NEW EMBO MEMBERS' REVIEW: The ErbB signaling network: receptor heterodimerization in development and cancer (original) (raw)
Introduction
Cells are continuously exposed to diverse stimuli ranging from soluble endocrine and paracrine factors, to signaling molecules on neighboring cells. It is of great importance that these extracellular signals are correctly interpreted by the cell, in order to achieve an appropriate developmental or proliferative response. Receptors of the tyrosine kinase family play pivotal roles in this process. By binding to specific peptide ligands they are able to integrate these external stimuli with internal signal transduction pathways, contributing in this fashion to the ability of the cell to respond correctly to its environment. In this review, we will concentrate on the role of ErbB receptors as normal signal transducers and their contribution to the process of malignant transformation during tumor development.
ErbB proteins belong to subclass I of the superfamily of receptor tyrosine kinases (RTKs). There are four members of the ErbB family: epidermal growth factor (EGF) receptor (also termed ErbB1/HER1), ErbB2/Neu/HER2, ErbB3/HER3 and ErbB4/HER4. We will refer to them, henceforth, as the ErbB receptors. All family members have in common an extracellular ligand-binding domain, a single membrane-spanning region and a cytoplasmic protein tyrosine kinase domain. A family of ligands, the EGF-related peptide growth factors, bind the extracellular domain of ErbB receptors leading to the formation of both homo- and heterodimers. Dimerization consequently stimulates the intrinsic tyrosine kinase activity of the receptors and triggers autophosphorylation of specific tyrosine residues within the cytoplasmic domain. These phosphorylated residues serve as docking sites for signaling molecules involved in the regulation of intracellular signaling cascades. Ultimately, downstream effects on gene expression determine the biological response to receptor activation.
ErbB receptors are expressed in a variety of tissues of epithelial, mesenchymal and neuronal origin, where they play fundamental roles in development, proliferation and differentiation. Moreover, deregulated expression of ErbB receptors, in particular ErbB1 and ErbB2, has been implicated in the development and malignancy of numerous types of human cancers. The ErbB family has evolved from a single ligand–receptor combination in Caenorhabditis elegans (Aroian et al., 1990), through Drosophila with one receptor and four ligands (Wasserman and Freeman, 1997), to vertebrates, where four ErbB receptors bind multiple EGF-related ligands. Consequently, numerous ErbB homo- and heterodimer combinations are possible in vertebrates, suggesting that the ErbB receptor family has evolved to provide a high degree of signaling diversity; an event that may have been necessary for the development of metazoans. With this in mind, we have attempted, in this short review, not only to discuss generally individual ErbB receptors and their signaling potential but also to provide examples of specific ErbB heterodimers in signaling and development. Our particular emphasis will be on their role in mammary gland biology, an organ in which the ErbB family and its ligands are critically involved in development, differentiation and cancer.
The ErbB receptors in development
The importance of ErbB receptors in development is proven from the analysis of genetically modified mice. Indeed, null mutations in individual ErbB loci are lethal. More specifically, depending upon the genetic background of the host, loss of ErbB1 leads to embryonic or perinatal lethality with mice showing abnormalities in multiple organs including the brain, skin, lung and gastrointestinal tract (Miettinen et al., 1995; Sibilia and Wagner, 1995; Threadgill et al., 1995; Sibilia et al., 1998). ErbB2 null mice die at midgestation (E10.5) due to trabeculae malformation in the heart (Lee et al., 1995), a phenotype that is shared by ErbB4 knockout mice (Gassmann et al., 1995). In addition, through genetic rescue of heart development via myocardial expression of an ErbB2 transgene, a further role for ErbB2 in peripheral nervous system development has been demonstrated (Morris et al., 1999). In the case of ErbB3, most knockout mice die by E13.5, displaying normal heart trabeculation but defective valve formation. Additionally, these animals show a generalized neural crest defect and lack Schwann cell precursors (Riethmacher et al., 1995; Erickson et al., 1997). From these data it is clear that ErbB receptors play critical roles in modulating specific aspects of vertebrate embryogenesis/development.
It is apparent, however, that ErbB receptors also play essential roles in the adult organism. The mammary gland is an organ that undergoes most of its proliferation and differentiation postnatally. At birth, the gland has a rudimentary system of ducts, which undergoes extensive development at puberty under the influence of steroid and peptide hormones. During pregnancy, lobuloalveolar proliferation occurs and, at parturition, the mammary fat pad is completely filled with milk-producing lobuloalveolar units (Hennighausen and Robinson, 1998). In the mammary gland, all four ErbB receptors are expressed in cell type- and developmental stage-specific patterns (Schroeder and Lee, 1998; Darcy et al., 2000). Embryonic or early lethality has prevented direct analyses of mammary glands from ErbB receptor negative mice. However, other approaches have revealed the importance of functional ErbB receptors in this organ. In the case of ErbB1, which is expressed at all stages of mammary gland development, mice (_wa_-2) harboring a mutation in the ErbB1 kinase domain exhibit sparse development of the mammary gland, indicative of defective ductal growth (Fowler et al., 1995). A major role for ErbB1 in ductal growth was further confirmed by expression of dominant-negative (DN) ErbB1 in the mammary gland (Xie et al., 1997), as well as by reconstitution experiments with ErbB1–/– neonatal mammary glands (Wiesen et al., 1999). In contrast, despite the fact that ErbB2 is also expressed at all developmental stages, transgenics expressing DN ErbB2 in the mammary gland display normal ductal growth. These mice do appear, however, to have defective lobuloalveoli and reduced milk protein secretion (Jones and Stern, 1999). As with ErbB2, transgenics expressing DN ErbB4 in the mammary gland exhibit normal ductal growth but impaired lactation (F.E.Jones et al., 1999). Consequently, a role in lactation has also been suggested for ErbB4, correlating with its elevated expression during pregnancy and lactation. ErbB3 is also expressed throughout development; however, a detailed analysis of its function has not been described. In summary, these data clearly suggest that the major function of ErbB1 in mammary gland development is in promoting ductal growth, while ErbB2 and ErbB4 appear to have key roles in lobuloalveolar differentiation and lactation.
ErbB ligands
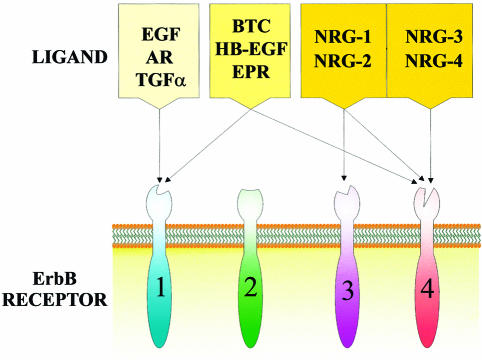
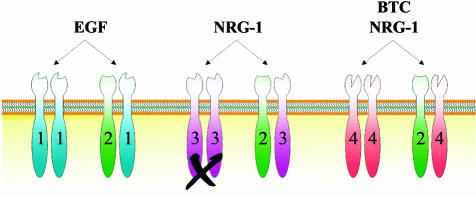
ErbB receptors are activated by a number of ligands, referred to as EGF-related peptide growth factors (reviewed in Peles and Yarden, 1993; Riese and Stern, 1998). These are produced as transmembrane precursors, and are processed and released by proteolysis (Massague and Pandiella, 1993). There are numerous ErbB-specific ligands, summarized in Figure 1, each with an EGF-like domain that is sufficient to confer binding specificity. These include EGF, amphiregulin (AR) and transforming growth factor-α (TGFα), which bind specifically to ErbB1, and betacellulin (BTC), heparin-binding EGF (HB-EGF) and epiregulin (EPR), which exhibit dual specificity in that they bind both ErbB1 and ErbB4. The neuregulins (NRG) comprise the third ligand family. NRG-1 and NRG-2 (Riese et al., 1995; Busfield et al., 1997; Carraway et al., 1997; Chang et al., 1997) both bind ErbB3 and ErbB4, whereas the more recent additions to the NRG family, NRG-3 (Zhang et al., 1997) and NRG-4 (Harari et al., 1999), bind ErbB4 but not ErbB3. It should be stated that NRG-1 is also known as neu differentiation factor, heregulin, acetylcholine receptor-inducing activity and glial growth factor, reflecting the different biological systems in which this ligand was first described (see Peles and Yarden, 1993). Despite the large number of ligands so far identified for ErbB1, 3 and 4, as well as intensive efforts, no direct ligand for ErbB2 has yet been discovered. However, increasing evidence suggests that the primary function of ErbB2 is as a coreceptor. In fact, ErbB2 is the preferred heterodimerization partner for all other ErbB family members (Figure 2) (Tzahar et al., 1996; Graus-Porta et al., 1997) and plays a role in the potentiation of ErbB receptor signaling (Beerli et al., 1995; Graus-Porta et al., 1995).
