in a dose-dependent manner (Fig. 1C). Mad2, which does not interact with Cdh1, did not (Fig. 1C), as described (Chen and Fang 2001; Pfleger et al. 2001b). As with Cdc20 (Reimann et al. 2001), the Emi1 C but not the N terminus is sufficient to block APCCdh1 activation (data not shown). Human Emi1 also inhibits both Cdc20 and Cdh1–APC activation in vitro and in vivo, indicating a conserved APC regulatory role for Emi1 (J. Hsu, J. Reimann, C. Sorensen, J. Lukas, and P. Jackson, in prep.). Neither Emi1 nor Mad2 inhibited the ubiquitylation activity of the core APC enzymatic components APC2/APC11 (Fig. 1D; Gmachl et al. 2000), further suggesting that both inhibitors act through Cdc20 or Cdh1.
Emi1 alignment with homologs from other organisms (Reimann et al. 2001) highlighted a conserved N-terminal KEN sequence, typically found in APCCdh1 substrates (Pfleger and Kirschner 2000). Emi1 is degraded in mitosis independent of the APC in the embryo (Reimann et al. 2001), but Cdh1 is not present in Xenopus embryos (Lorca et al. 1998). To test whether Emi1 is an APCCdh1 substrate, we assayed the stability of 35S-labeled Emi1 in Cdh1-supplemented interphase extracts. Cdh1 addition to extracts destabilized cyclin B but not Emi1 (Fig. 1E). Additionally, a KEN box mutant (KE71AA) did not stabilize Emi1 in mitotic extracts (Fig. 1E), and Emi1 was not ubiquitylated by APCCdh1 in vitro (data not shown). Thus, Emi1 does not appear to be an APCCdc20 or APCCdh1 substrate, but rather a Cdh1/Cdc20 regulator.
Emi1 but not Mad2 stabilizes cyclin A in Xenopus eggs
APC-dependent cyclin A destruction in prometaphase is not inhibited by the SC (Hunt et al. 1992; den Elzen and Pines 2001; Geley et al. 2001). In contrast, Emi1 prevents cyclin A destruction in Xenopus eggs (Fig. 2A; Reimann et al. 2001), whereas addition of GST–Mad2 to cycling extracts prevented cyclin B but not cyclin A destruction (Fig. 2B). Thus, unlike Emi1, Mad2 is not competent to stabilize cyclin A in either somatic or embryonic cells.
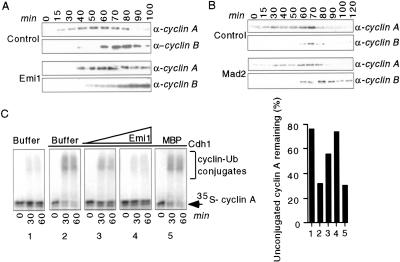
Figure 2.
Emi1 but not Mad2 inhibits cyclin A destruction in Xenopus eggs. (A,B) Emi1 prevents cyclin A and B destruction in egg extracts whereas Mad2 only stabilizes cyclin B. Activated Xenopus cycling egg extracts were incubated with buffer alone, MBP–Emi1, or GST–Mad2. Aliquots were removed at the indicated times and assayed for Xenopus cyclins A and B by immunoblotting. (C) Emi1 inhibits APCCdh1-mediated cyclin A ubiquitylation in vitro. IVT Cdh1 (panels 2_–_5) or rabbit reticulocyte lysate (1) was incubated with buffer (1 and 2), MBP–Emi1 (3, 3 μM; 4, 6 μM), or 6 μM MBP (5). APC immunopurified from interphase egg extracts was incubated with the Cdh1/protein mixtures. The ability of treated APC to ubiquitylate 35S-labeled cyclin A was assayed. The percentage of cyclin A remaining unconjugated to ubiquitin after 60 min was quantitated on a PhosphorImager (graph).
