Differential Cytopathology and Kinetics of Measles Oncolysis in Two Primary B-cell Malignancies Provides Mechanistic Insights (original) (raw)
Abstract
Clinical trials using vaccine measles virus (MV) as anticancer therapy are already underway. We compared the oncolytic potential of MV in two B-cell malignancies; adult acute lymphoblastic leukemia (ALL, an aggressive leukemia) and chronic lymphocytic leukemia (CLL, an indolent leukemia overexpressing Bcl-2) using patient-derived material. In vitro, distinct cytopathological effects were observed between MV-infected primary ALL and CLL cells, with large multinucleated syncytia forming in ALL cultures compared to minimal cell-to-cell fusion in infected CLL cells. Cell viability and immunoblotting studies confirmed rapid cell death in MV-infected ALL cultures and slower MV oncolysis of CLL cells. In cell lines, overexpression of Bcl-2 diminished MV-induced cell death providing a possible mechanism for the slower kinetic of MV oncolysis in CLL. In vivo, intratumoral MV treatment of established subcutaneous ALL xenografts had striking antitumor activity leading to complete resolution of all tumors. The antitumor activity of MV was also evident in disseminated ALL xenograft models. In summary, both ALL and CLL are targets for MV-mediated lysis albeit with different kinetics. The marked sensitivity of both primary ALL cells and ALL xenografts to MV oncolysis highlights the tremendous potential of MV as a novel replicating-virus therapy for adult ALL.
Introduction
Viruses that conditionally replicate and lyse transformed cells are promising agents for cancer therapy. Live attenuated measles virus (MV) derived from the Edmonston-B vaccine strain is particularly attractive as an oncolytic agent for human tumors.1 A long safety record derived from four decades of application as a vaccine against measles disease together with a viral genome that is readily engineered are attributes that favor its use for virotherapy. The clinical relevance of MV as a cancer therapeutic is further strengthened by a phase 1 trial which reported no dose limiting toxicities with this approach.2
The vaccine strain of MV is a negative strand RNA virus which enters cells predominantly through the CD46 receptor3 and has been previously shown to have specific cytotoxic activity in transformed cells from several tumor types.4,5,6 The most susceptible target cells are arguably B-cell malignancies.7,8 By contrast minimal cytopathic effects are seen in MV-infected normal peripheral blood lymphocytes.7 The efficiency of MV entry into neoplastic B-lymphocytes (targets relatively refractory to transduction by conventional vectors) is further highlighted by reports of high transduction rates in leukemic B-cells by lentiviral vectors pseudotyped with MV envelope proteins.9
The mode of action of MV oncolysis is not clear. CD46 overexpression on tumor cells is implicated in the selective lysis of malignant cells.10 In respect of how MV kills tumor cells, expression of the MV envelope glycoproteins, hemaglutinin (MV-H) and fusion (MV-F) in infected cells results in considerable cell-to-cell fusion which mediates a potent cytopathic effect (CPE) in vitro. By contrast, syncytia are rarely observed in excised MV-sensitive human tumor xenografts, hence there is debate over the necessity for multinucleated cell formation as a prelude to MV-induced death.8,11 Furthermore, most work has been carried out using cell lines, which may not be broadly representative of MV infection response in primary malignancies.
In this study, we have investigated the utility of MV oncolysis in two different B-cell malignancies, in both of which the need for novel agents is pressing.
Outcome for adults with acute lymphoblastic leukemia (ALL), a rapidly progressive acute malignancy—is poor with <40% of patients surviving long term.12 Chronic lymphocytic leukemia (CLL), although a much more indolent disease, is generally considered incurable.13 To get the most realistic insights possible, we aimed to use primary patient material for as much of our study as was feasible. In particular, we wished to determine the possible differences in the outcome of MV infection in two B-cell malignancies with very different clinical behaviors. CLL is a malignancy which overexpresses Bcl-2, a protein which confers a block to apoptosis. The key feature of CLL is the accumulation of mature and relatively indolent clonal B-cells whereas in ALL the malignant cells are rapidly proliferative cells derived from stem cells or cells early in the lymphoid differentiation pathway.14 We were interested in determining the relationship between cell killing, MV replication, protein expression, and syncytia formation in these two distinct B-cell malignancies, with the overall aims of gaining mechanistic insight into mechanisms of oncolysis and of determining in which B-cell leukemia to best focus future efforts at translating MV oncolysis into the clinic.
