An antigenic peptide produced by reverse splicing and double asparagine deamidation (original) (raw)
Abstract
A variety of unconventional translational and posttranslational mechanisms contribute to the production of antigenic peptides, thereby increasing the diversity of the peptide repertoire presented by MHC class I molecules. Here, we describe a class I-restricted peptide that combines several posttranslational modifications. It is derived from tyrosinase and recognized by tumor-infiltrating lymphocytes isolated from a melanoma patient. This unusual antigenic peptide is made of two noncontiguous tyrosinase fragments that are spliced together in the reverse order. In addition, it contains two aspartate residues that replace the asparagines encoded in the tyrosinase sequence. We confirmed that this peptide is naturally presented at the surface of melanoma cells, and we showed that its processing sequentially requires translation of tyrosinase into the endoplasmic reticulum and its retrotranslocation into the cytosol, where deglycosylation of the two asparagines by peptide-_N_-glycanase turns them into aspartates by deamidation. This process is followed by cleavage and splicing of the appropriate fragments by the standard proteasome and additional transport of the resulting peptide into the endoplasmic reticulum through the transporter associated with antigen processing (TAP).
Keywords: antigen processing, peptide splicing, tumor antigen
CD8+ cytotoxic T lymphocytes (CTLs) are the principal effectors recognizing, through their specific T cell receptor, peptide fragments bound to MHC class I molecules present on the surface of malignant cells. Most of the genes encoding class I-restricted tumor antigenic peptides were discovered by isolating T lymphocytes from melanoma patients. After in vitro stimulation by autologous melanoma cells, antitumor T lymphocytes were obtained and used to clone the genes encoding the tumor antigens. These genes included cancer germ-line genes, such as those of the melanoma antigen (MAGE) family, which encode tumor-specific antigens expressed in various tumors (1–4), but also differentiation genes encoding antigens expressed in both melanomas and normal melanocytes, such as Melan-A (MART-1), gp100, and tyrosinase (5–8). Precise identification of the antigenic peptides present at the surface of tumor cells is crucial to develop efficient immunotherapy strategies. These peptides can be used, for example, to generate melanoma-specific T cells for adoptive immunotherapy or in strategies using epitope-based vaccination. Understanding the processing and presentation pathways of such antigenic peptides may also help in designing appropriate protocols of immunotherapy. Class I-restricted antigenic peptides are usually made up of fragments of 8–11 aa directly derived from the degradation of the parental protein by the proteasome (9). Proteasomal substrates mostly comprise nuclear and cytosolic proteins but also include endoplasmic reticulum (ER) proteins returned to the cytosol during the process of ER-associated degradation (ERAD) (10, 11). In most cases, the sequence of the antigenic peptides can be easily predicted from that of the encoding gene or parental protein. However, a number of antigenic peptides were described in which the sequence could not be directly predicted from the encoded sequence. The study of the origin of such nonclassical peptides led to the description of unexpected biological mechanisms acting at a transcriptional, translational, or posttranslational level. Some peptides were found to arise from aberrant transcription of intronic sequences (12–14) or reverse-strand sequences (15). Others resulted from translation of alternative open-reading frames (16, 17), sometimes initiating at non-AUG codons (18, 19). Last, peptides were found to be modified at the posttranslational level undergoing, for example, deamidation of asparagine residues (20–22), phosphorylation of serine and threonine residues (23, 24), or peptide splicing (25–27). Deamidation was found to occur on asparagine residues corresponding to an _N_-glycosylation site and is thought to involve retrotranslocation from the ER into the cytosol and deglycosylation by peptide-_N_-glycanase (PNGase) (28). Peptide splicing allows the production of antigenic peptides made of two noncontiguous fragments of a given protein that are linked together after the excision of an intervening segment. These two fragments can be linked together either in the order in which they appear in the parental protein or in the reverse order. Three spliced antigenic peptides were described to date. One peptide is derived from fibroblast growth factor-5 (FGF-5) and is presented by HLA-A3 molecules. It is made of two peptide fragments of five and four residues located 40 aa apart in the parental protein (25). Another peptide is an HLA-A32–restricted peptide produced from the melanosomal protein gp100 by excision of 4 aa and splicing of the two flanking fragments of three and six residues (26). The third spliced peptide is a human minor histocompatibility antigen created by a polymorphism in the SP110 gene (27). This peptide, presented by HLA-A3 molecules, is made of two noncontiguous fragments of four and six residues separated by 6 aa in the sequence of SP110 protein. In this case, the two distant fragments are spliced together in the reverse order. The proteasome was found to be responsible for the splicing reaction leading to the production of the three spliced peptides (26, 27, 29). The catalytic mechanism proposed for the splicing reaction is a transpeptidation occurring at the active N-terminal threonine of a catalytic β-subunit of the proteasome. This transpeptidation involves the nucleophilic attack of the carbonyl carbon atom of an acyl-enzyme intermediate by the N-terminal amino group of a peptide fragment present at the active site of the enzyme. The efficiency of the splicing reaction is very low but is presumably increased in the catalytic chamber of the proteasome because of its confined space.
Here, we describe an antigenic peptide that contains several posttranslational modifications. The peptide, which derives from tyrosinase, is recognized by tumor-infiltrating lymphocytes (TIL) from a melanoma patient. It contains two deamidated asparagine residues and additionally, is made of two noncontiguous fragments that are spliced in the reverse order. We analyzed, in detail, the processing pathway leading to the production of this highly unusual peptide.
Results
Evidence for ER Processing of an HLA-A24–Restricted Tyrosinase Epitope.
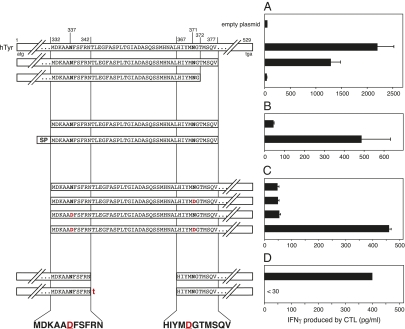
The adoptive transfer of TIL line TIL888 into the autologous melanoma patient was followed by tumor regression (30). TIL888 was found to recognize a tyrosinase-derived antigen presented by HLA-A24 molecules (30). To localize the peptide recognized by TIL888 and its representative clone CL62, we generated truncated constructs of the human tyrosinase gene and transfected them into COS-7 cells together with a plasmid encoding the HLA-A24 molecule. These cells were then tested for their ability to stimulate CTL CL62. Cells transfected with a construct encoding tyrosinase amino acids 1–377 were recognized by CTL CL62, whereas cells transfected with a shorter construct encoding amino acids 1–372 were not (Fig. 1_A_) in accordance with previous experiments (31). The tyrosinase gene encodes a signal peptide targeting translation into the ER where signal peptidase removes the N-terminal signal peptide. To evaluate the importance of this ER transit for production of the antigenic peptide, we generated two shorter constructs encoding amino acids 332–377 either alone or preceded by a signal peptide (Fig. 1_B_). Although COS-7 cells transfected with the construct containing the signal peptide were recognized by CTL CL62, those cells transfected with the plain construct, which is translated in the cytosol, were not recognized, indicating that translation into the ER was required for production of the antigen.
Fig. 1.
Localization of the minimal region of tyrosinase required to produce the antigen. (A–C) COS-7 cells were transiently transfected with a construct encoding HLA-A24 together with the indicated constructs derived from human tyrosinase (hTyr). The next day, these cells were tested for recognition by CTL CL62, a tyrosinase-reactive CTL clone isolated from line TIL888. IFNγ production was measured by ELISA in the culture supernatants after overnight incubation. In B, SP indicates that the plasmid construct encodes the signal peptide of IL-2. In C, where indicated, the Asn residues at position 337 or 371 were replaced by Asp residues by site-directed mutagenesis. (D) HEK293-A24 cells were transiently transfected with the indicated tyrosinase constructs containing an internal deletion. Transfected cells were tested for their recognition by TIL888 1 d later. Where indicated, an additional thymidine (t) was inserted between the codons corresponding to amino acids 342 and 367. The addition of this nucleotide results in a downstream frame shift after translation of the construct. The sequence of the minimal regions of tyrosinase required for recognition by the CTL is indicated below the sequences of the different constructs tested. Data shown are representative of at least two independent experiments. Error bars show SDs of triplicates.