Cyclin A is a key APCCdh1 target in G1 (Lukas et al. 1999; Sørensen et al. 2001), so we tested Emi1 inhibition of APCCdh1-mediated cyclin A ubiquitylation. Emi1 blocked APCCdh1 ubiquitylation of cyclin A in a dose-dependent manner (Fig. 2D). Human Emi1 also inhibits APCCdh1-mediated cyclin A ubiquitylation in vitro and in vivo (J. Hsu, J. Reimann, C. Sorensen, J. Lukas, and P. Jackson, in prep.), indicating conservation of Emi1's ability to regulate cyclin A stability.
Emi1 interacts with and inhibits Cdc20/Cdh1 already bound to the APC
Fractionation experiments show separate Emi1–Cdc20 and APCCdc20 complexes in eggs (Reimann et al. 2001). However, exogenously added Emi1 can inhibit the APC in mitotic egg extracts, where the APC is already activated by Cdc20. One possibility is that Cdc20–APC binding is dynamic, and exogenous Emi1 sequesters Cdc20 as it dissociates from the APC. An alternative explanation is that exogenous Emi1 binds and inhibits Cdc20 already associated with the APC. Emi1 may fail to bind APCCdc20 in mitotic extracts because Emi1 is already degraded before Cdc20 is fully bound to the APC (Reimann et al. 2001). We found that 35S-labeled Emi1 precipitated with APC prebound to either IVT Cdc20 or Cdh1, but not to APC preincubated with reticulocyte lysate or with control beads; Emi1 reproducibly bound APCCdh1 more strongly than it did APCCd20 (Fig. 3A). Emi1 did not prevent Cdc20 or Cdh1 binding to the APC in vitro; when IVT 35S-labeled Cdc20 or Cdh1 was incubated with APC beads, a similar amount of either protein was recovered in the presence or absence of Emi1 (Fig. 3B; data not shown).
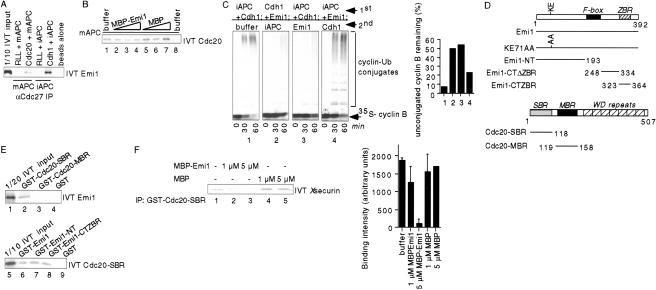
Figure 3.
Emi1 inhibits substrate binding to the APC. (A) Emi1 can bind Cdc20 and Cdh1 already associated with the APC. Mitotic (m), interphase (i), or no APC was immobilized on αCdc27 beads, bound to rabbit reticulocyte lysate, IVT Cdc20, or IVT Cdh1, and then incubated with 35S-labeled IVT Emi1. Bound Emi1 was analyzed by SDS/PAGE and autoradiography. (B) Emi1 does not prevent Cdc20 binding to the APC in vitro. 35S-labeled IVT Cdc20 was preincubated with buffer (lanes 1 and 8), MBP–Emi1 (2, 1 μM; 3, 3 μM; 4, 6 μM) or MBP (5, 1 μM; 6, 3 μM; 7, 6 μM) and then incubated with mitotic APC (1_–_7) or no APC (8) on αCdc27 beads. Bound Cdc20 was analyzed as in A. (C) Emi1 inhibits previously activated APC. Interphase APC (iAPC) complex immobilized on αCdc27 beads was first incubated with IVT Cdh1 (panels 1 and 3), buffer (2), or 10 μM MBP–Emi1 (4). Beads were then washed and incubated with buffer (1), IVT Cdh1 + 10 μM MBP–Emi1 (2), 10 μM MBP–Emi1 (3), or IVT Cdh1 (4). APC beads were washed and then assayed for cyclin B ubiquitylation activity. The percentage of cyclin B remaining unconjugated to ubiquitin after 60 min was quantitated on a PhosphorImager (graph). (D) Schematics of Emi1 and Cdc20 constructs used in this study. Emi1 F-box (residues 196–245), Emi1 zinc-binding region (ZBR, residues 323–364), Cdc20 substrate-binding region (SBR, residues 1–118), Cdc20 Mad2-binding region (MBR, residues 119–158), and Cdc20 WD-repeats (residues 181–480) are indicated. (E) Emi1 binds the substrate-binding region (SBR) of Cdc20. GST fusion proteins (lane 2, GST–Cdc20–SBR; 3, GST–Cdc20–MBR; 4 and 9, GST; 6, GST–Emil; 7, GST–Emi1–NT; 8, GST–Emi1–CTZBR) were incubated with 35S-labeled IVT Emi1 or Cdc20–SBR, bound to glutathione agarose, and analyzed as in A. (F) Emi1 can inhibit substrate binding to Cdc20. GST–Cdc20–SBR (1 μM) was prebound to glutathione agarose and then incubated with buffer (lane 1), MBP–Emi1 (lanes 2,3) or MBP (lanes 4,5). 35S-labeled IVT _X_securin was then added and the amount of securin bound to Cdc20 was analyzed as in A and was quantitated on a PhosphorImager (graph).