Fig. 1. Binding specificities of the EGF-related peptide growth factors. There are four categories of ligands that bind ErbB family receptors. EGF, AR and TGFα bind ErbB1; BTC, HB-EGF and EPR bind ErbB1 and ErbB4; NRG-1 and NRG-2 bind ErbB3 and ErbB4; and NRG-3 and NRG-4 bind ErbB4. See the text for more details.
Fig. 2. ErbB2 is the preferred dimerization partner for the other ErbB receptors. Ligand binding to ErbB1 (EGF), ErbB3 (NRG-1) or ErbB4 (NRG-1, BTC) induces the formation of receptor homodimers and ErbB2-containing heterodimers. ErbB3 homodimers do not signal (indicated by the X), since the receptor has impaired kinase activity. Only some of the possible ligand–receptor-induced combinations are indicated in the figure for the sake of simplicity.
Controlled expression determines ErbB ligand availability
Signaling diversity emanating from the ErbB family is generated by the repertoire of ErbB ligands and the combinatorial properties of induced receptor dimers. With the exception of EGF, which is found in many body fluids, ErbB ligands generally act over short distances as autocrine or paracrine growth factors. The availability of a specific ligand is, therefore, one way to control its signaling ability. In this respect, ErbB ligands demonstrate distinct expression patterns that are organ- and developmental stage-specific. Some ligands, such as NRG-1, are widely expressed (Meyer and Birchmeier, 1995) while others show more restricted expression profiles. For example, the pancreas has high amounts of NRG-4 (Harari et al., 1999), NRG-3 expression is restricted to developing and adult nervous system (Zhang et al., 1997) and EPR is high in macrophages and placenta (Toyoda et al., 1997). An extreme example for highly controlled expression, both with respect to tissue and time, is HB-EGF, which is induced in the uterine luminal epithelium solely at the site of blastocyst apposition 6–7 h prior to uterine implantation (Paria et al., 1999).
In the case of the mammary gland, ErbB1 ligands and NRG-1 are co-expressed at various developmental stages (Yang et al., 1995; Schroeder and Lee, 1998). In order to investigate their functional role during mammary gland development, mice with individual targeted disruption of EGF, AR and TGFα, as well as triple null mice, have been generated (Luetteke et al., 1999). Analysis of these mice has indicated that, while there is functional redundancy in the roles of these ErbB ligands, some ligands make specific contributions to mammary gland development. For example, of the three ligands, AR expression is highest in the developing ducts and only AR deficiency is associated with impaired ductal growth. This suggests that AR plays an essential, non-redundant role in this process. In contrast, however, lobuloalveolar development of lactating glands in AR null mice appears normal, whereas in triple null mice lactogenesis is abrogated (Luetteke et al., 1999). Taken together, these data imply that functional differentiation of the mammary gland requires the cooperation of multiple ErbB ligands.
Distinct biochemical properties of the ligands give rise to signaling diversity
Signaling diversity depends not only on the presence of a specific ErbB receptor and its ligand, but also on the biochemical characteristics of the individual ErbB ligands. First, ErbB ligands are bivalent, a property that determines which receptor dimers are formed, thereby influencing the signaling pathways activated (Beerli and Hynes, 1996; Riese et al., 1996). This biochemical feature bestows upon the ligands, therefore, the ability to diversify signaling possibilities. Ligand bivalency has been implied from biophysical (Lemmon et al., 1997), structure–function (Groenen et al., 1994) and immunological approaches (Katsuura and Tanaka, 1989). In the case of NRG-1, using a recombinant chimeric ligand (Barbacci et al., 1995; Tzahar et al., 1997), it has been demonstrated that major determinants required for high-affinity binding to the primary ErbB receptor (ErbB3 or ErbB4) are contained within the N-terminus, whilst the C-terminus recruits the second ErbB partner. The second partner is preferentially ErbB2 (Tzahar et al., 1997), which has also been shown to increase the affinity of ligand binding to all ErbB receptor heterodimers (J.Y.Jones et al., 1999). A second biochemical property of ErbB peptides is their differential binding affinities, a characteristic that influences signal strength and duration. In this respect, virally encoded, low-affinity ErbB-binding peptides have been demonstrated to hinder normal receptor downregulation and degradation (Tzahar et al., 1998). This implies that low-affinity ligands may be even more potent signal inducers than their high-affinity counterparts, a possibility that should be examined more thoroughly. A third property of ErbB ligand binding is the pH stability of the ligand–receptor interaction, which influences receptor trafficking. For example, ligands such as EGF, whose receptor interaction is relatively pH resistant, target ErbB1 to lysosomes, whereas TGFα and NRG-1 readily dissociate from their respective receptors at endosomal pH, resulting in receptor recycling (French et al., 1995; Waterman et al., 1998). Subsequent rerouting of the receptor to the plasma membrane may, therefore, play a major role in signal potentiation. These ligand-specific characteristics provide an extra level of control, contributing to the diversity and fine-tuning of cellular responses to ErbB receptor activation. Such elaborate mechanisms reflect the importance of this receptor family in proliferation and differentiation.
Major signaling pathways activated by ErbB receptors
Overlapping and specific ErbB substrates
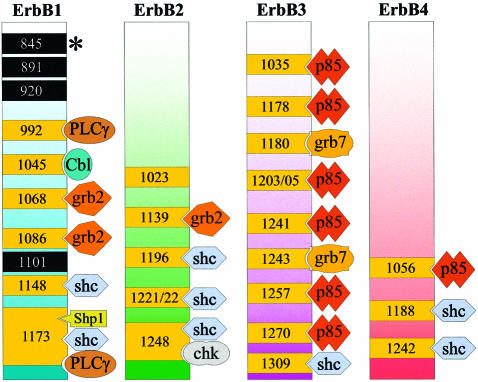
Ligand binding drives receptor dimerization, leading to activation of the intrinsic tyrosine kinase and autophosphorylation of specific, C-terminal tyrosine residues (reviewed in Heldin, 1995; Weiss and Schlessinger, 1998) that provide docking sites for proteins containing Src homology 2 (SH2) or phosphotyrosine binding (PTB) domains (reviewed in Shoelson, 1997; Sudol, 1998). These include adaptor proteins such as Shc, Crk, Grb2, Grb7 and Gab1; kinases such as Src, Chk and phosphatidylinositol 3-kinase (PI3K; via the p85 regulatory subunit); and the protein tyrosine phosphatases SHP1 and SHP2. Each ErbB receptor displays a distinct pattern of C-terminal autophosphorylation sites. Examination of the binding preferences of a variety of different SH2 and PTB domains, using BioCore or phosphopeptide competition assays, combined with studies utilizing receptor autophosphorylation site mutants and co-immunoprecipitation approaches, has enabled the identification of effector proteins that couple to specific ErbB phosphotyrosine residues (Figure 3). From these analyses, it has become evident that there is a great deal of overlap in the signaling pathways activated by the four ErbB receptors. Indeed, all ErbB family members, including the Drosophila and C.elegans homologs DER and Let23, couple via Shc and/or Grb2 to the mitogen-activated protein (MAP) kinase pathway. However, there are also examples of preferential modulation of specific pathways. For example, due to the presence of multiple binding sites for p85, ErbB3 is the most efficient activator of PI3K (Prigent and Gullick, 1994). In a similar vein, Eps15 and Cbl are examples of ErbB1-specific substrates (Fazioli et al., 1993; Levkowitz et al., 1996), both of which are involved in receptor downregulation. Eps15 binds the clathrin adaptor protein complex AP-2 and participates in coated-pit-mediated internalization (Torrisi et al., 1999). Interestingly, in contrast to other ErbB receptors, activated ErbB1 is rapidly internalized via clathrin-mediated endocytosis (Baulida et al., 1996), a phenomenon perhaps related to its specificity for Eps15. Moreover, Drosophila and C.elegans Cbl orthologs are known to regulate RTK signaling negatively (Yoon et al., 1995; Meisner et al., 1997); however, the mechanism by which this protein acts was only recently resolved. Cbl was identified as a RING finger domain-containing E3 ubiquitin protein ligase (Joazeiro et al., 1999; Yokouchi et al., 1999), required for ErbB1 ubiquitylation and targeting of the receptor to the lysosomal compartment (Levkowitz et al., 1999). These results help explain the dramatic decrease in ErbB1 levels caused by ligand activation, something not observed for the other ErbB receptors. Taken together, it is clear that ErbB receptors couple to specific downstream, intracellular pathways with differing efficiencies, thus affording greater signaling possibilities.
