Effects of Telmisartan on Glucose Levels in People at High Risk for Cardiovascular Disease but Free From Diabetes: The TRANSCEND study (original) (raw)
Abstract
OBJECTIVE
Several large clinical trials suggest that ACE inhibitors may reduce the incidence of diabetes. Less is known about the effects of angiotensin receptor blockers (ARBs) on reducing incident diabetes or leading to regression of impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) to normoglycemia.
RESEARCH DESIGN AND METHODS
Participants were 3,488 adults at high risk for cardiovascular disease but free from diabetes (mean age 67 years; 61% male) in the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) study. The participants were randomized to the ARB telmisartan 80 mg (n = 1,726) or placebo (n = 1,762) in addition to usual care.
RESULTS
During a median 56 months, 21.8% of participants treated with telmisartan and 22.4% of those on placebo developed diabetes (relative ratio 0.95 [95% CI 0.83–1.10]; P = 0.51). Participants originally diagnosed with IFG and/or IGT were equally likely to regress to normoglycemia (26.9 vs. 24.5%) or to progress to incident diabetes (20.1 vs. 21.1%; P = 0.59) on telmisartan or placebo.
CONCLUSIONS
There was no evidence that addition of the ARB telmisartan to usual care prevents incident diabetes or leads to regression of IFG or IGT in people at high risk for cardiovascular disease but free from diabetes.
Meta-analyses of hypertension studies show that blockade of the renin angiotensin system by ACE inhibitors and angiotensin receptor blockers (ARBs) is more effective than other classes of blood pressure–lowering medications for the prevention of incident diabetes (1). It is less clear whether renin angiotensin system blockade, as compared with placebo, reduces the risk of incident diabetes when added to usual care therapy in people at high risk for cardiovascular disease or for diabetes. In the Heart Outcomes Prevention Evaluation (HOPE) trial (2), the ACE inhibitor ramipril decreased the risk of self-reported incident diabetes (3.6 vs. 5.4%; relative ratio [RR] 0.66 [95% CI 0.51–0.85]; P < 0.001). In the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial (3), the ARB valsartan decreased the risk of incident diabetes (33.1 vs. 36.8%; RR 0.86 [0.80–0.92]; P < 0.001). In contrast, the Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication (DREAM) study (4), which was designed to specifically study the effects of renin angiotensin system blockade on diabetes prevention, found that inhibition with ramipril did not reduce incident diabetes in people with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) (17.1 vs. 18.5%; RR 0.91 [0.80–1.03]; P = 0.15).
The Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) (5) offers the opportunity to examine the impact of renin angiotensin system inhibition by the ARB telmisartan on incident diabetes and whether this drug may normalize IGT. Telmisartan is of particular interest in this respect because it is believed to be more effective than other ARBs in ameliorating glucose metabolism and insulin resistance (6,7). TRANSCEND contained 3,488 participants without diabetes at baseline. In these participants, we investigated whether addition of telmisartan to usual care reduced the risk of incident diabetes as compared with placebo, a prespecified secondary outcome of the trial. Our study hypothesis was that the ARB telmisartan would decrease incident diabetes. We also determined whether the use of telmisartan leads to regression of IFG and/or IGT, which was not a prespecified outcome.
RESEARCH DESIGN AND METHODS
The design of TRANSCEND has been previously described (5). In brief, all participants had a history of intolerance to ACE inhibitors and were men and women aged ≥55 years with coronary, peripheral, or cerebrovascular disease or diabetes with end organ damage (retinopathy, left ventricular hypertrophy, and albuminuria). Excluded were those in need of or unable to discontinue renin angiotensin system inhibition or who had known hypersensitivity to ARBs. People with symptomatic heart failure, significant primary valvular or cardiac outflow tract obstruction, constrictive pericarditis, complex congenital heart disease, syncope of unknown etiology, planned cardiac surgery or percutaneous coronary intervention within the previous 3 months, uncontrolled hypertension on treatment, heart transplantation, stroke as the result of subarachnoid hemorrhage, significant renal artery stenosis, creatinine levels ≥3.0 mg/dL (>265 μmol/L), hepatic dysfunction, and macroalbuminuria were excluded.