We next tested whether Emi1 could inhibit immunopurified APC already activated by Cdc20/Cdh1. Emi1 addition to preformed APCCdh1 complexes inhibited cyclin B ubiquitylation to a similar extent as when Cdh1 was preincubated with Emi1 (Fig. 3C). Preincubation of the APC with Emi1 reduced activation by Cdh1 somewhat, consistent with the small amount of Emi1 that associates with the APC in our binding assays (Fig. 2A). We obtained similar results with APCCdc20 (data not shown), indicating that Emi1 can inhibit the APC with either Cdc20 or Cdh1 already bound.
Emi1 inhibits substrate binding to Cdc20
The N-terminal 158 residues of Cdc20 are sufficient for binding to Emi1 (Reimann et al. 2001). This Cdc20 fragment contains both a Mad2-binding region (MBR, residues 118–158) and a substrate-binding region (SBR, residues 1– 118) (Luo et al. 2000; Pfleger et al. 2001a; Zhang and Lees 2001). We tested Emi1 binding to these domains, and found that Emi1 specifically bound the Cdc20 SBR, and not the MBR (Fig. 3E). Both Cdc20 binding domains of Emi1 (the Emi1 N terminus and zinc-binding region; Reimann et al. 2001) interact specifically with the Cdc20 SBR (Fig. 3E).
Because both Emi1 and substrates bind the Cdc20 SBR, we assayed the ability of Emi1 to inhibit substrate binding to Cdc20. MBP–Emi1 addition strongly reduced 35S-labeled securin from binding to the Cdc20 SBR in a dose-dependent manner (Fig. 3F). Using this substrate-binding assay, we find that Emi1 proteolytically cleaved and purified from MBP and a purified his-tagged Emi1 (which both inhibit APC activity similarly) also inhibit substrate–Cdc20 binding (data not shown). GST–Mad2 did not inhibit substrate binding to Cdc20 in our assay, consistent with earlier results (Pfleger et al. 2001b). Emi1 also blocks substrate binding to Cdh1 in vitro (data not shown), providing further evidence of the role of Emi1 as a general substrate inhibitor of APC activity.
Zinc is required for Emi1 to inhibit APC activity
A highly conserved cluster of cysteines and histidine in Emi1, a likely zinc-binding region (ZBR), is required for inhibiting APC activity (Reimann et al. 2001). Here, we found that at high concentrations, the Emi1 ZBR fragment is sufficient to inhibit APC activity in vitro (Fig. 4A).
