Mitochondrial Dysfunction in Diabetic Cardiomyopathy (original) (raw)
. Author manuscript; available in PMC: 2012 Jul 1.
Published in final edited form as: Biochim Biophys Acta. 2011 Jan 20;1813(7):1351–1359. doi: 10.1016/j.bbamcr.2011.01.014
Abstract
Cardiovascular disease is common in patients with diabetes and is a significant contributor to the high mortality rates associated with diabetes. Heart failure is common in diabetic patients, even in the absence of coronary artery disease or hypertension, an entity known as diabetic cardiomyopathy. Evidence indicates that myocardial metabolism is altered in diabetes, which likely contributes to contractile dysfunction and ventricular failure. The mitochondria are the center of metabolism, and recent data suggests that mitochondrial dysfunction may play a critical role in the pathogenesis of diabetic cardiomyopathy. This review summarizes many of the potential mechanisms that lead to mitochondrial dysfunction in the diabetic heart.
Keywords: diabetes, insulin resistance, heart failure, cardiomyopathy, mitochondria, oxidative stress, metabolism
1. Introduction
The prevalence of Type 2 diabetes is reaching pandemic proportions, with estimates that by the year 2025 nearly 300 million adults will be affected by diabetes mellitus [1, 2]. Cardiovascular disease is the leading cause of mortality in patients with diabetes mellitus [3, 4]. Patients with diabetes are at increased risk of hypertension, coronary artery disease and mortality following myocardial infarction [5, 6]. However, it has also been demonstrated that diabetic patients develop heart failure in the absence of risk factors such as hypertension and coronary artery disease[6-8]. The term “diabetic cardiomyopathy” is now used to refer to ventricular dysfunction in diabetic patients that is out of proportion to their underlying vascular disease [9, 10].
A number of investigators have used animal models to evaluate the mechanistic basis for cardiac dysfunction in the setting of obesity and Type 2 diabetes. The most commonly used models involve defects in leptin signaling, which is a critical hormone regulating energy intake and expenditure. These models include db/db mice and Zucker Diabetic Fatty (ZDF) rats (leptin receptor mutation), and ob/ob mice (leptin deficiency). All of these animals have the common phenotype of obesity, insulin resistance and hyperglycemia to varying degrees [11-13]. UCP-DTA animals, used less frequently, are a model in which the brown adipose tissue is ablated, leading to decreased metabolic rate, increased appetite, obesity, and progression from insulin resistance to diabetes [14]. Importantly, these animal models are resistant to development of atherosclerosis, allowing investigators to specifically evaluate heart failure without the presence of coronary artery disease[15, 16]. Echocardiographic data in these animal models has also demonstrated diastolic and later systolic dysfunction, similar to humans [17-21]. Thus, animal models support the human data that diabetes is associated with structural and functional abnormalities of the myocardium, leading to diabetic cardiomyopathy.
2. Metabolic abnormalities in the Diabetic Heart
Emerging data demonstrates that cardiac dysfunction in diabetic patients is linked to metabolic abnormalities. Obesity and diabetes are characterized by high levels of circulating fatty acids which results in increased cardiac fatty acid uptake, storage, and metabolism [22, 23]. Fatty acids taken up by the cardiomyocyte are normally catabolized in mitochondrial and in some circumstances, peroxisomal fatty acid β-oxidation (FAO) pathways. Fatty acids are also incorporated into triglycerides (TAG). There is a dynamic flux through the TAG pool, and recent data demonstrates that many of the fatty acids that are ultimately oxidized through β-oxidation flux through TAG [24]. In addition Banke et al. demonstrated evidence that peroxisome proliferator-activated receptor alpha (PPARα), which is upregulated in diabetic hearts, plays a role in modulating TAG flux [24]. The heart does not normally store significant amounts of lipid, but triglycerides can accumulate when fatty acid supply is high. Myocardial triglyceride content is notably increased in animal models and humans with obesity and Type 2 diabetes compared to healthy controls [25-27]. Additionally, myocardial energy substrate preference (glucose versus fatty acid) normally varies in a dynamic manner to meet the tremendous energy needs of the mammalian heart. In uncontrolled diabetes, cardiac energy substrate preference becomes constrained because of the need for insulin for myocardial glucose uptake. Glucose utilization in the diabetic heart is diminished at least in part because of insulin resistance, impaired pyruvate dehydrogenase activity, and reduced glucose transporter (e.g. Glut4) content. Thus, the diabetic heart relies almost exclusively on mitochondrial FAO for ATP synthesis. This reliance on FAO has potentially detrimental consequences, among which include impaired mitochondrial respiratory function.
3. Evidence for Mitochondrial Dysfunction in Diabetes
Mitochondria are the center of fatty acid and glucose metabolism, and thus are highly likely to be impacted by impaired metabolism associated with diabetes. A number of investigators have demonstrated mitochondrial abnormalities in skeletal muscle of insulin-resistant and diabetic humans. In two independent gene expression analyses, gene targets associated with mitochondrial oxidative phosphorylation (OXPHOS) have been reduced [28, 29]. Both groups found a decrease in peroxisome-proliferator-activated receptor (PPAR) gamma, coactivator-1α (PGC-1α), a master metabolic regulator that coordinates gene expression for pathways involved in mitochondrial biogenesis and respiratory function [30]. Additional studies of mitochondrial function and morphology in human skeletal muscle, further support a connection between mitochondrial dysfunction and diabetes. Shulman and colleagues demonstrated a reduction in ATP synthesis and mitochondrial content in severely insulin-resistant offspring of Type 2 diabetics [31, 32]. In addition, Kelley et al., noted impaired mitochondrial enzyme activities and reduced size and number of skeletal muscle mitochondria from diabetic patients [33, 34]. Pagel-Langenickel et al., demonstrated in isolated myocytes that disruption in insulin signaling results in dysregulation of mitochondrial biogenesis and impairment in mitochondrial membrane potential. These changes were restored by treatment with pioglitazone, to improve insulin signaling, and by overexpression of PGC-1α [35]. Taken together, these data strongly implicate impaired mitochondrial function in skeletal muscle of rodents and human diabetic patients.