TRANSCEND was conducted in 630 clinics in 40 countries. The study was coordinated at the Population Health Research Institute, McMaster University, and Hamilton Health Sciences, Hamilton, Ontario, Canada. The trial was approved by each center’s ethics committee. All participants provided written informed consent.
Run in and randomization
Eligible subjects were entered into a single blind run-in period involving placebo daily for 1 week followed by 2 weeks of telmisartan 80 mg daily. At the end of this period, they were randomized in a 1:1 ratio by use of an automated system to telmisartan or placebo. Of the 6,666 subjects who entered run in, 5,926 were randomized (2,954 to telmisartan and 2,972 to placebo). Follow-up visits were at 6 weeks, 6 months, and then every 6 months until study closeout. Both participants and trialists were blinded to treatment allocation.
Diagnosis of diabetes, IFG, and IGT
Diabetes was diagnosed by fasting glucose (FG) >125 mg/dL (>6.9 mmol/L) or 2-h glucose >199 mg/dL (>11.1 mmol/L) on an oral glucose tolerance test (OGTT) at clinic visit, or new diabetes reported by physician. OGTTs were performed at study entrance, year 2, and penultimate follow-up visit before scheduled closeout. Physician report of diabetes was based on FG level >125 mg/dL in the office, 2-h OGTT >199 mg/dL on OGTT done in the doctor’s office, A1C 1.1 times the upper limit of normal, or use of antihyperglycemic agents. The physician reports were not verified. Physician visits were every 6 months.
Participants with a clinic FG value 100–125 mg/dL (5.6–6.9 mmol/L) or FG 100–125 mg/dL on OGTT were considered to have IFG. Those who had a 2-h glucose level of 140–199 mg/dL (7.8–11.1 mmol/L) on OGTT were defined as having IGT.
Statistical analysis
Continuous data are given as mean and SDs and categorical data as actual frequencies and percentages. The primary analyses used a time-to-event approach and included all randomized participants without diabetes. The effect of treatment on incident diabetes was compared among participants with normal glucose and IFG and/or IGT at baseline. Treatment comparisons with regard to time-to-event related data are shown as hazard ratios (HRs) with 95% CI. Consistency of treatment effects in different subgroups was explored by Cox regression model, with tests for interaction. We used χ2 test to compare the proportions of participants with IFG and/or IGT at baseline who regressed to normal, remained IFG and/or IGT, and developed to diabetes at 2 years between telmisartan and placebo. For those participants who regressed to normal at 2 years, their status at 5 years was also compared. Analyses were carried out using SAS Version 8.2 (SAS Institute Inc., Cary, NC). A two-tailed P < 0.05 was considered statistically significant.
The study was designed and conducted by the steering committee. The study sponsor received the data only after the study had been completed. All data were received, checked, and analyzed independently by the Population Health Research Institute for the duration of the trial.
RESULTS
Of the 5,926 TRANSCEND participants, 2,438 (41.4%) were excluded because of a diagnosis of diabetes at baseline, an FG >125 mg/dL at the run-in visit, and/or a baseline FG >125 mg/dL and/or 2-h glucose >199 mg/dL on OGTT. The mean age of the cohort without diabetes at baseline was 67 years, 39% was female, the majority was European, 80% had a history of coronary artery disease, and 72% had hypertension (Table 1). There were no significant differences between subjects randomized to telmisartan (n = 1,726) or to placebo (n = 1,762) in terms of demographic, laboratory, national origin, glycemic status, prevalent cardiovascular disease, smoking status, and concomitant medication use.
Table 1.
