Incidence of Type 1 Diabetes in Sweden Among Individuals Aged 0–34 Years, 1983–2007: An analysis of time trends (original) (raw)
Abstract
OBJECTIVE
To clarify whether the increase in childhood type 1 diabetes is mirrored by a decrease in older age-groups, resulting in younger age at diagnosis.
RESEARCH DESIGN AND METHODS
We used data from two prospective research registers, the Swedish Childhood Diabetes Register, which included case subjects aged 0–14.9 years at diagnosis, and the Diabetes in Sweden Study, which included case subjects aged 15–34.9 years at diagnosis, covering birth cohorts between 1948 and 2007. The total database included 20,249 individuals with diabetes diagnosed between 1983 and 2007. Incidence rates over time were analyzed using Poisson regression models.
RESULTS
The overall yearly incidence rose to a peak of 42.3 per 100,000 person-years in male subjects aged 10–14 years and to a peak of 37.1 per 100,000 person-years in female subjects aged 5–9 years and decreased thereafter. There was a significant increase by calendar year in both sexes in the three age-groups <15 years; however, there were significant decreases in the older age-groups (25- to 29-years and 30- to 34-years age-groups). Poisson regression analyses showed that a cohort effect seemed to dominate over a time-period effect.
CONCLUSIONS
Twenty-five years of prospective nationwide incidence registration demonstrates a clear shift to younger age at onset rather than a uniform increase in incidence rates across all age-groups. The dominance of cohort effects over period effects suggests that exposures affecting young children may be responsible for the increasing incidence in the younger age-groups.
Over the past decades, childhood-onset type 1 diabetes has been reported to be increasing in most countries in the world (1). In Europe, an overall annual increase of up to 3.9% was reported, with a steeper rate of increase among children aged <5 years (2). In Sweden, childhood-onset diabetes incidence is, next to Finland, the highest reported in the world, and the increase has been steep and accelerating since the early 1990s (3). Such rapid increases in a chronic disease with potentially devastating late complications constitute a challenge for both pediatric and adult medical care. When comparing time trends among childhood-diagnosed cases and cases diagnosed between the ages of 15 and 34 years in Sweden between 1983 and 1998, no clear increase was notable in age-groups >15 years (4), and a recent report (5) covering this age-group indicated rather a decreasing trend of type 1 diabetes among young adults. Similar indications have been reported from small cohorts in Antwerp, Belgium (6), and in Yorkshire, U.K. (7). It has been speculated that the shift to younger age at onset is caused by risk factors accelerating an on-going β-cell destructive process and that even children with lower levels of genetic risk who are exposed to such factors are now progressing to clinical disease (8–10).
A number of population-based case-control studies have indicated that perinatal exposure to viral infections, blood-group incompatibility or its treatment, caesarean delivery, maternal age, parity, and preeclampsia are associated with childhood type 1 diabetes (11,12). If such factors impart a substantial increase in risk and vary over time, then birth cohort might be expected to be detectable.
This report describes the analysis of an exceptionally large nationwide dataset of prospectively registered cases over a 25-year period and covers a wide age range of up to 35 years. The aim of the analysis is to clarify whether the increase in childhood type 1 diabetes is mirrored by a decrease in older age-groups, suggesting that autoimmune type 1 diabetes has shifted to younger age at diagnosis. We also wanted to test whether cohort effects dominated over calendar-period effects, which would be consistent with the pattern of change being associated with exposures specifically affecting young children.
RESEARCH DESIGN AND METHODS
The study was conducted according to the guidelines of the Helsinki Declaration. Parents or patients gave individual informed consent to be registered, and the research registers were approved by the Swedish Data Inspection Board and the regional research ethics committees (Karolinska Institute, Stockholm and Umeå University, respectively).
This study merged data from two nationwide research registers: the Swedish Childhood Diabetes register, which was started on 1 July 1977, and the Diabetes Incidence in Sweden Study (DISS), which started in 1983.