Fig. 3. Specific phosphotyrosine residues and binding of signaling molecules to the ErbB RTKs. ErbB1: tyrosine residues that have been identified as autophosphorylation sites (Downward et al., 1984; Hsuan et al., 1989; Margolis et al., 1989; Walton et al., 1990) (in yellow) and sites for the Src kinase (Stover et al., 1995) (in black), including Y845 in the T loop of the kinase domain (shown by an asterisk) (Sato et al., 1995; Biscardi et al., 1999a), are indicated. Some of the proteins that have been shown to interact with specific tyrosine residues of ErbB1 are indicated: Shc binds via its PTB domain to Y1173 and Y1148, and via its SH2 domain to Y1173 (Batzer et al., 1994; Okabayashi et al., 1994; Sakaguchi et al., 1998); PLCγ binds to Y1173 (Chattopadhyay et al., 1999) and Y992 (Rotin et al., 1992); the major and minor binding sites for Grb2 are, respectively, Y1068 and Y1086 (Batzer et al., 1994; Okutani et al., 1994); Cbl binds Y1045 (Levkowitz et al., 1999); SHP1 binds to Y1173 (Keilhack et al., 1998). ErbB2: tyrosine residues that have been identified as autophosphorylation sites are indicated (Hazan et al., 1990; Segatto et al., 1990). Proteins that have been shown to interact with specific tyrosine residues are indicated: Shc binds via its PTB domain to Y1196 and Y1248, and via its SH2 domain to Y1248 and Y1221/2 (Ricci et al., 1995); the latter site has also been identified in Neu (Dankort et al., 1997); Grb2 binds Y1139 (Ricci et al., 1995) and the equivalent residue in Neu (Dankort et al., 1997); Chk binds Y1248 (Zrihan-Licht et al., 1998). ErbB3: phosphopeptide analyses of in vivo labeled ErbB3 have not been published. The p85 subunit of PI3K and the Shc binding sites have been mapped by phosphopeptide competition (Prigent and Gullick, 1994). Peptides encompassing Y1035 and Y1270 compete strongly, peptides encompassing Y1178, Y1203/05, Y1241 and Y1257 compete less strongly for p85 binding; a peptide encompassing Y1309 competes for Shc binding. The major and minor sites for Grb7 binding are, respectively, Y1180 and Y1243 (Fiddes et al., 1998). ErbB4: phosphopeptide analyses of in vivo labeled ErbB4 have not been published. Phosphopeptide competition analyses have shown that Shc binds Y1242 and Y1188 (Cohen et al., 1996b); the p85 subunit of PI3K binds Y1056 (Elenius et al., 1999). (Only the cytoplasmic domains of the four ErbB RTKS are shown; for graphic purposes, the receptors have not been aligned.)
ErbB-induced biological responses
In order to assess the role of autophosphorylation sites in a specific response, individual tyrosine residues have been restored to ErbB mutants lacking the major autophosphorylation sites. These are referred to as ‘add-back’ mutants. This approach has mainly been used to examine signaling from the Neu receptor, the rat ErbB2 equivalent. NeuT is the oncogenic variant of Neu, originally discovered in rat neuroectodermal tumors. This mutant receptor possesses a single mutation that alters the transmembrane domain (Bargmann et al., 1986), leading to constitutive receptor dimerization (Weiner et al., 1989). Mutation of NeuT autophosphorylation sites causes a dramatic reduction in its transforming ability. Surprisingly, four of the tyrosine residues when ‘added back’ individually fully restore the oncogenicity of NeuT. This demonstrates that, with respect to transformation potential, these tyrosines are functionally redundant (Dankort et al., 1997). In contrast, analyses of transgenic nematodes expressing Let23 add-back mutants revealed that individual tyrosines confer distinct biological functions. Viability and vulval differentiation were induced by each of three tyrosine residues in a Ras-dependent manner, whereas a single tyrosine was sufficient to confer fertility (Lesa and Sternberg, 1997). Comparison of the results from these two analyses suggests that once multiple biological responses can be surveyed, more roles for individual tyrosine residues and the pathways to which they couple will be uncovered.
The power of ErbB receptor heterodimerization
ErbB heterodimers provide signal diversification
The ability of ErbB ligands to induce not only receptor homodimers but also heterodimers expands ErbB signaling potential. Heterodimerization follows a strict hierarchical principle with ErbB2 representing the preferred dimerization partner of all other ErbB receptors (Figure 2) (Tzahar et al., 1996; Graus-Porta et al., 1997). This phenomenon can be explained, as discussed above, by the binding preferences of the bivalent ligands. Thus, the ErbB dimers formed are dictated by the nature of the ligand and the cell’s complement of ErbB receptors. ErbB2-containing heterodimers display increased ligand affinity due to a decelerated off-rate (Karunagaran et al., 1996) that can be correlated with prolonged activation of downstream signaling pathways (Beerli et al., 1995; Graus-Porta et al., 1995). Furthermore, biological responses such as proliferation (Beerli et al., 1995; Graus-Porta et al., 1995), morphological differentiation (Beerli et al., 1995) and migration/invasion (Spencer et al., 2000) are enhanced in cells expressing ErbB2.
ErbB heterodimerization is a means not only for signal amplification but also for signal diversification. The subsets of SH2- and PTB-binding signaling molecules recruited to an activated receptor are defined by the pattern of phosphorylated tyrosine residues in the C-terminus of the receptor (Figure 3). Based on our finding that the Cbl protein coupled only to EGF- but not to NRG-activated ErbB1 (Graus-Porta et al., 1997), we speculated that signal diversification arises at one level by differential transphosphorylation of a given receptor in distinct ErbB dimers. By phosphopeptide mapping of activated ErbB1 and ErbB2 from NIH 3T3 cells expressing single and pairwise combinations of ErbB receptors, we were able to prove that receptor phosphorylation is indeed modulated by the dimerization partner (Olayioye et al., 1998). This provides a biochemical explanation for the observation that EGF-activated ErbB1, but not ErbB1 heterodimerized with ErbB4, was able to recruit Grb2 (Olayioye et al., 1998). Furthermore, despite similar levels of total phosphotyrosine, ErbB2 constitutively dimerized due to a mutation in the transmembrane domain was considerably more potent in binding Shc than ErbB2 transactivated by EGF (Olayioye et al., 1998). Thus, the signal elicited by a receptor heterodimer is not simply the sum of the signaling properties of the individual dimerization partners, but is rather due to unique properties acquired by the heterodimer. For example, IL-3-dependent Ba/F3 cells engineered to co-express ErbB1 and ErbB4 demonstrate IL-3-independent proliferation in the presence of NDF or EGF. However, neither ligand promotes IL-3-independent proliferation of cells that individually express ErbB1 or ErbB4 (Riese et al., 1996). Similarly, NRG-induced ErbB2–ErbB4 heterodimers activate the transcription factor Stat5 while homodimers of either receptor fail to do so (Olayioye et al., 1999).