Baseline characteristics of TRANSCEND participants without diabetes at baseline categorized by assigned treatment
| Telmisartan (n = 1,726) | Placebo (n = 1,762) | |
|---|---|---|
| Demographic | ||
| Age (years) | 66.9 (7.5) | 67.1 (7.5) |
| Blood pressure (mmHg) | 140.0 (17.0)/82.0 (10.0) | 140.2 (16.3)/82.1 (10.1) |
| Sex (female) | 680 (39.4) | 689 (39.1) |
| BMI (kg/m2) | 27.6 (4.2) | 27.5 (4.2) |
| Waist-to-hip ratio | 0.9 (0.1) | 0.9 (0.1) |
| Heart rate (bpm) | 67.2 (11.5) | 67.2 (11.9) |
| Laboratory | ||
| Total cholesterol (mg/dL) | 195.0 (43.2) | 195.0 (43.2) |
| LDL cholesterol (mg/dL) | 116.2 (37.8) | 116.6 (38.2) |
| HDL cholesterol (mg/dL) | 49.8 (13.1) | 50.2 (14.7) |
| Triglycerides (mg/dL) | 145.1 (85.8) | 146.9 (83.2) |
| FG mean (mg/dL) | 95.9 (12.3) | 95.7 (12.1) |
| FG median (mg/dL) | 95.5 | 95.5 |
| FG interquartile range (mg/dL) | 87.7–102.9 | 88.3–104.0 |
| 2-h Glucose mean (mg/dL) | 120.9 (32.8) | 120.4 (32.6) |
| 2-h Glucose median (mg/dL) | 117.1 | 117.1 |
| 2-h Glucose interquartile range (mg/dL) | 96.9–142.3 | 95.5–142.3 |
| Creatinine (mg/dL) | 1.22 (0.3) | 1.22 (0.3) |
| Potassium (mg/dL) | 4.36 (0.44) | 4.34 (0.45) |
| Microalbuminuria (%) | 128 (7.4) | 114 (6.5) |
| Macroalbuminuria (%) | 8 (0.5) | 16 (0.9) |
| Ethnicity (%) | ||
| Asian | 335 (19.4) | 327 (18.6) |
| Arab | 15 (0.9) | 14 (0.8) |
| African | 23 (1.3) | 22 (1.2) |
| European | 1,125 (65.2) | 1,152 (65.4) |
| Native or Aboriginal | 208 (12.1) | 224 (12.7) |
| Other | 20 (1.2) | 23 (1.3) |
| Prevalent cardiovascular disease (%) | ||
| Coronary artery disease | 1,376 (79.7) | 1,412 (80.1) |
| Myocardial infarction | 887 (51.4) | 911 (51.7) |
| Angina pectoris | 868 (50.3) | 877 (49.8) |
| Stable | 679 (39.3) | 694 (39.4) |
| Unstable | 291 (16.9) | 257 (14.6) |
| Stroke or transient ischemic attack | 442 (25.6) | 428 (24.3) |
| Peripheral artery disease | 205 (11.9) | 190 (10.8) |
| Hypertension | 1,253 (72.6) | 1,255 (71.2) |
| Left ventricular hypertrophy | 193 (11.2) | 208 (11.8) |
| Coronary artery bypass grafting | 344(19.9) | 342 (19.4) |
| Percutaneous transluminal coronary angioplasty | 507 (29.4) | 513 (29.1) |
| Smoking status (%) | ||
| Current smoker | 187 (10.8) | 192 (10.9) |
| Past smoker | 799 (46.3) | 819 (46.5) |
| Medications at baseline (%) | ||
| Statin | 990 (57.4) | 1,012 (57.4) |
| Fibrate | 47 (2.7) | 61 (3.5) |
| Estrogens with or without progesterone (women) | 58 (8.5) | 63 (9.1) |
| β-Blocker | 1,062 (61.5) | 1,062 (60.3) |
| Aspirin | 1,360 (78.8) | 1,381 (78.4) |
| Clopidogrel or ticlopidine | 204 (11.8) | 202 (11.5) |
| Antiplatelet agent | 1,449 (84.0) | 1,470 (83.4) |
| Diuretic | 520 (30.1) | 509 (28.9) |
| Calcium channel blocker | 638 (37.0) | 679 (38.5) |
During a median follow-up of 56 months (interquartile range 51–64 months), 16.5% of telmisartan-treated and 19.1% of placebo-treated participants permanently discontinued study medication (P = 0.043). In addition, 5.1 and 7.0%, respectively, of participants were taking open-label, nonstudy ARB medications by study end (P = 0.027).