The Swedish Childhood Diabetes Register registers almost all incident cases of diabetes in age-groups 0–14.9 years in Sweden because all pediatric clinics participate and the Swedish health care system requires that all children aged <15 years with suspected diabetes are referred to pediatric departments. The cohort was restricted to subjects with a care-provider report of type 1 diabetes. The registration organization and format has been run using similar methods over the years, and ascertainment completeness using a secondary source has been shown to vary between 96 and 99% (13,14). During 2 years (1999 and 2000), three pediatric hospitals did not deliver data prospectively, but this has been adjusted afterward. In addition to the yearly internal validation procedures, as previously described (3), and the studies using external sources for validation, we have, since 2003, instituted a continuous validation with another source (i.e., the Swedish Quality Assessment Register for Childhood Diabetes) that covers the 0- to 18-year age-group. Children with type 1 diabetes are registered, and the onset date is the date of first insulin injection, normally the day of diagnosis and only exceptionally beyond the first week after diagnosis.
The DISS register covers incident cases in the age range of 15–34 years and collects data in collaboration with a contact person (a reporting physician and/or a diabetes nurse) at all (currently 120) departments of internal medicine, endocrinology, and pediatrics in Sweden. The classification into type 1, type 2, and unclassified diabetes in the DISS is based on the treating doctors’ clinical classification. During 1983–1991, the World Health Organization (WHO) classification was used, and since 1992 the American Diabetes Association (ADA) classification criteria were used. The reporting physicians annually receive a list of patients registered since the start of the study and a newsletter about the study’s progress. Using a computer-based patient administrative register as a secondary source, the level of ascertainment of type 1 diabetes during 1983–1987 was estimated as 91% in Västerbotten County in northern Sweden, covering 2.9% of the population, with no trend in ascertainment level during 1986–1997. The median level of ascertainment for type 1 diabetes for six hospitals, using the software DIABASE for their patients, was 82%. In the two southernmost counties, covering 9.2% of the population, the ascertainment rate was 86% and did not change during the study period (15).We have performed sensitivity analyses assuming a reduced ascertainment of 85% in all the older age-groups, and this did not affect the cohort effects shown in the results.
Statistical analysis
Age-specific incidence rates were calculated using population figures from Statistics Sweden. Poisson regression models were used to estimate rates of increase by calendar year. An age-period-cohort analysis using 5-year intervals was applied to the data, with a drift term used to describe linear trends that could well be equally ascribed to either period or cohort (16). Akaike information criterion and likelihood ratio tests were used to identify the best-fitting model.
RESULTS
Incidence rates by age and sex
Altogether, 12,880 (6,768 male and 6,112 female) cases in children aged <15 years and 7,369 (4,799 male and 2,570 female) cases in young adults aged 15–34 years were included in the analyses. The age-specific incidence rates per 100,000 person-years in 5-year intervals for both sexes and the cumulative incidence rates to age 35 years are shown in Table 1. The cumulative incidence rates increased for both male and female subjects during the 25-year period. Table 1 also shows that there is a consistent peak in incidence between the ages of 10 and 14 years in male subjects, but in female subjects the peak incidence was decreased, from aged 10 to 14 years at the start of the study period to aged 5 to 9 years at the end. For both sexes, there is a decrease after puberty, with a sustained higher rate in male relative to female subjects.
Table 1.
