Fat caves: caveolae, lipid trafficking and lipid metabolism in adipocytes (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 1.
Published in final edited form as: Trends Endocrinol Metab. 2011 May 17;22(8):318–324. doi: 10.1016/j.tem.2011.04.001
Abstract
Caveolae are subdomains of the eukaryotic cell surface that are so-called because they resemble little caves, small omega-shaped invaginations of the plasma membrane into the cytosol. They are present in many cell types and are especially abundant in adipocytes where they have been implicated as playing a role in lipid metabolism. Thus mice and humans lacking caveolae have small adipocytes and exhibit lipodystrophies along with other physiological abnormalities. Here we review the evidence supporting the role of caveolae in adipocyte lipid metabolism in the context of the protein and lipid composition of these structures.
INTRODUCTION
Although caveolae were first described as morphological features of the plasma membrane nearly 60 years ago, it was the discovery of caveolin-1 as an essential protein constituent that opened the floodgates for molecular studies of these structures [1]. Caveolin-1 proved to be identical to a 22 kDa protein that is the major tyrosine phosphorylated protein of src-transformed cells [2], an observation which suggested a link between cancer and caveolae. Despite the many years since these observations, the link between caveolae and cancer remains confusing and contradictory with some studies supporting oncogenic properties for this protein and other studies, tumor suppressor activity [3]. These apparent contradictions illustrate the current status concerning caveolar functions, which on the one hand, have been extensively studied, but on the other hand, are far from understood and are controversial [4]. As a possible exception to this general statement however, considerable in vivo and in vitro evidence supports a role for caveolae in adipocyte lipid metabolism, although many mechanistic details of this involvement remain uncertain, and these will be discussed herein. Additional aspects of caveolae biology such as recent advances in the nature of their protein composition and requirements for assembly have been reviewed in detail [5–8] or described elsewhere [9], and will be briefly summarized below.
CAVEOLAE STRUCTURAL COMPONENTS
The caveolins, the first known components of caveolae, comprise a family of 3 small (151–178 amino acids) integral membrane proteins with a 33 amino acid hydrophobic domain, roughly in the middle of the protein, which anchors them to the lipid bilayer by a loop structure that does not extend to the extracellular milieu [10]. Consequently all the functions and interactions of caveolins are thought to be in the cytosol or in the inner leaflet of the plasma membrane bilayer. The C-terminus of caveolins is palmitoylated and is modeled to have a helical structure, which is held close to the inner bilayer leaflet [11] and contains a number of positively charged residues (5 for caveolin-1) that could interact with fatty acid anions and modulate transmembrane fatty acid movement [8, 12]. Caveolins-1 and -2 are co-expressed in non-muscle tissue, mainly adipocytes, endothelial and epithelial cells, whereas caveolin-3 is expressed in cardiac, smooth and skeletal muscles [10]. Notably, caveolins and caveolae appear absent or are expressed at very low levels in brain and liver [13]. The caveolins form large hetero- and homo-oligomeric structures [14–16] that are capable of forming so-called lipid rafts, often operationally defined as detergent resistant membrane domains which criterion is often used to characterize caveolae [17]. As discussed below, detergent resistance per se is insufficient to implicate caveolae in a biological process. The essential requirements of caveolins-1 [18–20] and -3 [21] for caveolae formation in their respective cell types have been demonstrated by their targeted deletion in mice, whereas caveolin-2 appears dispensable in this regard, but is required for normal pulmonary function [22].
It was established early that caveolin -1 is a cholesterol binding protein [23] and that caveolae are rich in cholesterol, which is required for their structural integrity [24]. Thus the combination of cholesterol, sphingolipids whose expression mirrors the sterol [25], and caveolin oligomers likely accounts for the detergent resistance of caveolae [26]. Until recently, the experimental foci of most studies of caveolae were largely restricted to the caveolins and cholesterol, and their detergent resistance was the most often used criterion to define the behavior of caveolae upon physiological perturbation. This criterion is however imprecise as detergent resistant membrane domains exist apart from caveolae and may also be generated in situ [17] and much information about caveolae based on detergent resistance awaits confirmation by independent experimental approaches.