Involvement of Asparagine Deamidation in the Production of the Tyrosinase Epitope.
The previously described HLA-A*0201–restricted tyrosinase peptide 369YMDGTMSQV377 contains an Asp residue at position 371 instead of the Asn residue predicted by the nucleotide sequence of the gene (22). The Asn371 residue, which belongs to an _N_-linked glycosylation site, seems to be deglycosylated by the cytosolic enzyme PNGase (28), a process that occurs in the cytosol after retrotranslocation of tyrosinase from the ER (11). A construct lacking the signal sequence and containing a substitution of Asn371 with an aspartic acid was not recognized by CTL CL62 (Fig. 1_C_). However, this fragment contains, at position 337, another Asn residue within an _N_-linked glycosylation consensus sequence. A tyrosinase332–529 construct encoding the N337D substitution also failed to activate the CTL, whereas a construct encoding an Asp residue at both positions 337 and 371 was recognized by CTL CL62, indicating that both regions contributed to the epitope recognized by these T cells (Fig. 1_C_) and providing evidence that this epitope was processed in a similar way to the previously described HLA-A*0201–restricted 369YMDGTMSQV377 epitope (11, 22). Evaluation of tyrosinase variants containing internal deletions of sequences between Asn337 and Asn371 revealed that transfection of a construct lacking the region that encoded amino acids 343–366 stimulated cytokine release from TIL888 (Fig. 1_D_). A variant containing the same deletion but with an additional thymidine after codon 342, resulting in a downstream frame shift, was not able to sensitize cells to recognition by TIL888 (Fig. 1_D_). This result provided evidence that RNA splicing was not responsible for linking these two regions, because this process presumably would be unaffected by the addition of a single nucleotide at this position, and it raised the possibility that this antigenic peptide was produced by peptide splicing, as described recently (25–27).
Evidence for Peptide Splicing in Generating the Tyrosinase Epitope.
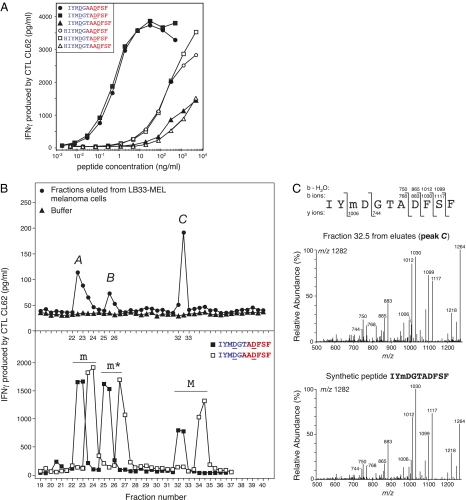
Target cells loaded with peptides of varying lengths containing combinations of amino acids between residues 332 and 342 followed by amino acids between residues 367 and 377 and containing the two aspartic residues D337 and D371 failed to be recognized by CTL CL62. These peptides only partially fit the HLA-A24 binding motif, namely Y or F at position 2 and F, I, or L at the C terminus (32, 33). However, we observed that many peptides made of two fragments assembled in the reverse order contain a more appropriate HLA-A24 binding motif. We then synthesized and tested the recognition of 180 peptides containing residues from the two tyrosinase regions determined above that were spliced in reverse order and in addition, included the two aspartate substitutions at residues 337 and 371 (Fig. S1). To facilitate the synthesis and subsequent analysis of these peptides, they were prepared in 30 pools of six peptides. Each pool was evaluated for its ability to stimulate the CTL. The peptides contained in each of the four positive pools were also fractionated by HPLC, and the fractions were tested for CTL recognition. Six fractions, corresponding to six different peptides, were able to stimulate CTL CL62 (Fig. S1). These six peptides were then synthesized separately, purified, and tested for CTL recognition after titration. Two of them, with sequences IYMDGTADFSF and IYMDGAADFSF, were efficiently recognized with a half-maximal activation at a peptide concentration between 0.1 and 1 ng/mL (Fig. 2_A_). The other purified peptides were able to stimulate the CTL but only at higher concentrations.
Fig. 2.
Identification of the antigenic peptide presented by melanoma cells and recognized by CTL CL62. (A) CTL recognition of synthetic peptides composed of two noncontiguous fragments of tyrosinase that are joined together in the reverse order to that of the tyrosinase sequence. The indicated peptides were loaded onto HLA-A24+ EBV B cells before the CTL assay. IFNγ production was measured after an overnight incubation. (B) Peptides were eluted from HLA class I molecules purified from melanoma cells LB33-MEL and were fractionated on a reversed-phase column by HPLC. The fractions were loaded onto presenting cells and tested for CTL recognition (Upper). IFNγ production was measured after an overnight incubation. Three peaks of CTL activity, indicated by A, B, and C on the graph, were detected. Buffer was run on the column before the eluted peptides to rule out any contamination of the HPLC system, and the fractions were tested similarly. Synthetic peptides IYMDGTADFSF and IYMDGAADFSF were oxidized by treatment with H2O2. The nonoxidized (M), methionine sulfoxide (m), and methionine sulfone (m*) forms of the peptides were separated and purified. About 10 ng each peptide form were injected together under the same HPLC conditions as those conditions used for eluates, and the fractions were tested for CTL recognition (Lower). (C) MS/MS fragmentation spectrum of the singly charged ion with m/z 1,282 detected in fraction 32.5 (peak C) from LB33-MEL eluates (Upper) and the fragmentation spectrum of the singly charged ion (m/z 1,282) of the synthetic peptide IYmDGTADFSF (Lower) are shown. The fragments that were detected are indicated above the peptide sequence for N-terminal b ions and their dehydrated forms and indicated below the sequence for C-terminal y ions. The ion with m/z 1,264 is the dehydrated form of ion 1,282, and the ion with m/z 1,218 corresponds to the loss of methanesulfenic acid from the methionine sulfoxide of ion 1,282 (55). Data shown are representative of at least two independent experiments (peaks A, B, and C).
Identification of the Naturally Processed Tyrosinase Peptide.
