Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes (original) (raw)
Abstract
The generation of reactive oxygen species (ROS) is inherent to immune responses. ROS are crucially involved in host defense against pathogens by promoting bacterial killing, but also as signaling agents coordinating the production of cytokines. Transient Receptor Potential Melastatin 2 (TRPM2) is a Ca2+-permeable channel gated via binding of ADP-ribose, a metabolite formed under conditions of cellular exposure to ROS. Here, we show that TRPM2-deficient mice are extremely susceptible to infection with Listeria monocytogenes (Lm), exhibiting an inefficient innate immune response. In a comparison with IFNγR-deficient mice, TRPM2−/− mice shared similar features of uncontrolled bacterial replication and reduced levels of inducible (i)NOS-expressing monocytes, but had intact IFNγ responsiveness. In contrast, we found that levels of cytokines IL-12 and IFNγ were diminished in TRPM2−/− mice following Lm infection, which correlated with their reduced innate activation. Moreover, TRPM2−/− mice displayed a higher degree of susceptibility than IL-12–unresponsive mice, and supplementation with recombinant IFNγ was sufficient to reverse the unrestrained bacterial growth and ultimately the lethal phenotype of _Lm_-infected TRPM2−/− mice. The severity of listeriosis we observed in TRPM2−/− mice has not been reported for any other ion channel. These findings establish an unsuspected role for ADP-ribose and ROS-mediated cation flux for innate immunity, opening up unique possibilities for immunomodulatory intervention through TRPM2.
Keywords: CXCL2, IL-12Rβ2−/−, NAD, pathogen-associated molecular pattern, NF-κB
Beyond their role as bacterial killing agents, reactive oxygen species (ROS) generated during immune responses have more recently been recognized for their crucial role in signaling (1, 2). Multiple studies have highlighted the role of ROS in mediating the production of proinflammatory cytokines, often requiring the activation of the redox-sensitive transcription factor NF-κB (3, 4).
The ion channel TRPM2 (Transient Receptor Potential Melastatin 2) is the unique fusion of a Ca2+-permeable nonselective cationic pore to an enzymatic region with residual ADP-ribose (ADPR)-hydrolase activity. TRPM2 channels are opened by direct binding of intracellular ADPR (5, 6) and are also activated indirectly under conditions of oxidative stress (7), a process shown to be dependent upon intracellular formation of ADPR (8). Gene expression studies and measurements of ADPR-activated, TRPM2-like currents have demonstrated the presence of TRPM2 channels in cells of the monocytic lineage and neutrophils (5, 6, 9). TRPM2’s sensitivity toward oxidants, acidification (10, 11), and elevated intracellular Ca2+ (12) combined with its expression profile suggested that it might be important for inflammatory processes.
TRPM2 knockdown in U937 human monocytes has shown a deficiency in H2O2-mediated Ca2+ influx and diminished activation of NF-κB–mediated transcription of the chemokine CXCL8 (13). In that same study, TRPM2−/− mice had greatly reduced CXCL2 (homolog of hCXCL8) production by monocytes, with a resultant reduction of neutrophilic infiltration and ulceration of the colon in a colitis mouse model (13). Furthermore, TRPM2 expression and currents were shown to be increased upon exposure of human monocytes to LPS, and knockdown of TRPM2 in human THP1 monocytes impaired LPS-induced production of several cytokines, including TNFα, IL-6, IL-8, and IL-10 (14). However, whether TRPM2 is necessary for cytokine production during a systemic immune response has not been demonstrated.
The bacterial pathogen Listeria monocytogenes (Lm) is widely used as a model of systemic infection in mice requiring both innate and adaptive immunity. Lm rapidly invades resident macrophages and nonphagocytic cells where recognition receptors of the Toll-like receptor and nucleotide oligomerization domain (Nod)-like receptor (TLR/NLR) families sense microbial components (15), resulting in the production of cytokines such as TNFα, IL-12, and IL-18. These cytokines act synergistically to promote IFNγ production by natural killer (NK), natural killer T (NKT), and CD8+ T cells, which transactivates bactericidal pathways in macrophages and neutrophils (16, 17) critical for restraining bacterial growth until adaptive immunity is generated. The contributions of ROS and Ca2+ channels during this innate immune response are not well understood.
As there is mounting evidence that TRPM2 may be involved in immune functions, we sought to investigate its role in fighting an infection in vivo. We found that TRPM2-deficient mice are highly susceptible to Lm infection, exhibiting impaired inflammatory cytokine production and uncontrolled bacterial growth. The severity of infection in TRPM2−/− mice was found to be greater than IL-12Rβ2−/− mice, similar to IFNγR−/− mice, and reversed by rIFNγ treatment. The only other ion channel currently known to be important for antibacterial defense is TRPV2, also a member of the TRP family of proteins (18). Notably, TRPV2−/− mice require a 1,000-fold higher Lm dose to exhibit lethality comparable to TRPM2−/− mice, further underscoring the unique and crucial function fulfilled by TRPM2 channels in combating Listeria infections as demonstrated in the present study.
Results
TRPM2 Is Required for Survival and Control of Bacterial Burden to Lm Infection.