Results
MV-induced cytopathology is distinct in primary ALL and CLL
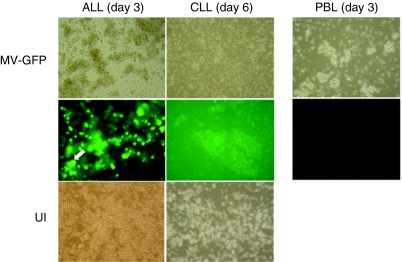
To confirm susceptibility of primary ALL (n = 6) and CLL (n = 5) cells to MV infection, we demonstrated cell surface expression of CD46 by fluorescence-activated cell sorting (data not shown). We infected primary ALL (n = 5) and CLL (n = 3) cells with MV expressing green fluorescent protein (MV-GFP) at a multiplicity of infection of 1.0 and compared CPE of the virus in culture. Photomicrographs of representative experiments are shown in Figure 1. Primary ALL specimens typically showed characteristic MV-induced cytopathic changes—including multinucleated syncytium formation which began very early postinfection and peaked at day 3, with rapid death thereafter. By contrast, the MV-GFP infected CLL cultures showed little or no evidence of cell-to-cell fusion even by day 6 postinfection. Despite a relative lack of syncytia in infected CLL cultures, cytopathic effects did eventually become apparent although only at later stages of infection when the vast majority of cells exhibited cell shrinkage and surface membrane contraction compared to mostly normal cytomorphological appearances in uninfected cultures at this time.
Figure 1.
Differential cytopathology between primary acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) cells following infection with attenuated measles virus (MV). Representative photomicrographs (×40 magnification) of primary ALL and CLL cells on the 3rd and 6th day following infection with MV-green fluorescent protein (GFP) (multiplicity of infection of 1.0). MV-GFP infected peripheral blood lymphocytes (PBL) controls at day 3 are also shown. The toptwo rows show transmission images and UV images of the same fields, respectively. The bottom row shows uninfected cells (UI) at corresponding timepoints. Large fluorescent syncytia (white arrow) are abundant in MV-infected ALL cultures. By contrast CLL cells exhibit little multinuclear aggregation.
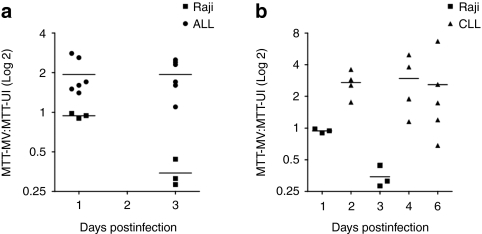
To compare the extent of MV-induced cell death in the infected ALL and CLL cultures we assessed MTT (CLL, n = 5) and MTS (ALL, n = 6) reduction in infected specimens and corresponding uninfected controls. The ability of viable cells to reduce tetrazolium to formazan in these assays is directly proportional to the number of living cells in culture. Raji B-cells which are highly susceptible to MV-oncolysis8 were used as a positive control. Figure 2a,b show the ratio of formazan product in infected cells:uninfected control cells. Surprisingly, bioreduction of MTT/MTS was always greater in infected primary cultures indicating an apparent enhancement of cell viability in MV-infected ALL (Figure 2a) and CLL (Figure 2b) cells. By contrast, rapid cell death ensued in MV-infected Raji cells as expected.
Figure 2.
MTS and MTT assays demonstrate enhanced production of formazan by measles virus (MV)-infected primary acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) cells. (a,b) show results of MTS/MTT testing following MV infection (multiplicity of infection 1.0) of primary ALL (n = 6) and CLL (n = 5) specimens, respectively, compared to a Raji cell line positive control. Datapoints represent the ratio of mean MTT/MTS absorbance in infected cells:uninfected control cells for each patient specimen. A ratio of <1 indicates reduced formazan production compared to control whereas a ratio of >1 indicates enhanced formazan production compared to control. MTT testing in Raji cells from three independent experiments are shown. Horizontal bars represent the means of independent experiments in each tumor group.
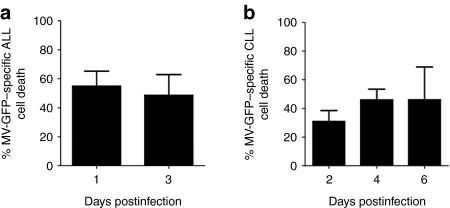
These unexpected results led us to assay cell viability postinfection by alternative methods: flow cytometric quantification of TO-PRO staining in ALL cells (n = 5) and direct counting of nonviable cells after Trypan blue staining in CLL specimens (n = 3). To exclude the influence of spontaneous cell death of primary cells in vitro, rates of “MV-specific cell death” were calculated and results are shown in Figure 3. Although a clear enhancement of cell death in both MV-infected ALL (Figure 3a) and CLL (Figure 3b) cultures was seen at all timepoints, the kinetics of this response appeared distinct with cell death ensuing rapidly in infected ALL cells (MV-specific cell death at day 1: 54.87 ± 10.37%; compared to 31.01 ± 13.06% enhancement of cell death in CLL cells at day 2. (P = 0.14).
Figure 3.
Cell viability following measles virus expressing green fluorescent protein (MV-GFP) infection of primary acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) cells. The percentage of MV-specific cell death is shown. (a) Primary ALL (n = 5) and (b) primary CLL (n = 3). Data represent the mean ± SEM.
