STAT6 Expression in Multiple Cell Types Mediates the Cooperative Development of Allergic Airway Disease (original) (raw)
. Author manuscript; available in PMC: 2012 Feb 15.
Published in final edited form as: J Immunol. 2011 Jan 17;186(4):2571–2583. doi: 10.4049/jimmunol.1002567
Abstract
Th2 cells induce asthma through the secretion of cytokines. Two such cytokines, IL-4 and IL-13, are critical mediators of many features of this disease. They both share a common receptor subunit, IL-4Rα, and signal through the STAT6 pathway. STAT6−/− mice have impaired Th2 differentiation and reduced airway response to allergen. Transferred Th2 cells were not able to elicit eosinophilia in response to OVA in STAT6−/− mice. To clarify the role of STAT6 in allergic airway inflammation, we generated mouse bone marrow (BM) chimeras. We observed little to no eosinophilia in OVA-treated STAT6−/− mice even when STAT6+/+ BM or Th2 cells were provided. However, when Th2 cells were transferred to STAT6×Rag2−/− mice, we observed an eosinophilic response to OVA. Nevertheless, the expression of STAT6 on either BM-derived cells or lung resident cells enhanced the severity of OVA-induced eosinophilia. Moreover, when both the BM donor and recipient lacked lymphocytes, transferred Th2 cells were sufficient to induce the level of eosinophilia comparable with that of wild-type (WT) mice. The expression of STAT6 in BM-derived cells was more critical for the enhanced eosinophilic response. Furthermore, we found a significantly higher number of CD4+CD25+ Foxp3+ T cells (regulatory T cells [Tregs]) in PBS- and OVA-treated STAT6−/− mouse lungs compared with that in WT animals suggesting that STAT6 limits both naturally occurring and Ag-induced Tregs. Tregs obtained from either WT or STAT6−/− mice were equally efficient in suppressing CD4+ T cell proliferation in vitro. Taken together, our studies demonstrate multiple STAT6-dependent and -independent features of allergic inflammation, which may impact treatments targeting STAT6.
CD4+ Th2 cells are a subset of T cells that secrete the characteristic cytokines IL-4, IL-5, and IL-13 (1). Studies in human and experimental mouse models of asthma have shown that these cells and their cytokines are critical for the development of this disease (1–5). IL-4 stimulation of CD4+ T cells acting through IL-4Rα leads to activation of the STAT6 signaling pathway, which is an important step in differentiation of Th2 effector cells (6–8). In vivo studies have shown that STAT6 signaling is critical for the development of airway eosinophilic inflammation, airway hyperresponsiveness (AHR), Th2 cytokines, mucus secretion, and allergen-specific IgE production in response to allergen challenge (6, 7, 9–11). This lack of the characteristic allergic response to OVA treatment in STAT6−/− mice can be overcome by intranasal eotaxin (11) or i.v. IL-5 (10) administration. However, other studies demonstrated that STAT6 is not absolutely required for Th2 response (12, 13). When treated with Schistosoma mansoni, either IL-4Rα−/− or STAT6−/− mice were able to develop Th2 cells that produced Th2 cytokines although at a lower level than that of wild-type (WT) mice (12). The authors suggested that IL-4Rα/STAT6 signaling is not required for Th2 cell development but is important for the final frequency of Th2 cells.
The effect of IL-4 on CD4+CD25+Foxp3+ T cells (regulatory T cells; Tregs) has been a subject of several recent studies (14–18) that showed controversial results. The in vitro inhibitory effect of IL-4 on inducible Tregs (iTregs) has been shown in naive CD4+ T cell cultures with anti-CD3, TGF-β, and/or IL-4 (16). TGF-β, as expected, induced a dose-dependent Foxp3 expression in stimulated CD4+ T cells. This effect was blocked by the addition of IL-4 to the cultures. In accord, the addition of IL-4 neutralizing Ab resulted in a potent Foxp3 enhancement. This inhibitory effect of IL-4 on iTregs was STAT6 dependent.
In contrast, the study of Maerten and associates (14) has shown that Tregs cultured in vitro with IL-4 were more potent in suppressing the proliferation of naive CD4+ T cells and inhibiting IFN-γ production by CD4+ T cells compared with Tregs cultured in medium. IL-4 prevented spontaneous apoptosis and enhanced CD25 and Foxp3 expression in the in vitro-cultured Tregs. A stimulatory effect of IL-4 on naturally occurring Tregs was also shown in a recent study by Pillemer and colleagues (17). IL-4 was noticed to be necessary to maintain Foxp3 expression by Tregs and promote their proliferation. This effect was IL-4Rα/STAT6-dependent as it was not found using either IL-4Rα- or STAT6-deficient cells. It was also shown that IL-4 could induce the formation of iTregs from naive CD4+ T cells (15). Therefore, the IL-4Rα/STAT6 pathway in Tregs has a complex effect and should be addressed in the context of the inflammatory environment.
In our current study, we addressed the complex role of STAT6 in allergic airway inflammation in vivo using the OVA model of the disease and radiation-induced bone marrow chimeras. We have found that in contrast to a classical allergic inflammatory response observed in WT mice (eosinophils compose >50% of broncho-alveolar lavage cells), STAT6−/− mice develop an alternative lung inflammatory pathology (mainly macrophages with small fractions of neutrophils and lymphocytes) in response to OVA treatment. We also found an increase in Tregs in spleens and lungs of PBS- and OVA-treated STAT6−/− mice compared with that in WT control mice. Our data suggest that this high number of functionally active Tregs presents one of the mechanism(s) of in vivo STAT6 regulation of allergic airway inflammation that may be related to a direct and indirect inhibition of Foxp3 by STAT6 (18–20). Indeed, our bone marrow chimeric studies have demonstrated that when endogenous lymphocytes that included Tregs are absent, transferred Th2 cells function effectively in inducing substantial eosinophilic inflammation in response to OVA. In addition, our studies also showed that the expression of STAT6 in bone marrow (BM)-derived inflammatory cells or in lung resident cells is not absolutely required for allergic response to OVA. However, whereas not required, STAT6 signaling in either cell type substantially potentiates the anti-OVA response. Moreover, we show in this study that STAT6 signaling in BM-derived cells is more critical for an optimal anti-OVA eosinophilic response than that in lung resident cells. Our studies provide new insights on the role of STAT6 in regulating the complex asthma phenotype and should be taken into account during the design of anti–IL-4 and anti–IL-13 therapies for asthma.
Materials and Methods
Mice
The generation, characterization, and genotyping of STAT6−/− mice on BALB/c genetic background were described in detail previously (9). STAT6−/− mice were crossed and back-crossed with Rag2−/− mice obtained from Taconic to generate STAT6×Rag2 double-knockout mice. BALB/c mice (referred to hereon as WT mice) and DO11.10×Rag2−/− mice were purchased from Taconic. Mice were bred and maintained under specific pathogen-free conditions within the animal facility at the University of Maryland School of Medicine. All procedures on mice were performed according to an animal protocol approved by the University of Maryland School of Medicine Animal Care and Use Committee.
Anesthetic
Avertin was used at doses of 0.3 mg/kg or 2 mg/kg by i.p. injection as previously described (21) to anesthetize or euthanize the mice, respectively.
BM preparation and Th2 cell differentiation
BM was isolated from both femur and tibia as previously described (22). Briefly, BM cells were flushed out with PBS followed by RBC lysis, cell count, and reconstitution with PBS to the appropriate volume for injections.
Th2 cells were generated in vitro according to a standard protocol (23). Briefly, DO11.10×Rag2−/− lymph node cells were stimulated with 1 μM OVA323–339 peptide (GenScript, Piscataway, NJ) in the presence of 20 ng/ml IL-2 and 50 ng/ml IL-4 (both from R&D Systems, Minneapolis, MN) plus 10 μg/ml anti–IL-12 and anti–IFN-γ (both from BD Biosciences, San Diego, CA). APCs were prepared by treating BALB/c spleen cells with anti–Thy-1.2 Ab hybridoma (HO1349) supernatant (1 h, 37°C) followed by rabbit complement (Low-Tox, Cedarlane) (30 min, 37°C). Twenty-fold excess of irradiated (3000 rad) APCs was used in cultures. Two 3-d steps of such stimulation were followed by IL-2–induced 3-d cell expansion (22). At the end of cultures, these cells were analyzed for in-tracellular IL-4 and IFN-γ content. As shown previously, these cells responded to in vitro stimulation with OVA323–339 peptide by producing IL-4 but not IFN-γ (22).