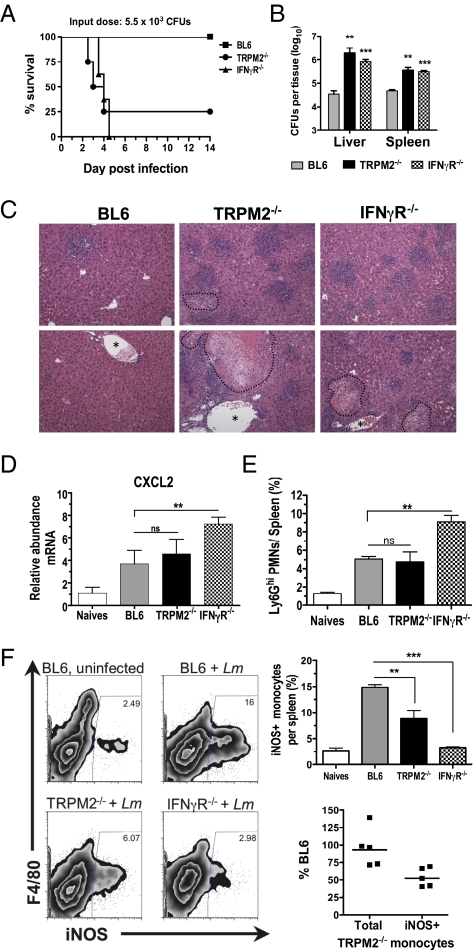
To evaluate whether TRPM2 is involved in mounting effective immunity against Lm infection, TRPM2−/− mice (Fig. S1) were infected i.v. with a sublethal dose of Lm in parallel with IFNγR−/− mice as a control strain exhibiting an elevated susceptibility to Lm. Whereas BL6 control mice survived a dose of 5.5 × 103 cfu and showed no outward signs of illness (Fig. 1_A_), 75% of TRPM2−/− mice rapidly succumbed to Lm infection, with a median survival time of 3.5 d, and it was fatal for 100% of IFNγR−/− mice (median survival time = 4 d) as previously reported (19). The death of TRPM2−/− mice beginning at 2.5 d postinfection (dpi), a time at which the specific immune response has not been established, indicated a severe defect in innate immunity and in the control of bacterial replication. To verify this conclusion, we infected TRPM2−/− mice and compared the bacterial burden in the liver and spleen 48 h postinfection (hpi) with that of BL6 and IFNγR−/− mice (Fig. 1_B_). The bacterial burden in the livers of TRPM2−/− mice was much higher than that of BL6 animals (P < 0.01) and even greater than that of IFNγR−/− mice, whereas the degree of expansion of Lm in the spleens of TRPM2−/− and IFNγR−/− mice was similar. Histopathology of the liver corroborated that Lm infection in TRPM2−/− mice appears grossly similar to IFNγR−/− mice, revealing a significant number of inflammatory abscesses (intense purple areas = dense cellular infiltrates) with an apparent parenchyma destruction and necrosis in perivascular regions, which was greatly increased from BL6 sections (Fig. 1_C_).
Fig. 1.
TRPM2 is required for survival and control of bacterial burden in L. monocytogenes infection. (A) TRPM2−/− mice are as susceptible to Lm infection as IFNγR−/− mice. Kaplan–Meier survival plot of mice i.v. infected with Lm. Eight BL6 (squares), TRPM2−/− (circles), or IFNγR−/− mice (triangles) were infected with 5.5 × 103 Lm. Median survival of TRPM2−/− mice closely matched IFNγR−/− mice and both are significant by log rank test (P < 0.005) compared with BL6. Data are representative of two independent experiments. (_B_) _Lm_ burden in liver and spleen during early infection. BL6, TRPM2−/−, and IFNγR−/− mice (three to five mice per group) infected with 2 × 103 _Lm_ and cfu determined 48 hpi. Mean value and SEM are indicated (**_P_ < 0.01, ***_P_ < 0.001). Data are representative of five experiments. (_C_) Comparison of liver histopathology of _Lm_ infected mice. Representative parenchyma (_Upper_) and perivascular (_Lower_) images from H&E stained BL6 livers infected with 5 × 103 _Lm_ compared with TRPM2−/− and IFNγR−/− moribund mice from _A_, day 4. Original magnification 200×; dotted lines outline areas of necrosis; asterisks denote blood vessel. (_D_) _cxcl2_ expression in response to _Lm_ infection. Collagenase digested splenocytes from naïve BL6 (_n_ = 3), naïve TRPM2−/− (_n_ = 2), or mice infected for 24 h with 4.5 x 103 _Lm_ (_n_ = 4 of each genotype) and _cxcl2_ mRNA quantified using qPCR. All naïve mice (BL6 and TRPM2−/−) had equivalent _cxcl2_ expression and were averaged together. Bars represent means ± SEM. NS indicates _P_ > 0.05, **P < 0.01. (E) Average frequency of Ly6Ghi gated neutrophils per spleen from naïve or mice infected as in B. Bars represent mean ± SEM, four mice per group and five independent experiments. (F) In vivo activation of iNOS by Ly6Glow Ly6C+ monocytes is impaired in response to Lm infection in TRPM2−/− mice. BL6, TRPM2−/−, and IFNγR−/− (n = 4 each) mice infected as in B. Representative density plots of iNOS expression in CD11b+/Ly6Glow/Ly6C+ gated monocytes. Average frequency of iNOS+ monocytes is significantly reduced in TRPM2−/−- and IFNγR−/−-infected mice. Bars represent mean frequency ± SEM **P < 0.01, ***P < 0.001. (Lower) Comparison of the mean absolute number of total or iNOS+ TRPM2−/− to total or iNOS+ BL6 monocytes at 48 hpi from five separate experiments (# TRPM2−/− cells ÷ # BL6 cells × 100).