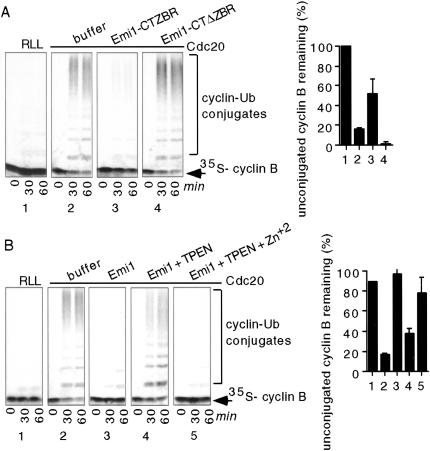
Figure 4.
Zinc is required for the APC inhibitory activity of Emi1 in vitro. (A) The zinc-binding region (ZBR) of Emi1 is sufficient to inhibit the APC in vitro. IVT Cdc20 (panels 2_–_4) or rabbit reticulocyte lysate (1) was preincubated with buffer (1 and 2), 80 μM GST–Emi1–CTZBR (3), or 80 μM GST–Emi1–CTΔZBR (4). APC immunopurified from mitotic egg extracts was incubated with the Cdc20/protein mixtures. The ability of treated APC to ubiquitylate 35S-labeled N terminus cyclin B fragment was assayed. The percentage of cyclin B unconjugated to ubiquitin by 60 min was quantitated on a PhosphorImager (graph). (B) Zn2+ chelation strongly reduces the ability of Emi1 to inhibit the APC in vitro, and addition of ZnCl2 rescues activity. MBP–Emi1 was incubated with TPEN and then dialyzed into XB− or XB− plus 50 μM ZnCl2. IVT Cdc20 (panels 2_–_5) or rabbit reticulocyte lysate (1) was incubated with buffer (1 and 2), untreated MBP–Emi1 (3), TPEN-treated MBP–Emi1 (4), or TPEN-treated MBP–Emi1 plus ZnCl2 (5). APC was immunopurified and cyclin B ubiquitylation was analyzed as in A.
To formally test whether zinc is required for Emi1's inhibitory activity, we chelated zinc from the Emi1 protein with the zinc chelator TPEN. The ability of TPEN-treated MBP–Emi1 to inhibit APC activity was strongly reduced and was restored by zinc addition (Fig. 4B). We see similar loss of activity with DPTA, another zinc chelator, and with zinc chelation from the Emi1 C terminus fragment.
Discussion
The APC is regulated by multiple mechanisms, including phosphorylation and binding of the Cdc20/Cdh1 activators or the Mad2 and Emi1 inhibitor proteins. Cdc20–APC activation is regulated by Emi1, Mad2, and the SC proteins including BubR1; Cdh1 activation of the APC is regulated by Mad2B and as shown here by Emi1. The activity of Emi1 toward the APC is partially controlled by its abundance in both the embryo (Reimann et al. 2001) and somatic cells, where Emi1 accumulates in late G1 much like cyclin A (J. Hsu, J. Reimann, C. Sorensen, J. Lukas, and P. Jackson, in prep.). Previous studies suggested that in addition to the ability of cyclin A/Cdk2 to phosphorylate Cdh1 and inactivate the APCCdh1 in S phase (Lukas et al. 1999; Sørensen et al. 2001), an additional E2F target might block APCCdh1 activity. Like cyclin A, Emi1 proves to be an E2F target and Emi1 inhibits Cdh1's ability to block cyclin A accumulation and S phase entry in vivo (J. Hsu, J. Reimann, C. Sorensen, J. Lukas, and P. Jackson, in prep.). The Drosophila Emi1 homolog Rca1 also blocks Cdh1 activity in flies (F. Sprenger, pers. comm.). As shown here, Emi1 binds Cdh1 to inhibit APCCdh1 activity in vitro. These data strongly suggest that Emi1 inactivates the APCCdh1 complex to promote cyclin A accumulation at the G1–S transition. Although it is unclear whether Emi1 continues to inhibit Cdh1 during S phase, as cells approach G2 Emi1 is available to inhibit Cdc20 as it is expressed, thereby promoting cyclin B accumulation and mitotic entry (Reimann et al. 2001).