Continuing in this context, it should be noted that ErbB3 is an impaired kinase due to substitutions in critical residues in its kinase domain (Guy et al., 1994). Hence, despite having multiple ligands, ErbB3 only functions as a signaling entity when complexed with another ErbB receptor. Therefore, the process of ErbB heterodimerization enables the integration of a ligand-less ErbB2 and a kinase-defective ErbB3 into signal transduction processes. In fact, a consensus is emerging that the ErbB2–ErbB3 heterodimer is the most potent ErbB signaling complex in terms of in vitro growth and transformation (Alimandi et al., 1995; Pinkas-Kramarski et al., 1996).
Biological effects of ErbB heterodimers
Phenotypes of ErbB receptor knock-out mice are the most striking proof of the power of receptor heterodimers. Mice individually null for ErbB2, ErbB4 or NRG-1 each demonstrate a lack of trabeculae formation in the heart (Gassmann et al., 1995; Lee et al., 1995; Meyer and Birchmeier, 1995; Kramer et al., 1996), showing that an important signaling moiety in the myocardium is the ErbB2–ErbB4 heterodimer stimulated in a paracrine fashion by NRG-1. The essential contribution of this receptor collaboration to heart development is clearly observed in ErbB2 null mice, where NRG-1-induced ErbB4 dimers cannot replace the function of the ErbB2–ErbB4 heterodimer. Ligand-induced ErbB2–ErbB3 heterodimers also play an important role in nervous system development. Indeed, mice individually mutant for ErbB2, ErbB3 or NRG-1 display a failure in neural crest cell migration, leading to impaired formation of the sympathetic nervous system (Britsch et al., 1998). A further example of in vivo ErbB cooperation is provided by a specific function for these receptors in the hypothalamus. Here, before the onset of puberty, NRG-mediated activation of ErbB2 and ErbB4 is required for the release of prostaglandin E2, which then controls the secretion of luteinizing hormone-releasing hormone (LHRH) from specific neuroendocrine sites. LHRH is essential for sexual development and adult reproductive function. In this respect, loss of ErbB2 function has been shown to delay the onset of puberty, by preventing LHRH secretion, despite the continued presence of ErbB4 and NRG (Ojeda and Ma, 1999). ErbB receptor heterodimers, therefore, play significant roles in a number of developmental processes—roles that cannot be performed by homodimers. This is indicative of the need to control receptor signaling precisely during development, and again reflects the evolutionary diversification of this family of receptors in metazoans.
ErbB receptors as signal integrators
In the past years it has become clear that there are also additional layers of complexity to the ErbB receptor system. For instance, ErbB RTKs integrate signaling events emanating from other receptor classes, such as G-protein-coupled receptors and cytokine receptors. Moreover, even in the absence of ErbB kinase activity, phosphorylation of specific residues in the cytoplasmic tail of the receptor by non-receptor kinases such as Jak and Src can provide docking sites for cytoplasmic signaling molecules. Thus, in these cases, the receptor is simply used as a scaffold protein, transmitting information to downstream signaling pathways. A detailed description of these interactions is outside the scope of this article and the reader is referred to recent reviews on the different modes of ErbB receptor activation (Biscardi et al., 1999b; Carpenter, 1999; Hackel et al., 1999; Luttrell et al., 1999).
To date, most work in the area of signal transduction has concentrated on membrane-proximal events. Consequently, downstream effects on gene expression, resulting in the modulation of specific biological/developmental programs, are still scantily described. New technologies such as nucleotide arrays and proteomics will help elucidate the issue by providing more information on how ErbB receptor signaling impinges on the expression of multiple genes and proteins. The ultimate goal will be to understand the interplay of ErbB RTKs and their ligands in the context of the entire signaling network present in an organ.
ErbB receptors in cancer: a special focus on ErbB cooperation
There is a wealth of clinical data demonstrating the importance of ErbB receptors, in particular ErbB1 and ErbB2, in the development and malignancy of human cancer. However, considering the brevity and the theme of this review only examples of ErbB receptor synergy will be discussed. The interested reader is referred to these reviews (Hynes and Stern, 1994; Salomon et al., 1995; Tang and Lippman, 1998) for more detailed information. Cooperation between ErbB receptors has been observed in oncogenic transformation, both in vitro in cultured cells and in primary human tumors. Following the initial observation that co-expression of ErbB2 with ErbB1 in NIH 3T3 fibroblasts augmented the effects of EGF on the transformed phenotype (Kokai et al., 1989), there have been numerous studies on the collaboration of ErbB receptors in transformation. For example, ErbB3 expression increases ErbB2-mediated transformation and tumorigenic growth in NIH 3T3 cells. Also, NRG-1-induced transformation of fibroblasts by ErbB4 requires co-expression of either ErbB1 or ErbB2 (Alimandi et al., 1995; Wallasch et al., 1995; Cohen et al., 1996a,b; Zhang et al., 1996). The enhanced transforming properties of cells expressing multiple ErbB receptors are presumably due to the diversity and signaling potency emanating from ErbB receptor combinations. Such synergies have clear potential in terms of their impact on the deregulation of cellular proliferation associated with tumor progression.
Overexpression of ErbB2 is observed in a significant proportion of breast and ovarian cancers, where it is associated with poor prognosis (Slamon et al., 1989). ErbB2 overexpression triggers ligand-independent activation of the kinase domain, apparently as a result of spontaneous dimer formation. Although ErbB2 homodimers alone may contribute to malignancy, a number of observations suggest that ErbB2 does indeed cooperate with other ErbB receptors during tumor development. Many human tumors that contain ErbB2 also exhibit autocrine stimulation of ErbB1 via expression of one of its numerous ligands (Salomon et al., 1995). Furthermore, mammary tumors derived from transgenic mice engineered to overexpress neu also exhibit co-overexpression of endogenous ErbB1 (DiGiovanna et al., 1998). The ability of ErbB2 to potentiate ErbB1 signaling, as discussed above, would provide tumor cells with a more potent growth stimulus and could lead to the activation of additional intracellular pathways. Such cooperation would, therefore, contribute to the maintenance of increased proliferation rates associated with tumor development. With this in mind, work from our own laboratory has shown that loss of ErbB2 function inhibits the proliferation of tumor cells displaying ErbB1 autocrine activation (Jannot et al., 1996).
In terms of other receptor combinations, ErbB2 and ErbB4 are also co-expressed in >50% of childhood medulloblastomas, and the ErbB4 ligand NRG-1 is found in a significant proportion of these same tumors. The simultaneous expression of all three appears to be of biological significance for the malignancy (Gilbertson et al., 1997), suggesting that co-expression of ErbB2 with ErbB4 can enhance the effects of autocrine or paracrine NRG-1 signaling.