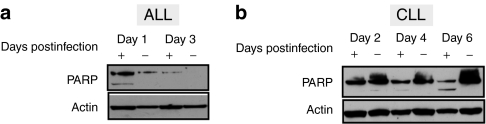
A difference in the rapidity of killing between MV-infected ALL and CLL cells was also apparent when apoptosis induction in these targets was examined. Cell lysates from primary ALL and CLL cells infected with MV-GFP were immunoblotted to assess cleavage of endogenous poly (ADP-ribose) polymerase cleavage at serial timepoints. Evidence of poly (ADP-ribose) polymerase cleavage was detected as early as day 1 postinfection in infected ALL specimens (Figure 4a) whereas in CLL samples significant poly (ADP-ribose) polymerase cleavage could only be detected at 6 (Figure 4b) and 4 days after infection. Taken together, these data suggest a slower induction of MV-mediated apoptotic cell death in CLL targets compared to human ALL tumors.
Figure 4.
Enhanced poly (ADP-ribose) polymerase (PARP) cleavage in primary acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) cells infected with measles virus expressing green fluorescent protein (MV-GFP). Immunoblotting for PARP cleavage (a) ALL cells. (b) CLL cells. Results for MV-GFP infected (+) or uninfected (−) cells are shown at various times postinfection. The data are representative of three and two experiments, respectively.
MV infection and replication is equivalent in primary ALL and CLL cells
To determine whether differences in viral genome replication and/or protein expression could be responsible for the difference in CPE and speed of killing between MV-infected ALL and CLL cells, we quantified viral genome replication in MV-infected primary cell cultures by relative-quantitative PCR. Figure 5a,b shows a 5–6 log increase in MV nucleocapsid (MV-N) RNA occurring over 6 days postinfection in specimens from both malignancies, confirming that differential viral genome replication rates were not responsible for the different cytopathology or timescale of cell death between ALL and CLL.
Figure 5.
Measles virus nucleocapsid (MV-N) RNA and proteins are produced in abundance after infection of primary acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) cells. Relative-quantitative PCR expression of MV-N mRNA in MV-green fluorescent protein (GFP) infected patient specimens (a) ALL (n = 3) (b) CLL (n = 3). The MV-N mRNA level is expressed relative to the expression of the housekeeping gene GAPDH. Data represent the mean values ± SEM. Representative immunoblots of lysates from MV-GFP infected (+) or uninfected (−) probed for MV-N, MV-fusion (F), and MV-hemaglutinin (H) at various days postinfection in (c) ALL (MV-N and MV-H only) and (d) CLL cells.
We next assessed expression of MV proteins, in infected CLL (MV-H, MV-N, and MV-F) and ALL cells (MV-H and MV-N only) by western blotting. Figure 5c,d show representative blots for ALL and CLL, respectively, and demonstrates abundant expression of MV-N and the envelope glycoprotein MV-H in both CLL and ALL cells after infection. MV-F protein expression was additionally sought in infected CLL cells which exhibited minimal cell-to-cell fusion. MV-F expression was confirmed in all cases indicating that lack of cell-to-cell fusion in these cells was not due to a failure of viral envelope protein expression. To assess whether MV production was responsible for the distinct antitumor kinetic of MV in our primary B-cell models, lysates and supernatants from MV-infected CLL (n = 6) and ALL cells (n = 2) were titrated by 50% end point dilution assay (TCID50) at serial timepoints (data not shown). In both cell types, <5 × 103 plaque forming unit (pfu)/ml of cell associated virus was detected at the day 3 postinfection timepoint alone. No virus was detected in the supernatant.
Overexpression of Bcl-2 reduces cell death in MV-infected cells
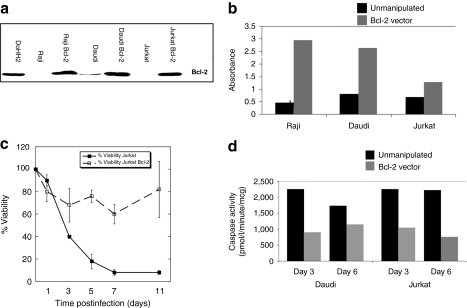
Elevated Bcl-2 expression is a major determinant of drug resistance in CLL15 but not in ALL.16,17 We thus hypothesized that overexpression of Bcl-2 diminishes MV-induced oncolysis in primary CLL. To investigate this hypothesis, we examined cell survival and activated caspase-3 activity in MV-infected cell lines overexpressing Bcl-2 compared with unmanipulated counterparts. Figure 6b shows MTS absorbance at day 3 post MV infection in Raji, Daudi, and Jurkat cells. In all three cell lines cell viability was greater in Bcl-2 overexpressing cell lines compared to unmanipulated controls. A timecourse experiment in Jurkat cells, where the difference was least pronounced, confirmed that this effect could be sustained up to 11 days postinfection (Figure 6c). Caspase-3 activity, quantified by enzyme-linked immunosorbent assay (Quantikine; R&D Systems, Abingdon, UK), was also consistently lower for Bcl-2 overexpressing cell lines compared to unmanipulated cell controls (Figure 6d). Thus, in all cell lines, overexpression of Bcl-2 conferred enhanced survival and reduction in apoptosis. Taken together, these data suggest that the ability of MV to lyse cells can be compromised by Bcl-2 overexpression which taken in the context of established Bcl-2 associated therapy resistance in human CLL provides a possible explanation for the slower speed of MV-mediated oncolysis in primary CLL cells.