Experimental protocols
To study the role of STAT6 in allergic airway inflammation, two experimental protocols were used either with or without a primary BM transplant. The protocols are depicted in Fig. 1.
FIGURE 1.
Experimental protocols used in this study (A) and a schematic representation of the BM chimeras (B). A and B, BM chimeras were generated by transferring BM isolated from WT, STAT6−/−, Rag2−/−, and STAT6×Rag2−/− mice into sublethally irradiated recipient mice as noted. Six weeks later, the mice received a priming injection of OVA in alum. In some cases, the mice received OVA-specific Th2 effectors derived from DO11.10 mice (as described in Materials and Methods) prior to immunization. A booster injection of Ag was administered 2 wk later. Then, the mice were challenged with aerosolized Ag on days 19, 21, and 26. Twenty-four hours after the last challenge, AHR measurements were performed. BAL was performed on euthanized mice 24 h later. Of note, nonchimeric groups of mice were treated with OVA as described above with omission of the irradiation and BM transfer steps.
- OVA treatment of mice. Three to five mice/group/experiment in three experiments were used. Nonchimeric 8- to 12-wk-old mice were treated with allergen as previously described (22). Briefly, mice were immunized with alum (Sigma) or 100 μg OVA (Sigma) in alum on day 1 and boosted on day 14. Mice were exposed to 1% OVA in PBS (Life Technologies) using an Invacare Envoy aerosol compressor for 20 min/day on days 19, 21, and 26. Mouse lung physiology measurements were performed 24 h later. Mice were euthanized 48 h after last OVA nebulization for the assessments.
- BM chimera generation and OVA treatment of mice. Three to seven mice/group/experiment in three experiments were used. BM-recipient 8- to 10-wk-old mice were sublethally irradiated (500 rad) using a Shepard Mark I model 68 irradiator, and 8 × 106 BM cells in 200 μl PBS was injected i.p. into each recipient. Six weeks were allowed for reconstitution. Reconstitution was analyzed by Western blotting or RT-PCR to detect STAT6 expression or by flow cytometry analysis of peripheral blood cells for B and T cell markers in the Rag2−/− system. Th2 cells were introduced by i.v. injection to different chimeras in concentration of 5 × 106 cells/50 μl PBS.
Western blot
Spleens were harvested from individual chimeric mice (Fig. 1) 6 wk after BM transfer. The spleens were disrupted using the frosted ends of microscope slides, and single-cell suspensions were prepared as described previously (21). Cells were then lysed, and 100 μg total protein from each individual mouse was loaded to the 7.5% SDS polyacrylamide gel and transferred to PVDF membrane (both from Bio-Rad) (24). After blocking and washing, the membranes were probed with rabbit polyclonal anti-mouse STAT6 Ab followed by goat anti-rabbit IgG-HRP secondary Ab (both from Santa Cruz) (24, 25). STAT6 binding was visualized by ECL detection reagents kit (Amersham Biosciences) according to the manufacturer’s instruction. The membranes were exposed to Kodak X-OMAT Scientific Imaging Film. The digital image analysis was performed using the AlphaImager system (α Innotech, San Leonardo, CA) with Alpha-EaseFC software. Densitometry was performed using National Institutes of Health Image software.
Lung RNA analysis by RT-PCR
Individual whole-mouse lungs were harvested into RNAzolB and quick frozen. Lung tissue was homogenized using a sonicator. Total RNA was precipitated and washed with 70% ethanol before reconstitution in DEPC-treated water. The total RNA from individual animals within a treatment group was pooled. Total pooled RNA (5 μg) was then subjected to reverse transcription and PCR using sense and antisense primers for either STAT6 or β2-microglobulin as described previously (24, 25).
Mouse lung physiology
Airway responses to methacholine challenge were determined 24 h after the last OVA nebulization. Respiratory pressure curves were recorded by whole-body plethysmography (Buxco Electronics), and the enhanced pause (PenH) was calculated as described (26).
Bronchoalveolar lavage fluid withdrawal, cell differential count, and lung tissue immunohistochemistry
Bronchoalveolar lavage (BAL) fluid and lung tissues were obtained from euthanized mice and processed as previously described (21, 22). Cytospin preparations were made with 200 μl BAL fluid (Cytospin 2; Shandon, Pittsburgh, PA) and stained with Diff-Quick (Dade Behring, Deerfield, IL). The differential cell counts were determined from four high-power fields.
Lung tissue was formalin-fixed, paraffin-embedded, and processed for H&E staining as described (22). In addition, for visualization of mucus production, periodic acid–Schiff (PAS) staining was performed, and alterations were scored as previously described ranging from 0 (no mucus) to 3 (maximum pathology) (22). For specific molecule immunohistochemistry, anti-F4/80 (Serotec, Raleigh, NC), anti–YM-1 (Stemcell Technologies, Vancouver, British Columbia, Canada), followed by appropriate biotinylated secondary Ab, and biotin–anti–Mac-2 (Cedarlane) Ab were used. Biotin-positive staining was revealed using either DAB peroxidase substrate kit (SK-4100; Vector) or NovaRED substrate for peroxidase (SK-4800; Vector).
Flow cytometry
To prepare single-cell suspensions from whole lungs, tissues were subjected to DNAse (Roche) and collagenase (Worthington Biochemical) treatment for 30 min at 37°C as described previously (27). Tissue digests then were passed through 40-μm cell strainers (BD Falcon). Spleen cell preparations were made as previously described (21). Cell surface expression of CD11c (Ab conjugated to fluorochrome allophycocyanin), CD11b (PerCP), I-Ad (PE or FITC), CD4 (PE or FITC), CD3 (FITC), CD8 (PerCP), CD25 (PE), and B220 (PE or FITC) was determined by flow cytometry analysis with a FACSCalibur flow cytometer using commercially available Abs from BD Biosciences. Anti-F4/80 (FITC) Ab was purchased from AbD Serotec (Raleigh, NC). Cells gated by forward- and side-scatter parameters were analyzed using either CELLQuest or FlowJo software at the Flow Cytometry Facility, Center for Vascular and Inflammatory Diseases, University of Maryland School of Medicine.
Cytokine analysis in BAL fluids
BAL fluids were analyzed for the content of IL-10, IL-17, IL-23, IL-5, IL-13, IFN-γ, eotaxin, keratinocyte-derived chemokine, monocyte chemotactic protein-1, macrophage-derived chemokine (MDC), MIP-1α, MIP-1β, RANTES, thymus and activation regulated chemokine (TARC), TNF-α, and TGF-β1 by using the Searchlight Proteome Array (Pierce Biotechnology, Woburn, MA). Data were analyzed using the ArrayVision software.
Intracellular staining
Intracellular cytokine staining was performed as described elsewhere (22). Unstimulated and stimulated cells were harvested, washed, and placed in FACS buffer with anti-CD16/CD32 Ab (BD Biosciences) to block non-specific binding. Cell surface staining for CD4 and CD25 expression was followed by incubation of cells in BD Cytofix/Cytoperm buffer for 18 h at 4°C. Then, cells were stained with either allophycocyanin–anti-Foxp3 or allophycocyanin-rat IgG2a isotype control (both from eBioscience).
CD4+CD25− and CD4+CD25+ T cell isolation for in vitro cultures
CD4+CD25+ T cells were isolated from spleens of either WT or STAT6−/− mice using the CD4+CD25+ T cell isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. The flow-through fraction of CD4+CD25− T cells obtained in the process of such isolation was further enriched using EasySep Negative Selection Mouse CD4+ T cell Enrichment Kit (Stemcell Technologies) according to the manufacturer’s instructions. The purity of CD4+CD25− and CD4+CD25+ T cells and the expression of Foxp3 were assessed by flow cytometry analysis using the Abs described above.
In vitro CD4+CD25− T cell proliferation suppression by Tregs assays
In vitro proliferation assay measured by 3H incorporation was performed as previously described (28) using a Packard Filtermate 96 cell harvester (Packard Instruments, Meriden, CT). Briefly, 100,000 CD4+CD25− T cells/well were cocultured in vitro with or without a decreasing number (from 100,000 to 25,000 cells/well) of either WT or STAT6−/− CD4+ CD25+ T cells. Cells were stimulated in vitro with Dynabeads Mouse T-Activator CD3/CD28 for cell activation and expansion (Invitrogen) according to the manufacturer’s instructions for 72 h, and 3H was added to the cultures 24 h before cell harvest.