Cardiac mitochondrial function has been less well studied in human subjects, likely secondary to difficulty obtaining appropriate human heart tissue samples for in depth investigations of mitochondrial functional capacity. However, a number of studies provide indirect evidence for altered cardiac mitochondrial function in diabetic patients. Diamant et al., studied high energy phosphate metabolism and cardiac function in asymptomatic well-controlled diabetic men and controls using MRI and 31P nuclear magnetic resonance spectroscopy. They demonstrated a reduction in multiple indexes of diastolic function by MRI in the diabetic patients; these functional changes were associated with a reduction in the cardiac phosphocreatine/ATP ratios [36]. More recently, the high energy phophate metabolism data was not confirmed in a study of non-ischemic diabetic cardiomyopathy using MRI and PET imaging, however there was also less difference in substrate metabolism changes in the study groups, which the authors suggested might contribute to the discrepancy in data [37]. A reduction in cardiac phosphocreatine/ATP ratios has also been demonstrated in hearts of diabetic patients with normal cardiac function by echocardiography [38], suggesting that changes in mitochondrial function may precede the reduction in contractility. Finally, Anderson et al., demonstrated in left atrial appendage tissue from type 2 diabetic patients undergoing coronary bypass surgery, that mitochondrial respiratory function was impaired and hydrogen peroxide emission was increased, suggesting an increase in oxidative stress [39]. Together with human data demonstrating altered lipid metabolism, these studies strongly implicate mitochondrial dysfunction in the human diabetic heart.
In contrast with human data, mitochondrial function has been directly studied in multiple animal models of diabetes (Type 1 and Type 2). In a model of chronic Type 1 diabetes (OVE26 mice), it was demonstrated that diabetic mice had evidence of mitochondrial biogenesis that was coupled to a reduction in mitochondrial function and mitochondrial ultrastructural abnormalities [40]. Animal models (db/db and ob/ob) of Type 2 diabetes have also provided evidence for reduced State 3 (ADP-stimulated or maximal) respiration [41, 42]. Additionally, target genes involved in mitochondrial function were noted to be decreased in ob/ob mice [42]. Furthermore, there is evidence for increased cardiac mitochondrial number (mitochondrial biogenesis) and ultrastructural defects by electron microscopy in two different obese animal models of insulin resistance and diabetes (UCP-DTA and ob/ob mice) [42, 43]. More recently Boudina et al., have also demonstrated a relationship between impaired cardiac insulin signaling and altered mitochondrial energetics by using mice with a cardiac-specific deletion of the insulin receptor [44]. The results from using this approach suggest that impairments in the insulin-signaling cascade in the absence of obesity or systemic metabolic abnormalities can affect mitochondrial form and function. In summary, the animal model investigations provide conclusive evidence that mitochondrial function is impaired in the hearts of animals with insulin resistance and diabetes. In the following sections, the potential mechanisms that contribute to mitochondrial impairment will be discussed.
4. Mechanisms of Mitochondrial Dysfunction in Diabetic Cardiomyopathy
4.1 Altered energy metabolism (including uncoupling)
As noted above, the heart has a high and continuous demand for oxidative metabolism in order to keep up with ATP production. Accordingly, the cardiomyocyte has a relatively high number of mitochondria compared to other tissues (approximately 40% of cardiomyocyte volume is mitochondria). Approximately 70% of the ATP generated by the heart is generated via oxidation of fatty acids. In healthy hearts, the rate of oxidative phosphorylation is tightly linked to the rate of ATP hydrolysis so that ATP content remains constant. Thus the heart relies on flexibility in its fuel source, preferentially using fatty acids or carbohydrates depending on physiologic or pathologic demands, including For changes supply in example, during workload, pathologic energyhypertrophy, substrate supply, and oxygen to heart failure and myocardial infarction the heart. the heart shifts fuel preference towards glucose oxidation [45]. This shift in fuel preference allows for continued ATP production with less oxygen consumed.
The diabetic heart has increased rates of FAO and decreased rates of glucose oxidation [46, 47]. Furthermore, the ability to shift fuel preference is constrained. These changes in FAO rates have been demonstrated in multiple mouse models [46] and in ZDF rats [48]. The increase in fatty acid oxidative capacity is mediated at least in part by increased activity of the nuclear receptor transcription factor, PPARα [49, 50]. PPARα is an important transcriptional regulator of fatty acid uptake and oxidation that controls expression of virtually every enzyme involved in the fatty acid utilization pathways [50, 51]. Mouse models in which PPARα is deficient (PPARα−/− mice) have reduced expression of genes involved in fatty acid utilization and reduced myocardial FAO rates [52-54]. In contrast, transgenic overexpression of PPARα in the heart (MHC-PPARα mice) results in markedly increased rates of fatty acid uptake, oxidation and TAG storage [55, 56]. Interestingly, we recently demonstrated that MHC-PPARα mice have mitochondrial ultrastructural abnormalities that were rescued when fatty acid uptake was limited by knocking out cardiac lipoprotein lipase (LpL) [57]. We found a reduction in several enzymes involved in mitochondrial OXPHOS, as well as a reduction in the PPARα co-activator PGC-1α in MHC-PPARα hearts; loss of LpL returned gene expression levels to normal [57]. PGC-1α is also known to co-activate other transcription factors that regulate mitochondrial number and mitochondrial OXPHOS [30, 58]. These data suggest that excessive fatty acid uptake has detrimental consequences on mitochondrial OXPHOS genes and mitochondrial ultrastructure. The specific role of PGC-1α in this process remains unclear. It has been noted by several investigators that PGC-1α is increased in insulin-resistant animal models [43, 46]. However, this may be a temporal phenomenon, as PGC-1α has also been found to be downregulated at later stages of diabetes (Duncan, unpublished data and [46]).