Peptides IYMDGTADFSF and IYMDGAADFSF, which correspond, respectively, to the reverse splicing of fragments ADFSF and IYMDGT or AADFSF and IYMDG, seemed, therefore, to be the best candidates for the natural antigenic peptide. To determine which candidate was identical to the natural peptide presented by melanoma cells, we isolated MHC class I molecules from melanoma cells LB33-MEL, which were efficiently recognized by CTL CL62. We eluted the peptides bound to MHC molecules with acid, fractionated them on a reversed-phase column by HPLC, and tested the collected fractions for CTL recognition. Surprisingly, the fractions recognized by CTL CL62 were distributed in three distinct peaks, designated peak A, peak B, and peak C (Fig. 2_B_ Upper). In parallel, we observed by HPLC-MS that the synthetic peptides IYMDGTADFSF and IYMDGAADFSF spontaneously oxidized partially on their methionine residue, where a sulfur atom acquired an oxygen and became a sulfoxide. Under strong oxidative conditions, we observed additional oxidation of the methionine sulfoxide into methionine sulfone, with two oxygen atoms on the same sulfur atom. The three forms of each of the two candidates (i.e., nonoxidized, sulfoxide, and sulfone) were prepared, purified, and chromatographed together under the HPLC conditions used for the peptides eluted from LB33-MEL cells. The collected fractions were then loaded onto target cells and tested for CTL recognition. We observed three peaks of CTL activity for each candidate. By comparing their retention times with the times of the purified peptides, we assigned the first peak to the sulfoxide form (m), the second peak to the sulfone (m*), and the third peak to the nonoxidized form (M) (Fig. 2_B_ Lower). This assignment indicated that the oxidized forms of the candidates were also recognized by CTL CL62. More importantly, the three peaks of CTL activity detected for synthetic peptide IYMDGTADFSF after fractionation corresponded exactly to the peaks obtained with the peptides eluted from melanoma cells (Fig. 2_B_). This correspondence was not observed for the positive peaks obtained after fractionation of the three oxidized forms of synthetic peptide IYMDGAADFSF. Thus, the profile of retention times of the antigenic peptide present at the surface of melanoma cells was identical to that of synthetic peptide IYMDGTADFSF. Notably, a titration of the three purified forms of synthetic peptide IYMDGTADFSF showed that they were equally recognized by the CTL (Fig. S2_A_). We also synthesized and tested spliced peptide ADFSFIYMDGT, which is made of the same noncontiguous fragments but assembled in the normal order, and peptide IYMNGTANFSF, which contains the two Asn residues of the original sequence of tyrosinase. As expected, these two peptides were not recognized by the CTL (Fig. S2_B_). Finally, peptide IYMDGTADFSF was clearly identified in peak C of the eluates by using HPLC coupled with tandem MS (HPLC-MS/MS) (Fig. 2_C_). Although the retention time of peak C clearly indicated the presence of the nonoxidized form of IYMDGTADFSF in the melanoma eluates, the HPLC-MS/MS analysis of peak C only detected this peptide under its sulfoxide form, with m/z equal to 1,282 and a fragmentation pattern identical to that of the corresponding synthetic sulfoxide peptide (Fig. 2_C_). The lack of detection of the nonoxidized form of IYMDGTADFSF in this fraction is likely because of its oxidation after the HPLC separation, probably during the drying step. We also detected, by MS/MS, the sulfoxide form of the peptide in peak A of LB33-MEL eluates as well as the sulfone form in peak B, although the latter was less abundant and close to the detection limit of our system (Fig. S3). Taken together, these results confirmed that the natural antigenic peptide presented by HLA-A24 to CTL CL62 is the 11-mer IYMDGTADFSF, which is a spliced and reordered peptide composed by the two noncontiguous fragments ADFSF and IYMDGT, each of them containing an Asp residue resulting from deamidation of an Asn residue.
Production of the Spliced and Reordered Peptide by the Proteasome.
The proteasome is responsible for the splicing reaction leading to the production of the three spliced antigenic peptides described so far (26, 27, 29). We evaluated its involvement in the production of the tyrosinase spliced peptide IYMDGTADFSF by treating melanoma cells LB39-MEL with the specific proteasome inhibitor epoxomicin. Treatment with epoxomicin decreased the recognition of melanoma cells by CTL CL62 (Fig. 3_A_ Top). As a control, we also evaluated, in the same experiment, the effect of epoxomicin on the presentation of two HLA-A2–restricted tyrosinase-derived antigenic peptides, one (369YMDGTMSQV377) being proteasome- and TAP-dependent and the other (1MLLAVLYCL9) being derived from the signal peptide and described as proteasome- and TAP-independent (34, 35). As expected, epoxomicin treatment decreased the presentation of the former but not the latter (Fig. 3_A_). Next, we showed that an HLA-A24+ tumor cell line, which does not express tyrosinase, was able to sensitize CTL CL62 when electroporated with the 25-residue precursor peptide ADFSFRNTLEHNALHIYMDGTMSQV (Fig. S4). The CTL activation was impaired when these cells were treated with epoxomicin before electroporation of the precursor. Collectively, these experiments strongly suggested an implication of the proteasome in the production of the spliced peptide IYMDGTADFSF.
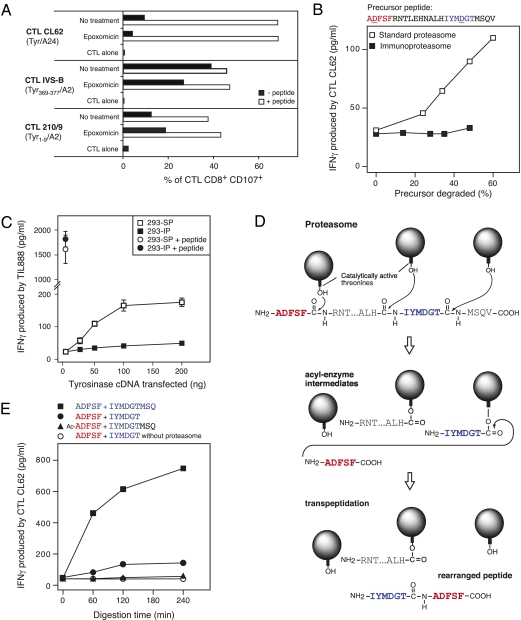
Fig. 3.
Production of the spliced antigenic peptide IYMDGTADFSF by the proteasome. (A) CTL recognition of melanoma cells treated or not treated with proteasome inhibitor. Melanoma cells LB39-MEL naturally express tyrosinase, HLA-A24, and HLA-A2. They were treated for 2 h with epoxomicin (0.5 μM) after acid elution of MHC-bound peptides. Cells were then washed and incubated for 1 more h with a lower dose of epoxomicin (0.01 μM) and where indicated, (white bars), with the corresponding synthetic antigenic peptide as a control. Recognition by three CTL clones was evaluated by flow cytometry in a degranulation assay performed after 5 h of incubation with LB39-MEL cells (SI Results explains the choice of this assay in this context). The percentage of CD8+ T cells expressing the degranulation markers CD107a and CD107b is shown. CTL CL62 recognizes tyrosinase/A24 spliced peptide IYMDGTADFSF, CTL IVS-B recognizes tyrosinase/A2 369YMDGTMSQV377, and CTL 210/9 recognizes tyrosinase/A2 1MLLAVLYCL9. One of three similar experiments is shown. (B) In vitro production of the tyrosinase spliced peptide by the standard proteasome. The indicated 25-mer precursor peptide was incubated with purified 20S standard proteasomes or immunoproteasomes. The reaction was stopped after 0, 1, 2, 3, and 4 h of incubation. Digests were then loaded onto HLA-A24+ EBV B cells. CTL CL62 was added and IFNγ production was measured after an overnight incubation. The degradation of the precursor peptide was determined by MS. One of two similar experiments is shown. (C) Processing of peptide IYMDGTADFSF by HEK293 cells expressing standard proteasomes (293-SP) or immunoproteasomes (293-IP). Both cell lines were transfected with an HLA-A24 plasmid construct and the indicated amounts of plasmid encoding tyrosinase. CTLs TIL888 were added and IFNγ production was measured in supernatants after an overnight incubation. Control cells were transfected with a plasmid coding for HLA-A24 and pulsed with the synthetic peptide IYMDGTADFSF for 1 h before addition of the CTLs. One of two similar experiments is shown. Error bars show SDs of triplicates. (D) Model for the catalytic mechanism producing the tyrosinase spliced peptide in the proteasome. The balls represent catalytically active β-subunits of the proteasome with the hydroxyl group of the side chain of their N-terminal threonine. (E) CTL recognition of digests obtained by incubating purified 20S standard proteasomes with various pairs of synthetic peptides during the indicated periods of time. The different digests were loaded onto presenting cells and tested for CTL stimulation. One of four similar experiments is shown. Ac-ADFSF, N-terminally acetylated peptide with sequence ADFSF.