Total serum IgE
An IgE-specific ELISA was used to quantitate total IgE Ab levels in serum using matching Ab pairs (R35-72 and R35-92) obtained from BD Biosciences–Pharmingen as described previously (21).
Statistics
Data are summarized as mean ± SEM. To calculate significance levels between experimental groups, Student t test (Microsoft Excel) and Mann–Whitney U test (Prizm-4) were performed.
Results
Alternative lung pathology is observed in allergen-treated STAT6 −/− mice
Whereas the role of STAT6 in mucus production is clearly established, its role in regulating eosinophilic inflammation is controversial (6–13). Therefore, to detail STAT6 function in allergen-induced inflammation, WT, STAT6−/−, Rag2−/−, and STAT6× Rag2−/− mice were subjected to allergen sensitization and challenges by a protocol depicted in Fig. 1_A_. Control animals were treated with alum alone and nebulized with PBS.
Analysis of BAL cell composition 48 h after the last OVA nebulization showed a classical allergic cellular response in WT mice with eosinophils dominating the BAL cellular infiltrates (47.5% of total BAL cells, 430,300 ± 152,552 cells; Fig. 2_A_). In contrast, significant numbers of neutrophils and lymphocytes were found in the BAL fluid from STAT6−/− mice (105,850 ± 62,603 and 41,966 ± 16,148). WT mouse BAL fluid showed the opposite, a higher number of lymphocytes (124,566 ± 22,785) and lower number of neutrophils (22,033 ±2,071). As expected, the BAL cell composition of OVA-treated Rag2−/− mice was not different from that of PBS-treated WT mice. Allergen-treated STAT6×Rag2−/− mice showed a slightly increased number of macrophages and neutrophils in the BAL fluid compared with that of PBS-treated counterparts and WT mice (Fig. 2_A_).
FIGURE 2.
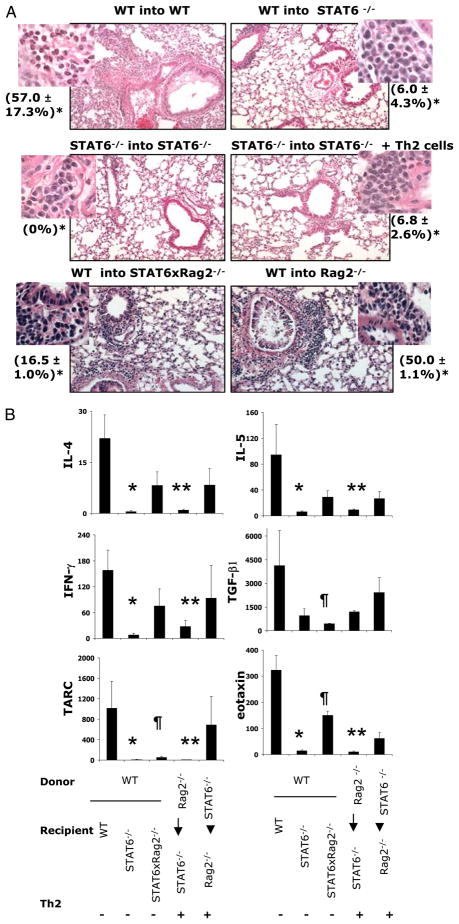
Alternative lung pathology in allergen-treated STAT6−/− mice (A, B). A, WT, Rag2−/−, STAT6−/−, and STAT6×Rag2−/− mice were immunized with OVA as described in Fig. 1_A_. BAL fluid was harvested, and the cells were analyzed by differential counting after cytospin. The average numbers (n = 3 to 5) of total cells (white bars), macrophages (black bars), eosinophils (right hatched bars), lymphocytes (left hatched bars), and neutrophils (gray bars) ±SEM are shown. *, ¶, **, †: p < 0.02 (differences in absolute numbers of total cells, eosinophils, lymphocytes, and neutrophils, respectively, in BAL fluid between OVA-treated WT and STAT6−/− mice). B, Lung sections were stained with H&E or PAS (inset) (original magnification ×4; insets ×100). *The percentage of eosinophils in the BAL fluid was calculated.
Examination of lung tissues obtained from OVA-treated WT mice showed severe, wide-spread inflammatory infiltrates localized predominately around airways and blood vessels (Fig. 2_B_). A significant feature of these infiltrates was the presence of eosinophils. In addition, lymphocytes and mononuclear cells were found in these inflammatory areas. Inflammatory infiltrates, although to a much lesser extent than in WT mice, were observed in the lungs of STAT6−/− mice but not in Rag2−/− mice and rarely in STAT6×Rag2−/− mice. In STAT6−/− lungs, the inflammatory infiltrates consisted of neutrophils, lymphocytes, and mononuclear cells but rare eosinophils. Significant airway tissue remodeling was found only in OVA-treated WT lungs with alterations of airway walls, intensive edema, epithelial cell shedding, and mucous plugs in some airways. This, however, was not observed in the lungs of other experimental animals used including STAT6−/− mice. Therefore, the inflammatory response to OVA treatment was observed in the airways in STAT6−/− mice, but it was different from that found in WT mice. In other words, it was not allergic (predominately eosinophilic) but rather alternative (monolymphocytic–neutrophilic) inflammation. To visualize goblet cells and mucus, PAS staining was performed on lung sections derived from the same mice. PAS+ cells were found in the airways of OVA-treated WT mice (Fig. 2_B_, inset). As expected, we detected a significant amount of total serum IgE in OVA-treated WT mice (3920 ± 1076 ng/ml) but not in STAT6−/−, Rag2−/−, or STAT6× Rag2−/− mice.
STAT6 deficiency affects lung macrophages and their subsets in allergic lungs
To study how STAT6 deficiency affects lung macrophages and their subsets in response to OVA treatment, we performed immunohistochemistry (IHC) and flow cytometry studies of the lung tissue and cells. We selected several markers for IHC staining of lung tissue sections such as F4/80 and Mac-2, markers for mature macrophages (29), and YM-1, a marker for alternatively activated macrophages (30). Expression of F4/80 and Mac-1 (CD11b), a marker for myeloid DCs (mDCs) and small lung macrophages (31), was analyzed by flow cytometry. Both these markers were detected in the lungs of PBS-treated mice without substantial difference between genotypes studied (data not shown). A strong F4/80 positivity was observed on inflammatory macrophages surrounding airways and blood vessels in allergen-treated WT lungs (Fig. 3_A_). In contrast, Mac-2 expression was mainly targeted to the cells in lung parenchyma (Fig. 3_B_). The numbers of both these cell subpopulations were reduced in STAT6−/− mice. Rare cells were found positive for both markers in Rag2−/− and STAT6× Rag2−/−mouse lungs. YM-1+ cells were found in abundance in the peribronchial areas of the airways of OVA-treated WT mouse lungs (Fig. 3_C_). Bronchial airway epithelial cells were also stained positively for this marker. Surprisingly, a few YM-1+ macrophages were found in the lung parenchyma of STAT6−/− mice. Rare YM-1+ cells with weak expression were noted in the lungs of STAT6×Rag2−/− mice suggesting that their accumulation is T and B cell dependent. YM-1 expression was not noted in alum–PBS–treated mouse lungs (data not shown).
FIGURE 3.
STAT6 regulates inflammatory macrophage accumulation in the lung tissue. WT, Rag2−/−, STAT6−/−, and STAT6×Rag2−/− mice were immunized with OVA as described in Fig. 1_A_. Control animals were injected with alum alone and challenged with PBS. A_–_C, Lung sections were stained with anti-F4/80, anti–Mac-2, or anti–YM-1 as noted and analyzed by immunohistochemistry (original magnification ×20; insets for Mac-2 are ×40). D and E, WT, STAT6−/−, and STAT6×Rag2−/− mice were immunized with OVA as described in Fig. 1_A_. Isolated lungs were subjected to enzymatic digestion to obtain single-cell suspensions. The cells were then stained with anti-CD11b and anti-F4/80 plus anti–MHC-II (D) or with anti-CD11b and anti-CD11c plus anti–MHC-II (E).
