Proliferative Capacity of Stem/Progenitor-like Cells in the Kidney may Associate with the Outcome of Patients with Acute Tubular Necrosis (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 1.
SUMMARY
Animal studies indicate that adult renal stem/progenitor cells can undergo rapid proliferation in response to renal injury, but whether the same is true in humans is largely unknown. To examine the profile of renal stem/progenitor cells responsible for acute tubular necrosis in human kidney, double- and triple-immunostaining was performed using proliferative marker and stem/progenitor protein markers on sections from ten kidneys with acute tubular necrosis and four normal adult kidneys. The immunopositive cells were recorded using two-photon confocal laser scanning microscopy. We found that dividing cells were present in the tubules of the cortex and medulla, as well as the glomerulus in normal human kidney. Proliferative cells in the parietal layer of Bowman’s capsule expressed CD133, and dividing cells in the tubules expressed immature cell protein markers paired box gene 2, vimentin and nestin. After acute tubular necrosis, Ki67-positive cells in the cortex tubules significantly increased compared to normal adult kidney. These Ki67-positive cells expressed CD133 and paired box gene 2, but not the cell death marker, activated caspase-3. In addition, the number of dividing cells increased significantly in patients with acute tubular necrosis, who subsequently recovered, compared to patients with acute tubular necrosis, who consequently developed protracted acute tubular necrosis or died. Our data suggest that renal stem/progenitor cells may reside not only in the parietal layer of Bowman’s capsule, but also in the cortex and medulla in normal human kidney, and the proliferative capacity of renal stem/progenitor cells after acute tubular necrosis may be an important determinant of a patient’s outcome.
Keywords: ATN, kidney, progenitor cells, stem cells, outcome, proliferation
INTRODUCTION
Acute tubular necrosis (ATN) is the most common cause of acute renal failure (ARF) in the hospital and intensive care units (ICU). It occurs after ischemic or nephrotoxic injury, or after a combination of both (mixed ATN). Animal studies show that cells in the tubules regenerate in response to renal injury, although the source of the cells that repopulate the injured nephron is unclear.
Previous studies have reported that Y-chromosome-positive cells that co-express epithelial protein markers are observed within the renal tubules in male patients transplanted with female kidneys [1, 2], and in lethally irradiated female mice transplanted with male bone marrow [1]. Purified mesenchymal stem cells (MSCs) expressing green fluorescent protein (GFP) can also integrate into developing tubules and glomeruli, and express appropriate tubular- and glomerulus-specific markers after injection into embryonic rats [3, 4]. These findings suggest that bone marrow-derived cells could transdifferentiate into tubular cells. However, recent studies show that Y-chromosome-positive cells that co-express tubule-specific markers are rarely observed in the damaged region of mouse kidney after unilateral renal ischemia [5–7], indicating that even if bone marrow-derived cells retain the capacity to transdifferentiate into renal epithelial cells, these cells may not significantly contribute to structural and functional repair in the acute tubule regenerative process [8], Instead, resident cells in the kidney may be the major source for renal regeneration.
Recent studies have focused on the possibility of residual renal stem/progenitor cells (RSCs) within the kidney. Oliver et al first reported the identification of slow dividing cells in the deepest zone of inner medulla of both rat and mouse adult kidney, as evidenced by immunostaining after administrating BrdU into animals [9, 10]. These BrdU-positive cells rapidly disappeared from the papilla following transient renal ischemia [9]. Clonal analysis reveals that these cells can differentiate into epithelial, neuronal and other uncharacterized cells under certain culture conditions, and incorporate into various tubule segments after injection under the renal capsule [11]. In addition, side population (SP) cells identified based on the cells’ ability to extrude dye Hoechst 33332 have been found in the renal interstitium of animals [12]. The SP cells are regulated by cells that reside in the intersitium of kidney and can further differentiate into multiple lineages in the presence of leukemia inhibitory factor (LIF) [12]. Pioneering work done by Bussolati and colleagues [13] shows the detection of a small number of cells expressing CD133, a stem/progenitor marker [14, 15], in the adult human kidney. Double immunostaining shows that CD133-positive cells also express paired box gene 2 (PAX2), an embryonic renal marker [13]. Renal tissue-derived CD133-positive cells are not only capable of expansion and differentiation into epithelial or endothelial cells in vitro, but also formation of tubular structures that express renal epithelial markers in vivo after subcutaneous implantation in SCID mice [13]. Other studies show that CD133 is predominantly expressed in a subset of parietal epithelial cells (PEC) in the adult kidney’s Bowman’s capsule, although CD133-positive cells confirmed the ability to differentiate into the kidney’s specialized structure and functionally recover after intravenous injection in SCID mice followed ARF [13, 16]. The fact that adult RSCs can undergo rapid proliferation in response to renal injury is mainly derived from the animal model, whether it can be applied to human largely unknown.
To examine the profile of RSCs responsible for ATN in adult human kidney, double- and triple-immunostaining was performed using proliferative marker and progenitor protein markers on sections from kidneys with ATN and normal kidneys. We found that the number of proliferative cells increased significantly in patients with ATN, who subsequently recovered, compared to ATN patients, who developed protracted ATN or death. Our data suggest that RSCs may reside not only in the parietal layer of Bowman’s capsule, but also in the cortex and medulla in normal human kidney, and that the proliferative capacity of RSCs in the kidney after ATN is an important determinant of the patient’s outcome.