The increase in FAO may be energetically detrimental, as there is a higher oxygen cost for oxidizing more fatty acids. Indeed, acute lipid infusion in healthy dogs is known to cause increased oxygen consumption [59, 60]. Animal models of Type 2 diabetes have similarly shown a reduction in cardiac efficiency, with increased myocardial oxygen consumption (_V_O2) associated with increased FAO [42, 46, 47, 61, 62]. Furthermore, we noted decreased ATP/oxygen consumption ratios in UCP-DTA mice, a model of diabetes, indicating reduced mitochondrial efficiency [43]. Finally, Peterson et al., have noted that obesity and insulin resistance affect cardiac efficiency in young women [63], suggesting similar physiology in the human diabetic heart. The increased demand for oxidizing fatty acids and the reduction in cardiac efficiency may contribute to contractile dysfunction in the diabetic heart. Furthermore, the altered substrate flexibility and the change in oxygen consumption potentially may contribute to increased mortality following ischemic damage in diabetic patients.
One potential explanation for increased _V_O2 and reduced cardiac efficiency (or mitochondrial ATP production for amount of oxygen consumed) is that mitochondrial uncoupling is increased. Studies with ob/ob and db/db mice demonstrated an increase in _V_O2, despite a reduction in gene expression for multiple of the OXPHOS complexes, indicating that oxygen was consumed in the setting of mitochondrial deficits. These data suggest that mitochondrial uncoupling is increased resulting in decreased ATP/oxygen ratios.
Mitochondrial _V_O2 is normally tightly coupled to ATP synthesis. Substrate oxidation results in reducing equivalents that deliver electrons across the mitochondrial electron transport chain. The energy that is produced during electron transfer is used to create an electrochemical gradient by pumping hydrogen ions (H+) from the mitochondrial matrix to the intermembrane space. These H+ generally reenter the matrix via the F0F1-ATPase, which uses the energy to produce ATP from ADP. It is possible for H+ to bypass the F0F1-ATPase, resulting in oxygen consumption that is not coupled to ATP production via the H+-translocase uncoupling protein (UCP)-1 [64]. There are four additional homologs for UCP1 (UCP2, 3, 4 and 5) [65]. Two of these homologs (UCP2 and UCP3) are also thought to mediate proton transfer back into the mitochondrial matrix, resulting in mitochondrial uncoupling [66-69]. Both UCP2 and UCP3 are expressed in the heart, although their specific role remains unclear [70, 71].
Recently, Boudina et al. demonstrated mitochondrial uncoupling in db/db mouse hearts [61]. They noted an increase in respiration in the setting of oligomycin, an inhibitor of the F0F1-ATPase and an increase in proton leak from cardiac mitochondria isolated from db/db mice. Adding guanosine diphosphate, an inhibitor of UCPs, resulted in restoration of proton leak to wild-type levels, strongly suggesting that the increased uncoupling was mediated by UCPs. A second potential mediator of mitochondrial uncoupling is the adenine nucleotide translocator (ANT). Boudina et al., also found that atractyloside, an inhibitor of ANT-mediated uncoupling, altered proton leak in db/db mitochondria, suggesting that ANT may also contribute to uncoupling in diabetic hearts [61].
4.2 Oxidative Stress
Mitochondria are a major source of reactive oxygen species (ROS) production. It is estimated that approximately 90% of basal cellular ROS is produced by the mitochondria in tissues with high rates of respiration, such as cardiomyocytes [72]. The electron transport chain generates superoxide radicals as an inevitable by-product at complex I and complex III in the respiratory chain [73, 74]. Although the mitochondria produce a large fraction of ROS, there are mechanisms that also likely contribute to increased cytosolic ROS in diabetes, including development of advanced glycation end (AGE) products and modulation of NADPH oxidase [75, 76]. Superoxide (O2•) within the mitochondrial intermembrane space, or in the cytosol, forms hydrogen peroxide (H2O2), which is involved in subsequent oxygen sensing modification via stabilization of the transcription factor hypoxia inducible factor-1 alpha (HIF-1α) [77]. Thus, ROS generated by the mitochondria, have the ability to modify multiple additional physiologic pathways. ROS can directly damage proteins by oxidation, or they can oxidize lipids to form lipid peroxidation products, which can induce protein or phospholipid damage. ROS are also involved in DNA damage, in particular damage to mitochondrial DNA [78]. Finally, ROS can generate peroxynitrite from nitric oxide (NO), causing intracellular nitrosylation [79] and further impairment of mitochondrial respiration. Mitochondria are also armed with ROS defense mechanisms, such that intrinsic ROS production is almost completely eliminated at baseline [80]. Manganese superoxide dismutase (MnSOD) facilitates the conversion of superoxide to H2O2, protecting cells from superoxide-induced damage [80]. Reduced glutathione (GSH) scavenges hydroxyl radicals preventing a free radical chain reaction [81, 82]. Interestingly, recent proteomic data suggests that cytosolic ROS defense mechanisms may be more significantly impacted by diabetes than mitochondrial defense mechanisms. Johnson et al., reported a reduction in cytosolic ROS scavenging proteins in diabetic rat tissue [83]. Thus, deficiencies in the antioxidant defense system may also contribute to oxidative damage in cells.
Oxidative stress has been implicated in the pathophysiology of diabetes and its complications [84, 85]. Cytosolic ROS production may play a role in inducing mitochondrial damage and even enhance mitochondrial ROS production. Cytosolic ROS are known to induce opening of the mitochondrial permeability transition pore (MPTP) during ischemia [86]. One proposed mechanisms for MPTP opening in diabetic kidney disease, is the production of AGE associated with hyperglycemia, which result in AGE receptor (RAGE)-induced production of cytosolic ROS, which in turn result in opening of the MPTP [75]. Recently, high levels of AGE products have been found in cardiac tissue of diabetic patients [87]. Furthermore, metformin, an anti-diabetic medication, has been shown to inhibit opening of the MPTP during ischemia reperfusion in diabetic rats [88].
