The global distribution and population at risk of malaria: past, present, and future (original) (raw)
. Author manuscript; available in PMC: 2011 Jul 28.
Abstract
The aim of this review was to use geographic information systems in combination with historical maps to quantify the anthropogenic impact on the distribution of malaria in the 20th century. The nature of the cartographic record enabled global and regional patterns in the spatial limits of malaria to be investigated at six intervals between 1900 and 2002. Contemporaneous population surfaces also allowed changes in the numbers of people living in areas of malaria risk to be quantified. These data showed that during the past century, despite human activities reducing by half the land area supporting malaria, demographic changes resulted in a 2 billion increase in the total population exposed to malaria risk. Furthermore, stratifying the present day malaria extent by endemicity class and examining regional differences highlighted that nearly 1 billion people are exposed to hypoendemic and mesoendemic malaria in southeast Asia. We further concluded that some distortion in estimates of the regional distribution of malaria burden could have resulted from different methods used to calculate burden in Africa. Crude estimates of the national prevalence of Plasmodium falciparum infection based on endemicity maps corroborate these assertions. Finally, population projections for 2010 were used to investigate the potential effect of future demographic changes. These indicated that although population growth will not substantially change the regional distribution of people at malaria risk, around 400 million births will occur within the boundary of current distribution of malaria by 2010: the date by which the Roll Back Malaria initiative is challenged to halve the world’s malaria burden.
“While keeping in mind the realities one can nevertheless be confident that malaria is well on its way towards oblivion. Already as a malariologist, I feel premonitory twinges of lonesomeness, and in my own organisation I am now a sort of ‘last survivor’. So perhaps it is fitting that I should take this backward glance at the fascinating pages of malaria history.”1
This extract from the 1955 preface of Paul Russell’s Man’s Mastery of Malaria1 now seems astonishing. In the 50 years that have passed since the series of lectures on which this book is based was given we have become less sanguine about the prospects for global malaria control. The purpose of this review is to document what happened to the global spatial limits of malaria risk during the past 100 years and use this to examine the task facing the global malaria-control community at the turn of this century.
Spatial distribution of malaria through time
The human race and malaria parasites have had a long evolutionary host–parasite association.2–4 Advances in bioinformatics5,6 largely support hypotheses inferred from changes in human ecology that around 10 000 years ago Plasmodium falciparum populations rapidly expanded in Africa and spread worldwide, coincident with human population growth and subsequent diasporas facilitated by the dawn of agriculture.7,8 It has also been suggested that this expansion followed an earlier smaller wave of migration in the pleistocene.4 The probable maximum preintervention distribution of malaria (around 1900)9–11 is shown in figure 1, reaching latitudinal extremes of 64° north and 32° south18 (corresponding approximately with the theoretical 15°C July and January isotherms, respectively, supporting Plasmodium vivax transmission).19 These maps represent risk from one or more of the four species of Plasmodium that cause malaria in human beings, hereafter referred to as all-cause malaria risk.20 We extend previous inquiry into this area7,21–24 by quantifying recorded changes in the global malaria distribution over a longer period of time, at more frequent intervals, and relating this distribution to the intensity of malaria risk.
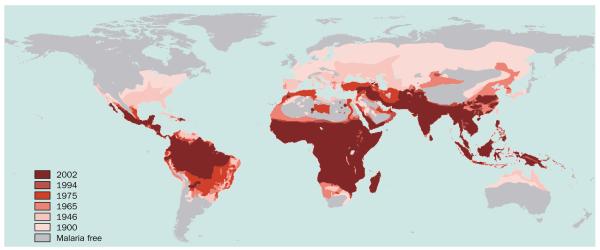
Figure 1.
The global distribution of malaria since preintervention (~1900–2002). All-cause malaria distribution maps for the preintervention distribution (circa 1900)9 and for the years 1946, 1965, 1975, 1992, 1994, and 200212–17 were georeferenced using ERDAS Imagine 8.5 (Leica Geosystems GIS & Mapping, Atlanta, GA, USA). Maps were then digitised on screen with MapInfo Professional 7.0 (MapInfo Corp, NY, USA). Areas of high and low risk were merged throughout to establish all-cause malaria transmission limits. The only modification of original maps was infilling areas labelled as unknown in China in the 1975 map14 with the distribution recorded in 1965.13 Each map was then overlaid to create a single global distribution map of malaria risk which illustrates range changes through time. Note that the 1992 distribution is excluded from the figure for clarity because it was so similar to that of 1994.
Human efforts to control malaria have markedly restricted its distribution during the 20th century.12–17 Distribution maps have been compiled largely from country reports and expert opinion arising from the network of regional offices of the WHO. Despite these maps being imperfect representations of global malaria-infection risk distribution in space and time, they nevertheless facilitate some insight into the progress of malaria control in the 20th century. We present results that were obtained using summary procedures in geographic information systems (GIS) on digitised (electronically redrawn and geographically referenced) versions of original maps, the exact methodology for which is explained in the relevant figure and table legends. These procedures show that since preintervention (about 1900–2002) development and control efforts have reduced the area of human malaria risk by around half, from 53% to 27% of the Earth’s land surface (table 1). The number of countries and territories (with populations of more than 100 000 inhabitants) exposed to some level of malaria risk fell from 140 to 88 during this period.
Table 1.
Global population at risk from malaria from preintervention to 2010 (~1900–2010)
| Time | Global population | Land area malarious | Countries at risk | Population exposed | ||
|---|---|---|---|---|---|---|
| Years | n | _km_2 | % | n | n | % |
| 1900 | 1 158 409 472 | 77 594 480 | 53·16 | 140 | 892 373 056 | 77·03 |
| 1946 | 2 391 400 960 | 58 565 752 | 40·12 | 130 | 1 635 815 808 | 68·40 |
| 1965 | 3 363 417 344 | 53 492 988 | 36·65 | 103 | 1 924 360 320 | 57·21 |
| 1975 | 4 085 759 488 | 48 075 780 | 32·93 | 91 | 2 121 086 592 | 51·91 |
| 1992 | 5 419 255 808 | 43 650 812 | 29·90 | 88 | 2 565 702 144 | 47·34 |
| 1994 | 5 582 432 256 | 39 537 020 | 27·08 | 87 | 2 570 555 136 | 46·05 |
| 2002 | 6 204 095 488 | 39 758 172 | 27·24 | 88 | 2 996 419 584 | 48·30 |
| 2010 | 6 807 085 056 | 39 758 172 | 27·24 | 88 | 3 410 862 080 | 50·11 |
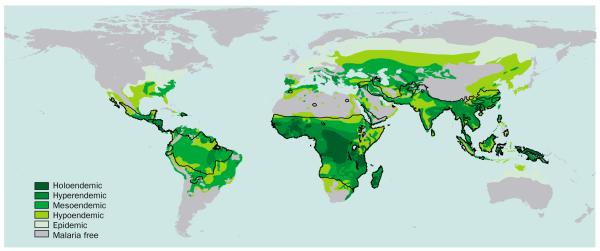
Despite a resurgence in the interest in mapping malaria endemicity in Africa,26–29 which has been used as an empirical basis to help estimate malaria burden,30,31 the only global map of malaria endemicity9 dates from Lysenko’s efforts in 1968 (figure 2). Endemicity as used by Lysenko9 was defined by the parasite rate in the 2–10-year age cohort (hypoendemic <0·1; mesoendemic 0·11–0·5; hyperendemic 0·51–0·75), except the holoendemic class (>0·75) where the parasite rate refers to the 1-year age group.32 This map was a major synthesis of historical records, documents, and maps of several malariometric indices (records of disease and vector presence and absence, spleen rates, parasite rates, sickle cell incidence, sporozoite rates, biting rates, etc) used to record malaria endemicity up until the late 1960s. These data were then interpolated globally for malaria at the peak of its assumed historical distribution, using a combination of expert opinion, global increase, temperature, and rainfall isohyets.9,33 The map is used here in its original form to frame a discussion on the regional variation in control effectiveness, since there is no modern global equivalent.