Age-specific incidence rates and cumulative incidence rate at the age of 35 years per 100,000 person-years by sex in 5-year periods during 1983–2007
| Age (years) | Period | Overall | ||||
|---|---|---|---|---|---|---|
| 1983–1987 | 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | ||
| Male subjects | ||||||
| 0–4 | 17.6 | 16.7 | 22.8 | 27.4 | 28.1 | 22.4 |
| 217 | 241 | 335 | 328 | 360 | 1,481 | |
| 5–9 | 28.1 | 28.6 | 31.2 | 39.8 | 46.4 | 34.8 |
| 352 | 361 | 462 | 590 | 570 | 2,335 | |
| 10–14 | 32.0 | 37.1 | 38.7 | 42.3 | 59.2 | 42.3 |
| 448 | 473 | 502 | 635 | 894 | 2,952 | |
| 15–19 | 20.2 | 20.0 | 17.1 | 22.2 | 20.4 | 20.0 |
| 301 | 285 | 223 | 293 | 312 | 1,414 | |
| 20–24 | 18.2 | 15.1 | 15.7 | 18.3 | 17.4 | 16.9 |
| 273 | 232 | 229 | 243 | 238 | 1,215 | |
| 25–29 | 16.2 | 18.0 | 14.4 | 15.8 | 14.6 | 15.8 |
| 232 | 283 | 229 | 235 | 204 | 1,183 | |
| 30–34 | 15.0 | 13.9 | 12.9 | 11.5 | 10.6 | 12.8 |
| 224 | 206 | 208 | 185 | 164 | 987 | |
| 35 | 736.9 | 747.1 | 763.7 | 886.1 | 983.7 | |
| Female subjects | ||||||
| 0–4 | 17.2 | 14.9 | 22.4 | 24.3 | 25.0 | 20.6 |
| 201 | 204 | 313 | 276 | 303 | 1,297 | |
| 5–9 | 28.8 | 27.6 | 35.9 | 40.0 | 53.3 | 37.1 |
| 343 | 331 | 504 | 564 | 621 | 2,363 | |
| 10–14 | 33.1 | 31.0 | 33.9 | 38.5 | 46.6 | 37.0 |
| 442 | 376 | 417 | 547 | 670 | 2,452 | |
| 15–19 | 12.4 | 12.3 | 11.9 | 11.9 | 12.4 | 12.2 |
| 176 | 166 | 148 | 149 | 180 | 819 | |
| 20–24 | 9.2 | 11.1 | 9.6 | 11.3 | 9.8 | 10.2 |
| 132 | 163 | 135 | 144 | 128 | 702 | |
| 25–29 | 9.8 | 7.9 | 8.2 | 8.6 | 6.2 | 8.2 |
| 135 | 118 | 125 | 124 | 83 | 585 | |
| 30–34 | 6.9 | 6.7 | 6.1 | 6.3 | 5.4 | 6.3 |
| 99 | 94 | 93 | 97 | 81 | 464 | |
| 35 | 587.6 | 557.6 | 640.4 | 704.3 | 793.5 |
Analysis of time trend
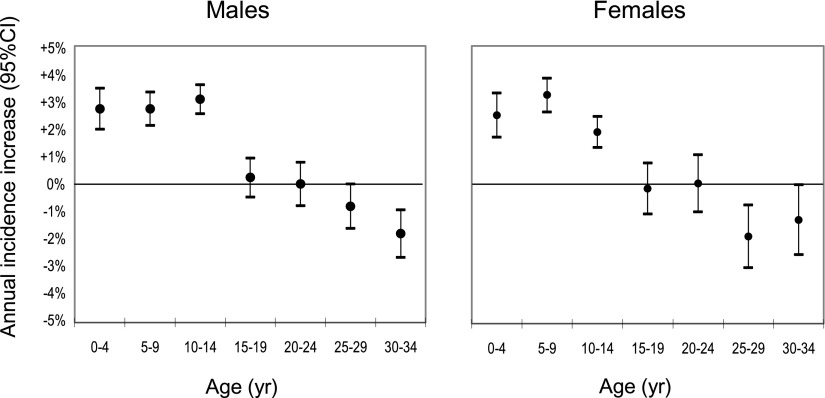
Figure 1 shows the trends in incidence of type 1 diabetes by 5-year age-group as percentage change per annum in both sexes. A clear increase in incidence is seen among the three youngest age-groups, and a decrease is seen among the two oldest age-groups. The average age at diagnosis decreased from 16.1 to 13.7 years in male subjects and from 14.1 to 10.9 years in female subjects during the 25-year period.