The ErbB2–ErbB3 signaling moiety in cancer
Another important observation pertaining to ErbB heterodimer collaboration during tumor development is that expression of ErbB3 is seen in many of the same tumor types that overexpress ErbB2, including breast, bladder and melanomas (Lemoine et al., 1992; Rajkumar et al., 1996; Bodey et al., 1997; Siegel et al., 1999). Furthermore, many ErbB2-overexpressing breast tumors display elevated levels of phosphotyrosine on ErbB3 (Alimandi et al., 1995), probably as a result of spontaneous dimerization with ErbB2. Moreover, mammary tumors of transgenic mice expressing transforming Neu mutants exhibit selective upregulation of ErbB3 expression and activity (Siegel et al., 1999), suggesting that there might be a selective advantage/pressure leading to co-expression of both receptors. Indeed, our own experiments support this viewpoint. Downregulation of membrane ErbB2 levels in ErbB2-overexpressing breast tumor cells has been achieved through the use of single-chain antibody (scFv) technology (Beerli et al., 1994; Neve et al., 2000). These experiments revealed that ErbB2 and ErbB3 function together to stimulate mitogenic signaling networks. This in turn contributes to uncontrolled tumor cell proliferation by a mechanism involving deregulation of the G1–S transition through modulation of the activation status of the essential G1–S regulator cyclin E-dependent kinase 2 (Cyclin E–Cdk2; Neve et al., 2000). The basis of this deregulation was demonstrated to be tumor cell dependency on elevated ErbB2–ErbB3 receptor signaling for the maintenance of proteins involved in the sequestration of the Cdk2 inhibitor p27_Kip1_ (see Sherr and Roberts, 1999 for overview of this field). Loss of ErbB2–ErbB3 activity, therefore, leads to redirection of p27_Kip1_ onto Cdk2 complexes, inhibiting its activity. A similar redirection of p27_Kip1_ was also observed if ErbB2 receptor signaling was impeded using an anti-ErbB2 growth inhibitory antibody (Lane et al., 2000), confirming the role of ErbB2 receptor signaling in the potentiation of breast tumor cell proliferation through deregulation of the G1–S transition.
ErbB2-directed therapies in the clinic
The ligand-independent activation of overexpressed ErbB2, combined with its preferred role as a partner for the other ErbB receptors, provides explanations for the oncogenic potential of ErbB2 and its involvement in so many human tumors. Considering the general importance of ErbB heterodimers and ErbB2 in particular, the latter has been under intense investigation as a target for cancer therapy. In this respect, a monoclonal antibody that targets the extracellular domain of ErbB2 (known as 4D5) specifically inhibits the in vitro growth of ErbB2-overexpressing tumor cells (Hudziak et al., 1989; Lewis et al., 1993, 1996). Excitingly, the humanized version of this antibody (Herceptin™) has been validated in the clinic as an ErbB2-directed therapeutic approach (Baselga et al., 1996; Pegram et al., 1998; Cobleigh et al., 1999). Moreover, this antibody is now being used to treat metastatic breast cancer patients with tumors overexpressing ErbB2. We have recently shown that treatment of ErbB2-overexpressing breast tumor cells with 4D5 results in a rapid reduction of ErbB2 phosphorylation, suggesting inhibition of ErbB2 receptor signaling (Lane et al., 2000). However, through comparison of a growth-inhibited cell line with a cell line unaffected by 4D5 treatment, receptor dephosphorylation was found to occur in both cases, whilst downstream effects were observed only in the case of the 4D5-sensitive cells. Hence, we postulate that antibody-induced inhibition of ErbB2 receptor activity in overexpressing tumor cells does not necessarily predict cellular response to antibody treatment (Lane et al., 2000). This phenomenon correlates with the fact that although all patients treated with Herceptin™ do have tumors exhibiting ErbB2 overexpression, not all respond to treatment (Baselga et al., 1996; Pegram et al., 1998; Cobleigh et al., 1999). From these data it is clear that the contribution of other ErbB receptors should be taken into account for future evaluations of ErbB2 as a target for tumor therapy. Through a clearer understanding of ErbB interactions, therefore, it is conceivable that future ErbB-directed approaches may prove to be even more beneficial for cancer treatment.
Acknowledgments
Acknowledgements
Thanks to Dr Ali Badache for helpful comments on the review and to the other members of the laboratory for many stimulating discussions. Dr Richard Neve was partially supported by a grant from the Krebsliga beider Basel. Dr Heidi Lane was partially supported by a grant from the Sshweizerische Krebsliga.
References
- Alimandi M., Romano,A., Curia,M.C., Muraro,R., Fedi,P., Aaronson,S.A., Di Fiore,P.P. and Kraus,M.H. (1995) Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation. Oncogene, 10, 1813–1821. [PubMed] [Google Scholar]
- Aroian R.V., Koga,M., Mendel,J.E., Ohshima,Y. and Sternberg,P.W. (1990) The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor family. Nature, 348, 693–699. [DOI] [PubMed] [Google Scholar]
- Barbacci E.G., Guarino,B.C., Stroh,J.G., Singleton,D.H., Rosnack,K.J., Moyer,J.D. and Andrews,G.C. (1995) The structure basis for the specificity of epidermal growth factor and heregulin binding. J. Biol. Chem., 270, 9585–9589. [DOI] [PubMed] [Google Scholar]
- Bargmann C.I., Hung,M.C. and Weinberg,R.A. (1986) Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell, 45, 649–657. [DOI] [PubMed] [Google Scholar]
- Baselga J. et al. (1996) Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol., 14, 737–744. [DOI] [PubMed] [Google Scholar]
- Batzer A.G., Rotin,D., Urena,J.M., Skolnik,E.Y. and Schlessinger,J. (1994) Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol., 14, 5192–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulida J., Kraus,M.H., Alimandi,M., Di Fiore,P.P. and Carpenter,G. (1996) All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem., 271, 5251–5257. [DOI] [PubMed] [Google Scholar]
- Beerli R.R. and Hynes,N.E. (1996) Epidermal growth factor-related peptides activate distinct subsets of erbB receptors and differ in their biological activities. J. Biol. Chem., 271, 6071–6076. [DOI] [PubMed] [Google Scholar]
- Beerli R.R., Wels,W. and Hynes,N.E. (1994) Intracellular expression of single chain antibodies reverts ErbB-2 transformation. J. Biol. Chem., 269, 23931–23936. [PubMed] [Google Scholar]
- Beerli R.R., Graus-Porta,D., Woods-Cook,K., Chen,X., Yarden,Y. and Hynes,N.E. (1995) Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol. Cell. Biol., 15, 6496–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscardi J.S., Maa,M.C., Tice,D.A., Cox,M.E., Leu,T.H. and Parsons,S.J. (1999a) c-Src-mediated phosphorylation of the EGF receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem., 274, 8335–8343. [DOI] [PubMed] [Google Scholar]
- Biscardi J.S., Tice,D.A. and Parsons,S.J. (1999b) c-Src, receptor tyrosine kinases and human cancer. Adv. Cancer Res., 76, 61–117. [DOI] [PubMed] [Google Scholar]
- Bodey B., Bodey,B.,Jr, Groger,A.M., Luck,J.V., Siegel,S.E., Taylor,C.R. and Kaiser,H.E. (1997) Clinical and prognostic significance of the expression of the c-erbB-2 and c-erbB-3 oncoproteins in primary and metastatic malignant melanomas and breast carcinomas. Anticancer Res., 17, 1319–1330. [PubMed] [Google Scholar]
