Structural Organization of the Actin Cytoskeleton at Sites of Clathrin-Mediated Endocytosis (original) (raw)
. Author manuscript; available in PMC: 2012 Jul 26.
Published in final edited form as: Curr Biol. 2011 Jun 30;21(14):1167–1175. doi: 10.1016/j.cub.2011.05.048
SUMMARY
Background
The dynamic actin cytoskeleton plays an important role in clathrin-mediated endocytosis (CME). However, its exact functions remain uncertain due to a lack of high resolution structural information regarding actin architecture at endocytic sites.
Results
Using platinum replica electron microscopy in combination with electron tomography we found that actin patches associated with clathrin-coated structures (CCSs) in cultured mouse cells consist of a densely branched actin network, in which actin filament barbed ends are oriented toward the CCS. The shape of the actin network varied from a small lateral patch at the periphery of shallow CCSs, to a collar-like arrangement around partly invaginated CCSs with actin filament barbed ends abutting the CCS neck, to a polarized comet tail in association with highly constricted or fully endocytosed CCSs.
Conclusions
Our data suggest that the primary role of the actin cytoskeleton in CME is to constrict and elongate the bud neck and drive the endocytosed vesicles from the plasma membrane. Moreover, in these processes barbed ends directly push onto the load, as in a conventional propulsion mechanism. Based on our findings we propose a model for initiation, evolution, and function of the dendritic actin network at CCSs.
INTRODUCTION
The dynamic actin cytoskeleton plays an important role in clathrin-mediated endocytosis (CME) [1, 2]. The formation of an endocytic vesicle during CME consists of: (i) assembly of the clathrin-based coat at the plasma membrane; (ii) invagination of the coated membrane to form a shallow pit; (iii) elongation of the coated pit and constriction of its neck to form a deeply invaginated bud; (iv) scission of the neck to form an endocytic vesicle; and finally (v) inward movement of the vesicle [3, 4]. All of these steps are energetically unfavorable and require force-generating machinery to occur. Actin polymerization has been proposed to provide force at multiple stages of CME [5–7].
The actin machinery functioning in CME is driven by Arp2/3 complex-dependent nucleation [3, 8]. When the Arp2/3 complex is activated by nucleation-promoting factors (NPFs), such as WASP family proteins [9, 10] and cortactin [11], it nucleates an actin filament as a branch on a pre-existing “mother” filament with a 70° angle between the barbed (plus) ends of both filaments [12]. The origin of the first mother filament remains unknown, but is thought to be nucleated by a different nucleator. Multiple Arp2/3 complex-dependent nucleation events result in the formation of a dendritic actin network, which generates pushing force by polymerizing barbed ends. The length of individual filaments and the number of elongating barbed ends are antagonistically controlled by capping protein (CP) and elongation factors, such as formins and Ena/VASP proteins [13–15]. In systems where Arp2/3 complex-dependent actin machinery is well-studied, such as lamellipodial protrusion and microbial comet tails, the barbed ends of branched actin filaments push onto the plasma membrane or the microbe in the direction of their movement [16–18].
F-actin assembly at clathrin-coated structures (CCSs) begins relatively late, after the coat has been assembled and peaks around the time of scission dissipating shortly after vesicle departure from the membrane [3, 8], suggesting a role in membrane deformation events. However, the exact role of the dynamic actin machinery during CME remains unknown, largely due to lack of high resolution structural information on small and transient actin patches associated with CCSs and a well-known difficulty of preserving dynamic actin filament networks for electron microscopy (EM). Consequently, various models predicting distinct modes of actin filament arrangement at CCSs have been proposed [3, 4, 19]. Understanding the structure of CCS-associated actin patches may distinguish between these models and shed light on specific roles of actin dynamics during CME.
In this study, we applied platinum replica EM in combination with electron tomography to analyze the three-dimensional structural organization of the actin cytoskeleton at CCSs. Our findings show that actin filaments at CCSs form a branched network with barbed ends oriented toward the CCS. The barbed ends abut the neck of partially invaginated CCSs and form a comet tail-like arrangements on deeply invaginated or fully endocytosed CCSs, suggesting that the main role of the actin cytoskeleton in CME is to mediate constriction of the bud neck and departure of the vesicle from the plasma membrane.
RESULTS
Structural organization of the CCS-associated actin cytoskeleton in detergent-extracted cells
A conventional way to analyze the cytoskeleton by platinum replica EM is to use detergent extraction to expose the cytoskeleton to metal shadowing [20, 21]. To ensure that detergent extraction preserves the CCS-associated dynamic actin network, we used fluorescence microscopy to test colocalization of clathrin with dendritic network components in detergent-extracted mouse B16F1 cells. Double staining of clathrin together with F-actin, the Arp2/3 complex, or CP revealed examples of CCSs localizing side-by-side or overlapping with punctate accumulations of actin network markers (Supplemental Figure S1A–C), as in living cells [6]. These results indicate that detergent extraction preserves the actin network at CCSs.
The structure of the CCS-associated actin cytoskeleton was investigated by correlative platinum replica EM utilizing cells expressing GFP-tagged clathrin light chain (GFP-CLC) as a coat marker (Figure 1), or GFP-CP as a marker of branched actin networks (Figure 2). Correlative EM helped locating individual CCSs or dendritic patches, respectively, which are detectable by EM only at high magnification.