Figure 1.
Change in average age at diagnosis by time period and 95% CI.
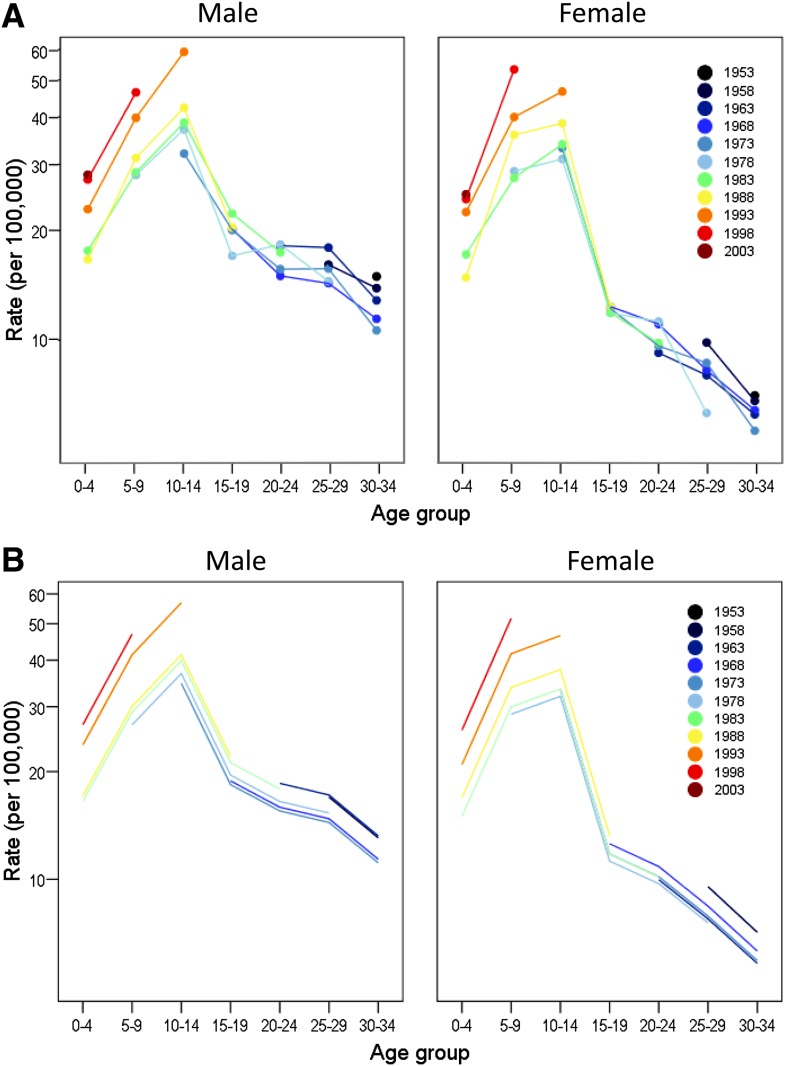
Table 2 shows the results of fitting Poisson regression models. The upper part of Table 2 describes the search for the best-fitting model (having the lowest Akaike information criterion) with P values that denote lack of fit. The lower part of Table 2 assesses the contribution of different terms when the models are tested relative to one another, as indicated. After first including age-group in the model, the analysis proceeds by next fitting the linear drift term that can well be equally ascribed to either period or cohort. Thereafter, the period and cohort effects, both of which subsume the drift effect, are added to the model. P values denote relative contributions to model fit. For both sexes, significant lack of fit is seen with the age-plus-period model (model 3) but not with the age-plus-cohort model (model 4). Cohort terms added to the age-plus-period model (model 5 vs. 3) provided a highly significant improvement in fit in both male and female subjects (P < 0.0001). In contrast, period terms added to the age-plus-cohort model (model 5 vs. 4) did not improve the fit in either male (P = 0.37) or female (P = 0.16) subjects. Akaike information criterion also achieved its minimum value in both sexes for the age-plus-cohort model. The fit of the age-plus-cohort model is illustrated in Fig. 2. Figure 2_A_ shows the observed incidence rate data and Fig. 2_B_ shows the fitted incidence rates obtained from the model. Each 5-year birth cohort is described by its midyear. Relative to the 1978 cohort, there were significantly increased rate ratios (P < 0.002) in both sexes for all four cohorts after 1983. Thus, rate ratios for the cohorts between 1988 and 2003 were 1.12, 1.54, 1.75, and 1.84, respectively, for male subjects and 1.18, 1.45, 1.80, and 1.74, respectively, for female subjects. Surprisingly, for the 1953 and 1958 cohorts, a tendency of an increased risk relative to the 1978 cohort of 1.27 (P < 0.005) and 1.11 (P = 0.10), respectively, also was found for male subjects and 1.26 (P = 0.06) and 1.28 (P = 0.01), respectively, was found for female subjects.