IFG and/or IGT
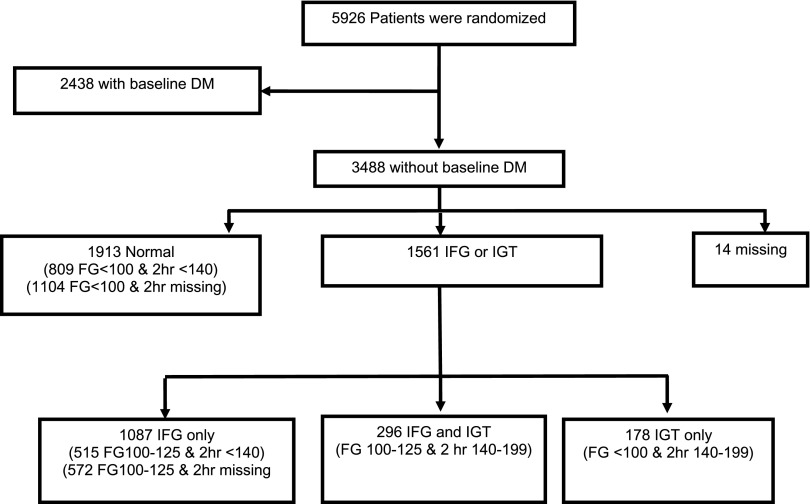
As shown in Fig. 1, of the 3,488 participants at baseline without diabetes, there were 1,383 (39.7%) with IFG, of whom 296 (21.4%) also had IGT. Of the 2,091 with baseline FG levels <100 mg/dL (59.9%), 178 (8.5%) had IGT. In total, there were 809 + 1,104 = 1,913 participants with FG <100 mg/dL and either a 2-h glucose <140 mg/dL or no evidence of raised 2-h glucose. There were 515 + 572 = 1,087 with IFG only, 296 with IFG and IGT, and 178 with IGT only. There was a total of 1,561 (44.8% of the cohort without diabetes at baseline) with IFG and/or IGT.
Figure 1.
Prevalence of IFG and/or IGT in the nondiabetic TRANSCEND cohort at baseline. DM, diabetes mellitus.
Incident diabetes
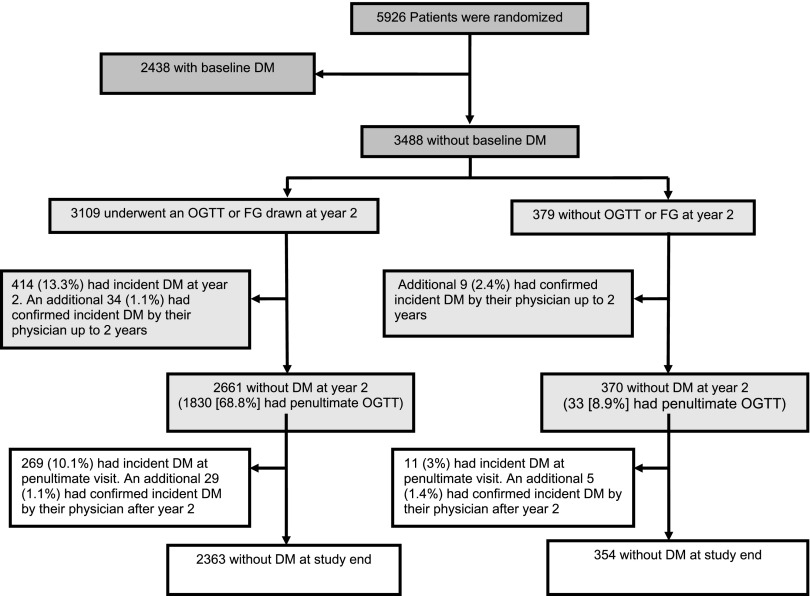
As shown in Fig. 2, of the 3,488 nondiabetic participants, 3,109 (89.1%) underwent an OGTT at year 2 of follow-up and/or had an FG level drawn at the year 2 clinic visit. Of these, 414 (13.3%) had incident diabetes. An additional 34 (1.1%) participants had incident diabetes based on a physician report by year 2. From among the remaining 2,661 participants, 269 (10.1%) were determined to have incident diabetes by OGTT or clinic FG level at the penultimate visit. Of the remaining 2,392 without diabetes (of whom 1,622 had a penultimate OGTT), an additional 29 (1.2%) were reported to have diabetes by their physician after the year 2 visit.