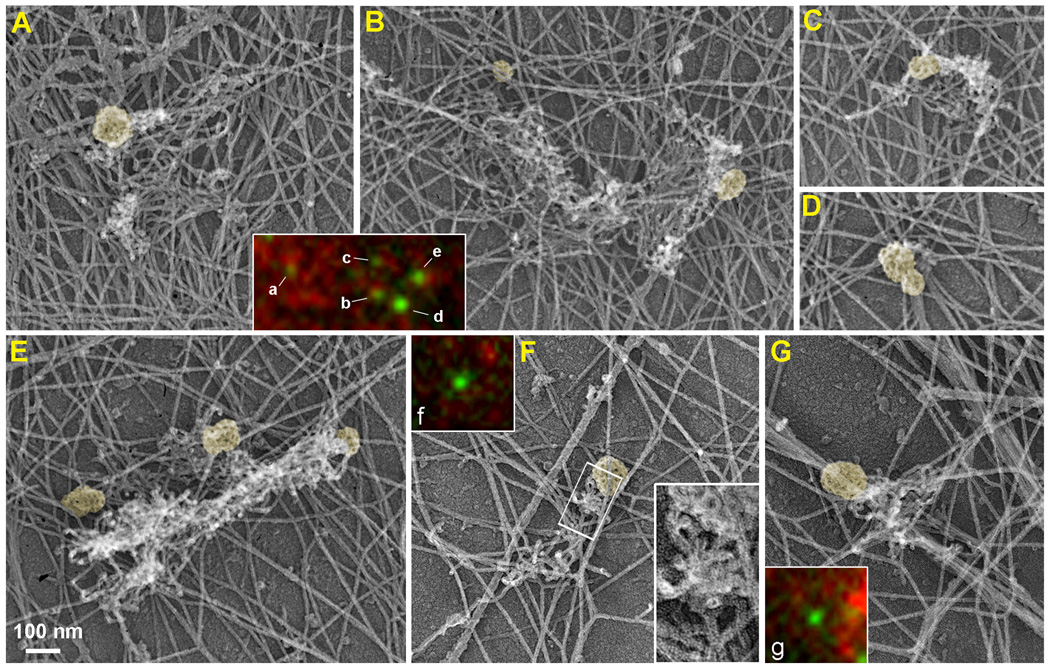
Figure 1. Correlative light and EM of GFP-CLC-expressing B16F1 cell.
(A–G) EM of a detergent-extracted cell showing CCSs (yellow) that peripherally associate with ruffle-like (A–C, E) or comet-tail-like (F, G) patches of dendritic network, whereas a double CCS (D) has very little, if any, dendritic actin network. Boxed region in F is enlarged in the inset to show branched filaments. Color insets show fluorescence images of GFP-CLC (green) and phalloidin staining (red) of the CCSs labeled by corresponding letters. The CCSs labeled d, f, and g do not show distinct F-actin enrichment in their vicinity by fluorescence staining, but associate with dendritic actin networks in EM images. See Supplemental figure S1 for additional images and stereo views of CCSs.
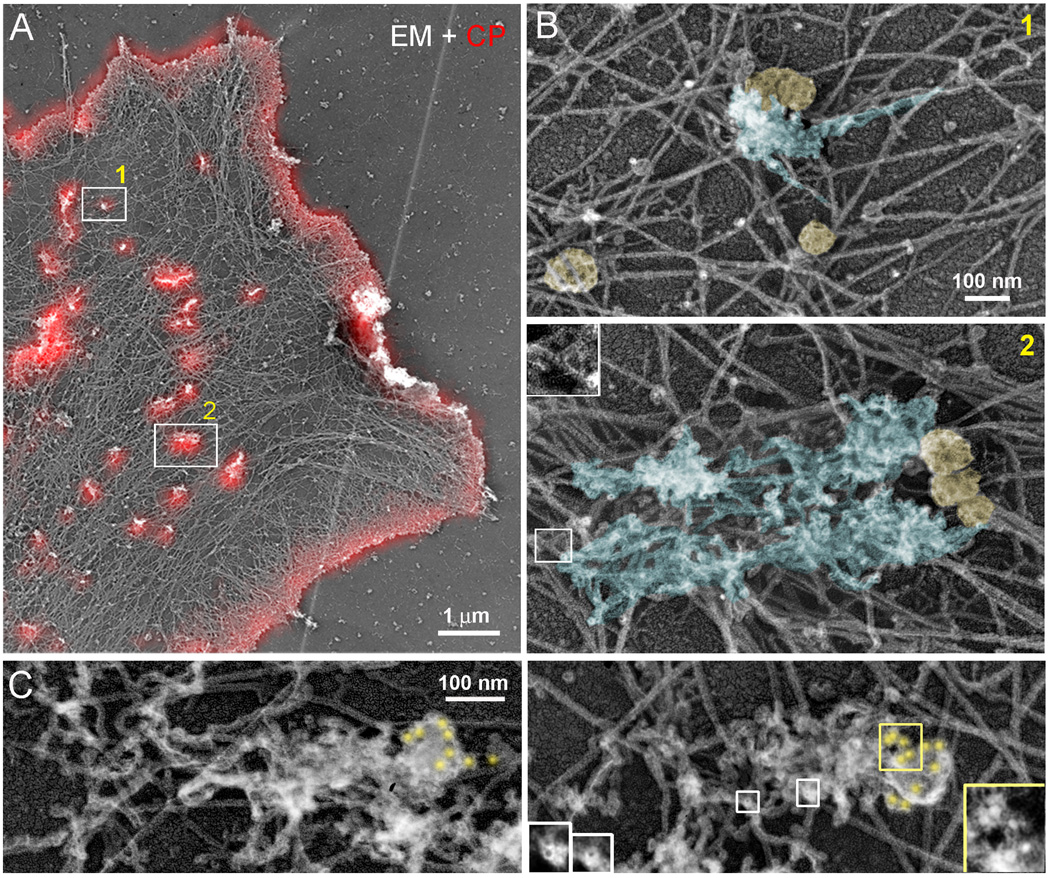
Figure 2. Correlative light and EM of GFP-CPβ-expressing B16F1 cell and clathrin immunogold staining of nonexpressing cells.
(A) EM of GFP-CPβ-expressing cell overlaid with GFP-CPβ fluorescence in red. (B) Enlarged EM images of boxed regions in B show dendritic comet-tails (blue) associated with CCSs (yellow). Boxed region in panel 2 is further enlarged in inset to show a branch. (C) Clathrin immunogold EM. Gold particles (yellow) accumulate at the tips of comet tails. Yellow and white boxes are enlarged in corresponding insets to show gold particles and tips of actin filaments, respectively.
Although many GFP-positive CCSs present in GFP-CLC-expressing live cells were removed by detergent extraction, many of them remained and were associated with local accumulations of F-actin (Supplemental Figure 1D). These CCSs could be subsequently located in correlative EM images of the same cell (Figure 1A–G and Supplemental Figure S1E). We found that CCS-associated F-actin puncta corresponded to small patches of actin networks that had very distinct morphology compared with the surrounding cytoskeletal network. The CCS-associated patches frequently looked like small ruffles or comet tails and consisted of short branched filaments displaying free filament ends. These features are especially well observed in stereo pairs of EM images (Supplemental Figure S1F). Free filament ends are predicted to be barbed ends based on the known geometry of branched networks [17]. CCSs usually associated with the periphery of the actin patch that was rich in filament ends. Interestingly, some CCSs that appeared as individual GFP-CLC dots by light microscopy were in fact several closely positioned CCSs (Figure 1D and 1E). It is important to keep this fact in mind while interpreting various events of CCS dynamics observed by light microscopy, such as splitting and merging of CCSs. Correlative EM also showed that some CCSs were associated with dendritic actin patches under EM investigation, but not with an obvious F-actin accumulation in corresponding fluorescence images (Figure 1F and 1G), suggesting that association of CCSs with actin may be underestimated by light microscopy. Some CCSs were not associated with dendritic actin patches, but could be bound to long lamellar actin filaments (Figure 1B and 2B).