Brownlee and colleagues have generated significant evidence that mitochondrial ROS activate multiple pathways for cellular damage in the setting of hyperglycemia [89-92]. An increase in 3-nitrotyrosine in association with increased cell death has been noted in human myocardial samples [93], as well as in a streptozotocin (STZ)-induced model of Type 1 diabetes [94]. In addition, there is evidence for tyrosine nitration of cardiac mitochondrial proteins in mice with Type 1 diabetes [79]. These data implicate peroxynitrite damage pathways in diabetic cardiomyopathy. Indeed, Cai et al., demonstrated a reduction in nitrosative damage with overexpression of metallothionein, an antioxidant protein, in STZ-treated mice [94]. In a different model of Type 1 diabetes (OVE26 mice), metallothionein overexpression in the heart rescued contractile dysfunction and returned levels of oxidized glutathione (GSSG) to normal [95]. Other studies have demonstrated that overexpression of superoxide scavengers, such as catalase and MnSOD, in mouse models of Type 1 diabetes, results in preservation of myocardial function and mitochondrial morphology [96, 97]. Data from Type 2 diabetic models is more limited. Boudina et al. have reported an increase in mitochondrial H2O2 in db/db mice in association with increased levels of MnSOD, suggesting that there is an increase in ROS production [61]. In addition, catalase overexpression in obese Ay mice resulted in normalization of cardiomyocyte contractility [96]. Thus, it appears that ROS mechanisms may be similar in Type 1 and Type 2 diabetic hearts. Taken together, these data implicate multiple pathways for oxidative stress in the diabetic myocardium, and suggest that each of these may contribute to myocardial dysfunction.
4.3 Impaired mitochondrial calcium handling
We have already discussed the proton gradient that is generated by oxidative phosphorylation in the electron transport chain. This proton gradient, also known as the transmembrane potential, is required for ATP production, but it also plays an important role in driving calcium accumulation in mitochondria. A uniporter carries calcium down the electrochemical gradient, and the accumulated calcium is removed via a Na+/Ca2+ exchanger. Calcium uptake into the mitochondrion now appears to be important for activation of the tricarboxylic acid (TCA) cycle, and possibly for increasing ATP production (see review [98]). The relative contribution of calcium uptake in ATP production remains to be defined in vivo. Nevertheless, it is proposed that calcium transfer from cytosol to mitochondria may represent a mechanism for coordinating ATP supply and demand for cardiomyocyte contraction [98, 99]. Mitochondrial calcium uptake may also act as a spatial buffering system, removing calcium locally and modulating cytosolic calcium concentrations, thus regulating activity of calcium-dependent processes [100]. The mitochondria lie in close proximity to sarcoplasmic reticulum and endoplasmic reticulum calcium release sites, thus mitochondrial calcium uptake likely plays a role in regulating calcium signaling from these organelles as well [98]. Interestingly, there may also be a role for mitochondrial and nuclear communication via calcium signaling. PGC-1α is known to play an important role in mitochondrial biogenesis and regulation of respiratory activity. PGC-1α is regulated in part by calcium dependent processes [30], and has been demonstrated to play a role in calcium signaling and calcium mediated cell death [101]. Given the potential impact of calcium signaling on mitochondrial energy metabolism, it is possible that altered calcium handling contributes to diabetic cardiomyopathy.
There are limited studies of mitochondrial calcium handling defects in models of Type 2 diabetes. Fauconnier and co-workers demonstrated impaired calcium handling in cardiomyocytes from ob/ob mice [102]. In addition Belke et al. found a reduction in calcium levels in isolated cardiomyocytes from db/db animals, and reduction in the rate of calcium decay, suggesting impaired mitochondrial calcium uptake [103]. Data on mitochondrial calcium handling deficits is more robust in Type 1 diabetes models. STZ-induced diabetic rats have lower rates of mitochondrial calcium uptake, a change that was associated with development of hyperglycemia [104]. Another cause of reduced mitochondrial calcium, is increased opening of the mitochondrial permeability transition pore (MPTP), resulting in release of accumulated calcium. In STZ-induced diabetic rats, it was observed that calcium uptake was similar in control versus diabetic animals, but that mitochondria in diabetic hearts were unable to retain the accumulated calcium. This phenomenon was not seen in the presence of an MPTP inhibitor, cylcosporin [105]. Thus, the data in these different animal models supports the notion that mitochondrial calcium handling is impaired in diabetic myocardium, resulting in compromised energy metabolism and thus reduced contractility.
4.4 Cell Death
A number of investigators have implicated cell death in the pathogenesis of diabetic cardiomyopathy in patients and in animal models [106-108]. The mechanisms contributing to apoptosis are not entirely clear, but likely involve mitochondrial and endoplasmic reticulum-stress responses, and oxidative stress. Cai et al. have demonstrated that metallothionein treatment of STZ-treated mice was able to reduce mitochondrial oxidative stress and attenuate cell death [109]. Li et al., demonstrated in STZ-induced diabetic rats that protein and mRNA expression of caspase 12 and Grp78 were induced in association with evidence of apoptosis, suggesting activation of an ER-stress response that contributed to cell death. Additionally, evidence of mitochondrial-induced apoptosis has been generated in experimental animals. Williamson et al., reported evidence of increased caspase activity in diabetic (STZ treated) mice in association with changes in mitochondrial membrane potential and MPTP opening, specifically in the interfibrillar mitochondrial population [110]. Additional reports from this same group have documented proteomic differences in mitochondrial proteins, including altered ANT1 and antioxidant expression, two potential contributors to mitochondrial-induced cell death. They have also noted changes in oxidative protein damage and mitochondrial cardiolipin content, which may alter mitochondrial membrane integrity and contribute to mitochondrial induced apoptosis [111, 112].