Figure 6.
Overexpression of Bcl-2 blunts an oncolytic response to measles virus (MV). Cell lines engineered to overexpress Bcl-2 were infected with MV (multiplicity of infection 1.0). (a) Western blot staining of Bcl-2 in Raji, Daudi, and Jurkat cell lines after retroviral transduction. The DoHH2 Bcl-2 expressing cell line is included as a positive control. (b) Cell viability as measured by MTS absorbance values carried out at day 3 post MV infection in Daudi, Raji, and Jurkat cells. Black shaded bars represent unmanipulated cells, gray bars represent Bcl-2 overexpressing cells. (c) Percentage cell viability in MV-infected Jurkat and Jurkat Bcl-2 expressing cell lines over an 11 day time course experiment. Data represent the mean ± SEM of four independent experiments. (d) Activated caspase-3 activity as determined by enzyme-linked immunosorbent assay at days 3 and 6 post infection in Daudi and Raji cells. Data show the means of one experiment performed in duplicate. Black shaded bars represent unmanipulated cells, gray bars represent Bcl-2 overexpressing cells.
Established Nalm-6 pre-B ALL xenografts regress after MV treatment
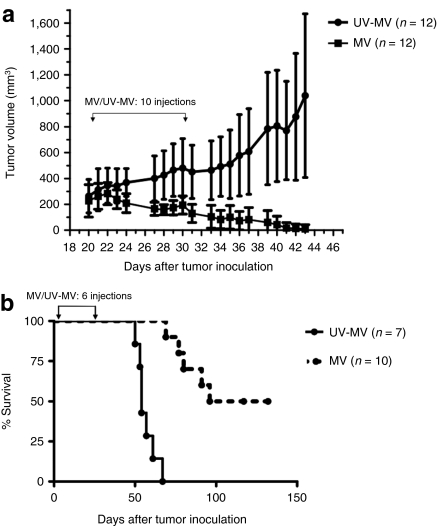
Next, we investigated whether the in vitro findings translated to in vivo antitumor activity. We studied specifically ALL since human xenotransplantation models for this disease are available. First, we tested the in vitro oncolytic efficacy of MV against four different B-precursor ALL-derived cell lines, REH, SEM, 697 and Nalm-6, all of which were readily killed (Figure 7a) by classic MV-induced cell-to-cell fusion with Nalm-6 being the slowest to respond. The less sensitive Nalm-6 cells was selected for in-vivo studies. A one-step MV growth curve on Nalm-6 cells is shown in Figure 7b. Mice bearing subcutaneous Nalm-6 tumors were treated with 10 intratumoral injections of 1 × 106 pfu of MV or UV inactivated MV (UV-MV). Median tumor volumes in the MV (215.7 mm3) and UV-MV (269.5 mm3) treated groups prior to the start of injections were not significantly different. As shown in Figure 8a, MV had striking antitumor activity. Complete resolution of all established subcutaneous ALL tumors was observed in 11/12 MV-treated mice by day 44 with the single remaining tumor fully regressing by day 62. In contrast, all UV-MV treated tumors progressed. The difference in tumor growth between the MV-treated and UV-MV control groups was highly statistically significant (P < 0.0001).
Figure 7.
Measles virus (MV) oncolysis in acute lymphoblastic leukemia (ALL) cell lines. (a) In order to determine which ALL cell line to use for the murine model, four B-ALL cell lines REH (closed triangles), SEM (closed squares), 697 (closed inverted triangles) and Nalm-6 (closed diamonds) were infected with MV-green fluorescent protein (GFP) and viability determined daily by Trypan blue estimation. The Y axis denotes percent viability normalized to control uninfected cells. (b) One-step growth curve showing the titer of cell-associated virus after MV infection of Nalm-6 cells over time.
Figure 8.
Measles virus (MV) oncolysis in a Nalm-6 subcutaneous model of acute lymphoblastic leukemia (ALL). (a) Tumor volumes in mm3 after intratumoral injection of MV (closed squares) or UV-MV(closed circles) into Nalm-6 ALL xenografts. (b) Kaplan–Meier survival curves of mice bearing disseminated Nalm-6 xenografts treated intravenously with a total dose of 6 × 106 plaque forming unit (pfu) of MV (broken line) or UV-MV control (solid line). Data represent the results from three independent experiments (n = 3–6 per group). Brackets with arrows indicate the period of MV/UV-MV administration.