As reported previously [22], GFP-CP localized to lamellipodia and small spots in the lamella (Figure 2A). However, the structure and function of CP spots has been previously unknown. We found that all GFP-CP-positive spots in the lamella corresponded to patches of dendritic actin network in EM images (Figure 2A and 2B). Remarkably, many patches were associated with CCSs, suggesting that a significant fraction of CP-positive actin patches mark the sites of CME. Similar to GFP-CLC-expressing cells, dendritic patches corresponding to GFP-CP spots frequently had a comet tail-like appearance with the CCS located at the tip of the tail (Figure 2B). The 70° branch angle, when clearly visualized within the dense tail, was oriented toward the CCS, similar to bacterial comet tails [18], suggesting that these comet tails may propel the CCSs.
In all cases when we observed association of a CCS with an actin patch in detergent-extracted cells, the CCS was peripherally associated with the actin network. To address the possibility that some CCSs could be hidden within or underneath the dendritic actin patch, we performed immunogold EM of clathrin (Figure 2C). The analysis of immunogold localization in EM samples was performed at high magnification to distinguish gold particles from vertically oriented filament ends. At low magnification, both structures appear as white dots in contrast-inverted images. However, at high magnification, gold particles have a solid white core surrounded by a grey halo of platinum-coated antibody layer, while filament ends have a dark core surrounded by a bright halo of the platinum layer (Figure 2C, insets). This approach revealed that when gold-labeled CCSs were found in association with the dendritic actin patch, they were peripherally located relative to the comet tail.
Structural organization of CCS-associated actin cytoskeleton in unroofed cells
Since the plasma membrane is an intrinsic structural constituent of CCSs, its removal by detergent extraction might affect the geometry and/or preservation of CCSs. Indeed, the majority of CCSs detected in the detergent-extracted samples had a nearly spherical shape, as expected for fully formed vesicles or highly invaginated CCSs, suggesting that shallow pits might be lost during extraction. Therefore, we investigated unroofed cells, in which most cellular components were removed by an ultrasonic burst, while the ventral plasma membrane remained preserved [23]. Both fluorescence microscopy and EM of unroofed B16F1 cells showed many CCSs associated with actin patches (Figure 3 and Supplemental Figure S2). In optimally processed samples, 43% of CCSs (N=426) was associated with a detectable dendritic actin network. However, this number is likely imprecise due to possible loss of CCSs and/or CCS-associated actin patches during unroofing.
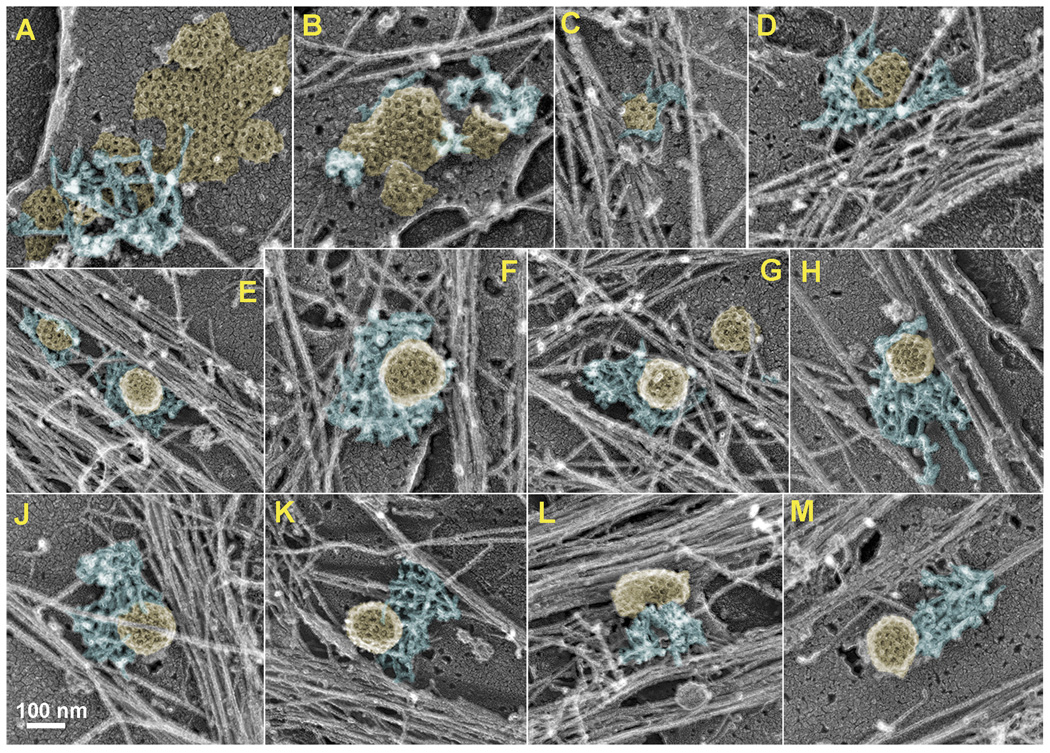
Figure 3. CCS-associated actin patches in unroofed cells.
(A–M) EM images showing clathrin plagues (A,B), shallow (C,D) and deeply invaginated (E–M) CCSs associated with dendritic actin patches of variable geometry, such as small pieces of dendritic network (A, B), collar-like (C–J), and comet tail-like (K–M) networks. See Supplemental figures S2 and S3 for fluorescence, EM and stereo EM images.