APC regulation by the Mad2 proteins is complex, involving additional factors. Mad2/Mad2B inhibit in vitro APC activation by Cdc20/Cdh1, but neither can inhibit preactivated APC complexes in vitro, despite being able to form ternary complexes with activator and APC (Fang et al. 1998a; Kallio et al. 1998; Chen and Fang 2001; Pfleger et al. 2001b; Sudakin et al. 2001; Tang et al. 2001). However, both Mad2 proteins can inhibit activated APC in Xenopus egg extracts and in vivo, suggesting that cellular factors activate Mad2 proteins to inhibit APC activity. Notably, APC inhibition by the SC requires Mad1 and BubR1 in vivo (Hwang et al. 1998; Jin et al. 1998; Chen et al. 1999; Sudakin et al. 2001; Tang et al. 2001). Although Mad2 and BubR1 are present and biochemically competent to inhibit the APC throughout the cell cycle, the APC is only sensitive when phosphorylated in mitosis and when spindle tension and/or microtubule attachment at kinetochores is lost (Abrieu et al. 2001; Skoufias et al. 2001; Sudakin et al. 2001).
In contrast, Emi1 can bind and inhibit activation of APC prebound to Cdc20 or Cdh1 in vitro and in vivo. The ability of Emi1 to inhibit an already activated APC would be a necessary feature for Emi1 to inactivate Cdh1 already bound to the APC at the G1–S transition. Thus, Emi1's APC inhibitory activity is likely controlled by Emi1 protein levels and its ability to bind Cdc20/Cdh1, and not at the level of APC phosphorylation.
APC-dependent cyclin A ubiquitylation is not inhibited by the SC or by Mad2, but is inhibited by Emi1 (den Elzen and Pines 2001; Geley et al. 2001; our present results). Consistently, Emi1 itself is destroyed in mitosis slightly before cyclin A levels drop, and Emi1 is not stabilized by SC activation (J. Hsu, J. Reimann, C. Sorensen, J. Lukas, and P. Jackson, in prep.). Moreover, Emi1 is not present in the purified Mad2 and BubR1-containing APC inhibitory complex (J. Hsu, V. Sudakin, T. Yen, P. Jackson, unpubl.). Thus, Emi1 activity is distinct from and independent of Mad2/BubR1.
Both the N and C termini of Emi1 bind the Cdc20 SBR. Mad2 binds just C-terminal to the SBR, and neither Mad2 nor Mad2B prevent Cdc20/Cdh1 substrate binding. Instead, they appear to inhibit substrate release from Cdc20/Cdh1 (Pfleger et al. 2001b), suggesting that Mad2 proteins prevent APC substrate turnover. Emi1 binding to the SBR directly inhibits substrate binding to Cdc20 in vitro. Thus, Emi1 appears to prevent substrate ubiquitylation by inhibiting substrate binding, although additional mechanisms may function in vivo.
Both the ZBR (Reimann et al. 2001) and, as we show here, zinc itself are required for the APC inhibitory activity of Emi1. Whether zinc fulfills a structural role, facilitates binding interactions, or has another role, such as a catalytic function, is unclear. We did find that zinc chelation did not appear to affect Emi1–Cdc20 binding in vitro (J.D.R. Reimann, B. Gardner, and P.K. Jackson, unpubl.).
The identification of other Cdc20/Cdh1-like proteins in various species (e.g., Cooper et al. 2000; Chu et al. 2001; Wan et al. 2001) suggests additional pathways of APC regulation. An attractive model is that Cdc20, Cdh1, and their homologs regulate the timing of APC activity by regulated binding of specific substrates. The ability of these APC adapters to bind and activate substrate ubiquitylation by the APC might in turn be restricted by a range of inhibitory proteins like Emi1 and the Mad2 proteins.
Materials and methods
Recombinant protein and construct preparation
Full-length, Emi1–NT, and Emi1–CTΔZBR constructs were described (Reimann et al. 2001). Emi1–CTZBR (amino acids 323–364) was cloned into pGEX6P1. Cdc20–SBR (amino acids 1–118) and Cdc20–MBR (amino acids 119–158) were cloned into pGEX6P1 and pCS2+ vectors. The Emi1 KE71AA site-directed mutant was cloned into pCS2+–5mt and verified by sequencing.