Since all MV-treated subcutaneous xenografts had completely regressed, three further mice with large, established Nalm-6 tumors were injected daily with 1 × 106 pfu MV for three consecutive days and tumors harvested at days 1, 2, and 5 after the 3rd injection in order to obtain evidence of in vivo viral replication. MV was recovered after Vero co-culture of washed tumor (but not from the wash supernatant) from tumors harvested at days 1 and 2 but not at day 5, confirming ongoing viral replication early after MV therapy in these Nalm-6 xenografts. Interestingly, in contrast to the CPE of MV-infected Nalm-6 cells in vitro, syncytia could not be identified in any of the harvested xenograft tumors.
Next, we evaluated the efficacy of intravenous administration of MV in a disseminated model of ALL. Severe combined immunodeficient mice injected intravenously with Nalm-6 cells received 1 × 106 pfu of MV or UV-MV control over a 4-week period and observed for signs of illness. Five of 10 mice receiving replication competent MV survived beyond 100 days whereas all mice receiving UV-MV died by 67 days. Fluorescence-activated cell sorting analysis of bone marrow of UV-MV treated mice demonstrated 50–100% of human CD19+/CD10+ cells, confirming death by leukemia. The median survival was 54 days in UV-MV controls and 114 days in the MV group. (P < 0.0001). A Kaplan–Meier analysis showing overall survival of the two groups is shown in Figure 8b. Interestingly, there was no evidence of Nalm-6 leukemia in bone marrows of the five surviving mice at experiment termination, 123 days after therapy, indicating a complete remission rate of 42% for MV-treatment in this model of disseminated ALL (data not shown).
Discussion
Our interest in development of MV as a therapy for B-cell leukemias led us to investigate oncolysis in primary patient-derived material to gain as much mechanistic information as possible. We found a number of differences between the replication and CPE of oncolytic MV in primary cells as compared to cell lines. An interesting and unexpected finding was the limited utility of MTT/MTS assay in determining MV-mediated cell death in primary ALL and CLL cells. There was a consistent enhancement of formazan production in MV-infected primary malignant B-cells compared to uninfected controls. Clearly, MV infection did not increase the number of viable primary cells in the culture (data not shown), instead it is likely that initial infection enhanced mitochondrial reductase activity, enabling infected cells to more effectively reduce MTT or MTS relative to “unactivated” controls. Hence, on a pragmatic note, we suggest that assays of cell death based on mitochondrial reductase activity might be misleading in the situation of MV infection of primary cells.
The heterogeneity in the extent of cell-to-cell fusion after MV infection in primary cells was unexpected. Variability of cell-to-cell fusion between different ALL samples was noted but the almost complete absence of syncytia in infected CLL cells despite clear evidence of infection and replication was unexpected. To date, published data on MV therapy in a variety of tumors consistently report substantial induction of fusion in tumor targets, at least in vitro4,5,6,7,8,18 and this has been regarded as a potential mechanism of MV-induced oncolysis. However, cell lines—as opposed to primary material—were the subject of study in all except one of these reports. Our own data show substantial differences in the levels of syncytia formation between cell lines and primary cells—Nalm-6, SEM, 697, and REH all readily formed very large multinucleated syncytia postinfection, a finding which is in sharp contrast with minimal cell-to-cell fusion in MV-infected primary CLL cells. A key point here is that, although the relative contributions of lysis versus fusion in inducing MV tumor cell death remain unclear, oncolytic activity after MV infection can certainly proceed without syncytia formation. Our finding, coupled with related reports of oncolytic equivalence between hypofusogenic and parent fusogenic MV strains against myeloma xenografts,19 suggest a mechanism for technical advances in refining and improving the efficacy of MV vectors for cancer therapy. Producing MV to high titers is difficult and the ability to concentrate the virus is limited by its relative fragility during centrifugation or dialysis. The limitations in titers are due in large part to the considerable cell-to-cell fusion engendered in producer cells, leading to their ultimate demise. Hypofusogenic MV vectors may be more readily produced at high titers and may therefore have greater clinical utility as an oncolytic agent for treating human tumor burdens.
The reasons underlying the lack of cell-to-cell fusion in primary CLL cells remain unclear. Western blotting showed an accumulation of MV-H and MV-F proteins as infection progressed, so absence of viral envelope protein production does not explain inability to form syncytia. One possible explanation we considered was lack of appropriate furin-mediated cleavage of MV-F0 to MV-F1, a phenomenon which has previously been reported in B-cell lines, in particular Daudi.20 However, the 42 kDa size of the MV-F band on the immunoblots and the lack of an F0 band at 64 kDa render this explanation unlikely. An alternative possibility is defective or absent transport of one or both glycoproteins to the cell surface in CLL cells. Cell surface expression of MV-H on the surface of MV-infected CLL cells was confirmed by flow cytometry in a subset of cases tested (data not shown) but cell surface expression of MV-F was not examined due to lack of suitable reagents. Failure of MV-F transport to the cell surface in CLL cells remains possible and has not been excluded.