Figure 2.
The Lysenko map of global malaria endemicity. This map was digitised from the original source9 using the method outlined in figure 1. Endemicity as used by Lysenko9 is defined by the parasite rate (PR) in the 2–10-year age cohort (hypoendemic <0·1; mesoendemic 0·11–0·5; hyperendemic 0·51–0·75) except the holoendemic class (0·75) where the PR refers to the 1-year age group.32 The black line represents the 2002 limit of malaria risk17. Note that the “epidemic” class is restricted to the temperate regions in these maps and that this term is used differently today.
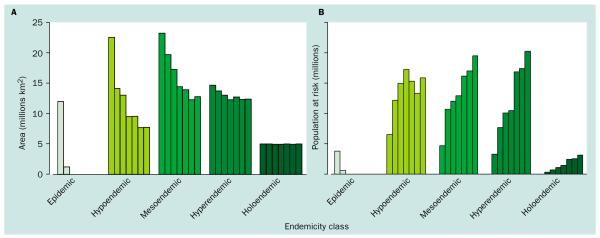
Using the contraction in the limits of all-cause malaria transmission (figure 1), assumed as a consequence of control efforts and development, and subdividing these changes by endemicity class (figure 2) shows that these gains have been most radical at lower endemicity rates with reductions of the epidemic, hypoendemic, and mesoendemic areas of 100%, 66%, and 45%, respectively, between 1900 and 2002 (table 2 and figure 3a). Conversely, there were negligible effects in areas of hyperendemic and holoendemic malaria with reductions of only 16% and 0%, respectively (table 2 and figure 3a). These numbers and the decreasing relative effect on the distribution over time (table 2) provide support for hypotheses of the increasing difficulty of malaria control with increasing intensity of malaria transmission.34–39 We now investigate how human populations have changed alongside this global restriction in the area of all-cause malaria.
Table 2.
The global area of malaria subdivided by endemicity class (1900–2002)
| Date | Epidemic | Hypoendemic | Mesoendemic | Hyperendemic | Holoendemic | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | _km_2 | % | _km_2 | % | _km_2 | % | _km_2 | % | _km_2 | % |
| 1900 | 11·99 | 15·45 | 22·57 | 29·08 | 23·30 | 30·03 | 14·69 | 18·94 | 5·05 | 6·50 |
| 1946 | 1·26 | 2·15 | 14·14 | 24·14 | 19·71 | 33·66 | 13·75 | 23·47 | 5·05 | 8·62 |
| 1965 | 0·02 | 0·04 | 13·04 | 24·38 | 17·25 | 32·25 | 13·07 | 24·42 | 4·98 | 9·31 |
| 1975 | 0 | 0·00 | 9·47 | 19·71 | 14·48 | 30·12 | 12·31 | 25·61 | 5·00 | 10·41 |
| 1992 | 0 | 0·00 | 9·55 | 21·88 | 13·94 | 31·94 | 12·75 | 29·21 | 5·00 | 11·47 |
| 1994 | 0 | 0·00 | 7·73 | 19·56 | 12·32 | 31·15 | 12·35 | 31·22 | 4·96 | 12·55 |
| 2002 | 0 | 0·00 | 7·75 | 19·50 | 12·81 | 32·21 | 12·39 | 31·17 | 5·01 | 12·60 |
Figure 3.
A histogram of (A) the global area (km2) and (B) population at risk of all-cause malaria risk subdivided by endemicity class (~1900–2002). Derivations of area and population at risk estimates are described in tables 1–3. The bars in each endemicity class show data for the years (left to right) 1900, 1946, 1965, 1975, 1992, 1994, and 2002.
Human populations at risk through time
The global human population has grown geometrically during the 20th century from approximately 1 to 6 billion (table 1). These demographics have important implications for the percentage of the human population exposed to all-cause malaria risk through time. The percentage of the global population at risk has decreased from 77% at the turn of the 20th century to a low of 46% in 1994. This figure increased to 48% in 2002 due to population growth in an unchanged geographic distribution. In absolute terms the numbers of people at risk have increased consistently from 0·9 to 3 billion over the same period (about 1900–2002; see table 1 for data and methods). At the turn of the 21st century, therefore, we estimate that 48% of the global population remain exposed to the risk of malaria, a situation that has deteriorated since the early 1990s and a figure substantially higher than the 40% widely cited.40–42
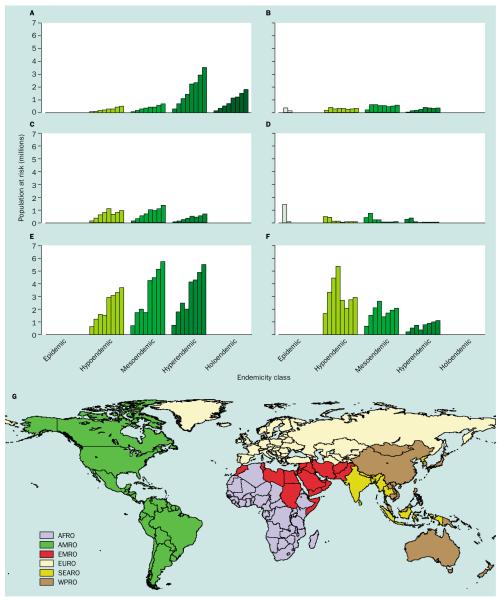
The changes in populations exposed to various rates of endemicity from around 1900–2002 are shown stratified by WHO region in figures 4a–g. These WHO regional groupings (figure 4g) are largely administrative but were originally defined to capture environmentally and epidemiologically coherent zones for public-health management.24 Certain anomalies exist, however, that make interpretation of regional malaria risk problematic, such as the inclusion of Somalia and Sudan in the Eastern Mediterranean Regional Office (EMRO). The European region (EURO) is the only grouping of countries to show a consistent decrease in populations at risk through time (figure 4d). The American region (AMRO) remained approximately stable in terms of populations at risk, as population growth compensated for substantial control gains during the 20th century (figure 4b). Limited growth in populations at risk are shown in the EMRO area (figure 4c), but the most striking changes are the sharp growth in populations at risk in the African Regional Office (AFRO) area (figure 4a) and particularly the South East Asia Regional Office (SEARO) area (figure 4e). In the AFRO area the population at risk grew from 0·06–0·65 billion during the 20th century, more than 80% of whom remain in areas of hyperendemic and holoendemic malaria. The SEARO area (dominated by India) has experienced even more dramatic growth from 0·2–1·5 billion people at risk, but unlike the AFRO area has only 37% of these populations in places defined as hyperendemic.9 Consistent growth in population at risk is not a feature of the Western Pacific region (WPRO; figure 4f) due to the marked reduction in the limits of transmission in China since 1975 (figure 1).44 These estimates of populations at risk, derived from crude maps of malaria endemicity, mask the very different public-health consequences of infection with P falciparum and P vivax,45 but do draw attention to a huge potential burden that is hard to quantify directly from official statistics.46 Furthermore, despite current emphasis on the AFRO area47 these analyses suggest that the SEARO region warrants greater international attention, an issue to which we return later. Finally, that much of this population growth will have been concentrated in urban areas is a significant confounder to global burden of malaria estimates and is the subject of significant contemporary research effort.
Figure 4.