All Emi1 and Cdc20 variants produced as MBP or GST fusion proteins were purified by standard protocols. Cdh1 baculovirus protein was as described (Kramer et al. 2000).
Binding assays
In vitro GST–Emi1 and GST–Cdc20 binding assays
First, 750 nM GST fusion protein was incubated with in vitro translated (IVT) 35S-labeled proteins (TNT Promega) in RIPB (100 mM NaCl, 50 mM β-glycerophosphate, 5 mM EDTA, 0.1% Triton X-100, 1 mM DTT) (1 h at 4°C). Samples were spun (14,000 rpm for 10 min), supernatant incubated with glutathione agarose (40 min at 4°C), beads washed 4× in RIPB, and bound proteins analyzed by SDS-PAGE and autoradiography.
Baculovirus reconstitution was performed as described (Reimann et al. 2001).
APC binding assays
APC was immunopurified from mitotic or interphase egg extracts on αCdc27 beads as described (Fang et al. 1998a) and incubated (room temperature for 1 h) with 10 μL IVT Cdc20, Cdh1 or rabbit reticulocyte lysate. Beads were washed 2× in XB− (20 mM HEPES, 100 mM KCl), incubated with 4 μL 35S-labeled IVT Emi1 diluted 1:38 in RIPB (4°C for 45 min), and washed 5× in Q-A buffer (20 mM HEPES, 500 mM KCl, 0.5% NP-40). Bound Emi1 was analyzed by SDS-PAGE and autoradiography. αCdc27 beads were subjected to identical binding and washing conditions. For testing the ability of Emi1 to inhibit Cdc20 APC binding, 2 μL of 35S-labeled IVT Cdc20 was prebound to MBP–Emi1 or MBP before binding to APC beads.
Substrate binding competition assay
GST–Cdc20–SBR (1 μM) prebound to glutathione agarose was incubated (4°C for 45 min) with 1 or 5 μM MBP–Emi1, his–Emi1, Emi1 with MBP removed, MBP, or BSA in NETN buffer (20 mM Tris-HCl at pH 7.5, 150 mM NaCl, 0.5% NP-40, 1 mM DTT, 1 mM EDTA, 1% aprotinin). Four microliters of 35S-labeled IVT securin was diluted 1:25 in NETN, then incubated with the above mixture (4°C for 45 min). Beads were washed 5× in NETN, and bound securin was analyzed by SDS-PAGE and autoradiography.
Zinc chelation experiments
MBP–Emi1 protein was incubated (4°C, 24 h) with two changes of XB− plus 2 mM TPEN, then incubated (4°C for 3 h) in either 50 μM ZnCl2 or XB−, and dialyzed into XB− (4°C for 18 h).
Degradation and ubiquitylation assays
Emi1 stability experiments in egg extracts
35S-labeled IVT Emi1, KE71AA, or N terminus sea urchin cyclin B substrate (Glotzer et al. 1991) was incubated at 23°C in Δ90 mitotic extracts (Reimann et al. 2001), interphase extracts with IVT Cdh1 (1:20 volume), or interphase extracts with unprogrammed reticulocyte lysate. Aliquots were removed and analyzed by SDS-PAGE and autoradiography.
Effect of Emi1 and Mad2 on cyclin A and B stability
Buffer, 1 μM MBP–Emi1, or 20 μM GST–Mad2 fusion protein was added to cycling extracts (Murray 1991). Aliquots were removed at the indicated times, and endogenous cyclin A and B levels were assayed by immunoblotting with αcyclin B2 or αcyclin A1 antibodies.