In the last 2–3 years, it was established in a number of laboratories that in addition to the caveolins, caveolae have a number (3–4 depending on the tissue) of peripheral membrane proteins now called cavins (see below, reviewed in [5–8]), at least one of which, cavin-1, is critical to caveolae structure [27–29]. Given the small size of caveolins as compared to cavins, the latter most probably represent the striated coat observed for caveolae in freeze etch electron micrographs, which was initially attributed to caveolin-1 [1]. The pattern of cavin-1 and -2 tissue expression closely resembles that of caveolin-1 and -2, and cavin-4 like caveolin-3, is expressed only in cardiac and skeletal muscle, particularly the latter. Cavin-1–3 had all been previously implicated as being associated with caveolae [5–8], but their prior names, cavin-1 as polymerase transcription releasing factor (PTRF), cavin-2 as serum deprivation response (SDR) protein and cavin-3 as SDR-related gene product that binds to C kinase (SRBC) gave little hint as to their localization in the case of the latter two., and possibly misleading information in the former case. Knockdown of cavin-1 expression in adipocytes [27] fibroblasts [27, 28], zebrafish [28] and a targeted gene knockout in mice [29] established an obligatory role for cavin-1 in the formation of caveolae in all tissues studied, including muscle. Cavin-1–3 are phospholipid binding proteins with leucine rich domains that likely mediate their interactions with one another, and they have been shown to form an endogenous trimeric complex in adipocytes by co-immuno-precipitation and by FRET when ectopically co-expressed [13]. All cavins, including cavin-4 (AKA muscle-restricted coil-coil protein or MURC), have PEST domains (peptide sequence which is rich in proline (P), glutamic acid (E), serine (S), and threonine (T)) of unknown physiological function(s) with regard to caveolae dynamics. Cavin-2 contributes to the curvature and formation of caveolae [30] and cavin-3 may function in the endocytosis of caveolae [31]. Knockdown of cavin-2 in vitro [13, 30], like cavin-1 knockdown [27, 28] results in lowered expression of caveolins-1 and -2 and cavin-1, hence reduced caveolae expression. Cavin-3 unlike cavin-1 &-2 is expressed at significant levels in liver and brain, and based on immunogold electron microscopy (EM) labeling in adipocytes, it may be present in caveolae to a lesser extent than the other cavins [13].
CAVEOLAE DEFICIENCY AND LIPID METABOLISM IN VIVO
The role of caveolae in organismal lipid metabolism first became apparent with the generation of caveolin-1 knockout mice. These animals lack caveolae in non-muscle tissues, are lean with small adipocytes, insulin resistant and they exhibit defects in insulin signaling [32, 33]. Mice lacking cavin-1 have a similar phenotype, being lean, hyperlipidemic and insulin resistant, but unlike the caveolin-1 null mice, they are markedly hyperinsulinemic at age 8–12 weeks [29]. As noted above, cavin-1 null mice lack caveolae in all tissues including muscle, and this most likely accounts for the phenotypic differences from caveolin-1 null mice. Caveolin-3 null mice, like cavin-1 nulls, develop insulin resistance at an early age [34] presumably due to a muscle-specific defect in insulin signaling and/or glucose uptake [29]. Determining the mechanistic reasons for the hyperlipidemic and insulin resistant phenotypes is complicated by the fact that the absence of caveolae results in marked vascular dysfunction [18–20] and adipocytes are highly vascularized. However, analysis of an endothelial cell knock-in rescue of caveolin-1 null mice reveals that the adipocytes themselves are abnormal when they lack caveolae, and restoring their vasculature does not reverse the lipodystrophic phenotype [35]. For caveolae-deficient animals, one presumes they are hyperlipidemic from birth or shortly thereafter and this can certainly explain their insulin resistance [36]. The hyperlipidemia itself is however harder to understand as the caveolin-1 [37] null animals have a blunted lipolytic response to adrenergic stimuli as well as reduced insulin signaling. Overall then, it could be the imbalance between insulin-dependent lipid storage and adrenergic-dependent lipolysis that gives rise to the observed hyperlipidemia as illustrated in Figure 1. Reduced lipolysis but even more reduced insulin-dependent lipid uptake in adipocytes could account for the observed lipodystrophic phenotype in this scenario resulting in increased levels of circulating fatty acids and consequent insulin resistance (Figure 1). An adaptive autophagic response in adipocytes from caveolin-1 null mice has been reported and this could also contribute to the lipoatrophy of these animals [38]. However, the inability of the caveolae null fat cells to store fat, by whatever the mechanism(s), and the hyperlipidemia, predict that fat must go somewhere, but its fate has yet to be determined. The reduced adiposity is apparently not reflected in ectopic lipid distribution, in the liver for example, and in the case of the cavin-1 null mouse, they are more sedentary than their wild type counterparts [29] so hyperactivity cannot explain the phenotype. Additional work will therefore be needed in this regard, for example, careful measurements of food intake.