Subcutaneous ALL xenografts were exquisitely sensitive to MV oncolysis. However, leukemias are rarely confined to a single site or solid mass, rather, they are almost overwhelmingly liquid, disseminated malignancies. Hence, the disseminated model is a more appropriate preclinical model for assessing the antitumor potential of MV in ALL. The highly significant prolongation of survival seen in mice with disseminated Nalm-6 leukemia treated with intravenous MV is the only demonstration of MV oncolysis in a disseminated cancer model other than myeloma.21 This, in addition to the finding of complete bone marrow responses in surviving mice in the MV-treated group, highlights not only the great potential for MV as a therapy for ALL but also the possibility for its therapeutic application in the setting of low tumor burdens or minimal residual disease. Minimal residual disease is easily and routinely determined in ALL by molecular or flow cytometric techniques and is significantly associated with ALL relapse.22
Although the apparent speed of onset of response of ALL to MV is promising, refinements in MV delivery methods in order to avoid a neutralizing antibody response will be still required in order to treat this disseminated malignancy successfully. Administration of the virus in infected cell carriers is one promising approach23,24 and has been recently demonstrated using an MV-infected irradiated myeloma cell line.21 The chemotherapeutic regimens used to treat ALL typically include multiple immunosuppressive drugs such as steroids and cyclophosphamide. Furthermore, monoclonal antibodies against B-cell antigens such as CD20,25 CD22,26 and CD1927 are gaining prominence in the armamentarium of therapy, leaving open the possibility that patients can receive virotherapy at a time of minimal residual disease during which they are also temporarily lacking the capacity to mount an anti-MV antibody response.
Both ALL and CLL are potential targets for MV-mediated lysis in vitro. However, the antitumor effects of MV, although present in CLL, are less clear cut than in ALL. In a putative clinical scenario, slow MV oncolysis may have the advantage of allowing tumor cells to produce a higher quantity of oncolytic virus thus offering the prospect of infecting and therefore eliminating more tumor burden. Mathematical models support this contention.28 However, this advantage could be offset by the progressively antiapoptotic phenotype that is typically associated with progressive CLL29 which might lead to eventual loss (versus slowing) of MV oncolysis. The relative abrogation of MV-induced cytotoxicity observed in Bcl-2 overexpressing cell lines (Figure 6) is in keeping with this hypothesis. The high level of gene expression achieved in CLL cells does, however, hold great promise toward using MV as gene transfer vectors for these cells. Limited CLL cell toxicity at the early stages of MV infection, absence of syncytia formation, and effects on mitochondrial function further strengthens the utility of MV vectors as a research tool in this context.
By contrast, the highly significant antineoplastic activity of MV against ALL—including in both subcutaneous and disseminated ALL xenografts—holds great promise toward developing attenuated MV as a therapeutic tool in adult ALL.
Materials and Methods
Primary cells and cell lines. 697, Nalm-6, were obtained from DSMZ (Braunschweig, Germany). SEM and REH cell lines were kind gifts from Dr Asim Kwaja (University College London). Cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum. Primary CLL cells were sourced entirely from fresh mononuclear preparations of peripheral blood, whereas ALL cells were isolated from frozen mononuclear derivatives of either bone marrow or peripheral blood. All specimens contained >90% neoplastic cells. Studies using primary patient material were approved by the Royal Free Hospital research ethics committee. Primary CLL cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum and primary ALL cells in AIM-V media supplemented with 20 ng/ml recombinant human interleukin-3, 10 ng/ml recombinant human interleukin-7 and 50 ng/ml stem cell factor (R&D Systems). Peripheral blood lymphocytes were isolated from healthy volunteers and used fresh. Vero cells (ATCC, Manassas,VA) were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum. Cell lines stably overexpressing Bcl-2 were generated by infection of Daudi, Raji, and Jurkat cells with a pBabePuro-bcl-2 retroviral vector. DNA encoding human Bcl-2 was PCR amplified from plasmid pB4 (ATCC) using primers tailed with EcoR1 sites and cloned into pBabePuro using EcoR1 digestion. Vector particles were produced by transient transfection of PA317 packaging cells. After retroviral vector transduction, cells were selected in puromycin. All Bcl-2 overexpressing cell lines were maintained in RPMI supplemented with 10% fetal bovine serum and puromycin.
MV production. MV was propagated on Vero cells as previously described8 and stored at −70 °C. Virus titers were determined by 50% TCID50 titration on Vero cells according to the Spearman–Karber method. Inactivation of virus stocks was performed by exposure to UV light for a period of 4 hours. MV inactivation was confirmed by titration on Vero cells.