A recent report demonstrated that TRPM2 mediates CXCL2 production necessary for neutrophil infiltration in a colitis model (13). Because CXCL2 is necessary for neutrophil recruitment in listeriosis, we tested whether TRPM2−/− mice had a similar dysregulation of cxcl2 expression and neutrophil trafficking in response to Lm. Thus, splenocytes were obtained from naïve or _Lm_-infected mice and the relative abundance of transcripts determined using quantitative RT-PCR. cxcl2 expression was induced similarly in TRPM2−/− and BL6-infected splenocytes 24 hpi, and more so in IFNγR−/− mice (Fig. 1_D_). In addition, neutrophil numbers assessed by flow cytometry were increased in the spleen in response to Lm infection in all strains (Fig. 1_E_) as was also seen by nuclear morphology of the H&E stained liver (Fig. 1_C_). Finally, neutrophils enriched from bone marrow of TRPM2−/− mice were infected in vitro with Lm, and the results show their intrinsic ability to kill Lm is comparable with BL6 neutrophils (Fig. S2_A_). These data suggest that the absence of functional TRPM2 channels does not prohibit Lm infection from inducing cxcl2 transcripts and the recruitment of functional neutrophils to the site of inflammation.
Because macrophages are central to the control of Lm replication by inhibiting vacuole escape and cell-to-cell spread of the bacterium through mechanisms initiated by IFNγ (19), we hypothesized that unrestricted growth of Lm in the TRPM2−/− mouse might be due to dysfunctional macrophage activation in vivo. To test this hypothesis, we analyzed the intracellular expression of iNOS in splenic monocytes by flow cytometry and measured an eightfold up-regulation in response to Lm infection of BL6 mice 2 dpi (Fig. 1_F_). In contrast, we observed a consistent 50% reduction in the frequency and number of iNOS+ Ly6Glow/CD11b+ cells in the TRPM2−/− spleens compared with control mice (P < 0.01), albeit not as severe as the 80% reduction observed in the IFNγR−/− mouse, and no difference in total frequency of monocytes recruited between groups was seen (Fig. 1_F_). To understand whether TRPM2−/− macrophages could respond to IFNγ, we examined several parameters in vitro using bone marrow-derived macrophages (BMDMs) and found that cultured TRPM2−/− BMDMs have functional IFNγR, TLR4, and TNFα signaling, normal iNOS expression, and intracellular Lm killing ability (Fig. S2 B_–_D). Together these data show that TRPM2−/− mice exhibit a significant monocyte/macrophage activation defect in response to Lm infection in vivo, which likely is occurring upstream of the IFNγ receptor.
TRPM2−/− Mice Have Diminished _Lm_-Induced Cytokine Production.
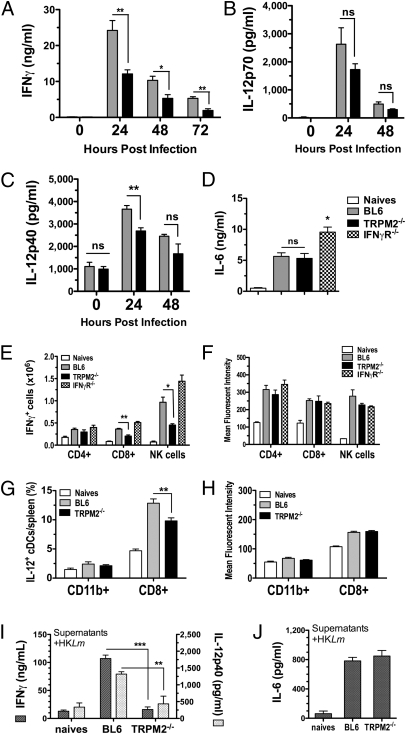
Survival to Lm infection and the transactivation of macrophage bactericidal activity occurs by an early antigen-independent production of IFNγ from NK cells and CD8+ T cells, a process dependent on IL-12 production (20, 21). Therefore, it was possible that cytokines leading to macrophage activation were not being produced in the TRPM2−/− mouse. To test this hypothesis, we first quantified the level of IFNγ in the serum following infection and consistently observed a 50% reduction of systemic IFNγ in TRPM2−/− mice compared with controls at 24 (P < 0.001) and 48 hpi, with only a 35% reduction at 72 hpi when serum IFNγ was waning (Fig. 2_A_). IL-12p70 and IL-12p40 were also found to be diminished ∼30% in the serum of TRPM2−/− mice following infection, a significant difference in IL-12p40 at 24 hpi (Fig. 2 B and C). However, not all inflammatory cytokines were reduced in the serum of TRPM2−/−-infected mice; IL-6 production was normally elevated in TRPM2−/− animals (Fig. 2_D_).
Fig. 2.