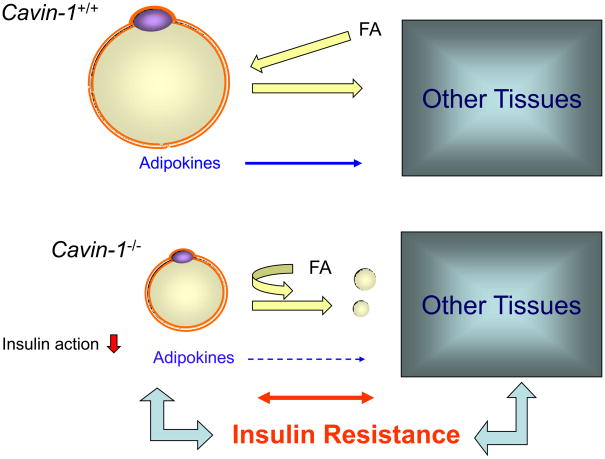
Figure 1. Insulin resistance in cavin-1 null mice.
Depicted are fat cells from wild type (W.T., [Ptrf (Cavin) +/+] and cavin-deficient (Ptrf −/−) sources. There is a balance of hormonally regulated fat (FA) storage and release in the W.T. tissue (equal sized arrows), but there is a decreased lipolytic response and decreased FA uptake in the knockout tissue and reduced adipokine levels. This, plus the loss of insulin responsiveness due to increased fatty acids in the circulation, causes a further metabolic imbalance and insulin resistance ensues. This process may be similar or identical in humans lacking functional cavin-1.
The physiological characteristics of humans exhibiting inactivating mutations in caveolin-1 [39, 40] and cavin-1 [41–44] have been recently described. These mutations have been identified in diverse ethnic groups worldwide, and like caveolin and cavin-1 null mice, subjects with these mutations are lipodystrophic and insulin resistant. The humans with cavin-1 mutations also exhibit myopathies and cardiac arrhythmias, amongst other abnormalities, and this phenotype is consistent with the cavin-1 requirement for caveolae formation in all tissues examined in mice including skeletal, cardiac and smooth muscle [29]. Interestingly, there have been no reports of a phenotype in either mice or humans in the heterozygous condition for either gene, but this might require some physiological stress such as exercise, cold exposure or a high fat diet in order to be manifest. In addition, there have yet to be any reports of cavin-2 mutations in the human population, but give that its expression most closely resembles that of cavin-1 [13], and its knockdown in vitro results in reduced cavin-1 and caveolin-1 expression [13, 30], the existence of such mutations in the human population seems quite probable. Cavin-3 is the outlier in this regard, and it is unclear if human mutations will be found for it because it is most highly expressed in the heart and it is expressed in liver and brain, and its expression in these tissues does not change in the cavin-1 null mouse [13].
CAVEOLIN, CAVEOLAE AND LIPID METABOLISM IN VITRO STUDIES
As expected, the majority of caveolin-1 and -2 in adipocytes is found at the cell surface (90+ %) [45, 46], but a significant amount can be found intracellularly as determined by cell fractionation of primary and cultured fat cells [45–47]. Of course, the biosynthesis of integral plasma membrane proteins like caveolin involves their translation in the endoplasmic reticulum (E.R.) (see below) and transit through the Golgi apparatus, which may account, in part, for the observed intracellular localization. In addition to caveloae, another defining property of the adipocyte is the lipid droplet (see Figure 3), the organelle where triglycerides are stored upon feeding and mobilized in the fasting state. The droplet is thought to arise from the intrabilayer space of the E.R. such that a phospholipid monolayer is generated around the droplet [48], which in turn is coated by members of the PAT (Perilipin, Adipophilin, Tip47) family of lipid droplet protein scaffolds [49]. In adipocytes, there are also a number of proteins involved in triglyceride assembly and hormonally-regulated disassembly (lipolysis) that are associated with the droplet [50, 51] (see Figure 3). These droplets are appreciated to represent a dynamic cellular organelle in adipocytes, although lipid droplets similar in appearance can be seen in any cell when exposed to sufficient levels of a fatty acid source. In cells other than adipocytes, however, the protein machinery required for robust triglyceride synthesis and hormonally-regulated lipolysis is generally lacking.
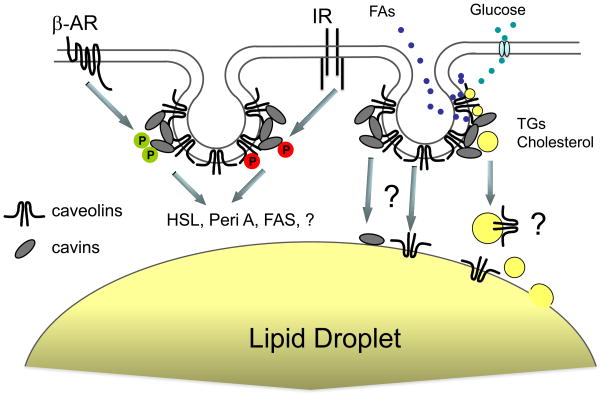
Figure 3. Possible molecular interactions in adipocytes involving caveolar proteins.