FACS analysis of lung digests showed two subpopulations of CD11b+ cells in mouse lungs based on the level of CD11b expression, namely CD11blow and CD11bhigh (Fig. 3_D_). The relative number of CD11bhigh cells (mean fluorescence intensity greater than 104) was the highest in PBS-treated WT mice (15.7–29.1% of lung cells, data not shown) compared with that of all other groups. OVA treatment downregulated this number (6.7–8.3%) but increased the number of CD11blow cells (mean fluorescence intensity between 101 and 103) from 4.6 to 8.7–12.4%. A similar trend for CD11blow but not for CD11bhigh expression was observed in the lungs of PBS- and OVA-treated STAT6−/− lungs (Fig. 3_D_). No significant changes between PBS or OVA treatments were found in the relative numbers of CD11b+ cells in STAT6×Rag2−/− mice. The number of CD11b+F4/80+ cells in OVA-treated WT mice showed a 3.8 times increase over that found in PBS-treated counterparts (Fig. 3_D_, upper panel). A slight increase in CD11b+F4/80+ cell number (1.3–1.5 times) was observed in allergen-treated STAT6−/− but not in STAT6×Rag2−/− lungs.
In contrast to macrophage numbers in the inflamed lungs, STAT6 deficiency did not affect either myeloid (CD11chigh subset) or plasmacytoid (CD11clow subset) dendritic cell (DC) numbers in the lung as assessed by flow cytometry using dual markers for DC, CD11c, and MHC class II (MHC-II) (Fig. 3_E_) and gating out CD11chigh autofluorescent macrophages from the analysis plot (data not shown). However, the number of CD11clow cells was significantly upregulated in STAT6−/− mice with Rag2 deficiency. Thus, STAT6 deficiency affects the accumulation of different inflammatory macrophage subpopulations in the lung but not mDCs, plasmacytoid DCs (pDCs), or small lung macrophages.
STAT6 deficiency affects airway responses
Despite multiple studies using STAT6−/− mice to define the requirement of IL-4 and/or IL-13 for airway physiology changes during allergic or antiviral response (6, 7, 9–11, 32), only two of these studies assessed the direct effect of STAT6 deficiency on airway responses (9, 32). In the first study, only a single dose of acetylcholine (50 mg/kg) was used for the AHR assessment, and a significant difference between WT and STAT6−/− mice was found. The second study used a different model of allergen sensitization. The STAT6−/− mice were not sensitized directly with allergen but rather nebulized for 7 consecutive days after a Th2 cell transfer. The authors showed upregulation of AHR to methacholine challenges in OVA-challenged STAT6−/− mice that was significant over PBS-challenged control animals. In contrast, Tomkinson and associates (10) compared dose-dependent AHR to methacholine between OVA-sensitized and challenged STAT6−/− mice and OVA-challenged only and found no difference in responsiveness. Hoshino and colleagues (11) used STAT6−/− mice together with Th2 cell transfer. We measured the response to methacholine challenge in PBS-treated or OVA-sensitized and challenged WT and STAT6−/− mice using whole-body plethysmography (26). Because many pathophysiological changes observed in the asthmatic airways are related to Th2 cytokines and elevated mucus production, we expected to see reduced responses to this nonspecific bronchoconstrictor in STAT6−/− mice compared with that in WT controls. However, we found the opposite (Fig. 4). STAT6−/− mice were more sensitive to methacholine than WT mice. When we compared OVA-treated with -untreated STAT6−/− mice, we observed a modest but statistically insignificant difference in PenH numbers over different methacholine concentrations. These results suggest that STAT6−/− deficiency alters lung responsiveness at baseline.
FIGURE 4.
STAT6 deficiency affects the baseline lung responsiveness to methacholine. WT and STAT6−/− mice were immunized with OVA as described in Fig. 1_A_. Control mice were left untreated. Mice were exposed to increasing concentrations of methacholine, and the change in PenH was measured using whole-body plethysmography. The percentage change in PenH is shown (meant ± SEM). *p < 0.05 (OVA-treated WT versus STAT6−/− mice).
STAT6 requirements for allergic airway inflammation
To delineate the role that STAT6 plays in allergic airway inflammation, we used a comprehensive strategy to generate chimeric mice using numerous combinations of BM donors and recipients (Fig. 1_B_). After reconstitution, the chimeric mice demonstrated a STAT6 phenotype closely matching the phenotype of the BM donor in the spleen indicating that the donor hematopoietic cells had successfully populated the periphery (Supplemental Fig. 1_A_). The levels of STAT6 mRNA in whole-lung tissue prior to any immunization or challenge was most similar to the phenotype of the recipient (Supplemental Fig. 1_B_). STAT6−/− mice receiving WT BM were weakly positive for STAT6 mRNA in the lung indicating that WT BM cells were able to enter the lung during the reconstitution period. Certain groups of chimeric mice received Th2 cell transfers 1 d prior to immunization with either alum alone or OVA in alum.
We initially analyzed the requirements for allergic airway inflammation using WT donor (d) BM into STAT6−/− recipient (r) chimeric mice and STAT6−/−(d)-STAT6−/−(r) chimera combinations with or without Th2 cells (Fig. 5). Using this approach, we found, as expected, that OVA treatment in WT(d)-WT(r) BM chimeric mice led to an increase in total number of cells in the BAL fluid compared with that by alum alone (data not shown). These mice demonstrated an increase in eosinophilia in response to OVA comparable with that observed in nonchimeric OVA-treated WT mice (57.0 ± 17.3% and 47.5 ± 10.5% of total BAL cells, respectively; Figs. 2_B_, 5_A_). In contrast, OVA-treated WT(d)-STAT6−/−(r) chimeras did not show significant lung eosinophilia (6.0 ± 4.3% of total BAL cells) even though they were clearly reconstituted with STAT6-expressing cells (Supplemental Fig. 1). This number was also comparable with that found in allergen-treated nonchimeric STAT6−/− mice (Figs. 2_B_, 5). STAT6−/−(d)-STAT6−/−(r) chimeras also were not able to mount a significant eosinophilic response to OVA even when Th2 cells were provided at the time of initiation of the OVA sensitization protocol. However, when WT BM was transferred into STAT6×Rag2−/− [WT(d)-STAT6×Rag2−/−(r)], OVA treatment induced a significant increase in eosinophilia (16.6%). These results suggest that in the absence of endogenous lymphocytes, STAT6 expression in lung resident cells is not necessary for the development of allergic airway inflammation. OVA-treated WT(d)-Rag2−/−(r) chimeras showed an enhanced lung eosinophilia (50.0 ± 1.1% of total BAL cells) indicating that, although not necessary, STAT6 expression in lung resident cells participates in the severity of the eosinophilic inflammatory response.
FIGURE 5.
STAT6-dependent allergic airway inflammation. The indicated BM chimeras were generated as described in Materials and Methods and depicted in Fig. 1_B_. Where indicated, OVA-specific Th2 effectors were transferred by i.v. injection 1 d prior to immunization with OVA/alum. A, Lungs were lavaged, and the percentage of eosinophils in the BAL (*) was determined by differential counting after cytospin. Lungs were harvested and stained with H&E (original magnification ×20; insets ×100). B, Cytokines and chemokines in the BAL fluid were measured using the Pierce Searchlight array. Data shown are mean ± SEM (n = 3 to 4 mice/group). *, **, ¶: p < 0.05 [WT(d)-WT(r) mice versus WT(d)-STAT6−/−(r), Rag2−/−(d)-STAT6−/−(r), and WT(d)-Rag2× STAT6−/−(r) mice, respectively].
It has been shown that STAT6 deficiency leads to a dysregulation of lung local chemokine production that involved eotaxin, TARC, and MDC (32). This dysregulation prevented transferred Th2 cells to migrate to the lung tissue. Our detailed analysis of cytokines and chemokines in the BAL fluid of chimeric mice has shown that, indeed, eotaxin and TARC were significantly attenuated in WT(d)-STAT6−/−(r) chimeras (Fig. 5_B_). Lymphocyte deficiency in STAT6−/− recipient mice led to a significant upregulation of BAL eotaxin although to a lower extent than that observed in WT(d)-WT(r) mice. BAL eotaxin and TARC content correlated with the levels of BAL and tissue eosinophilia observed in corresponding chimeras (Fig. 5_A_). In addition to lung chemokines, local levels of Th2 (IL-4 and IL-5, but not IL-13) and Th1 (IFN-γ) cytokines were attenuated by STAT6 deficiency and were partially rescued by the absence of endogenous lymphocytes in the WT BM recipient STAT6×Rag2−/− mice (Fig. 5_B_ and data not shown). Local TGF-β levels followed a similar trend with an exception for WT(d)-STAT6−/−(r) chimeras. Whereas the introduction of lymphocyte-deficient BM into STAT6−/− recipient was not efficient in inducing lung cytokine and chemokine expression, the opposite chimeras showed different results. A substantial increase in the levels of all cytokines assessed was found in STAT6−/−(d)-Rag2−/−(r) mice.