Effect of Emi1 on APCCdh1 activity in extracts
35S-labeled IVT Xl cyclin B1 (amino acids 2–97) fragment or securin was added (1:20 volume) to interphase extracts preincubated with either XB− buffer, IVT Cdh1 (1:25 volume) plus XB− buffer, or IVT Cdh1 (1:25 volume) plus 1 μM MBP–Emi1. Aliquots were removed and analyzed by SDS-PAGE and autoradiography.
APC2/APC11 substrate-independent ubiquitylation reaction
Twenty μM MBP, 20 μM MBP–Emi1, or 60 μM GST–Mad2 was incubated at room temperature in ULAA buffer (50 mM Tris at pH 7.5, 5 mM MgCl2, 2 mM NaF, 0.6 mM DTT) containing 1.5 ng/μL baculovirus expressed and purified APC2/APC11, 7.2 ng/μL Ubc5, 0.2 ng/μL E1 (Calbiochem), 2 mM ATP, 10 nM okadaic acid, and 3.2 ng/μL Flag-ubiquitin. Aliquots were removed at the indicated times and analyzed for polyubiquitin chains by immunoblotting with αFlag antibody (Sigma).
In vitro APC assays
Mitotic or interphase extract aCdc27 immunoprecipitates were incubated (25°C for 1 h) with 10 μL IVT Cdc20 or Cdh1 preincubated (4°C for 30 min) with protein or buffer as indicated in the figure legend, washed in XB−, and assayed for cyclin ubiquitylation as described (Fang et al. 1998b).
Acknowledgments
We thank C. Pfleger and M. Kirschner for securin cDNA and unpublished results, E. Kramer and J. Peters for Cdh1 constructs and antibodies, T. Hunt for _X_l cyclin A and B antibodies, M. Dobles and P. Sorger for GST–Mad2 cDNA, Jianing Huang and Ruby Daniel (Rigel Pharmaceuticals) for Flag-Ubiquitin, and R. Deshaies, G. Fang, A. Eldridge, J. Hsu, and D. Hansen for comments on the manuscript. This work was supported by the NIGMS Medical Scientists Training Grant GM07365 (J.R.), INSERM (F.M.), and NIH grants GM54811 and GM60439 (P.J).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL pjackson@cmgm.stanford.edu; FAX (650) 725-6902.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.945701.
References
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbé JC. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. doi: 10.1016/s0092-8674(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Burton JL, Solomon MJ. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes & Dev. 2001;15:2381–2395. doi: 10.1101/gad.917901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Fang G. MAD2B is an inhibitor of the anaphase-promoting complex. Genes & Dev. 2001;15:1765–1770. doi: 10.1101/gad.898701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Brady DM, Smith D, Murray AW, Hardwick KG. The spindle checkpoint of budding yeast depends on a tight complex between the Mad1 and Mad2 proteins. Mol Biol Cell. 1999;10:2607–2618. doi: 10.1091/mbc.10.8.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Henrion G, Haegeli V, Strickland S. Cortex, a Drosophila gene required to complete oocyte meiosis, is a member of the Cdc20/fizzy protein family. Genesis. 2001;29:141–152. doi: 10.1002/gene.1017. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O, Peters JM, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes & Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- Cooper KF, Mallory MJ, Egeland DB, Jarnik M, Strich R. Ama1p is a meiosis-specific regulator of the anaphase promoting complex/cyclosome in yeast. Proc Natl Acad Sci. 2000;97:14548–14553. doi: 10.1073/pnas.250351297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen N, Pines J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J Cell Biol. 2001;153:121–136. doi: 10.1083/jcb.153.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Zavitz KH, Thomas BJ, Lin M, Campbell S, Zipursky SL. Control of G1 in the developing Drosophila eye: rca1 regulates Cyclin A. Genes & Dev. 1997;11:94–105. doi: 10.1101/gad.11.1.94. [DOI] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner MW. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes & Dev. 1998a;12:1871–1883. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998b;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- Geley S, Kramer E, Gieffers C, Gannon J, Peters J, Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gmachl M, Gieffers C, Podtelejnikov AV, Mann M, Peters JM. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc Natl Acad Sci. 2000;97:8973–8978. doi: 10.1073/pnas.97.16.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilioti Z, Chung YS, Mochizuki Y, Hardy CF, Cohen-Fix O. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr Biol. 2001;11:1347–1352. doi: 10.1016/s0960-9822(01)00399-2. [DOI] [PubMed] [Google Scholar]