TRPM2−/− mice have diminished _L. monocytogenes_-induced cytokine production. TRPM2−/− mice exhibit reductions in serum cytokines IFNγ (A), IL-12p70 (B), and IL-12p40 (C) in response to Lm infection. BL6 and TRPM2−/− mice were infected with 5 × 103 Lm for the times indicated and serum assayed by ELISA. Bars represent mean ± SEM from three to five mice per group per time point. Data are representative of three independent experiments at 24 hpi, five experiments at 48 hpi, and two experiments at 72 hpi. (D) TRPM2−/− mice have normal serum IL-6 production in response to Lm infection. BL6, TRPM2−/−, and IFNγR−/− mice were infected with 8 × 103 Lm and serum IL-6 measured by ELISA at 24 hpi. Bars represent mean ± SEM from three to five mice per group and data are representative of two independent experiments. (E) TRPM2−/− mice have reduced numbers of IFNγ+ cells in response to Lm infection. BL6, TRPM2−/−, and IFNγR−/− mice infected with 8 × 103 Lm for 24 hpi and absolute numbers of gated NK1.1neg CD3+ T lymphocytes and NK1.1+ CD3neg NK cells expressing IFNγ are shown (Fig. S3 shows example of dot blots). Bars represent mean ± SEM for three to five mice per group. Data are representative of three independent experiments comparing BL6 with TRPM2−/− mice and two additional experiments including IFNγR−/− mice. (F) Mean fluorescent intensity (MFI) of IFNγ-gated cells is similarly increased in response to infection. Bars represent MFI ± SEM for IFNγ+-gated cells in E. (G) TRPM2−/− mice have a reduced frequency of IL-12+ CD8+ CD11chigh (cDCs) in response to Lm infection. BL6 and TRPM2−/− mice infected with 6 × 103 Lm for 24 hpi and frequency of gated CD11chigh CD8+ cDCs expressing IL-12p40 are shown. (Fig. S3 shows representative dot blots). Bars represent mean ± SEM for four to five mice per group. Data are representative of two independent experiments comparing BL6 with TRPM2−/− mice at 24 hpi and one experiment at 48 hpi. (H) MFI of IL-12p40–gated cells is similarly increased in response to infection. Bars represent MFI ± SEM for IL-12p40–gated cells in G. (I) Lm_-infected TRPM2−/− splenocytes have impaired IFNγ and IL-12p40 secretion in vitro. Collagenase digested splenocytes from BL6 and TRPM2−/− mice (pooled naïves, n = 2 of each genotype) or infected (n = 4, each) with 4.5 × 103 Lm for 24 h were cultured in vitro for 48 h with 108/mL HK_Lm and supernatants assayed for secreted cytokines by ELISA. Bars represent mean ± SEM and are representative of three separate experiments. (J) _Lm_-infected TRPM2−/− splenocytes secrete normal amounts of IL-6 in vitro. Collagenase digested splenocytes from BL6 and TRPM2−/− mice (pooled naïves, n = 3 each) or infected (n = 3–5, each) with 3 × 103 Lm for 48 h were cultured as in G. Bars represent mean ± SEM and are representative of three separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
To identify the source of the reduced IFNγ and IL-12 in vivo, we examined intracellular cytokine staining of lymphocytes and conventional dendritic cells (cDCs) in the spleens of infected animals (gating illustrated in Fig. S3 A, B, F, and G). We found a significant reduction of IFNγ+ CD8+ T cells (44%) and IFNγ+ NK cells (55%) in TRPM2−/− mice at 24 hpi with elevated numbers of IFNγ+ cells in IFNγR−/− mice (Fig. 2_E_). All three strains expressed similar levels of intracellular IFNγ on a per-cell basis, indicating they were not incapable of expressing IFNγ (Fig. 2_F_). Indeed, TRPM2−/− naïve lymphocytes up-regulated IFNγ similarly to BL6 lymphocytes when stimulated by IL-12 and IL-18 in vitro (Fig. S3 C_–_E), demonstrating that intrinsic IFNγ production is independent of TRPM2. We next aimed to determine whether cDCs or macrophages were responding normally to Lm infection and consistently observed a fourfold increase in the frequency of IL-12 expressing CD8+ (but not CD11b+) cDCs in infected BL6 mice (Fig. 2_G_), similar to previous reports (22). Importantly, we identified a 20–25% reduction in the frequency (but not mean fluorescent intensity, MFI) of IL-12 expressing TRPM2−/− CD8+ cDCs in response to infection (Fig. 2 G and H). In contrast, we were unable to detect significant levels or changes in intracellular IL-12 from macrophages of infected animals; however, when we stimulated BMDMs in vitro, these cells were capable of secreting IL-12p40 (Fig. S3_H_). Finally, when infected splenocytes were restimulated ex vivo with heat-killed (HK)Lm, TRPM2−/− cells demonstrated a striking inability to secrete IFNγ or IL-12p40 (Fig. 2_G_) but normal IL-6 (Fig. 2_H_). Collectively, these data suggest that during a complex Lm infection, TRPM2 plays an important role in the production of the early inflammatory cytokine IL-12 from CD8+ cDCs (and potentially other innate populations) necessary to initiate IFNγ-mediated innate immunity.
IL-12 Is Indispensable for Low-Dose Infection of TRPM2−/− Mice.