Cell survival assays. Primary cells were inoculated with MV at a multiplicity of infection of 1.0 in 1 ml of Optimem (Invitrogen, Paisley, UK) at 37 °C for 2 hours, uninfected controls were incubated with Optimem alone. At the end of the incubation the viral inoculum/Optimem was removed and the cells maintained in their standard medium. Thereafter, at various timepoints, the cells were photographed using transmission microscopy and fluorescent light. Viability was assessed by Trypan blue staining followed by cell counting in a Neubauer chamber and flow cytometric analysis of TO-PRO-3 nuclear staining. Data from these assays were used to calculate MV-specific cell death rates using the following formula30 [(% cell death in MV treated cells−% cell death in control cultures)/(100−% cell death in control group) × 100]. MTT (Promega, Southampton, UK) or MTS (Promega) assays were carried out according to manufacturers' instructions. All colorimetric assays were performed in triplicate.
Real-time quantitative PCR of MV-N mRNA. RNA was extracted from primary ALL and CLL cells with RNAeasy Plus micro kit (Qiagen, Valencia, CA) and Trizol reagent (Invitrogen, San Diego, CA), respectively. Complementary DNA was synthesized with Superscript III Reverse Transcriptase (Invitrogen). Real-time quantitative PCR for MV-N and GAPDH transcripts was performed in duplicate in a 25 µl reaction using a custom Taqman gene expression assay (Applied Biosystems, Warrington, UK). Relative quantification analysis was performed using the comparative _C_T (2−ΔΔCT) method with GAPDH as a reference gene and uninfected control cycle threshold values as the calibrator. MV-N primer sequences were
5′-GTATCCTGCTCTTGGACTGCAT-3′ (forward),
5′-GTTCATCAAGGACTCAAGTGTGGAT-3′ (reverse) and Taqman probe sequence was 5′-TCACCAGCAAATTC-3′.
Assessment of MV replication in tumor cells. Five million CLL and 1 × 106 ALL cells derived from primary material or cell lines were infected as described above with MV at a multiplicity of infection of 1.0 and supernatants and cell lysates harvested daily. Viral titers were determined by 50% TCID50 titration on Vero cells in a 96 well plate.
Western blotting. Proteins were separated on a 12% polyacrylamide gel (Invitrogen) and transferred to nitrocellulose membranes according to previously published procedures.31 The following primary antibodies were used for MV protein expression: MV-H (Chemicon, Temicula, CA), MV-F (kind gift from Robert Cattaneo, Mayo Clinic College of Medicine), antibodies against actin (Sigma-Aldrich, Dorset, UK) were used as loading controls. Immunoreactive bands were visualized using appropriate horseradish peroxidase–linked secondary antibodies (Dako, Cambridge, UK) followed by enhanced chemiluminescence (GE Healthcare, Little Chalfont, UK).
Apoptosis was assayed by western blotting for poly(ADP-ribose) polymerase, (Alexis Biochemicals, Exeter, UK) cleavage. Bcl-2 protein expression was investigated in retrovirally transduced and control nontransduced Raji, Daudi, and Jurkat cell lines using the anti-Bcl-2 primary antibody, (1:2500; Pharmingen, San Diego, CA). Total protein levels were determined for both the transduced and nontransduced specimens using the BioRad DC protein assay and equivalent amounts of protein loaded onto polyacrylamide gels.
In vivo experiments. Six- to eight-week-old CB17 severe combined immunodeficient mice (Charles Rivers, Margate, UK) were housed in a barrier facility and cared for according to protocols approved by the UK home office. To establish subcutaneous ALL xenografts 1 × 107 viable Nalm-6 cells were premixed with 2 mg of Matrigel in a volume of 200 µl and injected subcutaneously, into the right flank. Once the tumors were palpable and measurable in two diameters by caliper assessment (~2–3 weeks after tumor cell injection) 1 × 106 pfu of MV was administered intratumorally for 10 doses beginning at day 20 over a 2-week period. Control mice received identical treatment with UV-MV. Tumor measurements were made five times a week and the tumor volume calculated according to the formula V =a2b/2 where a is the shortest and b the longest diameter. Mice were euthanized when tumors reached a volume of 2.5 cm3 or when invasion into local tissue was observed.
Disseminated ALL xenografts were established by tail vein injection of 1 × 106 Nalm-6 cells. Three days after tumor cell transfer 1 × 106 pfu of MV was intravenously administered by tail vein injection and repeated at weekly intervals for a total of six doses. Control mice were injected with UV-MV. Mice were monitored daily and euthanized when predefined humane end points were reached.
Statistical analysis. Data are presented as the mean values ± SEM. The significance of differences between subcutaneous xenograft groups was determined by Wilcoxon rank test. Survival curves were compared using the log rank test.