Histograms of population at all-cause malaria risk (~1900–2010) subdivided by malaria endemicity and WHO regional grouping. (A) AFRO, (B) AMRO, (C) EMRO, (D) EURO, (E) SEARO, (F) WPRO, and (G) a map of WHO regional groupings. Derivations of area and population at risk estimates are described in tables 1–3. The WHO regional grouping map was generated from global administrative boundaries (Environmental Systems Research Institute Inc, Redlands, CA, USA) and county tables in annexes of the 2002 World Health Report43. The bars in each endemicity class show data for the years (left to right) 1900, 1946, 1965, 1975, 1992, 1994, and 2002.
Global malaria control from ~1900 to 2002
During the 19th century great improvements in the control of several communicable diseases were realised, chiefly as a result of environmental improvements.48–52 In parallel, improved social conditions (particularly housing) and changing land use (particularly agricultural practices) contributed significantly to the global reduction in the distribution of malaria.8,21,53–56 These gains from malaria control were often coincidental with economic and social development, forces that although spatially heterogeneous, have remained undiminished throughout the 20th century. Our analyses (figure 1 and table 1) show that, counterintuitively, all-cause malaria is not an obligate tropical disease but more precisely one that we have progressively restricted to the tropics in the 20th century through development and control.1,3,8,21,37,40,57–59 This global reduction followed several distinct phases.
The first “sanitation era” of malaria intervention focused primarily on environmental control of mosquito breeding sites,1,60–63 once Ross64–66 had discovered the importance of anophelene mosquitoes in the life-history of avian malaria in 1898 and Grassi showed the full transmission cycle of the human malarias later that year.67–69 The well-documented success in mosquito control in the Panama Canal,70–72 Indonesia,73 Malaysia,74 the mines and plantations of the Zambian copper belt,75,76 and the eradication of A_nopheles gambiae_ in Brazil77,78 and Egypt78,79 validated such approaches (despite some conspicuous failures in Sardinia,80 Sierra Leone, and India1,57,64). To generalise, the species sanitation approach was generally adopted where it was logistically feasible and there was a commercial incentive for investment.53,54,81 This type of intervention was broadly responsible for the preintervention (about 1900) to 1946 contraction in the global malaria distribution (figure 1 and table 1).
The discovery of the residual insecticide properties of dichlorodiphenyltrichloroethane (DDT) in the 1940s enabled, for the first time, large-scale, wide-area approaches to malaria control82 and its effect on those engaged in malaria interventions should not be underestimated. The WHO endorsed this approach through the global malaria-eradication programme (from approximately 1955–1969)83 using DDT to interrupt transmission in an “attack phase” and chemoprophylaxis to eradicate malaria in the later “consolidation phase” of intervention.36,84–87 The successes, long-term benefits, and problems of consolidation and maintenance have been well documented,57,81,88 along with the subsequent resurgence of malaria in India89 and the complete disregard of sub-Saharan Africa in the “global” eradication efforts.84 The shrinkage in the global malaria distribution from 1946–1965 (figure 1 and table 1) largely coincides with this eradication era.
Since 1965 the further restriction of the global distribution of malaria has resulted from continued national efforts in the developing economies of meso-America and South America, the latitudinal extremes of Africa,90 the middle east, and China.44 Despite these local successes no significant impact was made on the global limits of malaria risk between 1992 and 2002 (figure 1 and table 1).
In 1998 the Roll Back Malaria movement was launched as a mentoring, coordinating, and advocacy vehicle for international malaria control.91–94 Its widely publicised mandate is to reduce the global malaria burden of risk, morbidity, and mortality by half by 2010. To realise this bold ambition Roll Back Malaria has four main targets: to achieve a 60% coverage of children and pregnant women with insecticide treated nets (ITNs), to have 60% of malaria cases receive effective treatment within 24 h of the onset of symptoms, for 60% of pregnant women to receive intermittent presumptive therapy (IPT), and for 60% of epidemics to be detected within 2 weeks of onset and then responded to appropriately within a further 2 weeks.47 Implicit in the strategy used to formulate these targets is a focus on malaria in the highly endemic areas of sub-Saharan Africa where most of the remaining global burden of malaria is thought to be.95,43 We explore this assumption and its implication for international malaria-control priorities in the following section.
The consequences of changing global population at risk
Malaria burden
There has been a renewed interest in establishing precise estimates of morbidity and mortality as part of the Global Burden of Disease Programme.96,97 Almost all of this work, including studies on acute respiratory-tract infections,98 HIV/AIDS,99 and malaria,30,31 has been driven by the use of empirical survey data, modelled and extrapolated to wider areas. These studies are all critically dependent on the denominator population at risk. For a vector-borne disease such as malaria, the population at risk is a function of the coincidence of human population and malaria infection risk and endemicity.30,31 Because such an empirical approach has not been developed for regions outside AFRO, the most widely cited estimate of the global proportion of malaria morbidity and mortality borne by AFRO is 90%,43,95 with estimates ranging from 62 to 93%.24,43,100,101 The variation in these estimates has wide-ranging implications for policy and the strategic emphasis of the Roll Back Malaria movement.
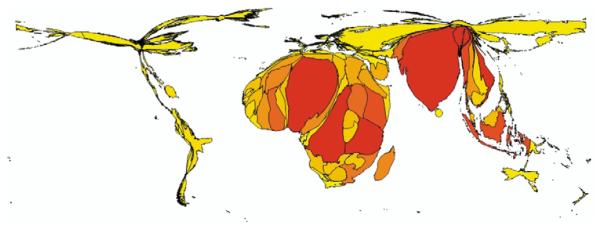
To highlight this issue we extended our descriptions of populations at risk of all-cause malaria to make preliminary estimates of regional variation in malaria exposure globally. To achieve this it is necessary both to assess regional variation in populations exposed to the various rates of malaria endemicity and to quantify the important distinction between P falciparum and P vivax burden.45 Using the 2002 malaria distribution (figure 1),17 the 1968 Lysenko malaria endemicity map (figure 2),9 and population distribution projected to 2002 (table 1) a national prevalence index has been computed. This national prevalence index was estimated at the country level by assuming each endemicity class was described by its midpoint parasite rate value as follows: (population exposed to hypoendemic × 0·05) + (population exposed to mesoendemic × 0·305) + (population exposed to hyperendemic × 0·63) + (population exposed to holoendemic × 0·875). We then used the product of the national prevalence and a P falciparum index (the proportion of malaria cases reported nationally in 1993 that were due to P falciparum)15,102 to create a national falciparum prevalence (N_f_P). This approach makes several assumptions: first, that the underlying maps used to generate these metrics are relatively precise; second, that the national prevalence may be based on the total population (since national data on the variation in age-specific infection rates are not readily available); third, that the P falciparum index estimated in 1993 is compatible with endemicity estimates from the late 1960s; and fourth, that the P falciparum index is the same across all endemicity classes in a country. Given these assumptions we use the N_f_P metric to explore malaria-risk distribution only at the regional level (table 4) and have displayed the resulting N_f_P metric as a cartogram,103 a graphic that depicts countries in proportion to some attribute other than area, to help visualise these variations (figure 5).
Table 4.