To directly evaluate the ability of the proteasome to produce this spliced peptide, we incubated the precursor peptide used in the electroporation experiment with either purified 20S standard proteasomes or purified 20S immunoproteasomes containing the three inducible catalytic subunits β1i, β2i, and β5i (36, 37). The resulting digests were loaded onto presenting cells and tested for their ability to stimulate CTL CL62. Digestion of the precursor peptide by standard proteasomes led to a CTL activation increasing with the degradation of the precursor peptide (Fig. 3_B_). In contrast, the digests obtained with immunoproteasomes failed to activate the CTL. Efficient degradation of the precursor peptide by both proteasome types was verified by MS. These results showed that the spliced peptide IYMDGTADFSF is produced in vitro by the standard proteasome but not by the immunoproteasome. Consistent with this finding, we observed that cells containing exclusively standard proteasomes were efficiently recognized by CTL CL62 after transfection of constructs encoding tyrosinase and HLA-A24 (Fig. 3_C_). In contrast, cells previously shown to exclusively contain immunoproteasomes were not recognized by the CTL after transfection (38). According to the catalytic mechanism proposed for the previously described spliced peptides, cleavage at the C terminus of fragment IYMDGT would be required to form an acyl-enzyme intermediate, which would be further attacked by the N-terminal amino group of fragment ADFSF to form, by transpeptidation, the rearranged spliced peptide (Fig. 3_D_). To validate this mechanism, we incubated various pairs of synthetic peptides together with purified standard proteasomes. Digests obtained with peptides ADFSF and IYMDGTMSQ were efficiently recognized by CTL CL62 (Fig. 3_E_), and the presence of the spliced peptide IYMDGTADFSF was confirmed by MS/MS in the 240-min digest. However, when we incubated proteasomes with peptides ADFSF and IYMDGT, the digests were weakly recognized by the CTL. These results indicated that cleavage of the peptide bond after the Thr of fragment IYMDGTMSQ is required to obtain an efficient production of the spliced peptide IYMDGTADFSF, which was predicted by the transpeptidation model. We also observed that production of the spliced peptide was abolished by blocking the N-terminal amino group of fragment ADFSF with an acetyl group (Fig. 3_E_). This finding indicated that fragment ADFSF is responsible, with its N-terminal amino group, for the nucleophilic attack of the acyl-enzyme intermediate. Collectively, these results confirmed the essential role of the proteasome in the transpeptidation reaction, leading to the spliced and reordered peptide IYMDGTADFSF.
A detailed MS analysis of digests obtained by incubating the long precursor peptide with standard proteasomes or immunoproteasomes indicated that the cleavages required to produce fragments IYMDGT and ADFSF were performed less efficiently by the immunoproteasome (Fig. S5). Hence, the better production of the spliced peptide by the standard proteasome seems to result from a better production of the fragments to splice. This conclusion is in line with our recent study of the differential processing of the three other spliced antigenic peptides (39).
Deamidation of Asparagine Residues by PNGase.
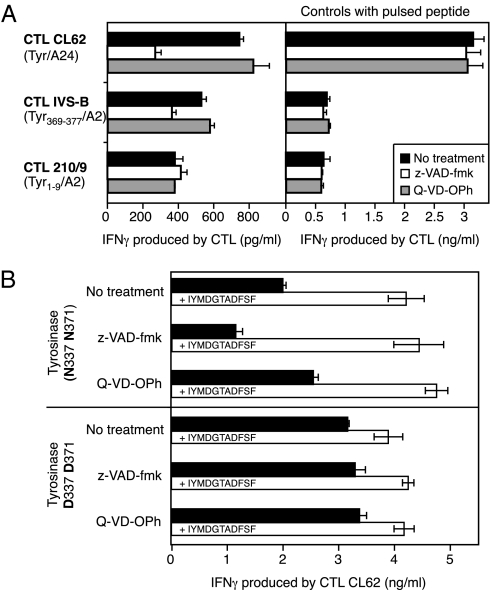
Next, we verified whether this spliced antigenic peptide, which contains two Asp residues instead of the two Asn residues present in the tyrosinase sequence, followed the same processing pathway as the tyrosinase antigenic peptide 369YMDGTMSQV377, which contains one of these two deamidated residues. We showed that the production of spliced peptide IYMDGTADFSF also required translation of tyrosinase into the ER (Fig. 1_B_) followed by proteasomal degradation in the cytosol (Fig. 3 and Fig. S4). We then evaluated the role of PNGase in the deamidation process leading to spliced peptide IYMDGTADFSF. We treated melanoma cells LB39-MEL with the caspase inhibitor z-VAD-fmk, which is also a potent inhibitor of PNGase (28, 40), and tested them for recognition by the three CTL clones used previously. Treatment with z-VAD-fmk strongly inhibited presentation of peptide IYMDGTADFSF and to a lesser extent, peptide YMDGTMSQV (Fig. 4_A_). However, it did not affect presentation of peptide MLLAVLYCL, which does not contain a deamidated residue. Treatment of tumor cells with Q-VD-OPh, a general caspase inhibitor that does not block PNGase activity (40), had no effect on CTL recognition (Fig. 4_A_). This result indicated that PNGase plays a role in the processing of spliced peptide IYMDGTADFSF. To clarify the role of PNGase in the generation of this epitope, we transfected COS-7 cells with an HLA-A24–encoding construct and a construct encoding either a full-length WT tyrosinase (Tyr N337 N371) or a full-length tyrosinase in which Asn at positions 337 and 371 were replaced by Asp (Tyr D337 D371). After transfection, we treated cells with z-VAD-fmk or Q-VD-OPh and tested their recognition by CTL CL62. In line with the results reported above with melanoma cells, z-VAD-fmk but not Q-VD-OPh reduced recognition of cells transfected with Tyr N337 N371 (Fig. 4_B_ Upper). In contrast, z-VAD-fmk did not affect recognition of cells transfected with Tyr D337 D371 (Fig. 4_B_ Lower). We obtained similar results when we transfected a pair of shorter constructs, coding for tyrosinase332–337 preceded by a signal peptide (SP-Tyr332-377) and containing no other _N_-glycosylation sites than N337 and N371 (Fig. S6). Taken together, these results indicate that PNGase is responsible for the conversion of Asn residues into Asp during the processing of the spliced and doubly deamidated peptide IYMDGTADFSF.
Fig. 4.
Role of PNGase in the processing of the tyrosinase spliced peptide. (A) Melanoma cells LB39-MEL, expressing both HLA-A24 and HLA-A2, were treated for 5 h with the indicated compounds (50 μM) after acid elution of MHC-bound peptides. Both z-VAD-fmk and Q-VD-OPh are caspase inhibitors. Only z-VAD-fmk blocks PNGase activity. Cells were then tested for recognition by three different CTL clones (Left). CTL CL62 recognizes the tyrosinase/A24 spliced peptide IYMDGTADFSF, CTL IVS-B recognizes tyrosinase/A2 369YMDGTMSQV377, and CTL 210/9 recognizes tyrosinase/A2 1MLLAVLYCL9. IFNγ production was measured after an overnight incubation. Cells were pulsed with the corresponding synthetic antigenic peptide before addition of the CTL to check their antigen-presenting capacity (Right). Data shown are representative of at least two independent experiments. (B) COS-7 cells were transiently transfected with plasmids encoding HLA-A24 and a full-length tyrosinase construct encoding either Asn residues at positions 337 and 371 (N337 N371) or Asp residues at the same positions (D337 D371); 5 h after transfection, cells were incubated overnight with 50 μM of the indicated compounds. Supernatants were then removed, and CTL CL62 was added. Where indicated (white bars), cells were pulsed for 1 h with the antigenic peptide before the addition of the CTL. Data shown are representative of four independent experiments. In all panels, error bars show SDs of triplicates.
We next evaluated the role of TAP in the processing pathway of the tyrosinase spliced peptide using ICP47, a herpex simplex viral protein that blocks TAP transport (41). We transiently transfected COS-7 cells with plasmids encoding tyrosinase, ICP47, and the appropriate HLA molecule. Presentation of the spliced peptide IYMDGTADFSF to CTL CL62 was inhibited by ICP47 in a dose-dependent manner (Fig. S7). As expected, a similar inhibition was observed for the presentation of peptide YMDGTMSQV, whereas presentation of signal sequence-derived peptide MLLAVLYCL was not affected (35).