4.5 Altered Mitochondrial Dynamics and Biogenesis
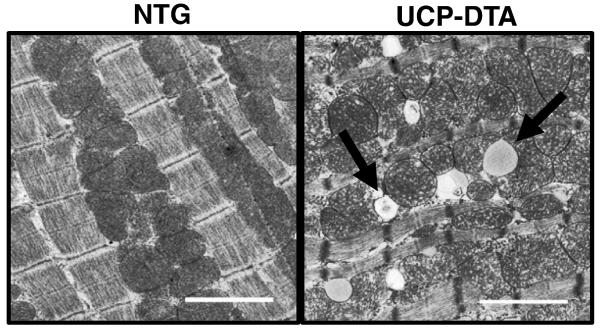
Recent studies by our group and others have demonstrated an increase in cardiac mitochondria in multiple mouse models of diabetes [42, 43, 113]. In each of these models, despite mitochondrial biogenesis, mitochondrial respiratory function and/or ATP production was impaired. Thus, mitochondrial biogenesis was not coupled to an increase in mitochondrial function. These studies have raised the question as to whether mitochondrial biogenesis is an adaptive or maladaptive process. We observed in UCP-DTA mice an increase in PGC-1α expression in insulin-resistant animals [43]. In addition, there was an absence of mitochondrial biogenesis in UCP-DTA animals in a PGC-1α-deficient background, suggesting that PGC-1α was necessary for the increase in mitochondrial number. However, PGC-1α was not increased in ob/ob animals, despite an increase in mitochondrial DNA and mitochondrial number [42]. In addition, we have also observed a downregulation of PGC-1α with the onset of overt diabetes in UCP-DTA animals (unpublished data), despite evidence of persistently increased mitochondrial number (Figure 2). These observations, suggest that PGC-1α-independent mechanisms may play a role in the mitochondrial biogenic response, and that upregulation of PGC-1α is a temporal phenomenon, possibly related to hyperglycemia. The specific role for mitochondrial biogenesis in the diabetic heart remains unclear. While it is tempting to speculate that excess fatty acid uptake stimulates an increase in mitochondrial number, it is clear from these animal models that mitochondrial respiratory capacity is not enhanced [42, 43, 113]. It is possible perhaps that mitochondrial proliferation itself is a trigger for mitochondrial dysfunction, but at this time there is no clear data to support this hypothesis.
Figure 2. Evidence of mitochondrial biogenesis and ultrastructural changes in diabetic myocardium.
Representative electron micrographic images of papillary muscle from non-transgenic (NTG) and diabetic, UCP-DTA hearts showing increased mitochondrial number. Arrows represent areas of mitochondrial architecture damage (left) and lipid accumulation (right).
Mitochondria are dynamic organelles that frequently change shape and subcellular distribution. The number and morphology of mitochondria are regulated by rates of mitochondrial fission and fusion. Thus the change in mitochondrial number in diabetic hearts may reflect alterations in rates of mitochondrial fission and/or fusion. In addition, mitochondrial fission and fusion have also recently been associated with mitochondrial fragmentation and apoptosis [114, 115]. Thus, an additional potential mechanism for mitochondrial impairment and contractile dysfunction may be altered mitochondrial fission/fusion machinery. There is limited data regarding the role of mitochondrial dynamics in diabetes. Impaired mitochondrial fission has been implicated in mitochondrial failure in pancreatic β-cells [116, 117]. Mitofusin 2, an important regulator of mitochondrial fusion, was reduced in skeletal muscle of obese ZDF rats [118]. Studies of cardiac mitochondrial fission and fusion are even more limited. In isolated cardiac cells, Yu et al., demonstrated that mitochondrial fission contributed to high-glucose induced apoptosis [119]. In multiple different cardiac-derived cells exposed to high glucose, it was demonstrated that mitochondria were fragmented and that cell death was increased. Inhibition of mitochondrial fission, by overexpression of a dominant-negative DLP1 (dynamin-like protein) protein, resulted in normalization of mitochondrial morphology, ROS levels and cell death [119]. The role of mitochondrial fission and fusion in intact animals remains to be elucidated.
4.6 Mitochondrial lipid content
Mitochondrial membranes, like those of other organelles, are comprised of a lipid bilayer. The mitochondria are characterized by a highly specialized phospholipid, cardiolipin, which is almost exclusively localized to the inner mitochondrial membrane where it is synthesized [120]. Evidence suggests that cardiolipin plays a key role in regulating mitochondrial bioenergetics, by optimizing activities of various mitochondrial inner membrane proteins involved in OXPHOS [121-123]. Cardiolipin has a role in maintaining contact sites between the inner and outer mitochondrial membranes [124]. Cardiolipin has also been noted to be required for electron transfer in the mitochondrial respiratory chain [125]. Because of its location in the inner mitochondrial membrane, cardiolipin interacts with numerous other inner membrane proteins to optimize mitochondrial respiratory activity and anion transport. It is also speculated to play a role in mitochondrial fission and fusion (see reviews [120, 122]). In addition, cardiolipin is susceptible to oxidative damage by ROS and has been shown to play a critical role in calcium handling and cell death [120]. The importance of cardiolipin is underscored by the pathology seen in Barth syndrome, an X-linked recessive disorder with mutations in tafazzin, an essential enzyme for cardiolipin remodeling; patients with Barth syndrome suffer from variable degrees mitochondrial dysfunction and cardiomyopathy [126]. Alterations in cardiolipin content or composition have also been demonstrated in a several other disease states [127]. Given the dramatic changes in lipid uptake and storage seen in diabetic patients, it has been hypothesized that mitochondrial lipids may also be altered, and that this could contribute to mitochondrial dysfunction. Thus far, there are limited data regarding cardiolipin content in models of diabetes. Han et al. found that both ob/ob mice and STZ-induced diabetic mice had significantly decreased levels of cardiolipin in the myocardium [128]. More recently, it was demonstrated that STZ-treated mice had significant changes to the interfibrillar mitochondrial population, which included reduced mitochondrial size, cardiolipin content and electron transport activity [112]. These findings were associated with a decrease in contractile function using an isolated working heart model [112]. Thus, these data suggest that diabetes is another pathologic state in which mitochondrial cardiolipin content is altered, which may have important implications for altered mitochondrial bioenergetics.
5. Conclusion
The heart requires a continuous source of substrate and optimal mitochondrial metabolism to meet its high-energy demands. In the setting of diabetes, substrate flexibility is limited and mitochondrial function is compromised, which we propose results in contractile dysfunction and thus contributes to diabetic cardiomyopathy. This review summarizes multiple possible mechanisms that may contribute to mitochondrial dysfunction in the diabetic heart. Many of these mechanisms have an impact that overlaps with those of other systems. Thus, more than likely a combination of these different mechanisms play a role in the decline of mitochondrial function during the progression from insulin resistance to full blown diabetes and during the progression from compensated to decompensated heart failure. As the diabetes pandemic continues and cardiovascular disease rates increase, it is imperative that investigators pursue additional mechanistic studies in human subjects as well as begin to explore potential therapies targeted at specific mitochondrial defects that may contribute to diabetic cardiomyopathy.
