Distinct functional outputs of PTEN signalling are controlled by dynamic association with β-arrestins (original) (raw)
Abstract
The tumour suppressor PTEN (phosphatase and tensin deleted on chromosome 10) regulates major cellular functions via lipid phosphatase-dependent and -independent mechanisms. Despite its fundamental pathophysiological importance, how PTEN's cellular activity is regulated has only been partially elucidated. We report that the scaffolding proteins β-arrestins (β-arrs) are important regulators of PTEN. Downstream of receptor-activated RhoA/ROCK signalling, β-arrs activate the lipid phosphatase activity of PTEN to negatively regulate Akt and cell proliferation. In contrast, following wound-induced RhoA activation, β-arrs inhibit the lipid phosphatase-independent anti-migratory effects of PTEN. β-arrs can thus differentially control distinct functional outputs of PTEN important for cell proliferation and migration.
Keywords: Akt, β-arrestin, cell migration, cell proliferation, PTEN
Introduction
The tumour suppressor PTEN (phosphatase and tensin deleted on chromosome 10) regulates numerous cellular functions including proliferation and migration (Salmena et al, 2008; Chalhoub and Baker, 2009). Underscoring its critical importance as a major cellular regulator, the PTEN gene is frequently deleted or mutated in a broad range of human cancers. PTEN is a dual-specificity phosphatase that is capable of dephosphorylating lipids and peptides. The crystal structure of PTEN revealed the presence of two major functional domains: an N-terminal catalytic phosphatase domain and a C-terminal C2 domain (Lee et al, 1999). PTEN also contains a short N-terminal phosphatidylinositol 4,5 bisphosphate (PIP2) binding sequence and a C-terminal tail region, which contains multiple phosphorylation sites, implicated in regulating PTEN conformation and function (Vazquez et al, 2000; Odriozola et al, 2007; Rahdar et al, 2009). The major physiological substrate of PTEN is phosphatidylinositol 3,4,5 trisphosphate (PIP3; Maehama and Dixon, 1999). PTEN, therefore, acts to inhibit the PI3K/Akt pathway through its lipid phosphatase catalytic activity towards PIP3. By converting PI3K-generated PIP3 into PIP2, PTEN thus negatively regulates the proliferative signals depending on activated Akt. The fact that >40% of naturally occurring PTEN mutations found in cancer lie within the C-terminal region (Waite and Eng, 2002), outwith the catalytic domain, suggests that PTEN has a wider role than inhibition of the PI3K/Akt pathway in cellular regulation and tumour suppression. For example, a phosphatase-dead point mutant of PTEN can still maintain increased p53 protein levels and activity, comparable to those elicited by wild-type PTEN (Freeman et al, 2003). In addition, an RNA-dependent protein kinase (PKR)-eukaryotic translation initiation factor 2 (eIF2) phosphorylation pathway that inhibits protein synthesis is regulated by PTEN and does not require its phosphatase activity (Mounir et al, 2009). Also, PTEN can inhibit cell migration through its C2 domain, independent of its lipid phosphatase activity (Raftopoulou et al, 2004). This anti-migratory activity appears to involve instead the protein phosphatase activity of PTEN and the dephosphorylation of a single residue, Thr383, in its C-terminal regulatory tail.
Contrasting with the large amount of data available on the role of PTEN in tumourigenesis, how PTEN is regulated by other factors and how this impacts on cellular function has only been partially elucidated (Wang and Jiang, 2008), particularly with regard to signal transduction pathways emanating from receptor stimulation at the cell surface. β-Arrestins (β-arr1 and β-arr2) are two ubiquitously expressed proteins that were initially appreciated for the roles they play in the regulation of G protein coupled receptors (GPCRs; Dewire et al, 2007; Moore et al, 2007). Emerging evidence has, however, shown that β-arrs also act as dynamic scaffolds to regulate the activities and subcellular distribution of multiple signalling proteins. Through their scaffolding properties downstream of diverse cell surface receptors, β-arrs modulate signal transduction in many essential biological events that often overlap with cellular functions regulated by PTEN (DeFea, 2007; Dewire et al, 2007; Xiao et al, 2007; Luttrell and Gesty-Palmer, 2010). In this study, we uncover a functional direct interaction between β-arrs and the tumour suppressor PTEN. We demonstrate that β-arrs serve as important upstream regulators to modulate both lipid phosphatase-dependent and -independent functions of PTEN, which respectively impact on cell proliferation and migration.
Results
_β_-Arrestin directly interacts with PTEN and enhances its lipid phosphatase activity
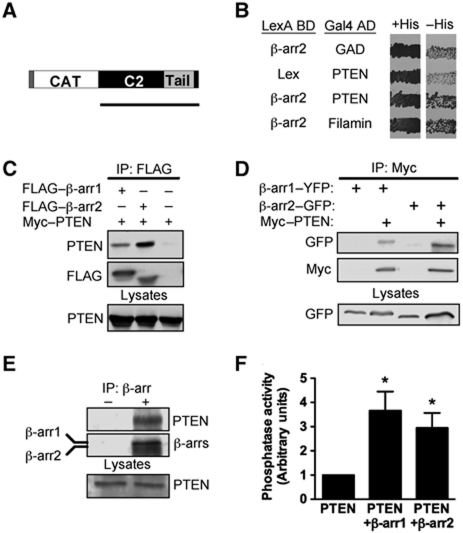
Using a cytoplasmic yeast two-hybrid screen (Sos recruitment system) with β-arr2 as bait (Supplementary Figure S1a), we isolated a clone corresponding to a C-terminal fragment of PTEN (amino acids 203–403) from a human thymus cDNA library (Figure 1A). The interaction between β-arr2 and PTEN was subsequently validated with full-length proteins using the L40 yeast reporter strain cotransformed with pLexABD-β-arr2 and pGal4AD-PTEN (Figure 1B). This putative association was confirmed in mammalian cells by co-immunoprecipitation experiments of various forms of tagged PTEN and βarrs from lysates of transfected COS and HEK293 cells (Figure 1C and D). We subsequently demonstrated the presence of endogenous PTEN and β-arrs in the same molecular complex in HEK293 cells, in which PTEN was found in immunoprecipitates of a pan-β-arr1/2 antibody (Figure 1E). Taken together, the above results indicate that both β-arr1 and β-arr2 associate with PTEN. As the co-immunoprecipitation experiments detailed above cannot rule out the possibility that the interaction between β-arrs and PTEN occurs indirectly due to the presence of other proteins, we performed in vitro binding experiments using purified recombinant forms of PTEN and β-arrs. A GST–PTEN fusion protein was incubated with either His6–β-arr1 or His6–β-arr2 and western blotting with anti-β-arr antibodies demonstrated that the interaction of PTEN with either β-arr1 or β-arr2 is indeed direct (Figure 2A and B).
Figure 1.