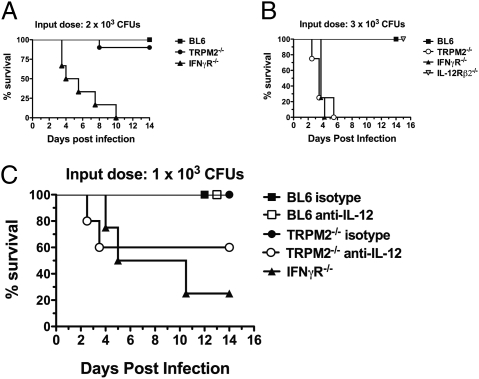
Although IFNγ responsiveness is required for resistance to Lm infection at very low doses (19), the major IFNγ-inducing cytokine, IL-12p70, has been shown to be dispensable for immunity to doses ≤2 × 103 cfu (23). Because TRPM2−/− mice exhibited a reduced (but not absent) production of inflammatory cytokines and monocyte activation, we sought to determine whether there were sufficient innate mechanisms to survive doses of Lm infection where IL-12p70 is dispensable. For this evaluation, TRPM2−/− mice were infected with ≤2 × 103 cfu of Lm in parallel with BL6 and IFNγR−/− mice (Fig. 3_A_). As expected, IFNγR−/− mice succumbed to this lower-dose Lm infection (4.75 d, P < 0.0001), albeit with slightly delayed kinetics. In contrast, TRPM2−/− mice faired well, with only 10% of infected mice succumbing to listeriosis compared with the 100% mortality seen at three times this amount (Fig. 1_A_). On the basis of these results, we wanted to directly compare the apparent dispensability of TRPM2 to low-dose infection with that of IL-12Rβ2−/− mice (24), which lack responsiveness to IL-12 but not IL-23 (25). When mice were infected with 3 × 103 cfu, 100% mortality was observed in TRPM2−/− mice, with median survival times (3.5 d, P < 0.0001) exactly matching IFNγR−/− mice (Fig. 3_B_). In striking contrast, IL-12Rβ2−/− mice completely resisted Lm infection at this dose, confirming that IL-12p70–independent mechanisms of IFNγ production exist to initiate innate immunity sufficiently to overcome low-dose infection (23).
Fig. 3.
IL-12 is indispensable for low-dose infection of TRPM2−/− mice. (A) TRPM2−/− mice can survive a lower dose Lm infection. Kaplan–Meier survival plot of 10 BL6 (squares), 10 TRPM2−/− (circles), or 6 IFNγR−/− mice (triangles) infected i.v. with 2 × 103 Lm. Median survival of IFNγR−/− mice (4.75 d) was significantly reduced by log rank test (P < 0.0001) compared with BL6 and TRPM2−/− mice. Data representative of four separate experiments. (B) TRPM2−/− mice are susceptible to a low-dose Lm infection where IL-12Rβ2−/− mice are resistant. Kaplan–Meier survival plot of eight BL6 (squares), four TRPM2−/− (open circles), or four IL-12Rβ2−/− mice (open triangles), or five IFNγR−/− mice (closed triangles) infected with 3 × 103 Lm. Median survival of TRPM2−/− mice closely matched IFNγR−/− mice (3.5 d) and both are significant by log rank test (P < 0.0001) compared with BL6 and IL-12Rβ2−/− mice. Data are representative of two separate experiments. (C) IL-12p40 is indispensable in TRPM2−/− mice infected with very low doses of Lm. Kaplan–Meier survival plot of BL6 (squares), TRPM2−/− (circles), or IFNγR−/− mice (triangles) infected i.v. with 1 × 103 Lm (five to eight mice each group). Three hours before infection, BL6 or TRPM2−/− mice were i.p. injected with 1.5 mg neutralizing mAb against IL-12p40 (white) or rat isotype control IgG2A (black). Median survival of IFNγR−/− mice (7.75 d) was significant by log rank test (P < 0.005) compared with BL6 and TRPM2−/−. Data are representative of two separate experiments.
To better understand this “all-or-nothing” switch in sensitivity of TRPM2−/− mice to these low doses, we aimed to deplete endogenous IL-12 in TRPM2−/− mice and monitor susceptibility at a dose where they would normally survive. For this experiment, 1.5 mg of neutralizing IL-12p40 or isotype control mAb was injected into mice 3 h before infection with 1 × 103 cfu of Lm, and survival was monitored. As expected, BL6 and TRPM2−/− isotype antibody-treated mice survived this low-dose infection (Fig. 3_C_). Importantly, TRPM2−/− mice treated with anti–IL-12p40 antibody displayed incomplete resistance with 40% mortality within 4 d of infection to the 1 × 103 cfu dose, contrasting the complete survival of BL6 mice depleted of IL-12p40. Thus, unlike IL-12p35−/− mice (23) and IL-12Rβ2−/− mice (Fig. 3_B_), IL-12 becomes essential for survival to low-dose Lm infection in the absence of TRPM2 (Fig. 3_C_). These results place the susceptibility of TRPM2−/− mice between that of IFNγ and IL-12–unresponsive animals and further suggest that TRPM2−/− mice have additional defects in IL-12–independent antilisterial pathways.
Recombinant IFNγ Treatment Is Sufficient to Reverse TRPM2−/− Susceptibility to High-Dose Lm Infection.