Detailed EM analysis of ventral membranes revealed a larger variety of CCS shapes as compared with detergent-extracted cells (Figure 3 and Supplemental Figure S3). In addition to nearly spherical CCSs, we observed relatively flat or shallow CCSs. CCS-associated dendritic actin patches also displayed greater variation in their geometry, as compared with detergent-extracted samples. In addition to comet tail-like shapes (Figure 3K–M), we also observed collar-like actin patches that encircled the CCS over a large fraction of its perimeter (Figure 3C–J), as well as very small lateral actin patches (Figure 3A, 3B and Figure 4). However, the dome of CCSs remained free of actin filaments with the occasional exception of actin filaments extending over the peripheral portion of a CCS (Figure 3B, 3D, and 3J). As in detergent-extracted cells, CCS-associated dendritic patches consisted of very short and densely branched actin filaments. We estimated an average length of actin filaments in the patches to be ~50–100 nm. However, this value is probably an overestimation, as we observed many small bumps on filament sides likely representing branches, which were too short to be reliably measured. The outer diameter of CCSs found in association with actin patches was 129±13 nm (N=42) including the thickness of clathrin coat (~17 nm) and platinum layer (~2 nm), suggesting a typical size of the membrane vesicle (~90 nm) within CCSs. Large clathrin lattices, likely corresponding to “clathrin plaques” [24], were rarely observed in these cells. Although dendritic actin patches were observed in association with 83% of clathrin plagues (N=18), they were unproportionally small and interacted with only 11±3% of the plaque perimeter (Figure 3A and 3B).
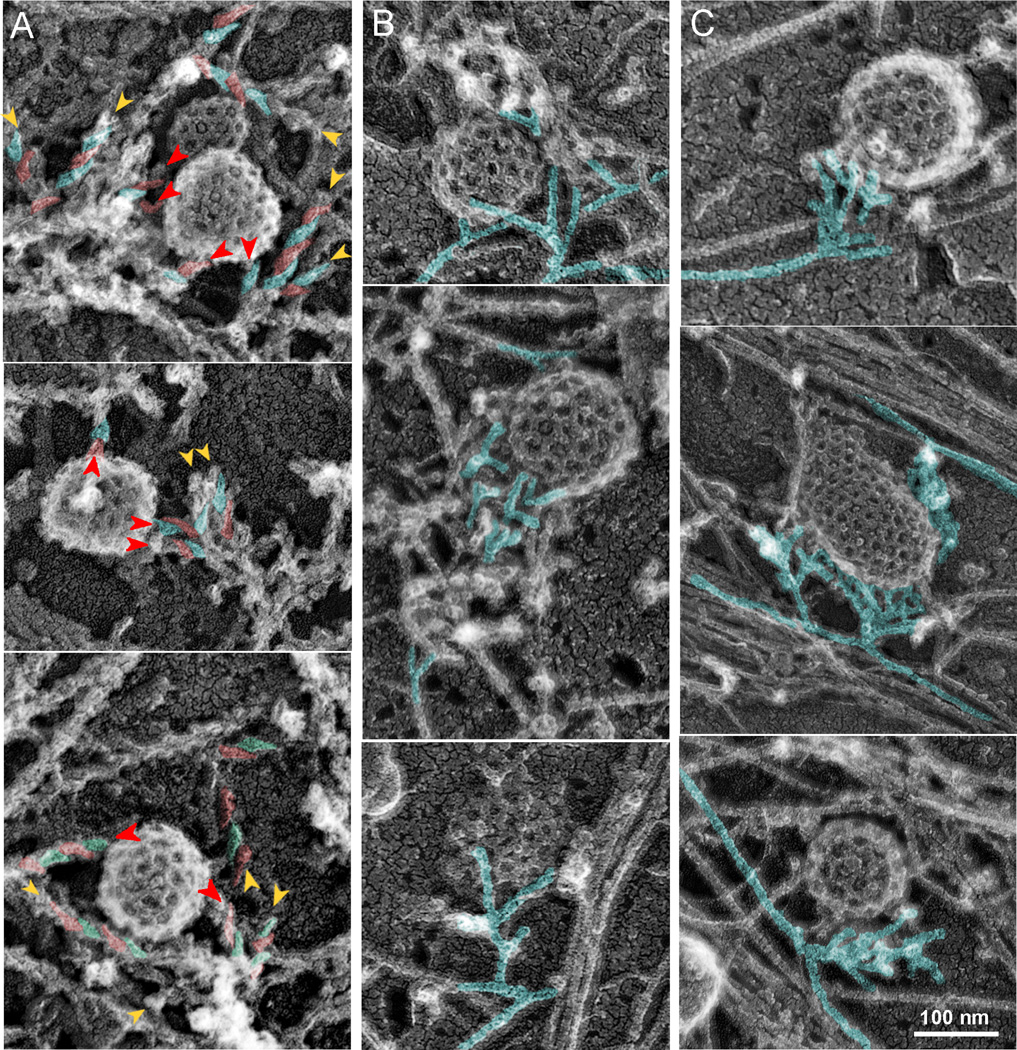
Figure 4. Orientation and origin of actin filaments in CCS-associated dendritic patches.
EM of unroofed cells with (A) or without (B,C) S1 decoration. (A) Asymmetric “comma-shaped” units of S1 decoration revealing the filament polarity are highlighted in alternating red and cyan colors on some filaments. The head of the “comma” is oriented toward the pointed end. Barbed ends (arrowheads) face the CCS. Red arrowheads mark actin filaments terminating on CCSs. (B) The 70° angle between branched filaments (cyan) is predominantly oriented toward the CCS. (C) Dendritic actin network appears to originate from one (top and bottom) or two (middle) points on nearby long actin filaments (cyan). See Supplemental figure S3 for stereo views of CCSs.
Polarity and origin of actin filaments in CCS-associated dendritic patches
A key unanswered question regarding the organization of the actin cytoskeleton at CCSs is the orientation of actin filaments. We determined the actin filament polarity in the CCS-associated actin patches by decorating actin filaments with myosin subfragment 1 (S1) in unroofed cells (Figure 4A and Supplemental Figure S3B). We found that the majority of actin filaments terminating on a CCS were oriented with their barbed ends toward the CCS (84%, N=151 filaments from 48 CCSs). Because of high density and short lengths of actin filaments in dendritic patches, we were only able to determine polarity of a relatively small fraction of actin filaments within each dendritic patch using S1 decoration. Therefore, we additionally estimated the orientation of actin filaments relative to CCSs based on the 70° angle between barbed ends of Arp2/3 complex-nucleated filaments [25, 26]. We found that the 70° angle usually faced the CCS (Figure 4B and Supplemental Figure S3C), indicating that the barbed ends are oriented toward the CCS.