1. MATERIALS AND METHODS
1.1. Human Renal Specimens
Ten renal specimens from patients with ATN, who were administrated at the Sir Run Run Shaw Hospital of Zhejiang University School of Medicine between 2005–2008, were obtained by renal biopsy. All biopsies had been performed by puncture, guided by ultrasound, at 2–5 days after the ATN diagnosis. The renal function of these patients was monitored up to 60 days. The patients ranged in age from 31 to 74 years with a mean of 51.8 years (median 50). Studies of acute kidney injury usually lack data on pre-admission kidney function [17]. The outpatient serum creatinine (from 0.7mg—1.3mg/dl) was thus obtained from their medical history records within one year prior to hospital administration, which was used as a reference of the baseline renal function of the patients. In addition, ATN patients with history of chronic kidney disease, diabete melitus, hepertension, congestive heart failure and liver disease were not selected in this study. Patient information is summarized in Table 1. Four renal specimens from patients without clinical or postmortem evidence of renal disease were obtained from the unmatched donor organs in this hospital and from NICHD Brain and Tissue Bank for Developmental Disorders (Baltimore, MD, USA). The patients ranged in age from 28 to 46 years with a mean of 40 years. The study was performed according to the Declaration of Helsinki and protocols approved by the Institutional Research Review Board at Sir Run Run Shaw Hospital of Zhejiang University School of Medicine, China.
Table 1.
Characteristics of all studied patients
| Patient | Sex | Age | Eitilogy | Creatinine(mg/dl) | Ureanitrogen(mg/dl) | Biopsy | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | F | 37 | Amikacin | 8.1 | 51 | ATN | Recovery |
| 2 | F | 74 | Gentamicin | 7.1 | 65 | ATN | Recovery |
| 3 | F | 74 | Amikacin | 8.3 | 68 | ATN | Chronic |
| 4 | F | 46 | Gentamicin | 6.5 | 41 | ATN | Recovery |
| 5 | M | 63 | Aristolochic Acid | 10.2 | 108 | ATN | Chronic |
| 6 | M | 54 | Biliary tract infection | 5.3 | 106 | ATN | Recovery |
| 7 | M | 31 | Biliary tract infection | 6.2 | 98 | ATN | Death |
| 8 | F | 52 | Biliary tract infection | 8.4 | 116 | ATN | Chronic |
| 9 | M | 53 | Traumatic hepatic rupture | 8.5 | 98 | ATN | Death |
| 10 | M | 34 | Epidemic hemorrhagic fever | 11 | 120 | ATN | Recovery |
1.2. Immunohistochemistry
After the initial fixation with alcoholic 10% Formalin, renal tissues were postfixed in paraformaldehyde for 24 hr, incubated with 30% sucrose for 3 days, and embedded in paraffin; 6-µm sections were cut on a microtome and stored at room temperature. Sections were deparaffinized with xylene and rehydrated with ethanol. The sections were subjected to an antigen retrieval procedure according to the manufacture instruction (Vector Laboratories). In brief, Na-Citrate buffer (10 mM, pH 6.5) was pre-hearted in the microwave. The sections were then soaked in the solution and cooked for 15 min in the microwave, then cool down at the room temperature for 20 min [18]. Endogenous peroxidase activity was blocked by 30 min incubation at room temperature in 1% H2O2. After several washes with PBS, sections were incubated in blocking solution (2% goat serum, 0.1% Triton X-100, 1% bovine serum albumin in PBS) for 1 hr at room temperature. Primary antibodies (Table 2) used were 1) mouse monoclonal anti-proliferation-related Ki-67 antigen (Ki-67; Novocastra, Newcastle upon Tyne, UK; 1:50), 2) rabbit polyclonal anti-Ki-67 antigen (Zymed, Southern San Francisco, CA; 1:100), 3) goat polyclonal anti-minichromosome maintenance 2 (MCM2; Santa Cruz Biotechnology, Santa Cruz, CA; 1:100), 4) goat polyclonal anti-human-specific Vimentin (Chemicon, Billerica, MA; 1:200), 5) rabbit polyclonal anti-PAX2 antigen (Zymed; 1:500) and 6) mouse monoclonal anti-CD133 (Miltenyi Biotc, Germany; 1:100). Primary antibodies were added in blocking buffer and incubated with sections at 4°C overnight. After the incubation, sections were washed with PBS, followed by incubation with biotinylated goat anti-rabbit or goat antibody (1:200) (for polyclonal antibodies) or biotinylated horse anti-mouse antibody (1:200) (for monoclonal antibodies) for 1 hr at room temperature. Avidin–biotin complex (Vector Elite; Vector Laboratories, Burlingame, CA) and a diaminobenzidine solution or nickel solution (Vector Laboratories) were used to obtain a visible reaction product. Sections were dehydrated, sealed, and coverslipped. A Nikon microscope and a Magnifire digital color camera were used for the observations and photography of the slides, respectively.
Table 2.