Estimates of malaria morbidity and mortality by WHO administrative region
| WHO regions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Year | AFRO | AMRO | EMRO | EURO | SEARO | WPRO | Total | |
| WHO (2002) 43 | ||||||||
| 2001 | Morbidity | 342 814 347 | 3 798 292 | 14 894 969 | 0 | 32 930 363 | 2 238 314 | 396 676 285 |
| Percentage | 86·4 | 1·0 | 3·8 | 0·0 | 8·3 | 0·6 | 100·0 | |
| Mortality | 962 736 | 1 445 | 54 570 | 160 | 94 380 | 10 474 | 1 123 764 | |
| Percentage | 85·7 | 0·1 | 4·9 | 0·0 | 8·4 | 0·9 | 100·0 | |
| Scenario A | ||||||||
| 2002 | NP | 334 028 822 | 38 504 919 | 73 799 354 | 4 736 720 | 482 235 352 | 134 712 740 | 1 068 042 048 |
| Percentage | 31·3 | 3·6 | 6·9 | 0·4 | 45·2 | 12·6 | 100·0 | |
| NfP | 334 028 822 | 7 475 574 | 35 976 263 | 0 | 206 563 944 | 48 408 818 | 632 453 421 | |
| Percentage | 52·8 | 1·2 | 5·7 | 0·0 | 32·7 | 7·7 | 100·0 | |
| 2010 | NP | 403 084 278 | 43 575 514 | 90 974 069 | 5 186 586 | 541 603 144 | 146 320 691 | 1 230 774 481 |
| Percentage | 32·8 | 3·5 | 7·4 | 0·4 | 44·0 | 11·9 | 100·0 | |
| NfP | 403 084 278 | 8 310 592 | 44 297 793 | 0 | 231 365 224 | 54 311 995 | 741 369 882 | |
| Percentage | 54·4 | 1·1 | 6·0 | 0·0 | 31·2 | 7·3 | 100·0 | |
| Scenario B | ||||||||
| 2002 | NP | 334 028 822 | 12 810 398 | 22 857 751 | 1 633 218 | 175 340 349 | 40 328 276 | 586 998 814 |
| Percentage | 56·9 | 2·2 | 3·9 | 0·3 | 29·9 | 6·9 | 100·0 | |
| NfP | 334 028 822 | 1 706 170 | 10 709 060 | 0 | 75 602 014 | 17 938 499 | 439 984 565 | |
| Percentage | 75·9 | 0·4 | 2·4 | 0·0 | 17·2 | 4·1 | 100·0 | |
| 2010 | NP | 403 084 278 | 14 613 880 | 28 530 282 | 1 778 624 | 197 290 551 | 44 128 430 | 689 426 046 |
| Percentage | 58·5 | 2·1 | 4·1 | 0·3 | 28·6 | 6·4 | 100·0 | |
| NfP | 403 084 278 | 1 901 788 | 13 405 644 | 0 | 84 798 879 | 20 150 770 | 523 341 360 | |
| Percentage | 77·0 | 0·4 | 2·6 | 0·0 | 16·2 | 3·9 | 100·0 |
Figure 5.
The national falciparum prevalence (NfP) cartogram for 2002. The NfP was calculated using the method outlined in table 4. These continuous area cartograms103 were generated using MAPresso (http://www.mapresso.com), a public domain Java applet. Ten iterations were used.
It is hard to be unimpressed by the scale of the problem in AFRO (53% of global N_f_P). The absolute magnitude of the P falciparum malaria-infection burden in SEARO (33% of global N_f_P) is also compelling (table 4 and figure 5) and not incompatible with recent analyses, which report a significant proportion of global childhood mortality in the SEARO area.104 The implications of such analyses cannot be dismissed simply by arguing that control and development have changed so fundamentally the endemicity in regions outside AFRO since the late 1960s. If we hypothesise this to be the case and recalculate N_f_P by assuming that in all areas outside AFRO endemicity has been so radically reduced by development and control that all areas have stepped down one endemicity class9 (ie, from hyperendemic to mesoendemic, from mesoendemic to hypoendemic, and from hypoendemic to zero risk), we still have 25% of the N_f_P outside AFRO (table 4, scenario b).
There are differences between P falciparum malaria risk and resulting morbidity and mortality outcomes experienced in different populations of the world.105 It is beyond the scope of this review to attempt to model morbidity and mortality globally. Reconciling N_f_P estimates with national level malaria reporting is a logical and important extension of this work. It is perhaps worth noting, however, that while mortality rates from P falciparum infection are on average nine per 1000 in the under-five populations of AFRO,31,106 they range from 0·1–1 per 1000 and 0·01–0·1 per 1000 (in all age groups) in SEARO countries such as Myanmar (Burma) and Sri Lanka, respectively.105 This order of magnitude difference in mortality risk means that most malaria mortality is likely still to be in AFRO. The absolute magnitude of populations at risk outside of AFRO (table 2 and table 4), however, indicate that malaria-attributable mortality will not be trivial, particularly in SEARO, and that morbidity is likely to be substantial. What these analyses suggest therefore is that reliance on WHO country reports for disease-burden estimates outside AFRO in calculating the global malaria burden must be augmented with alternative approaches used to estimate the burden in AFRO30,31 to enable sensible comparisons. The two most important implications of regional variations in the distributions of risk relate to the current status of antimalarial drug management and the extent to which vector control is likely to be effective outside AFRO.
Drug resistance
The idea that populations living in areas of low malaria transmission are catalysts for the development of antimalarial drug resistance is now supported on both theoretical107–109 and empirical grounds.110–113 These analyses have shown that almost 30% of the global population at risk from malaria reside in areas of hypoendemic and mesoendemic transmission in the SEARO region (table 3), including Thailand, the focus for the origin of drug-resistant malaria.6,114,115 The spread of drug-resistant malaria parasites from SEARO to AFRO has provided an explanation for the rising mortality from malaria in this region since 1990.106,116–118 It is possible therefore that aggressive efforts to limit transmission outside of AFRO might have a larger than expected global effect on public health by helping delay the development of drug resistance.
Table 3.
Population by malaria endemicity class (1900–2010)
| Date | Epidemic | Hypoendemic | Mesoendemic | Hyperendemic | Holoendemic | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | n | % | n | % | n | % | n | % | n | % |
| 1900 | 183·81 | 20·60 | 313·22 | 35·10 | 223·14 | 25·01 | 158·51 | 17·76 | 13·76 | 1·54 |
| 1946 | 22·38 | 1·37 | 583·46 | 35·67 | 512·56 | 31·33 | 366·47 | 22·40 | 34·30 | 2·10 |
| 1965 | 0·07 | 0·00 | 720·21 | 37·43 | 577·78 | 30·02 | 484·30 | 25·17 | 52·62 | 2·73 |
| 1975 | 0·09 | 0·00 | 830·13 | 39·14 | 619·08 | 29·19 | 503·21 | 23·72 | 69·64 | 3·28 |
| 1992 | 0 | 0·00 | 736·74 | 28·72 | 775·70 | 30·23 | 811·34 | 31·62 | 115·77 | 4·51 |
| 1994 | 0 | 0·00 | 638·86 | 24·85 | 815·08 | 31·71 | 836·36 | 32·54 | 122·55 | 4·77 |
| 2002 | 0 | 0·00 | 761·72 | 25·42 | 937·52 | 31·29 | 974·30 | 32·52 | 150·28 | 5·02 |
| 2010 | 0 | 0·00 | 849·95 | 24·92 | 1 051·86 | 30·84 | 1 126·52 | 33·03 | 181·95 | 5·33 |
Types of vector control
The difficulty of malaria intervention in areas of high transmission is a tenet of malariology34–39,119,120 and the debate has often centred on the holoendemic areas of sub-Saharan Africa. The relatively high anthropophily,121 longevity, and density of the A gambiae complex and Anopheles funestus and their resulting efficiency as malaria vectors in sub-Saharan Africa3,122–124 results in an average annual entomological inoculation rate for the continent (based on 159 samples of malarious areas) of 121 infected bites per person per annum.29,125 Furthermore, these high rates of transmission combined with the dominance of P falciparum in sub-Saharan Africa15,102 warn against naive extrapolation of control successes in temperate and subtropical parts of the world, before considering a host of other economic, logistic, and social constraints to control.126
Despite the widely accepted intransigence of malaria in holoendemic areas, opinions on appropriate control strategies in such areas differ.34,37,39,61,78,127,128 We do not attempt to reignite this debate but several aspects of these analyses are noteworthy. First, any optimistic prospect derived from the reductions in the total area malarious by endemicity class is confounded by demographic changes. Numbers at risk have increased relentlessly in all endemicity classes except the epidemic one (table 3 and figures 3a–b). Second, more than half of the 2002 malaria distribution is hypoendemic (19·5%) or mesoendemic (32·2%; table 3). Regardless of the position adopted on the choice of eradication versus control in hyperendemic or holoendemic areas (and/or the exact suite of interventions that could be applied), more than 50% of those at risk of malaria live in areas where sustained control is inherently epidemiologically feasible. In the SEARO area such control has a strong historical precedent for success89 and more ready access to the resources needed to implement control than the AFRO area.