The Arp2/3 complex, which initiates the actin assembly at CCSs, requires a pre-existing actin filament to nucleate a branch [27]. It remains unclear how the first actin filament is produced. A small size and distinct morphology of the CCS-associated actin patches may have shed light on this issue. We noticed that dendritic actin patches frequently formed a single-point contact at their pointed-end side with a long linear actin filament belonging to the surrounding lamellar actin network or a stress fiber (Figure 4C and Supplemental Figure S3D). These observations suggest that when the Arp2/3 complex is activated by NPFs accumulated at the CCS, it may take advantage of a randomly available actin filament to initiate nucleation of the dendritic network.
Electron tomography of actin patches associated with CCSs of different shapes
Branched actin networks exert force when their growing barbed ends push onto the plasma membrane. Having established that barbed ends in the CCS-associated actin patches are generally oriented toward the CCS, we sought to determine more precisely the surfaces abutted by barbed ends, as they would likely correspond to sites of force application. Thus, we combined platinum replica EM with electron tomography by collecting images at the tilt angles up to ±70° relative to the plane of the sample (Figure 5A–F and Supplemental Movies S1–4). This approach allowed us to obtain additional three-dimensional information, as compared to stereo pairs. By analyzing electron tomography series, we determined the degree of CCS invagination and the position of the actin-CCS contacts and correlated them with each other. To perform correlation at a quantitative level (Figure 5G, H), we classified CCSs into three categories according to degree of invagination: (i) shallow CCS with a hemispherical or less invaginated shape and a broad base; (ii) deeply invaginated CCSs with a more rounded shape and a reduced diameter of the base; and (iii) constricted CCSs or fully formed vesicles with a spherical shape and a narrow or undetectable neck. The position of the actin patch relative to the CCS was assessed both in the plane of the plasma membrane (Figure 5G) and in Z-direction (Figure 5H).
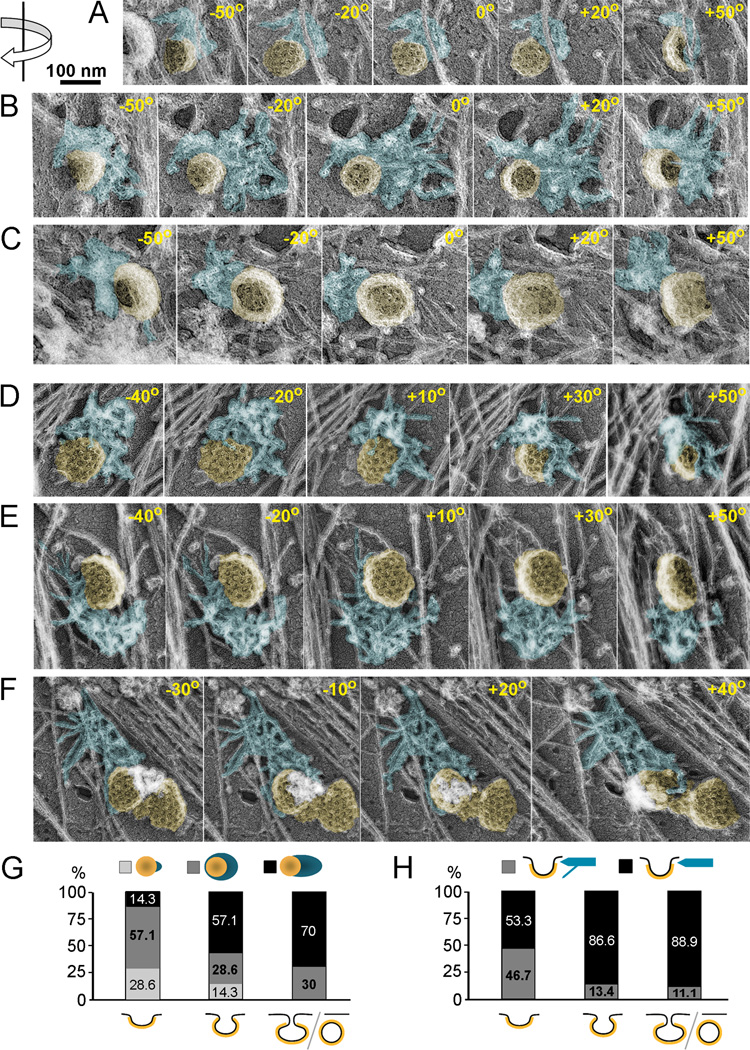
Figure 5. Electron tomography of CCS-associated dendritic patches in unroofed cells.
(A–F) Examples of actin patches (blue) associated with CCSs (yellow) shown as montages of selected frames from single axis tilt series acquired every 1° using FEI CM300-FEG electron microscope (A–C) or every 10° using Philips CM120 electron microscope (D–F). The tilt angles are shown in each frame. Direction of rotation is indicated by arrow at the upper left corner. Shallow (A, D), deeply invaginated (B, C, E), and constricted (F) CCSs are associated with actin patches primarily at their necks; some filaments in D hang over the CCS dome. (G, H) Correlation of three dimensional actin patch geometry with the degree of CCS invagination. (G) Actin patch geometry in the plane of the plasma membrane. Percentage of CCSs associated with a small patch (light grey), collar-shaped patch (dark grey) or comet tail (black) is plotted for each degree of invagination (shallow, deep, or constricted/endocytosed). (H) Actin patch geometry in the Z direction. Percentage of CCSs interacting with actin patch exclusively at the neck (black) or predominantly at the neck with few filaments extending over the dome (grey) are plotted for each degree of CCS invagination. Percentage numbers for each category are shown in plots (G and H). At least 11 CCSs are scored for each CCS category. See Supplemental movies 1–4 for video versions of tilt series shown in A–F.
We found that shallow CCSs (Figure 5A, D and Supplemental Movies S1 and S2) were frequently associated with a collar-like actin patch, which contacted the CCS over a large part of its perimeter, although the shape of the actin patch was frequently asymmetric and biased to one side (Figure 5D,G and Supplemental Movie S2). However, some shallow CCSs interacted with a small piece of the actin network only at one side (Figure 5A,G and Supplemental Movie S1). Deeply invaginated (Figure 5B, C, E and Supplemental Movies S1 and S3) and constricted (Figure 5F and Supplemental Movie S4) CCSs, when viewed en face, most commonly displayed unilateral association with an elongated actin patch reminiscent of a comet tail, but collar-like arrangements were also observed (Figure 5G).