β-Arrestin associates with PTEN in mammalian cells and enhances its lipid phosphatase activity. (A) Schematic representation of PTEN indicating catalytic (CAT), C2 and C-terminal regulatory region. Black bar shows the PTEN clone isolated from the SRS yeast two-hybrid screen performed in Cdc25H yeast. (B) The L40 yeast reporter strain containing the indicated plasmids was tested for histidine auxotrophy. Transformants were patched on selective medium containing histidine (+His) and subsequently replica plated onto medium lacking histidine (−His) and incubated for 4–5 days. Growth in the absence of histidine indicates interaction between full-length β-arr2 and full-length PTEN. Filamin (repeats 22–24) was used as positive control. (C) Western blot of FLAG immunoprecipitates from COS cells coexpressing PTEN with FLAG–β-arr1, FLAG–β-arr2 or vector control. (D) Western blot of Myc immunoprecipitates from HEK293 cells coexpressing Myc–PTEN with β-arr1–YFP or β-arr2–GFP. (E) Co-immunoprecipitation of endogenous β-arrs with endogenous PTEN from HEK293 lysates using a rabbit monoclonal β-arr1/2 antibody D24H9 (+) or a control anti-GST antibody (−). Western blots of immunoprecipitations were performed using β-arr1/2 antibody D24H9 or PTEN 138G6 rabbit monoclonal antibodies and secondary anti-Rabbit Trueblot HRP antibodies (eBioscience). (F) PTEN immunoprecipitates from HEK cells expressing PTEN, PTEN and β-arr1 or PTEN and β-arr2 were analysed for the capacity to dephosphorylate water-soluble diC8-PIP3 (100 μM). *P<0.05. Vector control values (CTL) were subtracted and samples normalized to PTEN alone. Data shown represent mean±s.e.m. of three independent experiments.
Figure 2.
β-Arrestin directly binds and activates PTEN lipid phosphatase activity in vitro. (A, B) GST control or GST–PTEN bound to glutathione-agarose beads was incubated with His6–β-arr1 or His6–β-arr2. GST pulldown samples were analysed on western blot using anti-β-arr1/2 (A), anti-β-arr2 (B) or anti-GST antibodies. Ten percent of His6–β-arr1 and His6–β-arr2 used in the pulldown was loaded as input control. (C) His6–PTEN (40 nM) was incubated alone or with GST, GST–β-arr1 or GST–β-arr2 (100 nM) and assessed for dephosphorylation of diC8-PIP3 (100 μM). Samples were normalized to PTEN alone. Data shown represent mean±s.e.m. of four independent experiments. **P<0.01.
To determine if the association with β-arrs can modulate the principal function of PTEN, namely its lipid phosphatase catalytic activity, we used an in vitro assay that measures the amount of free phosphate liberated from PIP3 (Georgescu et al, 1999; Galan-Moya et al, 2011). The catalytic activity of immunoprecipitated PTEN was increased three-fold in lysates of cells containing PTEN coexpressed with either β-arr1 or β-arr2, indicating that both β-arrs can enhance PTEN's lipid phosphatase activity (Figure 1F; Supplementary Figure S1b). Furthermore, the catalytic activity of purified PTEN towards PIP3 was also enhanced in the presence of either recombinant purified β-arr1 or β-arr2, demonstrating that the direct interaction of β-arrs with PTEN is sufficient to stimulate its lipid phosphatase activity (Figure 2C).
_β_-Arrestin binds to the C2 domain of PTEN
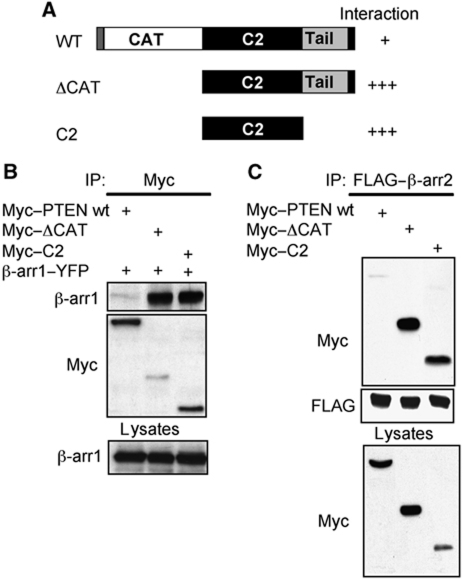
PTEN contains an N-terminal catalytic domain with dual lipid and protein phosphatase activity, and a C-terminal C2 domain that interacts with membrane lipids (Figure 3A; Chalhoub and Baker, 2009) and regulates the effects of PTEN on cell migration (Raftopoulou et al, 2004). In addition, a C-terminal tail region includes multiple phosphorylation sites that regulate PTEN conformation and function (Vazquez et al, 2000; Odriozola et al, 2007; Rahdar et al, 2009). Using truncations of PTEN, we found that β-arr1 and β-arr2 interact in co-immunoprecipitation experiments with both a C-terminal portion of PTEN lacking its catalytic domain (ΔCAT) and the isolated C2 domain (Figure 3A–C). Notably, association between β-arrs and the isolated C2 domain of PTEN was stronger than that observed with the full-length protein, suggesting that the interaction site(s) for β-arrs are ‘masked’ to some extent by PTEN intramolecular interactions under basal conditions, and may be subject to signal-dependent regulation.
Figure 3.
β-Arrestin binds to the C2 domain of PTEN. (A) Schematic representation of PTEN truncations indicating relative association with β-arr. WT: amino acids 1–403; ΔCAT: amino acids 179–403; C2: amino acids 179–355. (B) Western blot of β-arr1 contained in Myc–PTEN truncation immunoprecipitates from lysates of co-transfected COS cells. (C) Western blot of Myc–PTEN truncations contained in FLAG–β-arr2 immunoprecipitates from lysates of co-transfected COS cells.
The RhoA/ROCK signalling pathway controls _β_-arrestin–PTEN interaction
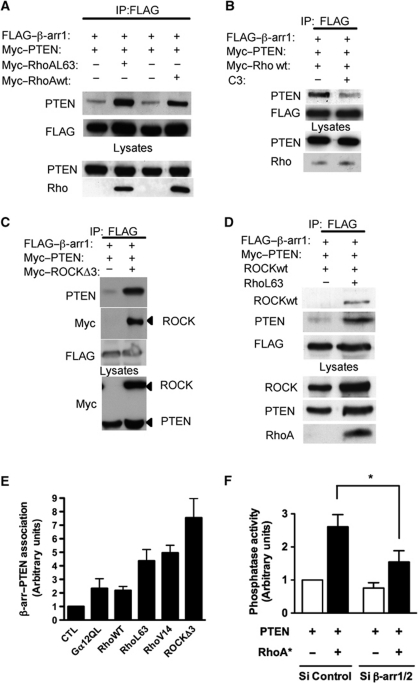
The small GTPase, RhoA was so far one of the few other proteins known to increase PTEN lipid phosphatase activity, via its downstream effector Rho kinase (ROCK) (Li et al, 2005; Papakonstanti et al, 2007). We, therefore, tested if RhoA could modulate PTEN–β-arr interaction. In the presence of constitutively active forms of RhoA (RhoA L63 and RhoA V14), the association between β-arr1 and PTEN was markedly enhanced (Figure 4A and E; Supplementary Figure S2a). Wild-type RhoA also enhanced β-arr–PTEN association, although to a lesser extent than constitutively active RhoA (Figure 4A and E), suggesting that the active state of RhoA promotes this association. Supporting this hypothesis, a cell permeable Rho inhibitor (C3 transferase) decreased β-arr1–PTEN complex formation in the presence of wild-type RhoA (Figure 4B). An active form of the Rho effector ROCK (ROCKΔ3) also produced a marked enhancement in the PTEN–β-arr association and was found to be part of the same molecular complex (Figure 4C and E). Similar data were obtained with wild-type ROCK in the presence of constitutively active RhoA L63 (Figure 4D). We then assessed if β-arrs, in turn, might participate in RhoA-dependent activation of PTEN catalytic activity. In the experiments showing that active RhoA increased PTEN lipid phosphatase activity, the inhibition of endogenous β-arr1/2 expression using RNAi (Supplementary Figure S2b) markedly reduced this effect, indicating that β-arrs are indeed required for RhoA-dependent upregulation of PTEN catalytic activity (Figure 4F).