Implications for rolling back malaria
Despite the international support and political will for malaria control having improved in the past 5 years,91–94,129–131 doubts about the efficacy, focus, and particularly the financing of international initiatives have been raised132–134 with a concomitant push for strategic changes in the direction and emphasis of research and control.135–137 The Commission for Macroeconomics and Health has estimated that an immediate injection of at least US$1 billion per annum is needed to enable the Roll Back Malaria movement to start to work towards its goals138 and that this should be boosted to between $1·5–2·5 billion annually by 2007 if it is to have any chance of meeting them. Recent analyses of donor expenditure suggest that these financial targets are far from being met.133,134
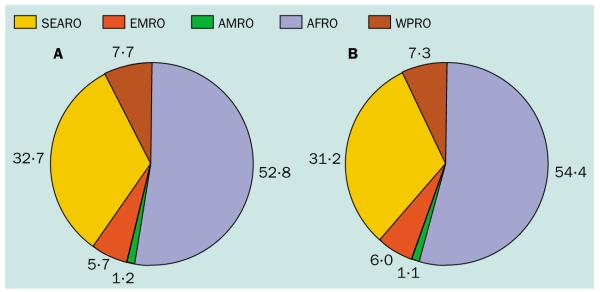
In this environment, the central goal of the Roll Back Malaria movement91–94 to decrease by 50% the global malaria burden (risk, infection, morbidity, or mortality?) by 2010 by meeting targets on ITN distribution, IPT in pregnant women, prompt and effective treatment and epidemic preparedness, looks increasingly difficult. We emphasise here that its implementation and effect monitoring are made more problematic by a lack of accurate information on the global distribution of populations and risk at various stages of malaria endemicity and the resulting distribution of malaria mortality and morbidity. Addressing this issue is a priority. We have also presented here, through analyses of the N_f_P distribution between WHO regions (table 4 and figure 6a), a preliminary attempt to define the problem facing Roll Back Malaria in the future. Such analyses have illustrated how human demographics continually shift public-health goalposts.
Figure 6.
Pie charts of the national falciparum prevalence (NfP) by WHO region for 2002 (A) and projected to 2010 (B). Data derived as for figure 5 and estimated using population projected for 2010 as described in table 1.
Extending these analyses of the regional distribution of N_f_P to 2010 in a hypothetical world where population has grown according to current projections (and all else is equal including risk and control) we have also made various predictions (table 4 and figure 6b). The higher population growth rates in the tropics will increase the percentage of the global population exposed to infection risk to above 50% (table 1), but, surprisingly, the proportion of the N_f_P outside AFRO will remain largely unchanged at approximately half (46%; figure 6b).
Conclusions
No recent global maps of malaria endemicity have been developed since those of Lysenko in 1968,9 despite significant advances in the collection of empirical data, global environmental information from satellites, and the statistical techniques that can be used to integrate them.28,29 In addition, given the poor health information systems in the AFRO area it is paradoxical that some of the best information on malaria endemicity and burden exists for this region.27–29 We suggest therefore that updating global maps of malaria endemicity is a priority and that it would provide regionally consistent measures of population at risk that could contribute to continuing efforts to refining global burden of disease estimates for malaria. Preliminary analyses of existing endemicity maps indicate the probable extent of malaria infection risk outside the AFRO area, and particularly in the SEARO region, conclusions that remain robust even under very optimistic scenarios of endemicity reduction. These populations are of particular political importance given the historical success of aggressive vector control in areas of low-to-medium malaria endemicity and the dangers of inaction in facilitating the emergence of drug-resistant malaria parasites. We stress, however, that the global burden of malaria is still dominated by the AFRO countries which are least able to raise financial resources to tackle their high rates of malaria death and disability.31 In summary, while international priorities seem broadly justified in their focus on the AFRO area, there is an urgent need to define the global extent of malaria risk and disease to enable effective and equitable prioritisation of investment and strategic direction by Roll Back Malaria and others.
Search strategy and selection criteria.
Data for this review were identified through PubMed Medline, the Bodleian library at Oxford, manual searches of the WHO’s Weekly Epidemiological Record (http://www.who.int/wer/en/), suggestions of reviewers (formal and informal), and the bibliographies of the resulting articles. We used the following Boolean search statement: “malaria” and (“distribution” and “maps”), “malaria” and (“burden” or “risk”), “malaria” and (“control” and “campaign”). Articles in all languages were selected and Lysenko (1968) translated from Russian.
Acknowledgments
We thank Alastair Graham, Sarah Hay, Eline Korenromp, Francois Nosten, Kevin Marsh, Ellis McKenzie, Sarah Randolph, David Rogers, Dennis Shanks, Nick White, and two anonymous referees for comments on earlier drafts of the manuscript. We are also grateful to Adrian Herzog for Mapresso and help in the production of cartograms, Andy Nelson for advice on the projection of human population figures, and Nora Markova for helping translate the relevant sections of Lysenko (1968) into English. SIH is funded by a Research Career Development Fellowship from the Wellcome Trust (#069045). RWS is a Wellcome Trust Senior Research Fellow (#058992) and acknowledges the support of the Kenyan Medical Research Institute (KEMRI). This paper is published with the permission of the director of KEMRI.
Footnotes
Conflicts of interest
Since completing this research SIH, CAG, and RWS have undertaken an agreement to perform work (APW), for which remuneration will be received, with RBM/WHO to estimate global, regional, national, and subnational population at risk of various levels of malaria endemicity, by age group and parasite type in countries outside Africa.