Antibodies used for immunocytochemistry
| Antibody | Dilution | Manufacturer | Catalog # |
|---|---|---|---|
| Mouse anti-Ki67 | 1:50 | Novocastra, Newcastle upon Tyne, UK | NCL-L-Ki67-MM1 |
| Rabbit anti-Ki67 | 1:100 | Zymed, Southern San Francisco, CA, USA | 180191 |
| Goat anti-MCM2 | 1:100 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | Sc-9839 |
| Goat anti-Vimentin | 1:500 | Chemicon, Billerica, MA, USA | AB1620 |
| Rabbit anti-PAX2 | 1:500 | Zymed, Southern San Francisco, CA, USA | 76-6000 |
| Mouse anti-CD133 | 1:100 | Miltenyi Biotec, Germany | 130-090-422 |
| Mouse anti-human specific nestin | 1:500 | Chemicon, Billerica, MA, USA | MAB5326 |
| Rabbit anti-Notch1 | 1:200 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | Sc-6014R |
| Mouse anti-cleavaged caspase-3 | 1:500 | Cell signaling, Danvers, MA, USA | #9661 |
1.3. Double- and Triple-label Immunostaining
Double- or triple-label immunostaining was performed on renal sections as previously described [19]. The primary antibodies used, in addition to those listed above, were 1) mouse monoclonal anti-human specific nestin (Chemicon; 1:200), 2) rabbit polyclonal anti-Notch1 (Santa Cruz Biotechnology; 1:200), and 3) mouse monoclonal anti-cleavaged caspase-3 (Cell signaling, Danvers, MA; 1:500). The secondary antibodies were Alexa Fluor 488-, 594-, or 647-conjugated donkey anti-mouse, anti-goat, or anti-rabbit IgG (1:200–500; Molecular Probes, Carlsbad, CA). Nuclei were counterstained with DAPI using prolong Gold antifade reagent (Molecular Probes). Fluorescence signals were detected using an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss Ltd) equipped with a two-photon Chameleon laser (Coherent Inc.). Images were acquired using LSM 510 Imaging Software (Carl Zeiss Ltd). Two, triple or four color images were scanned using argon, 543 HeNe, 633 HeNe, and Chameleon (750–780 nm for DAPI) lasers. Selected images were viewed at high magnification, and 3D images were constructed using Imaris software version 7.0 (Bitplane AG, Zurich, Switzerland). Controls will include omitting either the primary or secondary antibody or preabsorbing primary antibody.
1.4. Cell counting
Quantification of immunopositive cells were determined by counting in the renal tissue. immune-positive cells in a sequence of ten consecutive computer images of 400x high power fields-0.0047 mm2 each. Only immunoreactive cells with the clear identifiable nuclei were counted. The results were expressed as a mean number of immunopositive cells per mm2. In each specimen staining intensity was recorded by two independent observers.
2. RESULTS
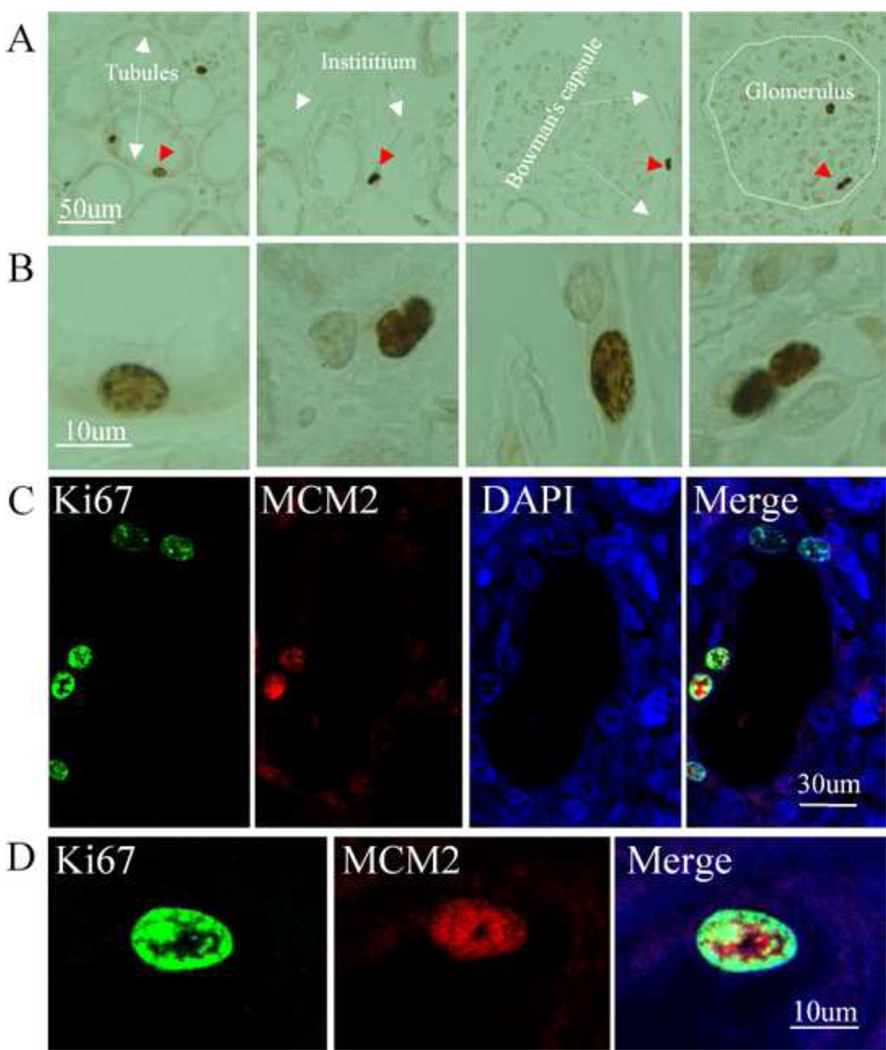
Immunocytochemistry was first performed using antibody against Ki67, a marker of cell cycling and proliferation, to investigate the profile of proliferative cells in normal human kidney. We found that Ki67 was expressed in cells in the proximal and distal tubules, as well as in the intersititium of both cortex and medulla of adult normal human kidney. Some of them were located near the tubular basement membrane of the intersititium. Ki67-positive cells were also found in the renal glomerulus and the parietal layer of Bowman’s capsule (Fig. 1A). High magnification views show that Ki67 was expressed in the cell nuclei, which was confirmed by counterstaining of nuclei with DAPI (Fig. 1A and B). About 1.6 ± 0.2/mm2 Ki67-positive cells were randomly distributed throughout the normal human kidney. Some Ki67-positive cells co-expressed MCM2, a protein expressed in G1 phase of the cell cycle, confirming that Ki67-positive cells were indeed proliferating (Fig. 1B).