Figure 2.
Incident diabetes in the nondiabetic TRANSCEND cohort. The criteria for physician diagnosis of diabetes (during every 6-month visit) were predefined as one or more of the following: 1) use of glucose-lowering agents, 2) HbA1c 1.1 times the upper limit of normal, and 3) FG >125 mg/dL (>6.9 mmol/L) with performance of a confirmatory OGTT with cutoff criteria identical to those in the main study. Participants who did not satisfy any of the OGTT criteria or the FG criterion were considered free of diabetes. DM, diabetes mellitus.
From among the 379 participants without an OGTT or FG value at the year 2 visit, 9 (2.4%) were reported to have diabetes by their physician up until the year 2 visit. Of the remaining 370 participants, 11 (3.0%) had incident diabetes based on OGTT or FG at the penultimate visit. An additional five participants were reported to have incident diabetes by their treating physician after year 2.
In total, there were 771 (22.1%) participants in the baseline nondiabetic cohort who developed incident diabetes.
Risk of incident diabetes categorized by medication assignment
Table 2 shows that 21.8% of participants treated with telmisartan and 22.4% on placebo developed incident diabetes (HR 0.95 [95% CI 0.83–1.10]; P = 0.51) during follow-up. In subgroup analysis, participants with normal glucose status and those with IFG and/or IGT had similar HR estimates (interaction P = 0.19). Supplementary Fig. 1 shows the cumulative rates of incident diabetes categorized by risk factors for diabetes—waist-to-hip ratio, BMI, presence of hypertension, presence of albuminuria, and use of β-blockers or diuretics. There were no differences between subjects randomized to telmisartan or placebo.
Table 2.
Cumulative incidence of diabetes and regression of IFG and/or IGT in TRANSCEND participants without diabetes at baseline
| Telmisartan | Placebo | HR (95% CI) | P value | |
|---|---|---|---|---|
| Total nondiabetic cohort at baseline | n = 1,726 376 (21.8) | n = 1,762 395 (22.4) | 0.95 (0.83–1.10) | 0.51 |
| FG <100 mg/dL and 2-h glucose <140 mg/dL at baseline* | n = 951 118 (12.4) | n = 962 134 (13.9) | 0.85 (0.67–1.09) | 0.21 |
| IFG and/or IGT at baseline | n = 769 258 (33.6) | n = 792 259 (32.7) | 1.05 (0.88–1.24) | 0.61 |
Regression or progression of IFG and/or IGT
Table 3 shows that at year 2, there was no significant difference in the proportion of participants with IFG and/or IGT who regressed to normal glucose status on telmisartan (26.9%) as compared with placebo (24.5%). Likewise, a similar proportion progressed to incident diabetes in the two treatment groups (20.1 vs. 21.1%, respectively). Of those with IFG and/or IGT at baseline who regressed to normal glucose at year 2, there were no differences in glucose status at year 5 between treatment groups.
Table 3.