- Britsch S., Li,L., Kirchhoff,S., Theuring,F., Brinkmann,V., Birchmeier,C. and Riethmacher,D. (1998) The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev., 12, 1825–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busfield S.J. et al. (1997) Characterization of a neuregulin-related gene, Don-1, that is highly expressed in restricted regions of the cerebellum and hippocampus. Mol. Cell. Biol., 17, 4007–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. (1999) Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J. Cell Biol., 146, 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K.L. III, Weber,J.L., Unger,M.J., Ledesma,J., Yu,N., Gassmann,M. and Lai,K. (1997) Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature, 387, 512–516. [DOI] [PubMed] [Google Scholar]
- Chang H., Riese,D.J.,II, Gilbert,W., Stern,D.F. and McMahan,U.J. (1997) Ligands for ErbB-family receptors encoded by a neuregulin-like gene. Nature, 387, 509–512. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., Vecchi,M., Ji,Q., Mernaugh,R. and Carpenter,G. (1999) The role of individual SH2 domains in mediating association of phospholipase C-γ1 with the activated EGF receptor. J. Biol. Chem., 274, 26091–26097. [DOI] [PubMed] [Google Scholar]
- Cobleigh M.A. et al. (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol., 17, 2639–2648. [DOI] [PubMed] [Google Scholar]
- Cohen B.D., Kiener,P.A., Green,J.M., Foy,L., Fell,P. and Zhang,K. (1996a) The relationship between human epidermal growth-like factor receptor expression and cellular transformation in NIH3T3 cells. J. Biol. Chem., 271, 30897–30903. [DOI] [PubMed] [Google Scholar]
- Cohen B.D., Green,J.M., Foy,L. and Fell,H.P. (1996b) HER4-mediated biological and biochemical properties in NIH3T3 cells. J. Biol. Chem., 271, 4813–4818. [DOI] [PubMed] [Google Scholar]
- Dankort D.L., Wang,Z., Blackmore,V., Moran,M.F. and Muller,W.J. (1997) Distinct tyrosine autophosphorylation sites negatively and positively modulate Neu-mediated transformation. Mol. Cell. Biol., 17, 5410–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy K.M., Zangani,D., Wolhueter,A.L., Huang,R.Y., Vaughan,M.M., Russell,J.A. and Ip,M.M. (2000) Changes in ErbB2 (HER2/neu), ErbB3 and ErbB4 during growth, differentiation and apoptosis of normal rat mammary epithelial cells. J. Histochem. Cytochem., 48, 63–80. [DOI] [PubMed] [Google Scholar]
- DiGiovanna M.P., Lerman,M.A., Coffey,R.J., Muller,W.J., Cardiff,R.D. and Stern,D.F. (1998) Active signaling by Neu in transgenic mice. Oncogene, 17, 1877–1884. [DOI] [PubMed] [Google Scholar]
- Downward J., Parker,P. and Waterfield,M.D. (1984) Autophosphorylation sites on the epidermal growth factor receptor. Nature, 311, 483–485. [DOI] [PubMed] [Google Scholar]
- Elenius K., Choi,C.J., Paul,S., Santiestevan,E., Nishi,E. and Klagsbrun,M. (1999) Characterization of a naturally occurring ErbB4 isoform that does not bind or activate phosphatidyl inositol 3-kinase. Oncogene, 18, 2607–2615. [DOI] [PubMed] [Google Scholar]
- Erickson S.L., O’Shea,K.S., Ghaboosi,N., Loverro,L., Frantz,G., Bauer,M., Lu,L.H. and Moore,M.W. (1997) ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2- and heregulin-deficient mice. Development, 124, 4999–5011. [DOI] [PubMed] [Google Scholar]
- Fazioli F., Minichiello,L., Matoskova,B., Wong,W.T. and Di Fiore,P.P. (1993) Eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol. Cell. Biol., 13, 5814–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddes R.J., Campbell,D.H., Janes,P.W., Sivertsen,S.P., Sasaki,H., Wallasch,C. and Daly,R.J. (1998) Analysis of Grb7 recruitment by heregulin-activated erbB3 receptors reveals a novel target selectivity for erbB3. J. Biol. Chem., 273, 7717–7724. [DOI] [PubMed] [Google Scholar]
- Fowler K.J. et al. (1995) A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc. Natl Acad. Sci. USA, 92, 1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A.R., Tadaki,D.K., Niyogi,S.K. and Lauffenburger,D.A. (1995) Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J. Biol. Chem., 270, 4334–4340. [DOI] [PubMed] [Google Scholar]
- Gassmann M., Casagranda,F., Orioli,D., Simon,H., Lai,C., Klein,R. and Lemke,G. (1995) Aberrant neural and cardiac development in mice lacking the erbB4 neuregulin receptor. Nature, 378, 390–394. [DOI] [PubMed] [Google Scholar]
- Gilbertson R.J., Perry,R.H., Kelly,P.J., Pearson,A.D.J. and Lunee,J. (1997) Prognostic significance of HER2 and HER4 co-expression in childhood medulloblastoma. Cancer Res., 57, 3272–3280. [PubMed] [Google Scholar]
- Graus-Porta D., Beerli,R.R. and Hynes,N.E. (1995) Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol. Cell. Biol., 15, 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D., Beerli,R.R., Daly,J.M. and Hynes,N.E. (1997) ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J., 16, 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenen L.C., Nice,E.C. and Burgess,A.W. (1994) Structure–function relationships for the EGF/TGF-α family of mitogens. Growth Factors, 11, 235–257. [DOI] [PubMed] [Google Scholar]
- Guy P.M., Platko,J.V., Cantley,L.C., Cerione,R.A. and Carraway,K.L. (1994) Insect cell-expressed p180ErbB3 possesses an impaired tyrosine kinase activity. Proc. Natl Acad. Sci. USA, 91, 8132–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel P.O., Zwick,E., Prenzel,N. and Ullrich,A. (1999) Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr. Opin. Cell Biol., 11, 184–189. [DOI] [PubMed] [Google Scholar]
- Harari D., Tzahar,E., Romano,J., Shelly,M., Pierce,J.H., Andrews,G.C. and Yarden,Y. (1999) Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene, 18, 2681–2689. [DOI] [PubMed] [Google Scholar]
- Hazan R., Margolis,B., Dombalagian,M., Ullrich,A., Zilberstein,A. and Schlessinger,J. (1990) Identification of autophosphorylation sites of HER2/neu. Cell Growth Differ., 1, 3–7. [PubMed] [Google Scholar]
- Heldin C.-H. (1995) Dimerization of cell surface receptors in signal transduction. Cell, 80, 213–223. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. and Robinson,G.W. (1998) Think globally, act locally: the making of a mouse mammary gland. Genes Dev., 12, 449–455. [DOI] [PubMed] [Google Scholar]
- Hsuan J.J., Totty,N. and Waterfield,M.D. (1989) Identification of a novel autophosphorylation site (P4) on the epidermal growth factor receptor. Biochem. J., 262, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak R.M., Lewis,G.D., Winget,M., Fendly,B.M., Shepard,H.M. and Ullrich,A. (1989) p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol., 9, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N.E. and Stern,D.F. (1994) The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim. Biophys. Acta, 1198, 165–184. [DOI] [PubMed] [Google Scholar]
- Jannot C.B., Beerli,R.R., Mason,S., Gullick,W.J. and Hynes,N.E. (1996) Intracellular expression of a single-chain antibody directed to the EGFR leads to growth inhibition of tumor cells. Oncogene, 13, 275–282. [PubMed] [Google Scholar]
- Joazeiro C.A.P., Wing,S.S., Huang,H.K., Leverson,J.D., Hunter,T. and Liu,Y.C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science, 286, 309–311. [DOI] [PubMed] [Google Scholar]
- Jones F.E. and Stern,D.F. (1999) Expression of dominant-negative ErbB2 in the mammary gland of transgenic mice reveals a role in lobuloalveolar development and lactation. Oncogene, 18, 3481–3490. [DOI] [PubMed] [Google Scholar]
- Jones F.E., Welte,T., Fu,X.Y. and Stern,D.F. (1999) ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J. Cell Biol., 147, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.Y., Akita,R.W. and Sliwkowski,M.X. (1999) Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett., 447, 227–231. [DOI] [PubMed] [Google Scholar]
- Karunagaran D., Tzahar,E., Beerli,R.R., Chen,X., Graus-Porta,D., Ratzkin,B.J., Seger,R., Hynes,N.E. and Yarden,Y. (1996) ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J., 15, 254–264. [PMC free article] [PubMed] [Google Scholar]