Fig. 1.
Presence of Proliferative cells in the adult normal human kidney. Immunocytocehmistry was performed on the sections from normal adult human kidney. Ki67-positive cells were found in cells located in the tubule, intersititium, the parietal layer of Bowman’s capsule and glomerulus as indicated (A). High magnification of images from panel A (B). Double-immunostaining was performed using anti-Ki67 and MCM2, and images were recorded using a two photon laser-scanning confocal microscope. Ki67-positive cells (green) co-expressed MCM2 (red). DAPI (blue) was used to counterstain the nuclei (C). High magnification of images from panel C (D).
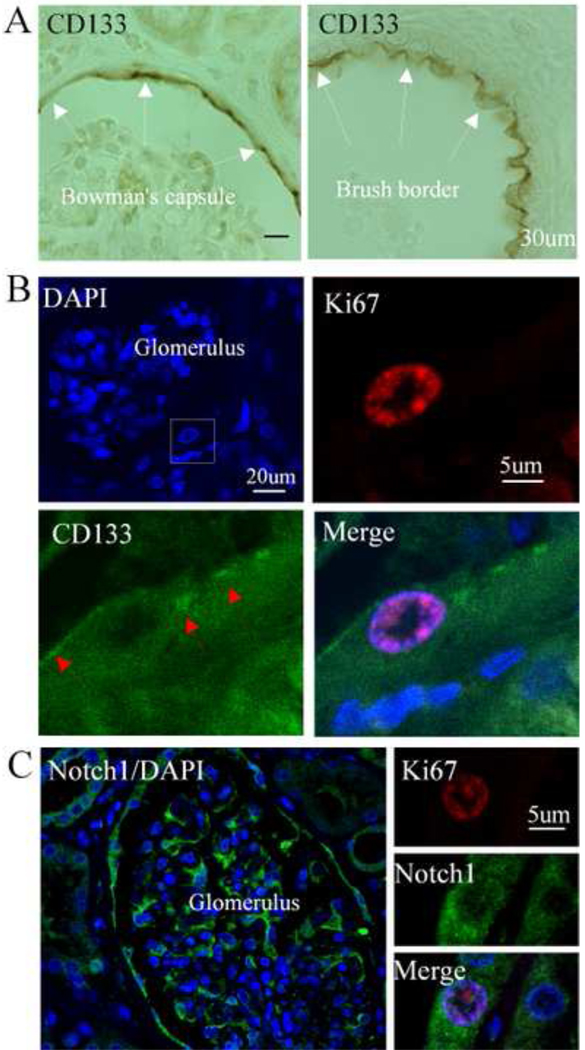
To determine the phenotype of Ki67-positive cells in the normal kidney, we performed immunostaining using anti-CD133 antibody, since CD133 has been considered a marker of renal progenitor cells. As shown in Figure 2A, we confirmed that CD133 were predominantly expressed in the subpopulations of cells in the parietal layer of Bowman’s capsule in the juxtamedullary region of the cortex, and in the brush border of cuboidal epithelial cells lining the proximal convoluted tubule. Some of the Ki67-positive cells in the parietal layer of Bowman’s capsule expressed CD133. Notch1 was abundantly expressed in a subset of cells in the parietal layer of Bowman’s capsule, tubule, and glomerulus. Other Notch1-positive cells located in the tubule expressed Ki67 (Fig. 2C).
Fig. 2.
Co-expression of CD133 and Notch1 with Ki67 in the adult normal human kidney. CD133 was expressed in cells located in the parietal layer of Bowman’s capsule (left panel) and brush border of tubule in normal human kidney (right panel) (A). Low magnification of the glomerulus staining with DAPI (blue) (top left panel). Double labeling showed that Ki67-positive cells (red) expressed CD133 (green) (B). Notch1 (green) was highly expressed in the cells located in the parietal layer of Bowman’s capsule and the glomerulus (left panel). Double immunolabeling indicated that Ki67-positive cells (red) in the tubule of kidney expressed Notch1 (green) (right panel) (C). DAPI was used for nuclei counterstaining.
