The aneuploidy paradox in cell growth and tumorigenesis (original) (raw)
. Author manuscript; available in PMC: 2011 Jul 10.
Published in final edited form as: Cancer Cell. 2008 Dec 9;14(6):431–433. doi: 10.1016/j.ccr.2008.11.011
Summary
Aneuploidy, an abnormal chromosome number, is a frequent characteristic of malignant cells, leading to the suggestion that aneuploidy drives tumorigenesis. In a recent issue of Science, Williams et al. demonstrated that chromosome gains in primary cells cause proliferative defects, which is paradoxical since chromosome gains frequently occur in human tumors.
Aneuploidy is a hallmark of tumor cells
Aneuploidy occurs in ~90% of solid human tumors and ~75% of hematopoietic cancers (Weaver and Cleveland, 2006). Aneuploidy was recognized as a common characteristic of tumor cells 118 years ago. This finding led to Boveri’s hypothesis that aneuploid cells are the progenitors of tumors in 1902 and 1914.
An experimental test of Boveri’s idea has been hampered by the difficulty of generating aneuploidy without causing additional, confounding defects. Early experiments used drugs to induce aneuploidy, many of which were subsequently shown to induce DNA damage. More recent experiments have used mouse models that generate high degrees of whole chromosomal aneuploidy from reduced levels of components of the mitotic checkpoint (also known as the spindle assembly checkpoint). This checkpoint is the major cell cycle control pathway in mitosis; it acts to prevent aneuploidy by delaying cell cycle advance through mitosis prior to successful attachment of every chromosome to the mitotic spindle (Musacchio and Salmon, 2007). Mice that express reduced levels of mitotic checkpoint components develop aneuploidy, but have divergent tumor phenotypes. Some are tumor prone, while others are not. These divergent outcomes are likely due to the fact that most of these mitotic checkpoint components are expressed throughout the cell cycle and participate in diverse cellular processes, including apoptosis, transcriptional repression, and chromosomal rearrangements (Weaver and Cleveland, 2006). However, CENP-E, a tether between spindle microtubules and centromeres, is a component of the mitotic checkpoint that apparently functions exclusively in chromosome segregation. Reduction in CENP-E promotes some types of tumors and inhibits others (Weaver et al., 2007).
Chromosome gains, but not losses, cause a proliferative disadvantage
Following this preceding work, Williams et al created aneuploid Murine Embryonic Fibroblasts (MEFs) of a defined karyotype (Williams et al., 2008). These cells contain a single extra copy of chromosome 1, 13, 16 or 19 and were created by mating animals containing two different Robertsonian translocations involving the same chromosome with wild type animals. In each case, gain of a single whole chromosome yielded trisomic MEFs that grew more slowly than diploid MEFs (Williams et al., 2008). These results from cells that have a stable 2n + 1 aneuploid genome are consistent with results from cells that exhibit Chromosomal INstability (CIN). CIN cells are aneuploid due to recurring gains and losses of chromosomes during multiple divisions. In vivo, splenocytes and peripheral blood lymphocytes that exhibit aneuploidy and CIN due to reduction in CENP-E proliferate well after loss of one or a few chromosomes. However, cells that have gained one or a few chromosomes are almost completely eliminated from the cycling population. This proliferative disadvantage is observable, albeit more subtle, in MEFs with reduced levels of CENP-E, where chromosome losses outnumber gains in the cells that continue cycling (Weaver et al., 2007).
A proliferative disadvantage following chromosome gain, but not chromosome loss, seems to be a consistent lesson. Developing neuronal precursors (Kaushal et al., 2003), ES cells lacking the BRCA1 tumor suppressor (Shen et al., 1998), MEFs that express a mutant form of the Adenomatous Polyposis Coli (APC) tumor suppressor, and MEFs that are heterozygous for the mitotic checkpoint component BubR1 (Rao et al., 2005), have all been seen to grow more slowly after chromosome gain. This is also true after gain of even a single chromosome in budding yeast (Torres et al., 2007). Thus, chromosome gains consistently cause a growth defect in multiple species and contexts.