References
- 1.Russell PF. Man’s mastery of malaria. Oxford University Press; Oxford: 1955. [Google Scholar]
- 2.Wiesenfeld SL. Sickle-cell trait in human biological and cultural evolution. Science. 1967;157:1134–40. doi: 10.1126/science.157.3793.1134. [DOI] [PubMed] [Google Scholar]
- 3.Coluzzi M. The clay feet of the malaria giant and its African roots: hypotheses and inferences about origin, spread and control of Plasmodium falciparum. Parassitologia. 1999;41:277–83. [PubMed] [Google Scholar]
- 4.Joy DA, Feng XR, Mu JB, et al. Early origin and recent expansion of Plasmodium falciparum. Science. 2003;300:318–21. doi: 10.1126/science.1081449. [DOI] [PubMed] [Google Scholar]
- 5.Hume JCC, Lyons EJ, Day KP. Human migration, mosquitoes and the evolution of Plasmodium falciparum. Trends Parasitol. 2003;19:144–49. doi: 10.1016/s1471-4922(03)00008-4. [DOI] [PubMed] [Google Scholar]
- 6.Hartl DL. The origin of malaria: mixed messages from genetic diversity. Nature Reviews Microbiol. 2004;2:15–22. doi: 10.1038/nrmicro795. [DOI] [PubMed] [Google Scholar]
- 7.De Zulueta J. Changes in the geographical distribution of malaria throughout history. Parassitologia. 1987;29:193–205. [PubMed] [Google Scholar]
- 8.De Zulueta J. Malaria and ecosystems: from prehistory to posteradication. Parassitologia. 1994;36:7–15. [PubMed] [Google Scholar]
- 9.Lysenko AJ, Semashko IN. Geography of malaria. A medico-geographic profile of an ancient disease. In: Lebedew AW, editor. Itogi Nauki: Medicinskaja Geografija. Academy of Sciences, USSR; Moscow: 1968. pp. 25–146. [Google Scholar]
- 10.Russell PF. The present status of malaria in the world. Am J Trop Med Hyg. 1952;1:111–23. doi: 10.4269/ajtmh.1952.1.111. [DOI] [PubMed] [Google Scholar]
- 11.Russell PF. World-wide malaria distribution, prevalence and control. Am J Trop Med Hyg. 1956;5:937–56. doi: 10.4269/ajtmh.1956.5.937. [DOI] [PubMed] [Google Scholar]
- 12.Pampana EJ, Russell PF. Malaria: a world problem. Chronicle World Health Organ. 1955;9:31–96. [Google Scholar]
- 13.WHO Malaria eradication in 1965. Chronicle World Health Organ. 1966;20:286–300. [PubMed] [Google Scholar]
- 14.WHO Information on the world malaria situation: January to December 1975. Wkly Epidemiol Rec. 1977;52:21–34. [Google Scholar]
- 15.WHO World malaria situation in 1992. Part II. Wkly Epidemiol Rec. 1994;69:317–21. [PubMed] [Google Scholar]
- 16.WHO World malaria situation in 1994. Part I. Wkly Epidemiol Rec. 1997;72:269–74. [PubMed] [Google Scholar]
- 17.WHO . Worldwide malaria distribution in 2002. Public Health Mapping Group, World Health Organization; Geneva: 2003. [Google Scholar]
- 18.Snow RW, Gilles HM. The epidemiology of malaria. In: Warrell DA, Gilles HM, editors. Essential malariology. 4th edn Arnold; London: 2002. pp. 85–106. [Google Scholar]
- 19.Dutta HM, Dutt AK. Malaria ecology: a global perspective. Soc Sci Med. 1978;12:69–84. [PubMed] [Google Scholar]
- 20.Sindon RE, Gilles HM. The malaria parasites. In: Warrell DA, Gilles HM, editors. Essential malariology. 4th edn Arnold; London: 2002. pp. 8–34. [Google Scholar]
- 21.Haworth J. The global distribution of malaria and the present control effort. In: Wernsdorfer WH, McGregor I, editors. Malaria: principles and practice of malariology. Churchill Livingstone; Edinburgh: 1988. pp. 1379–420. [Google Scholar]
- 22.Hamoudi A, Sachs JD. The changing global distribution of malaria: a review. Centre for International Development at Harvard University; Harvard, MA: 1999. [Google Scholar]
- 23.Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64:85–96. doi: 10.4269/ajtmh.2001.64.85. [DOI] [PubMed] [Google Scholar]
- 24.Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev. 2002;15:564–94. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diechmann U, Balk D, Yetman G. Transforming population data for interdisciplinary usages: from census to grid. The Center for International Earth Science Information Network (CIESIN); New York: 2001. [Google Scholar]
- 26.Snow RW, Marsh K, Sueur D Le. The need for maps of transmission intensity to guide malaria control in Africa. Parasitol Today. 1996;12:455–57. [Google Scholar]
- 27.Craig MH, Snow RW, Sueur D le. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol Today. 1999;15:105–11. doi: 10.1016/s0169-4758(99)01396-4. [DOI] [PubMed] [Google Scholar]
- 28.Hay SI, Omumbo JA, Craig MH, Snow RW. Earth observation, geographic information systems and Plasmodium falciparum malaria in sub-Saharan Africa. Adv Parasitol. 2000;47:173–215. doi: 10.1016/s0065-308x(00)47009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers DJ, Randolph SE, Snow RW, Hay SI. Satellite imagery in the study and forecast of malaria. Nature. 2002;415:710–15. doi: 10.1038/415710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snow RW, Craig M, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant population. Bull World Health Organ. 1999;77:624–40. [PMC free article] [PubMed] [Google Scholar]
- 31.Snow RW, Craig MH, Newton CRJC, Steketee RW. The public health burden of Plasmodium falciparum malaria in Africa: deriving the numbers. The Disease Control Priorities Project (DCPP); Washington DC: 2003. p. 75. Working Paper Number 11. [Google Scholar]
- 32.Metselaar D, Van Thiel PH. Classification of malaria. Trop Geogr Med. 1959;11:157–61. [Google Scholar]
- 33.Lysenko AJ, Beljaev AE. An analysis of the geographical distribution of Plasmodium ovale. Bull World Health Organ. 1969;40:383–94. [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson DB, Garnham PCC, Swellengrebel NH. A review of hyperendemic malaria. Trop Dis Bull. 1950;47:677–98. [PubMed] [Google Scholar]
- 35.Macdonald G. Epidemiological basis of malaria control. Bull World Health Organ. 1956;15:613–26. [PMC free article] [PubMed] [Google Scholar]
- 36.Macdonald G. Eradication of malaria. Public Health Rep. 1965;80:870–80. [PMC free article] [PubMed] [Google Scholar]
- 37.Onori E, Beales PF, Gilles HM. From malaria eradication to malaria control: the past, present and the future. In: Gilles HM, Warrell DA, editors. Essential malariology. 3rd edn Edward Arnold; London: 1993. pp. 267–82. [Google Scholar]
- 38.Mouchet T, Carnevale P. Malaria endemicity in the various phytogeographic and climatic areas of Africa, south of Sahara. Southeast Asian J Trop Med Public Health. 1981;12:439–40. [Google Scholar]
- 39.Mouchet J, Manguin S, Sircoulon J, et al. Evolution of malaria in Africa for the past 40 years: impact of climatic and human factors. J Am Mosq Control Assoc. 1998;14:121–30. [PubMed] [Google Scholar]
- 40.WHO . The world health report 1999: making a difference. World Health Organization; Geneva: 1999. Rolling back malaria; pp. 49–63. [Google Scholar]
- 41.WHO . WHO expert committee on malaria: twentieth report. World Health Organization; Geneva: 2000. [Google Scholar]
- 42.Trigg PI, Kondrachine AV. The current global malaria situation. In: Sherman IW, editor. Malaria: parasite biology, pathogenesis, and protection. American Society for Microbiology Press; Washington, DC: 1998. pp. 11–22. [Google Scholar]
- 43.WHO . The world health report 2002: reducing risks, promoting healthy life. World Health Organization; Geneva: 2002. [DOI] [PubMed] [Google Scholar]
- 44.Yip K. Antimalaria work in China: a historical perspective. Parassitologia. 1998;40:29–38. [PubMed] [Google Scholar]
- 45.Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 46.Sharma VP. Determinants of malaria in South Asia. In: Casman EA, Dowlatabadi H, editors. The contextual determinants of malaria. Resources for the Future Press; Washington, DC: 2002. pp. 110–132. [Google Scholar]
- 47.WHO/UNICEF . The African malaria report 2003. World Health Organization / United Nations Children’s Fund; Geneva/New York: 2003. p. 120. [Google Scholar]