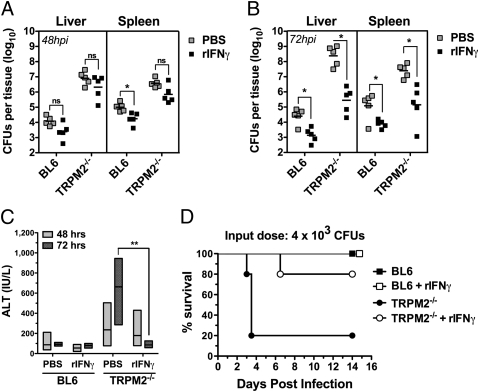
Beyond IL-12, TNFα and IL-18 are important costimulators of IFNγ production (21, 26, 27), but they also provide IFNγ-independent protection against listeriosis (28, 29). Because TRPM2−/− mice depleted of endogenous IL-12p40 were more susceptible to Lm infection than IL-12Rβ2−/− mice, it is possible that IFNγ-independent pathways are modulated by TRPM2, contributing to the Lm sensitivity. To address this question, we injected recombinant mouse IFNγ (rIFNγ) before Lm infection to determine whether we could ameliorate the bacterial burden observed in TRPM2−/− mice during a higher sublethal infection (6 × 103 Lm) (Fig. 4 A and B). When bacterial burden was measured at 48 and 72 hpi, we observed the typical uncontrolled Lm growth in the liver and spleen of PBS-treated TRPM2−/− mice, approaching values observed in moribund mice by 72 hpi (109 cfu). At 48 hpi, rIFNγ treatment provided a slight but consistent improvement for both groups, although the result was not significant (Fig. 4_A_). However, at 72 hpi the response to rIFNγ treatment was significant for both TRPM2−/− and BL6 animals (P < 0.05) with TRPM2−/− mice exhibiting a dramatic 600-fold recovery in cfu burden in the liver versus the modest 17-fold improvement for BL6 mice (Fig. 4_B_). rIFNγ treatment also activated control mechanisms for Lm growth in the spleen of TRPM2−/− mice, albeit the benefit was comparable with that seen in BL6 mice (∼35-fold for both groups) (Fig. 4_B_). Because bacterial colonization is particularly severe in the liver of TRPM2−/− mice, as illustrated by the hepatic damage observed by histology (Fig. 1_C_), we examined whether rIFNγ treatment had an effect on hepatocyte survival by measuring serum alanine aminotransferase (ALT) as an indicator of liver damage. In response to Lm infection, TRPM2−/− mice exhibit increasing amounts of ALT in the serum over time, whereas BL6 mice showed no systemic signs of liver damage (Fig. 4_C_). In contrast, ALT levels in rIFNγ-treated TRPM2−/− mice were reduced to concentrations similar to BL6 mice at 72 hpi, indicating that TRPM2−/− liver damage was reversed by administration of rIFNγ (Fig. 4_C_). Finally, when we analyzed the effect of rIFNγ supplementation on the overall survival of TRPM2−/− mice, we found that indeed, most TRPM2−/− mice having received rIFNγ were now resistant to this higher-dose infection (Fig. 4_D_). Because rIFNγ was sufficient to rescue TRPM2−/− mice, these data suggest that TRPM2 is not crucial for IFNγ-independent pathways of protection against Lm infection.
Fig. 4.
Recombinant IFNγ treatment is sufficient to reverse TRPM2−/− susceptibility to high-dose Lm infection. (A and B) Pretreating mice with rIFNγ improves Lm burden in liver and spleen. BL6 and TRPM2−/− mice were i.v. injected with either recombinant mouse IFNγ (105 U/each) in PBS or control PBS and 18 h later, infected i.v. with 6 × 103 Lm. Colony-forming units in both liver and spleen were determined 48 hpi (A) and 72 hpi (B). Mean value and SEM are indicated (NS indicates P > 0.05, *P < 0.05). Data are representative of three experiments. (C) Pretreating mice with rIFNγ reverses serum ALT elevation in _Lm_-infected TRPM2−/− mice. Serum was obtained from BL6 and TRPM2−/− mice treated as in A and B (n = 5 each group) and ALT quantified using a modified Reitman Frankel colorimetric endpoint reaction. Floating bars represent range, and the line is the mean. TRPM2−/− mice receiving rIFNγ had a significant reversal of ALT, **P < 0.01. (D) Recombinant IFNγ treatment is sufficient to reverse mortality of TRPM2−/− mice to Lm infection. Kaplan–Meier survival plot of mice i.v. infected with Lm. BL6 (squares) and TRPM2−/− (circles) were i.v. injected with mouse rIFNγ (105 U/each) in PBS (white), or control PBS (black) and 18 h later, infected i.v. with 4 × 103 Lm. Median survival of rIFNγ-treated TRPM2−/− mice was significantly improved from untreated TRPM2−/− mice (3.5 d) by log rank test (P < 0.05).
Discussion
We show here that the TRPM2 ion channel is critical for innate immunity in response to Lm infection. Efficient _Lm_-induced IL-12 and IFNγ production, resultant macrophage transactivation, and control of bacterial replication are dependent on functional TRPM2 channels. Most significantly, TRPM2−/− mice are more susceptible to Lm than IL-12-unresponsive animals (IL-12Rβ2−/−), succumb to listeriosis similarly to mice deficient in IFNγR when the input dose is >2 × 103 cfu, and morbidity and mortality of TRPM2−/− mice can be completely reversed by administering recombinant IFNγ. Importantly, we found that the recruitment of neutrophils and monocytes does not appear to require TRPM2 during Lm infection, in contrast to a previous report in a chemical injury model where TRPM2 is involved in chemokine production and required for neutrophil influx to the colon (13). Instead, the present data unveil TRPM2−/− mice as the ion channel-deficient model with the highest susceptibility to listeriosis, identifying TRPM2 as an essential ion entry pathway required for adequate inflammatory cytokine production in response to intracellular bacterial infection.