A previous study from our laboratory had shown that the expression of IL-4Rα in lung resident cells was not required for allergic airway inflammation in WT(d)–IL-4Rα ×Rag2−/−(r) mice (22). In other words, when endogenous lymphocytes were absent in WT BM recipient IL-4Rα ×Rag2−/− chimeric mice, these mice mounted even higher levels of eosinophilia than those of WT (d)-WT(r) counterparts. Therefore, in our next set of experiments, we analyzed the role of STAT6 in the context of Rag2 deficiency (Fig. 6). We found that OVA treatment of Rag2−/−(d)-STAT6× Rag2−/−(r) chimeras, which also received Th2 cell transfer, induced an increase in the number of eosinophils found in the BAL fluid to levels that were comparable with those observed in Rag2−/− (d)-Rag2−/−(r) (Fig. 6_A_). Moreover, in both these chimeras, the eosinophilia was significantly higher than that in control OVA-treated nonchimeric mice. OVA treatment of STAT6×Rag2−/− (d)-Rag2−/−(r) chimeras induced an increase in the number of eosinophils found in the BAL fluid, but these numbers were significantly less than those induced in the Rag2−/−(d)-Rag2−/−(r) mice. In the absence of STAT6 in both the BM donor and recipient, OVA still induced eosinophilia, albeit at a significantly reduced level compared with that of Rag2−/−(d)-Rag2−/−(r) mice. Similar patterns were observed when analyzing the percentage of eosinophils in the BAL fluid and the lung histology (Fig. 6_B_). Taken together, these results suggest that when endogenous lymphocytes are absent, STAT6 is not absolutely required for OVA-induced, Th2-driven airway inflammation.
FIGURE 6.
STAT6-independent allergic airway inflammation (A, B). The indicated BM chimeras were generated as described in Materials and Methods and depicted in Fig. 1_B_. Non-irradiated WT mice and the chimeras were immunized with alum or OVA/alum and challenged as indicated. A, Lungs were lavaged, and the cells in the BAL fluid were analyzed by differential counting. The average numbers of eosinophils are shown ± SEM (n = 3 to 4 mice/group). B, Representative lung histology (H&E, original magnification ×20; left insets ×100) from OVA-primed mice is shown. Right insets, PAS, original magnification ×40. *Percentage of eosinophils in BAL fluid. C, BAL cytokines and chemokines were measured as described in Materials and Methods. Data shown are mean ± SEM (n = 3 to 4 mice/group). *p < 0.05 [levels of BAL IL-4, TGF-β1, and eotaxin in Rag2×STAT6−/−(d)-Rag2×STAT6−/−(r) mice versus all other experimental groups of mice with an exception for comparable BAL TGF-β1 in Rag2−/−(d)-Rag2×STAT6−/−(r) mice]; **p < 0.05 [Rag2−/−(d)-Rag2−/−(r) mice versus WT mice]; †p < 0.05 [Rag2−/−(d)-Rag2×STAT6−/−(r) versus WT mice]; ¶p < 0.05 [Rag2−/−(d)-Rag2×STAT6−/− (r) mice versus WT mice].
Analysis of goblet cell metaplasia and mucus secretion was performed using PAS staining of lung sections derived from the same mice. No PAS+ cells were present in the bronchi of PBS-treated chimeric mice (data not shown). The most severe degree of goblet cell metaplasia (PAS score = 3) was seen in OVA-treated Rag2−/−(d)-Rag2−/−(r) that received Th2 cells (Fig. 6_B_, inset). Mucous cell differentiation was even more pronounced in these chimeric mice than that observed in OVA-treated WT control mice (PAS score = 3, data not shown). No PAS+ cells were present in recipient mice lacking STAT6 [Rag2−/−(d)-STAT6×Rag2−/−(r)], even though these mice contained STAT6+ BM-derived cells (PAS score = 0, data not shown). In the absence of STAT6 in BM-derived cells [STAT6×Rag2−/−(d)-Rag2−/−(r)], there was some rare goblet cell differentiation with a low number of mucus-producing cells (PAS score = 1; Fig. 6_B_, inset). These results are consistent with those previously reported using STAT6−/− mice (9, 10, 12, 32–34) showing the absence of mucus production after allergen treatment. Furthermore, these results clearly establish that even in the presence of STAT6+ BM cell types, goblet cell metaplasia and mucus production are dependent upon expression of STAT6 in non-BM-derived cell types, most likely the lung epithelial cells (35).
Our results clearly indicate that the expression of STAT6 in BM-derived and non-BM-derived cells is not absolutely required for the Th2-driven recruitment of eosinophils into the lung tissue and the local expression of chemokines and cytokines critical for the allergic response. However, although not required, STAT6 expression in either BM-derived cells or lung resident cells amplifies the inflammatory response. Moreover, the expression of STAT6 in BM-derived cells appears to be more critical for maintaining an efficient lung allergic response than that in resident lung cells. In contrast, lung local STAT6 expression was less critical for inflammation but was absolutely required for mucus production in response to allergen. These results are consisted with our previous observations using IL-4Rα−/− chimeras (22).
Analysis of local cytokine and chemokine expression in STAT6−/− lungs
As already mentioned, the study by Mathew et al. (32) had shown that when Th2 cells are introduced to STAT6−/− mice, these mice were unable to mount an allergic inflammatory response to OVA due to dysregulation of lung local chemokine production. Mainly, expression of key chemokines implicated in Th2 cell and eosinophil trafficking, such as eotaxin-1, eotaxin-2, TCA-3, TARC, and MDC, was markedly attenuated in the lungs of OVA-challenged STAT6−/− mice. Th2 cells in this model did not migrate to the lung tissue. Analysis of chemokines and cytokines in the BAL fluid of chimeric mice showed that the levels of IL-4 and eotaxin were the lowest in STAT6×Rag2−/−(d)-STAT6×Rag2−/−(r) mice (Fig. 6_C_). Low BAL IFN-γ and TARC contents in this group were not significantly different compared with those of other mice. IL-17 was very low in all groups (<10 pg/ml).
Although STAT6−/− mice do not mount a Th2 response, they do have competent lymphocytes that can respond to Ag by producing Th1 cells capable of secreting IFN-γ (10, 36, 37). Indeed, the levels of IFN-γ in STAT6−/− BM chimeras were comparable with those observed in nonchimeric WT mice (Fig. 6_C_). When STAT6−/− mice were treated with OVA, we found a decrease in the level of cellular infiltrate present in the lungs compared with that of WT mice, and the infiltrate was mainly composed of monocytes, lymphocytes, and neutrophils (Fig. 2_A_, 2_B_). The presence of such infiltrate is consistent with the production of Th1 cytokines (38). In addition to that, Th17 cells can dampen Th2 response (39) and induce similar infiltration into the airways as we observed in OVA-treated STAT6−/− mice (40). However, we did not find any significant differences in IFN-γ or IL-17 production in BAL fluids obtained from OVA-treated WT or STAT6−/− mice (data not shown). Moreover, their concentration was very low (<10 pg/ml).
Altered number of functionally active Tregs in STAT6-deficient mice
To identify which lymphocyte subsets infiltrate into the lungs of WT and STAT6−/− mice after OVA treatment, we examined B220+, CD3+, CD4+, and CD8+ cells in lung digests by flow cytometry. Analysis of single-cell suspensions obtained from OVA-treated mouse lungs showed no differences in B220+ cell numbers between OVA-treated WT and STAT6−/− mice (11.5 ± 5.3% versus 10.5 ± 1.6% of total cell numbers, respectively). As expected, these cells were absent in STAT6×Rag2−/− mice (0.65 ± 0.32%).
Surprisingly, we found more T cells, including both CD4+ and CD8+ T cell subsets, in the lungs of PBS-treated STAT6−/− mice compared with that in similarly treated WT mice (Fig. 7_A_). Although aerosolized OVA challenges induced significant increases in CD4+ and CD8+ T cells in WT mice (from 1.1 ±0.4% to 6.5 ±1.3% and from 0.4 ± 0% to 4.3 ± 1.5% of total lung cells, respectively), the relative numbers of these cells in OVA-treated STAT6−/− mice were significantly higher (9.1 ± 2.4% and 6.5 ± 1.3% of total lung cells, p < 0.05; Fig. 7_A_). As expected, neither CD4+ nor CD8+ T cells were detected in the lungs of STAT6×Rag2−/− mice.