- 48.Omran AR. The epidemiologic transition: a theory of the epidemiology of population change. Milbank Quarterly. 1971;49:509–38. [PubMed] [Google Scholar]
- 49.Mackenbach JP. The epidemiologic transition theory. J Epidemiol Community Health. 1994;48:329–31. doi: 10.1136/jech.48.4.329-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtin PD. Death by migration: Europe’s encounter with the tropical world in the nineteenth century. Cambridge University Press; Cambridge: 1989. Sanitation and tropical hygiene midcentury; pp. 40–61. [Google Scholar]
- 51.Doll R. Health and the environment in the 1990s. Am J Public Health. 1992;82:933–41. doi: 10.2105/ajph.82.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 53.Bradley GH. A review of malaria control and eradication in the United States. Mosquito News. 1966;26:462–70. [Google Scholar]
- 54.Bruce-Chwatt LJ, De Zulueta J. The rise and fall of malaria in Europe. A historico-epidemiological study. Oxford University Press; Oxford: 1980. [Google Scholar]
- 55.Kitron U. Malaria, agriculture, and development—lessons from past campaigns. Int J Health Serv. 1987;17:295–326. doi: 10.2190/68UG-BAWQ-YXCT-HFKT. [DOI] [PubMed] [Google Scholar]
- 56.Dobson MJ. Contours of death and disease in early modern England. Cambridge University Press; Cambridge: 1997. The epidemiological landscapes of the past; pp. 493–539. [Google Scholar]
- 57.Harrison G. Mosquitoes, malaria and man: a history of hostilities since 1880. John Murray; London: 1978. [Google Scholar]
- 58.De Zulueta J. Dealing with malaria in the last 60 years. A personal experience. Parassitologia. 2000;42:87–90. [PubMed] [Google Scholar]
- 59.Gilles HM. Historical outline. In: Warrell DA, Gilles HM, editors. Essential Malariology. 4th edn Arnold; London: 2002. pp. 1–7. [Google Scholar]
- 60.Covell G. Malaria control by anti-mosquito measures. 2nd edn Thacker Spink; London: 1941. [Google Scholar]
- 61.Bradley DJ. Malaria: old infections, changing epidemiology. Health Trans Rev. 1992;2(suppl):137–52. [Google Scholar]
- 62.Gilles HM, Lucas AO. Tropical medicine: 100 years of progress. Br Med Bull. 1998;54:269–80. doi: 10.1093/oxfordjournals.bmb.a011687. [DOI] [PubMed] [Google Scholar]
- 63.Konradsen F, van der Hoek W, Amerasinghe FP, Mutero C, Boelee E. Engineering and malaria control: learning from the past 100 years. Acta Tropica. 2004;89:99–108. doi: 10.1016/j.actatropica.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 64.Ross R. Memoirs, with a full account of the great malaria problem and its solution. John Murray; London: 1923. [Google Scholar]
- 65.Nye ER, Gibson ME. Ronald Ross: malariologist and polymath: a biography. Macmillan Press Ltd; London: 1997. The great malaria problem; pp. 57–76. [Google Scholar]
- 66.Bynum WF. Ronald Ross and the malaria-mosquito cycle. Parassitologia. 1999;41:49–52. [PubMed] [Google Scholar]
- 67.Sherman IW. A brief history of malaria and discovery of the parasite’s life cycle. In: Sherman IW, editor. Malaria: parasite biology, pathogenesis, and protection. American Society for Microbiology Press; Washington, DC: 1998. pp. 3–10. [Google Scholar]
- 68.Fantini B. The concept of specificity and the Italian contribution to the discovery of the malaria transmission cycle. Parassitologia. 1999;41:39–47. [PubMed] [Google Scholar]
- 69.Dobson MJ. The malaria centenary. Parassitologia. 1999;41:21–32. [PubMed] [Google Scholar]
- 70.Gorgas WC. Sanitation in Panama. Appleton; London: 1915. [Google Scholar]
- 71.Simmons JS. Malaria in Panama. Johns Hopkins University Press; Baltimore: 1939. [Google Scholar]
- 72.McCullough D. The path between the seas: the creation of the Panama canal, 1870–1914. Simon and Schuster; New York: 1977. [Google Scholar]
- 73.Swellengrebel NH. How the malaria service in Indonesia came into being: 1898–1948. J Hyg. 1950;48:146–57. doi: 10.1017/s0022172400014972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watson M. The prevention of malaria in the Federated Malay States. John Murray; London: 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watson M. African highway: the battle for health in central Africa. John Murray; London: 1953. [Google Scholar]
- 76.Utzinger J, Tozan Y, Doumani F, Singer BH. The economic payoffs of integrated malaria control in the Zambian copperbelt between 1930 and 1950. Trop Med Int Health. 2002;7:657–77. doi: 10.1046/j.1365-3156.2002.00916.x. [DOI] [PubMed] [Google Scholar]
- 77.Soper FL, Wilson DB. Anopheles gambiae in Brazil, 1930–1940. Rockefeller Foundation; New York: 1943. [Google Scholar]
- 78.Killeen GF, Fillinger U, Kiche I, Gouagna LC, Knols BGJ. Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa? Lancet Infect Dis. 2002;2:618–27. doi: 10.1016/s1473-3099(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 79.Shousha AT. Species eradication; eradication of Anopheles gambiae from Upper Egypt, 1942–1945. Bull World Health Organ. 1948;1:309–52. [PMC free article] [PubMed] [Google Scholar]
- 80.Logan JA. The Sardinian Project: an experiment in the eradication of an indigenous malaria vector. Johns Hopkins Press; Baltimore: 1953. [Google Scholar]
- 81.De Zulueta J. The end of malaria in Europe: an eradication of the disease by control measures. Parassitologia. 1998;40:245–46. [PubMed] [Google Scholar]
- 82.Najera JA. Malaria and the work of WHO. Bull World Health Organ. 1989;67:229–43. [PMC free article] [PubMed] [Google Scholar]
- 83.Litsios S. Malaria control and the future of international public health. In: Casman EA, Dowlatabadi H, editors. The contextual determinants of malaria. Resources for the Future; Washington DC: 2002. pp. 292–328. [Google Scholar]
- 84.Litsios S. The tomorrow of malaria. Pacific Press; Wellington: 1966. [Google Scholar]
- 85.Pampana EJ. A textbook of malaria eradication. Second edn Oxford University Press; Oxford: 1969. [Google Scholar]
- 86.Beales PF, Gilles HM. Rationale and technique of malaria control. In: Warrell DA, Gilles HM, editors. Essential malariology. 4th edn Arnold; London: 2002. pp. 107–90. [Google Scholar]
- 87.Spielman A, D’Antonio M. Mosquito: a natural history of our most persistent and deadly foe. Faber and Faber; London: 2001. The great mosquito crusade; pp. 141–78. [Google Scholar]
- 88.Kitron U, Spielman A. Suppression of transmission of malaria through source reduction—antianopheline measures applied in Israel, the United States, and Italy. Rev Infect Dis. 1989;11:391–406. doi: 10.1093/clinids/11.3.391. [DOI] [PubMed] [Google Scholar]
- 89.Sharma VP. Re-emergence of malaria in India. Indian J Med Res. 1996;103:26–45. [PubMed] [Google Scholar]
- 90.Smith A, Hansford CF, Thomson JF. Malaria along the southernmost fringe of its distribution in Africa: epidemiology and control. Bull World Health Organ. 1977;55:95–103. [PMC free article] [PubMed] [Google Scholar]
- 91.Nabarro D. International health beyond 2000. Nat Med. 1998;4:762–63. doi: 10.1038/nm0798-762. [DOI] [PubMed] [Google Scholar]
- 92.Nabarro DN, Tayler EM. The roll back malaria campaign. Science. 1998;280:2067–68. doi: 10.1126/science.280.5372.2067. [DOI] [PubMed] [Google Scholar]
- 93.Nabarro DN. Roll back malaria. Parassitologia. 1999;41:501–04. [PubMed] [Google Scholar]
- 94.Nabarro DN, Mendis KN. Roll back malaria is unarguably both necessary and possible. Bull World Health Organ. 2000;78:1454–55. [PMC free article] [PubMed] [Google Scholar]
- 95.WHO . The world health report 1999: making a difference. World Health Organization; Geneva: 1999. [Google Scholar]
- 96.Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 97.Murray CJL, Lopez AD, editors. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020. Harvard University Press; Cambridge: 1996. [Google Scholar]