The green P represents serine/threonine phosphorylation of cavin-1 and the red P, tyrosine phosphorylation of caveolin-1, which have been experimentally documented. How the mechanism by which these events affect caveolar function and lipolysis remains unclear. Other somewhat speculative events of protein and lipid movement are indicated by question marks. Note that the indicated proteins are not drawn to scale and the two caveolae represented are not necessarily meant to have different functions, rather they are drawn this way for clarity. Abbreviations are: FAs, fatty acids; βAR, beta-adrenergic receptor; IR insulin receptor; TG, triglycerides; HSL, hormone sensitive lipase; Peri A, perilipin A; FAS, fatty acylCoA synthase.
A possible role for caveolins in lipid droplets, hence lipid flux and metabolism, was first observed in a variety of cells expressing either mutated caveolins [52–54], caveolin-2 alone [54] or as a result of brefeldin A exposure [53, 54]. In these circumstances, caveolin was observed to traffic from the cell surface and/or E.R. to the lipid droplet. However, these and other studies [55] were primarily designed to follow the membrane trafficking of caveolins and did not involve adipocytes. Moreover the role of the cavin proteins in caveolae biology was not known at that time. Subsequent studies of fat cells identified caveolin-1 as a lipid droplet protein by proteomic analysis of purified droplets [56], immuno E.M. [57] and by cell fractionation and immunofluorescence (I.F.) [57, 58]. Some caveats apply to the identification of caveolin-1 in droplets, namely that it is a exceptionally abundant protein in adipocytes and the lipid droplets derive from the E.R., the route(s) of caveolin trafficking noted above. However the cited data, taken together, support a lipid droplet localization for caveolin- and its possible functional role there, and the presence and possible role(s) of the cavins in lipid droplets remains to be determined.
One can envision a number of possibilities mechanistic roles for caveolae in adipocyte lipid metabolism: i. cell surface caveolins and/or caveolae can influence transmembrane fatty acid flux [12, 57, 59]; ii. they may influence and/or regulate triglyceride synthesis [57]; iii. they may be involved in vesicular trafficking and/or endocytosis, in this case related to cholesterol homeostasis [58]; iv. they may regulate signaling pathways involved in lipid metabolism, namely those mediated by insulin [60] and adrenergic receptors [37]. These activities are summarized in Figures 2 & 3. Concerning i., adipocytes traffic fatty acids in and out of the cell as their most quantitatively robust activity and they do so very rapidly [61]. Fatty acid anions are weak detergents and caveolins form detergent resistant domains. These facts are consistent with the proposed role for caveolins and caveolae in protecting cells from lipotoxicity [12, 57, 59]. Data to support this hypothesis come from experiments in transfected HEK293 cells, which revealed that cells lacking caveolin expression have greater susceptibility to fatty acid-mediated lipotoxicty as compared to those where caveolins were ectopically expressed [59]. In addition, the presence of caveolae seems to enhance fatty acid uptake and accumulation in adipocytes [62] and HEK293 cells [59]. We interpret this result to be due, at least in part, to enhanced fatty acid availability to acylCoA ligases, i.e., vectorial acylation and mass action, as suggested by Masek and Coleman [63]. Thus, the structure of caveolin-1 [11] as discussed above would allow the concentration and stabilization of fatty acid anions at the cytoplasmic face of caveolae where they can be further metabolized (Figure 3). Moreover, this function does not preclude the other proposed roles of adipocyte caveolae. Thus the notion that caveolae are a locus for triglyceride synthesis (ii), based on the ability of isolated caveolae to perform this process [57], is teleologically attractive considering their proposed role as sites of concentrated fatty acid entry into the cell, but it is one that needs further experimental support.
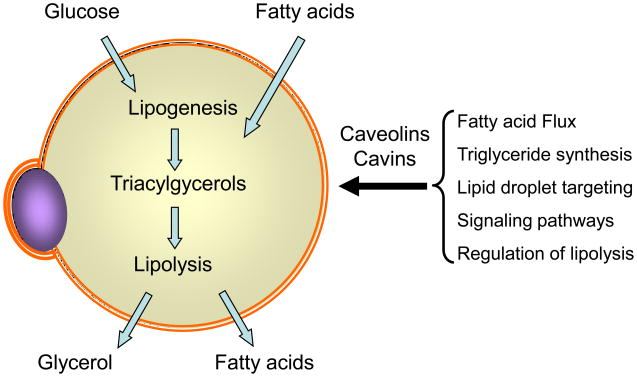
Figure 2. Possible adipocyte metabolic functions affected by caveolae and their protein constituents.