- Hunt T, Luca FC, Ruderman JV. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992;116:707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang LH, Lau LF, Smith DL, Mistrot CA, Hardwick KG, Hwang ES, Amon A, Murray AW. Budding yeast Cdc20: A target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- Jin DY, Spencer F, Jeang KT. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ. Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J Cell Biol. 1998;141:1393–1406. doi: 10.1083/jcb.141.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lin DP, Matsumoto S, Kitazono A, Matsumoto T. Fission yeast Slp1: An effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- Kramer ER, Gieffers C, Hölzl G, Hengstschläger M, Peters JM. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr Biol. 1998;8:1207–1210. doi: 10.1016/s0960-9822(07)00510-6. [DOI] [PubMed] [Google Scholar]
- Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell. 2000;11:1555–1569. doi: 10.1091/mbc.11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T, Castro A, Martinez AM, Vigneron S, Morin N, Sigrist S, Lehner C, Dorée M, Labbé JC. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 1998;17:3565–3575. doi: 10.1093/emboj/17.13.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C, Sørensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, Bartek J, Lukas J. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- Luo X, Fang G, Coldiron M, Lin Y, Yu H, Kirschner MW, Wagner G. Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20. Nat Struct Biol. 2000;7:224–229. doi: 10.1038/73338. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Page AM, Hieter P. The anaphase-promoting complex: New subunits and regulators. Annu Rev Biochem. 1999;68:583–609. doi: 10.1146/annurev.biochem.68.1.583. [DOI] [PubMed] [Google Scholar]
- Pfleger CM, Kirschner MW. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes & Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Pfleger CM, Lee E, Kirschner MW. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes & Dev. 2001a;15:2396–2407. doi: 10.1101/gad.918201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger CM, Salic A, Lee E, Kirschner MW. Inhibition of Cdh1–APC by the MAD2-related protein MAD2L2: A novel mechanism for regulating Cdh1. Genes & Dev. 2001b;15:1759–1764. doi: 10.1101/gad.897901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz S, Hwang ES, Visintin R, Amon A. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr Biol. 1998;8:750–760. doi: 10.1016/s0960-9822(98)70298-2. [DOI] [PubMed] [Google Scholar]
- Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;105:645–655. doi: 10.1016/s0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- Shah JV, Cleveland DW. Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell. 2000;103:997–1000. doi: 10.1016/s0092-8674(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- Skoufias DA, Andreassen PR, Lacroix FB, Wilson L, Margolis RL. Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc Natl Acad Sci. 2001;98:4492–4497. doi: 10.1073/pnas.081076898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, Lukas J. A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex-Cdh1 and cyclin A–Cdk2 during cell cycle progression. Mol Cell Biol. 2001;21:3692–3703. doi: 10.1128/MCB.21.11.3692-3703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Bharadwaj R, Li B, Yu H. Mad2-independent inhibition of APC–Cdc20 by the mitotic checkpoint protein BubR1. Dev Cell. 2001;1:227–237. doi: 10.1016/s1534-5807(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Visintin R, Prinz S, Amon A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- Wan Y, Kirschner MW. Identification of multiple CDH1 homologues in vertebrates conferring different substrate specificities. Proc Natl Acad Sci. 2001;98:13066–13071. doi: 10.1073/pnas.231487598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae W, Nasmyth K. Whose end is destruction: Cell division and the anaphase-promoting complex. Genes & Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Schwab M, Nasmyth K, Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282:1721–1724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lees E. Identification of an overlapping binding domain on Cdc20 for Mad2 and anaphase-promoting complex: Model for spindle checkpoint regulation. Mol Cell Biol. 2001;21:5190–5199. doi: 10.1128/MCB.21.15.5190-5199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]