- 98.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 99.Walker N, Schwartlander B, Bryce J. Meeting international goals in child survival and HIV/AIDS. Lancet. 2002;360:284–89. doi: 10.1016/S0140-6736(02)09550-8. [DOI] [PubMed] [Google Scholar]
- 100.Stürchler D. How much malaria is there worldwide? Parasitol Today. 1989;5:39–40. doi: 10.1016/0169-4758(89)90188-9. [DOI] [PubMed] [Google Scholar]
- 101.Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64:1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- 102.WHO World malaria situation in 1993. Part II. Wkly Epidemiol Rec. 1996;71:25–29. [PubMed] [Google Scholar]
- 103.Dougenik JA, Chrisman NR, Niemeyer DR. An algorithm to construct continuous area cartograms. Professional Geographer. 1985;37:75–81. [Google Scholar]
- 104.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 105.Alles HK, Mendis KN, Carter R. Malaria mortality rates in south Asia and in Africa: implications for malaria control. Parasitol Today. 1998;14:369–75. doi: 10.1016/s0169-4758(98)01296-4. [DOI] [PubMed] [Google Scholar]
- 106.Korenromp EL, Williams BG, Gouws E, Dye C, Snow RW. Measurement of trends in childhood malaria mortality in Africa: an assessment of progress toward targets based on verbal autopsy. Lancet Infect Dis. 2003;3:349–58. doi: 10.1016/s1473-3099(03)00657-1. [DOI] [PubMed] [Google Scholar]
- 107.Hastings IM. A model for the origin and spread of drug resistant malaria. Parasitology. 1997;115:133–41. doi: 10.1017/s0031182097001261. [DOI] [PubMed] [Google Scholar]
- 108.Hastings IM, Watkins WM, White NJ. The evolution of drug-resistant malaria: the role of drug elimination half-life. Philos Trans R Soc Lond B Biol Sci. 2002;357:505–19. doi: 10.1098/rstb.2001.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.White NJ, Pongtavornpinyo W. The de novo selection of drug-resistant malaria parasites. Proc R Soc Lond B Biol Sci. 2003;270:545–54. doi: 10.1098/rspb.2002.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Talisuna AO, Langi P, Bakyaita N, et al. Intensity of malaria transmission, antimalarial-drug use and resistance in Uganda: what is the relationship between these three factors? Trans R Soc Trop Med Hyg. 2002;96:310–17. doi: 10.1016/s0035-9203(02)90108-2. [DOI] [PubMed] [Google Scholar]
- 111.Talisuna AO, Langi P, Mutabingwa TK, et al. Intensity of transmission and spread of gene mutations linked to chloroquine and sulphadoxine-pyrimethamine resistance in falciparum malaria. Int J Parasitol. 2003;33:1051–58. doi: 10.1016/s0020-7519(03)00156-5. [DOI] [PubMed] [Google Scholar]
- 112.Hastings IM. Malaria control and the evolution of drug resistance: an intriguing link. Trends Parasitol. 2003;19:70–73. doi: 10.1016/s1471-4922(02)00017-x. [DOI] [PubMed] [Google Scholar]
- 113.Ariey F, Robert V. The puzzling links between malaria transmission and drug resistance. Trends Parasitol. 2003;19:158–60. doi: 10.1016/s1471-4922(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 114.Kidson C, Singhasivanon P, Supavej S. Mekong malaria: malaria, multi-drug resistance and economic development in the greater Mekong subregion of southeast Asia. Southeast Asian J Trop Med Public Health. 1999;30(suppl 4):1–101. [PubMed] [Google Scholar]
- 115.White NJ. Delaying antimalarial drug resistance with combination therapy. Parassitologia. 1999;41:301–08. [PubMed] [Google Scholar]
- 116.Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trend Parasitol. 2001;17:593–97. doi: 10.1016/s1471-4922(01)02031-1. [DOI] [PubMed] [Google Scholar]
- 117.Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64(suppl 1–2):12–17. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 118.Hay SI, Cox J, Rogers DJ, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–09. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Macdonald G. The epidemiology and control of malaria. Oxford University Press; London: 1957. [Google Scholar]
- 120.Oaks SC, Mitchell VS, Pearson GW, Carpenter CCJ. Epidemiologic approaches to malaria control. Malaria: obstacles and opportunities. National Academy Press; Washington DC: 1991. pp. 211–36. [PubMed] [Google Scholar]
- 121.Bruce-Chwatt LJ, Garret-Jones C, Weitz B. Ten year study (1955–64) of host selection by Anopheline mosquitoes. Bull World Health Organ. 1966;35:405–39. [PMC free article] [PubMed] [Google Scholar]
- 122.Coluzzi M. Heterogeneities of the malaria vectorial system in tropical Africa and their significance in malaria epidemiology and control. Bull World Health Organ. 1984;62:107–13. [PMC free article] [PubMed] [Google Scholar]
- 123.Lindsay SW, Parson L, Thomas CJ. Mapping the ranges and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and An arabiensis, using climate data. Proc R Soc Lond B Biol Sci. 1998;265:847–854. doi: 10.1098/rspb.1998.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Service MW, Townson H. The Anopheles vector. In: Warrell DA, Gilles HM, editors. Essential malariology. 4th edn Arnold; London: 2002. pp. 59–84. [Google Scholar]
- 125.Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans R Soc Trop Med Hyg. 2000;94:113–27. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hay SI, Rogers DJ, Randolph SE, et al. Hot topic or hot air? Climate change and malaria resurgence in East African highlands. Trends Parasitol. 2002;18:530–34. doi: 10.1016/s1471-4922(02)02374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. The potential impact of integrated malaria transmission control on entomologic inoculation rate in highly endemic areas. Am J Trop Med Hyg. 2000;62:545–51. doi: 10.4269/ajtmh.2000.62.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Snow RW, Marsh K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol. 2002;52:235–64. doi: 10.1016/s0065-308x(02)52013-3. [DOI] [PubMed] [Google Scholar]
- 129.Davies CS. The Multilateral Initiative on Malaria: co-ordination and co-operation in international malaria research. Parassitologia. 1999;41:497–500. [PubMed] [Google Scholar]
- 130.Ramsay S. Global Fund makes historic first round of payments. Lancet. 2002;359:1581–82. doi: 10.1016/s0140-6736(02)08531-8. [DOI] [PubMed] [Google Scholar]
- 131.Sachs JD. A new global effort to control malaria. Science. 2002;298:122–24. doi: 10.1126/science.1077900. [DOI] [PubMed] [Google Scholar]
- 132.Yamey G. Global campaign to eradicate malaria—Roll Back Malaria has achieved a high profile but little real action. BMJ. 2001;322:1191–92. doi: 10.1136/bmj.322.7296.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Teklehaimanot A, Snow RW. Will the Global Fund help roll back malaria in Africa? Lancet. 2002;360:888–89. doi: 10.1016/s0140-6736(02)11069-5. [DOI] [PubMed] [Google Scholar]
- 134.Narasimhan V, Attaran A. Roll Back Malaria? The scarcity of international aid for malaria control. Malaria J. 2003;2:8. doi: 10.1186/1475-2875-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Toure YT, Coluzzi M. The challenges of doing more against malaria, particularly in Africa. Bull World Health Organ. 2000;78:1376–76. [PMC free article] [PubMed] [Google Scholar]
- 136.Remme JHF, Blas E, Chitsulo L, et al. Strategic emphases for tropical diseases research: a TDR perspective. Trends Parasitol. 2002;18:421–26. doi: 10.1016/s1471-4922(02)02387-5. [DOI] [PubMed] [Google Scholar]
- 137.Guerin PJ, Olliaro P, Nosten F, et al. Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect Dis. 2002;2:564–73. doi: 10.1016/s1473-3099(02)00372-9. [DOI] [PubMed] [Google Scholar]
- 138.Sachs JD, Ahluwalia IJ, Amoako KY, et al. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health, World Health Organization; Geneva: 2001. [Google Scholar]