In the Z-direction, the actin patch contacted shallow CCSs only at the base, where the clathrin coat meets the plasma membrane (Figure 5A, D and Supplemental Movies S1 and S2). In about half of analyzed shallow CCSs (Figure 5H), some actin filaments extended over the clathrin coat, but never covered a significant portion of the CCS dome (Figure 5D and Supplemental Movie S2). Deeply invaginated CCSs had actin patches interacting almost exclusively with their necks (Figure 5B, C, E, H and Supplemental Movies S1 and S3) and only rare actin filaments suspended over the CCS periphery (Figure 5H). We did not observe extensive interaction of actin filaments with clathrin coats in any of the CCS categories. Although we cannot exclude a possibility that actin filaments interacting with the dome of CCSs were torn away by the unroofing procedure, the total absence of such examples favors the idea that the CCS neck is a primary site of force application from polymerizing actin filaments.
DISCUSSION
Actin polymerization was proposed to function at multiple stages of CME including formation, invagination, constriction, and lateral movement of clathrin-coated pits, as well scission and departure of clathrin-coated vesicles [3, 5–7, 28–30]. For all of these functions, ambiguity exists as to how actin polymerization applies the pushing force [3, 4]. Resolving the actin architecture relative to the CCS may distinguish or prioritize the existing models. In this study, we have succeeded in characterizing the high resolution 3D structure of CCS-associated actin patches in mammalian cells by platinum replica EM and electron tomography. We have found that CCS-associated actin patches represent small pieces of the dendritic actin network, consisting of unusually short and densely branched actin filaments, as also observed in yeast [31] and Hip1R-depleted HeLa cells [32], suggesting high activity of capping proteins in these patches.
Importantly, we have determined the polarity of actin filaments in actin patches and found that barbed ends are oriented toward the CCS. These data suggest that actin generates force directed toward the CCS, thus resolving the existing uncertainty on this point [3, 4]. The opposite end of the patch, predicted to be the oldest part, is usually anchored to a long actin filament of the lamellar cytoskeleton. This organization suggests that when NPFs are recruited to and activated at the CCS, the Arp2/3 complex employs a nearby actin filament as a co-activator [27], so that the autocatalytic process of dendritic nucleation can begin without a need for additional actin nucleators. The anchorage of the dendritic patch to the surrounding cytoskeleton may also enhance the effect of generated force by providing traction to the assembling dendritic network.
Electron tomography provided further insights into the pathway of actin patch development over time. Thus, very small actin patches are most frequently found in association with shallow CCSs that represent relatively early stages of CME, whereas more invaginated CCSs are associated with larger actin patches that tend to encircle the CCS. These findings suggest that the actin patch may start locally and then spread around the CCS forming a collar-like shape. The comet tail-like shape appears to be the subsequent stage of patch morphogenesis, as it becomes the dominant feature of spherical CCSs. We suppose that the collar-to-comet tail reorganization may be a consequence of neck constriction, during which barbed ends become consolidated, at the narrowing neck of the constricted CCS and then propagate into the same direction forming a comet tail.
Furthermore, electron tomography allowed us to precisely define the CCS surfaces interacting with barbed ends, thus gaining insight into actin cytoskeleton functions during CME. Our data suggest that neck constriction is one of the key roles of actin polymerization in this process, as proposed previously [6, 7, 28]. Indeed, at partially invaginated CCSs (shallow or deep), barbed ends almost exclusively abut the neck of the CCS, suggesting that the neck is the major site of force application. The constriction is expected to be especially efficient, when the actin network encircles the CCS in a collar-like fashion, whereas more asymmetric, baby-bib-like configurations may produce lateral movements of CCSs [6]. The circumferential arrangement of actin filament barbed ends and their position close to the plane of the plasma membrane suggests that actin filaments push onto the plasma membrane next to the clathrin coat rather than on the coat itself. This idea is consistent with cortactin localization at the perimeter of the clathrin cage [11], where it supposedly activates the Arp2/3 complex and induces actin assembly. With such arrangement, the energy of actin polymerization can be more efficiently directed toward the emerging neck.
Our data are also consistent with the idea that the actin network promotes CCS invagination [3, 7, 29]. However, a lack of massive association of actin filaments with the clathrin coat suggests that CCSs unlikely invaginate by riding on the retrograde flow [3]. Instead, constant branching of actin filaments within dendritic patches associated with constricted CCSs may cause network expansion in Z-direction, which would drive the CCS from the plasma membrane [19]. In this scenario, barbed ends would form a hemispherical front and push on all three surfaces, the plasma membrane, the neck, and the CCS, thus integrating the existing models [3, 4, 19].
Finally, the comet tail-like morphology of actin patches suggests that the actin network can drive the departure of endocytosed vesicles from the plasma membrane after scission [1, 2, 29]. At this stage, actin may push directly onto the clathrin coat and may be more strongly bound to it, as suggested by better preservation of comet tail-associated CCSs after detergent extraction. This strong binding may be mediated by Hip1R, a protein interacting with both actin and the clathrin coat [3].
Another unanswered question is how much actin is needed for CME in mammalian cells. Discrepant results on this point have been attributed to the conditional requirement of actin in mammalian CME when the endocytic process encounters challenges, such as high turgor pressure [29], excessive membrane tension [33, 34], or bulky cargo [35], including large flat clathrin-coated plaques versus regularly sized (~100 nm) clathrin-coated vesicles [24]. Our study provided some insights into this question. First, we found that dendritic actin patches detectable by EM may be missed by fluorescence imaging of detergent-extracted phalloidin-stained samples. The problem may be exacerbated during live imaging in the presence of soluble pools of fluorescent reporters. Second, large clathrin plaques displayed scarce association with the dendritic actin network in our samples, in contrast to regularly sized CCSs. Although it is unknown whether clathrin plaques undergo endocytosis in this cell type, we were unable to confirm that large clathrin plaques have greater requirement for the actin cytoskeleton [24]. Third, much evidence regarding dispensability of actin in mammalian CME resulted from the application of actin depolymerizing drugs [24, 36, 37]. Following treatment with latrunculin B or cytochalasin D at comparable concentrations, we found multiple remaining F-actin patches extensively colocalizing with CCSs (Supplemental figure S4), suggesting that the actin polymerization machinery at CCSs successfully competes for scarce actin resources remaining following drug treatment. Therefore, disruption of prominent F-actin structures, such as stress fibers, by actin drugs is not an indicator of total inhibition of the CCS-associated actin machinery. Together our findings suggest that the involvement of the actin cytoskeleton in mammalian CME may be greater than currently appreciated. In any case, whether actin polymerization is needed constantly or conditionally, the mechanism of actin functions in CME is unclear and our study has provided critical insights into this problem.