Adipocytes store triglycerides derived from fatty acids and glucose (lipogenesis) and release glycerol and fatty acids upon lipolysis. Data from in vivo and in vitro studies support a role for caveolar proteins in the processes indicated in the bracket, although many of the mechanistic details by which this occurs await further studies.
Regarding caveolar endocytosis in adipocytes [58] (possibility iii above), this process is not generally considered to be rapid or quantitatively robust in most instances [64], and the mechanistic basis of caveolae endocytosis is not at all clear in terms of what might happen to the cavins and caveolins during this process. In primary adipocytes, we addressed this issue, and showed that the major ectodomain proteins of this structure in fat cells (see below) exhibited little or no endocytic behavior over a 15 min. period in the presence and absence of insulin [46]. In studying cholesterol internalization [58], Le Lay et al. did not determine the extent caveolin-1 endocytosis with regard to the total cell surface caveolin/caveolae, rather just the appearance of caveolin in intracellular loci. Moreover these data are consistent with the prior studies involving caveolin mutants and other perturbations noted above [52–54]. Finally, caveolae are highly enriched in cholesterol as previously noted, and the adipocyte lipid droplet is the largest cholesterol reservoir in the body [65], which may well be filled by the described [58] endocytic route.
The role of caveolae as centers for cell signaling involving numerous and diverse signaling pathways (possibility iv above) has generated numerous reviews (e.g., [66]) and the insulin [60] and β– adrenergic signaling [37] pathways of adipocytes are amongst those implicated as being involved, at least in part, with caveolae. On the other hand, we have failed to find evidence for the presence of insulin receptors (IRs) and heterotrimeric G proteins in immuno-purified caveolae from adipocytes [46] and varying the caveolin expression over 40 fold in HEK cells is without effect on insulin signaling [67]. We attribute the apparent discrepancies in many of the signaling studies to the different methodologies used for caveolae characterization (reviewed in [68]), and the association of a given signaling pathway with caveolae should be established by multiple independent experimental approaches as has also been recommended elsewhere [5]. On the other hand, as depicted in Figure 3, signaling from both the β–AR and IR appears to impinge on caveolae and their protein constituents by altering their phosphorylation states.
OTHER PROTEIN COMPONENTS OF CAVEOLAE AND LIPID METABOLISM
Caveolae express cavin-1 at levels roughly comparable to caveolin-1 as suggested by immuno-gold double labeling of these proteins and E.M. [69], and the question arises as to whether or not cavin-1 can affect adipocyte lipid metabolism directly or secondarily as a component of caveolae. Cavin-1 knockdown in vitro diminishes the acute uptake of labeled oleate [27], and the in vivo effects of cavin-1 loss are much more dramatic resulting in small fat cells and lipodystrophy [29]. A recent paper has demonstrated that cavin-1 (PTRF) knockdown in cultured adipocytes inhibits lipolysis, and in mice_,_ cavin-1 levels are up-regulated upon fasting and isoproterenol exposure and down-regulated by insulin treatment [70]. The in vitro and fasting data can be explained by changes in caveolins, hence caveolae, due to loss of cavin-1 as previously discussed. However, a striking finding in this study [70] is that cavin-1 is robustly phosphorylated on 5 serine and one threonine residues as a result of adrenergic activation of PKA, and expression in cultured adipocytes of the mutants for three of these sites, S42A, T304A and S368A, inhibits isoproterenol-stimulated lipolysis compared to over-expressed wild type protein, although not compared to control cells [70]. This result suggests a direct role of cavin-1, possibly apart from caveolae and caveolin-1, as the levels of caveolin-1 are apparently unchanged upon transfection of the cavin-1 constructs. It will therefore be interesting to determine where in the cell the phosphorylation site mutants of cavin-1 are targeted, to caveolae or elsewhere, if caveolae composition or amount are themselves altered when phosphorylation site mutant cavin-1 is expressed, and if the interaction of mutant cavin-1 with proteins of the hormonally-regulated lipolytic machinery occurs and/or is altered compared to wild type protein. Indeed, caveolins have been implicated to have biological roles apart from caveolae as has been shown in a number of contexts [71], including studies showing that they modulate membrane lipid content and alter transmembrane fatty acid flux in the absence of caveolae formation [12, 59, 67], and it will be worthwhile to explore if such is also the case for cavin-1 and -2, as is likely to be for cavin-3 due to its pattern of tissue expression.