To determine whether the complex processing of this antigenic peptide only takes place in tumor cells or also takes place in normal cells, we tested recognition of normal melanocytes, which express tyrosinase. CTL CL62 was strongly activated by HLA-A24–positive but not -negative melanocytes (Fig. S8). This result indicated that normal cells produced the deamidated spliced peptide, in line with the fact that PNGase and the standard proteasome are present in most normal cells.
Discussion
In the present study, we identified a tumor antigenic peptide derived from tyrosinase and made of two spliced and reordered peptide fragments, each one containing an Asp residue resulting from Asn deamidation. Tyrosinase is a membrane-associated _N_-linked glycoprotein, which is synthesized, folded, and glycosylated in the ER before being transported through the _trans_-Golgi network to melanosomes (42). Unfolded or misfolded tyrosinase molecules frequently accumulate in melanoma cells (43) and represent an important source of MHC class I-restricted antigenic peptides recognized by melanoma-specific T cells (31, 34). These misfolded tyrosinase molecules are retained in the ER through prolonged interaction with lectin chaperones implicated in ER quality control and are also targeted for retrotranslocation to the cytosol through the ERAD pathway (44–46). Here, we show that the presentation to CD8+ T lymphocytes of the spliced peptide derived from tyrosinase requires translation of tyrosinase molecules into the ER and depends on proteasome activity and TAP transport. We also observed that this epitope contains two Asp residues, one in each peptide fragment to be spliced, instead of the two Asn at positions 337 and 371 that were predicted by the sequence of the tyrosinase gene. This posttranslational deamidation was already described for the Asn residue at position 371 during the processing of the tyrosinase369–377 epitope (22). It was shown that deamidation of this Asn was partially caused by its glycosylation in the ER and subsequent deglycosylation by PNGase in the cytosol (28). Here, by inhibiting PNGase activity with z-VAD-fmk in melanoma cells naturally expressing tyrosinase, we confirmed the involvement of this enzyme in the processing of the tyrosinase369–377 epitope and showed that PNGase also contributes to the processing of the spliced antigenic peptide IYMDGTADFSF. We specified the role of PNGase by comparing the presentation of the spliced peptide between cells expressing WT tyrosinase and cells expressing a tyrosinase variant in which Asn at positions 337 and 371 was replaced by Asp. In both cases, transfected cells efficiently presented the antigenic peptide. However, we observed that inhibition of PNGase activity in cells expressing WT tyrosinase decreased the presentation of the antigen, whereas no effect was observed in cells expressing the tyrosinase variant with the Asp residues. This observation confirmed that PNGase was responsible for the conversion of these Asn residues into Asp. This does not exclude, however, the existence of a second pathway, which would be responsible for deamidation of unglycosylated Asn residues present on some tyrosinase molecules, as suggested for the tyrosinase369–377 peptide (28, 47).
The presence of antigenic peptide IYMDGTADFSF under three different forms in the melanoma eluates was unexpected. These forms corresponded to three oxidation states of the methionine: a nonoxidized, a sulfoxide, and a sulfone form (Fig. 2_B_). We wondered whether the oxidized forms of the spliced peptide were naturally produced in melanoma cells or were produced in vitro during the process of peptide elution. We observed that synthetic peptide IYMDGTADFSF spontaneously oxidized in vitro into the sulfoxide form. This oxidation is favored by drying. It was, therefore, not possible to determine whether the cells naturally presented the sulfoxide form of the peptide. Concerning the sulfone form of the peptide, we were surprised to detect its presence in the eluates, because such a form was only obtained in vitro after treatment of the synthetic peptide with strong oxidative conditions. Additionally, intracellular proteins or peptides containing methionine sulfone were never described in the literature to our knowledge. To determine the origin of the sulfone form of the natural peptide, we repeated the experiment of HLA-peptide complexes extraction using HLA-A24+ EBV B cells exogenously loaded with a preparation of synthetic peptide IYMDGTADFSF containing the nonoxidized form with small amounts of the sulfoxide form. The extract was separated on an HPLC column, and the resulting fractions were tested in a CTL assay. Despite the absence of the sulfone form in the initial peptide preparation, three peaks of CTL activity were detected, corresponding to the three forms of the peptide. This finding indicated that the sulfone form of the peptide could be produced during the elution process. The factor responsible for this oxidation is unclear. One candidate is sodium azide, which was present in our lysis buffer and might cause oxidation of cysteine and methionine residues (48). In conclusion, although showing that oxidation of the peptide can occur during the elution process, our results do not exclude the production of oxidized forms of the antigenic peptide by melanoma cells.
The direct role of the proteasome in the production of spliced peptide IYMDGTADFSF was confirmed by in vitro experiments in which a precursor peptide was digested with purified 20S proteasomes. Standard proteasomes were able to produce the antigenic peptide IYMDGTADFSF, whereas immunoproteasomes were not. Such differences between the two proteasome types to produce spliced peptides were already observed for the three known spliced peptides and resulted from a different capacity to produce the peptidic partners of the splicing reaction (39).
The catalytic mechanism of the splicing reaction seems identical to that proposed for the other spliced peptides, involving a transpeptidation at the active N-terminal threonine of a catalytic β-subunit of the proteasome (Fig. 3_D_). Fragment IYMDGT is involved in the formation of an acyl-enzyme intermediate linking, by an ester bond, the C terminus of this fragment to the hydroxyl group of the side chain of an active threonine of the proteasome. During proteolysis, this intermediate is hydrolyzed, and fragment IYMDGT is liberated. In the splicing reaction, the N-terminal amino group of fragment ADFSF competes with water molecules and performs the nucleophilic attack on the acyl-enzyme intermediate, leading to the creation of a new peptide bond and the production of spliced peptide IYMDGTADFSF. This transpeptidation model was validated by incubating various peptide pairs with purified proteasomes (Fig. 3_E_). Nevertheless, we also observed a weak activation of the CTL in response to digests obtained by incubating proteasomes with ADFSF and IYMDGT. According to the transpeptidation model, such digests should not contain the spliced antigenic peptide and activate the CTL, because the appropriate acyl-enzyme intermediate cannot form. The weak activation of CTL CL62 did not result from a transpeptidation producing the shorter spliced peptides IYMDGADFSF or IYMDADFSF, because they are not recognized by CTL CL62 (Fig. S1). If the tyrosinase spliced peptide is indeed produced in this digest, this production can only occur by a condensation reaction between ADFSF and IYMDGT within the proteasome, because the incubation of these two fragments in the absence of proteasomes did not lead to any detectable CTL activation (Fig. 3_E_). The mechanism of this reaction would involve, therefore, the interaction of peptide fragments with the proteasome independently of proteolytic cleavage. A comparable phenomenon was described by studying the incorporation of 18O into peptide fragments after protease digestion, a technique commonly used for protein sequencing and quantitative proteomic studies (49, 50). In these experiments, proteins were digested with endoproteases like trypsin in highly 18O-enriched water, and because proteolysis proceeds through the incorporation of oxygen from water into the newly generated C-terminal carboxyl group of peptide fragments, proteolytic cleavage could be used to generate a set of 18O-labeled peptides. However, in addition to incorporating one 18O atom, peptide fragments were found to incorporate two atoms of 18O in their C-terminal carboxyl group. This observation indicated that peptide fragments must continue to interact with the enzyme and can undergo repeated binding/hydrolysis cycles caused by the reversibility of the reaction liberating the acyl-enzyme intermediate. In our case, this would imply that fragment IYMDGT is accepted as a pseudosubstrate by the proteasome to form a covalent ester intermediate with the activated hydroxyl group of a catalytic threonine. This intermediate would be further hydrolyzed to give back fragment IYMDGT or sometimes, aminolyzed by the N-terminal group of fragment ADFSF to give rise to the spliced peptide IYMDGTADFSF. If such a mechanism of reverse proteolysis really occurred in our in vitro experiment, it would contribute to a minor fraction of the spliced peptide produced (Fig. 3_E_). Remarkably, this condensation reaction seemed to also take place in living cells, because we observed that electroporation of the splicing partners ADFSF and IYMDGT triggered CTL activation, although about six times less than electroporation of fragments ADFSF and IYMDGTMSQ.