Table 2.
Poisson regression model fit and tests on model terms
| Model fit | Male subjects | Female subjects | ||||
|---|---|---|---|---|---|---|
| Akaike information criterion | Lack-of-fit P value | Akaike information criterion | Lack-of-fit P value | |||
| Model 1: age | 616.6 | <0.0001 | 521.8 | <0.0001 | ||
| Model 2: age + drift | 495.3 | <0.0001 | 412.0 | <0.0001 | ||
| Model 3: age + period | 482.6 | <0.0001 | 402.7 | <0.0001 | ||
| Model 4: age + cohort | 326.2 | 0.07 | 312.2 | 0.06 | ||
| Model 5: age + cohort + period | 329.1 | 0.05 | 313.1 | 0.09 | ||
| Test on model terms* | Likelihood ratio test | Likelihood ratio test | ||||
| χ2 | df | P value | χ2 | df | P value | |
| Drift | age (2 vs. 1) | 123.3 | 1 | <0.0001 | 111.8 | 1 | <0.0001 |
| Period | age, drift (3 vs. 2) | 18.7 | 3 | 0.0003 | 15.3 | 3 | 0.002 |
| Cohort | age, drift (4 vs. 2) | 187.1 | 9 | <0.0001 | 117.8 | 9 | <0.0001 |
| Period | age, cohort (5 vs. 4) | 3.1 | 3 | 0.37 | 5.2 | 3 | 0.16 |
| Cohort | age, period (5 vs. 3) | 171.5 | 9 | <0.0001 | 107.7 | 9 | <0.0001 |
Figure 2.
Observed incidence rates by 5-year birth cohort (A) and fitted incidence rates obtained from the age-cohort model (B) for male and female subjects, respectively.
CONCLUSIONS
From the population-based nationwide research registers in Sweden, including >20,000 incident cases with age at diagnosis <35 years and birth years between 1948 and 2007, it was possible to clearly show that the pattern of change over time in type 1 diabetes reflects a shift to younger age at diagnosis. In terms of cumulative incidence at age 35 years, there still is an increase over time, which possibly reflects that a shift to younger age is ongoing also among older-age-at-diagnosis groups. A study covering all age-groups would be necessary to answer the question of whether the cumulative lifetime incidence of type 1 diabetes is stable over time.
Our data provide strong support for static or declining type 1 diabetes incidence rates in adults aged ≤35 years, as reported in our previous Swedish nationwide study (4) as well as in studies from regional registers in Antwerp, Belgium (6), and Yorkshire, U.K. (7), that combined childhood-onset and young-onset cases of type 1 diabetes. In contrast, a study from the low-incidence province of Turin, Italy, found that the incidence from 1984 to 1996 increased not only in children but also in young adults (17).