Glucose status at 2 years and 5 years of TRANSCEND participants with IFG and/or IGT at baseline
| Telmisartan | Placebo | P value | |
|---|---|---|---|
| Glucose status at 2 years of participants with IFG/IGT at baseline | |||
| IFG/IGT at baseline | 769 | 792 | |
| Known glucose status at 2 years | 706 | 705 | |
| Regression to normal at 2 years | 190 (26.9) | 173 (24.5) | |
| Remains IFG/IGT at 2 years | 374 (53.0) | 383 (54.3) | |
| Develops diabetes at 2 years | 142 (20.1) | 149 (21.1) | 0.59 |
| Glucose status at 5 years of participants with IFG/IGT at baseline and regression to normal at 2 years | |||
| IFG/IGT at baseline with regression to normal at 2 years | 190 | 173 | |
| Known glucose status at 5 years | 169 | 145 | |
| Remains normal at 5 years | 59 (34.9) | 57 (39.3) | |
| Develops IFG/IGT at 5 years | 95 (56.2) | 72 (49.7) | |
| Develops diabetes at 5 years | 15 (8.9) | 16 (11.0) | 0.49 |
CONCLUSIONS
In this study of nondiabetic adults at high risk for cardiovascular disease, there was no evidence that the addition of telmisartan to ongoing therapy prevented the development of incident diabetes. This finding was not consistent with our study hypothesis. Telmisartan also neither prevented a deterioration of glucose tolerance over time nor improved glucose status in participants with IFG and/or IGT.
Our findings contrast with those of NAVIGATOR (3), the only other ARB-based study to examine the impact of renin angiotensin system blockade on incident diabetes. Several factors may explain this discrepancy. NAVIGATOR, comprising >4,600 participants in each arm, was much larger than TRANSCEND, which limited the power to detect a small risk reduction. We estimated there was only 36% power to detect a 10% risk reduction of diabetes with telmisartan compared with placebo. Second, NAVIGATOR was a study of people with IGT, whereas the present population contained a majority of people with normal glucose tolerance and thereby at lower risk for incident diabetes. Third, participants in TRANSCEND were more likely than those in NAVIGATOR to be on β-blockers (∼60 vs. 40%), which are known to raise blood glucose (8), possibly hindering any glucose sparing effect of telmisartan. Fourth, TRANSCEND included older individuals (by ∼4 years) with cardiovascular disease (∼80%) than did NAVIGATOR (∼24%). Both age and cardiovascular disease increase insulin resistance (9,10), making it more difficult to detect an effect of an ARB on glucose metabolism. Last, TRANSCEND included more ethnic groups at higher risk of glucose disorders (e.g., Asians) (11) than NAVIGATOR, which enrolled predominantly Caucasians.
Our results also contrast with those of hypertension studies that reported lower incident diabetes rates with renin angiotensin system blockade, mainly ACE inhibitors (4,12). It should be noted that many of these studies were post hoc analyses in which incident diabetes was not a prespecified outcome. The results relied on physician-reported or self-reported incident diabetes and did not measure FG or obtain 2-h postchallenge levels. This makes it likely that many subjects with seemingly normal glucose metabolism would have been diagnosed with new-onset diabetes if thoroughly investigated (13,14). Last, several studies reported lower incident diabetes rates with renin angiotensin system blockade compared with β-blockers and thiazide diuretics, medications which are known to increase glucose levels.
ARBs are believed to be insulin sensitizing through their effects on muscle, microcirculation, and pancreatic β-cells (12). Telmisartan is also believed to be able to activate peroxisome proliferator–activated receptor-γ, a nuclear receptor regulator of carbohydrate metabolism (6). This effect is due to telmisartan’s structural similarity with pioglitazone, a proliferator–activated receptor-γ agonist used for the treatment of diabetes (7). Other ARBs do not have this structural characteristic. Despite these potential advantages, we found no statistically significant impact of telmisartan on glucose disorders in this study. These findings are supported by a recent clinical study of individuals with insulin resistance (15), which failed to demonstrate an effect of telmisartan on measures of glucose metabolism and insulin sensitivity. In addition, another study of the ARB valsartan did not show improvement in insulin responsiveness in subjects with IGT (16). As such, our results are consistent with other studies.
TRANSCEND has advantages, including rigorous determination of diabetes status, complete ascertainment of end points, and ethnic diversity. A limitation should be noted. There was a relatively high rate of discontinuations from assigned medications. The rate of discontinuation was also unequal (16.5% of telmisartan-treated vs. 19.1% of placebo-treated participants). This would have the effect of muting any clinical effect and diluting our ability to detect small differences between telmisartan and placebo therapies.