Only four spliced peptides were identified to date as antigenic peptides recognized by specific T cells. Many other spliced peptides probably remain to be discovered, maybe with other relevant functions. Supporting this possibility, a recent study analyzing MS data of in vitro proteasomal products with an algorithm that predicts all theoretically possible spliced peptides from a given polypeptide revealed that the proteasome produces a substantial number of spliced peptides (51). Therefore, despite the intrinsically low efficiency of the splicing reaction, it seems that the splicing activity of the proteasome is more important than initially thought and is presumably facilitated by the catalytic and structural properties of this enzyme, as we previously suggested. Liepe et al. (51) proposed that peptide splicing is “an intrinsic additional catalytic property of the proteasome.” We have to keep in mind, however, that all proteases are theoretically able to catalyze peptide ligation. Peptide splicing has already been described in plants and was found, in some cases, to be catalyzed by vacuolar asparaginyl endopeptidase (52, 53). Because this protease is involved in the production of antigenic peptides presented by MHC class II molecules (54), future studies may reveal the existence of spliced antigenic peptides that are not produced by the proteasome.
Materials and Methods
Isolation and Fractionation of MHC-Bound Peptides Present on Melanoma Cells.
MHC class I peptide complexes were immunopurified using monoclonal antibody W6/32 after lysis of LB33-MEL cells. After elution with acetic acid (pH 2.6), peptides were also fractionated by HPLC after injection on a C18 column. Fractions were collected, dried, and resuspended in water. Each fraction was diluted in X-vivo 10 medium and loaded onto 20,000 HLA-A24+ EBV B cells before addition of 15,000 CTL CL62 for the stimulation assay. More details are provided in SI Materials and Methods.
Supplementary Material
Supporting Information
Acknowledgments
We thank Luc Pilotte and Aline Depasse for excellent technical assistance and Julie Klein for editorial assistance. This work was supported by the European Community under Sixth Framework Program Grant LSHC-2006-518234 (Cancer Immunotherapy), the Walloon Region Programme d'Excellence CIBLES, the Fonds J. Maisin (Belgium), the Fondation contre le Cancer (Belgium), and the Fonds National de la Recherche Scientifique (FNRS; Belgium). A.D. is supported by a Télévie fellowship from the FNRS, and N.V. is a post-doctoral researcher with the FNRS.
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 11741.
This article is a Direct Submission. J.W.Y. is a guest editor invited by the Editorial Board.
References
- 1.Heidecker L, et al. Cytolytic T lymphocytes raised against a human bladder carcinoma recognize an antigen encoded by gene MAGE-A12. J Immunol. 2000;164:6041–6045. doi: 10.4049/jimmunol.164.11.6041. [DOI] [PubMed] [Google Scholar]
- 2.Ma W, et al. Two new tumor-specific antigenic peptides encoded by gene MAGE-C2 and presented to cytolytic T lymphocytes by HLA-A2. Int J Cancer. 2004;109:698–702. doi: 10.1002/ijc.20038. [DOI] [PubMed] [Google Scholar]
- 3.van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 4.Gaugler B, et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker ABH, et al. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brichard V, et al. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami Y, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulie PG, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rock KL, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 10.Bacik I, et al. Introduction of a glycosylation site into a secreted protein provides evidence for an alternative antigen processing pathway: Transport of precursors of major histocompatibility complex class I-restricted peptides from the endoplasmic reticulum to the cytosol. J Exp Med. 1997;186:479–487. doi: 10.1084/jem.186.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosse CA, et al. The class I antigen-processing pathway for the membrane protein tyrosinase involves translation in the endoplasmic reticulum and processing in the cytosol. J Exp Med. 1998;187:37–48. doi: 10.1084/jem.187.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulie PG, et al. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc Natl Acad Sci USA. 1995;92:7976–7980. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilloux Y, et al. A peptide recognized by human cytolytic T lymphocytes on HLA-A2 melanomas is encoded by an intron sequence of the N-acetylglucosaminyltransferase V gene. J Exp Med. 1996;183:1173–1183. doi: 10.1084/jem.183.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins PF, et al. The intronic region of an incompletely spliced gp100 gene transcript encodes an epitope recognized by melanoma-reactive tumor-infiltrating lymphocytes. J Immunol. 1997;159:303–308. [PubMed] [Google Scholar]
- 15.Van den Eynde BJ, et al. A new antigen recognized by cytolytic T lymphocytes on a human kidney tumor results from reverse strand transcription. J Exp Med. 1999;190:1793–1800. doi: 10.1084/jem.190.12.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Probst-Kepper M, et al. An alternative open reading frame of the human macrophage colony-stimulating factor gene is independently translated and codes for an antigenic peptide of 14 amino acids recognized by tumor-infiltrating CD8 T lymphocytes. J Exp Med. 2001;193:1189–1198. doi: 10.1084/jem.193.10.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R-F, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malarkannan S, Horng T, Shih PP, Schwab S, Shastri N. Presentation of out-of-frame peptide/MHC class I complexes by a novel translation initiation mechanism. Immunity. 1999;10:681–690. doi: 10.1016/s1074-7613(00)80067-9. [DOI] [PubMed] [Google Scholar]
- 19.Ronsin C, et al. A non-AUG-defined alternative open reading frame of the intestinal carboxyl esterase mRNA generates an epitope recognized by renal cell carcinoma-reactive tumor-infiltrating lymphocytes in situ. J Immunol. 1999;163:483–490. [PubMed] [Google Scholar]
- 20.Ferris RL, et al. Processing of HIV-1 envelope glycoprotein for class I-restricted recognition: Dependence on TAP1/2 and mechanisms for cytosolic localization. J Immunol. 1999;162:1324–1332. [PubMed] [Google Scholar]
- 21.Selby M, et al. Hepatitis C virus envelope glycoprotein E1 originates in the endoplasmic reticulum and requires cytoplasmic processing for presentation by class I MHC molecules. J Immunol. 1999;162:669–676. [PubMed] [Google Scholar]
- 22.Skipper JCA, et al. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarling AL, et al. Phosphorylated peptides are naturally processed and presented by major histocompatibility complex class I molecules in vivo. J Exp Med. 2000;192:1755–1762. doi: 10.1084/jem.192.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarling AL, et al. Identification of class I MHC-associated phosphopeptides as targets for cancer immunotherapy. Proc Natl Acad Sci USA. 2006;103:14889–14894. doi: 10.1073/pnas.0604045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanada K, Yewdell JW, Yang JC. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature. 2004;427:252–256. doi: 10.1038/nature02240. [DOI] [PubMed] [Google Scholar]
- 26.Vigneron N, et al. An antigenic peptide produced by peptide splicing in the proteasome. Science. 2004;304:587–590. doi: 10.1126/science.1095522. [DOI] [PubMed] [Google Scholar]
- 27.Warren EH, et al. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science. 2006;313:1444–1447. doi: 10.1126/science.1130660. [DOI] [PubMed] [Google Scholar]