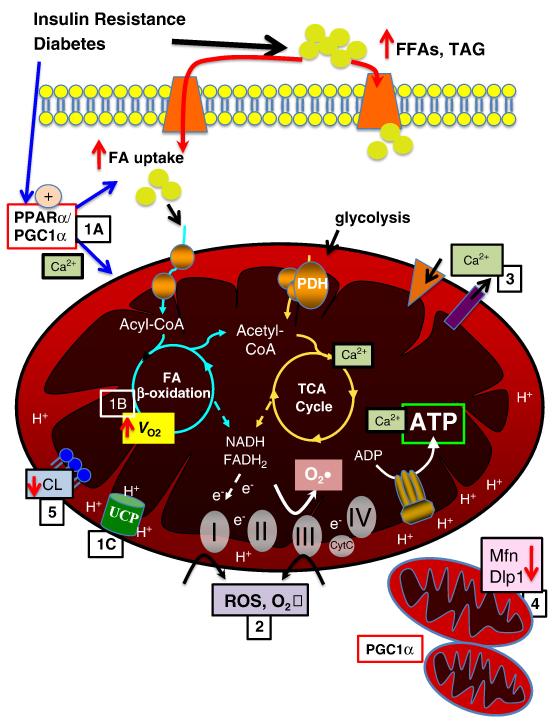
Figure 1. Mechanisms contributing to mitochondrial dysfunction.
Insulin resistance and diabetes lead to increased circulating free fatty acids (FFA) and triglyceride (TAG). There is increased fatty acid (FA) uptake via FA transporters (e.g. FATP, CD36), in part mediated by increased activity of PPARα and PGC-1α (1A). Increased FA uptake and PPARα contribute to increased FAO and oxygen consumption (_V_O2) (1B). In addition, proton transfer via uncoupling proteins (UCPs) contributes to uncoupling, reducing ATP production and contributing to cardiac inefficiency (1C). Increased FAO and oxidative phosphorlyation and impairment in the electron transport chain contribute to increased ROS and superoxide (O2•) production at complexes I and III (2), in addition a reduction in ROS scavenging enzymes may contribute to oxidative protein damage. Calcium transport across mitochondrial membranes is altered (3), impacting TCA cycle enzyme activity and ATP production. Emerging data suggest altere mitochondrial dynamics, with evidence of mitochondrial biogenesis, perhaps mediated in part by PGC-1α, as well as decreased levels of important regulators of mitochondrial fission, such as mitofusin 2 (Mfn) and dynamin-like protein (Dlp) (4). Mitochondrial membrane phospholipid content is altered, with evidence of decreased cardiolipin content, which may impact mitochondrial function via multiple modalities (5).
ACKNOWLEDGEMENTS
The author would like to acknowledge Dr. Daniel Kelly for his support and mentorship and all the members of the Duncan lab for their contributions to our work. Dr. Duncan is supported by a NHLBI K08 award (HL084093) and is a scholar in the Pediatric Critical Care Scientist Development Program (HD047349).
REFERENCES
- [1].Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- [2].King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- [3].Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- [4].Rayner SPM, Buckley C, Press V. In: Coronary heart disease statistics: Diabetes Supplement. B.H.F.H.P.R. Group, editor. University of Oxford; Oxford: 2001. [Google Scholar]
- [5].Abbott RD, Donahue RP, Kannel WB, Wilson PW. The impact of diabetes on survival following myocardial infarction in men vs. women. JAMA. 1988;260:3456–3460. [PubMed] [Google Scholar]
- [6].Cohen-Solal A, Beauvais F, Logeart D. Heart failure and diabetes mellitus: epidemiology and management of an alarming association. J Card Fail. 2008;14:615–625. doi: 10.1016/j.cardfail.2008.04.001. [DOI] [PubMed] [Google Scholar]
- [7].Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- [8].Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- [9].Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- [10].Fein FS, Sonnenblick EH. Diabetic cardiomyopathy. Cardiovascular Drugs Therapeutic. 1994;8:65–73. doi: 10.1007/BF00877091. [DOI] [PubMed] [Google Scholar]
- [11].Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochimica et Biophysica Acta. 2005;1734:112–126. doi: 10.1016/j.bbalip.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [12].Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15:318–330. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- [13].Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2:454–466. doi: 10.1242/dmm.001941. [DOI] [PubMed] [Google Scholar]
- [14].Lowell BB, Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- [15].Kjerrulf M, Berke Z, Aspegren A, Umaerus M, Nilsson T, Svensson L, Hurt-Camejo E. Reduced cholesterol accumulation by leptin deficient (ob/ob) mouse macrophages. Inflamm Res. 2006;55:300–309. doi: 10.1007/s00011-006-0087-8. [DOI] [PubMed] [Google Scholar]
- [16].Wu KK, Wu TJ, Chin J, Mitnaul LJ, Hernandez M, Cai TQ, Ren N, Waters MG, Wright SD, Cheng K. Increased hypercholesterolemia and atherosclerosis in mice lacking both ApoE and leptin receptor. Atherosclerosis. 2005;181:251–259. doi: 10.1016/j.atherosclerosis.2005.01.029. [DOI] [PubMed] [Google Scholar]
- [17].Aasum E, Belke DD, Severson DL, Riemersma RA, Cooper M, Andreassen M, Larsen TS. Cardiac function and metabolism in Type 2 diabetic mice after treatment with BM 17.0744, a novel PPARα activator. Am J Physiol Heart Circ Physiol. 2002;283:H949–H957. doi: 10.1152/ajpheart.00226.2001. [DOI] [PubMed] [Google Scholar]
- [18].Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- [19].Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- [20].Yue TL, Bao W, Gu JL, Cui J, Tao L, Ma XL, Ohlstein EH, Jucker BM. Rosiglitazone treatment in zucker diabetic fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes. 2005;54:554–562. doi: 10.2337/diabetes.54.2.554. [DOI] [PubMed] [Google Scholar]
- [21].Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–982. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- [22].Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- [23].Rodrigues B, Cam MC, McNeill JH. Myocardial substrate metabolism: implications for diabetic cardiomyopathy. J Mol Cell Cardiol. 1995;27:169–179. doi: 10.1016/s0022-2828(08)80016-8. [DOI] [PubMed] [Google Scholar]
- [24].Banke NH, Wende AR, Leone TC, O’Donnell JM, Abel ED, Kelly DP, Lewandowski ED. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARalpha. Circ Res. 2010;107:233–241. doi: 10.1161/CIRCRESAHA.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- [26].Murthy VK, Shipp JC. Accumulation of myocardial triacylglycerols in ketotic diabetes. Diabetes. 1977;26:222–229. doi: 10.2337/diab.26.3.222. [DOI] [PubMed] [Google Scholar]
- [27].Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- [28].Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- [29].Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Finck BN, Kelly DP. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. NEJM. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- [34].Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- [35].Pagel-Langenickel I, Bao J, Joseph JJ, Schwartz DR, Mantell BS, Xu X, Raghavachari N, Sack MN. PGC-1alpha integrates insulin signaling, mitochondrial regulation, and bioenergetic function in skeletal muscle. J Biol Chem. 2008;283:22464–22472. doi: 10.1074/jbc.M800842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42:328–335. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- [37].Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524–1532. doi: 10.1016/j.jacc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- [38].Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040–3046. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- [39].Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54:1891–1898. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM, Jr, Klein JB, Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type I diabetes. Am J Physiol Endocrinol Metab. 2004;287:E896–E905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- [41].Kuo TH, Moore KH, Giacomelli F, Wiener J. Defective oxidative metabolism of heart mitochondria from genetically diabetic mice. Diabetes. 1983;32:781–787. doi: 10.2337/diab.32.9.781. [DOI] [PubMed] [Google Scholar]