FIGURE 7.
STAT6 deficiency alters the number of Tregs in the lung. WT and STAT6−/− mice were immunized with alum or OVA/alum as described in Fig. 1_A_. The mice were challenged as indicated. Lungs were digested with collagenase–DNAse, and cell suspensions were analyzed by FACS for expression of CD4 and CD8 on T cells (A) and for expression of CD4, CD25, and Foxp3 (B). Foxp3 staining in the CD4+, CD25hi population is shown.
To define further CD4+ T cells in STAT6−/− mice, we performed an additional flow cytometry analysis for surface CD25 and intracellular Foxp3 expression. We found more CD4+CD25high cells in PBS- and OVA-treated STAT6−/− lungs compared with that in similarly treated WT counterparts (Fig. 7_B_). More than 90% of these cells coexpressed Foxp3 suggesting that these were Tregs. As expected, neither T cells nor Tregs were found in STAT6×Rag2−/− mice (data not shown). Notably, lungs of PBS-treated STAT6−/− mice had more Foxp3+CD25+CD4− cells, which further increased with allergen treatment (Fig. 7_B_).
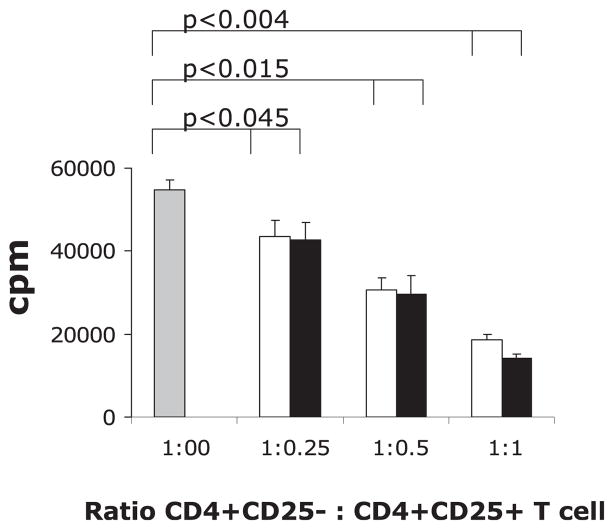
To study the functional activity of Tregs, we performed in vitro proliferation suppression assays using purified WT CD4+ T cells proliferating in response to anti-CD3/anti-CD28 beads (Fig. 8). The WT CD4+ T cells were cocultured with or without increasing number of either WT or STAT6−/− CD4+CD25+ cells. We found that both WT and STAT6−/− Tregs were similarly active in suppression of T cell proliferation measured by thymidine incorporation (Fig. 8). These results suggest that an additional mechanism by which STAT6 controls allergic airway inflammation is by limiting the number of Tregs (20).
FIGURE 8.
STAT6-deficient Tregs are functionally active. Tregs were obtained from the spleens of untreated WT (open bars) and STAT6−/− (black bars) mice as described in Materials and Methods. CD4+CD25− WT T cells were obtained and used as effectors. The effector T cells were incubated with increasing amounts of WT or STAT6−/− Tregs. Cells were stimulated with Dynabeads (CD3/CD28) for 72 h, and 3H was added to the cultures for the last 24 h before cell harvest. Data are presented as mean cpm ± SEM of 3H incorporation in 72-h cultures.
Discussion
The studies reported here show several roles for STAT6 in allergic airway inflammation. Although previous reports indicated an important role for STAT6 in Th2 cell differentiation and allergen-induced asthma-like lung responses (6–11), we show here that STAT6 is not absolutely required for the OVA-induced, Th2-driven inflammatory response. Moreover, using BM chimeras, we clearly demonstrate that STAT6 in both BM-derived cells and non-BM-derived cells, although not required, contributes to the disease progression. Additionally and importantly, we demonstrated that the expression of STAT6 in BM-derived cells was more critical for maintaining an efficient lung eosinophilic response than that in lung resident cells. Whereas lung local STAT6 expression was not critical for inflammation, it was absolutely required for efficient mucus production. Finally, our studies suggest that another mechanism by which STAT6 regulates in vivo allergic responses is through Treg inhibition.
Previous studies showed that STAT6−/− mice have greatly reduced AHR to a nonspecific bronchoconstrictor challenge, airway mucus hypersecretion, and allergen-specific IgE response (9–11). However, one study using STAT6−/− mice demonstrated increased CD8+ T cell lung infiltration in response to allergen treatment compared with that in similarly treated WT mice (34). Because the studies mentioned above used STAT6−/− mice with different genetic backgrounds (C57BL/6, BALB/c, or B6/129J) as well as different protocols for the allergen treatment, they provided important but inconsistent information about the exact role of STAT6 in the disease pathogenesis. For example, STAT6 was shown to be absolutely required for BAL Th2 cytokine induction in the B6×129J/STAT6−/− experimental asthma model (10), but it was not that essential in the BALB/c/STAT6−/− model with Th2 cell transfer followed by allergen nebulizations (11). In addition, the importance of STAT6 signaling in regulating IFN-γ–mediated Th1 response is still questionable as an induction of BAL IFN-γ to allergen was (9) or was not (10) observed in STAT6−/− mice with different treatment protocols. Nevertheless, all studies underlined a critical role of STAT6 signaling in the induction of AHR. We show in this study that STAT6−/− mice on the BALB/c background demonstrated an increase in PenH in response to methacholine without any allergen exposure (Fig. 4) compared with that in WT mice. This reactivity was modestly, but not significantly, enhanced by allergen treatment. We used noninvasive, barometric plethysmography as a measure of lung responses (26). Some lung physiologists do not believe the noninvasive technique directly detects changes in airway mechanics and that the PenH value is not a measure of airway resistance (41). However, other studies argue that the PenH value is influenced by and related to airway resistance (42). The use of the invasive technique to measure directly airway resistance in anesthetized, intubated, and ventilated mice is thought to be the most accurate measure of airway function. However, the invasive technique has its own limitations related to the use of anesthesia, restraint, and paralysis of tested animals (42). Thus, although we did not directly measure airway resistance, our results suggest that the lack of STAT6 influences lung responsiveness to methacholine. Additional in-depth studies will be required to determine the mechanism by which STAT6 regulates such responses and whether STAT6 directly influences smooth muscle cell contractility.
In our study, we used a more chronic protocol of allergen treatment (Fig. 1_A_) compared with the published studies on STAT6−/− mice with either one allergen challenge (9) or a Th2 cell transfer with following Ag nebulizations (11). The study by Tomkinson and colleagues (10) used a similar protocol of chronic allergen exposure but in B6×129J/STAT6−/− mice. The mouse genetic background significantly affects AHR outcome (43). We also show here that indeed STAT6−/− mice were unable to mount a classical allergic cellular response (Fig. 2_A_, 2_B_) where eosinophils dominate BAL cellular infiltrate. However, in contrast to the previously reported data on the absence of inflammation in allergen-treated STAT6−/− mice (9–11), we measured the lung pathology with an alternative monolymphocytic-neutrophilic type of inflammation in STAT6−/− mice. Our other interesting observation included STAT6×Rag2−/− mice, which, in contrast to allergen-unresponsive Rag2−/− mice, also showed some foci of OVA-induced local lung inflammation (Fig. 2_A_, 2_B_ and Fig. 3_A_, 3_B_). This led us to conclude that a T cell-independent allergic disease-suppressive regulatory mechanism might work specifically in the absence of STAT6. This regulatory mechanism might include F4/80+CD11b+ cells and/or pDCs, which both were found in higher numbers in PBS-treated STAT6×Rag2−/− mice compared with that in WT and STAT6−/− mice (Fig. 3_D_, 3_E_). However, in both WT mice and STAT6−/− mice, the relative number of F4/80+CD11b+ cells went up after OVA treatment. This increase might represent the allergen-induced compensatory mechanism for allergic inflammation downregulation. The number of lung pDCs was the highest in OVA-exposed STAT6×Rag2−/− mice. It is known that pDCs can downregulate the allergic response by inducing Tregs with involvement of multiple pathways (44) or exclusively through PD1–PDL1 interaction (45). The latter might be the case for these double-knockout mice with T cell deficiency. Therefore, in the absence of T/B cells (Rag2 deficiency) and STAT6, there might be another mechanism of disease regulation that is not readily apparent in STAT6−/− mice.