Figure 4.
A Rho/ROCK signalling pathway controls β-arrestin–PTEN association. (A) Co-immunoprecipitation of FLAG–β-arr1 with PTEN in the presence or absence of wild-type or active RhoA (Q63L) from lysates of co-transfected COS cells. (B) Co-immunoprecipitation of FLAG–β-arr1 with PTEN in the presence or absence of a cell permeable Rho inhibitor (C3 transferase, 2 μg/ml for 6 h). (C) Co-immunoprecipitation of FLAG–β-arr1 with PTEN in the presence or absence of active ROCK (ROCKΔ3) from lysates of co-transfected COS cells. (D) Co-immunoprecipitation of FLAG–β-arr1 with PTEN and wild-type ROCK in the presence or absence of active RhoA-Q63L from lysates of co-transfected COS cells. (E) Graph shows relative β-arr1–PTEN association in the presence of indicated Gα12, Rho or ROCK constructs versus co-immunoprecipitation of β-arr1–PTEN alone (CTL, control). (F) In vitro phosphatase assay of PTEN immunoprecipitated from HEK293 cells expressing PTEN alone or coexpressing PTEN and RhoA-Q63L, transfected with control (CTL) or siRNA directed against β-arrs (β-arr1/2). *P<0.05.
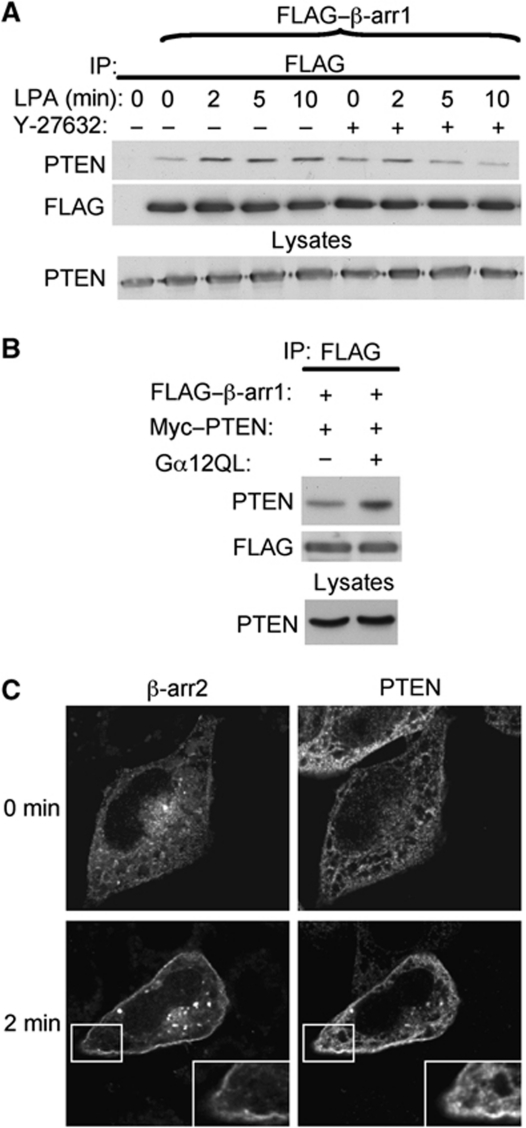
As β-arrs are known to translocate to activated GPCRs and subsequently modulate downstream signalling pathways, we next sought to determine if a receptor that activates RhoA could alter PTEN–β-arr complex formation as well as their subcellular localization. We used as a receptor model the lysophosphatidic acid receptor 1 (LPA1-R), a GPCR that couples to Gα12 and activates RhoA (Ishii et al, 2004), and which is known to recruit β-arr (Urs et al, 2005; Li et al, 2009). Stimulation of exogenous LPA1-R in HEK293 cells expressing Gα12 increased the association between β-arr1 and PTEN within 2 min. The ROCK inhibitor, Y-27632, blunted this effect (Figure 5A) further confirming the facilitating effect of the Rho/ROCK pathway on PTEN–β-arr association. Also, expression of an active form of Gα12 increased PTEN–β-arr association (Figures 4E and 5B). In cells expressing exogenous receptor, PTEN and β-arr2, under basal conditions β-arr2 showed its expected cytoplasmic distribution and PTEN was also predominantly cytoplasmic. Following 2 min LPA1-R stimulation, both β-arr2 and PTEN translocated to the plasma membrane (Figure 5C). Taken together, these data indicate that a receptor-activated RhoA/ROCK pathway positively regulates the β-arr–PTEN interaction and controls the subcellular region where this association occurs.
Figure 5.
Activation of the LPA1-R recruits β-arrestin and PTEN to the plasma membrane and increases association. (A) Co-immunoprecipitation of FLAG–β-arr1 with PTEN following LPA1-R stimulation (10 μM) for the indicated times (min) in HEK cells expressing LPA1-R and Gα12. Cells were also pre-incubated or not with the ROCK inhibitor Y-27632 (10 μM). (B) Co-immunoprecipitation of FLAG–β-arr1 with PTEN in the presence or absence of active Gα12 from lysates of COS cells. (C) HeLa cells transfected with LPA1-R, β-arr2–GFP and PTEN were fixed, permeabilized and processed for fluorescence microscopy using an anti-PTEN antibody (138G6) and revealed with AlexaFluor-568 secondary antibodies. Fluorescence emitted by GFP and red fluorescence corresponding to PTEN staining before and after LPA1-R stimulation are indicated.
_β_-Arrestin cooperates with PTEN to counter regulate the PI3K/Akt signalling pathway
As the lipid phosphatase activity of PTEN, by converting PIP3 to PIP2, prevents Akt activation (pAkt), we next examined the effect of β-arr-mediated PTEN regulation on pAkt levels, using both endogenous and reconstituted cell systems. Coexpression of either β-arr1 or β-arr2 potentiated the inhibition of Akt activation caused by reintroducing PTEN in PTEN-null prostate cancer PC-3 cells (Supplementary Figure S3a, lanes 1–4). As both β-arrs can inhibit PI3K activity in vitro (Wang et al, 2007) and β-arr2 (but not β-arr1) has been shown to form a complex with Akt and its negative regulator PP2A (Beaulieu et al, 2005), we performed experiments to confirm that the inhibitory effect of β-arrs on Akt activity in PC-3 cells does require PTEN. In these cells, a lipid phosphatase-dead mutant of PTEN (G129E) failed to inhibit pAkt levels, and co-transfection of either β-arr1 or β-arr2 had no additional effect on pAkt levels, indicating that the impact of β-arrs on PTEN requires the integrity of its lipid phosphatase activity (Supplementary Figure S3a, lanes 5–7). In addition, transfection of β-arr1 or β-arr2 alone, in the absence of PTEN, had no substantial effect on Akt activity in PC-3 cells (Supplementary Figure S3a, lanes 8–9). Thus, in this cell model both β-arrs do indeed inhibit Akt activation by enhancing the lipid phosphatase activity of PTEN.