A cohort effect dominating over a period effect implies that responsible exposures are operating early in life or perhaps even during the prenatal period. A cohort effect also has been reported from Finland among cohorts from 1951 to 1996 in age-groups ≤15 years (18). Likewise, a Danish study, also covering age-groups ≤15 years, showed a steep increase in age-at-onset groups <5 years, explained by an increased risk for cohorts born at the beginning of the 1980s (19). Other studies (14,20,21), including an earlier report from Sweden (14), failed to show a cohort effect using similar methods. Most likely, the different results are explained by the fact that cohort effects are more readily detected by studies of large populations over long periods covering a broad age range. In agreement with our data, a large number of studies have shown minimal differences between the sexes in incidence in those <15 years of age, whereas some European centers have shown the rate in male subjects aged >15 years to be almost twice that found in female subjects (22).
The main strengths of our study are the size, the nationwide coverage, and the 25-year period of prospective registration covering a wide range of birth cohorts and age-groups facilitating powerful analyses. Crucial for time-trend analysis is a sustained high level of ascertainment over time. In Sweden, despite some changes in hospital organization during the last decade, which provided for greater choice of hospital care, the national childhood diabetes care program requires that all recently diagnosed children be cared for at pediatric inpatient clinics, and we ascertain cases from all hospitals with pediatric departments. Ascertainment procedures show a consistently high ascertainment rate close to 100%. The variability over time is thus assessed to be a maximum of 4%, which should not affect the time-trend analysis in this age-group. For the age-group >15 years in Sweden, as in other countries (22), the ascertainment level is lower because of the more complicated and varied routines for care of newly diagnosed patients. Our sensitivity analyses suggest that modest changes in ascertainment, such as a reduction to 85% in all the adult age-groups, are unlikely to explain the cohort effects we have observed. Our time-trend analyses might still be somewhat affected by short time variability of either ascertainment or incidence rate, but this would not seriously affect our main results and the conclusions of this study. Another potential problem is that, in the adult case group, disease classification was based on the WHO clinical classification in the first years of the study period and on ADA criteria subsequently. However, the proportion of cases in the DISS classified as type 1 diabetes has not significantly changed over time (4).
The fact that the age-cohort model provided the best fit led us to present our data pictorially in Fig. 2, with cohorts individually identified, but there are some important issues to consider. Our analysis of cohort effects suggests, rather surprisingly, that some of the earliest cohorts were at moderately increased risk relative to the middle (1978) cohort. Higher ascertainment or misclassification of diabetes type in older age-groups in the early years of the registers is a likely possibility. A number of type 2 diabetic patients in the oldest age-groups in the early years of the study may thus have been registered as type 1 diabetic, but in the later years, when the ADA criteria were used and validation procedures were more strongly stressed, this tendency may have disappeared. The relationship between age, period, and cohort means that linear trends across cohorts cannot be distinguished from linear trends across periods (16). This so-called drift can well be equally ascribed to either period effects or cohort effects, but to account for the raised risk in the early cohorts would require a decreasing rather than an increasing linear trend across periods, which seems rather implausible.
What does our analysis suggest about future rates of type 1 diabetes in Sweden? Caution is always advisable in extrapolating models into the future. Extrapolation of the age-cohort model depicted in the lower half of Fig. 2 would suggest that the youngest cohorts will maintain high incidence rates throughout their lifetimes. Although we added terms specifying different linear tends over periods in each age-group, we found that these did not significantly improve on the fit of our age-cohort models. Resolution of this issue will require longer observation of the cohorts.
The explanation of the pattern of change with time is probably multifactorial but indicates association with changing lifestyle habits. It has been proposed earlier that overeating, having a high growth rate, being overweight, and having a sedentary lifestyle, all characteristics that have increased also in Sweden and that lead to an increased insulin demand, may accelerate autoimmune β-cell destruction and lead to earlier clinical diagnosis (8,10,23). Besides the striking pubertal incidence peak confirmed in this study, a high growth rate as well as increased BMI in early childhood was shown to increase the risk of childhood type 1 diabetes in a case-control study involving a number of European countries (24). Of interest, an inverse association was found between age at onset of type 1 diabetes and BMI in a U.S. study but only in children with signs of reduced β-cell function (25). Thus, the shift to a younger age at onset, as opposed to a cohort-on-cohort increase across the entire age spectrum, is compatible with the idea that exposures act by accelerating the β-cell destructive process.