REFERENCES
- Fielding AK. Measles as a potential oncolytic virus. Rev Med Virol. 2005;15:135–142. doi: 10.1002/rmv.455. [DOI] [PubMed] [Google Scholar]
- Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA.et al. (2010Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer Cancer Res 70875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y. The cellular receptor for measles virus–elusive no more. Rev Med Virol. 2001;11:149–156. doi: 10.1002/rmv.308. [DOI] [PubMed] [Google Scholar]
- Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC., and, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ.et al. (2003Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme Cancer Res 632462–2469. [PubMed] [Google Scholar]
- Msaouel P, Iankov ID, Allen C, Morris JC, von Messling V, Cattaneo R.et al. (2009Engineered measles virus as a novel oncolytic therapy against prostate cancer Prostate 6982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R., and, Russell SJ. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–2007. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA.et al. (2001Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice Blood 973746–3754. [DOI] [PubMed] [Google Scholar]
- Frecha C, Costa C, Lévy C, Nègre D, Russell SJ, Maisner A.et al. (2009Efficient and stable transduction of resting B lymphocytes and primary chronic lymphocyte leukemia cells using measles virus gp displaying lentiviral vectors Blood 1143173–3180. [DOI] [PubMed] [Google Scholar]
- Anderson BD, Nakamura T, Russell SJ., and, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- Grote D, Cattaneo R., and, Fielding AK. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–6468. [PubMed] [Google Scholar]
- Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK.et al. (2008In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood 1111827–1833. [DOI] [PubMed] [Google Scholar]
- Chiorazzi N, Rai KR., and, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H.et al. (2008_WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues_4th edn. IARC: Lyon, France [Google Scholar]
- Buggins AG., and, Pepper CJ. The role of Bcl-2 family proteins in chronic lymphocytic leukaemia. Leuk Res. 2010;34:837–842. doi: 10.1016/j.leukres.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Uckun FM, Yang Z, Sather H, Steinherz P, Nachman J, Bostrom B.et al. (1997Cellular expression of antiapoptotic BCL-2 oncoprotein in newly diagnosed childhood acute lymphoblastic leukemia: a Children's Cancer Group Study Blood 893769–3777. [PubMed] [Google Scholar]
- Coustan-Smith E, Kitanaka A, Pui CH, McNinch L, Evans WE, Raimondi SC.et al. (1996Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia Blood 871140–1146. [PubMed] [Google Scholar]
- Allen C, Paraskevakou G, Liu C, Iankov ID, Msaouel P, Zollman P.et al. (2008Oncolytic measles virus strains in the treatment of gliomas Expert Opin Biol Ther 8213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Russell SJ, Dingli D.2010Fusion Versus Lysis: Non-Fusogenic Measles Viruses as Oncolytic Agents [abstract]American Society of Gene Therapy 2009 Meeting:S3
- Fujinami RS., and, Oldstone MB. Failure to cleave measles virus fusion protein in lymphoid cells. J Exp Med. 1981;154:1489–1499. doi: 10.1084/jem.154.5.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Russell SJ., and, Peng KW. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol Ther. 2010;18:1155–1164. doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Rai L, Buck G, Richards SM, Mortuza Y, Mitchell W.et al. (2010Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993 Br J Haematol 14880–89. [DOI] [PubMed] [Google Scholar]
- Munguia A, Ota T, Miest T., and, Russell SJ. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 2008;15:797–806. doi: 10.1038/gt.2008.45. [DOI] [PubMed] [Google Scholar]
- Iankov ID, Blechacz B, Liu C, Schmeckpeper JD, Tarara JE, Federspiel MJ.et al. (2007Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy Mol Ther 15114–122. [DOI] [PubMed] [Google Scholar]
- Thomas DA, O'Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W.et al. (2010Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia J Clin Oncol 283880–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussai F, Campana D, Bhojwani D, Stetler-Stevenson M, Steinberg SM, Wayne AS.et al. (2010Cytotoxicity of the anti-CD22 immunotoxin HA22 (CAT-8015) against paediatric acute lymphoblastic leukaemia Br J Haematol 150352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S.et al. (2008Tumor regression in cancer patients by very low doses of a T cell-engaging antibody Science 321974–977. [DOI] [PubMed] [Google Scholar]
- Dingli D, Offord C, Myers R, Peng KW, Carr TW, Josic K.et al. (2009Dynamics of multiple myeloma tumor therapy with a recombinant measles virus Cancer Gene Ther 16873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grever MR, Lucas DM, Dewald GW, Neuberg DS, Reed JC, Kitada S.et al. (2007Comprehensive assessment of genetic and molecular features predicting outcome in patients with chronic lymphocytic leukemia: results from the US Intergroup Phase III Trial E2997 J Clin Oncol 25799–804. [DOI] [PubMed] [Google Scholar]
- Friesen C, Herr I, Krammer PH., and, Debatin KM. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- Jones DT, Ganeshaguru K, Virchis AE, Folarin NI, Lowdell MW, Mehta AB.et al. (2001Caspase 8 activation independent of Fas (CD95/APO-1) signaling may mediate killing of B-chronic lymphocytic leukemia cells by cytotoxic drugs or gamma radiation Blood 982800–2807. [DOI] [PubMed] [Google Scholar]