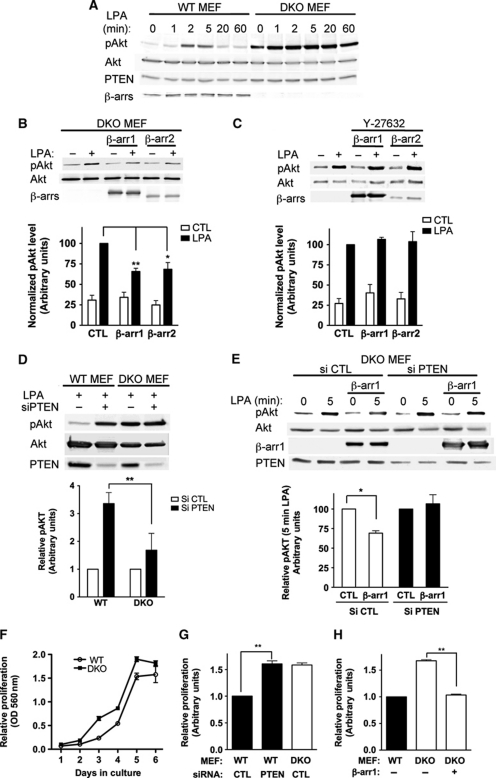
β-arr1/2 knock-out (DKO) MEFs (Kohout et al, 2001) were used to analyse more precisely the effect of endogenous β-arr expression on Akt activity. Following activation of endogenous LPA-R, pAkt levels (both pSer473 and pThr308) increased in WT MEFs, peaking at 2–5 min and then rapidly decreased (Figure 6A; Supplementary Figure S3b). In DKO MEFs, lacking β-arr expression, not only were pAkt levels elevated before stimulation, compared with WT MEFs, they rapidly reached a maximal value after LPA-R stimulation (Figure 6A), which was maintained throughout the time course of the experiment. Thus, loss of endogenous β-arr results in increased Akt activation, downstream of the LPA-R. Importantly, reintroduction of either β-arr1 or β-arr2 into DKO MEFs reduced Akt activation following LPA-R stimulation (Figure 6B; Supplementary Figure S3c), providing further evidence that β-arrs participate in blunting Akt activation downstream of the LPA-R. In addition, pre-incubation of cells with the ROCK inhibitor Y-27632 abrogated the inhibiting effect of β-arrs on Akt activity downstream of LPA-R activation, demonstrating the requirement of a functional Rho/ROCK pathway to achieve this effect (Figure 6C).
Figure 6.
β-Arrestin and PTEN cooperate to inhibit Akt signalling and cell proliferation. (A) Activation of Akt (pSer473) in wild-type or β-arr1/2 KO (DKO) MEFs in response to LPA stimulation (10 μM) for the indicated times (min). (B) Active Akt was monitored in DKO MEFs following transfection with control vector, β-arr1 or β-arr2 plasmids and stimulation with LPA (5 min). Data shown represent the mean±s.e.m. calculated from three independent experiments (**P<0.01; *P<0.05). (C) Active Akt was monitored in DKO MEFs after transfection with control vector, β-arr1 or β-arr2 plasmids and pre-incubation of cells with the ROCK inhibitor (Y-27632, 10 μM). Cells were stimulated with LPA for 5 min. Data shown represent the mean±s.e.m. calculated from three independent experiments. (D) MEF WT and DKO cells were transfected with the indicated siRNA 48 h before LPA stimulation (5 min) and pAkt levels monitored. Data shown represent the mean±s.e.m. calculated from four independent experiments and are normalized to stimulated control cells transfected with control siRNA (**P<0.01). (E) DKO MEFs were co-transfected with the indicated siRNA and control vector or β-arr1-encoding plasmids and pAkt levels were monitored. Data shown represent the mean±s.e.m. calculated from four independent experiments and are normalized to stimulated control cells transfected with control siRNA (*P<0.05). (F) Proliferation of wild-type and DKO MEFs was examined using crystal violet staining. Representative experiment performed in triplicate. (G) MEFs were nucleofected with the indicated siRNA. Proliferation was increased in PTEN siRNA WT MEFs versus CTL transfected cells between day 2 and day 6. Graph shows proliferation at day 4 of the experiment. **P<0.01. (H) MEFs were nucleofected with control or β-arr1-encoding plasmids. Proliferation was inhibited in DKO MEFs transfected with β-arr1. **P<0.01.
In WT MEFs, expressing endogenous β-arr1 and β-arr2, knockdown of PTEN led to an increased pAkt response after LPA stimulation, comparable to that observed in DKO MEFs expressing endogenous PTEN (Figure 6D), indicating that termination of LPA-promoted Akt activation in MEFs necessitates both β-arrs and PTEN. Supporting the cooperation of β-arrs and PTEN in this process, the increased pAkt response in DKO MEFs was not amplified further by siRNA-mediated reduction of PTEN levels (Figure 6D). Finally, in DKO MEFs, reintroduction of β-arr1 caused a decrease in pAkt levels, compared with control transfected cells, but this effect was lost in the case of simultaneous siRNA-mediated reduction of PTEN levels (Figure 6E), confirming that the reduction of pAkt signal elicited by β-arrs does indeed pass through PTEN. To investigate the functional importance of PTEN–β-arr cooperation in inhibiting Akt signalling, we carried out proliferation studies in MEFs. DKO MEFs proliferated faster than their wild-type counterpart over a 6-day time course (Figure 6F). Based on the data above, we hypothesized that if this was caused by enhanced PI3K/Akt signalling, due to the absence of β-arr-dependent PTEN activation, then reducing PTEN expression in WT MEFs would shift their proliferation rate towards that of DKO MEFs. We found that inhibition of PTEN expression did indeed significantly increase the proliferation of WT MEFs (Figure 6G). Conversely, recomplementation of DKO MEFs with β-arr1 significantly inhibited the proliferation of DKO MEFs, shifting them towards the proliferation of WT MEFs (Figure 6H). Taken together, the above data show that β-arrs enhancement of PTEN lipid phosphatase activity can functionally result in the negative regulation of Akt activation and cell proliferation.
_β_-Arrestin regulates the lipid phosphatase-independent effect of PTEN on cell migration
PTEN can also regulate cell function independently of its lipid phosphatase activity, as in the case of its inhibitory effect on glioma cell migration, which occurs through its C2 domain (Raftopoulou et al, 2004). Given that β-arrs interact with this C2 domain, we assessed if β-arrs could also modulate the anti-migratory effect of PTEN, using a wound-healing assay and U373 glioma cells lacking endogenous PTEN. In U373 cells reconstituted with physiological levels of PTEN (U373-PTEN; Kotelevets et al, 2001; Supplementary Figure S4a), and β-arr1, an increased association between PTEN and β-arr1 was observed following wounding and during migration (Figure 7A). Interestingly, the increased β-arr1–PTEN association paralleled an increase in active GTP-bound RhoA levels, documented by the enhanced interaction of RhoA with the Rho binding domain of its effector rhotekin that only captures the GTP-bound form of RhoA (compare Figure 7A and B). Similar results were obtained with endogenous β-arrs and PTEN in primary rat astrocytes (Supplementary Figure S4b). In migration assays, confluent monolayers of wild-type PTEN-null U373 cells growing on glass coverslips were wounded to induce migration, and cells at the leading edge of the wound were microinjected, with a control GFP plasmid, β-arr1–YFP, β-arr2–GFP or wild-type Myc-tagged PTEN. After overnight migration, GFP-, β-arr1–YFP- and β-arr2–GFP-expressing cells remained at the leading edge of the wound, whereas cells injected with PTEN failed to migrate and were overtaken by non-injected control cells (Figure 7C and E). When expression vectors for PTEN and either β-arr were injected in combination, cells regained the capacity to migrate, indicating that both β-arrs could overcome the inhibitory effect of PTEN. Furthermore, both β-arrs also overcame the previously reported (Raftopoulou et al, 2004) inhibitory effect of the isolated C2 domain on U373 cell migration (Figure 7D and E). Taken together, these data demonstrate that both β-arr1 and β-arr2 interact with the C2 domain of PTEN to block its inhibitory effect and thus rescue migration.