Another covalent modification on caveolar proteins is phospho-tyrosine, first reported in adipocytes as an insulin-dependent event for caveolin [72] although it is unlikely that it is directly mediated by the insulin receptor (reviewed in [68]). Subsequently, insulin-dependent tyrosine phosphorylation of caveolin and cavin-1–3 has been shown in a number of independent studies in several cell types [68, 73–75]. Because cavin-3 has no tyrosine residues, its identification as a phospho-tyrosine species [75] most likely derives from its co-immunprecipitation in a cavin complex. The physiological role of tyrosine phosphorylation of caveolar proteins with regard to insulin’s actions remains to be determined.
Adipocyte caveolae express high levels of two ectodomain proteins, semicarbazide sensitive amine oxidase (SSAO) and FAT/CD36 (FAT for fatty acid translocase), as determined both by Western blotting [46] and by proteomic analysis [76]. These two proteins apparently account for the great majority of the ectodomain protein mass of caveolae as they were the only such proteins identified by mass spectrometry of purified caveolae from rats and humans [46, 76], and by ectodomain labeling and protein staining protocols [46]. Both FAT/CD36 and SSAO have potential roles in adipocyte lipid metabolism as the former is widely described as a fatty acid transporter [77], and the latter can mimic insulin action due to peroxide generated upon amine oxidation [78]. It is unclear if physiologically significant levels of peroxide can be generated from an endogenous amine such as norepineprine, and if this were the case, the result would be the simultaneous lipolytic stimulation from the catecholamine and anti-lipolytic action from the resultant peroxide, a physiologically unlikely possibility. On the other hand, while the function of CD36 as a fatty acid transporter is controversial [79], mice engineered to lack this protein are hyperlipidemic and show abnormalities in lipoprotein uptake [80]. In fact, the phenotype of the CD36 null mouse is not dissimilar from that of cavin-1 and caveolin-1 deficient mice, and indeed, the aortas of this last animal have greatly diminished CD36 expression [81]. CD36 is widely expressed throughout the body including in skeletal muscle and its loss there due to caveolae loss could further contribute to the dyslipidemic phenotype of the animals.
CONCLUSIONS AND FUTURE DIRECTIONS
There is now a large body of data from in vitro and in vivo experimental approaches, as well as from experiments of nature, that support a role for caveolae in lipid trafficking and metabolism. The apparently identical phenotypes of mice with targeted deletions that eliminate caveolae to that of humans with naturally occurring inactivating mutations of caveolar proteins provide ample proof of principal for the physiological importance of caveolae, and the use of a “model” organism to gain insight for the human situation. With regard to lipid metabolism, and indeed with other physiological abnormalities resulting from caveolae deficiency, the pleiotropic nature of the defects complicates determination of the biochemical and cell biological mechanisms that give rise to the phenotypes. It is however completely feasible to address these mechanistic questions by a variety of experimental approaches, which will include tissue-specific knockouts of the caveolar proteins, conditional mutations, as well the use of cultured adipocytes derived from the embryonic fibroblasts of caveolin-1 and cavin-1 knockout mice. No doubt such approaches are currently underway in many laboratories around the world. In addition, other new roles for caveolar proteins aside from metabolism are being identified. For example, cavin-1 plays a role in the response of Hela and endothelial cells to swelling and stretching as has very recently been shown, and the authors of this study suggest caveolae can act as membrane reservoirs [82]. This role may also be true for adipocytes, as caveolae greatly add to their cell surface area and may facilitate lipid (FA) trafficking in this way.