In accord with the previously published data (9–11), we did not find any OVA-induced lung remodeling in STAT6−/− mice including significant alterations to the lung tissue structure with epithelial cell shedding and mucous plugs in the airways. Our BM chimeras clearly demonstrated that lung STAT6 expression was absolutely required for the mucus secretion as BM-recipient mice lacking STAT6 showed no PAS+ cells in the airways. These results are consistent with those previously reported (9, 10, 12, 32–34) showing the absence of mucus production in allergen-treated STAT6−/− mice. In addition, we show here that STAT6 in BM-derived cells is also important for mucous cell hyperplasia. In the absence of STAT6 in BM-derived cells, we could see rare goblet cell differentiation in OVA-treated recipient STAT6+ mice. Furthermore, these results clearly establish that even in the presence of STAT6+ BM cell types, goblet cell metaplasia and mucus production are dependent upon expression of STAT6 in non-BM-derived cell types, most likely the lung epithelial cells (35).
A previous study from our laboratory demonstrated that the expression of IL-4Rα on a nonlymphoid, MHC-II+, BM-derived cell type significantly contributed to the severity of allergic airway inflammation and mucus production (22). When IL-4Rα+ BM was administered to Rag2−/− mice and Th2 cells provided, there was a strong expansion of MHC-II+ cells in spleens and lungs of these mice. This was not observed in IL-4Rα ×Rag2−/−(d)-IL-4Rα × Rag2−/−(r) chimeras. These MHC-II+ cells were also CD11b+, which suggested their macrophage nature as CD11c+MHC-II+ cells did not change significantly in number between the experimental groups. We performed IHC of lung tissues for specific macrophage markers and found that Mac-2+ cells and F4/80+ cells were reduced in number in STAT6−/− mice compared with those in WT mice (Fig. 3_A_, 3_B_). Notably, we found a differential distribution of these markers in the inflamed lung tissue suggesting that the non-overlapping subtypes of inflammatory macrophages were induced in the lung in response to OVA. Despite that, some cells, especially those around airways, were obviously positive for both markers on IHC slides. Our flow cytometry data (Fig. 3_D_) clearly demonstrated a significant increase in CD11b+F4/80+ cell number in allergen-treated WT mice compared with that in PBS-treated counterparts and some increase in this macrophage subset cell number in STAT6−/− but not in STAT6×Rag2−/− lungs. Both these molecules, F4/80 and CD11b, can be specific markers for the tissue-infiltrating classically activated macrophages (CAMs), which usually localize in the active sites of the disease (46) like peribronchial areas for allergic asthma. Alternatively activated macrophages (AAMs) are F4/80+ and express YM-1 (47). We observed large numbers of YM-1+ cells in OVA-treated WT mice; however, we did not expect to see these cells in STAT6−/− mice as their differentiation is typically IL-4Rα/STAT6-dependent (47–49). Nevertheless, we noted a few scattered YM-1+ macrophages in the lung parenchyma of STAT6−/− mice, whereas lung bronchial epithelial cells were positive for YM-1 only in WT mice (Fig. 3_A_). Although it is well established that IL-4 and IL-13 directly induce AAMs, other factors such as IL-33 (an IL-1 family member), IL-25 (an IL-17 family member), and LPS can amplify AAM differentiation (48, 49). It is possible this low level of YM-1 expression in STAT6−/− mice is mediated by such factors.
The F4/80+CD11b+ cells are not CD11b+ myeloid suppressor cells, which generally lack F4/80 (reviewed in Ref. 50). If we consider that there is a higher possibility for Th1 response development with STAT6 deficiency (10, 36, 37) leading in its turn to a functional Th2 deficiency, we would expect to find a higher number of IFN-γ–dependent CAMs in the lungs of OVA-treated STAT6−/− mice. We showed here that neither F4/80+CD11b+ macrophage number (Fig. 3) nor BAL IFN-γ (Fig. 5_B_ and data not shown) were upregulated in STAT6−/− mice. IFN-γ was not increased in the STAT6−/− chimeras in response to allergen compared with that in WT mice (Figs. 5_B_, 6_C_). Therefore, with these experiments we ruled out the possibility of the inhibition of allergic airway inflammation in STAT6−/− mice by allergic disease-suppressive (reviewed in Refs. 48, 49) CAMs. As mentioned above, lung DCs (gated as CD11c+MHC-II+ cells) did not change in numbers between IL-4Rα chimeric mice with or without Rag2 deficiency (22). In accord with these data, STAT6 deficiency did not affect myeloid (CD11chigh subset) or plasmacytoid (CD11clow subset) DC numbers in the lung (Fig. 3_E_). There were no changes in the mouse lung immature and mature mDC numbers (out of CD11chigh cells, CD11c+MHC-IIlow and CD11c+MHC-II+ cells, respectively) between two groups (Fig. 3_E_). These were surprising findings as it is currently well established that lung mDCs are absolutely required for the initiation and progression of allergic airway inflammation (51, 52) and that lung pDCs are the cells suppressing this response through the activation of Tregs by different mechanisms (44, 45). Nevertheless, based on this observation, we conclude that STAT6 deficiency does not affect lung DC number. However, it might affect their activation state.
Surprisingly, we found more T cells, including both CD4+ and CD8+ T cell subsets, in the lungs of PBS-treated STAT6−/− mice compared with that in similarly treated WT mice (Fig. 7_A_). The increased number of BAL lymphocytes has been noted in allergen-treated STAT6−/− mice by other investigators (9, 10); however, no relevant statistics and cell phenotype analysis were done. We found a 2-fold increase in the number of functionally active Tregs in PBS-and OVA-treated STAT6−/− lungs compared with that in similarly treated WT counterparts (Figs. 7_B_, 8). Although these changes are small, Tregs are very potent in their regulatory/suppressor function. Thus, even a small increase in number may be sufficient to mediate a significant in vivo effect. Other groups have noted a reciprocal relationship between STAT6 and Tregs; STAT6 suppresses Foxp3 expression and Treg differentiation, and Foxp3 blocks GATA3-mediated transcription of IL-5 (20). We also found more Foxp3+ CD25+CD4− cells in STAT6−/− mice (Fig. 7_B_), which could potentially be the recently identified regulatory CD8+ T cells (53, 54) and/or Foxp3+ regulatory DCs (55).
In summary, we show here that STAT6 plays a unique and complex role in regulation of allergic airway inflammation depending on whether it is expressed in BM-derived inflammatory cells or non-BM-derived lung resident cells. Our multiple BM chimeras clearly show that the cells expressing IL-4Rα, responsive to IL-4 and/or IL-13 through STAT6 signaling, act cooperatively to induce a full spectrum of pathologies found in allergic asthma. Our results on the in vivo STAT6 inhibitory effect on Tregs should be taken in account when designing IL-4/IL-13/IL-4Rα/STAT6-based asthma immunotherapy.
Supplementary Material
Supplemental Data
Acknowledgments
This work was supported by National Institutes of Health Grants RO1AI59775 and RO1AI038985 (to A.D.K.).
We thank Dr. Scott Strome for use of the laboratory cell harvester and Xiulan Qi for technical assistance.