Figure 7.
β-Arrestin interacts with the C2 domain of PTEN to regulate U373 glioma cell migration. (A) Western blot of FLAG immunoprecipitates from wounded U373-PTEN cells transiently expressing FLAG–β-arr1. Time following wounding is indicated (h). (B) Relative active RhoA levels were assayed following wounding of U373-PTEN cells transiently expressing β-arr1. Data shown represent the mean±s.e.m. calculated from three independent experiments (C) U373 cells at the leading edge of wounds were microinjected with plasmids encoding GFP; β-arr1–YFP; Myc–PTEN; β-arr1–YFP and Myc–PTEN or GFP and Myc–PTEN, and left to migrate overnight. Cells expressing relevant constructs were detected following staining with a polyclonal anti-Myc antibody and/or direct fluorescence emitted by GFP. All cells were observed by staining with rhodamine-conjugated phalloidin and/or DAPI. The red dotted line indicates the wound edge. The number of cells that were still at the wound edge following migration overnight was quantified. (D) U373 cells at the leading edge of wounds were microinjected with plasmids encoding Myc–PTEN-C2 or β-arr1–YFP and Myc–PTEN-C2. The red dotted line indicates the wound edge. (E) Graph showing quantification of U373 cells found at the edge of the wound after migration overnight. Between 150 and 400 cells were counted for each condition. Data shown represent mean±s.e.m. of 4–6 independent experiments.
A naturally occurring lipid phosphatase defective point mutant of PTEN, Gly129Glu (G129E), was still capable of inhibiting U373 cell migration, whereas a mutant lacking both lipid and protein phosphatase activities, Cys124Ser (C124S), lost the anti-migratory phenotype (Figure 8A and B; Supplementary Figure S5), demonstrating that in addition to the C2 domain, the protein phosphatase activity of PTEN, not the lipid phosphatase activity, is important for the regulation of cell migration (Raftopoulou et al, 2004). A group of phosphorylation sites at Ser380, Thr382, Thr383 and Ser385 in the carboxy-regulatory tail region have previously been reported to control PTEN's conformation and function (Vazquez et al, 2000; Odriozola et al, 2007; Rahdar et al, 2009). In the C124S protein phosphatase-dead form of PTEN, Thr383 is phosphorylated in its regulatory phosphorylation motif and it was therefore proposed that auto-dephosphorylation of Thr383 controls the effect of the C2 domain on migration. Indeed, mutations of Ser380, Thr382, Thr383 and Ser385 together or Thr383 alone in the carboxy-terminal regulatory phosphorylation motif of the catalytically dead C124S PTEN (CS-A4 and CS-T383A, respectively) restored the capacity to inhibit migration by PTEN (Raftopoulou et al, 2004; Figure 8A and B; Supplementary Figure S5). This suggests that in these mutants the C2 domain is active and able to block migration. As β-arrs interact strongly with the C2 domain of PTEN, we next examined β-arr1 association with CS-T383A and CS-A4. Interestingly, whereas, CS-T383A showed increased binding to β-arr1 in co-immunoprecipitation experiments, compared with wild-type PTEN, CS-A4 was defective in β-arr1 binding (Figure 8C). We subsequently tested the effect of β-arr1 on CS-T383A and CS-A4 in the migration assay. Microinjection of β-arr1 with CS-T383A PTEN but not the β-arr-binding defective CS-A4 PTEN was found to restore migration of U373 cells (Figure 8B and D), establishing that β-arr interaction with PTEN is necessary for the release of the brake on cell migration. Taken together, these data suggest that residues in the regulatory motif of PTEN, which control PTEN conformation and C2 domain anti-migratory effect, are also capable of controlling β-arr binding and subsequent effects on migration.
Figure 8.
The carboxy-terminal regulation motif of PTEN controls β-arr1 binding and effects on migration. (A) U373 cells at the leading edge of wounds were microinjected with plasmids encoding β-arr1–YFP; PTEN-G129E; PTEN-C124S; C124S-T383A; C124S-A4; β-arr1–YFP and C124S-T383A or β-arr1–YFP and C124S-A4, and left to migrate overnight. Cells expressing relevant constructs were detected following staining with a polyclonal anti-Myc or anti-PTEN antibody. All cells were observed by staining with rhodamine- or Alexa488-conjugated phalloidin and DAPI. The red dotted line indicates the wound edge. The number of cells that were still at the wound edge following migration overnight was quantified. (B) Graph showing quantification of U373 cells found at the edge of the wound after migration overnight. Between 150 and 200 cells were counted for each condition. Data shown represent mean±s.e.m. of 3–6 independent experiments. (C) Western blot of wild-type PTEN, C124S-T383A or C124S-A4 contained in β-arr1 immunoprecipitates. Data shown represent the mean±s.e.m. calculated from four independent experiments. (D) Cells expressing indicated constructs were detected as in Figure 7A. The red dotted line indicates the wound edge. The number of cells that were still at the wound edge following migration overnight was quantified.
Discussion
The data described here demonstrate that β-arrs are able to interact with and exert control on distinct functional signalling outputs of tumour suppressor PTEN, modulating its effects on cell proliferation and migration. Moreover, while it is now appreciated that PTEN can function through lipid phosphatase-dependent and -independent mechanisms (Chalhoub and Baker, 2009), this is the first time, to our knowledge, that a single PTEN interaction partner has been shown to modulate both events. Recently, the unique arrestin orthologue of C. elegans was found in the same molecular complex with DAF-18, the PTEN orthologue in this organism (Palmitessa and Benovic, 2010). Our present data support the idea that the arrestin–PTEN association is evolutionarily conserved. We further demonstrate that both β-arr1 and β-arr2 interact directly with PTEN to enhance its catalytic lipid phosphatase activity and also show that β-arr–PTEN association is dynamically regulated in mammalian cells.
Our findings establish an important link between cell surface receptor activation and PTEN function. We demonstrate that β-arrs and PTEN are co-recruited to the plasma membrane following LPA-R activation, and that β-arrs act downstream of the LPA-R/Gα12-regulated RhoA/ROCK pathway to enhance the lipid phosphatase activity of PTEN towards PIP3. Following ligand stimulation, other GPCRs, such as the sphingosine 1-phosphate 2 receptor (Sanchez et al, 2005), the thromboxane A2 receptor (Song et al, 2009) and the follicle-stimulating hormone receptor (Dupont et al, 2010), were reported to enhance PTEN lipid phosphatase activity. Interestingly, both the sphingosine 1-phosphate 2 receptor and the thromboxane A2 receptor were shown to regulate PTEN through RhoA. Our data suggest that β-arrs are a key component in the cascade of molecular events involved in GPCR-promoted regulation of PTEN. Since a large number of GPCRs couple to Gα12/13 proteins and activate RhoA (Siehler, 2009), the β-arr-mediated activation of PTEN downstream of GPCRs could physiologically occur in many different tissues. It was recently shown that downstream of angiotensin II receptor stimulation, β-arr1 binds to the RhoA GAP ARHGAP21 to inhibit its RhoA GAP function, promoting an increase in RhoA activity (Barnes et al, 2005; Anthony et al, 2011). An intriguing prospect, therefore, emerges that β-arrs could act both as upstream RhoA regulators and also as downstream RhoA effectors.