Acknowledgments
This work was supported by NIH grants R01DK056935 & R01DK030425 to PFP
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rothberg KG, et al. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 2.Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–20166. [PubMed] [Google Scholar]
- 3.Goetz JG, et al. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27:715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 4.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 5.Bastiani M, Parton RG. Caveolae at a glance. J Cell Sci. 2010;123:3831–3836. doi: 10.1242/jcs.070102. [DOI] [PubMed] [Google Scholar]
- 6.Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 2010;20:177–186. doi: 10.1016/j.tcb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Briand N, et al. Cavin proteins: New players in the caveolae field. Biochimie. 2011;93:71–77. doi: 10.1016/j.biochi.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Pilch PF, et al. Caveolae and lipid trafficking in adipocytes. Current Lipidology. 2011;6:49–58. doi: 10.2217/clp.10.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayer A, et al. Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic. 2010;11:361–382. doi: 10.1111/j.1600-0854.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 10.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spisni E, et al. Structural insights into the function of human caveolin 1. Biochem Biophys Res Commun. 2005;338:1383–1390. doi: 10.1016/j.bbrc.2005.10.099. [DOI] [PubMed] [Google Scholar]
- 12.Meshulam T, et al. Role of caveolin-1 and cholesterol in transmembrane fatty acid movement. Biochemistry. 2006;45:2882–2893. doi: 10.1021/bi051999b. [DOI] [PubMed] [Google Scholar]
- 13.Bastiani M, et al. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J Cell Biol. 2009;185:1259–1273. doi: 10.1083/jcb.200903053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monier S, et al. Oligomerization of VIP21-caveolin in vitro is stabilized by long chain fatty acylation or cholesterol. FEBS Lett. 1996;388:143–149. doi: 10.1016/0014-5793(96)00519-4. [DOI] [PubMed] [Google Scholar]
- 15.Das K, et al. The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. Implications for the assembly of caveolae membranes in vivo. J Biol Chem. 1999;274:18721–18728. doi: 10.1074/jbc.274.26.18721. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez I, et al. Mechanism of caveolin filament assembly. Proc Natl Acad Sci U S A. 2002;99:11193–11198. doi: 10.1073/pnas.172196599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- 18.Drab M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 19.Razani B, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 20.Zhao YY, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galbiati F, et al. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 22.Razani B, et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata M, et al. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothberg KG, et al. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridgway ND. Interactions between metabolism and intracellular distribution of cholesterol and sphingomyelin. Biochim Biophys Acta. 2000;1484:129–141. doi: 10.1016/s1388-1981(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 26.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Pilch PF. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem. 2008;283:4314–4322. doi: 10.1074/jbc.M707890200. [DOI] [PubMed] [Google Scholar]
- 28.Hill MM, et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, et al. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8:310–317. doi: 10.1016/j.cmet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen CG, et al. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol. 2009;11:807–814. doi: 10.1038/ncb1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon KA, et al. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009;28:1001–1015. doi: 10.1038/emboj.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razani B, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 33.Cohen AW, et al. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol. 2003;285:C222–235. doi: 10.1152/ajpcell.00006.2003. [DOI] [PubMed] [Google Scholar]
- 34.Capozza F, et al. Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol. 2005;288:C1317–1331. doi: 10.1152/ajpcell.00489.2004. [DOI] [PubMed] [Google Scholar]
- 35.Briand N, et al. Distinct roles of endothelial and adipocyte caveolin-1 in macrophage infiltration and adipose tissue metabolic activity. Diabetes. 2010;60:448–453. doi: 10.2337/db10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guilherme A, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen AW, et al. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–1270. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- 38.Le Lay S, et al. The lipoatrophic caveolin-1 deficient mouse model reveals autophagy in mature adipocytes. Autophagy. 2010;6:754–763. doi: 10.4161/auto.6.6.12574. [DOI] [PubMed] [Google Scholar]
- 39.Cao H, et al. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis. 2008;7:3. doi: 10.1186/1476-511X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim CA, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93:1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi YK, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119:2623–2633. doi: 10.1172/JCI38660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dwianingsih EK, et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol Genet Metab. 2010;101:233–237. doi: 10.1016/j.ymgme.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Rajab A, et al. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 2010;6:e1000874. doi: 10.1371/journal.pgen.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shastry S, et al. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am J Med Genet A. 2010;152A:2245–2253. doi: 10.1002/ajmg.a.33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandror KV, et al. Expression and compartmentalization of caveolin in adipose cells: coordinate regulation with and structural segregation from GLUT4. J Cell Biol. 1995;129:999–1006. doi: 10.1083/jcb.129.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Souto RP, et al. Immunopurification and characterization of rat adipocyte caveolae suggest their dissociation from insulin signaling. J Biol Chem. 2003;278:18321–18329. doi: 10.1074/jbc.M211541200. [DOI] [PubMed] [Google Scholar]