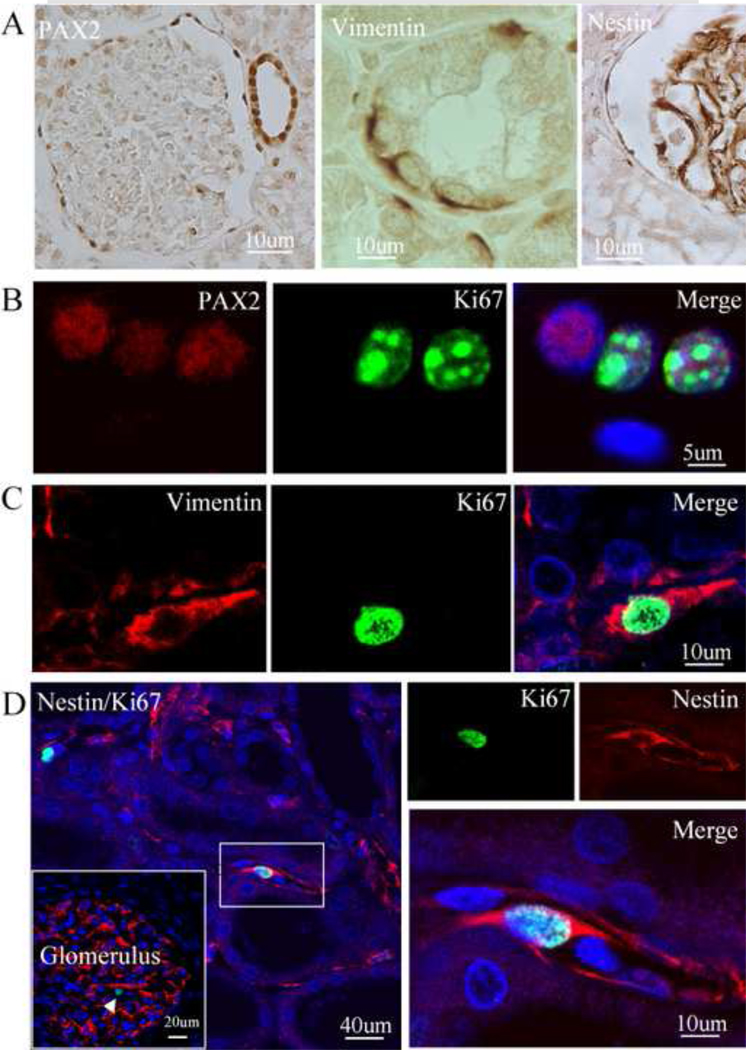
In light of the insubstantial expression of CD133 in the tubule and intersititium, we next asked whether immature proteins other than CD133 were expressed in dividing cells in these normal regions. We found that PAX2 was highly expressed in the cells located in the intersititium and the parietal layer of Bowman’s capsule, as well as in the tubule and the glomerulus (Fig. 3A). Some of them expressed Ki67 (Fig. 3B and C). Nestin, an immature marker, was expressed in the interstitial and glomerular cells (Fig. 3D, left panel). As shown in Figure 3D, some interstitial nestin-positive cells expressed Ki67.
Fig. 3.
Co-expression of PAX2, vimentin and nestin with Ki67 in the adult normal human kidney. PAX2 (right panel), vimentin (middle panel) and nestin (right panel) were expressed in cells located in the tubule and intersititium and in the parietal layer of Bowman's capsule and glomerulus of normal human kidney (A). PAX2-positive cells (red) in the tubule co-expressed Ki67 (green) (B). Vimentin-positive cells (red) in the tubule co-expressed Ki67 (green) (C). Immunocytochemistry showed that nestin (red) was expressed in the interstitial cells and glomerular cells (insert). Some of nestin-positive cells in the intersititium co-expressed Ki67 (red). Left panel: low magnification; right panel: high magnification. Nuclei were counterstained with DAPI (blue) (D).
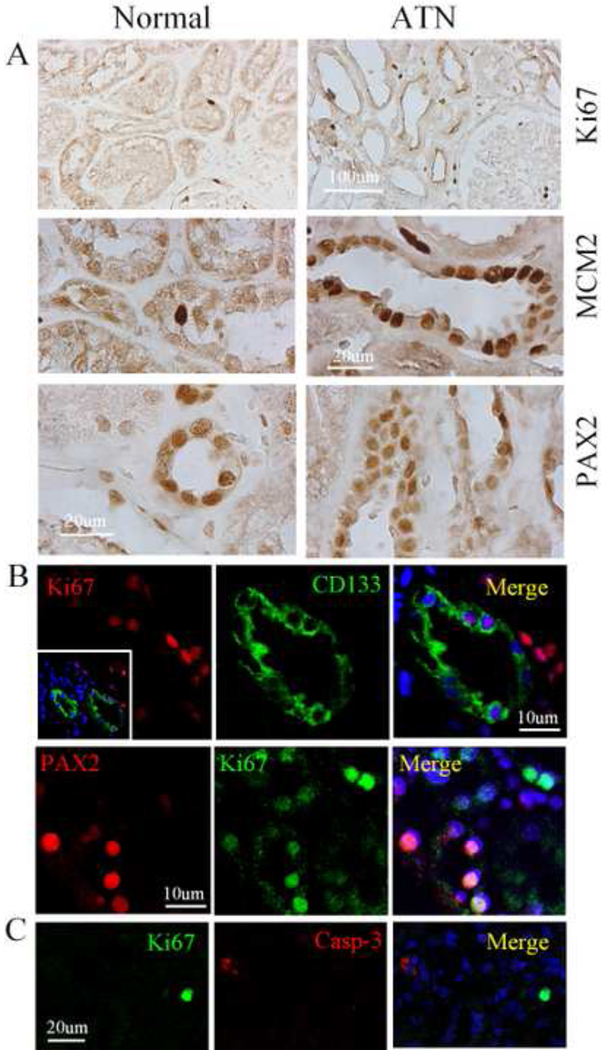
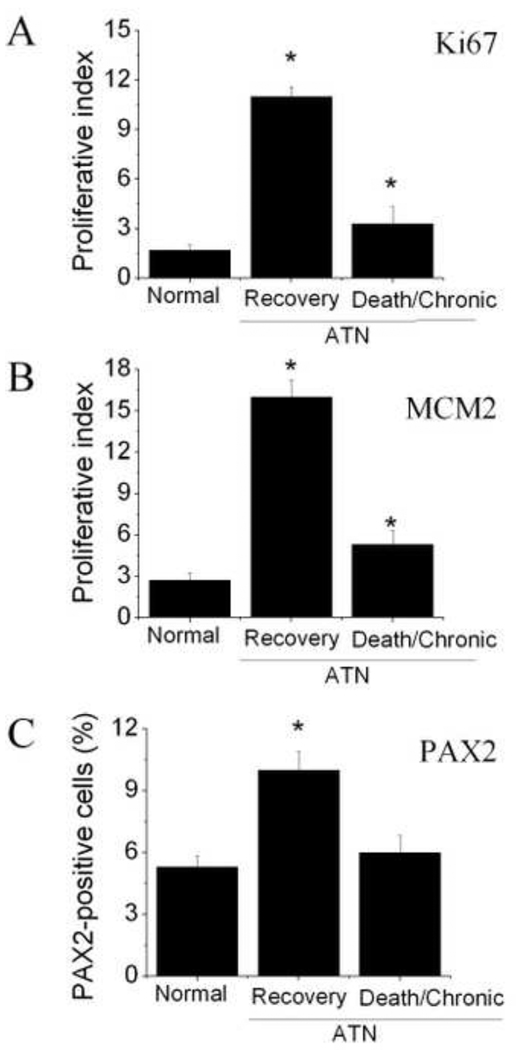
Next, we examined the biopsies of ten patients with ATN. As shown in Figure 4A, we found that the number of Ki67- and MCM2-positive cells, as well as PAX2-positive cells in the tubular regions of the cortex in the ATN kidney, was significantly higher than that of the normal control group. In addition, CD133 was induced in the tubular cells after ANT. Two photon confocal images show that Ki67-positive cells in tubular sections in ATN kidney expressed CD133 as well as PAX2 (Fig. 4B). In addition, these Ki67-positive cells expressed MCM2, but not activated caspase-3, a cell death protein marker (Fig. 4C). Interestingly, Ki67-positive cells were present in significantly higher numbers in patients with ATN, who subsequently recovered, relative to patients with ATN, who eventually developed protracted ATN or died (Fig. 5).