Functionally, the β-arr-mediated enhancement of PTEN's lipid phosphatase activity inhibits Akt signalling and cell proliferation. It has already been established that in certain contexts β-arr2 can form a complex with Akt and its negative regulator PP2A (Beaulieu et al, 2005), and that both isoforms may directly regulate PI3K activity (Povsic et al, 2003; Wang et al, 2007). Thus, β-arrs appear as central regulators of the PI3K/Akt pathway, operating at different key nodes of this signalling axis, which modulates both cell growth and survival.
β-arrs are also important modulators of directional migration both in vitro and in vivo (Fong et al, 2002; Walker et al, 2003; Buchanan et al, 2006; DeFea, 2007), interacting with and regulating proteins involved in actin cytoskeleton reorganization (Scott et al, 2006; Coureuil et al, 2010) and polymerization (Zoudilova et al, 2007). Here, the finding that β-arrs interact with the C2 domain of PTEN to release a brake on U373 cell movement, independently of the lipid phosphatase activity of PTEN, represents a new mechanism by which β-arrs control migration. This anti-migratory activity of PTEN is regulated by the protein phosphatase activity of PTEN, which might control the auto-dephosphorylation of Thr383, in its C-terminal regulatory tail. Experiments with the C124S catalytically dead mutant of PTEN allowed us to correlate binding of β-arr with Thr383-dephosphorylated PTEN with the negative control exerted by β-arr on the anti-migratory effect of PTEN. Since the anti-migratory activity of the C2 domain is under control of the C-terminal regulatory tail of PTEN and previous studies have demonstrated extensive intramolecular interactions between the C-tail and the C2 domain (Odriozola et al, 2007; Rahdar et al, 2009) this may indicate that dephosphorylation of Thr383 unmasks β-arr-binding sites(s) on PTEN. However, additional studies are necessary to fully understand the complex mechanisms involved in changes of PTEN conformation and control of cell migration, since the mutation of all four regulatory phosphorylation sites (Ser380, Thr382, Thr383 and Ser385) in catalytically inactive PTEN inhibits β-arr binding.
Additional functional convergence of PTEN- and β-arr-mediated signalling concerns the tumour suppressor p53. PTEN can regulate p53 protein levels and activity through both phosphatase-dependent and -independent mechanisms (Freeman et al, 2003). Several studies have shown that β-arr2 can regulate the Mdm2-dependent inhibition of tumour suppressor p53. Binding of β-arr2 to Mdm2 reduces Mdm2-mediated p53 ubiquitination and degradation (Wang et al, 2003a). Furthermore, β-arr2, via its nuclear export activity (Scott et al, 2002b), titrates Mdm2 out of the nucleus and increases p53 activity (Wang et al, 2003b; Boularan et al, 2007). In light of the interaction between β-arrs and PTEN identified here, β-arrs could also possibly control p53 levels/activity via PTEN-dependent mechanisms and thus serve as major regulators of the p53/PTEN/Mdm2 tumour suppressor–oncoprotein network (Mayo and Donner, 2002). Interestingly, several recent studies have documented alterations of β-arr mRNA and protein levels during breast cancer progression that correlate with poor clinical outcome (Li et al, 2009; Michal et al, 2011).
In summary, our data indicate that β-arrs serve as versatile molecular scaffolds to regulate distinct functional outputs of PTEN that impact on cell proliferation and migration. Finally, as PTEN is a major tumour suppressor that is frequently deregulated in a broad range of human cancers, our findings that β-arrs serve as upstream signalling regulators of PTEN may have important consequences for cancer progression and metastasis.
Materials and methods
Plasmids and reagents
Previously described Myc–PTEN (Raftopoulou et al, 2004) and Myc–Rho constructs in pMyc–RK5 were obtained from Alan Hall. β-arr constructs used in these studies have been described previously (Scott et al, 2002a, 2002b). The β-arr A1CT antibody was a kind gift from RJ Lefkowitz. β-arr1/2 (D24H9), β-arr2 (C16D9), PTEN (138G6), pAkt and total Akt antibodies were from Cell Signaling. GFP and Myc (9E10) antibodies were from Roche. Rabbit polyclonal Myc antibody (ab9106) was from Abcam. LPA, Rabbit polyclonal FLAG antibody and EZview Red anti-FLAG and -Myc agarose Affinity gels were from Sigma. Anti-PTEN mAb (A2B1) was from Santa Cruz and anti-PTEN mAb clone 6H2.1 was from Millipore. Cell penetrating C3 transferase, which inhibits RhoA by ADP ribosylation in the effector-binding domain of the GTPase, was from Cytoskeleton Inc.
Cell culture and transfection
COS, HEK293, HeLa, U373, WT and β-arr1/2 null MEF cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin. PC-3 cells were maintained in RPMI supplemented with 10% FBS and penicillin/streptomycin. The MEF lines were kindly provided by RJ Lefkowitz. COS and HEK293 cells were transfected using GeneJuice (Novagen) and MEF lines with Fugene HD (Roche). The siRNA was transfected either by nucleofection or with DharmaFECT (Dharmacon) and for co-transfection of plasmid and siRNA, X-tremeGENE (Roche) was used. Targeting of PTEN was performed using an siRNA SMARTPOOL from Dharmacon. The double-stranded 5′-ACCUGCGCCUUCCGCUAUG-3′ siRNA sequence was used to simultaneously target both β-arr1 and β-arr2 as previously described (Molla-Herman et al, 2008).
Sos recruitment system yeast two-hybrid screen
Sos recruitment system (Aronheim et al, 1997) library screening was performed as previously described (Scott et al, 2006). Briefly, Cdc25H yeast cells expressing a C-terminally truncated β-arr2 bait, pSosGlyβ-arr2ΔC1 containing amino acids 1–337 (Supplementary Figure S1a) and mGAP (to reduce Ras GTPase false positives obtained in the screen) were transformed with a human thymus cDNA library fused to a myristylation signal (Stratagene) under the control of the galactose-induced pGal1 promoter. Following library transformation, yeast cells were plated onto Leu-/Ura-/Trp-agar plates containing 2% galactose/1% raffinose and placed in an incubator at 25°C for 40 h before being transferred to the non-permissive temperature of 37°C. Clones that grew at 37°C after 5–7 days were considered as potential β-arr interactors, and plasmid DNA was subsequently extracted and sequenced. Using this approach, ∼1.5 × 106 clones were screened. A total of 135 β-arr2-interacting clones were identified, of which one corresponded to a C-terminal clone PTEN (Figure 1A).
Classical yeast two-hybrid assay
The L40 yeast reporter strain containing a LexA-inducible gene, HIS3, was cotransformed with pLexABD and pGal4AD hybrid expression vectors and plated on selective medium. Transformants were subsequently assayed for histidine auxotrophy.