- [42].Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- [43].Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-α/PGC-1α gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- [46].Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun U. Jeong, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- [47].Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- [48].Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2102–2110. doi: 10.1152/ajpheart.00935.2004. [DOI] [PubMed] [Google Scholar]
- [49].Duncan JG, Finck BN. The PPARalpha-PGC-1alpha Axis Controls Cardiac Energy Metabolism in Healthy and Diseased Myocardium. PPAR Res. 2008;2008:253817. doi: 10.1155/2008/253817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Finck BN, Kelly DP. Peroxisome proliferator-activated receptor alpha (PPARalpha) signaling in the gene regulatory control of energy metabolism in the normal and diseased heart. J Mol Cell Cardiol. 2002;34:1249–1257. doi: 10.1006/jmcc.2002.2061. [DOI] [PubMed] [Google Scholar]
- [51].Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends in Cardiovascular Medicine. 2000;10:238–245. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- [52].Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARα) in the cellular fasting response: The PPARα-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- [54].Campbell FM, Kozak R, Wagner A, Altarejos JY, Dyck JR, Belke DD, Severson DL, Kelly DP, Lopaschuk GD. A role for peroxisome proliferator-activated receptor alpha (PPARalpha ) in the control of cardiac malonyl-CoA levels: reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking PPARalpha are associated with higher concentrations of malonyl-CoA and reduced expression of malonyl-CoA decarboxylase. J Biol Chem. 2002;277:4098–4103. doi: 10.1074/jbc.M106054200. [DOI] [PubMed] [Google Scholar]
- [55].Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Finck BN. The role of the peroxisome proliferator-activated receptor alpha pathway in pathological remodeling of the diabetic heart. Curr Opin Clin Nutr Metab Care. 2004;7:391–396. doi: 10.1097/01.mco.0000134371.70815.32. [DOI] [PubMed] [Google Scholar]
- [57].Duncan JG, Bharadwaj KG, Fong JL, Mitra R, Sambandam N, Courtois MR, Lavine KJ, Goldberg IJ, Kelly DP. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-alpha transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-alpha activators. Circulation. 2010;121:426–435. doi: 10.1161/CIRCULATIONAHA.109.888735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- [59].Kjekshus JK, Mjos OD. Effect of free fatty acids on myocardial function and metabolism in the ischemic dog heart. J Clin Invest. 1972;51:1767–1776. doi: 10.1172/JCI106978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mjos OD. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest. 1971;50:1386–1389. doi: 10.1172/JCI106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- [62].How OJ, Aasum E, Severson DL, Chan WY, Essop MF, Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes. 2006;55:466–473. doi: 10.2337/diabetes.55.02.06.db05-1164. [DOI] [PubMed] [Google Scholar]
- [63].Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- [64].Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- [65].Ledesma A, de Lacoba MG, Rial E. The mitochondrial uncoupling proteins. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-12-reviews3015. REVIEWS3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- [67].Gimeno RE, Dembski M, Weng X, Deng N, Shyjan AW, Gimeno CJ, Iris F, Ellis SJ, Woolf EA, Tartaglia LA. Cloning and characterization of an uncoupling protein homolog: a potential molecular mediator of human thermogenesis. Diabetes. 1997;46:900–906. doi: 10.2337/diab.46.5.900. [DOI] [PubMed] [Google Scholar]
- [68].Stuart JA, Brindle KM, Harper JA, Brand MD. Mitochondrial proton leak and the uncoupling proteins. J Bioenerg Biomembr. 1999;31:517–525. doi: 10.1023/a:1005456725549. [DOI] [PubMed] [Google Scholar]
- [69].Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- [70].Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. Lancet. 2004;364:1786–1788. doi: 10.1016/S0140-6736(04)17402-3. [DOI] [PubMed] [Google Scholar]
- [71].Murray AJ, Panagia M, Hauton D, Gibbons GF, Clarke K. Plasma free fatty acids and peroxisome proliferator-activated receptor alpha in the control of myocardial uncoupling protein levels. Diabetes. 2005;54:3496–3502. doi: 10.2337/diabetes.54.12.3496. [DOI] [PubMed] [Google Scholar]
- [72].Conn PM. Handbook of Models of Human Aging. First ed Elsevier Academic Press; 2006. [Google Scholar]
- [73].Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- [74].Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, Forbes JM. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Li L, Renier G. Activation of nicotinamide adenine dinucleotide phosphate (reduced form) oxidase by advanced glycation end products links oxidative stress to altered retinal vascular endothelial growth factor expression. Metabolism. 2006;55:1516–1523. doi: 10.1016/j.metabol.2006.06.022. [DOI] [PubMed] [Google Scholar]
- [77].Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- [78].Wallace DC. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992;256:628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- [79].Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem. 2003;278:33972–33977. doi: 10.1074/jbc.M303734200. [DOI] [PubMed] [Google Scholar]