Fig. 4.
Expression pattern of proliferative cells in normal and ATN kidney. Expression pattern of Ki67, MCAM2 and PAX2 in normal and ATN kidney (A). Double immunostaining shows that Ki67-positive cells (red) expressed CD133 (green) (top panel) and Ki67-positive cells (green) expressed PAX2 (red) on the section from ATN (bottom panel) (B). Double immunostaining shows that Ki67-positive cells (green) did not express cell death marker, activated caspase-3 form (red) (C).
Fig. 5.
Proliferative index in normal and ATN kidney. The ratio of Ki67 positively immunostained nuclei to total nuclei per each section of normal and ATN kidney was considered as a proliferative index (A). The ratio of MCM2 positively immunostained nuclei to total nuclei per each section of normal and ATN kidney was considered as a proliferative index (B). The percentage of PAX2-positive cells in normal and ATN kidney with different outcome (C).
3. DISCUSSION
In this study, we find that proliferating cells are present not only in the proximal and distal tubules, but also in the glomerulus of adult human kidney. We also confirm that CD133 is abundantly expressed in a small subpopulation of cells located in the parietal layer of Bowman’s capsule. The dividing cells in this parietal layer mainly express CD133 and predominantly express PAX2, vinmetin and nestin in the tubules. After ATN, CD133 is induced in the tubule. In addition, proliferative cells in the cortex tubules are significantly higher compared to normal adult kidney as well. These ki67-positive cells expressed CD133 and PAX2, but not the cell death marker, activated caspase-3. Moreover, the number of dividing cells increased significantly in patients with ATN, who had subsequent recovery, compared to patients with ATN, who consequently developed protracted ATN or death, suggesting that the proliferative capacity of RSCs after ATN may be an important determinant of the patient’s outcome.