Regulated production of cytokines is absolutely essential for an efficacious immune response to infection, involving multiple pathogen recognition signaling pathways and an organized sequence of cellular activation. The diminished but not absent production of IL-12 and IFNγ in TRPM2−/− mice is reminiscent of the reduced bacteria_-_induced cytokine response of mice deficient in TLR2 (16), Rip-2 (30), and MyD88 (31–33), suggesting that TRPM2 ion-conducting activity may contribute to similar pathogen-associated pattern recognition signaling pathways. In fact, Ca2+ is a universal second messenger necessary for many signaling pathways including TLRs, which potently induce IL-12 and TNFα in macrophages and dendritic cells (34). Additionally, cytosolic localization of Lm is a prerequisite for TLR2-independent IL-12 and TNFα induction (31), as well as early IFNγ production from CD8+ T cells (20), highlighting the importance of NLRs for initiating cytokine production in this infection model. Finally, the redistribution of Lm within the microarchitecture of infected tissues such as the spleen (35, 36) and the spatial and hierarchical coordination of cells and inflammatory cytokine production by DCs (37) suggest that the innate immune response and inflammatory cytokine production is more complex than the linear model of TLR- or NLR-signaling cascades within a single cell type.
However, given that TLR and NLR bacterial recognition pathways use the transcription factor NF-κB to initiate proinflammatory cytokine production (38, 39), and NF-κB activity is enhanced by Ca2+ (40, 41), TRPM2 may participate in signaling pathways leading to NF-κB–dependent gene expression. This notion is supported by the defective clearance of Lm in mice deficient in NF-κB (42), the importance of TRPM2 for LPS-induced TNFα production in human monocytes (14), as well as the attenuated NF-κB activation and reduced IL-12 and IFNγ expression during chemically induced colitis using a different TRPM2−/− mouse strain (13). It is noteworthy that although it has been shown in the murine DSS colitis model that IL-6 production is mediated by NF-κB (p65) in the colon (43), DSS-treated TRPM2−/− mice show no alterations in IL-6 levels (13). We similarly found IL-6 levels to be equivalent in TRPM2−/− and wild-type mice following Lm infection, suggesting that TRPM2’s involvement may not uniformly impact NF-κB–dependent cytokine production. Additionally, our characterization of systemic cytokine levels in the serum correlated with intracellular staining of key cells involved in anti-Listeria defense in vivo, but were disparate compared with in vitro responses. We interpret this systemic cytokine profile and overall survival to infection as a reflection of the summation of TRPM2-dependent and -independent pathways, as well as differential expression of functional TRPM2 channels in various tissues and cell types. Moreover, the metabolic capabilities of a particular environment to produce ADPR will ultimately determine the extent of TRPM2’s activation and involvement, particularly in response to oxidant exposure (6, 8) where activation of the redox-sensitive NF-κB transcription factor occurs (44). Thus, in vitro studies may be limited in their ability to recapitulate the required factors for TRPM2-activated ion conductance and function during an immune response, whereas dissection of delicately balanced signaling events during a systemic in vivo infection comes with its own set of weaknesses.
The causality of the extreme susceptibility of TRPM2−/− mice to listeriosis presented here appears to be multilayered and only partially explained by the reduced IL-12 and IFNγ production in vivo. Mice deficient in the common cytokine receptor γ-chain (γc−/−) also have very low levels of IFNγ (due to the absence of NK cells), but better control of bacterial replication during the innate stage (45), as do TLR2−/− mice (16). IL-12 has been shown to be dispensable to low-dose infections (23), which we have confirmed, except when TRPM2 is missing. Thus, IL-12–independent mechanisms that would normally compensate for IL-12 deficiency, such as TNFα or IL-18, are also affected in TRPM2−/− mice. Indeed, it has been reported elsewhere that TRPM2 contributes to LPS-induced TNFα production in human monocytes (14) and such a TNFα deficiency would contribute to the elevated susceptibility in TRPM2−/− mice. However, because recombinant IFNγ can reverse the effects of depleting IL-12, but not TNFα (29), we used a similar experimental design to address this possibility. Our result that rIFNγ completely reversed the Lm susceptibility caused by TRPM2 deficiency therefore indicates that TNFα production is not abrogated in TRPM2−/− mice, although we cannot exclude that TNFα levels might be diminished. As previously reported, TNFα is extremely difficult to capture in the serum of _Lm_-infected mice (46), and we were unable to measure TNFα serum levels using commercial ELISAs. Future studies will thus need to answer this question by assessing TNFα concentrations locally in relevant organs such as spleen and liver.
The uniqueness of TRPM2-mediated ion fluxes resides in their activation in response to the accumulation of the NAD metabolite ADPR. Therefore, TRPM2 is expected to only participate in biological processes downstream of events resulting in ADPR production, such as situations of oxidative stress encountered during immune responses. In sum, our results suggest that the TRPM2 ion channel and its gating molecule ADPR are previously unsuspected players necessary for robust cytokine production and innate cell activation during intracellular bacterial infection. These findings highlight the potential of the metabolic manipulation of ADPR levels or other modes of TRPM2 modulation as unexplored and promising immunomodulatory strategies.
Materials and Methods
Mice.