- 47.Scherer PE, et al. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol. 1994;127:1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohsaki Y, et al. Biogenesis of cytoplasmic lipid droplets: from the lipid ester globule in the membrane to the visible structure. Biochim Biophys Acta. 2009;1791:399–407. doi: 10.1016/j.bbalip.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Bickel PE, et al. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy S, et al. Lipid droplet-organelle interactions; sharing the fats. Biochim Biophys Acta. 2009;1791:441–447. doi: 10.1016/j.bbalip.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann R, et al. Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta. 2009;1791:494–500. doi: 10.1016/j.bbalip.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Pol A, et al. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J Cell Biol. 2001;152:1057–1070. doi: 10.1083/jcb.152.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ostermeyer AG, et al. Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets. J Cell Biol. 2001;152:1071–1078. doi: 10.1083/jcb.152.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujimoto T, et al. Caveolin-2 is targeted to lipid droplets, a new “membrane domain” in the cell. J Cell Biol. 2001;152:1079–1085. doi: 10.1083/jcb.152.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu P, et al. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 56.Brasaemle DL, et al. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 57.Ost A, et al. Triacylglycerol is synthesized in a specific subclass of caveolae in primary adipocytes. J Biol Chem. 2005;280:5–8. doi: 10.1074/jbc.C400429200. [DOI] [PubMed] [Google Scholar]
- 58.Le Lay S, et al. Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: a role for caveolar endocytosis. Traffic. 2006;7:549–561. doi: 10.1111/j.1600-0854.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 59.Simard JR, et al. Caveolins sequester FA on the cytoplasmic leaflet of the plasma membrane, augment triglyceride formation, and protect cells from lipotoxicity. J Lipid Res. 2010;51:914–922. doi: 10.1194/jlr.M900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saltiel AR, Pessin JE. Insulin signaling in microdomains of the plasma membrane. Traffic. 2003;4:711–716. doi: 10.1034/j.1600-0854.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 61.Kamp F, et al. Rapid flip-flop of oleic acid across the plasma membrane of adipocytes. J Biol Chem. 2003;278:7988–7995. doi: 10.1074/jbc.M206648200. [DOI] [PubMed] [Google Scholar]
- 62.Pohl J, et al. Long-chain fatty acid uptake into adipocytes depends on lipid raft function. Biochemistry. 2004;43:4179–4187. doi: 10.1021/bi035743m. [DOI] [PubMed] [Google Scholar]
- 63.Mashek DG, Coleman RA. Cellular fatty acid uptake: the contribution of metabolism. Curr Opin Lipidol. 2006;17:274–278. doi: 10.1097/01.mol.0000226119.20307.2b. [DOI] [PubMed] [Google Scholar]
- 64.Sandvig K, et al. Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem Cell Biol. 2008;129:267–276. doi: 10.1007/s00418-007-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krause BR, Hartman AD. Adipose tissue and cholesterol metabolism. J Lipid Res. 1984;25:97–110. [PubMed] [Google Scholar]
- 66.Patel HH, et al. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wharton J, et al. Dissociation of insulin receptor expression and signaling from caveolin-1 expression. J Biol Chem. 2005;280:13483–13486. doi: 10.1074/jbc.M413891200. [DOI] [PubMed] [Google Scholar]
- 68.Pilch PF, et al. Cellular spelunking: exploring adipocyte caveolae. J Lipid Res. 2007;48:2103–2111. doi: 10.1194/jlr.R700009-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Vinten J, et al. A 60-kDa protein abundant in adipocyte caveolae. Cell Tissue Res. 2001;305:99–106. doi: 10.1007/s004410100389. [DOI] [PubMed] [Google Scholar]
- 70.Aboulaich N, et al. Polymerase I and Transcript Release Factor Regulates Lipolysis Via a Phosphorylation-Dependent Mechanism. Diabetes. 2011 doi: 10.2337/db10-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Head BP, Insel PA. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 2007;17:51–57. doi: 10.1016/j.tcb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Mastick CC, Saltiel AR. Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3-L1 cells. J Biol Chem. 1997;272:20706–20714. doi: 10.1074/jbc.272.33.20706. [DOI] [PubMed] [Google Scholar]
- 73.Ibarrola N, et al. A novel proteomic approach for specific identification of tyrosine kinase substrates using [13C]tyrosine. J Biol Chem. 2004;279:15805–15813. doi: 10.1074/jbc.M311714200. [DOI] [PubMed] [Google Scholar]
- 74.Schmelzle K, et al. Temporal dynamics of tyrosine phosphorylation in insulin signaling. Diabetes. 2006;55:2171–2179. doi: 10.2337/db06-0148. [DOI] [PubMed] [Google Scholar]
- 75.Kruger M, et al. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc Natl Acad Sci U S A. 2008;105:2451–2456. doi: 10.1073/pnas.0711713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aboulaich N, et al. Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem J. 2004;383:237–248. doi: 10.1042/BJ20040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zorzano A, et al. Semicarbazide-sensitive amine oxidase activity exerts insulin-like effects on glucose metabolism and insulin-signaling pathways in adipose cells. Biochim Biophys Acta. 2003;1647:3–9. doi: 10.1016/s1570-9639(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 79.Eyre NS, et al. FAT/CD36 expression alone is insufficient to enhance cellular uptake of oleate. Biochem Biophys Res Commun. 2008;370:404–409. doi: 10.1016/j.bbrc.2008.02.164. [DOI] [PubMed] [Google Scholar]
- 80.Febbraio M, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 81.Frank PG, et al. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 82.Sinha B, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]