Purification of His6–_β_-arr proteins, GST–PTEN, GST–_β_-arrs and in vitro binding assays
Overnight cultures of BL21(DE3)pLysS (Novagen) transformed with His6-TAT-HA-βarr1 or His6-TAT-HA-βarr2 plasmids were diluted 1/4 and grown for 4 h at 37°C after IPTG induction. Bacteria were resuspended and lysed by sonication in 50 mM Tris, 0.1 mM EDTA, 0.1 mM DTT, 2.5% glycerol, 20 mM imidazole, pH 7.4 supplemented with protease inhibitors (lysis buffer). Insoluble material was eliminated by centrifugation at 10 000 g for 15 min, and the supernatant was applied to Ni-NTA agarose beads (Biorad). The columns were washed with lysis buffer, and bound proteins were eluted with 200 mM imidazole in lysis buffer. Eluted proteins were dialysed into 100 mM Tris using a dialysis membrane (MWCO: 12–14 000). GST–PTEN, GST–β-arr1 or GST–β-arr2 fusion proteins were expressed in BL21(DE3)pLysS (Novagen) and purified on a GSTrap High-Performance column (GE Healthcare) according to the manufacturer's instructions. Products eluted with 10 mM glutathione were desalted on a HiTrap desalting column (GE Healthcare) in PBS. Proteins were characterized by SDS–PAGE and Coomassie staining.
For in vitro binding assays, 1.5 μg of GST–PTEN was immobilized on 20 μl glutathione-agarose beads (Sigma) for 1 h at 4°C in TEN 300 (20 mM Tris, 0.1 mM EDTA, 300 mM NaCl, pH 7.4, supplemented with protease inhibitors). Beads were washed three times in TEN 300. 1.5 μg of His6–β-arr1 or His6–β-arr2 was then added in a final volume of 500 μl of TEN 300. After 1 h at 4°C, beads were washed four times with 1 ml of TEN 300. Complexes were applied on 10% SDS–PAGE gels, and proteins were revealed by western blotting with anti-β-arr1/2 (D24H9) or β-arr2 (C16D9).
PTEN phosphatase assay
Phosphatase assays were performed as described previously (Georgescu et al, 1999; Galan-Moya et al, 2011). For phosphatase assays using purified recombinant proteins, eluted GST–β-arrs were dialysed into 100 mM Tris using a dialysis membrane (MWCO: 12–14 000) and characterized by SDS–Page and Coomassie staining. Purified His6–PTEN (Enzo) was used at 40 nM with 100 nM of purified GST–β-arr1 or GST–β-arr2 in a final volume of 47.5 μl of phosphatase buffer (100 mM Tris, 10 mM DTT). Proteins were incubated 30 min at 4°C before 100 μM water-soluble di-C8-PIP3 (Echelon Biosciences) was added. After 45 min incubation at 37°C, released phosphate was measured using Biomol Green reagent. A similar strategy was used to assess PTEN phosphatase activity following immunoprecipitation from cells.
Proliferation assay
MEF cells were seeded in triplicate at a density of 10 000 cells/well of a 12-well plate. Cells were fixed in cold methanol and stained for 30 min with crystal violet (0.1% in PBS), rinsed repeatedly with dH2O before being dried and solubilized in 10% acetic acid (Corsi et al, 2009). Cell number was estimated by measuring optical density at 560 nm.
Migration assay
Human glioma U373 cells were plated onto coverslips in 4-well plates and grown until confluency. Monolayers were scratched (wounded) with a sterile pipette tip and 1 h later plasmids (0.1–0.35 μg/μl in PBS) were microinjected directly into the nucleus of cells in the first row at the wound edge. An Eppendorf Femtojet and Injectman micromanipulator attached to a Leica DMI 3000 microscope were used for microinjections. Following microinjection, cells were returned to a 5% CO2 incubator and left to migrate for 18 h before being fixed in 4% paraformaldehyde for 10 min and processed for immunofluorescence.
Immunofluorescence
Cells growing on coverslips were fixed in 4% paraformaldehyde-PBS and quenched with 50 mM NH4Cl for 10 min. A 1% BSA-0.05% saponin-PBS (HeLa cells) or a 1%BSA-0.2% Triton X-100-PBS (U373 cells) solution was used to block and permeabilize cells. Cells were incubated with primary and secondary antibodies and/or Alexa Fluor (488 or 594) as indicated in figure legends before being mounted on slides. Confocal images of HeLa cells were taken using a Leica spinning-disk microscope (× 63 oil immersion lens) equipped with a CoolSnap HQ2 CCD camera. Representative images of migrating U373 cells were taken using a Zeiss Axio Observer.Z1 microscope with a dry × 20 objective and CoolSNAP HQ2 CCD camera.
Immunoprecipitation and immunoblotting
Cells growing in 10 cm dishes were lysed in 1 ml of cold lysis buffer (50 mM Hepes (pH 7.4), 250 mM NaCl, 2 mM EDTA, 0.5% NP-40, 10% glycerol supplemented with protease inhibitors; Roche) and clarified by centrifugation at 13 000 r.p.m. for 20 min at 4°C. Following immunoprecipitation, immune complexes were washed extensively, and immunoprecipitated proteins were detected by western blot.
Rho activation assay
RhoA activation was monitored using a commercially available ELISA kit (Cytoskeleton) according to the manufacturer's instructions.
Statistical analysis
All data are expressed as mean±s.e.m. Data were analysed using Student's _t_-test and P<0.05 (at least) was considered as statistically significant.
Supplementary Material
Supplementary Information
Review Process File
Acknowledgments
We thank A Hall, S Narumiya, AJ Ridley and RJ Lefkowitz for providing reagents and J Liotard, O Muntaner and A Thuret for technical assistance. This work was funded by grants from the Association pour la Recherche sur le Cancer (ARC 4954 to MGHS and ARC 3918 to EC), the Agence Nationale pour la Recherche (‘ANR’ BLAN07-3-187842 to SM and ‘ANR’ BLAN07-1-191659 to SEM), Ligue Contre le Cancer (comité de l’Oise to MGHS and SM), The Royal Society (International Joint Project Scheme to MGHS and GSB), CNRS and INSERM.