In closing, it would be of benefit were treatment of diabetes-related comorbidities or risk factors able to lower glucose levels or prevent diabetes from developing. This would be especially attractive for “nondiabetic drugs” (17). In this study, no evidence for such was found with the addition of telmisartan to usual therapy in people without diabetes but at high risk for cardiovascular disease.
Supplementary Material
Supplementary Data
Acknowledgments
Boehringer Ingelheim funded the study. No other potential conflicts of interest relevant to this article were reported.
J.I.B. conceptualized and designed the study; acquired, analyzed, and interpreted data; and drafted the manuscript. P.G. analyzed and interpreted data. L.R. conceptualized and designed the study, acquired data, and drafted the manuscript. H.S. conceptualized and designed the study and analyzed and interpreted data. J.P. conceptualized and designed the study and critically revised the manuscript for important intellectual content. P.C., A.D., R.F., M.K., and E.P. acquired data and critically revised the manuscript for important intellectual content. S.Y. conceptualized and designed the study; acquired, analyzed, and interpreted data; and drafted and critically revised the manuscript for important intellectual content. K.T. conceptualized and designed the study; acquired, analyzed, and interpreted data; and drafted the manuscript.
The authors thank Hertzel Gerstein, MD, Department of Medicine and Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Ontario, Canada, for critical review of the manuscript and for helpful comments.
Footnotes
References
- 1.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2007;369:201–207 [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Gerstein H, Hoogwerf B, et al. ; HOPE Study Investigators. Ramipril and the development of diabetes. JAMA 2001;286:1882–1885 [DOI] [PubMed] [Google Scholar]
- 3.Holman RR, Haffner SM, McMurray JJ, et al. ; NAVIGATOR Study Group. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1463–1476 [DOI] [PubMed] [Google Scholar]
- 4.Bosch J, Yusuf S, Gerstein HC, et al. ; DREAM Trial Investigators. Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551–1562 [DOI] [PubMed] [Google Scholar]
- 5.Teo K, Yusuf S, Sleight P, et al. ; ONTARGET/TRANSCEND Investigators. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J 2004;148:52–61 [DOI] [PubMed] [Google Scholar]
- 6.Vitale C, Mercuro G, Castiglioni C, et al. Metabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndrome. Cardiovasc Diabetol 2005;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamana A, Arita M, Furuta M, Shimajiri Y, Sanke T. The angiotensin II receptor blocker telmisartan improves insulin resistance and has beneficial effects in hypertensive patients with type 2 diabetes and poor glycemic control. Diabetes Res Clin Pract 2008;82:127–131 [DOI] [PubMed] [Google Scholar]
- 8.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med 2000;342:905–912 [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA. The Claude Bernard Lecture 2009: Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links.. Diabetologia 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr 2007;2:183–189 [DOI] [PubMed] [Google Scholar]
- 11.Bhopal RS, Rafnsson SB. Could mitochondrial efficiency explain the susceptibility to adiposity, metabolic syndrome, diabetes and cardiovascular diseases in South Asian populations? Int J Epidemiol 2009;38:1072–1081 [DOI] [PubMed] [Google Scholar]
- 12.Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens 2005;23:463–473 [DOI] [PubMed] [Google Scholar]
- 13.Norhammar A, Tenerz Å, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 2002;359:2140–2144 [DOI] [PubMed] [Google Scholar]
- 14.Bartnik M, Rydén L, Ferrari R, et al. ; Euro Heart Survey Investigators. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. Eur Heart J 2004;25:1880–1890 [DOI] [PubMed] [Google Scholar]
- 15.Hsueh W, Davidai G, Henry R, Mudaliar S. Telmisartan effects on insulin resistance in obese or overweight adults without diabetes or hypertension. J Clin Hypertens (Greenwich) 2010;12:746–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bokhari S, Israelian Z, Schmidt J, Brinton E, Meyer C. Effects of angiotensin II type 1 receptor blockade on β-cell function in humans. Diabetes Care 2007;30:181. [DOI] [PubMed] [Google Scholar]
- 17.Paulweber B, Valensi P, Lindström J, et al. A European evidence-based guideline for the prevention of type 2 diabetes. Horm Metab Res 2010;42(Suppl. 1):S3–S36 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data