In conclusion, this exceptionally large nationwide population-based study using prospectively recorded incidence data over a 25-year period confirms previous indications of a shift to a younger age at onset of type 1 diabetes with an increase in the pediatric age-group but with a decrease in the older-age-at-onset groups. A cohort effect seems to predominate over a period effect, indicating that exposures operating in early childhood may be responsible.
Supplementary Material
Supplementary Data
Acknowledgments
The Swedish Childhood Diabetes Register is funded by the Swedish Research Council Project no. 07531 and the Västerbotten County Council and the DISS by the Swedish Research Council Project no. 14531 and the Swedish Diabetes Association. The funding sources played no part in the study design; collection, analysis, and interpretation of data; writing of the manuscript; or in the decision to submit the manuscript for publication.
No potential conflicts of interest relevant to this article were reported.
G.G.D. has been the coordinator of the Swedish Childhood Diabetes Study since 1978, is responsible for data collection and validation, initiated this study, and wrote the first draft of the manuscript. L.N. is responsible for the DISS database, contributed to discussions on analysis, and commented on the manuscript. C.C.P. undertook the statistical analysis, advised on the content of the manuscript, and commented on drafts of the manuscript.
Participants in the Swedish Childhood Diabetes Study Group (SCDSG) (diabetes nurses and doctors) and DISS (doctors) are responsible for sending and correcting patient record sheets for the respective register and for contributing to the validation procedures.
Footnotes
References
- 1.Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of type I diabetes: the analysis of the data on published incidence trends. Diabetologia 1999;42:1395–1403 [Erratum in Diabetologia 1999;43:685] [DOI] [PubMed] [Google Scholar]
- 2.Patterson C, Dahlquist G, Gyürus E, Green A, Soltesz G; the EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-2020: a multicenter prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 3.Dahlquist G, Mustonen L; Swedish Childhood Diabetes Study Group. Analysis of 20 years of prospective registration of childhood onset diabetes time trends and birth cohort effects. Acta Paediatr 2000;89:1231–1237 [DOI] [PubMed] [Google Scholar]
- 4.Pundziute-Lyckå A, Dahlquist G, Nyström L, et al. ; Swedish Childhood Diabetes Study Group. The incidence of type I diabetes has not increased but shifted to a younger age at diagnosis in the 0-34 years group in Sweden 1983-1998. Diabetologia 2002;45:783–791 [DOI] [PubMed] [Google Scholar]
- 5.Östman J, Lönnberg G, Arnqvist HJ, et al. Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983-2002. J Intern Med 2008;263:386–394 [DOI] [PubMed] [Google Scholar]
- 6.Weets I, De Leeuw IH, Du Caju MV, et al. ; Belgian Diabetes Registry. The incidence of type 1 diabetes in the age group 0-39 years has not increased in Antwerp (Belgium) between 1989 and 2000: evidence for earlier disease manifestation. Diabetes Care 2002;25:840–846 [DOI] [PubMed] [Google Scholar]
- 7.Feltbower RG, McKinney PA, Parslow RC, Stephenson CR, Bodansky HJ. Type 1 diabetes in Yorkshire, UK: time trends in 0-14 and 15-29-year-olds, age at onset and age-period-cohort modelling. Diabet Med 2003;20:437–441 [DOI] [PubMed] [Google Scholar]
- 8.Dahlquist G. Environmental risk factors in human type 1 diabetes: an epidemiological perspective. Diabetes Metab Rev 1995;11:37–46 [DOI] [PubMed] [Google Scholar]