Author contributions: ELF, HE, EC, LK, CB, LCDG, ECh and MGHS performed experiments; ELF, HE, SEM, SM and MGHS designed experiments; ELF, HE, EC, LK, CB, LA, AB, LCDG, GSB, JAP, ECh, SEM, SM and MGHS analysed data; LK and ECh provided reagents; SM and MGHS wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Anthony DF, Sin YY, Vadrevu S, Advant N, Day JP, Byrne AM, Lynch MJ, Milligan G, Houslay MD, Baillie GS (2011) beta-Arrestin 1 inhibits the GTPase-activating protein function of ARHGAP21, promoting activation of RhoA following angiotensin II type 1A receptor stimulation. Mol Cell Biol 31: 1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronheim A, Zandi E, Hennemann H, Elledge SJ, Karin M (1997) Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Mol Cell Biol 17: 3094–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ (2005) beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem 280: 8041–8050 [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG (2005) An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122: 261–273 [DOI] [PubMed] [Google Scholar]
- Boularan C, Scott MG, Bourougaa K, Bellal M, Esteve E, Thuret A, Benmerah A, Tramier M, Coppey-Moisan M, Labbe-Jullie C, Fahraeus R, Marullo S (2007) beta-arrestin 2 oligomerization controls the Mdm2-dependent inhibition of p53. Proc Natl Acad Sci USA 104: 18061–18066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN (2006) Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci USA 103: 1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub N, Baker SJ (2009) PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 4: 127–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi JM, Houbron C, Billuart P, Brunet I, Bouvree K, Eichmann A, Girault JA, Enslen H (2009) Autophosphorylation-independent and -dependent functions of focal adhesion kinase during development. J Biol Chem 284: 34769–34776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureuil M, Lecuyer H, Scott MG, Boularan C, Enslen H, Soyer M, Mikaty G, Bourdoulous S, Nassif X, Marullo S (2010) Meningococcus Hijacks a beta2-adrenoceptor/beta-Arrestin pathway to cross brain microvasculature endothelium. Cell 143: 1149–1160 [DOI] [PubMed] [Google Scholar]
- DeFea KA (2007) Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol 69: 535–560 [DOI] [PubMed] [Google Scholar]
- Dewire SM, Ahn S, Lefkowitz RJ, Shenoy SK (2007) beta-Arrestins and cell signaling. Annu Rev Physiol 69: 483–510 [DOI] [PubMed] [Google Scholar]
- Dupont J, Musnier A, Decourtye J, Boulo T, Lecureuil C, Guillou H, Valet S, Fouchecourt S, Pitetti JL, Nef S, Reiter E, Crepieux P (2010) FSH-stimulated PTEN activity accounts for the lack of FSH mitogenic effect in prepubertal rat Sertoli cells. Mol Cell Endocrinol 315: 271–276 [DOI] [PubMed] [Google Scholar]
- Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD (2002) Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci USA 99: 7478–7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H (2003) PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3: 117–130 [DOI] [PubMed] [Google Scholar]
- Galan-Moya EM, Le Guelte A, Fernandes EL, Thirant C, Dwyer J, Bidere N, Couraud PO, Scott MG, Junier MP, Chneiweiss H, Gavard J (2011) Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Rep 12: 470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H (1999) The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA 96: 10182–10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii I, Fukushima N, Ye X, Chun J (2004) Lysophospholipid receptors: signaling and biology. Annu Rev Biochem 73: 321–354 [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ (2001) beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA 98: 1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelevets L, van Hengel J, Bruyneel E, Mareel M, van Roy F, Chastre E (2001) The lipid phosphatase activity of PTEN is critical for stabilizing intercellular junctions and reverting invasiveness. J Cell Biol 155: 1129–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP (1999) Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99: 323–334 [DOI] [PubMed] [Google Scholar]
- Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, Mills GB, Babwah AV, Bhattacharya M (2009) Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol Cancer Res 7: 1064–1077 [DOI] [PubMed] [Google Scholar]
- Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D (2005) Regulation of PTEN by Rho small GTPases. Nat Cell Biol 7: 399–404 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Gesty-Palmer D (2010) Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev 62: 305–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE (1999) PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol 9: 125–128 [DOI] [PubMed] [Google Scholar]
- Mayo LD, Donner DB (2002) The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci 27: 462–467 [DOI] [PubMed] [Google Scholar]
- Michal AM, Peck AR, Tran TH, Liu C, Rimm DL, Rui H, Benovic JL (2011) Differential expression of arrestins is a predictor of breast cancer progression and survival. Breast Cancer Res Treat (e-pub ahead of print 12 February 2011; doi:10.1007/s10549-011-1374-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla-Herman A, Boularan C, Ghossoub R, Scott MG, Burtey A, Zarka M, Saunier S, Concordet JP, Marullo S, Benmerah A (2008) Targeting of beta-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS ONE 3: e3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL (2007) Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69: 451–482 [DOI] [PubMed] [Google Scholar]
- Mounir Z, Krishnamoorthy JL, Robertson GP, Scheuner D, Kaufman RJ, Georgescu MM, Koromilas AE (2009) Tumor suppression by PTEN requires the activation of the PKR-eIF2alpha phosphorylation pathway. Sci Signal 2: ra85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odriozola L, Singh G, Hoang T, Chan AM (2007) Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem 282: 23306–23315 [DOI] [PubMed] [Google Scholar]
- Palmitessa A, Benovic JL (2010) Arrestin and the multi-PDZ domain-containing protein MPZ-1 interact with phosphatase and tensin homolog (PTEN) and regulate Caenorhabditis elegans longevity. J Biol Chem 285: 15187–15200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstanti EA, Ridley AJ, Vanhaesebroeck B (2007) The p110delta isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J 26: 3050–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povsic TJ, Kohout TA, Lefkowitz RJ (2003) Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem 278: 51334–51339 [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A (2004) Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303: 1179–1181 [DOI] [PubMed] [Google Scholar]
- Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN (2009) A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci USA 106: 480–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Carracedo A, Pandolfi PP (2008) Tenets of PTEN tumor suppression. Cell 133: 403–414 [DOI] [PubMed] [Google Scholar]
- Sanchez T, Thangada S, Wu MT, Kontos CD, Wu D, Wu H, Hla T (2005) PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc Natl Acad Sci USA 102: 4312–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MG, Benmerah A, Muntaner O, Marullo S (2002a) Recruitment of activated G protein-coupled receptors to pre-existing clathrin-coated Pits in living cells. J Biol Chem 277: 3552–3559 [DOI] [PubMed] [Google Scholar]
- Scott MG, Le Rouzic E, Perianin A, Pierotti V, Enslen H, Benichou S, Marullo S, Benmerah A (2002b) Differential nucleocytoplasmic shuttling of beta-arrestins. Characterization of a leucine-rich nuclear export signal in beta-arrestin2. J Biol Chem 277: 37693–37701 [DOI] [PubMed] [Google Scholar]
- Scott MG, Pierotti V, Storez H, Lindberg E, Thuret A, Muntaner O, Labbé-Jullié C, Pitcher JA, Marullo S (2006) Cooperative regulation of ERK activation and cell shape change by Filamin A and ß-arrestins. Mol Cell Biol 26: 3432–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehler S (2009) Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J Pharmacol 158: 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Zhang M, Wang S, Xu J, Choi HC, Zou MH (2009) Thromboxane A2 receptor activates a Rho-associated kinase/LKB1/PTEN pathway to attenuate endothelium insulin signaling. J Biol Chem 284: 17120–17128 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Urs NM, Jones KT, Salo PD, Severin JE, Trejo J, Radhakrishna H (2005) A requirement for membrane cholesterol in the beta-arrestin- and clathrin-dependent endocytosis of LPA1 lysophosphatidic acid receptors. J Cell Sci 118: 5291–5304 [DOI] [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers WR (2000) Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol 20: 5010–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite KA, Eng C (2002) Protean PTEN: form and function. Am J Hum Genet 70: 829–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ (2003) Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest 112: 566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Gao H, Ni Y, Wang B, Wu Y, Ji L, Qin L, Ma L, Pei G (2003a) Beta-arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. J Biol Chem 278: 6363–6370 [DOI] [PubMed] [Google Scholar]
- Wang P, Kumar P, Wang C, Defea KA (2007) Differential regulation of class IA phosphoinositide 3-kinase catalytic subunits p110 alpha and beta by protease-activated receptor 2 and beta-arrestins. Biochem J 408: 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wu Y, Ge X, Ma L, Pei G (2003b) Subcellular localization of beta-arrestins is determined by their intact N domain and the nuclear export signal at the C terminus. J Biol Chem 278: 11648–11653 [DOI] [PubMed] [Google Scholar]
- Wang X, Jiang X (2008) Post-translational regulation of PTEN. Oncogene 27: 5454–5463 [DOI] [PubMed] [Google Scholar]
- Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR III, Lefkowitz RJ (2007) Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA 104: 12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA (2007) Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem 282: 20634–20646 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File