- 28.Altrich-VanLith ML, et al. Processing of a class I-restricted epitope from tyrosinase requires peptide N-glycanase and the cooperative action of endoplasmic reticulum aminopeptidase 1 and cytosolic proteases. J Immunol. 2006;177:5440–5450. doi: 10.4049/jimmunol.177.8.5440. [DOI] [PubMed] [Google Scholar]
- 29.Dalet A, Vigneron N, Stroobant V, Hanada K, Van den Eynde BJ. Splicing of distant peptide fragments occurs in the proteasome by transpeptidation and produces the spliced antigenic peptide derived from fibroblast growth factor-5. J Immunol. 2010;184:3016–3024. doi: 10.4049/jimmunol.0901277. [DOI] [PubMed] [Google Scholar]
- 30.Robbins PF, et al. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994;54:3124–3126. [PubMed] [Google Scholar]
- 31.Kang X, et al. Identification of a tyrosinase epitope recognized by HLA-A24-restricted, tumor-infiltrating lymphocytes. J Immunol. 1995;155:1343–1348. [PubMed] [Google Scholar]
- 32.Kubo RT, et al. Definition of specific peptide motifs for four major HLA-A alleles. J Immunol. 1994;152:3913–3924. [PubMed] [Google Scholar]
- 33.Maier R, et al. Peptide motifs of HLA-A3, -A24, and -B7 molecules as determined by pool sequencing. Immunogenetics. 1994;40:306–308. doi: 10.1007/BF00189978. [DOI] [PubMed] [Google Scholar]
- 34.Wölfel T, et al. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 35.Wölfel C, et al. Transporter (TAP)- and proteasome-independent presentation of a melanoma-associated tyrosinase epitope. Int J Cancer. 2000;88:432–438. [PubMed] [Google Scholar]
- 36.Akiyama K, et al. Replacement of proteasome subunits X and Y by LMP7 and LMP2 induced by interferon-gamma for acquirement of the functional diversity responsible for antigen processing. FEBS Lett. 1994;343:85–88. doi: 10.1016/0014-5793(94)80612-8. [DOI] [PubMed] [Google Scholar]
- 37.Groettrup M, et al. A third interferon-gamma-induced subunit exchange in the 20S proteasome. Eur J Immunol. 1996;26:863–869. doi: 10.1002/eji.1830260421. [DOI] [PubMed] [Google Scholar]
- 38.Chapiro J, et al. Destructive cleavage of antigenic peptides either by the immunoproteasome or by the standard proteasome results in differential antigen presentation. J Immunol. 2006;176:1053–1061. doi: 10.4049/jimmunol.176.2.1053. [DOI] [PubMed] [Google Scholar]
- 39.Dalet A, Stroobant V, Vigneron N, Van den Eynde BJ. Differences in the production of spliced antigenic peptides by the standard proteasome and the immunoproteasome. Eur J Immunol. 2011;41:39–46. doi: 10.1002/eji.201040750. [DOI] [PubMed] [Google Scholar]
- 40.Misaghi S, Pacold ME, Blom D, Ploegh HL, Korbel GA. Using a small molecule inhibitor of peptide: N-glycanase to probe its role in glycoprotein turnover. Chem Biol. 2004;11:1677–1687. doi: 10.1016/j.chembiol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 41.York IA, et al. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 42.Jimbow K, et al. Assembly, target-signaling and intracellular transport of tyrosinase gene family proteins in the initial stage of melanosome biogenesis. Pigment Cell Res. 2000;13:222–229. doi: 10.1034/j.1600-0749.2000.130403.x. [DOI] [PubMed] [Google Scholar]
- 43.Halaban R, et al. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J Biol Chem. 2002;277:14821–14828. doi: 10.1074/jbc.M111497200. [DOI] [PubMed] [Google Scholar]
- 44.Popescu CI, Paduraru C, Dwek RA, Petrescu SM. Soluble tyrosinase is an endoplasmic reticulum (ER)-associated degradation substrate retained in the ER by calreticulin and BiP/GRP78 and not calnexin. J Biol Chem. 2005;280:13833–13840. doi: 10.1074/jbc.M413087200. [DOI] [PubMed] [Google Scholar]
- 45.Svedine S, Wang T, Halaban R, Hebert DN. Carbohydrates act as sorting determinants in ER-associated degradation of tyrosinase. J Cell Sci. 2004;117:2937–2949. doi: 10.1242/jcs.01154. [DOI] [PubMed] [Google Scholar]
- 46.Wang N, Daniels R, Hebert DN. The cotranslational maturation of the type I membrane glycoprotein tyrosinase: The heat shock protein 70 system hands off to the lectin-based chaperone system. Mol Biol Cell. 2005;16:3740–3752. doi: 10.1091/mbc.E05-05-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostankovitch M, Altrich-Vanlith M, Robila V, Engelhard VH. N-glycosylation enhances presentation of a MHC class I-restricted epitope from tyrosinase. J Immunol. 2009;182:4830–4835. doi: 10.4049/jimmunol.0802902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manneberg M, Lahm HW, Fountoulakis M. Oxidation of cysteine and methionine residues during acid hydrolysis of proteins in the presence of sodium azide. Anal Biochem. 1995;224:122–127. doi: 10.1006/abio.1995.1016. [DOI] [PubMed] [Google Scholar]
- 49.Angel PM, Orlando R. Trypsin is the primary mechanism by which the (18)O isotopic label is lost in quantitative proteomic studies. Anal Biochem. 2006;359:26–34. doi: 10.1016/j.ab.2006.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnölzer M, Jedrzejewski P, Lehmann WD. Protease-catalyzed incorporation of 18O into peptide fragments and its application for protein sequencing by electrospray and matrix-assisted laser desorption/ionization mass spectrometry. Electrophoresis. 1996;17:945–953. doi: 10.1002/elps.1150170517. [DOI] [PubMed] [Google Scholar]
- 51.Liepe J, et al. The 20S proteasome splicing activity discovered by SpliceMet. PLoS Comput Biol. 2010;6:e1000830. doi: 10.1371/journal.pcbi.1000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillon AD, et al. Biosynthesis of circular proteins in plants. Plant J. 2008;53:505–515. doi: 10.1111/j.1365-313X.2007.03357.x. [DOI] [PubMed] [Google Scholar]
- 53.Min W, Jones DH. In vitro splicing of concanavalin A is catalyzed by asparaginyl endopeptidase. Nat Struct Biol. 1994;1:502–504. doi: 10.1038/nsb0894-502. [DOI] [PubMed] [Google Scholar]
- 54.Watts C, et al. Creation versus destruction of T cell epitopes in the class II MHC pathway. Ann N Y Acad Sci. 2003;987:9–14. doi: 10.1111/j.1749-6632.2003.tb06028.x. [DOI] [PubMed] [Google Scholar]
- 55.Lagerwerf FM, van de Weert M, Heerma W, Haverkamp J. Identification of oxidized methionine in peptides. Rapid Commun Mass Spectrom. 1996;10:1905–1910. doi: 10.1002/(SICI)1097-0231(199612)10:15<1905::AID-RCM755>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11741–11742.
Author Summary
Cytolytic T lymphocytes (CTL) are a class of immune cells that kill diseased cells such as cancerous or virus-infected cells. To do so, they must recognize peptide fragments produced when viral or cancer-related proteins located inside the cell are broken down by the proteasome, a catalytic complex responsible for the bulk of protein degradation in the cytosol of animal cells. The peptide fragments are presented at the surface of diseased cells by a different group of immune proteins called MHC class I molecules. Peptides recognized by CTL usually consist of fragments of 8 to 11 amino acids that are transported into the endoplasmic reticulum (ER), an organelle surrounding the nucleus, by a protein dubbed “transporter associated with antigen processing” (TAP). Once inside the ER, the peptides are loaded onto MHC class I molecules. Stable peptide/MHC complexes can then exit the ER and reach the cell surface, where they can be recognized by surrounding lymphocytes. Most antigenic peptides consist of a fragment of the parental protein. However, some antigenic peptides undergo modifications resulting in a change of their sequence. The extent of such modifications and their significance remain unclear. Here, we describe a highly unexpected peptide, which combines two complex modifications. One is the splicing of two peptide fragments that are not contiguous in the parental protein. The other is a conversion of asparagine amino acid residues into aspartic acid residues.