So what underlies the growth disadvantage from an extra copy of a single chromosome? For the four mouse chromosomes tested by Williams et al (2008), the simplest view is that increased expression of each of the many genes on the additional chromosome (the smallest of which encodes 678 known genes) disrupts the balance of gene products required for normal cell growth and cycling. It is also possible that chromosome gains cause a proliferative defect due to increased levels of DNA damage or unfolded proteins. Whatever the mechanism, the available data suggest that it is surprisingly sensitive, since gain of a single, small chromosome is sufficient to engage it. It is also unclear at what stage of the cell cycle chromosome gains cause delay, as accumulation at a particular cell cycle stage was not observed (Williams et al., 2008). Nor did trisomic MEFs display an increase in cell death or senescence. Interestingly, MEFs that have gained chromosomes continue to cycle after loss of p53, suggesting that the proliferative disadvantage caused by chromosome gain is (at least partly) p53 dependent (Matijasevic et al., 2008).
Aneuploid tumor cells predominantly exhibit chromosome gains
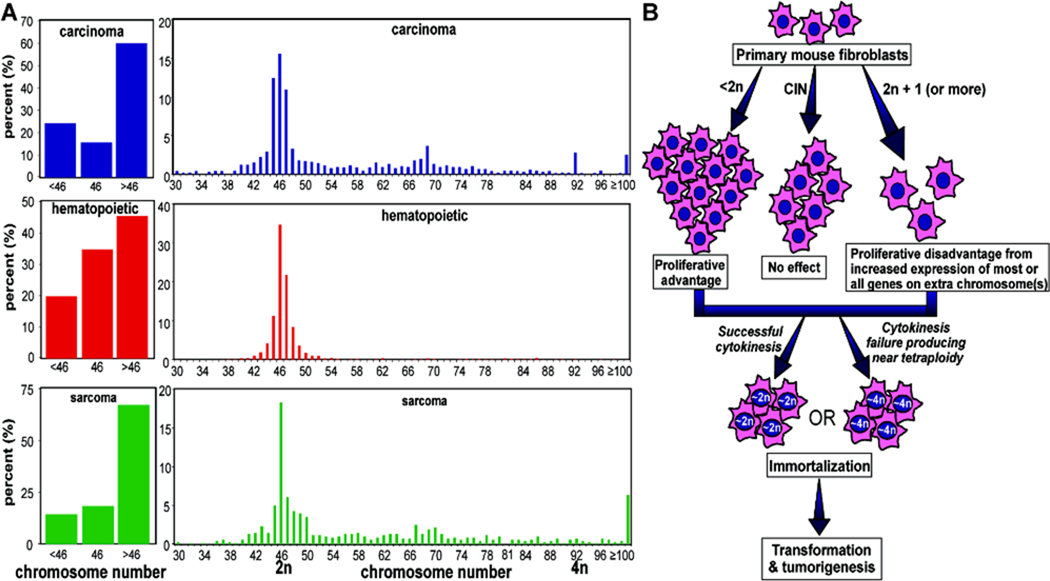
The fact that chromosome gains, but not chromosome losses, cause a proliferative defect raises a paradox for their contribution to tumorigenesis, because most tumor cells exhibit chromosome gains (Fig. 1A). For all tumors, including carcinomas, hematopoietic cancers and sarcomas, >70% of aneuploid tumor cells exhibit chromosome gains (Fig. 1A, left panels). While spontaneous immortalization of each of the MEFs triploid for defined chromosomes was accompanied by acquisition of a near tetraploid state (Williams et al, 2008), what this means for tumorigenesis is unsettled, as this is not the case for hematopoeitic malignacies and only a minority of carcinomas or sarcomas are near tetraploid (Fig. 1A, right panels).
Figure 1. Chromosome gains commonly occur in human tumors, but cause proliferative defects in primary cells.
A. Chromosome gains are the most common type of aneuploidy found in human tumors. The number of chromosomes per cell in carcinomas (top), hematopoietic cancers (middle) and sarcomas (bottom) are shown. Chromosome numbers were obtained from the Mitelman database of cancer chromosomes (Mitelman et al., 2006). B. Different types of aneuploidy have distinct effects on cell growth and tumorigenesis. Cells that have (left) lost chromosomes or (middle) exhibit continuing Chromosomal INstability (CIN) do not have a growth defect. (Right) Chromosome gains cause a proliferative disadvantage in primary cells, probably from increased levels of expression of the many genes encoded by the additional chromosome. Spontaneous immortalization can be accompanied by tetraploidization, which is probably the result of cytokinesis failure, as observed by Williams et al. However, tetraploidization is not a requirement for immortalization, as p19ARF−/− MEFs are immortal and contain a near-diploid karyotype (Weaver and Cleveland, unpublished).