- Katsuura M. and Tanaka,S. (1989) Topographic analysis of human epidermal growth factor by monospecific antibodies and synthetic peptides. J. Biochem., 106, 87–92. [DOI] [PubMed] [Google Scholar]
- Keilhack H., Tenev,T., Nyakatura,E., Godovac-Zimmermann,J., Nielsen,L., Seedorf,K. and Bohmer,F.D. (1998) Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SHP-1 to the epidermal growth factor receptor and attenuation of receptor signaling. J. Biol. Chem., 273, 24839–24846. [DOI] [PubMed] [Google Scholar]
- Kokai Y., Myers,J.N., Wada,T., Brown,V.I., LeVea,C.M., Davis,J.G., Dobashi,K. and Greene,M.I. (1989) Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell, 58, 287–292. [DOI] [PubMed] [Google Scholar]
- Kramer R., Bucay,B., Kane,D.J., Martin,L.E., Tarpley,J.E. and Theill,L.E. (1996) Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc. Natl Acad. Sci. USA, 93, 4833–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H.A., Beuvink,I., Motoyama,A.B., Daly,J.M., Neve,R.M. and Hynes,N.E. (2000) ErbB2 potentiates breast tumor proliferation through modulation of p27Kip1/Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol. Cell. Biol., 20, 3210–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.F., Simon,H., Chen,H., Bates,B., Hung,M.C. and Hauser,C. (1995) Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature, 378, 394–398. [DOI] [PubMed] [Google Scholar]
- Lemmon M.A., Bu,Z., Ladbury,J.E., Zhou,M., Pinchasi,D., Lax,I., Engelman,D.M. and Schlessinger,J. (1997) Two EGF molecules contribute additively to stabilization of the EGFR dimer. EMBO J., 16, 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine N.R., Barnes,D.M., Hollywood,D.P., Hughes,C.M., Smith,P., Dublin,E., Prigent,S.A., Gullick,W.J. and Hurst,H.C. (1992) Expression of the c-_erb_B3 gene product in breast cancer. Br. J. Cancer, 66, 1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesa G.M. and Sternberg,P.W. (1997) Positive and negative tissue-specific signaling by a nematode epidermal growth factor receptor. Mol. Biol. Cell, 8, 779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G., Klapper,L.N., Tzahar,E., Freywald,A., Sela,M. and Yarden,Y. (1996) Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene, 12, 1117–1125. [PubMed] [Google Scholar]
- Levkowitz G. et al. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell, 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- Lewis G.D., Figari,I., Fendly,B., Wong,W.L., Carter,P., Gorman,C. and Shepard,H.M. (1993) Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol. Immunother., 37, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G.D., Lofgren,J.A., McMurtrey,A.E., Huijens,A., Fendly,B.M., Bauer,K.D. and Sliwkowski,M.X. (1996) Growth regulation of human breast and ovarian tumor cells by heregulin: evidence for the requirement of ErbB2 as a critical component in mediating heregulin responsiveness. Cancer Res., 56, 1457–1465. [PubMed] [Google Scholar]
- Luetteke N.C., Qiu,T.H., Fenton,S.E., Troyer,K.L., Riedel,R.F., Chang,A. and Lee,D.C. (1999) Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF ligands in mouse mammary gland development. Development, 126, 2739–2750. [DOI] [PubMed] [Google Scholar]
- Luttrell L.M., Daaka,Y. and Lefkowitz,R.J. (1999) Regulation of tyrosine kinase cascades by G-protein coupled receptors. Curr. Opin. Cell Biol., 11, 177–183. [DOI] [PubMed] [Google Scholar]
- Margolis B.L., Lax,I., Kris,R., Dombalagian,M., Honegger,A.M., Howk,R., Givol,D., Ullrich,A. and Schlessinger,J. (1989) All autophosphrylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. Identification of a novel site in EGF receptor. J. Biol. Chem., 264, 10667–10671. [PubMed] [Google Scholar]
- Massague J. and Pandiella,A. (1993) Membrane anchored growth factors. Annu. Rev. Biochem., 62, 515–541. [DOI] [PubMed] [Google Scholar]
- Meisner H., Daga,A., Buxton,J., Fernandez,B., Chawla,A., Banerjee,U. and Czech,M.P. (1997) Interactions of Drosophila Cbl with epidermal growth factor receptors and role of Cbl in R7 photoreceptor cell development. Mol. Cell. Biol., 17, 2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. and Birchmeier,C. (1995) Multiple essential functions of neuregulin in development. Nature, 378, 386–390. [DOI] [PubMed] [Google Scholar]
- Miettinen P.J., Berger,J.E., Meneses,J., Phung,P., Pedersen,R.A., Werb,Z. and Derynk,R. (1995) Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature, 376, 337–341. [DOI] [PubMed] [Google Scholar]
- Morris J.K., Weichun,L., Hauser,C., Marchuk,Y., Getman,D. and Lee,K.F. (1999) Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron, 23, 273–283. [DOI] [PubMed] [Google Scholar]
- Neve R.M., Sutterlüty,H., Pullen,N., Lane,H.A., Daly,J.M., Krek,W. and Hynes,N.E. (2000) Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumour cells. Oncogene, 19, 1647–1656. [DOI] [PubMed] [Google Scholar]
- Ojeda J. and Ma,Y.J. (1999) Glial–neuronal interactions in the neuroendocrine control of mammalian puberty: facilitatory effects of gonadal steroids. J. Neurobiol., 40, 528–540. [DOI] [PubMed] [Google Scholar]
- Okabayashi Y., Kido,Y., Okutani,T., Sugamoto,Y., Sakaguchi,K. and Kasuga,M. (1994) Tyrosine 1148 and 1173 of activated human epidermal growth factor receptors are binding sites of Shc in intact cells. J. Biol. Chem., 269, 18674–18678. [PubMed] [Google Scholar]
- Okutani T., Okabayashi,Y., Kido,Y., Sugimoto,Y., Sakaguchi,K., Matuoka,K., Takenawa,T. and Kasuga,M. (1994) Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human epidermal growth factor receptor in intact cells. J. Biol. Chem., 269, 31310–31314. [PubMed] [Google Scholar]
- Olayioye M.A., Graus-Porta,D., Beerli,R.R., Rohrer,J., Gay,B. and Hynes,N.E. (1998) ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol. Cell. Biol., 18, 5042–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye M.A., Beuvink,I., Horsch,K., Daly,J.M. and Hynes,N.E. (1999) ErbB receptor-induced activation of Stat transcription factors is mediated by Src tyrosine kinases. J. Biol. Chem., 274, 17209–17218. [DOI] [PubMed] [Google Scholar]
- Paria B.C., Elenius,K., Klagsbrun,M. and Dey,S.K. (1999) Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development, 126, 1997–2005. [DOI] [PubMed] [Google Scholar]
- Pegram M.D. et al. (1998) Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol., 16, 2659–2671. [DOI] [PubMed] [Google Scholar]
- Peles E. and Yarden,Y. (1993) Neu and its ligands: from an oncogene to neural factors. BioEssays, 15, 815–824. [DOI] [PubMed] [Google Scholar]
- Pinkas-Kramarski R. et al. (1996) Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J., 15, 2452–2467. [PMC free article] [PubMed] [Google Scholar]
- Prigent S.A. and Gullick,W. (1994) Identification of c-erbB-3 binding sites for phosphatidylinositol 3′-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J., 13, 2831–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar T., Stamp,G.W.H., Pandha,H.S., Waxman,J. and Gullick,W.J. (1996) Expression of the type 1 tyrosine kinase growth factor EGF receptors c-_erb_B-2 and c-_erb_B-3 in bladder cancer. J. Pathol., 179, 381–385. [DOI] [PubMed] [Google Scholar]
- Ricci A., Lanfrancone,L., Chiari,R., Belardo,G., Pertica,C., Natali,P.G., Pelicci,P.G. and Segatto,O. (1995) Analysis of protein–protein interactions involved in the activation of the Shc/Grb-2 pathways by the ErbB-2 kinase. Oncogene, 11, 1519–1529. [PubMed] [Google Scholar]
- Riese D.J. II and Stern,D.F. (1998) Specificity within the EGF family/ErbB receptor family signaling network. BioEssays, 20, 41–48. [DOI] [PubMed] [Google Scholar]