Three antigenic peptides produced by peptide splicing in the proteasome have been described so far, and the biochemical mechanism of splicing has been detailed (1–4). In the course of protein degradation by the proteasome, each peptide fragment resulting from peptide bond cleavage transiently forms an intermediate in which the peptide fragment remains attached to the proteasome catalytic subunit by an ester bond. Usually, this intermediate is rapidly hydrolyzed by surrounding water molecules. However, during peptide splicing, the amino terminus of other peptide fragments present in the catalytic chamber competes with water molecules and occasionally leads to the formation of a new peptide bond that links the two fragments. This process is called transpeptidation. Interestingly, the noncontiguous peptide fragments can be assembled either in the same or reverse order to that in which they appear in the parental protein (3).
Three antigenic peptides have been described where an asparagine residue was converted into an aspartic acid. This conversion was analyzed in detail for one of the peptides, and it was shown to occur through the removal of the amide group of the side chain of the asparagine (5). As a result, the peptide contains an aspartic acid (D in the one-letter amino acid code) at position 3 of its sequence, which reads YMDGTMSQV, whereas the sequence of the parental protein has an asparagine at this position (YMNGTMSQV). This asparagine is a site where sugar moieties are normally added to some cellular proteins, a process known as _N_-glycosylation. Deamidation of the asparagine of the peptide was found to result from the removal of the attached sugar moiety before the breakdown of the protein by the proteasome. This deglycosylation is performed by an enzyme called peptide-_N_-glycanase (PNGase), which removes not only the sugar moiety but also the nitrogen of the side chain of the asparagine where the sugar was attached, thereby converting the amide into a carboxylate group. Hence, the asparagine becomes an aspartic acid (5).
In the present study, we describe an antigenic peptide that combines these two posttranslational modifications. It is recognized by CD8+ cytotoxic T lymphocytes isolated from a human melanoma, a potentially fatal skin cancer. The parental protein, the enzyme tyrosinase, is responsible for the production of melanin in pigmented cells. The peptide, with the sequence IYMDGTADFSF, is made up of the fragments ADFSF and IYMDGT, which are separated by 27 amino acids in the parental protein. In the peptide, these fragments are spliced in the reverse order to that in which they appear in the tyrosinase protein. In addition, the peptide IYMDGTADFSF contains, in positions 4 and 8, two aspartate residues resulting from deamidation of the asparagines encoded by the parent gene. CTL assays and tandem mass spectrometry analyses on peptides eluted from the surface of melanoma cells confirmed that the peptide IYMDGTADFSF was naturally presented by antigen-presenting MHC molecules on melanoma cells.
Using a proteasome inhibitor, we showed that the proteasome was required for the production of the peptide IYMDGTADFSF. Purified proteasomes incubated with an extended precursor peptide containing the two aspartates were able to produce the spliced peptide in vitro. The splicing reaction producing the reordered peptide IYMDGTADFSF seemed to occur by transpeptidation, as in the previous examples of spliced peptides (2–4).
Chemical inhibition of PNGase impaired the presentation of the spliced peptide in both melanoma cells and transfected cells expressing the wild-type tyrosinase but not in cells expressing a modified tyrosinase containing aspartic residues instead of asparagines at the relevant positions. This result confirmed that PNGase was responsible for the conversion of the encoded asparagine residues into aspartates, presumably through removal of the sugar moieties attached to these _N_-glycosylation sites. Because PNGase is located in the cytosol and protein _N_-glycosylation occurs in the ER, the parental protein seems to be retrotranslocated from the ER to the cytosol before removal of the sugar moieties. Such retrotranslocation is known as a critical step of ER-associated degradation (ERAD), a process allowing proteasomal degradation in the cytosol of misfolded proteins located in the ER.
Presentation of the spliced antigenic peptide was also impaired by blocking the activity of TAP, the transporter channeling the antigenic peptides back to the ER for assembly with MHC molecules. This result further confirmed the production of this peptide in the cytosol by PNGase and the proteasome, followed by its transport into the ER by the TAP transporter.
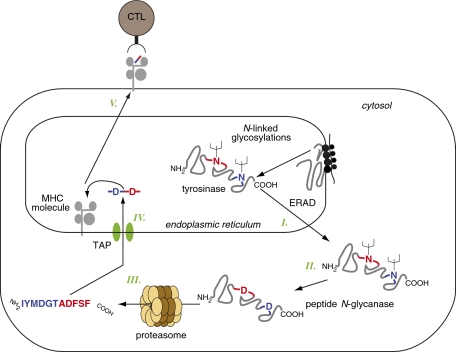
Together, our findings indicate that the processing of this unusual antigenic peptide requires the translation and the glycosylation of the parental protein in the ER followed by its retrotranslocation into the cytosol, where PNGase converts the two glycosylated asparagine residues into aspartates (Fig. P1). This process is followed by cleavage and reverse splicing of the appropriate fragments by the proteasome, and additional transport of the resulting peptide into the ER through the TAP transporter.
Fig. P1.
Production of the deamidated reverse-spliced antigenic peptide derived from tyrosinase. Misfolded _N_-glycosylated tyrosinase proteins are retrotranslocated from the ER into the cytosol (I), where peptide _N_-glycanase converts two asparagines into aspartates upon removal of the asparagine-associated sugars (II). This process is followed by cleavage and reverse splicing of the deamidated protein by the proteasome (III), leading to production of the final antigenic peptide IYMDGTADFSF, which is then transported back into the ER by the TAP transporter (IV). The peptide/MHC complex then migrates to the cell surface where it can be recognized by the CTL (V). ERAD, ER-associated degradation.
The description of this antigenic peptide and the characterization of its mode of production expand our knowledge on modified antigenic peptides. In particular, this fourth example of spliced peptide suggests that spliced peptides are not exceptional and may represent a significant part of the repertoire of antigenic peptides presented to T lymphocytes by antigen-presenting molecules. Moreover, the relevance of such modified peptides is strengthened by the fact that the peptide described here is the target of T lymphocytes whose infusion into a melanoma patient was followed by tumor regression, indicating that this spliced peptide is a relevant target of antitumor immune responses and might be useful for cancer immunotherapy.
Footnotes
The authors declare no conflict of interest.
This article is a Direct Submission.
See full research article on page E323 of www.pnas.org.
References
- 1.Hanada K, Yewdell JW, Yang JC. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature. 2004;427:252–256. doi: 10.1038/nature02240. [DOI] [PubMed] [Google Scholar]
- 2.Vigneron N, et al. An antigenic peptide produced by peptide splicing in the proteasome. Science. 2004;304:587–590. doi: 10.1126/science.1095522. [DOI] [PubMed] [Google Scholar]
- 3.Warren EH, et al. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science. 2006;313:1444–1447. doi: 10.1126/science.1130660. [DOI] [PubMed] [Google Scholar]
- 4.Dalet A, Vigneron N, Stroobant V, Hanada K, Van den Eynde BJ. Splicing of distant peptide fragments occurs in the proteasome by transpeptidation and produces the spliced antigenic peptide derived from fibroblast growth factor-5. J Immunol. 2010;184:3016–3024. doi: 10.4049/jimmunol.0901277. [DOI] [PubMed] [Google Scholar]
- 5.Altrich-VanLith ML, et al. Processing of a class I-restricted epitope from tyrosinase requires peptide N-glycanase and the cooperative action of endoplasmic reticulum aminopeptidase 1 and cytosolic proteases. J Immunol. 2006;177:5440–5450. doi: 10.4049/jimmunol.177.8.5440. [DOI] [PubMed] [Google Scholar]
Supplementary Materials
Supporting Information