Abbreviations used in this article
AAM
alternatively activated macrophage
AHR
airway hyperresponsiveness
BAL
bronchoalveolar lavage
BM
bone marrow
CAM
classically activated macrophage
(d)
donor
IHC
immunohistochemistry
iTreg
inducible Treg
mDC
myeloid DC
MDC
macrophage-derived chemokine
MHC-II
MHC class II
PAS
periodic acid–Schiff
pDC
plasmacytoid DC
PenH
enhanced pause
(r)
recipient
TARC
thymus and activation regulated chemokine
Treg
regulatory T cell
WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 3.Coyle AJ, Le Gros G, Bertrand C, Tsuyuki S, Heusser CH, Kopf M, Anderson GP. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995;13:54–59. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- 4.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 7.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 8.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 9.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomkinson A, Kanehiro A, Rabinovitch N, Joetham A, Cieslewicz G, Gelfand EW. The failure of STAT6-deficient mice to develop airway eosinophilia and airway hyperresponsiveness is overcome by interleukin-5. Am J Respir Crit Care Med. 1999;160:1283–1291. doi: 10.1164/ajrccm.160.4.9809065. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino A, Tsuji T, Matsuzaki J, Jinushi T, Ashino S, Teramura T, Chamoto K, Tanaka Y, Asakura Y, Sakurai T, et al. STAT6-mediated signaling in Th2-dependent allergic asthma: critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. Int Immunol. 2004;16:1497–1505. doi: 10.1093/intimm/dxh151. [DOI] [PubMed] [Google Scholar]
- 12.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 13.Finkelman FD, Morris SC, Orekhova T, Mori M, Donaldson D, Reiner SL, Reilly NL, Schopf L, Urban JF., Jr Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 14.Maerten P, Shen C, Bullens DM, Van Assche G, Van Gool S, Geboes K, Rutgeerts P, Ceuppens JL. Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. J Autoimmun. 2005;25:112–120. doi: 10.1016/j.jaut.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25-CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 16.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pillemer BB, Qi Z, Melgert B, Oriss TB, Ray P, Ray A. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. J Immunol. 2009;183:155–163. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapoval S, Dasgupta P, Dorsey NJ, Keegan AD. Regulation of the T helper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J Leukoc Biol. 2010;87:1011–1018. doi: 10.1189/jlb.1209772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Rückert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapoval SP, Nabozny GH, Marietta EV, Raymond EL, Krco CJ, Andrews AG, David CS. Short ragweed allergen induces eosinophilic lung disease in HLA-DQ transgenic mice. J Clin Invest. 1999;103:1707–1717. doi: 10.1172/JCI6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly-Welch AE, Melo ME, Smith E, Ford AQ, Haudenschild C, Noben-Trauth N, Keegan AD. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. J Immunol. 2004;172:4545–4555. doi: 10.4049/jimmunol.172.7.4545. [DOI] [PubMed] [Google Scholar]
- 23.Fitch FW, Gajewski TF, Hu-Li J. Production of TH1 and TH2 cell lines and clones. Curr Protoc Immunol Chapter. 2006;3(Unit 3.13) doi: 10.1002/0471142735.im0313s72. [DOI] [PubMed] [Google Scholar]
- 24.Chapoval SP, Al-Garawi A, Lora JM, Strickland I, Ma B, Lee PJ, Homer RJ, Ghosh S, Coyle AJ, Elias JA. Inhibition of NF-kappaB activation reduces the tissue effects of transgenic IL-13. J Immunol. 2007;179:7030–7041. doi: 10.4049/jimmunol.179.10.7030. [DOI] [PubMed] [Google Scholar]
- 25.Ford AQ, Heller NM, Stephenson L, Boothby MR, Keegan AD. An atopy-associated polymorphism in the ectodomain of the IL-4R(alpha) chain (V50) regulates the persistence of STAT6 phosphorylation. J Immunol. 2009;183:1607–1616. doi: 10.4049/jimmunol.0803266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 27.Chapoval SP, Lee CG, Tang C, Keegan AD, Cohn L, Bottomly K, Elias JA. Lung vascular endothelial growth factor expression induces local myeloid dendritic cell activation. Clin Immunol. 2009;132:371–384. doi: 10.1016/j.clim.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapoval SP, Neeno T, Krco CJ, Marietta EV, Harders J, David CS. HLA-DQ6 and HLA-DQ8 transgenic mice respond to ragweed allergens and recognize a distinct set of epitopes on short and giant ragweed group 5 antigens. J Immunol. 1998;161:2032–2037. [PubMed] [Google Scholar]
- 29.Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 30.Raes G, De Baetselier P, Noël W, Beschin A, Brombacher F, Hassanzadeh GhG. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 31.Gonzalez-Juarrero M, Shim TS, Kipnis A, Junqueira-Kipnis AP, Orme IM. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003;171:3128–3135. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- 32.Mathew A, MacLean JA, DeHaan E, Tager AM, Green FH, Luster AD. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J Exp Med. 2001;193:1087–1096. doi: 10.1084/jem.193.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tekkanat KK, Maassab HF, Cho DS, Lai JJ, John A, Berlin A, Kaplan MH, Lukacs NW. IL-13-induced airway hyperreactivity during respiratory syncytial virus infection is STAT6 dependent. J Immunol. 2001;166:3542–3548. doi: 10.4049/jimmunol.166.5.3542. [DOI] [PubMed] [Google Scholar]
- 34.Miyata S, Matsuyama T, Kodama T, Nishioka Y, Kuribayashi K, Takeda K, Akira S, Sugita M. STAT6 deficiency in a mouse model of allergen-induced airways inflammation abolishes eosinophilia but induces infiltration of CD8+ T cells. Clin Exp Allergy. 1999;29:114–123. doi: 10.1046/j.1365-2222.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- 35.McCusker CT, Wang Y, Shan J, Kinyanjui MW, Villeneuve A, Michael H, Fixman ED. Inhibition of experimental allergic airways disease by local application of a cell-penetrating dominant-negative STAT-6 peptide. J Immunol. 2007;179:2556–2564. doi: 10.4049/jimmunol.179.4.2556. [DOI] [PubMed] [Google Scholar]
- 36.Stamm LM, Räisänen-Sokolowski A, Okano M, Russell ME, David JR, Satoskar AR. Mice with STAT6-targeted gene disruption develop a Th1 response and control cutaneous leishmaniasis. J Immunol. 1998;161:6180–6188. [PubMed] [Google Scholar]
- 37.Chitnis T, Najafian N, Benou C, Salama AD, Grusby MJ, Sayegh MH, Khoury SJ. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J Clin Invest. 2001;108:739–747. doi: 10.1172/JCI12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohn L, Herrick C, Niu N, Homer R, Bottomly K. IL-4 promotes airway eosinophilia by suppressing IFN-gamma production: defining a novel role for IFN-gamma in the regulation of allergic airway inflammation. J Immunol. 2001;166:2760–2767. doi: 10.4049/jimmunol.166.4.2760. [DOI] [PubMed] [Google Scholar]
- 39.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 40.Fujiwara M, Hirose K, Kagami S, Takatori H, Wakashin H, Tamachi T, Watanabe N, Saito Y, Iwamoto I, Nakajima H. T-bet inhibits both TH2 cell-mediated eosinophil recruitment and TH17 cell-mediated neutrophil recruitment into the airways. J Allergy Clin Immunol. 2007;119:662–670. doi: 10.1016/j.jaci.2006.12.643. [DOI] [PubMed] [Google Scholar]
- 41.Bates J, Irvin C, Brusasco V, Drazen J, Fredberg J, Loring S, Eidelman D, Ludwig M, Macklem P, Martin J, et al. The use and misuse of Penh in animal models of lung disease. Am J Respir Cell Mol Biol. 2004;31:373–374. doi: 10.1165/ajrcmb.31.3.1. [DOI] [PubMed] [Google Scholar]
- 42.Finkelman FD. Use of unrestrained, single-chamber barometric plethysmography to evaluate sensitivity to cholinergic stimulation in mouse models of allergic airway disease. J Allergy Clin Immunol. 2008;121:334–335. doi: 10.1016/j.jaci.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 43.Levitt RC, Mitzner W. Expression of airway hyperreactivity to acetylcholine as a simple autosomal recessive trait in mice. FASEB J. 1988;2:2605–2608. doi: 10.1096/fasebj.2.10.3384240. [DOI] [PubMed] [Google Scholar]
- 44.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Köhl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, Smit JJ, Coyle A, Clausen BE, Hoogsteden HC, Lambrecht BN, Hammad H. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol. 2009;183:1074–1082. doi: 10.4049/jimmunol.0900471. [DOI] [PubMed] [Google Scholar]
- 46.Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, van Rooijen N, Davidson A, Ivashkiv LB. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci USA. 2010;107:3012–3017. doi: 10.1073/pnas.0914902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loke P, MacDonald AS, Robb A, Maizels RM, Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur J Immunol. 2000;30:2669–2678. doi: 10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 49.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–4097. [PubMed] [Google Scholar]
- 52.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakagawa T, Tsuruoka M, Ogura H, Okuyama Y, Arima Y, Hirano T, Murakami M. IL-6 positively regulates Foxp3+CD8+ T cells in vivo. Int Immunol. 2010;22:129–139. doi: 10.1093/intimm/dxp119. [DOI] [PubMed] [Google Scholar]
- 54.Tsai YG, Yang KD, Niu DM, Chien JW, Lin CY. TLR2 agonists enhance CD8+Foxp3+ regulatory T cells and suppress Th2 immune responses during allergen immunotherapy. J Immunol. 2010;184:7229–7237. doi: 10.4049/jimmunol.1000083. [DOI] [PubMed] [Google Scholar]
- 55.Lipscomb MW, Taylor JL, Goldbach CJ, Watkins SC, Wesa AK, Storkus WJ. DC expressing transgene Foxp3 are regulatory APC. Eur J Immunol. 2010;40:480–493. doi: 10.1002/eji.200939667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data