- [80].Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- [81].Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- [82].Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Johnson DT, Harris RA, French S, Aponte A, Balaban RS. Proteomic changes associated with diabetes in the BB-DP rat. Am J Physiol Endocrinol Metab. 2009;296:E422–432. doi: 10.1152/ajpendo.90352.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- [85].Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- [86].Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;290:H2024–2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- [87].Willemsen S, Hartog JW, Hummel YM, van Ruijven MH, van der Horst IC, van Veldhuisen DJ, Voors AA. Tissue advanced glycation end products are associated with diastolic function and aerobic exercise capacity in diabetic heart failure patients. Eur J Heart Fail. 2010 doi: 10.1093/eurjhf/hfq168. [DOI] [PubMed] [Google Scholar]
- [88].Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- [89].Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- [90].Giardino I, Edelstein D, Brownlee M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J Clin Invest. 1996;97:1422–1428. doi: 10.1172/JCI118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- [94].Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54:1829–1837. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- [95].Ye G, Metreveli NS, Ren J, Epstein PN. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes. 2003;52:777–783. doi: 10.2337/diabetes.52.3.777. [DOI] [PubMed] [Google Scholar]
- [96].Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- [97].Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- [98].Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- [99].Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53(Suppl 1):S96–102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- [100].Parekh AB. Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiol. 2003;547:333–348. doi: 10.1113/jphysiol.2002.034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bianchi K, Vandecasteele G, Carli C, Romagnoli A, Szabadkai G, Rizzuto R. Regulation of Ca2+ signalling and Ca2+-mediated cell death by the transcriptional coactivator PGC-1alpha. Cell Death Differ. 2006;13:586–596. doi: 10.1038/sj.cdd.4401784. [DOI] [PubMed] [Google Scholar]
- [102].Fauconnier J, Lanner JT, Zhang SJ, Tavi P, Bruton JD, Katz A, Westerblad H. Insulin and inositol 1,4,5-trisphosphate trigger abnormal cytosolic Ca2+ transients and reveal mitochondrial Ca2+ handling defects in cardiomyocytes of ob/ob mice. Diabetes. 2005;54:2375–2381. doi: 10.2337/diabetes.54.8.2375. [DOI] [PubMed] [Google Scholar]
- [103].Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes. 2004;53:3201–3208. doi: 10.2337/diabetes.53.12.3201. [DOI] [PubMed] [Google Scholar]
- [104].Flarsheim CE, Grupp IL, Matlib MA. Mitochondrial dysfunction accompanies diastolic dysfunction in diabetic rat heart. Am J Physiol. 1996;271:H192–202. doi: 10.1152/ajpheart.1996.271.1.H192. [DOI] [PubMed] [Google Scholar]
- [105].Oliveira PJ, Seica R, Coxito PM, Rolo AP, Palmeira CM, Santos MS, Moreno AJ. Enhanced permeability transition explains the reduced calcium uptake in cardiac mitochondria from streptozotocin-induced diabetic rats. FEBS Lett. 2003;554:511–514. doi: 10.1016/s0014-5793(03)01233-x. [DOI] [PubMed] [Google Scholar]
- [106].Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, Kittleson MM, Minhas KM, Berkowitz DE, Wei C, Hare JM. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- [107].Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- [108].Li Z, Zhang T, Dai H, Liu G, Wang H, Sun Y, Zhang Y, Ge Z. Involvement of endoplasmic reticulum stress in myocardial apoptosis of streptozocin-induced diabetic rats. J Clin Biochem Nutr. 2007;41:58–67. doi: 10.3164/jcbn.2007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48:1688–1697. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- [110].Williamson CL, Dabkowski ER, Baseler WA, Croston TL, Alway SE, Hollander JM. Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am J Physiol Heart Circ Physiol. 2010;298:H633–642. doi: 10.1152/ajpheart.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, Hollander JM. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol. 2010;299:H529–540. doi: 10.1152/ajpheart.00267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dabkowski ER, Williamson CL, Bukowski VC, Chapman RS, Leonard SS, Peer CJ, Callery PS, Hollander JM. Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am J Physiol Heart Circ Physiol. 2009;296:H359–369. doi: 10.1152/ajpheart.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology. 2006;21:250–258. doi: 10.1152/physiol.00008.2006. [DOI] [PubMed] [Google Scholar]
- [114].Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- [115].Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- [116].Dlaskova A, Spacek T, Santorova J, Plecita-Hlavata L, Berkova Z, Saudek F, Lessard M, Bewersdorf J, Jezek P. 4Pi microscopy reveals an impaired three-dimensional mitochondrial network of pancreatic islet beta-cells, an experimental model of type-2 diabetes. Biochim Biophys Acta. doi: 10.1016/j.bbabio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- [117].Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- [119].Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009;45:643–650. doi: 10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- [121].Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- [122].Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Robinson NC. Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr. 1993;25:153–163. doi: 10.1007/BF00762857. [DOI] [PubMed] [Google Scholar]
- [124].Ardail D, Lerme F, Louisot P. Further characterization of mitochondrial contact sites: effect of short-chain alcohols on membrane fluidity and activity. Biochem Biophys Res Commun. 1990;173:878–885. doi: 10.1016/s0006-291x(05)80868-x. [DOI] [PubMed] [Google Scholar]
- [125].Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- [126].Hauff KD, Hatch GM. Cardiolipin metabolism and Barth Syndrome. Prog Lipid Res. 2006;45:91–101. doi: 10.1016/j.plipres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- [127].Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- [128].Han X, Yang J, Cheng H, Yang K, Abendschein DR, Gross RW. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry. 2005;44:16684–16694. doi: 10.1021/bi051908a. [DOI] [PubMed] [Google Scholar]