C57BL/6J (BL6) were obtained from The Jackson Laboratory. IFNγR1−/− mice were kindly provided by Dr. P. Marrack (National Jewish Health, Denver, CO), and IL-12Rβ2−/− mice were a gift from Dr. R. Kedl (University of Colorado, Denver, CO). All mice were housed under pathogen-free conditions at the National Jewish Health Biological Resource Center. Mice matched for sex and age (6–12 wk) were used for all experiments, which were approved by the institutional animal care and use committee. All studies were conducted after review by the GlaxoSmithKline (GSK) Institutional Animal Care and Use Committee and in accordance with the GSK Policy on the Care, Welfare and Treatment of Laboratory Animals.
Additional Methods.
Detailed experimental protocols are provided in SI Materials and Methods.
Supplementary Material
Supporting Information
Acknowledgments
We thank Dave Riches and Leonard Dragone for carefully reviewing the manuscript. We are grateful to Andrew Scharenberg and Ben Buelow for technical advice. We further acknowledge Laboratory Animal Sciences at GlaxoSmithKline.This work was supported by an Early Excellence Award of the Sandler Program in Asthma Research (to A.-L.P.), National Institutes of Health (NIH) Training Grant 5 T32 AI07405 (to H.K.), and NIH Grants AI065638 and AI055701 (to L.L.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Nathan C. Specificity of a third kind: Reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111:769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wink DA, et al. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89:873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulua AC, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temkin V, Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: The receptor-interacting protein 1 odyssey. Immunol Rev. 2007;220:8–21. doi: 10.1111/j.1600-065X.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 5.Perraud AL, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 6.Sano Y, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 7.Hara Y, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 8.Perraud AL, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- 9.Heiner I, et al. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: Evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J. 2003;371:1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J, Xie J, Yue L. Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J Gen Physiol. 2009;134:471–488. doi: 10.1085/jgp.200910254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starkus JG, Fleig A, Penner R. The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J Physiol. 2010;588:1227–1240. doi: 10.1113/jphysiol.2010.187476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starkus J, Beck A, Fleig A, Penner R. Regulation of TRPM2 by extra- and intracellular calcium. J Gen Physiol. 2007;130:427–440. doi: 10.1085/jgp.200709836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto S, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wehrhahn J, Kraft R, Harteneck C, Hauschildt S. Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J Immunol. 2010;184:2386–2393. doi: 10.4049/jimmunol.0902474. [DOI] [PubMed] [Google Scholar]
- 15.Corr SC, O'Neill LA. Listeria monocytogenes infection in the face of innate immunity. Cell Microbiol. 2009;11:703–709. doi: 10.1111/j.1462-5822.2009.01294.x. [DOI] [PubMed] [Google Scholar]
- 16.Seki E, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 17.Xie QW, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 18.Link TM, et al. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai WJ, et al. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5297–5304. [PubMed] [Google Scholar]
- 20.D'Orazio SE, Troese MJ, Starnbach MN. Cytosolic localization of Listeria monocytogenes triggers an early IFN-gamma response by CD8+ T cells that correlates with innate resistance to infection. J Immunol. 2006;177:7146–7154. doi: 10.4049/jimmunol.177.10.7146. [DOI] [PubMed] [Google Scholar]
- 21.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell LM, et al. Distinct responses of splenic dendritic cell subsets to infection with Listeria monocytogenes: Maturation phenotype, level of infection, and T cell priming capacity ex vivo. Cell Immunol. 2011;268:79–86. doi: 10.1016/j.cellimm.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brombacher F, et al. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int Immunol. 1999;11:325–332. doi: 10.1093/intimm/11.3.325. [DOI] [PubMed] [Google Scholar]
- 24.Wu C, et al. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J Immunol. 2000;165:6221–6228. doi: 10.4049/jimmunol.165.11.6221. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 26.Okamura H, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 27.Wherry JC, Schreiber RD, Unanue ER. Regulation of gamma interferon production by natural killer cells in scid mice: Roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991;59:1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neighbors M, et al. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on Interferon gamma production. J Exp Med. 2001;194:343–354. doi: 10.1084/jem.194.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 30.Chin AI, et al. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–194. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- 31.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: No role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 32.Scanga CA, et al. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72:2400–2404. doi: 10.1128/IAI.72.4.2400-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fremond CM, et al. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–1799. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aki D, et al. Peptidoglycan and lipopolysaccharide activate PLCgamma2, leading to enhanced cytokine production in macrophages and dendritic cells. Genes Cells. 2008;13:199–208. doi: 10.1111/j.1365-2443.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 35.Aoshi T, et al. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur J Immunol. 2009;39:417–425. doi: 10.1002/eji.200838718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoshi T, et al. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity. 2008;29:476–486. doi: 10.1016/j.immuni.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29:819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 39.Ting JP, Duncan JA, Lei Y. How the noninflammasome NLRs function in the innate immune system. Science. 2010;327:286–290. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 41.Frantz B, et al. Calcineurin acts in synergy with PMA to inactivate I kappa B/MAD3, an inhibitor of NF-kappa B. EMBO J. 1994;13:861–870. doi: 10.1002/j.1460-2075.1994.tb06329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 43.Murano M, et al. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–58. doi: 10.1046/j.1365-2249.2000.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Andersson A, Dai WJ, Di Santo JP, Brombacher F. Early IFN-gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol. 1998;161:5600–5606. [PubMed] [Google Scholar]
- 46.Nakane A, Numata A, Minagawa T. Endogenous tumor necrosis factor, interleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect Immun. 1992;60:523–528. doi: 10.1128/iai.60.2.523-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information