Aneuploidy can promote and inhibit immortalization and tumorigenesis
The effect of chromosome gain on spontaneous immortalization appears to depend on which chromosome is involved. An extra copy of chromosome 13 delays immortalization, an extra copy of chromosome 16 may accelerate immortalization, and an extra copy of chromosome 19 has no effect (Williams et al., 2008). This is reminiscent of the finding that aneuploidy due to reduction in CENP-E promotes spontaneous spleen and lung tumors, but inhibits genetically and chemically induced tumors (Weaver et al., 2007). Similarly, increasing the rate of chromosome missegregation in animals expressing the Multiple Intestinal Neoplasia allele of APC by reducing levels of the mitotic checkpoint component BubR1 causes increased levels of colon tumors, but decreased rates of small intestine tumors (Rao et al., 2005). Thus, a consistent insight is that aneuploidy can both promote and inhibit immortalization, transformation and tumorigenesis, depending on the context of other genetic changes. Unsurprisingly, the effects of aneuploidy are likely to be dependent on the specific chromosomes that have been gained and lost (Fig. 1B).
Conclusions – Not all aneuploid cells are created equal
Aneuploidy has been proposed as a cause of cancer for over 100 years, based on the fact that aneuploidy is a strikingly common characteristic of tumors. It is now clear that the effects of aneuploidy are more complex than initially proposed. Aneuploidy can drive tumorigenesis, but it does not necessarily do so. In some contexts, aneuploidy actually suppresses tumors. It is not yet clear why aneuploidy has different effects in distinct contexts, but at least three factors are almost certainly relevant: 1) the specific combination of chromosomes present in a given cell; 2) whether the cell is stably aneuploid or contains a karyotype that is evolving due to further chromosomal missegregation; and 3) the additional mutations, particularly in oncogenes and tumor suppressors, that are retained (or lost) in the cell. Further experiments will be required to determine which types of aneuploidy promote versus inhibit tumors in a given context.
Contributor Information
Beth A. Weaver, Email: baweaver@wisc.edu.
Don W. Cleveland, Email: dcleveland@ucsd.edu.
References
- Kaushal D, Contos JJ, Treuner K, Yang AH, Kingsbury MA, Rehen SK, McConnell MJ, Okabe M, Barlow C, Chun J. Alteration of gene expression by chromosome loss in the postnatal mouse brain. J Neurosci. 2003;23:5599–5606. doi: 10.1523/JNEUROSCI.23-13-05599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matijasevic Z, Steinman HA, Hoover K, Jones SN. MdmX promotes bipolar mitosis to suppress transformation and tumorigenesis in p53-deficient cells and mice. Mol Cell Biol. 2008;28:1265–1273. doi: 10.1128/MCB.01108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman F, Johansson B, Mertens FE. Mitelman Database of Chromosome Aberrations in Cancer (2006) 2006 http://cgapncinihgov/Chromosomes/Mitelman.
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nature reviews. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Rao CV, Yang YM, Swamy MV, Liu T, Fang Y, Mahmood R, Jhanwar-Uniyal M, Dai W. Colonic tumorigenesis in BubR1+/−ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proc Natl Acad Sci U S A. 2005;102:4365–4370. doi: 10.1073/pnas.0407822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen SX, Weaver Z, Xu X, Li C, Weinstein M, Chen L, Guan XY, Ried T, Deng CX. A targeted disruption of the murine Brca1 gene causes gamma-irradiation hypersensitivity and genetic instability. Oncogene. 1998;17:3115–3124. doi: 10.1038/sj.onc.1202243. [DOI] [PubMed] [Google Scholar]
- Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317:916–924. doi: 10.1126/science.1142210. [DOI] [PubMed] [Google Scholar]
- Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007;11:25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]