- 9.Gale EA. Spring harvest? Reflections on the rise of type 1 diabetes. Diabetologia 2005;48:2245–2250 [DOI] [PubMed] [Google Scholar]
- 10.Dahlquist G. Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia 2006;49:20–24 [DOI] [PubMed] [Google Scholar]
- 11.Vehik K, Dabelea D. The changing epidemiology of type 1 diabetes: why is it going through the roof? Diabetes Metab Res Rev 2011;27:3–13 [DOI] [PubMed] [Google Scholar]
- 12.Dahlquist GG, Patterson C, Soltesz G. Perinatal risk factors for childhood type 1 diabetes in Europe: the EURODIAB Substudy 2 Study Group. Diabetes Care 1999;22:1698–1702 [DOI] [PubMed] [Google Scholar]
- 13.Dahlquist G, Mustonen L. Childhood onset diabetes: time trends and climatological factors. Int J Epidemiol 1994;23:1234–1241 [DOI] [PubMed] [Google Scholar]
- 14.Nyström L, Dahlquist G, Rewers M, Wall S. The Swedish Childhood Diabetes Study: an analysis of the temporal variation in diabetes incidence 1978-1987. Int J Epidemiol 1990;19:141–146 [DOI] [PubMed] [Google Scholar]
- 15.Littorin B, Sundkvist G, Scherstén B, et al. Patient administrative system as a tool to validate the ascertainment in the Diabetes Incidence Study in Sweden (DISS). Diabetes Res Clin Pract 1996;33:129–133 [DOI] [PubMed] [Google Scholar]
- 16.Clayton D, Schifflers E. Models for temporal variation in cancer rates: II: age-period-cohort models. Stat Med 1987;6:469–481 [DOI] [PubMed] [Google Scholar]
- 17.Bruno G, Merletti F, Biggeri A, et al. ; Piedmont Study Group for Diabetes Epidemiology. Increasing trend of type I diabetes in children and young adults in the province of Turin (Italy): analysis of age, period and birth cohort effects from 1984 to 1996. Diabetologia 2001;44:22–25 [DOI] [PubMed] [Google Scholar]
- 18.Moltchanova E, Penttinen A, Karvonen M. A hierarchical Bayesian birth cohort analysis from incomplete registry data: evaluating the trends in the age of onset of insulin-dependent diabetes mellitus (T1DM). Stat Med 2005;24:2989–3004 [DOI] [PubMed] [Google Scholar]
- 19.Svensson J, Carstensen B, Mølbak A, et al. Increased risk of childhood type 1 diabetes in children born after 1985. Diabetes Care 2002;25:2197–2201 [DOI] [PubMed] [Google Scholar]
- 20.Tuomilehto J, Rewers M, Reunanen A, et al. Increasing trend in type 1 (insulin-dependent) diabetes mellitus in childhood in Finland: analysis of age, calendar time and birth cohort effects during 1965 to 1984. Diabetologia 1991;34:282–287 [DOI] [PubMed] [Google Scholar]
- 21.Staines A, Bodansky HJ, Lilley HE, Stephenson C, McNally RJ, Cartwright RA. The epidemiology of diabetes mellitus in the United Kingdom: the Yorkshire Regional Childhood Diabetes Register. Diabetologia 1993;36:1282–1287 [DOI] [PubMed] [Google Scholar]
- 22.Kyvik KO, Nystrom L, Gorus F, et al. The epidemiology of type 1 diabetes mellitus is not the same in young adults as in children. Diabetologia 2004;47:377–384 [DOI] [PubMed] [Google Scholar]
- 23.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia 2001;44:914–922 [DOI] [PubMed] [Google Scholar]
- 24.EURODIAB Substudy 2 Study Group. Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 2002;25:1755–1760 [DOI] [PubMed] [Google Scholar]
- 25.Dabelea D, D’Agostino RB, Jr, Mayer-Davies EJ, et al. ; SEARCH for Diabetes in Youth Study Group. Testing the accelerator hypothesis: body size, β-cell function, and age at onset of type 1 (autoimmune) diabetes. Diabetes Care 2006;29:290–294 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data