In theory, damaged or dead cells in renal diseases can be replaced by transplanted renal stem cells derived from human embryonic stem cells (hESCs), since ESCs are capable of unlimited self-renewal and can differentiate into a diverse range of specialized cell types. The use of hESCs for clinical applications, however, remains one of biggest challenges in the 21st century due to technical problems as well as ethical and moral concerns. Efforts have been made to explore whether endogenous RSCs also reside in adult kidney, as is the case in other adult organs such as the brain. With no unique and definitive biochemical and histochemical markers to label stem cells in general, RSCs remain difficult to identify definitively by a single marker. The identification of adult stem cells is thus largely determined by in vitro assays to examine their capacity for self-renewal and differentiation. Studies suggest that renal tissue-derived CD133+ cells may be renal stem-like cells based on their capability of self-renewal, in vitro differentiation into endothelial cells, and formation of tubular structures expressing renal epithelial markers after transplantation into SCID mice in vivo [13, 14]. Previous studies show that CD133 is mainly expressed in the interstitial cells and the subpopulations of parietal epithelial cells of Bowman’s capsule of adult kidney [16, 20], as well as on the apical surface of neuroepithelial cells and the brush border of kidney tubules [22]. Using human specific anti-CD133 antibody [21], we confirmed that CD133-positive cells were mainly located in the parietal layer of Bowman’s capsule and brush border [13, 22], but were rarely found in other regions including the intersititium and tubules. Ki67 antigen is the prototypic cell cycle related nuclear protein and expressed by proliferating cells in all phases of the active cell cycle (G1, S, G2 and M phase), but otherwise absent in resting (G0) cells. It is thus routinely used as a marker of cell cycling and proliferation [21, 23]. We found that Ki67-positive cells were scattered throughout the renal tissues including the intersititium, tubule, glomerulus and parietal layer of Bowman’s capsule. These Ki67-positive cells also expressed MCM2, another specific marker for actively cycling cells, confirming the presence of proliferating cells in the adult normal human kidney. We noted that the number of Ki67-positive cells was greater than that of MCM2 in normal human kidney. A possible explanation is that while Ki67 is expressed in G1, S, G2 and M phase, MCM2 is mainly expressed in early G1 phase. It has been noted that only a few of CD133/Ki67-positive cells were observed in the parietal layer of Bowman’s capsule, and that most Ki67-positive cells in other renal regions do not express CD133. Such an observation raises a debate as to whether CD133-positive cells are the only origin of RSCs and whether the parietal layer of Bowman’s capsule is the only niche for RSCs in normal adult human kidney. A recent study suggests that many of the epithelial cells in the proximal tubule are not in the G0 phase but rather in the G1 phase of the cell cycle. This prolonged G1 phase correlates with the low number of mitotic cells in adult rats and the large gap between consecutive cell cycles [24]. Another possibility is that most RSCs in normal kidney are quiescent or slow cycling cells [24]. Interestingly, CD133-positive cells are observed in the tubule of the cortex after ATN and co-express Ki67. These cells are likely immature cells, although it remains unclear whether they come from bone marrow or from resident cells, including the dedifferentiation of the tubular cells.
Vimentin is not only the most ubiquitous intermediate filament protein, but also the first protein to be expressed during cell differentiation. It is thus used as a marker for neural stem/progenitor cells (NSCs) in adult brain [25]. Recent studies show that renal tissue-derived CD133-positive cells express vimentin before they fully differentiate into epithelial cells [13], suggesting that vimentin is an immature marker of RSCs [8]. We found that vimentin was expressed in interstitial cells, parietal epithelial cells in the parietal layer of Bowman’s capsule, and tubular cells of adult normal human kidney. Some of them also expressed Ki67, suggesting that these cells most likely are immature cells. As for other intermediate filament proteins, nestin was originally identified in neuroepithelial stem cells. This protein has been widely used as a predominant marker for neural progenitor cells, glioma cells, and tumor endothelial cells in the mammalian central nervous system. Additionally, nestin is also expressed in some non-neuronal organs including metanephric blastema, podocytic cells at all stages of glomerular development and some stem/progenitor cells that originated from neuroectodermal and mesenchymal lineages [26]. Nestin has recently received a lot of attention in the field as a marker for detecting newly formed endothelial cells [27]. Moreover, nestin is induced in renal tubular and interstitial cells in the adult rat kidney following unilateral ureteral obstruction [28]. It was shown that nestin is highly expressed in glomerular cells and interstitial cells in normal adult human kidney. This finding is consistent with the recent discovery that nestin is expressed in podocytes of normal mature human glomeruli [29]. Studies show that nestin-positive cells co-expressed with vimentin [29], and we found that Ki67-positive cells expressed nestin and vimentin, suggesting that these cells are most likely renal progenitor-like cells. We noted that nestin is abundantly expressed in the glomeruli, but since only a few of Ki67-positive cells are found in this region, it is possible that the cells in normal glomeruli have a very slow turnover rate and that most nestin-positive cells remain at the quiescent stage (G0). A similar reasoning can be applied to cells located in the parietal layer of Bowman’s capsule.
Recovery of renal function following ATN is dependent on the replacement of necrotic tubular cells with functional tubular epithelium. In line with this, we found a higher number of dividing cells and PAX2-positive cells in the tubular region of kidney in patients with ATN, who subsequently recovered, compared to patients who eventually developed protracted ATN or died, suggesting that the number of dividing cells or RSCs is associated with the outcome of patients with ATN. A current explanation for the development of chronic renal tubular necrosis is an imbalance between injurious mechanism and regenerative repair. Therefore, the contribution of RSCs to the repair of tubular damage after ATN will determine the outcome of patients with ATN. The source of RSCs in response to ATN remains unclear. These cells may result from the proliferation of surviving dedifferentiated cells, renal stem cells that reside inside the kidney and migrate to the site of regeneration, or bone marrow cells that gain access to the injured epithelium and differentiate into mature cells. Since only ten kidney biopsies were used in this study, further studies, including increased number of samples, should be performed to determine whether the proliferation rate of the tubular epithelium in patients who recover from ATN is higher than in patients who do not recover.
Acute renal failure is a life-threatening disease with a high mortality rate. Over the last 50 years, there has been an ever-increasing shortage of donor kidney, but renal transplantation is the only available therapy for end stage renal diseases. Endogenous RSCs provide exciting therapeutic potential in the treatment of acute and chronic kidney diseases. Therefore, identifying, isolating and characterizing endogenous adult RSCs and understanding the molecular mechanisms underlying the regulation of RSC biological behaviors is crucial for furthering the search for better treatments.