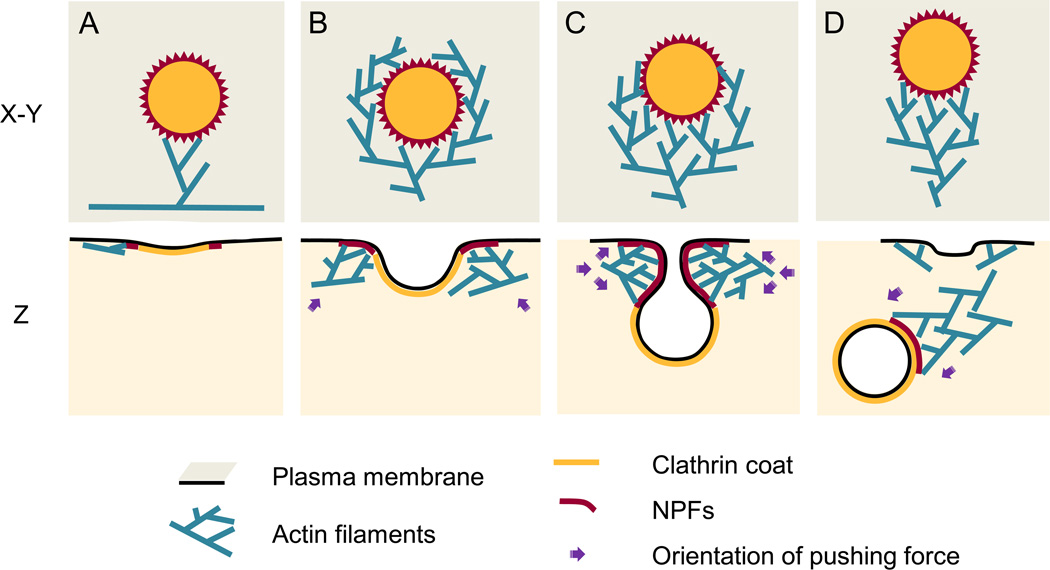
Based on our data, we propose a model for the actin cytoskeleton assembly and functions at CCSs (Figure 6). A forming CCS recruits and activates NPFs, such as N-WASP [9, 10] and cortactin [11], which accumulate at the plasma membrane around the CCS perimeter. An inactive Arp2/3 complex is activated by these NPFs and a nearby actin filament and begins branched nucleation. Newly formed actin filaments support subsequent rounds of Arp2/3 complex-dependent branched nucleation leading to expansion of the network. Filaments positioned close to the CCS, and thus to NPFs, have increased probability to bind an NPF-activated Arp2/3 complex and serve as mother filaments for subsequent rounds of nucleation. As a result, the expanding dendritic network gradually encircles the CCS, although it frequently remains asymmetric. During the growth of the network, the barbed ends push onto the plasma membrane at the boundary with the clathrin coat and promote invagination of the bud and constriction of the neck. Consolidation of the pushing front at the narrowing CCS neck results in reorganization of a collar into a comet tail, which drives the departure of the clathrin-coated vesicle from the plasma membrane after scission.
Figure 6. Model for actin assembly, organization, and function during CME.
Individual steps of assembly (A–D) are shown en face (X–Y) or in profile (Z). (A) Actin assembly begins when the Arp2/3 complex is activated by NPFs (red) recruited to the periphery of the CCS and binds an actin filament in the cytoplasm. (B) Actin network expands and gradually encircles the CCS through multiple rounds of dendritic nucleation guided by NPFs. The force generated by actin polymerization at this stage may drive lateral movements of the shallow CCS. (C) Dendritic actin network surrounds the neck of the deeply invaginated CCS and promotes constriction and elongation of its neck. (D) Actin network reorganizes into a comet tail and after scission drives the vesicle away from the plasma membrane.
EXPERIMENTAL PROCEDURES
Cells and reagents
B16F1 mouse melanoma cells were cultured as described [15]. Monoclonal clathrin heavy chain antibody (clone X-22) was from Abcam (Cambridge, MA), polyclonal rabbit antibody against p16 subunit of the Arp2/3 complex was described [38], and polyclonal rabbit antibody against β-subunit of capping protein was a gift from D. Schafer (University of Virginia). Fluorescently labeled secondary antibodies, phalloidins and Deep-Red CellMask membrane dye were from Invitrogen. Secondary rabbit antibodies conjugated to 12-nm colloidal gold were from Jackson Immuno Research Laboratories (West Grove, PA). Myosin S1 was a gift from Dr. Y. Goldman (University of Pennsylvania). Latrunculin B was from Calbiochem (La Jolla, CA), and all other reagents were from Sigma-Aldrich unless indicated otherwise. EGFP-tagged clathrin light chain α (EGFP-CLC) was a gift from Dr. J. Keen (Jefferson University) and pEGFP-tagged β2 subunit of heterodimeric capping protein (EGFP-CP) was a gift from D. Schafer (University of Virginia).
Light Microscopy
Immunofluorescence staining of detergent-extracted cells, transient transfection, and fluorescence light microscopy were described [15]. Clathrin immunofluorescence staining was performed after paraformaldehyde fixation, as X-22 antibody does not recognize glutaraldehyde-fixed samples.
Electron Microscopy
Platinum replica EM procedures were described [20, 21]. ImmunoEM of clathrin was performed as described for myosin II. Cell “unroofing” was performed as described [23]. Unroofed cells were immediately fixed with 2% glutaraldehyde and processed for EM.
Electron tomography series from platinum replica samples were prepared by two approaches. First, single axis tilt series were acquired every 3° using increments according to the Saxton scheme over a range of ±70° using FEI CM300-FEG electron microscope operating at an accelerating voltage of 300 kV and equipped with a Gatan Model 670 High Tilt Analytical Holder and a TVIPS F415MP 4k×4k CCD camera. The tomograms were processed using PROTOMO software package [39]. Figures and movies were constructed using CHIMERA [40]. Second, single axis tilt series with images acquired every 10° over a range of ±50° were obtained using Philips CM120 transmission electron microscope operating at an accelerating voltage of 80 kV. Images were manually aligned using Adobe Photoshop. Movies were produced from aligned and cropped images using Metamorph.