- Riese D.J. II, van Raaij,T.M., Plowman,G.D., Andrews,G.C. and Stern,D.F. (1995) The cellular response to neregulins is governed by complex interactions of the erbB receptor family. Mol. Cell. Biol., 15, 5770–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese D.J. II, Bermingham,Y., van Raaij,T.M., Buckley,S., Plowman,G.D. and Stern,D.F. (1996) Betacellin activates the epidermal growth factor receptor and ErbB4 and induces cellular response patterns distinct from those stimulated by epidermal growth factor or neuregulin-β. Oncogene, 12, 345–353. [PubMed] [Google Scholar]
- Riethmacher D., Sonnenberg-Riethmacher,E., Brinkmann,V., Yamaai,T., Lewin,G.R. and Birchneier,C. (1995) Severe neuropathies in mice with targeted mutations in the erbB3 receptor. Nature, 389, 725–730. [DOI] [PubMed] [Google Scholar]
- Rotin D. et al. (1992) SH2 domains prevent tyrosine phosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase Cγ. EMBO J., 11, 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi K., Okabayashi,Y., Kido,Y., Kimura,S., Matsumura,Y., Inushima,K. and Kasuga,M. (1998) Shc phosphotyrosine-binding domain dominantly interacts with epidermal growth factor receptors and mediates ras activation in intact cells. Mol. Endocrinol., 12, 536–543. [DOI] [PubMed] [Google Scholar]
- Salomon D.S., Brandt,R., Ciardiello,F. and Normanno,N. (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol., 19, 183–232. [DOI] [PubMed] [Google Scholar]
- Sato K., Sato,A., Aoto,M. and Fukami,Y. (1995) c-Src phosphorylates EGF receptor on tyrosine 845. Biochem. Biophys. Res. Commun., 215, 1078–1087. [DOI] [PubMed] [Google Scholar]
- Schroeder J.A. and Lee,D.C. (1998) Dynamic expression and activation of ERBB receptors in the developing mouse mammary gland. Cell Growth Differ., 9, 451–464. [PubMed] [Google Scholar]
- Segatto O., Lonardo,F., Pierce,J.H., Bottaro,D.P. and Di Fiore,P.P. (1990) The role of autophosphorylation in modulation of erbB-2 transforming function. New Biol., 2, 187–195. [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Shoelson S.E. (1997) SH2 and PTB domain interactions in tyrosine kinase signal transduction. Curr. Opin. Chem. Biol., 1, 227–234. [DOI] [PubMed] [Google Scholar]
- Sibilia M. and Wagner,E.F. (1995) Strain-dependent epithelial defects in mice lacking the EGF receptor. Science, 269, 234–238. [DOI] [PubMed] [Google Scholar]
- Sibilia M., Steinbach,J.P., Stingl,L., Aguzzi,A. and Wagner,E.F. (1998) A strain-dependent postnatal neurodegeneration in mice lacking EGF receptor. EMBO J., 17, 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel P.M., Ryan,E.D., Cardiff,R.D. and Muller,W. (1999) Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J., 18, 2149–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D.J. et al. (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science, 244, 707–712. [DOI] [PubMed] [Google Scholar]
- Spencer K.S.R., Graus-Porta,D., Leng,J., Hynes,N.E. and Klemke,R.L. (2000) ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J. Cell Biol., 148, 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover D.R., Becker,M., Liebetanz,J. and Lydon,N.B. (1995) Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and p85α. J. Biol. Chem., 270, 15591–15597. [DOI] [PubMed] [Google Scholar]
- Sudol M. (1998) From Src homology domains to other signaling modules: proposal of the ‘protein recognition code’. Oncogene, 17, 1469–1474. [DOI] [PubMed] [Google Scholar]
- Tang C.K. and Lippman,M.E. (1998) EGF family receptors and their ligands in human cancer. In O’Malley,B.W. (ed.), Hormones and Signaling, Vol. I. Academic Press, San Diego, CA, pp. 113–165. [Google Scholar]
- Threadgill D.W. et al. (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science, 269, 230–234. [DOI] [PubMed] [Google Scholar]
- Torrisi M.R., Lotti,L.V., Belleudi,F., Gradini,R., Salcini,A.E., Confalonieri,S., Pelicci,P.G. and Di Fiore,P.P. (1999) Eps15 is recruited to the plasma membrane upon epidermal growth factor receptor activation and localizes to components of the endocytic pathway during receptor internalization. Mol. Biol. Cell, 10, 417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., Komurasaki,T., Uchida,D. and Morimoto,S. (1997) Distribution of mRNA for human epiregulin, a differentially expressed member of the epidermal growth factor family. Biochem. J., 326, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahar E., Waterman,H., Chen,X., Levkowitz,G., Karunagaran,D., Lavi,S., Ratzkin,B.J. and Yarden,Y. (1996) A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol., 16, 5276–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahar E. et al. (1997) Bivalence of EGF-like ligands drives the ErbB signaling network. EMBO J., 16, 4938–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahar E. et al. (1998) Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO J., 17, 5948–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallasch C., Weiss,F.U., Niederfellner,G., Jallal,B., Issing,W. and Ullrich,A. (1995) Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J., 14, 4267–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton G.M., Chen,W.S., Rosenfeld,M.G. and Gill,G.N. (1990) Analysis of deletions of the carboxyl terminus of the EGFR reveals self-phosphorylation at tyrosine 992 and enhanced in vivo tyrosine phosphorylation of cell substrates. J. Biol. Chem., 265, 1750–1754. [PubMed] [Google Scholar]
- Wasserman J.D. and Freeman,M. (1997) Control of EGF receptor activation in Drosophila. Trends Cell Biol., 7, 431–436. [DOI] [PubMed] [Google Scholar]
- Waterman H., Sabanai,I., Geiger,B. and Yarden,Y. (1998) Alternative intracellular routing of ErbB receptors may determine signaling potency. J. Biol. Chem., 273, 13819–13827. [DOI] [PubMed] [Google Scholar]
- Weiner D.B., Liu,J., Cohen,J.A., Williams,W.V. and Greene,M.I. (1989) A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature, 339, 230–231. [DOI] [PubMed] [Google Scholar]
- Weiss A. and Schlessinger,J. (1998) Switching signals on or off by receptor dimerization. Cell, 94, 277–280. [DOI] [PubMed] [Google Scholar]
- Wiesen J.F., Young,P., Werb,Z. and Cunha,G.R. (1999) Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development, 126, 335–344. [DOI] [PubMed] [Google Scholar]
- Xie W., Paterson,A.J., Chin,E., Nabell,L.M. and Kudlow,J.E. (1997) Targeted expression of a dominant negative epidermal growth factor receptor in the mammary gland of transgenic mice inhibits pubertal mammary duct development. Mol. Endocrinol., 11, 1766–1781. [DOI] [PubMed] [Google Scholar]
- Yang Y., Spitzer,E., Meyer,D., Sachs,M., Niemann,C., Hartmann,G., Weidner,K.M., Birchmeier,C. and Birchmeier,W. (1995) Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J. Cell Biol., 131, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi M., Kondo,T., Houghton,A., Bartkiewicz,M., Horne,W.C., Zhang,H., Yoshimura,A. and Baron,R. (1999) Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J. Biol. Chem., 274, 31707–31712. [DOI] [PubMed] [Google Scholar]
- Yoon C.H., Lee,J., Jongeward,G.D. and Sternberg,P.W. (1995) Similarity of sli-1, a regulator of vulval development in C.elegans, to the mammalian proto-oncogene c-Cbl. Science, 269, 1102–1105. [DOI] [PubMed] [Google Scholar]
- Zhang D. et al. (1997) Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc. Natl Acad. Sci. USA, 94, 9562–9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Sun,J., Liu,N., Wen,D., Chang,D., Thomason,A. and Yoshinaga,S.K. (1996) Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 and HER2. J. Biol. Chem., 271, 3884–3890. [PubMed] [Google Scholar]
- Zrihan-Licht S., Deng,B., Yarden,Y., McShan,G., Keydar,I. and Avraham,H. (1998) Csk homologous kinase, a novel signaling molecule, directly associates with the activated ErbB-2 receptor in breast cancer cells and inhibits their proliferation. J. Biol. Chem., 273, 4065–4072. [DOI] [PubMed] [Google Scholar]