ACKNOWLEDGEMENTS
This work was supported by Buck Institute for Age Research to K.J. and by Sir Run Run Shaw Hospital, Zhejiang University School of Medicine to Y.Y. Some human tissues were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD. The role of the NICHD Brain and Tissue Bank is to distribute tissue and, therefore, it cannot endorse the studies performed or the interpretation of results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62:1285–1290. doi: 10.1111/j.1523-1755.2002.kid569.x. [DOI] [PubMed] [Google Scholar]
- 3.Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci. 2004;117:5655–5664. doi: 10.1242/jcs.01488. [DOI] [PubMed] [Google Scholar]
- 4.Yokoo T, Ohashi T, Shen JS, Sakurai K, Miyazaki Y, Utsunomiya Y, Takahashi M, Terada Y, Eto Y, Kawamura T, Osumi N, Hosoya T. Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci U S A. 2005;102:3296–3300. doi: 10.1073/pnas.0406878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szczypka MS, Westover AJ, Clouthier SG, Ferrara JL, Humes HD. Rare incorporation of bone marrow-derived cells into kidney after folic acid-induced injury. Stem Cells. 2005;23:44–54. doi: 10.1634/stemcells.2004-0111. [DOI] [PubMed] [Google Scholar]
- 6.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause D, Cantley LG. Bone marrow plasticity revisited: protection or differentiation in the kidney tubule? J Clin Invest. 2005;115:1705–1708. doi: 10.1172/JCI25540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantley LG. Adult stem cells in the repair of the injured renal tubule. Nat Clin Pract Nephrol. 2005;1:22–32. doi: 10.1038/ncpneph0021. [DOI] [PubMed] [Google Scholar]
- 9.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003;14:3138–3146. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- 11.Al-Awqati Q, Oliver JA. The kidney papilla is a stem cells niche. Stem Cell Rev. 2006;2:181–184. doi: 10.1007/s12015-006-0046-3. [DOI] [PubMed] [Google Scholar]
- 12.Hishikawa K, Marumo T, Miura S, Nakanishi A, Matsuzaki Y, Shibata K, Ichiyanagi T, Kohike H, Komori T, Takahashi I, Takase O, Imai N, Yoshikawa M, Inowa T, Hayashi M, Nakaki T, Nakauchi H, Okano H, Fujita T. Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J Cell Biol. 2005;169:921–928. doi: 10.1083/jcb.200412167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 17.Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR, Go AS, Parikh CR, Peterson JF. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 77:536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi V, Chandy KG. Immunohistochemistry: paraffin sections using the Vectastain ABC kit from vector labs. J Vis Exp. 2007;308 doi: 10.3791/308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. PNAS. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Florek M, Haase M, Marzesco AM, Freund D, Ehninger G, Huttner WB, Corbeil D. Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res. 2005;319:15–26. doi: 10.1007/s00441-004-1018-z. [DOI] [PubMed] [Google Scholar]
- 21.Mao Y, Zhou L, Zhu W, Wang X, Yang G, Xie L, Mao X, Jin K. Proliferative status of tumor stem cells may be correlated with malignancy grade of human astrocytomas. Front Biosci. 2007;12:2252–2259. doi: 10.2741/2227. [DOI] [PubMed] [Google Scholar]
- 22.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burger PC, Shibata T, Kleihues P. The use of the monoclonal antibody Ki-67 in the identification of proliferating cells: application to surgical neuropathology. Am J Surg Pathol. 1986;10:611–617. doi: 10.1097/00000478-198609000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walder S, Zhang F, Ferretti P. Up-regulation of neural stem cell markers suggests the occurrence of dedifferentiation in regenerating spinal cord. Dev Genes Evol. 2003;213:625–630. doi: 10.1007/s00427-003-0364-2. [DOI] [PubMed] [Google Scholar]
- 26.Lobo MV, Arenas MI, Alonso FJ, Gomez G, Bazan E, Paino CL, Fernandez E, Fraile B, Paniagua R, Moyano A, Caso E. Nestin, a neuroectodermal stem cell marker molecule, is expressed in Leydig cells of the human testis and in some specific cell types from human testicular tumours. Cell Tissue Res. 2004;316:369–376. doi: 10.1007/s00441-003-0848-4. [DOI] [PubMed] [Google Scholar]
- 27.Ohike N, Sato M, Hisayuki T, Imataka H, Sato S, Wada Y, Saito K, Takahashi M, Tajiri T, Kunimura T, Morohoshi T. Immunohistochemical analysis of nestin and c-kit and their significance in pancreatic tumors. Pathol Int. 2007;57:589–593. doi: 10.1111/j.1440-1827.2007.02143.x. [DOI] [PubMed] [Google Scholar]
- 28.Sakairi T, Hiromura K, Yamashita S, Takeuchi S, Tomioka M, Ideura H, Maeshima A, Kaneko Y, Kuroiwa T, Nangaku M, Takeuchi T, Nojima Y. Nestin expression in the kidney with an obstructed ureter. Kidney Int. 2007;72:307–318. doi: 10.1038/sj.ki.5002277. [DOI] [PubMed] [Google Scholar]
- 29.Perry J, Ho M, Viero S, Zheng K, Jacobs R, Thorner PS. The intermediate filament nestin is highly expressed in normal human podocytes and podocytes in glomerular disease. Pediatr Dev Pathol. 2007;10:369–382. doi: 10.2350/06-11-0193.1. [DOI] [PubMed] [Google Scholar]