For additional experimental details, see Supplemental Experimental procedures.
Supplementary Material
01
02
03
04
05
ACKNOWLEDGEMENTS
We are grateful to Drs. Dorothy Schafer, Yale Goldman, and James Keen for generous gifts of reagents and Dr. Roger Craig for permission to use Philips CM120 electron microscope. This work was supported by NIH grants GM087253 and RR 22482 to TS and the NIH Cell Migration Consortium (NIH GM64346) to KT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental information includes Supplemental Experimental Procedures, 4 figures and 4 movies.
REFERENCES
- 1.Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- 2.Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 3.Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 4.Galletta BJ, Mooren OL, Cooper JA. Actin dynamics and endocytosis in yeast and mammals. Curr Opin Biotechnol. 2010;21:604–610. doi: 10.1016/j.copbio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005;16:964–975. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson S, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, Destaing O, Ko G, Takasaki J, Cremona O, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell. 2009;17:811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrais D, Merrifield CJ. Dynamics of endocytic vesicle creation. Dev Cell. 2005;9:581–592. doi: 10.1016/j.devcel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci. 2005;118:3103–3115. doi: 10.1242/jcs.02444. [DOI] [PubMed] [Google Scholar]
- 10.Innocenti M, Gerboth S, Rottner K, Lai FP, Hertzog M, Stradal TE, Frittoli E, Didry D, Polo S, Disanza A, et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol. 2005;7:969–976. doi: 10.1038/ncb1304. [DOI] [PubMed] [Google Scholar]
- 11.Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol. Cell. Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 14.Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery; pivotal role of the filament barbed end. Cell. 2004;118:363–373. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5:e317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small JV, Isenberg G, Celis JE. Polarity of actin at the leading edge of cultured cells. Nature. 1978;272:638–639. doi: 10.1038/272638a0. [DOI] [PubMed] [Google Scholar]
- 17.Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron LA, Svitkina TM, Vignjevic D, Theriot JA, Borisy GG. Dendritic organization of actin comet tails. Curr Biol. 2001;11:130–135. doi: 10.1016/s0960-9822(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 19.Suetsugu S. The direction of actin polymerization for vesicle fission suggested from membranes tubulated by the EFC/F-BAR domain protein FBP17. FEBS Lett. 2009;583:3401–3404. doi: 10.1016/j.febslet.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Svitkina T. Electron microscopic analysis of the leading edge in migrating cells. Methods Cell Biol. 2007;79:295–319. doi: 10.1016/S0091-679X(06)79012-4. [DOI] [PubMed] [Google Scholar]
- 21.Svitkina T. Imaging cytoskeleton components by electron microscopy. Methods Mol Biol. 2009;586:187–206. doi: 10.1007/978-1-60761-376-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer DA, Welch MD, Machesky LM, Bridgman PC, Meyer SM, Cooper JA. Visualization and molecular analysis of actin assembly in living cells. J Cell Biol. 1998;143:1919–1930. doi: 10.1083/jcb.143.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heuser J. The production of 'cell cortices' for light and electron microscopy. Traffic. 2000;1:545–552. doi: 10.1034/j.1600-0854.2000.010704.x. [DOI] [PubMed] [Google Scholar]
- 24.Saffarian S, Cocucci E, Kirchhausen T. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol. 2009;7:e1000191. doi: 10.1371/journal.pbio.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, Pollard TD. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci U S A. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourne J, Morgan JR, Pieribone VA. Actin polymerization regulates clathrin coat maturation during early stages of synaptic vesicle recycling at lamprey synapses. J Comp Neurol. 2006;497:600–609. doi: 10.1002/cne.21006. [DOI] [PubMed] [Google Scholar]
- 29.Aghamohammadzadeh S, Ayscough KR. Differential requirements for actin during yeast and mammalian endocytosis. Nat Cell Biol. 2009;11:1039–1042. doi: 10.1038/ncb1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu M, Huang B, Graham M, Raimondi A, Heuser JE, Zhuang X, De Camilli P. Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nat Cell Biol. 2010;12:902–908. doi: 10.1038/ncb2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young ME, Cooper JA, Bridgman PC. Yeast actin patches are networks of branched actin filaments. J Cell Biol. 2004;166:629–635. doi: 10.1083/jcb.200404159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engqvist-Goldstein AE, Zhang CX, Carreno S, Barroso C, Heuser JE, Drubin DG. RNAi-mediated Hip1R silencing results in stable association between the endocytic machinery and the actin assembly machinery. Mol Biol Cell. 2004;15:1666–1679. doi: 10.1091/mbc.E03-09-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu AP, Loerke D, Schmid SL, Danuser G. Global and local regulation of clathrin-coated pit dynamics detected on patterned substrates. Biophys J. 2009;97:1038–1047. doi: 10.1016/j.bpj.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batchelder EM, Yarar D. Differential requirements for clathrin-dependent endocytosis at sites of cell-substrate adhesion. Mol Biol Cell. 2010;21:3070–3079. doi: 10.1091/mbc.E09-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cureton DK, Massol RH, Whelan SP, Kirchhausen T. The length of vesicular stomatitis virus particles dictates a need for actin assembly during clathrin-dependent endocytosis. PLoS Pathog. 2011;6:e1001127. doi: 10.1371/journal.ppat.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1:161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- 37.Boucrot E, Saffarian S, Massol R, Kirchhausen T, Ehrlich M. Role of lipids and actin in the formation of clathrin-coated pits. Exp Cell Res. 2006;312:4036–4048. doi: 10.1016/j.yexcr.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vignjevic D, Yarar D, Welch MD, Peloquin J, Svitkina T, Borisy GG. Formation of filopodia-like bundles in vitro from a dendritic network. J Cell Biol. 2003;160:951–962. doi: 10.1083/jcb.200208059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkler H, Taylor KA. Accurate marker-free alignment with simultaneous geometry determination and reconstruction of tilt series in electron tomography. Ultramicroscopy. 2006;106:240–254. doi: 10.1016/j.ultramic.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03
04
05